1. Introduction
AKT signaling pathways are related to the
suppression of apoptosis, proliferation and metastasis of cells,
angiogenesis and various other processes (1). AKT is involved in the proliferation
of cells via its phosphorylation of Cyclin D1, and promotes the
phosphorylation of BAD and caspase-9 which is associated with
apoptosis. By inhibiting these molecules (BAD and caspase-9), AKT
suppresses apoptosis. AKT plays an important role in the
chemosensitivity to various agents, because it prevents the normal
apoptotic response to treatment. AKT is also deeply involved in
angiogenesis and the invasion of cancer cells into surrounding
tissues through vascular endothelial growth factor (VEGF) and MMP
(2,3). The mechanism regulating
phosphatidylinositol-3 kinase (PI3K)/AKT has been shown to be
abnormal in various solid carcinomas and hematological neoplasms.
The overexpression of AKT at the DNA or protein level has been
reported in breast cancer and stomach cancers (1); thus, AKT has drawn attention as a
target for the development of new molecular-targeted cancer
therapeutic agents. In this review, a basic overview of the role of
AKT in normal and malignant tissues is provided, along with an
in-depth discussion of its role in gastric cancer and its potential
as a target for therapy.
2. What is AKT?
AKT is a serine/threonine kinase activated at
downstream of integrin, which is a receptor for various
pro-proliferation and bioactive substances (receptor tyrosine
kinases and G protein-coupled receptors) as well as extracellular
matrix receptor. AKT has three isoforms, with AKT1 and AKT2 being
expressed ubiquitously, while the expression of AKT3 occurs in
limited tissues (brain and testes). All three isoforms are composed
of a pleckstrin homology (PH) domain, linker domain, kinase domain
and C-terminal hydrophobic domain. Following the activation of PI3
kinase, the phosphorylation of both threonine 308 and serine 473
through PDKI and mTORC2 is required for complete activation of AKT
(4,5). Activated AKT phosphorylates various
substrates to exert its functions in cell proliferation, growth,
anti-apoptosis and cell cycle progression.
3. AKT and the pro-survival effect
In cancer cells, the activation of various receptors
occurs as a result of stimulation from proliferators and adhesion
to the extracellular matrix, and AKT is subsequently activated
downstream of PI3 kinase. As noted above, the PI3K/AKT signaling
pathway is involved in the suppression of apoptosis, the
proliferation and metastasis of cells, as well as in angiogenesis
(1). AKT regulates cell
proliferation by phosphorylating Cyclin D1 and promotes the
phosphorylation of BAD and caspase-9, which are associated with
apoptosis. By inhibiting the functions of these proteins, AKT
suppresses apoptosis, clearly showing that AKT plays an important
role in chemosensitivity, because many chemotherapeutic agents
exert their effects by inducing caspase-mediated apoptosis. AKT is
also deeply involved in angiogenesis and the invasion of cancer
cells into surrounding tissues through VEGF and MMP (2,3). In
the phosphatase and tensin homolog deleted on chromosome 10 (PTEN)
heterozygous knockout mice, PI3K/AKT signaling is activated
continually, which causes tumor formation (6).
4. AKT signal transduction in cancer
cells
AKT is a protein in the PI3K pathway. Stimulation of
receptor tyrosine kinases or G-coupled proteins activates PI3K,
which in turn activates AKT. AKT phosphorylation is maintained by
heat shock protein 90, and AKT is dephosphorylated by protein
phosphatase 2A. Thus, AKT is involved in signaling mediated by
various growth factors and cytokines. In particular, insulin-like
growth factor 1 (IGF-1), epidermal growth factor (EGF) receptor and
human EGF receptor 2, which are important in cancer progression,
activate AKT (7,8). Based on these functions, AKT
activation or overexpression can serve as a biomarker for
predicting the metastasis of human gastrointestinal cancer
(9).
The phosphorylation of AKT modulates signals from
PTEN, and the mammalian target of rapamycin (mTOR) to exert diverse
effects on cells (10). In this
regard, AKT1 is recognized as an apoptotic inhibitor, which
enhances cancer promotion. Phosphorylation via AKT inactivates
Bcl-2 antagonist of cell death (Bad), resulting in its dissociation
from Bcl-2. Nuclear factor (NF) κB is also activated by AKT, which
in turn upregulates the transcription of many survival genes
(11). AKT also induces
angiogenesis through the upregulation of VEGF (12). The existence of an extensive
AKT-microRNA regulatory network suggests that microRNA-mediated
gene regulation interacts with the AKT signaling pathway (13). Hence, the expression of AKT is a
pivotal tumorigenic factor, and AKT is recognized as a relevant
molecular target for cancer therapy (8).
5. The activion of various cancer cells by
AKT
It has been reported that AKT has some involvement
in the motor activity of various cells, including cancer cells. For
example, AKT increases the motor activity of mammary epithelial
cells by increasing the expression of MMP-2 (14). In addition, an examination using
cells derived from fibrosarcoma and pancreatic cancer showed that
increased expression of the receptor for MMP-9 and IGF-1 led to
enhanced metastasis of cells, and this effect depended on the
activation of AKT kinase (15,16).
It was also reported that high expression of AKT induced
epithelial-mesenchymal transition (EMT) and enhanced the invasive
potential of squamous cell carcinoma cells (17). AKT gene amplification and mutations
cause AKT activation in many solid carcinomas and hematological
neoplasms, and mutations of PTEN, which has a negative effect on
PI3 kinase and growth factor receptors upstream of AKT activation,
occur frequently, which cause the abnormal activation of AKT
(5,18). AKT signaling consolidates various
intercellular signals; hence, it is essential to understand the
molecular mechanism for future studies of carcinogenesis and cancer
therapy.
6. The PI3K-AKT-mTOR pathway and its
activation in gastric cancer
mTOR is a serine/threonine kinase of 289 kD that is
activated by AKT and Rheb. mTOR exists as two separate complexes in
the cytoplasm (TORC1 and TORC2). TORC1 contains an mTOR binding
protein, Raptor, which facilitates the transcription and
translation of the protein through phosphorylation of S6K1 (p70 S6
kinase l = 40S ribosomal protein S6 kinase l) and eukaryotic
translation initiation factor 4E-binding protein 1 (4EBP1) in the
presence of amino acids, and accelerates cell proliferation. In
addition, the activation of hypoxia-inducible factor-1 (HIF-1)
leads TORC1 to induce angiogenesis through VEGF and in the
facilitation of progression of the cell cycle from the G1 to S
phase through Cyclin D. At the same time, TORC2, which contains
Rictor, provides positive feedback to activate AKT, and facilitates
cell survival.
The PI3K-AKT-mTOR pathway has been reported to be
activated in many malignant tumors due to abnormalities in various
genes such as EGFR, HER2, PTEN, PIK3CA and TSC1 (19,20).
In gastric cancer, it was reported that gene mutations and gene
amplifications of PIK3CA, AKT gene amplification and a loss of PTEN
can all cause activation of the PI3K-AKT-mTOR pathway, and that
such mutations or amplifications are present in 30–60% of cases
(21). Moreover, HIF-1 is involved
in cell proliferation, angiogenesis and blood vessel maturation in
gastric cancer. These fundamental findings suggest that the
PI3K-AKT-mTOR pathway is important as a target for antineoplastic
agents (22).
7. hTERT
The activity of human telomerase reverse
transcriptase (hTERT) is regulated by its expression and
phosphorylation. Protein kinase C and AKT can both phosphorylate
hTERT (23,24). AKT-mediated phosphorylation of
hTERT induces the intranuclear translocation of hTERT, and
subsequently, activates hTERT. In contrast, ring finger protein 1,
an E3 ubiquitin ligase, decreases the activity of hTERT by inducing
its ubiquitylation (25).
Dysregulated PTEN/PI3K/AKT signaling interacts with
the Wingless-INT pathway to induce EMT, which is usually associated
with a cancer stem cell-phenotype and a poor prognosis (26). It has recently been reported that
hTERT promotes transforming growth factor-β and β-catenin-induced
EMT by inducing the nuclear translocation of β-catenin and
increasing its transcriptional activity for vimentin expression
(27). Therefore, PTEN/PI3K/AKT
signaling enhances EMT and stem cell phenotypes. In one of our
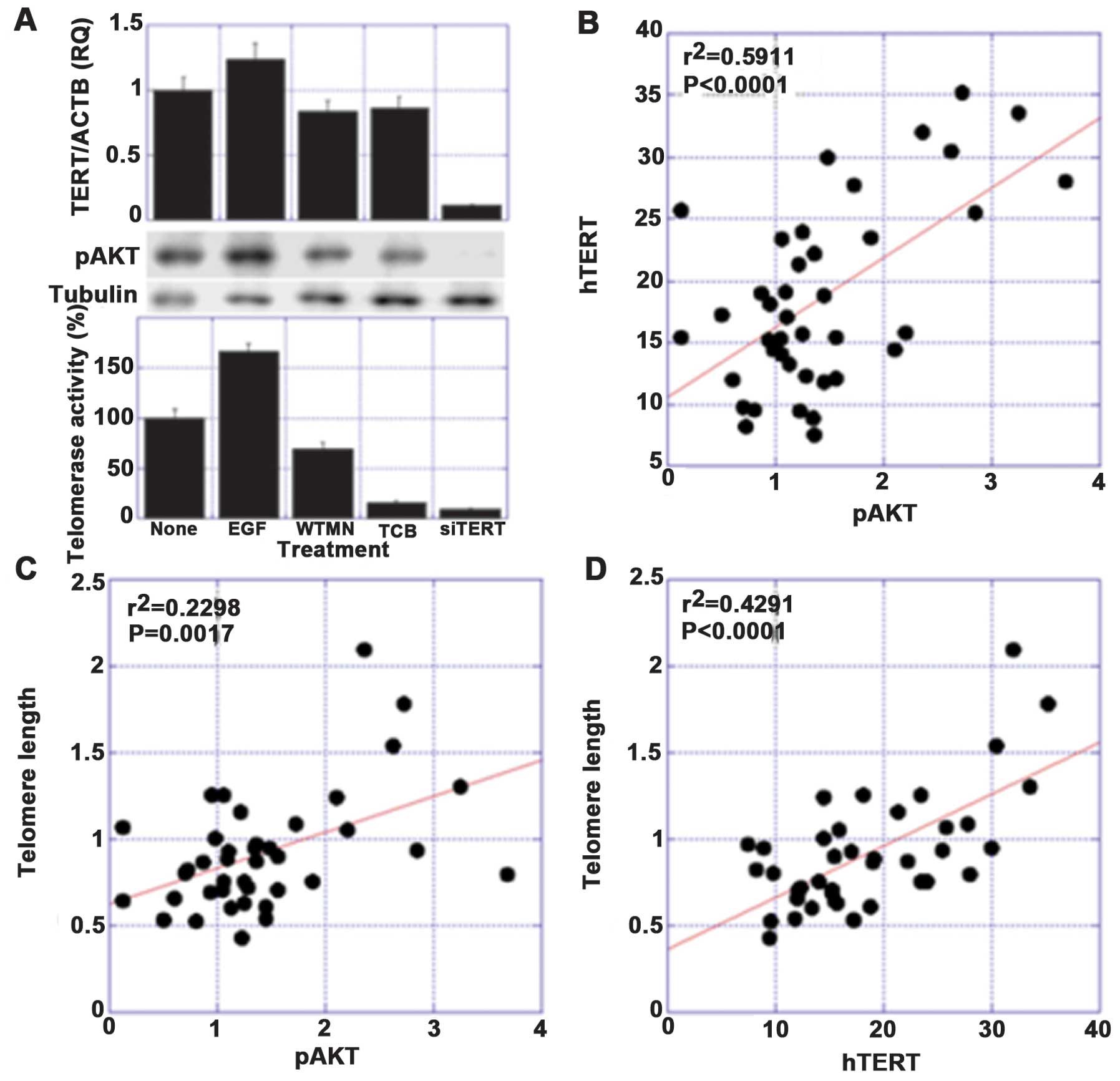
studies, significant correlations were found among the levels of
phosphorylated AKT (pAKT), hTERT expression and the telomere length
(Fig. 1).
8. The concurrent effects of pAKT and hTERT
on the progression of gastric cancer
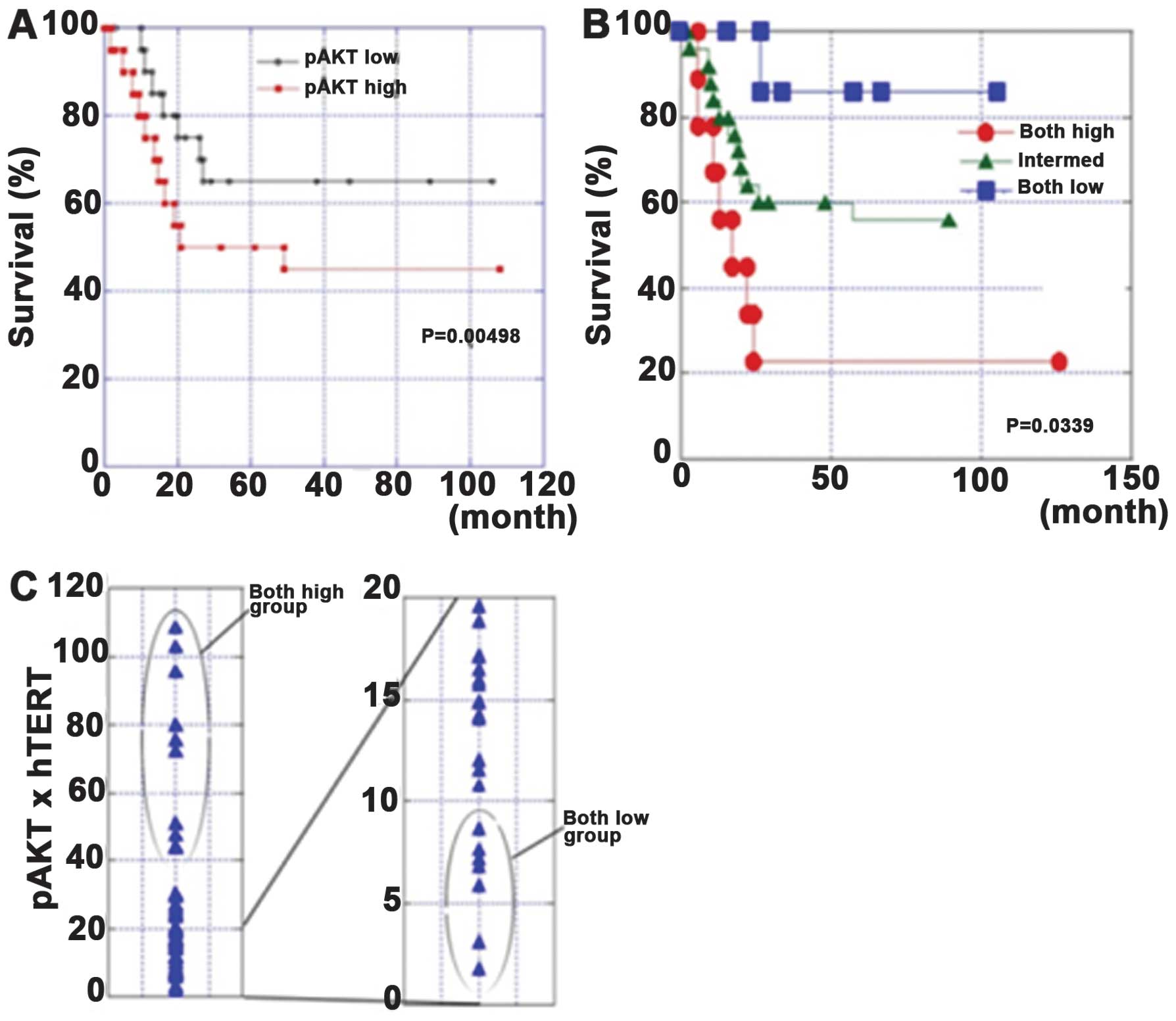
In our study, the association of AKT
phosphorylation, TERT expression and telomerase activity was
confirmed in tissue specimens from 40 patients with gastric cancer.
The survival rates of the pAKT-high patients or the pAKT-high and
hTERT-high patients were significantly poorer than those in other
patients. These associations resulted in poor prognoses in cases
with high pAKT levels or high pAKT/hTERT levels. A multivariate
analysis revealed that the pAKT levels or pAKT/hTERT levels were
independent prognostic factors. The examination of more gastric
cancer cases is required to confirm the hypothesis that the
EMT/stem cell phenotype affects disease progression.
Angiogenesis is associated with cancer progression
(28). VEGF expression is closely
related to neovascularization and cancer progression in many
malignancies. The PI3K/AKT pathway is one of the inducers of a VEGF
response, which includes other inducers, such as mitogen-activated
protein kinase (extracellular signal-regulated kinases or p38),
Src, focal adhesion kinase, Rho family GTPases and endothelial
nitric oxide (29). The PI3K/AKT
pathway increases the secretion of VEGF from cancer cells by
HIF-1-dependent and -independent mechanisms (30). Therefore, AKT suppression could
result in an anti-angiogenic effect on gastric cancer.
Our data showed that AKT and hTERT were widely
expressed in gastric cancer. The concurrent expression of these two
proteins at high levels is associated with a poor prognosis
(31). These results suggest that
AKT and hTERT are good molecular targets, and that inhibiting them
could be useful for the treatment of gastric cancer (Fig. 2).
9. Relationships among the levels of pAKT
and iNOS, NT and hTERT in gastric mucosa
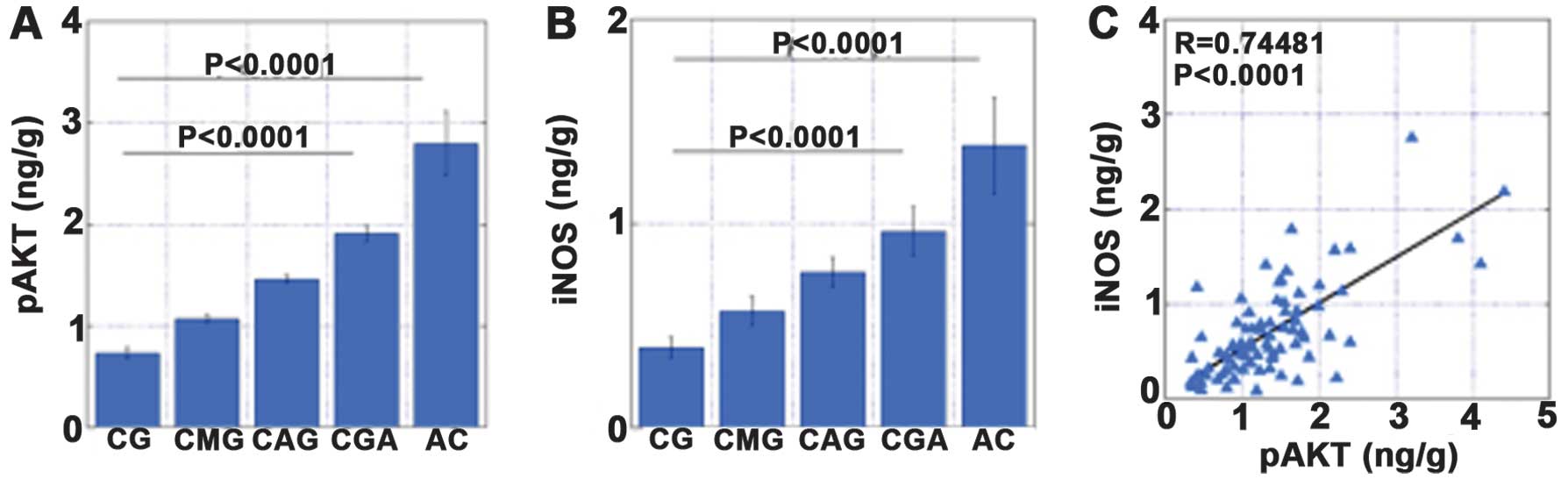
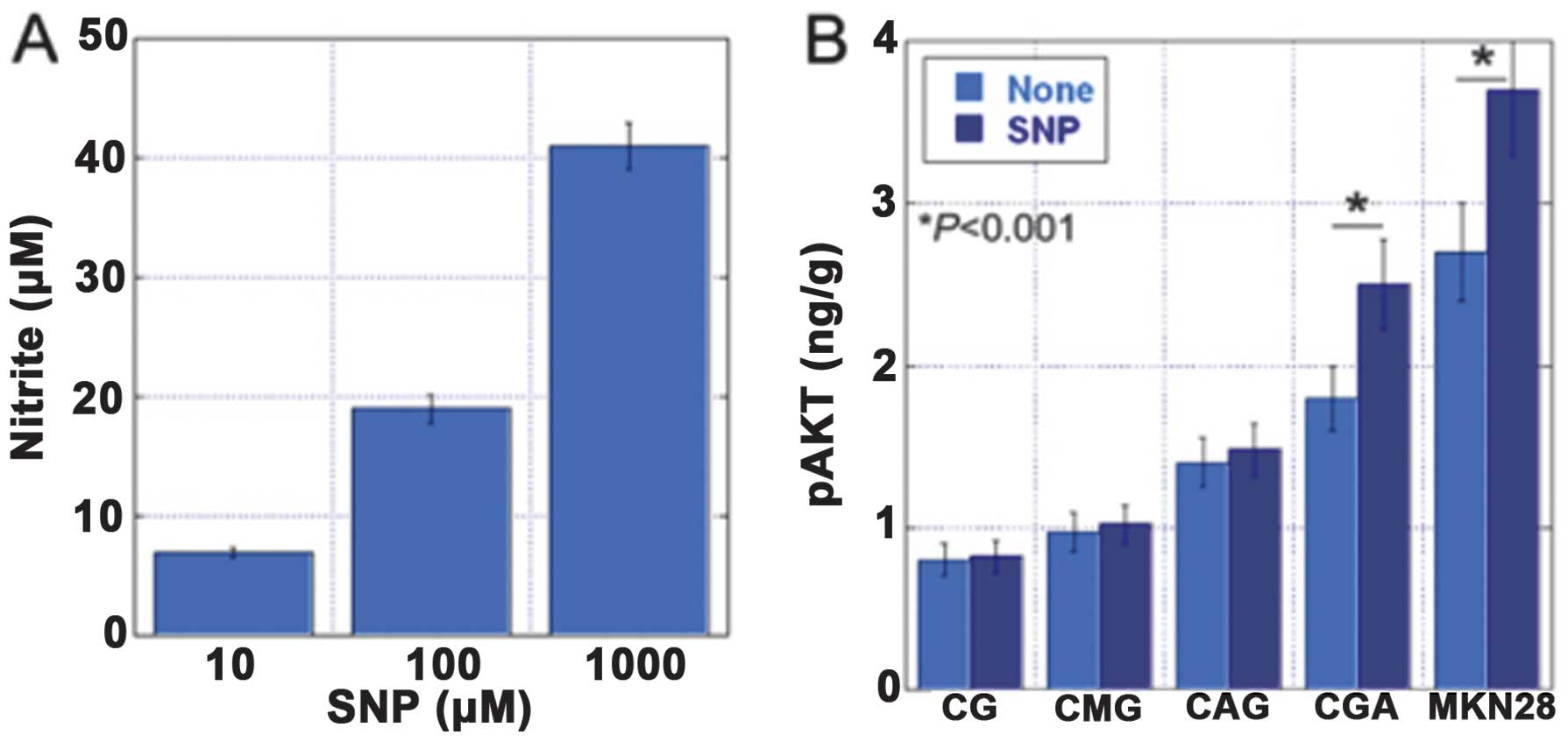
AKT phosphorylation was associated with the
expression levels of inducible nitric oxide synthase (iNOS) and
hTERT, and with increased levels of nitrotyrosine (NT). To
establish the role of oxidative stress and v-akt murine thymoma
viral oncogene homolog (AKT) activation in the development of
gastric cancer, we examined the levels of pAKT, iNOS, NT and hTERT
by an enzyme-linked immunosorbent assay in 73 non-cancerous gastric
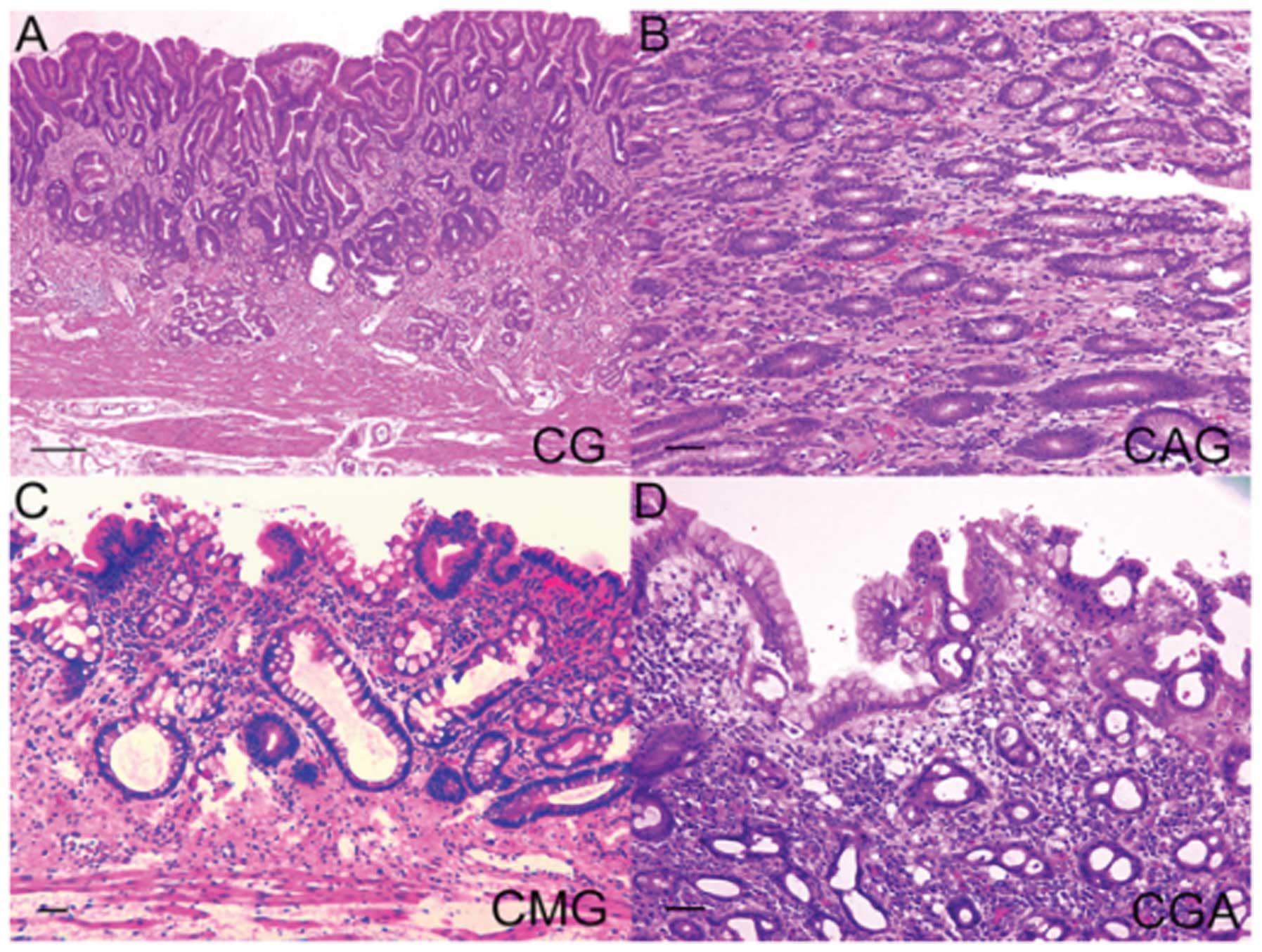
mucosa samples and 10 gastric carcinoma specimens (32). The gastric mucosa was classified
into four categories: chronic gastric mucosa without
Helicobacter pylori (H. pylori) (CG), chronic active
gastritis with H. pylori (CAG), chronic metaplastic
gastritis without H. pylori (CMG) and chronic gastritis with
atypia without H. pylori (CGA) (Fig. 3). The levels of pAKT were
associated with the levels of iNOS, NT and hTERT. In addition, the
levels of pAKT, iNOS and NT increased in the order CG, CAG, CMG and
CGA (Figs. 4–7).
10. Conclusions
The role of AKT signal transduction in gastric
cancer was discussed. It is important to analyze the role of AKT
while taking into consideration the microenvironment and individual
variations. The AKT signal transduction in gastric cancer may
represent a biomarker for the diagnosis of gastric cancer and a
target for treatment in the future.
Abbreviations:
|
PI3K
|
phosphatidylinositol-3 kinase
|
|
hTERT
|
human telomerase reverse
transcriptase
|
|
pAKT
|
phosphorylated AKT
|
|
EGF
|
epidermal growth factor
|
|
PTEN
|
phosphatase and tensin homolog deleted
on chromosome 10
|
|
mTOR
|
mammalian target of rapamycin
|
|
NF
|
nuclear factor
|
|
EMT
|
epithelial-mesenchymal transition
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Tokunaga E, Oki E, Egashira A, Sadanaga N,
et al: Deregulation of the Akt pathway in human cancer. Curr Cancer
Drug Targets. 8:27–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stiles B, Gilman V, Khanzenzon N, et al:
Essential role of AKT-1/protein kinase B alpha in PTEN-controlled
tumorigenesis. Mol Cell Biol. 22:3842–3851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellacosa A, Testa JR, Moore R and Larue
L: A portrait of AKT kinases: human cancer and animal models depict
a family with strong individualities. Cancer Biol Ther. 3:268–275.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Manning BD and Cantley LC: AKT/PKB
signaling: navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engelman JA, Luo J and Cantley LC: The
evolution of phosphatidylinositol 3-kinases as regulators of growth
and metabolism. Nat Rev Genet. 7:606–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sukawa Y, Yamamoto H, Nosho K, et al:
Alterations in the human epidermal growth factor receptor
2-phosphatidylinositol 3-kinase-v-Akt pathway in gastric cancer.
World J Gastroenterol. 18:6577–6586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berg M and Soreide K: EGFR and downstream
genetic alterations in KRAS/BRAF and PI3K/AKT pathways in
colorectal cancer: implications for targeted therapy. Discov Med.
14:207–214. 2012.PubMed/NCBI
|
|
9
|
Ng L, Poon RT and Pang R: Biomarkers for
predicting future metastasis of human gastrointestinal tumors. Cell
Mol Life Sci. 70:3631–3656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheung M and Testa JR: Diverse mechanisms
of AKT pathway activation in human malignancy. Curr Cancer Drug
Targets. 13:234–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Downward J: PI 3-kinase, Akt and cell
survival. Semin Cell Dev Biol. 15:177–182. 2004. View Article : Google Scholar
|
|
12
|
Radisavljevic Z: AKT as locus of cancer
angiogenic robustness and fragility. J Cell Physiol. 228:21–24.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu M and Mo YY: The Akt-associated
microRNAs. Cell Mol Life Sci. 69:3601–3612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park BK, Zeng X and Glazer RI: Akt1
induces extracellular matrix invasion and matrix
metalloproteinase-2 activity in mouse mammary epithelial cells.
Cancer Res. 61:7647–7653. 2001.PubMed/NCBI
|
|
15
|
Kim D, Kim S, Koh H, et al: Akt/PKB
promotes cancer cell invasion via increased motility and
metalloproteinase production. FASEB J. 15:1953–1962. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanno S, Tanno S, Mitsuuchi Y, Altomare
DA, Xiao GH and Testa JR: AKT activation up-regulates insulin-like
growth factor I receptor expression and promotes invasiveness of
human pancreatic cancer cells. Cancer Res. 61:589–593. 2001.
|
|
17
|
Grille SJ, Bellacosa A, Upson J, et al:
The protein kinase Akt induces epithelial mesenchymal transition
and promotes enhanced motility and invasiveness of squamous cell
carcinoma lines. Cancer Res. 63:2172–2178. 2003.PubMed/NCBI
|
|
18
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Granville CA, Memmott RM, Gills JJ and
Dennis PA: Handicapping the race to develop inhibitors of the
phosphoinositide 3-kinase/Akt/mammalian target of rapamycin
pathway. Clin Cancer Res. 12:679–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cully M, You H, Levine AJ and Mak TW:
Beyond PTEN mutations: the PI3K pathway as an integrator of
multiple inputs during tumorigenesis. Nat Rev Cancer. 6:184–192.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Markman B, Atzori F, Pérez-García J,
Tabernero J and Baselga J: Status of PI3K inhibition and biomarker
development in cancer therapeutics. Ann Oncol. 21:683–691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yuan R, Kay A, Berg WJ and Lebwohl D:
Targeting tumorigenesis: development and use of mTOR inhibitors in
cancer therapy. J Hematol Oncol. 2:452009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li H, Zhao L, Yang Z, Funder JW and Liu
JP: Telomerase is controlled by protein kinase Calpha in human
breast cancer cells. J Biol Chem. 273:33436–33442. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kang SS, Kwon T, Kwon DY and Do SI: Akt
protein kinase enhances human telomerase activity through
phosphorylation of telomerase reverse transcriptase subunit. J Biol
Chem. 274:13085–13090. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JH, Park SM, Kang MR, et al: Ubiquitin
ligase MKRN1 modulates telomere length homeostasis through a
proteolysis of hTERT. Genes Dev. 19:776–781. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karamitopoulou E: Tumor budding cells,
cancer stem cells and epithelial-mesenchymal transition-type cells
in pancreatic cancer. Front Oncol. 2:2092013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Z, Li Q, Li K, et al: Telomerase
reverse transcriptase promotes epithelial-mesenchymal transition
and stem cell-like traits in cancer cells. Oncogene. 32:4203–4213.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fidler IJ: The pathogenesis of cancer
metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003.
|
|
29
|
Claesson-Welsh L and Welsh M: VEGFA and
tumour angiogenesis. J Intern Med. 273:114–127. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sasaki T, Kuniyasu H, Luo Y, et al: AKT
activation and telomerase reverse transcriptase expression are
concurrently associated with prognosis of gastric cancer.
Pathobiology. 81:36–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasaki T, Kuniyasu H, Luo Y, et al:
Increased phosphorylation of AKT in high-risk gastric mucosa.
Anticancer Res. 33:3295–3300. 2013.PubMed/NCBI
|