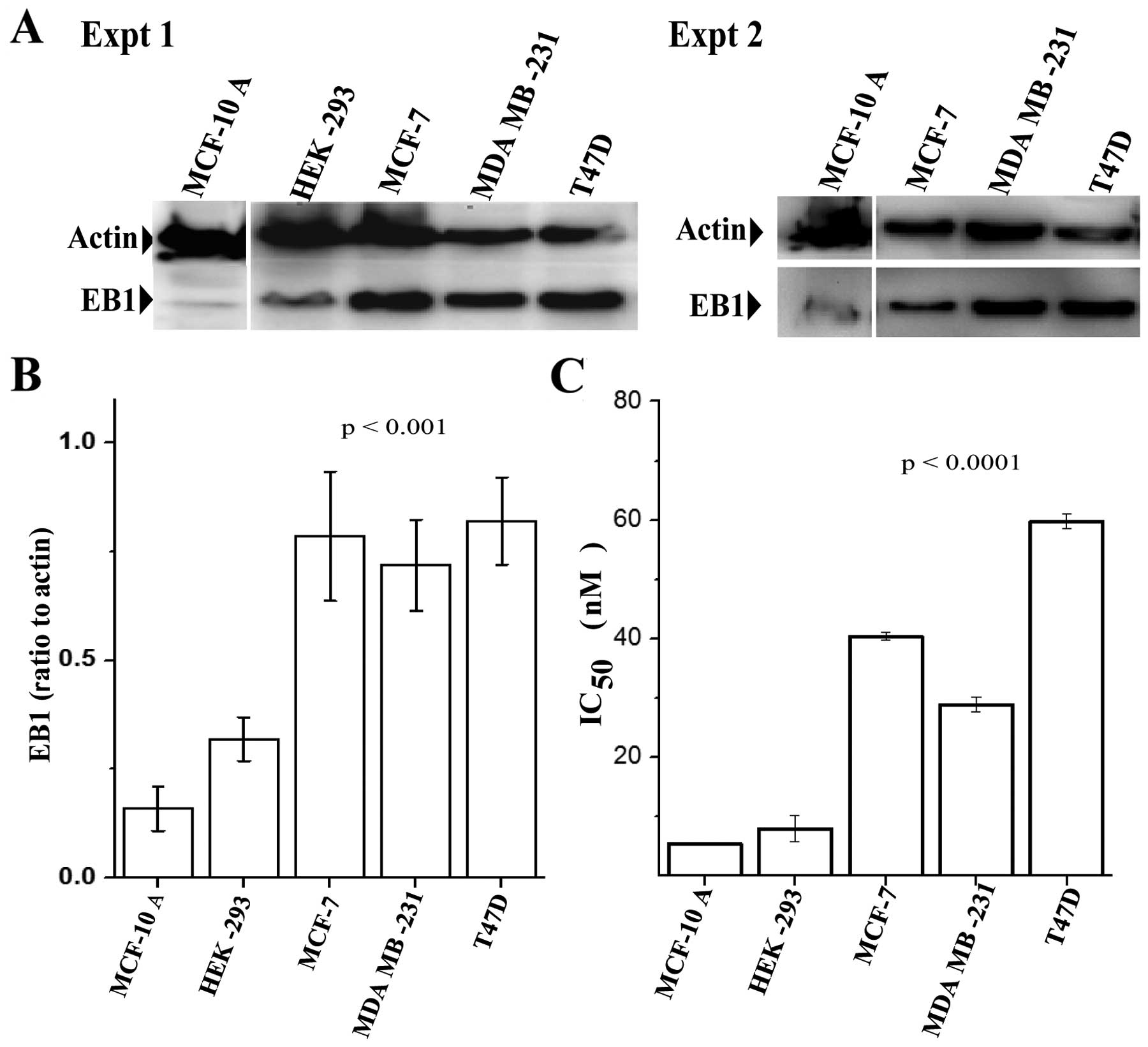
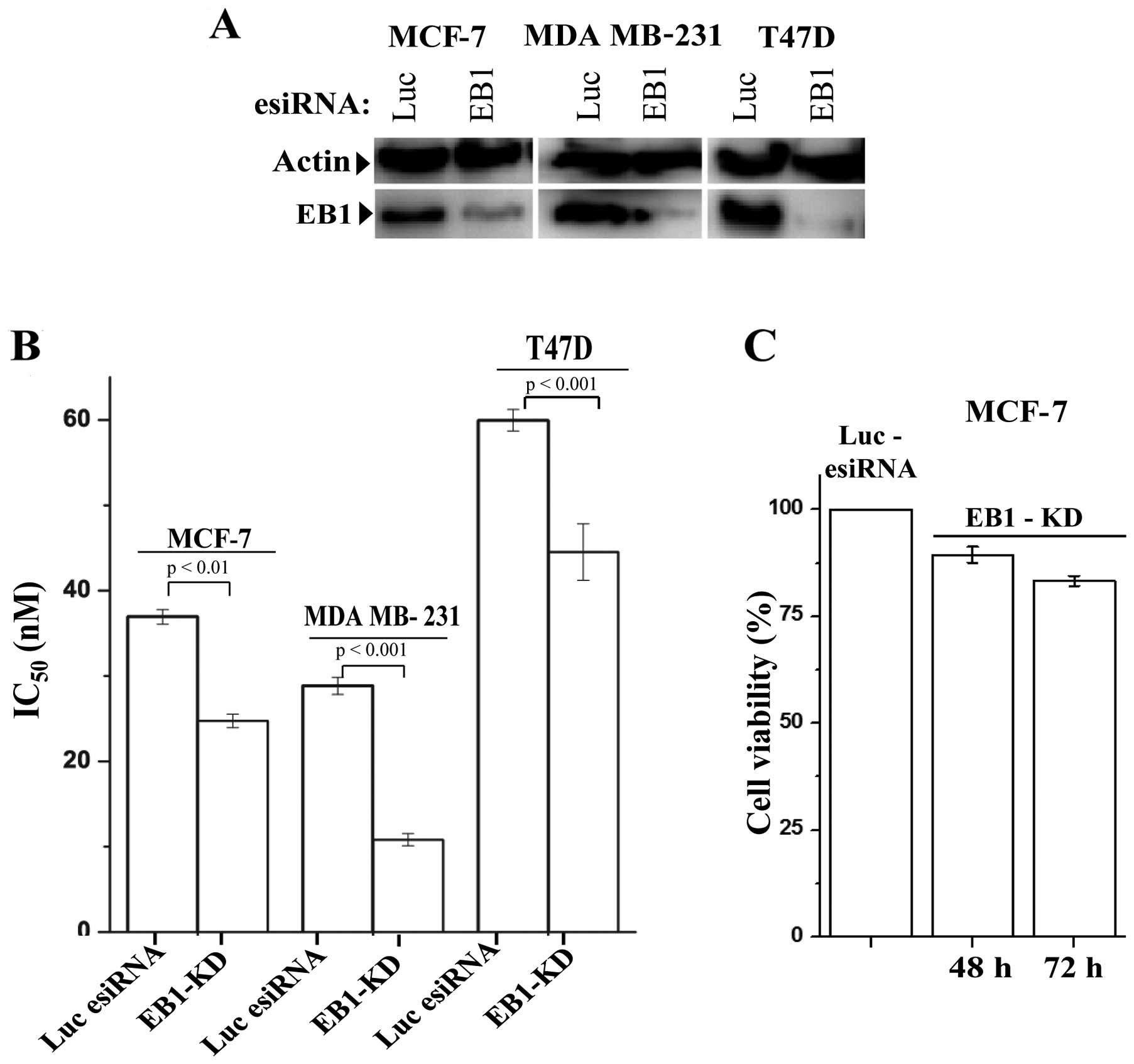
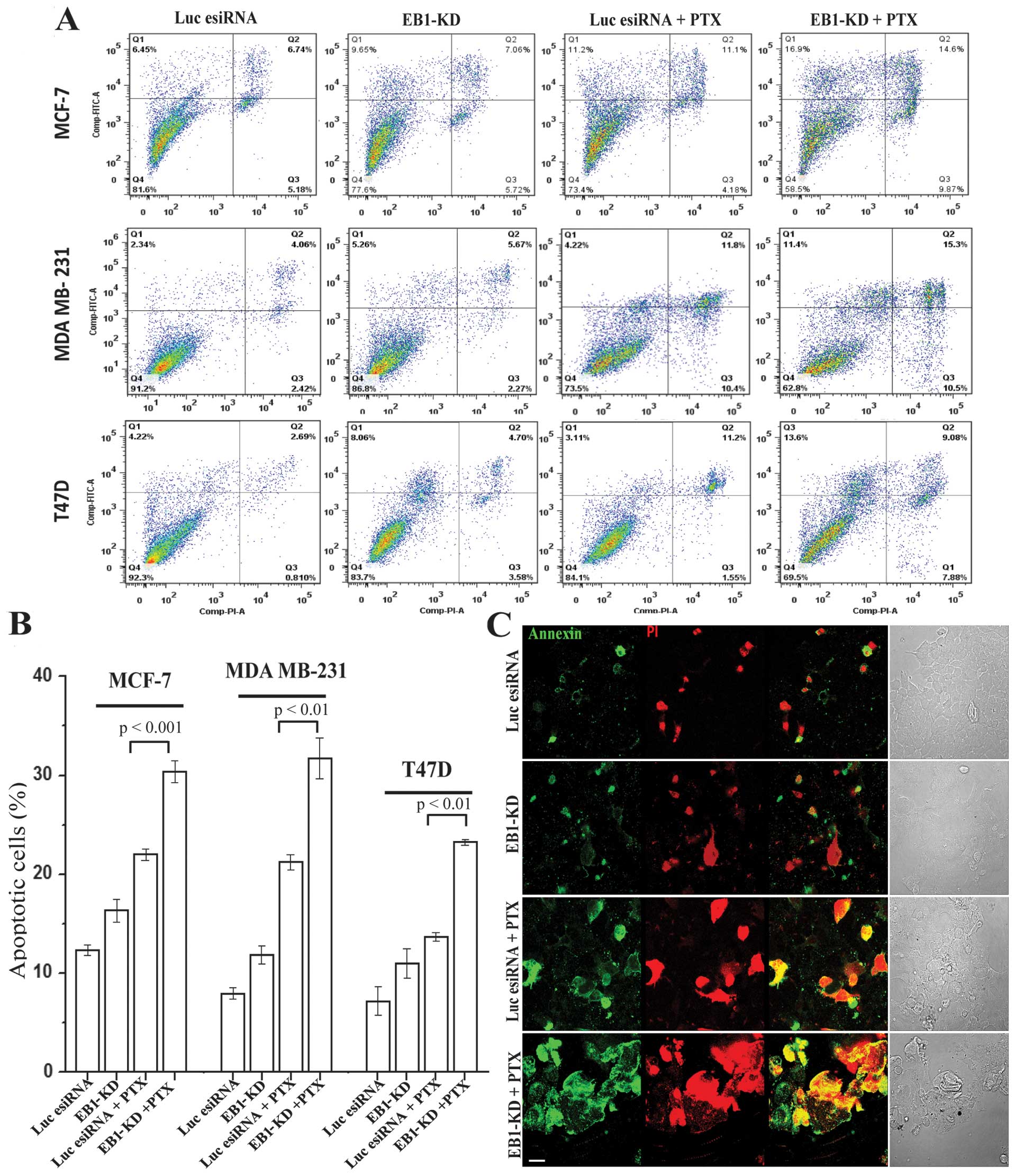
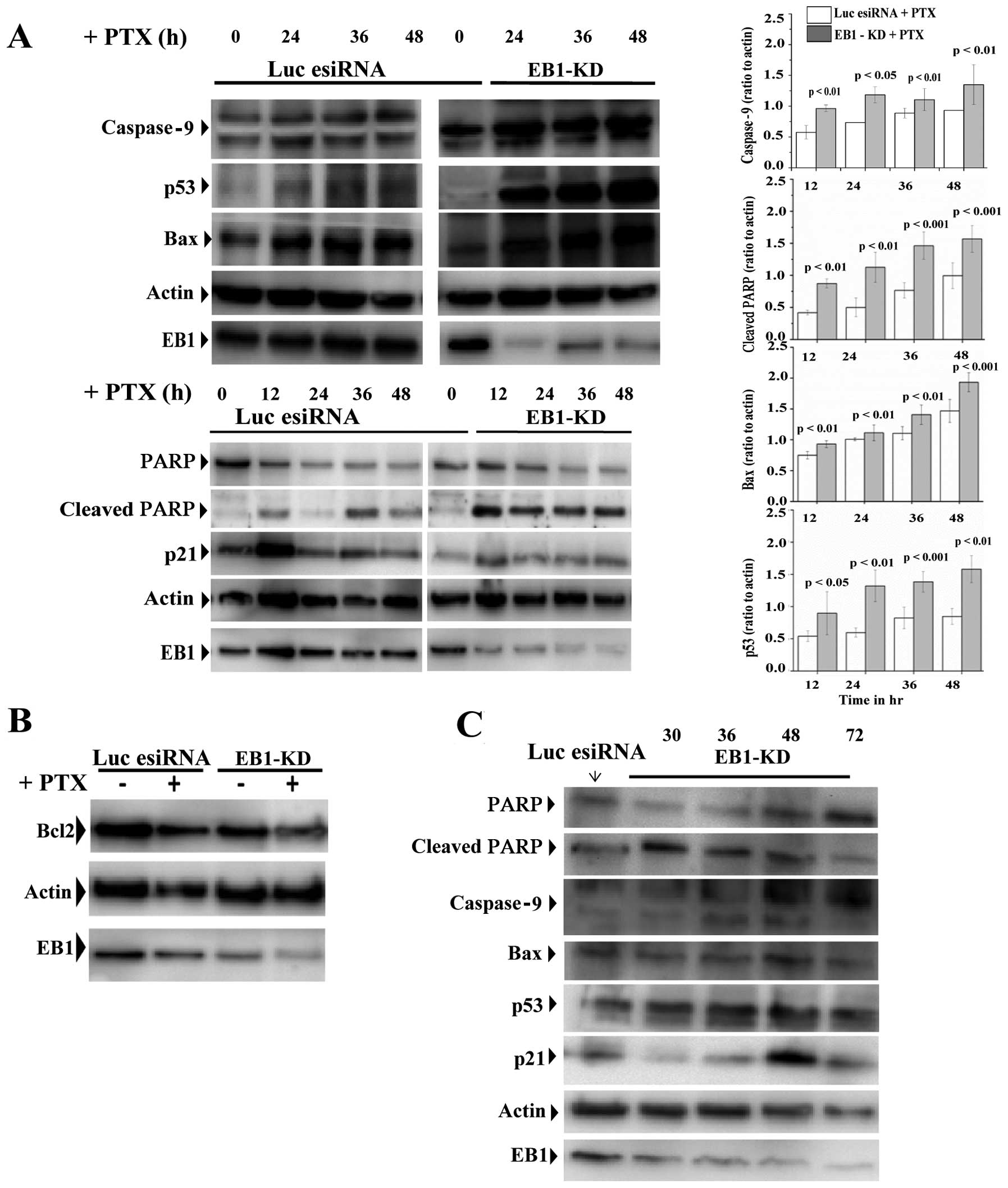
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographical regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2
|
Mamounas EP, Bryant J, Lembersky B,
Fehrenbacher L, Sedlacek SM, Fisher B, Wickerham DL, Yothers G,
Soran A and Wolmark N: Paclitaxel after doxorubicin and
cyclophosphamide as adjuvant chemotherapy for node-positive breast
cancer: results from NSABP B-28. J Clin Oncol. 23:3686–3696. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin M, Seguí MA, Antón A, Ruiz A, Ramos
M, Adrover E, Aranda I, Rodríguez-Lescure A, Grosse R, Calvo L, et
al: GEICAM 9805 Investigators: Adjuvant docetaxel for high-risk,
node-negative breast cancer. N Engl J Med. 363:2200–2210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Henderson IC, Berry DA, Demetri GD,
Cirrincione CT, Goldstein LJ, Martino S, Ingle JN, Cooper MR, Hayes
DF, Tkaczuk KH, et al: Improved outcomes from adding sequential
Paclitaxel but not from escalating Doxorubicin dose in an adjuvant
chemotherapy regimen for patients with node-positive primary breast
cancer. J Clin Oncol. 21:976–983. 2003. View Article : Google Scholar
|
|
5
|
McGrogan BT, Gilmartin B, Carney DN and
McCann A: Taxanes, microtubules and chemoresistant breast cancer.
Biochim Biophys Acta. 1785:96–132. 2008.PubMed/NCBI
|
|
6
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manfredi JJ, Parness J and Horwitz SB:
Taxol binds to cellular microtubules. J Cell Biol. 94:688–696.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giannakakou P, Gussio R, Nogales E,
Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC
and Fojo T: A common pharmacophore for epothilone and taxanes:
molecular basis for drug resistance conferred by tubulin mutations
in human cancer cells. Proc Natl Acad Sci USA. 97:2904–2909. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Orr GA, Verdier-Pinard P, McDaid H and
Horwitz SB: Mechanisms of Taxol resistance related to microtubules.
Oncogene. 22:7280–7295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kavallaris M, Kuo DY, Burkhart CA, Regl
DL, Norris MD, Haber M and Horwitz SB: Taxol-resistant epithelial
ovarian tumors are associated with altered expression of specific
beta-tubulin isotypes. J Clin Invest. 100:1282–1293. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Derry WB, Wilson L and Jordan MA:
Substoichiometric binding of taxol suppresses microtubule dynamics.
Biochemistry. 34:2203–2211. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jordan MA, Toso RJ, Thrower D and Wilson
L: Mechanism of mitotic block and inhibition of cell proliferation
by taxol at low concentrations. Proc Natl Acad Sci USA.
90:9552–9556. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blagosklonny MV, Robey R, Sheikh MS and
Fojo T: Paclitaxel-induced FasL-independent apoptosis and slow
(non-apoptotic) cell death. Cancer Biol Ther. 1:113–117. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ganguly A, Yang H, Pedroza M, Bhattacharya
R and Cabral F: Mitotic centromere-associated kinesin (MCAK)
mediates paclitaxel resistance. J Biol Chem. 286:36378–36384. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung M and Giannakakou P: BRCA1 regulates
microtubule dynamics and taxanes-induced apoptotic cell signaling.
Oncogene. 33:1418–1428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Liu B, Zhang C, Peng G, Liu M, Li
D, Gu F, Chen Q, Dong JT, Fu L and Zhou J: Parkin regulates
paclitaxel sensitivity in breast cancer via a microtubule-dependent
mechanism. J Pathol. 218:76–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alli E, Bash-Babula J, Yang JM and Hait
WN: Effect of stathmin on the sensitivity to antimicrotubule drugs
in human breast cancer. Cancer Res. 62:6864–6869. 2002.PubMed/NCBI
|
|
18
|
Sun X, Li D, Yang Y, Ren Y, Li J, Wang Z,
Dong B, Liu M and Zhou J: Microtubule-binding protein CLIP-170 is a
mediator of paclitaxel sensitivity. J Pathol. 226:666–673. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Q and Luduena RF: Removal of beta III
isotype enhances taxol induced microtubule assembly. Cell Struct
Funct. 18:173–182. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kamath K, Wilson L, Cabral F and Jordan
MA: Beta-tubulin induces paclitaxel resistance in association with
reduced effects on microtubule dynamic instability. J Biol Chem.
280:12902–12907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akhmanova A and Steinmetz MO: Tracking the
ends: a dynamic protein network controls the fate of microtubule
tips. Nat Rev Mol Cell Biol. 9:309–322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Honnappa S, Okhrimenko O, Jaussi R,
Jawhari H, Jelesarov I, Winkler FK and Steinmetz MO: Key
interaction modes of dynamic +TIP networks. Mol Cell. 23:663–671.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Komarova Y, Lansbergen G, Galjart N,
Grosveld F, Borisy GG and Akhmanova A: EB1 and EB3 control CLIP
dissociation from the ends of growing microtubules. Mol Biol Cell.
16:5334–5345. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Askham JM, Vaughan KT, Goodson HV and
Morrison EE: Evidence that an interaction between EB1 and
p150(Glued) is required for the formation and maintenance of a
radial microtubule array anchored at the centrosome. Mol Biol Cell.
13:3627–3645. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dixit R, Barnett B, Lazarus JE, Tokito M,
Goldman YE and Holzbaur EL: Microtubule plus-end tracking by
CLIP-170 requires EB1. Proc Natl Acad Sci USA. 106:492–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su LK and Qi Y: Characterization of human
MAPRE genes and their proteins. Genomics. 71:142–149. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tirnauer JS, Grego S, Salmon ED and
Mitchison TJ: EB1-microtubule interactions in Xenopus egg extracts:
role of EB1 in microtubule stabilization and mechanisms of
targeting to microtubules. Mol Biol Cell. 13:3614–3626. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manna T, Honnappa S, Steinmetz MO and
Wilson L: Suppression of microtubule dynamic instability by the
+TIP protein EB1 and its modulation by the CAP-Gly domain of
p150glued. Biochemistry. 47:779–786. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vitre B, Coquelle FM, Heichette C, Garnier
C, Chrétien D and Arnal I: EB1 regulates microtubule dynamics and
tubulin sheet closure in vitro. Nat Cell Biol. 10:415–421. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Komarova Y, De Groot CO, Grigoriev I,
Gouveia SM, Munteanu EL, Schober JM, Honnappa S, Buey RM,
Hoogenraad CC, Dogterom M, et al: Mammalian end binding proteins
control persistent microtubule growth. J Cell Biol. 184:691–706.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mohan R, Katrukha EA, Doodhi H, Smal I,
Meijering E, Kapitein LC, Steinmetz MO and Akhmanova A: End-binding
proteins sensitize microtubules to the action of
microtubule-targeting agents. Proc Natl Acad Sci USA.
110:8900–8905. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maurer SP, Cade NI, Bohner G, Gustafsson
N, Boutant E and Surrey T: EB1 accelerates two conformational
transitions important for microtubule maturation and dynamics. Curr
Biol. 24:372–384. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Draviam VM, Shapiro I, Aldridge B and
Sorger PK: Misorientation and reduced stretching of aligned sister
kinetochores promote chromosome missegregation in EB1- or
APC-depleted cells. EMBO J. 25:2814–2827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brüning-Richardson A, Langford KJ, Ruane
P, Lee T, Askham JM and Morrison EE: EB1 is required for spindle
symmetry in mammalian mitosis. PLoS One. 6:e288842011.PubMed/NCBI
|
|
35
|
Ward T, Wang M, Liu X, Wang Z, Xia P, Chu
Y, Wang X, Liu L, Jiang K, Yu H, et al: Regulation of a dynamic
interaction between two microtubule-binding proteins, EB1 and
TIP150, by the mitotic p300/CBP-associated factor (PCAF)
orchestrates kinetochore microtubule plasticity and chromosome
stability during mitosis. J Biol Chem. 288:15771–15785. 2013.
View Article : Google Scholar
|
|
36
|
Green RA, Wollman R and Kaplan KB: APC and
EB1 function together in mitosis to regulate spindle dynamics and
chromosome alignment. Mol Biol Cell. 16:4609–4622. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun L, Gao J, Dong X, Liu M, Li D, Shi X,
Dong J, Lu X, Liu C and Zhou J: EB1 promotes Aurora-B kinase
activity through blocking its inactivation by protein phosphatase
2A. Proc Natl Acad Sci USA. 105:7153–7158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dong X, Liu F, Sun L, Liu M, Li D, Su D,
Zhu Z, Dong JT, Fu L and Zhou J: Oncogenic function of microtubule
end-binding protein 1 in breast cancer. J Pathol. 220:361–369.
2010.PubMed/NCBI
|
|
39
|
Jaïs P, Sabourin JC, Bombled J, Rougier P,
Lasser P, Duvillard P, Bénard J and Bressac-de Paillerets B:
Absence of somatic alterations of the EB1 gene adenomatous
polyposis coli-associated protein in human sporadic colorectal
cancers. Br J Cancer. 78:1356–1360. 1998.PubMed/NCBI
|
|
40
|
Nishigaki R, Osaki M, Hiratsuka M, Toda T,
Murakami K, Jeang KT, Ito H, Inoue T and Oshimura M: Proteomic
identification of differentially-expressed genes in human gastric
carcinomas. Proteomics. 5:3205–3213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Zhou X, Zhu H, Liu S, Zhou C,
Zhang G, Xue L, Lu N, Quan L, Bai J, et al: Overexpression of EB1
in human esophageal squamous cell carcinoma (ESCC) may promote
cellular growth by activating beta-catenin/TCF pathway. Oncogene.
24:6637–6645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Soule HD, Maloney TM, Wolman SR, Peterson
WD Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF and Brooks
SC: Isolation and characterization of a spontaneously immortalized
human breast epithelial cell line, MCF-10. Cancer Res.
50:6075–6086. 1990.PubMed/NCBI
|
|
43
|
Kittler R, Heninger AK, Franke K,
Habermann B and Buchholz F: Production of endoribonuclease-prepared
short interfering RNAs for gene silencing in mammalian cells. Nat
Methods. 2:779–784. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Calegari F, Haubensak W, Yang D, Huttner
WB and Buchholz F: Tissue-specific RNA interference in
post-implantation mouse embryos with endoribonuclease-prepared
short interfering RNA. Proc Natl Acad Sci USA. 99:14236–14240.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Manna T, Thrower DA, Honnappa S, Steinmetz
MO and Wilson L: Regulation of microtubule dynamic instability in
vitro by differentially phosphorylated stathmin. J Biol Chem.
284:15640–15649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang L, Lau YK, Xia W, Hortobagyi GN and
Hung MC: Tyrosine kinase inhibitor emodin suppresses growth of
HER-2/neu-overexpressing breast cancer cells in athylic mice and
sensitizes these cells to the inhibitory effects of paclitaxel.
Clin Cancer Res. 5:343–353. 1999.
|
|
48
|
Gireesh KK, Rashid A, Chakraborti S, Panda
D and Manna T: CIL-102 binds to tubulin at colchicine binding site
and triggers apoptosis in MCF-7 cells by inducing monopolar and
multinucleated cells. Biochem Pharmacol. 84:633–645. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Young A, Dictenberg JB, Purohit A, Tuft R
and Doxsey SJ: Cytoplasmic dynein-mediated assembly of pericentrin
and gamma tubulin onto centrosomes. Mol Biol Cell. 11:2047–2056.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ciardiello F, Caputo R, Pomatico G, De
Laurentiis M, De Placido S, Bianco AR and Tortora G: Resistance to
taxanes is induced by c-erbB-2 overexpression in human MCF-10A
mammary epithelial cells and is blocked by combined treatment with
an antisense oligonucleotide targeting type I protein kinase A. Int
J Cancer. 85:710–715. 2000. View Article : Google Scholar
|
|
51
|
Giannakakou P, Sackett DL, Ward Y, Webster
KR, Blagosklonny MV and Fojo T: p53 is associated with cellular
microtubules and is transported to the nucleus by dynein. Nat Cell
Biol. 2:709–717. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Luo Y, Li D, Ran J, Yan B, Chen J, Dong X,
Liu Z, Liu R, Zhou J and Liu M: End-binding protein 1 stimulates
paclitaxel sensitivity in breast cancer by promoting its actions
toward microtubule assembly and stability. Protein Cell. 5:469–479.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Skube SB, Chaverri JM and Goodson HV:
Effect of GFP tags on the localization of EB1 and EB1 fragments in
vivo. Cytoskeleton (Hoboken). 67:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Giannakakou P, Nakano M, Nicolaou KC,
O’Brate A, Yu J, Blagosklonny MV, Greber UF and Fojo T: Enhanced
microtubule-dependent trafficking and p53 nuclear accumulation by
suppression of microtubule dynamics. Proc Natl Acad Sci USA.
99:10855–10860. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rathinasamy K and Panda D: Kinetic
stabilization of microtubule dynamic instability by benomyl
increases the nuclear transport of p53. Biochem Pharmacol.
76:1669–1680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Carney BK, Caruso Silva V and Cassimeris
L: The microtubule cytoskeleton is required for a G2 cell cycle
delay in cancer cells lacking stathmin and p53. Cytoskeleton
(Hoboken). 69:278–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tao W, South VJ, Zhang Y, Davide JP,
Farrell L, Kohl NE, Sepp-Lorenzino L and Lobell RB: Induction of
apoptosis by an inhibitor of the mitotic kinesin KSP requires both
activation of the spindle assembly checkpoint and mitotic slippage.
Cancer Cell. 8:49–59. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Maurer SP, Fourniol FJ, Bohner G, Moores
CA and Surrey T: EBs recognize a nucleotide dependent structural
cap at growing microtubule ends. Cell. 149:371–382. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sandblad L, Busch KE, Tittmann P, Gross H,
Brunner D and Hoenger A: The Schizosaccharomyces pombe EB1
homolog Mal3p binds and stabilizes microtubule lattice seam. Cell.
127:1415–1424. 2006. View Article : Google Scholar
|
|
60
|
Pagano A, Honoré S, Mohan R, Berges R,
Akhmanova A and Braguer D: Epothilone B inhibits migration of
glioblastoma cells by inducing microtubule catastrophes and
affecting EB1 accumulation at microtubule plus ends. Biochem
Pharmacol. 84:432–443. 2012. View Article : Google Scholar : PubMed/NCBI
|