Introduction
SIRT1 is a nicotinamide adenine dinucleotide
(NAD)-dependent histone deacetylase that has been reported to play
an important role in a variety of physiological processes including
aging, metabolism, cell survival and carcinogenesis (1–6).
Moreover, there is growing evidence strongly suggesting that SIRT1
has a role in cancer progression (7–14).
SIRT1 suppresses apoptosis and cell senescence and also promotes
cell migration and invasion (14–18),
angiogenesis (19,20), and drug resistance in multiple
models (7–10). In vivo studies show that
SIRT1 inhibition suppresses tumor growth, metastasis and
progression in several cancer types, including prostate, breast and
neuroblastoma (11,12,14).
In addition, SIRT1 overexpression has been shown to correlate with
poor prognosis in several cancer types, including large B-cell
lymphoma (21), prostate cancer
(18,22), pancreatic cancer (23), gastric cancer (24,25),
breast cancer (26),
hepatocellular carcinoma (27),
colorectal cancer (28) and lung
cancer (29). Collectively, these
results suggest an important role for SIRT1 in cancer growth and
progression. SIRT1 inhibitors are therefore of significant interest
as potential therapeutic agents.
Several inhibitors of SIRT1 have been reported,
including nicotinamide (30),
sirtinol (31), cambinol (32), EX-527 (33), Tenovin-6 (34), splitomycin (35), toxoflavin (36), salermide (37), 2-anilinobenzamides (38) among other compounds. These SIRT1
inhibitors can induce selective cytotoxicity in cancer cells in
vitro (31,32,34–36,38,39).
In addition, several SIRT1 inhibitors have been tested in cancer
xenograft mouse models (32,34,40).
Cambinol was well tolerated in mice and significantly inhibited the
growth of Burkitt lymphoma xenografts (32). Tenovin-6 suppressed tumorigenesis
of melanoma and N-Myc-induced neuroblastoma (34), and inauhzin, a phenothiazine,
reduced colon xenograft growth (40). These results provide
proof-of-concept examples that SIRT1 inhibition may be an effective
modality in cancer therapy.
Here we report the identification of a new SIRT1
inhibitor, JQ-101, which induces cancer cell apoptosis and
senescence, suppresses cancer cell invasion, and exerts
cancer-specific cytotoxity, repressing tumor cell growth.
Materials and methods
Cells, antibodies and reagents
All cancer and normal cells lines were obtained from
the American Type Culture Collection (Manassas, VA). LNCaP, PC3,
Ramos, Jurkat, H1299 and MRC5 cells were maintained in RPMI-1640
medium with 10% FBS (HyClone, CO). H460, A549, ZR75 and MDA231
cells were maintained in DMEM medium with 10% FBS. PZ-HPV-7 cells
were maintained in Keratinocyte Serum-Free Medium supplemented with
Epidermal Growth Factor (Invitrogen, Carlsbad, CA).
Antibodies to SIRT1 (sc-74504) were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies to Ac-p53,
p53, Ac-Histone H4 and H4 were purchased from Millipore (Billerica,
MA). Antibodies to β-actin were purchased from Sigma-Aldrich (St.
Louis, MO). Sirtinol was purchased from Sigma-Aldrich.
Chemical synthesis of polyprenylated
acylphloroglucinol (PPAP) analogues
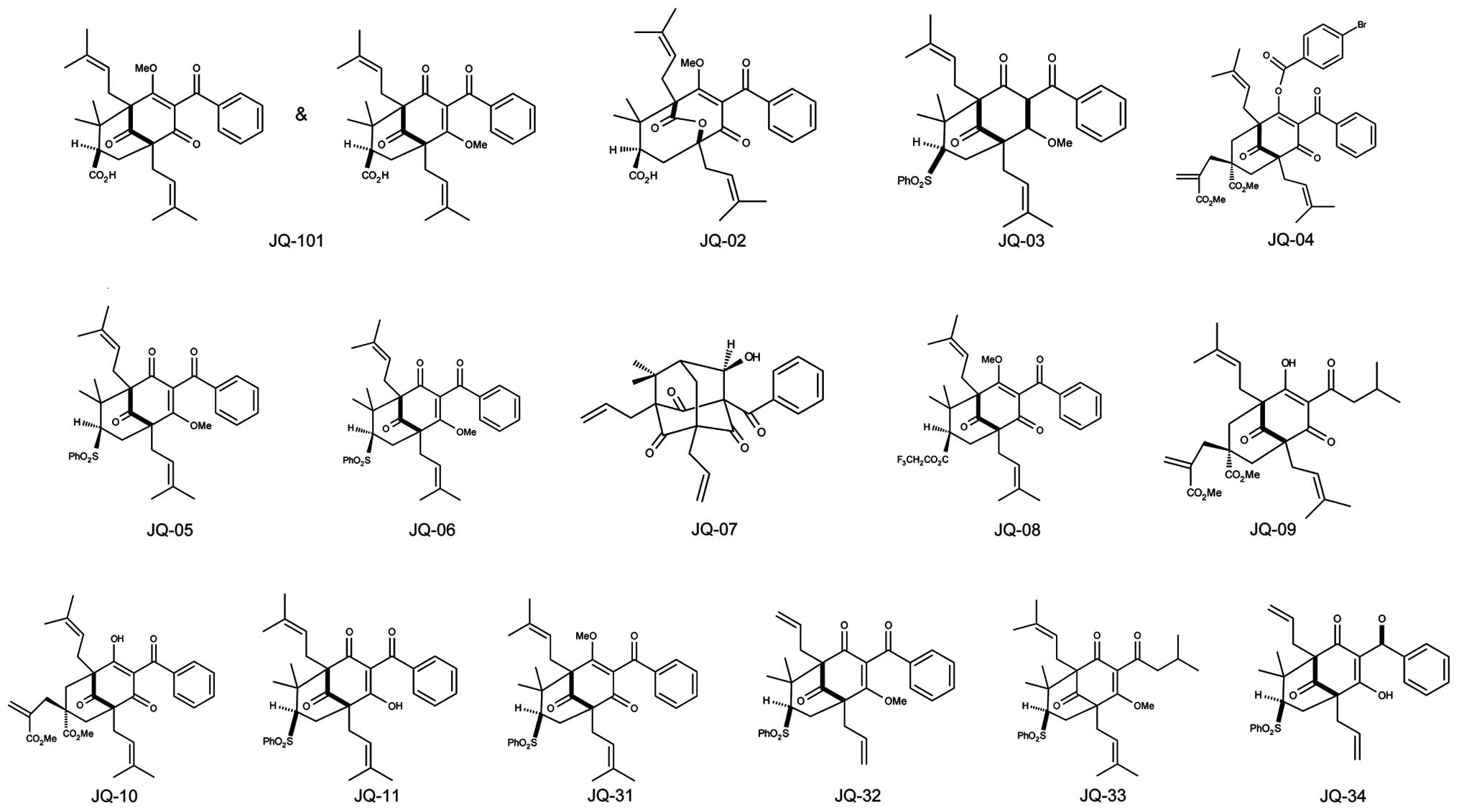
JQ-101, JQ-2, JQ-3, JQ-4, JQ-5, JQ-6, JQ-7, JQ-8,
JQ-9, JQ-10, JQ-11, JQ-31, JQ-32, JQ-33 and JQ-34 (Fig. 1) are simplified analogues of the
type B PPAP natural product clusianone and were synthesized using
our reported procedure involving tandem alkylative
dearomatization-annulation of acylphloroglucinols to rapidly
construct the bicyclo[3.3.1] nonane-1,3,5-trione core (41). BM001, BM002, BM003, BM004, BM005,
BM006, BM007, BM008, BM01810, BM01817, BM01847, BM-01-1005,
BM-01-1013F2, BM-01-1011, BM-01-1022 and related bicyclo[2.2.2]
octadiones (Table I) were
synthesized using the reported method involving
Mn(III)/Cu(II)-mediated oxidative radical cyclizations of
dearomatized phloroglucinol substrates (42). Compounds QZ-2001-2005, analogues of
the type A PPAP nemorosone, were prepared as intermediates during
the course of our chemical synthesis of 7-epi-nemorosone (43).
 | Table ICytotoxicity measurement of JQ-101 in
multiple cancer/normal cell lines. |
Table I
Cytotoxicity measurement of JQ-101 in
multiple cancer/normal cell lines.
| Cancer cell
lines | IC50
(μM) |
|---|
| LNCaP | 20 |
| Jurkat | 25 |
| H460 | 30 |
| Ramos | 33 |
| A549 | 40 |
| MDA-MB-231 | 60 |
| ZR75 | 66 |
| H1299 | 85 |
| PC3 | 119 |
| Normal cell
lines |
| PZ-HPV-7 | 250 |
| MRC5 | 300 |
SIRT1 and SIRT2 activity analysis and
small molecule screening
The Cayman hSIRT1 activity assay kit (SIRT1 Direct
Fluorescent Screening Assay Kit, cat. no. 10010401) and hSIRT2
activity assay kit (SIRT2 Direct Fluorescent Screening Assay Kit,
cat. no. 700280) were used to quantitate the IC50s of
the SIRT inhibitors. The assay was carried out according to the
manufacturer’s instructions. All compounds were preincubated with
the hSIRT1/hSIRT2 proteins before commencing the reaction through
the addition of the ‘Fluor de Lys’ deacetylase substrate.
Deacetylation of K382-p53/K320-p53 was used as a marker of HDAC
activity. Fluorescence was read (Ex 360 nm/Em 460 nm) using Synergy
HT Multi-Mode Microplate Reader (BioTek). Assays were repeated in
triplicate. Quantitation of acetylation was derived from the levels
of fluorescence (displayed in arbitrary fluorescence units), and
the percentages of inhibition were calculated from the arbitrary
fluorescence unit of the treated assays and non-treated controls.
The IC50 was determined using GraphPad Prism 6.0.
Establishment of stable SIRT1-knockdown
cell lines
The Phoenix packaging cell line was transfected with
shRNA expression plasmids pSUPER.retro.puro-SIRT1
(5′-GATGAAGTTGACCTCCTCA-3′) or pSUPER.retro.puro-non-targeting
shRNA control separately, using Lipofectamine 2000 (Invitrogen).
After 48 h, the medium containing retrovirus was collected,
filtered, treated with polybrene and transferred to LNCap or H460
cell cultures. Infected cells were selected with puromycin and the
stably-infected colonies were pooled.
Immunoblot analysis
Cells were lysed with 1% Nonidet P-40 lysis buffer
(1% NP-40, 150 mM NaCl, 50 mM Tris-HCl) with protease inhibitor
(Roche Diagnostics). Protein samples were subsequently separated on
an 8% SDS-polyacrylamide gel and analyzed with anti-Ac-p53,
anti-p53, anti-SIRT1, anti-Ac-Histone H4, anti-H4 or β-actin
antibodies.
Cell viability assay
MTS (Promega, G3580) assays were used to quantify
cell viability. Cells were plated at a density of 104
cells/well on 96-well plates and exposed to various concentrations
of JQ-101, ranging from 5 to 125 μM, for 72 h. A total of 20 μl MTS
CellTiter96Aqueous one solution reagent was added to the medium for
1 h, and the absorbance was read at 490 nm.
Apoptosis analysis by FACS
The cultured cells were trypsinized, collected and
labeled with Annexin V antibody and PI. The procedure for cell
labeling followed the Annexin V kit instructions (Annexin V-PE
Apoptosis Detection Kit I, BC Pharmingen, cat. no. 559763). The
labeled samples were further analyzed with flow cytometry.
SA-β-gal staining
The cells were seeded at 30% confluence, and JQ-101
was added to a final concentration 50 μM. At 4 days after the
addition of JQ-101 to the culture media, the cells were washed with
phosphate-buffered saline (PBS) and fixed with PBS containing 2%
formaldehyde and 0.2% glutaraldehyde for 10 min. The cells were
then incubated at 37°C overnight with staining solution [40 mM
citric acid sodium phosphate, pH 6.0, 1 mg/ml
5-bromo-4-chloro-3-isolyl-β-D-galactoside (X-gal, Fisher,
Pittsburgh, PA), 5 mM potassium ferricyanide, 150 mM NaCl, 2 mM
MgCl2]. SA-β-gal-positive cells were enumerated by
microscopy.
Cell invasion assay
Cell invasion activity of cancer cells was assessed
using BD BioCoat Matrigel Invasion Chambers (BD cat. no. 354480).
Briefly, the cells were trypsined, washed with PBS, and resuspended
in media containing 1% FBS at a density of 5×104
cells/ml. A total of 500 μl of cells suspended in medium containing
JQ-101 or DMSO (vehicle control) was placed in the upper chamber
coated with matrigel and 500 μl of culture media was added to the
lower chamber of the transwell. After 48 h of incubation at 37°C,
the transwells were removed from the 24-well plates and stained
with Diff-Quick solution. The invading cells were enumerated by
microscopy.
Results
Design, synthesis and evaluation of SIRT1
inhibitors based on polyprenylated acylphloroglucinol (PPAP)
natural products
Polyprenylated acylphloroglucinols (PPAPs) are a
family of natural products that possess a wide range of different
biological activities (44). There
are more than 100 PPAPs discovered to date. Most of these were
isolated from plants and trees from Clusiaceae (Guttiferae). PPAPs
are divided into three types (A, B and C), depending on the
position of the acyl group on the bicyclic core (44). It has been reported that the PPAPs
guttiferone G and aristoforin have SIRT1 inhibitory activity,
although little biological function has been shown (39). We synthesized a panel of
derivatives of PPAP compounds using our reported procedure
involving tandem alkylative dearomatization/annulation of
acylphloroglucinols to rapidly construct the bicyclo[3.3.1]
nonane-1,3,5-trione core (41) or
employing Mn(III)/Cu(II)-mediated oxidative radical cyclizations of
dearomatized phloroglucinol substrates (42).
Using a fluorogenic substrate, we performed a
biochemical-based inhibitory assay with recombinant SIRT1 and
SIRT2. We synthesized and screened over 35 synthesized analogues of
the type B PPAP natural product clusianone and the type A PPAP
natural product nemorosone (see Fig.
1 for details and structures). Among the synthesized PPAP
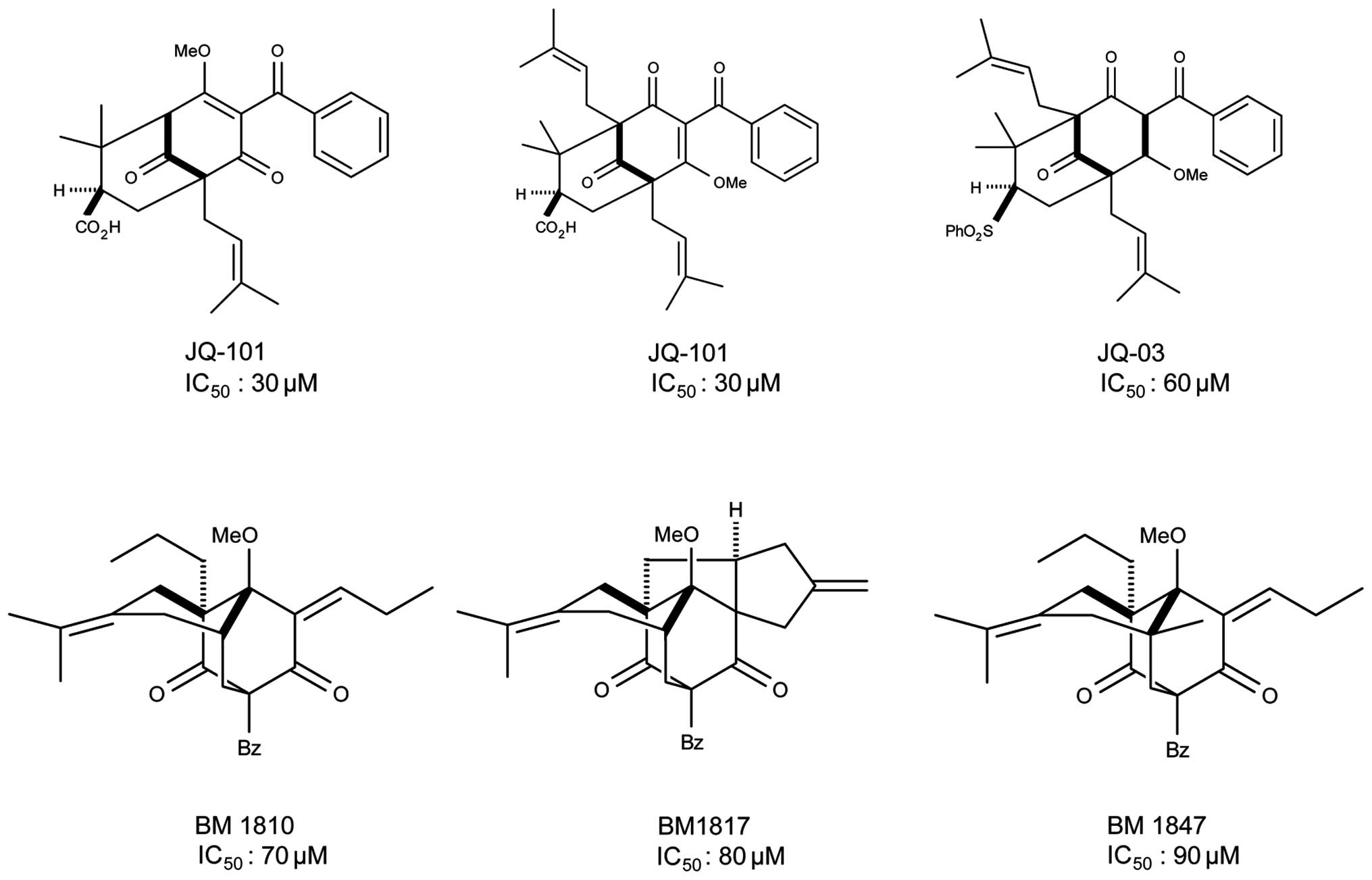
analogues, we identified five compounds with SIRT1 inhibitory
activity with IC50 ranging from 30 to 90 μM, including
JQ-101, JQ-3, BM1810, BM1817 and BM1847 (Fig. 2). One of these compounds,
designated JQ-101, which showed the best in vitro activity
for inhibition of SIRT1, forms the major focus of this report.
Inhibition of SIRT1 deacetylase activity
by JQ-101 in vitro and in vivo
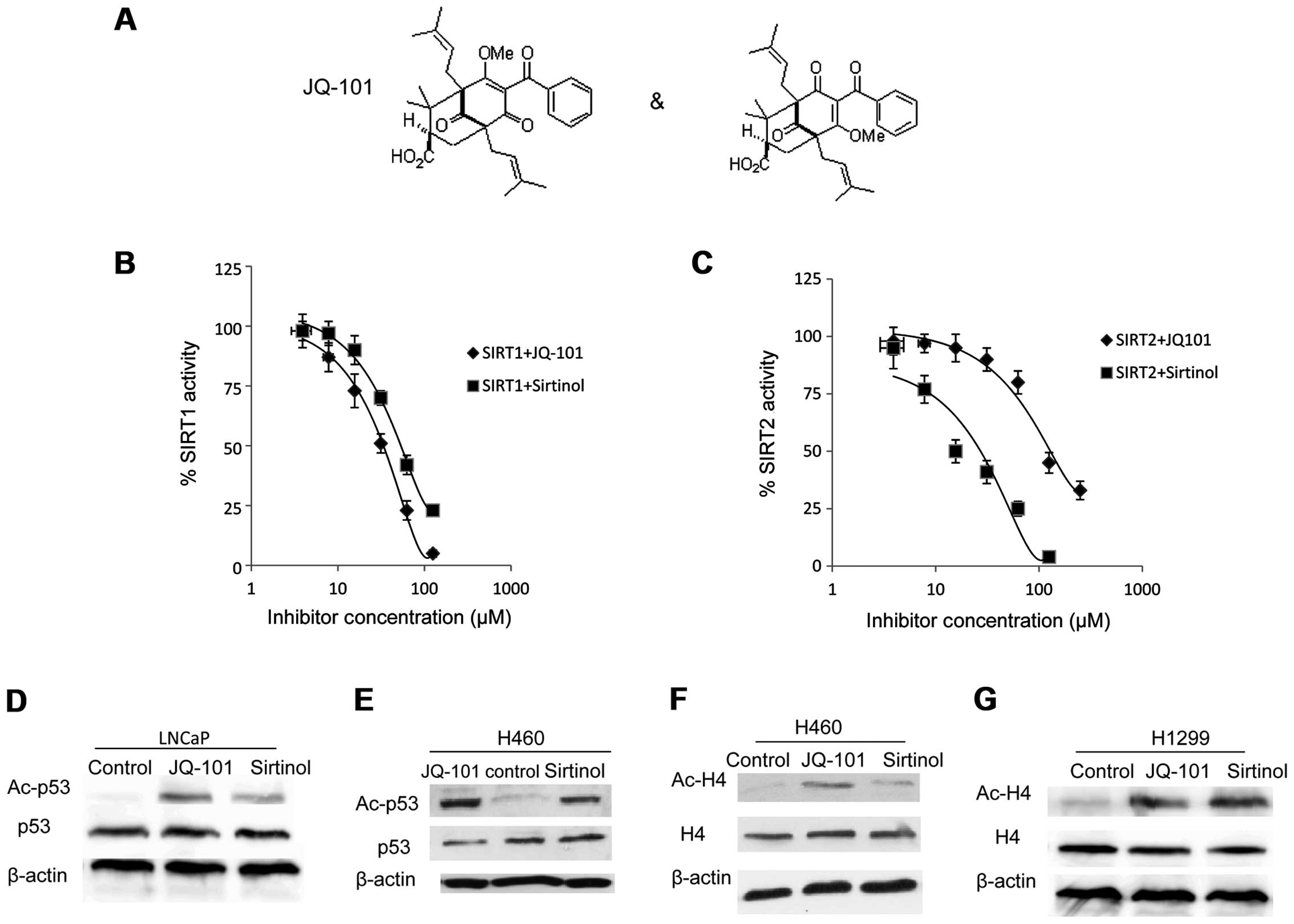
Using a fluorogenic substrate, we performed
biochemical-based inhibition assays with recombinant SIRT1 and
SIRT2. JQ-101 inhibited SIRT1 deacetylase activity with an
IC50 of 30 μM (Fig. 3A and
B). JQ-101 also inhibited the closely-related class III HDAC
SIRT2, with an IC50 of 150 μM (Fig. 3C). Thus, JQ-101 has 5-fold
selectivity in inhibiting SIRT1 over SIRT2. Sirtinol was used as a
positive control for the assay, with an IC50 value of 60
μM for SIRT1 (Fig. 3B) and 20 μM
for SIRT2 (Fig. 3C), respectively,
in good agreement with reported values.
We next examined whether JQ-101 can target SIRT1 in
a cell-based system. It is well established that lysine 382 of p53
is a cellular substrate of SIRT1 (46). We therefore assessed the levels of
lysine 382-acetylated p53 in p53 wild-type cells exposed to JQ-101
compared to vehicle. Treatment with JQ-101 increased the
acetylation levels of p53 in LNCaP and H460 cells (Fig. 3D and E). We also assessed the level
of histone H4 acetylation at lysine 16, another confirmed substrate
for SIRT1. JQ-101 treatment increased the levels of histone H4
acetylation in both H460 (p53 wt) and H1299 (p53 null) cells
(Fig. 3E and F). These findings
suggest that JQ-101 can prevent the deacetylation of known
biological substrates of SIRT1, thereby demonstrating on-target
SIRT1 inhibitory activity of JQ-101 in vivo.
JQ-101 suppresses cancer cells
growth
Several SIRT1 inhibitors have been shown to produce
antitumor activity in vitro and in mouse xenograft models.
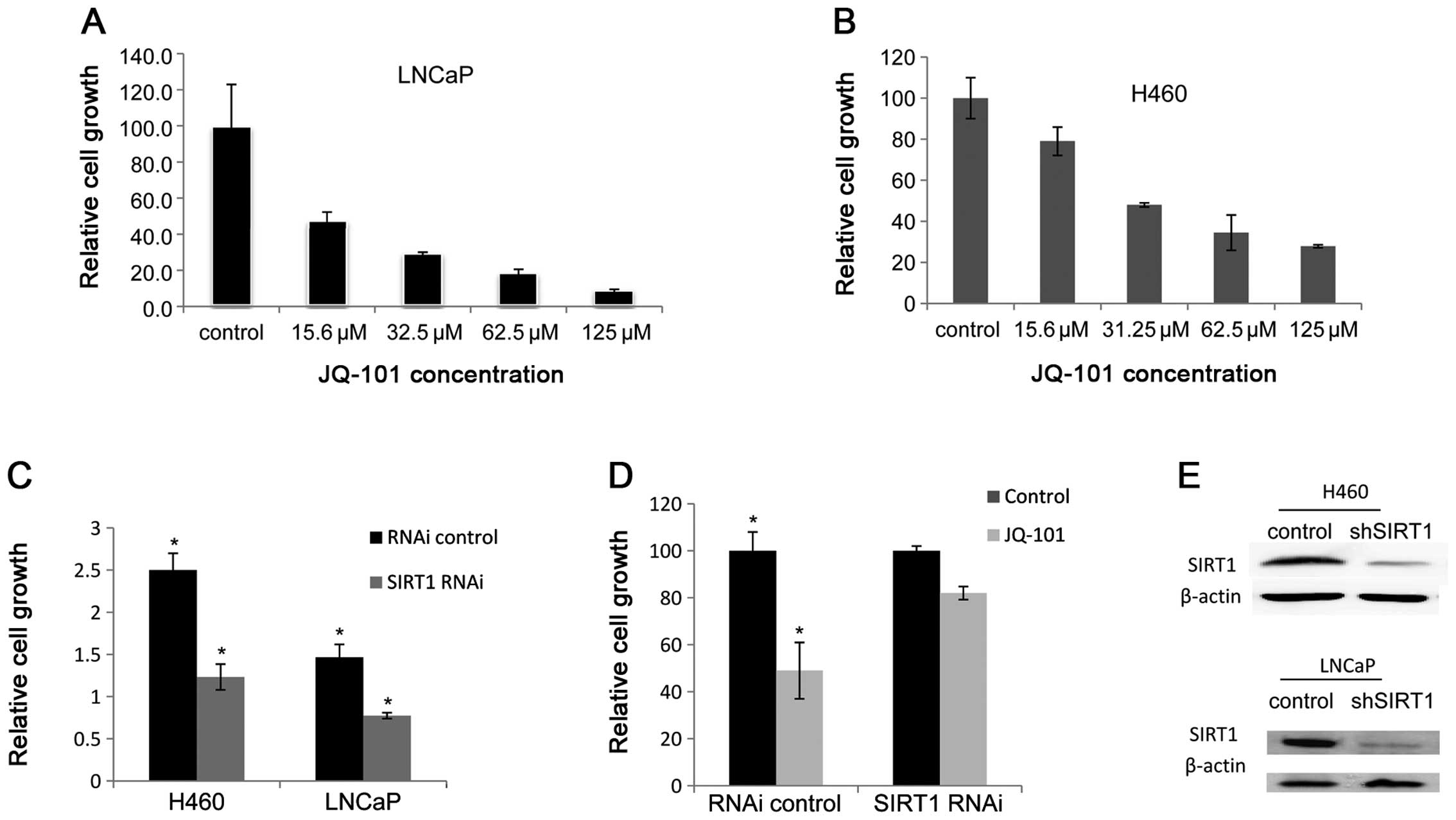
To investigate the effects of JQ-101 on cancer cell growth and
survival inhibition, we determined the inhibitory ability of JQ-101
on a panel of human cancer cell lines, with normal human cell lines
as serving as non-cancer controls. As shown in Fig. 4A and B, JQ-101 suppressed LNCaP and
H460 tumor cell growth in a dose-dependent manner. We screened
eight different cancer cell lines in total, including lines derived
from prostate cancers (LNCaP and PC3), lung cancers (H460 and
A549), leukemia (Jurkat), lymphoma (Ramos), and breast cancers
(ZR75 and MDA231). JQ-101 suppressed tumor cell growth at
IC50 values ranging from 20 to 119 μM (Table I). By comparison, JQ-101 was much
less toxic in normal human cells, including one normal human
prostate epithelial cell line (PZ-HPV-7) and one human lung
fibroblast cell line (MRC-5) (Table
I). These results suggest that JQ-101 exerts cancer
cell-selectivity in inhibiting the growth of cells.
To more specifically study the role of SIRT1 in
control of cancer cell growth, SIRT1 was knocked down with SIRT1
RNAi in LNCaP and H460 cells. SIRT1 silencing resulted in cancer
cell growth suppression (Fig. 4C).
This suggests that SIRT1 is important in control of growth in these
cancer cells. To further study the specificity of JQ-101 mediated
cancer cell growth, we treated the both SIRT1-silenced and vector
control-transfected H460 cancer cell lines with JQ-101. The effect
of JQ-101 on cancer cell growth is greatly attenuated in the
SIRT1-silenced cells (Fig. 4D).
This suggests that JQ-101 suppresses cancer cell growth by
targeting SIRT1.
JQ-101 induces both apoptosis and cell
senescence
To further elucidate the molecular mechanisms of
this tumor-selective growth suppression, Annexin V and β-gal
staining were carried out to study the effects of JQ-101 in
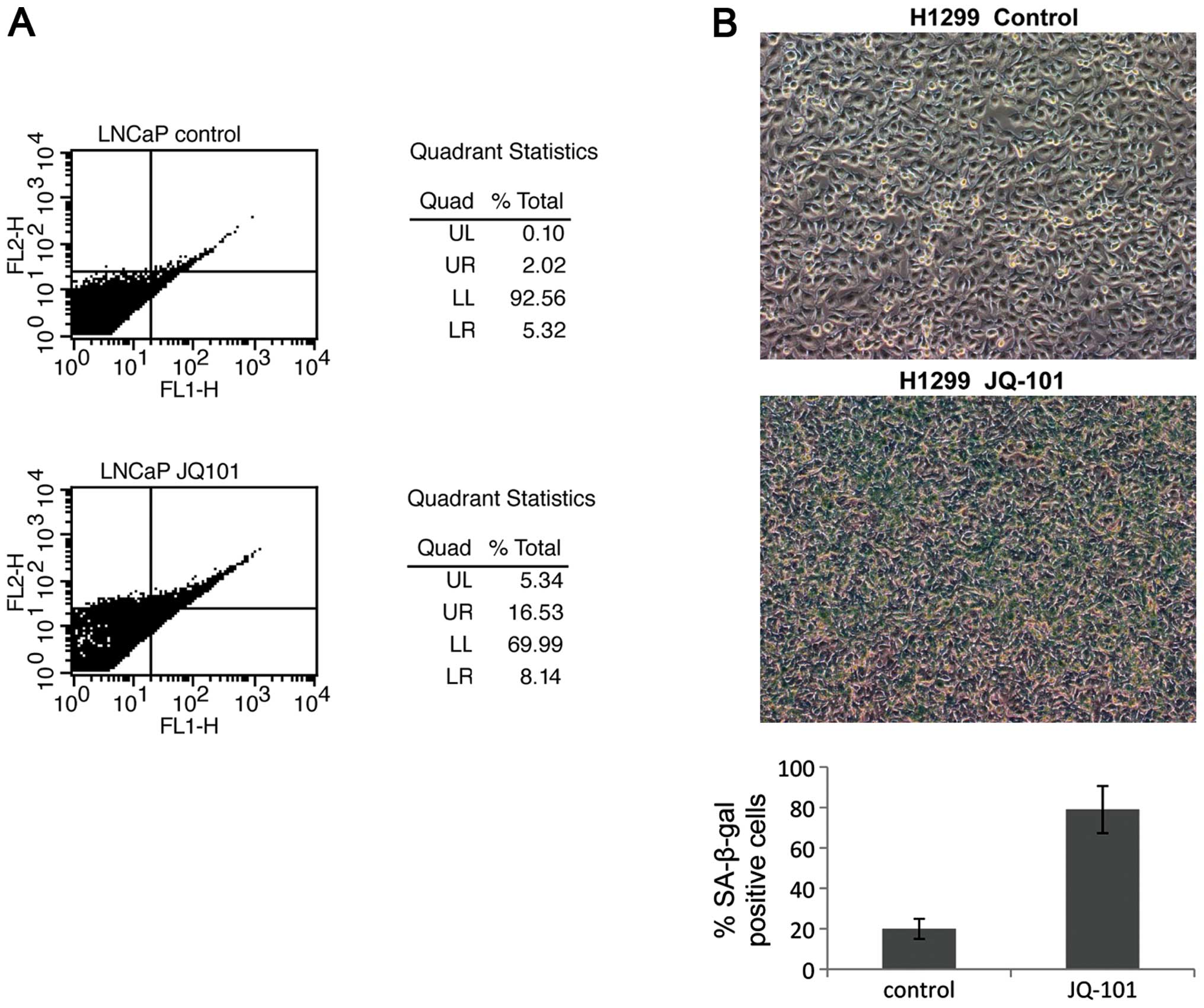
inducing apoptosis or cell senescence. JQ-101 treatment induced
cell death and apoptosis in LNCaP cells; proportions of cells in
both late stage (14.5%) and early stage (3.2%) apoptosis increased
after JQ-101 treatment (Fig. 5A).
In contrast, apoptosis was not observed in H1299 cells (data not
shown); instead, JQ-101 treatment induced cell senescence in H1299
cells (Fig. 5B) but not in LNCaP
cells (data not shown). This suggests that the induction of either
apoptosis or cell senescence, in a context-dependent fashion, are
the mechanisms whereby JQ-101 suppresses cancer cell growth and
survival.
JQ-101 inhibits cancer cell invasion
Recent studies have shown that SIRT1 plays an
important role in cancer cell migration and invasion (14,16,46).
We therefore determined whether SIRT1 inhibition by JQ-101 could
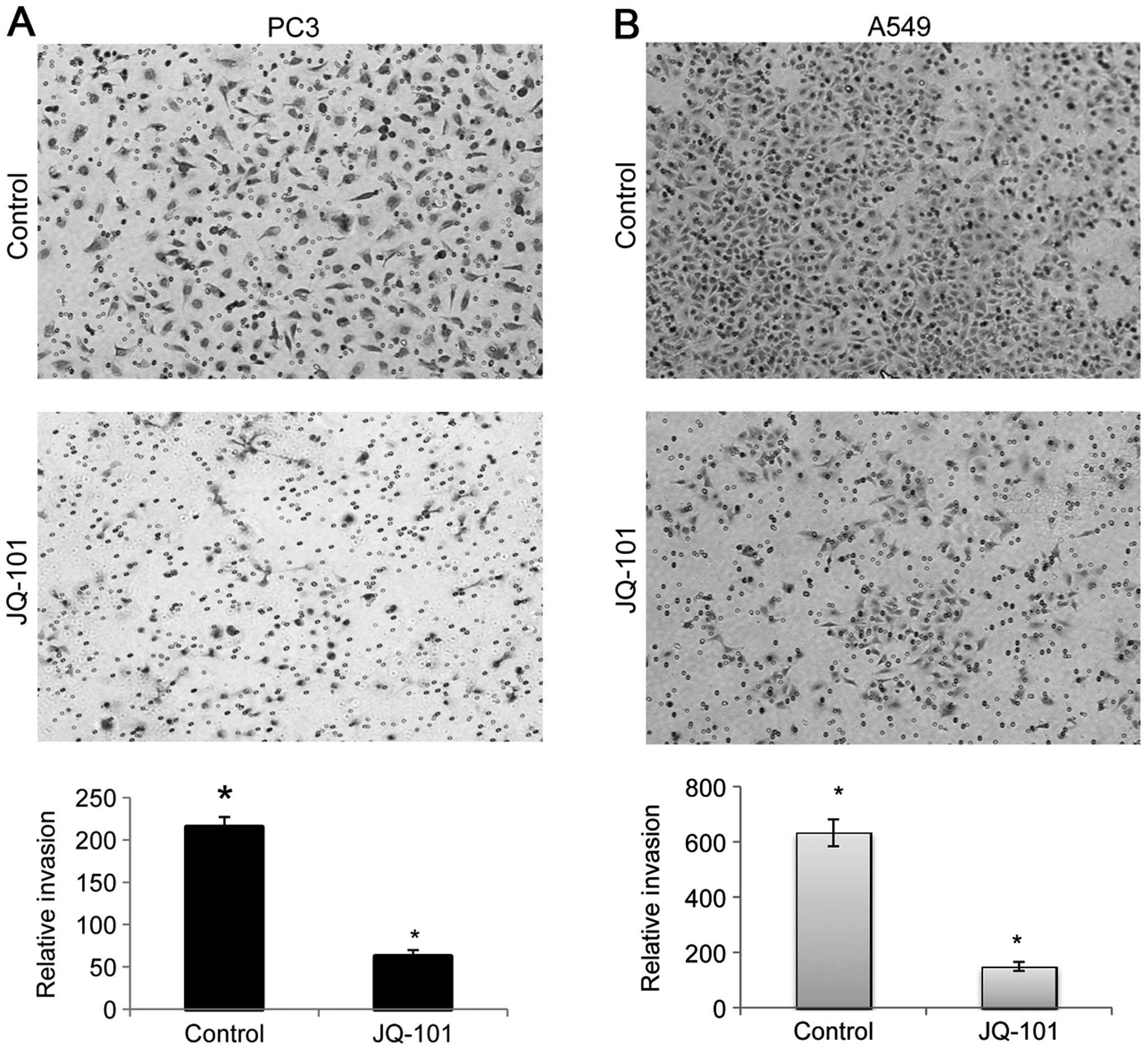
inhibit cancer cell invasion. PC3 prostate cancer and A549 lung
cancer cells, which exhibit invasive behavior, were exposed to
JQ-101, and the effects on cancer cell invasion were quantitated,
using a Transwell chamber assay system with matrigel coated on the
top of an ECM-like membrane to prevent the transmigration of
non-invasive cells. Exposure to JQ-101 significantly decreased
invasion compared to vehicle control, producing a 3.5-fold
reduction in PC3 cell invasion and a 4.2-fold reduction in A549
cells (Fig. 6A and B), suggesting
that JQ-01 exerts an inhibitory effect on cancer cell invasion.
Discussion
SIRT1 has attracted much attention for its
functional roles in cancer (47).
We report here a new SIRT1 inhibitor JQ-101, a compound possessing
a polyprenylated acylphloroglucinol (PPAP) core, which exhibits
selective inhibition of tumor cell line growth and survival though
induction of apoptosis and cellular senescence, and significantly
suppresses cancer cell invasion.
To date, several SIRT1 inhibitors have been
developed with a broad range of potency in SIRT1 inhibition
(32–36). The IC50 for JQ-101
against STIR1 is approximately 30 μM, a potency similar to several
of the previously reported SIRT1-inhibitory compounds, including
sirtinol (31), cambinol (32), salermide (37), tenovin-6 (34) and splitomycin (35). Most of the SIRT1 inhibitors
identified previously also possess SIRT2 inhibitory activity, due
to the high level of sequence similarity between SIRT1 and SIRT2.
While JQ-101 also exhibited inhibitory effects on SIRT2, 5-fold
higher concentrations were necessary, making JQ-101 somewhat
SIRT1-specific. Our results show that JQ-101 can inhibit the growth
and survival of a panel of cancer cell lines. Although we cannot
rule out the possibility that minor inhibitory effects of SIRT 2
activity may contribute in part to the observed selective
cytotoxicity of JQ-101, these studies were carried out under
conditions far below the IC50 for SIRT2. In addition,
our results show that SIRT1 knockdown greatly impairs the effect of
JQ-101 on cell growth and survival (Fig. 4D), demonstrating that SIRT1 is the
major target of JQ-101.
It has been shown that SIRT1 inhibition can induce
apoptosis in many cancer cell types, in particular p53-wild-type
cells (45,48–50);
alternatively, SIRT1 inhibition can also induce cell senescence
(51–53). Our results show that JQ-101 can
trigger either apoptosis in p53-wild-type LNCaP cells or induce
cell senescence in p53-null H1299 cells (Fig. 5). Depending upon the specific
genetic background of the cancer cell, SIRT1 inhibition by JQ-101
may utilize different downstream targets of SIRT1 to induce either
apoptosis or cell senescence, to suppress cancer cell growth and
survival.
We also show in this study that JQ-101 treatment can
greatly reduce cancer cell invasion (Fig. 6). We have previously identified
SIRT1 as a positive regulator of epithelial-to-mesenchymal
transition (EMT), cell invasion, and metastatic growth of prostate
cancer cells, and put forward SIRT1 as a potential therapeutic
target to reverse EMT and to prevent prostate cancer progression
(8,19). Consistent with our findings, it has
been shown that SIRT1 inhibition reduces cancer cell migration and
invasion while SIRT1 activation promotes cancer metastasis
(12,15,23,54).
These results suggest that SIRT1 plays an important role in cancer
invasion and metastasis, and that development of SIRT1 inhibitors
may represent a critical new targeted strategy to prevent cancer
metastasis and progression.
Given our findings that JQ-101 can selectively
suppress cancer cell growth by inducing tumor cell apoptosis and
senescence, and significantly inhibit cancer cell invasion, these
results suggest a potential role of JQ-101 or a derivative in a
SIRT1-targeted approach to cancer therapy. Studies are under way to
delineate the effects of JQ-101 on tumor formation, growth and
metastasis in mouse models in vivo. JQ-101 will also serve
as a novel chemical scaffold for future development of more potent
SIRT1 inhibitors to be used in cancer treatment.
Acknowledgements
We sincerely thank Dr F. Picard (Laval University,
Canada) for providing SIRT1 shRNA vector. We thank Branko Mitasev
(Department of Chemistry, Boston University) for compound
synthesis, and thank Heba Ijaz, Nadine Aziz, Marina Amaro, and
Elizabeth Cho (Cancer Center, Boston University) for their
technical support. This study was supported by grants from the
National Cancer Institute (CA141036 and CA129046) (Y.D.), (CA101992
and CA164245) (D.V.F.), (GM-073855 and GM-067041) (J.A.P.Jr), the
Department of Defense (PC100093) (D.V.F.), National Science
Foundation (CHE-0848082) (J.A.P.Jr), the Clinical and Translational
Science Institute award of NIH (UL1RR025771) (Y.D.), and the
Department of Medicine Pilot Project Grant (Y.D.).
References
|
1
|
Bordone L and Guarente L: Calorie
restriction, SIRT1 and metabolism: understanding longevity. Nat Rev
Mol Cell Biol. 6:298–305. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inoue T, Hiratsuka M, Osaki M and Oshimura
M: The molecular biology of mammalian SIRT proteins: SIRT2 in cell
cycle regulation. Cell Cycle. 6:1011–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saunders LR and Verdin E: Sirtuins:
critical regulators at the crossroads between cancer and aging.
Oncogene. 26:5489–5504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donmez G and Guarente L: Aging and
disease: connections to sirtuins. Aging Cell. 9:285–290. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bosch-Presegue L and Vaquero A: The dual
role of sirtuins in cancer. Genes Cancer. 2:648–662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knight JR and Milner J: SIRT1, metabolism
and cancer. Curr Opin Oncol. 24:68–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu F, Chou PM, Zheng X, Mirkin BL and
Rebbaa A: Control of multidrug resistance gene mdr1 and cancer
resistance to chemotherapy by the longevity gene sirt1. Cancer Res.
65:10183–10187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kojima K, Ohhashi R, Fujita Y, et al: A
role for SIRT1 in cell growth and chemoresistance in prostate
cancer PC3 and DU145 cells. Biochem Biophys Res Commun.
373:423–428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SM, Bae JH, Kim MJ, et al:
Bcr-Abl-independent imatinib-resistant K562 cells show aberrant
protein acetylation and increased sensitivity to histone
deacetylase inhibitors. J Pharmacol Exp Ther. 322:1084–1092. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang XJ, Finkel T, Shen DW, Yin JJ,
Aszalos A and Gottesman MM: SIRT1 contributes in part to cisplatin
resistance in cancer cells by altering mitochondrial metabolism.
Mol Cancer Res. 6:1499–1506. 2008. View Article : Google Scholar
|
|
11
|
Marshall GM, Liu PY, Gherardi S, et al:
SIRT1 promotes N-Myc oncogenesis through a positive feedback loop
involving the effects of MKP3 and ERK on N-Myc protein stability.
PLoS Genet. 7:e10021352011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki K, Hayashi R, Ichikawa T, et al:
SRT1720, a SIRT1 activator, promotes tumor cell migration, and lung
metastasis of breast cancer in mice. Oncol Rep. 27:1726–1732.
2012.PubMed/NCBI
|
|
13
|
Gabr AG, Goto H, Hanibuchi M, et al:
Erlotinib prevents experimental metastases of human small cell lung
cancer cells with no epidermal growth factor receptor expression.
Clin Exp Metastasis. 29:207–216. 2012. View Article : Google Scholar
|
|
14
|
Byles V, Zhu L, Lovaas JD, et al: SIRT1
induces EMT by cooperating with EMT transcription factors and
enhances prostate cancer cell migration and metastasis. Oncogene.
31:4619–4629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Zhang M, Dong H, et al:
Deacetylation of cortactin by SIRT1 promotes cell migration.
Oncogene. 28:445–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakane K, Fujita Y, Terazawa R, et al:
Inhibition of cortactin and SIRT1 expression attenuates migration
and invasion of prostate cancer DU145 cells. Int J Urol. 19:71–79.
2012. View Article : Google Scholar
|
|
17
|
Holloway KR, Calhoun TN, Saxena M, et al:
SIRT1 regulates Dishevelled proteins and promotes transient and
constitutive Wnt signaling. Proc Natl Acad Sci USA. 107:9216–9221.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lovaas JD, Zhu L, Chiao CY, Byles V,
Faller DV and Dai Y: SIRT1 enhances matrix metalloproteinase-2
expression and tumor cell invasion in prostate cancer cells.
Prostate. 73:522–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Potente M, Ghaeni L, Baldessari D, et al:
SIRT1 controls endothelial angiogenic functions during vascular
growth. Genes Dev. 21:2644–2658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Potente M and Dimmeler S: Emerging roles
of SIRT1 in vascular endothelial homeostasis. Cell Cycle.
7:2117–2122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jang KY, Hwang SH, Kwon KS, et al: SIRT1
expression is associated with poor prognosis of diffuse large
B-cell lymphoma. Am J Surg Pathol. 32:1523–1531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huffman DM, Grizzle WE, Bamman MM, et al:
SIRT1 is significantly elevated in mouse and human prostate cancer.
Cancer Res. 67:6612–6618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao G, Cui J, Zhang JG, et al: SIRT1 RNAi
knockdown induces apoptosis and senescence, inhibits invasion and
enhances chemosensitivity in pancreatic cancer cells. Gene Ther.
18:920–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cha EJ, Noh SJ, Kwon KS, et al: Expression
of DBC1 and SIRT1 is associated with poor prognosis of gastric
carcinoma. Clin Cancer Res. 15:4453–4459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
DiMeo TA, Anderson K, Phadke P, et al: A
novel lung metastasis signature links Wnt signaling with cancer
cell self-renewal and epithelial-mesenchymal transition in
basal-like breast cancer. Cancer Res. 69:5364–5373. 2009.
View Article : Google Scholar
|
|
26
|
Lee H, Kim KR, Noh SJ, et al: Expression
of DBC1 and SIRT1 is associated with poor prognosis for breast
carcinoma. Hum Pathol. 42:204–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Zhang B, Wong N, et al: Sirtuin 1
is upregulated in a subset of hepatocellular carcinomas where it is
essential for telomere maintenance and tumor cell growth. Cancer
Res. 71:4138–4149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kriegl L, Vieth M, Kirchner T and Menssen
A: Up-regulation of c-MYC and SIRT1 expression correlates with
malignant transformation in the serrated route to colorectal
cancer. Oncotarget. 3:1182–1193. 2012.PubMed/NCBI
|
|
29
|
Tseng RC, Lee CC, Hsu HS, Tzao C and Wang
YC: Distinct HIC1-SIRT1-p53 loop deregulation in lung squamous
carcinoma and adenocarcinoma patients. Neoplasia. 11:763–770.
2009.PubMed/NCBI
|
|
30
|
Bitterman KJ, Anderson RM, Cohen HY,
Latorre-Esteves M and Sinclair DA: Inhibition of silencing and
accelerated aging by nicotinamide, a putative negative regulator of
yeast sir2 and human SIRT1. J Biol Chem. 277:45099–45107. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grozinger CM, Chao ED, Blackwell HE,
Moazed D and Schreiber SL: Identification of a class of small
molecule inhibitors of the sirtuin family of NAD-dependent
deacetylases by phenotypic screening. J Biol Chem. 276:38837–38843.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Heltweg B, Gatbonton T, Schuler AD, et al:
Antitumor activity of a small-molecule inhibitor of human silent
information regulator 2 enzymes. Cancer Res. 66:4368–4377. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Solomon JM, Pasupuleti R, Xu L, et al:
Inhibition of SIRT1 catalytic activity increases p53 acetylation
but does not alter cell survival following DNA damage. Mol Cell
Biol. 26:28–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lain S, Hollick JJ, Campbell J, et al:
Discovery, in vivo activity, and mechanism of action of a
small-molecule p53 activator. Cancer Cell. 13:454–463. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bedalov A, Gatbonton T, Irvine WP,
Gottschling DE and Simon JA: Identification of a small molecule
inhibitor of Sir2p. Proc Natl Acad Sci USA. 98:15113–15118. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi G, Lee J, Ji JY, et al: Discovery of
a potent small molecule SIRT1/2 inhibitor with anticancer effects.
Int J Oncol. 43:1205–1211. 2013.PubMed/NCBI
|
|
37
|
Lara E, Mai A, Calvanese V, et al:
Salermide, a Sirtuin inhibitor with a strong cancer-specific
proapoptotic effect. Oncogene. 28:781–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suzuki T, Imai K, Nakagawa H and Miyata N:
2-Anilinobenzamides as SIRT inhibitors. ChemMedChem. 1:1059–1062.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gey C, Kyrylenko S, Hennig L, et al:
Phloroglucinol derivatives guttiferone G, aristoforin, and
hyperforin: inhibitors of human sirtuins SIRT1 and SIRT2. Angew
Chem Int Ed Engl. 46:5219–5222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Zhang Q, Zeng SX, Zhang Y, Mayo
LD and Lu H: Inauhzin and Nutlin3 synergistically activate p53 and
suppress tumor growth. Cancer Biol Ther. 13:915–924. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Qi J and Porco JA Jr: Rapid access to
polyprenylated phloroglucinols via alkylative
dearomatization-annulation: total synthesis of (+/−)-clusianone(1).
J Am Chem Soc. 129:12682–12683. 2007.PubMed/NCBI
|
|
42
|
Mitasev B and Porco JA Jr:
Manganese(III)-mediated transformations of phloroglucinols: a
formal oxidative [4 + 2] cycloaddition leading to
bicyclo[2.2.2]octadiones. Org Lett. 11:2285–2288. 2009.PubMed/NCBI
|
|
43
|
Zhang Q and Porco JA Jr: Total synthesis
of (+/−)-7-epi-nemorosone. Org Lett. 14:1796–1799. 2012.
|
|
44
|
Biber N, Mows K and Plietker B: The total
synthesis of hyper-papuanone, hyperibone L, epi-clusianone and
oblongifolin A. Nat Chem. 3:938–942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vaziri H, Dessain SK, Ng Eaton E, et al:
hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell.
107:149–159. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Noh SJ, Baek HA, Park HS, et al:
Expression of SIRT1 and cortactin is associated with progression of
non-small cell lung cancer. Pathol Res Pract. 209:365–370. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu T, Liu PY and Marshall GM: The
critical role of the class III histone deacetylase SIRT1 in cancer.
Cancer Res. 69:1702–1705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li L, Wang L, Li L, et al: Activation of
p53 by SIRT1 inhibition enhances elimination of CML leukemia stem
cells in combination with imatinib. Cancer Cell. 21:266–281. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kalle AM, Mallika A, Badiger J, Alinakhi,
Talukdar P and Sachchidanand: Inhibition of SIRT1 by a small
molecule induces apoptosis in breast cancer cells. Biochem Biophys
Res Commun. 401:13–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sun Y, Sun D, Li F, et al: Downregulation
of Sirt1 by antisense oligonucleotides induces apoptosis and
enhances radiation sensitization in A549 lung cancer cells. Lung
Cancer. 58:21–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ota H, Tokunaga E, Chang K, et al: Sirt1
inhibitor, Sirtinol, induces senescence-like growth arrest with
attenuated Ras-MAPK signaling in human cancer cells. Oncogene.
25:176–185. 2006.PubMed/NCBI
|
|
52
|
Huang J, Gan Q, Han L, et al: SIRT1
overexpression antagonizes cellular senescence with activated
ERK/S6k1 signaling in human diploid fibroblasts. PLoS One.
3:e17102008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jang SY, Kim SY and Bae YS: p53
deacetylation by SIRT1 decreases during protein kinase CKII
downregulation-mediated cellular senescence. FEBS Lett.
585:3360–3366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Saxena M, Dykes SS, Malyarchuk S, Wang AE,
Cardelli JA and Pruitt K: The sirtuins promote Dishevelled-1
scaffolding of TIAM1, Rac activation and cell migration. Oncogene.
Dec 23–2013.(Epub ahead of print).
|