Introduction
Galectin-3 (Gal-3) is a chimera-type member of the
carbohydrate-binding protein family with high affinity for
β-galactosides (1). Gal-3 is
located in the nucleus, cytosol, mitochondria, cell surface and
extracellular space and is also secreted. Gal-3 has been shown to
be involved in extracellular interactions between the cell surface
and extracellular matrix glycoproteins and glycolipids, and
intracellular interactions between nuclear and cytoplasmic proteins
to regulate signaling pathways (2). Gal-3 contributes to various cellular
processes, including cell growth, malignant transformation,
metastasis, angiogenesis, adhesion, migration and drug resistance
(3,4). Gal-3 expression in pancreatic cancer
and gastric cancer cells increases cell migration and invasion
(5,6). Gal-3 in breast cancer cells greatly
enhances adhesion to endothelial cells (7). Overexpression of Gal-3 through the
introduction of Gal-3 cDNA increases proliferation of oral tongue
squamous cell carcinoma (TSCC) cells and induces invasion and
epithelial-to-mesenchymal transition (EMT) (8). Gal-3 also promotes β-catenin/Wnt
signaling and induces cyclin D1 and c-Myc in TSCC and breast cancer
cells (8,9).
β-catenin is a multi-functional protein that plays a
key role at adherent junctions and in Wnt signaling, and
deregulation of β-catenin is associated with the development of
various human malignancies (10).
Binding of canonical Wnts to frizzled (Fz) receptor and low-density
lipoprotein 5 or 6 (LRP5/6) co-receptors leads to inhibition of
β-catenin phosphorylation and subsequent translocation into the
nucleus and activates the expression of Wnt-responsive genes
(11). Wnt signaling increases
osteoprogenitor cell proliferation and prevents apoptosis (12,13).
Based on gene expression profiling of human osteosarcoma,
deregulated Wnt signaling is also involved in high metastatic rate
and poor long-term survival (14).
Osteosarcoma (OS) is the most extensive malignant
bone tumor, occurring primarily in children and adolescents and
accounting for ~35% of all cases of bone cancer and the survival
rate of OS patients with localized disease is ~65% (15). However, despite significant
progress in OS treatment in recent years, because of the high rate
of metastasis and relapse and the poor response to combination
chemotherapy and radiation therapy, this disease has a poor
prognosis and the survival rate of patients with metastasis is ~25%
(16). Despite aggressive
chemotherapeutic treatment strategies, metastatic lesions develop
rapidly and the molecular mechanisms that are involved in
osteosarcoma growth and metastasis are not fully understood.
EMT is a major phenotype of cancer metastasis and
invasion. Wnt/β-catenin and focal adhesion signaling pathways (FAK,
Src and paxillin) cooperatively regulate the overall process of EMT
(17). Mechanical loading on
osteocyte also activates the β-catenin signaling pathway by a
mechanism involving nitric oxide, focal adhesion kinase and the Akt
signaling pathway (18). Increased
β-catenin-mediated activity has been frequently reported in
osteosarcoma (19,20). However, the correlation between
Gal-3 and β-catenin signaling in OS remained unclear. In the
present study, we provide the first demonstration that Gal-3 is
implicated in the regulation of migration and invasion of OS cells
subjected to expression of β-catenin. We also focus on discovering
the molecular mechanism underlying OS metastasis.
Materials and methods
Cell culture
The human OS cell line HOS was purchased from the
American Type Culture Collection (ATCC, Manassas, VA, USA). Cells
were maintained in RPMI-1640 medium (HyClone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone) and
antibiotics under a humidified atmosphere with 5%
CO2.
Chemicals
Cisplatin (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in sterile dimethyl sulfoxide (DMSO). PD98059 was
purchased from Calbiochem (San Diego, CA, USA). LY294002 was
purchased from Cell Signaling Technology (Beverly, MA, USA).
Transfection with small interfering RNA
(siRNA)
Experimentally verified human Gal-3-siRNA duplex,
FAK-siRNA duplex, and negative control-siRNA were obtained from
Bioneer Corp. (Daejeon, Korea) and siRNA targeting human β-catenin
was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cells were transiently transfected by electroporation under
optimized conditions. Briefly, cells were electroporated with 300
nM siRNA in serum-free medium in a 0.4-cm electroporation cuvette
using the Bio-Rad Gene Pulser Xcell System (Bio-Rad Laboratories,
Hercules, CA, USA).
Cell proliferation assay
Cell proliferation was measured using the AlamarBlue
assay. After 24-h transfection, cells were plated in triplicate in
96-well plates at a density of 5×103 cells/well. After
incubation for 24 h, AlamarBlue (Serotec Ltd., Kidlington, Oxford,
UK) was added (10% by volume) to each well and relative
fluorescence was determined 9 h later using a SpectraMax M2e
Multi-Detection Microplate Reader (Molecular Devices, Sunnyvale,
CA, USA; excitation, 530 nm; emission, 590 nm). Relative
fluorescence unit (RFU) values were expressed as mean ± standard
deviation (SD) of three determinations.
Migration and invasion assay
EBV-infected HCECs transendothelial migration assay
was performed using CytoSelect™ tumor transendothelial migration
assay kit (Cell Biolabs, Inc., San Diego, CA, USA), according to
the manufacturer’s protocol. Migrated cells were measured the
fluorescence (RFU) by microplate reader. The invasion assay was
determined using the CultreCoat 96 Well Medium BME Cell Invasion
Assay kit (R&D Systems, Minneapolis, MN, USA), according to the
manufacturer’s protocol. Invaded cells were stained with calcein AM
and quantified by microplate reader.
Wound healing assay
Wound healing assays were used to measure the
migration ability of bone cancer cells. Transfected HOS cells were
plated in 6-well plates. After the cell layers had reached
confluence, we inflicted a uniform wound in a straight line in each
monolayer using a 200-μl micropipette tip and washed the plates
with PBS to remove all cell debris. The cells were cultured in 5%
CO2 at 37°C and images were taken at 0 and 48 h after
scratching using an inverted phase contrast microscope at ×100
magnification.
ELISA
Concentrations of IL-6 and IL-8 in the culture
supernatants were quantified using a single cytokine ELISA kit
(Single Analyte ELISArray; Qiagen, Hilden, Germany). VEGF and Gal-3
were quantified using a single cytokine ELISA assay kit from
R&D Systems. The data are expressed as the average of
biological replicates ± SD.
Analysis of apoptosis and cell cycle by
flow cytometry
The percentage of human HOS cells undergoing
apoptosis was determined by flow cytometry using FITC-labeled
Annexin V (BD Biosciences, San Diego, CA, USA) and
7-amino-acti-nomycin D (7-AAD; BD Biosciences). After transfection
for 24 h, cells were treated with 1, 2, 5 or 10 μM cisplatin for 24
h and then harvested, rinsed with PBS, and incubated with Annexin V
and 7-AAD in Annexin V binding buffer at room temperature for 15
min in the dark. For cell cycle analysis, cells were harvested
after 36-h transfection, washed twice with PBS (2% FBS), fixed with
70% cold aqueous ethanol, and stored at 4°C for at least 1 h. Cell
pellets were stained with staining solution containing RNase A (10
mg/ml) and propidium iodide (PI; 2 mg/ml) in PBS for 30 min in the
dark at room temperature. The percentage of apoptotic cells and the
DNA content were measured using a FACSCalibur flow cytometer (BD
Biosciences) equipped with the CellQuest Pro software (BD
Biosciences).
Real-time PCR
Total RNA was extracted using an RNeasy Mini kit
(Qiagen) according to the manufacturer’s protocol and transcribed
into cDNA using oligo(dT) (Bioneer) and reverse transcriptase
(Bioneer). mRNA levels were quantified using an ECO Real-Time PCR
system (Illumina, Inc., San Diego, CA, USA) and a SYBR-Green Master
Mix kit (Takara, Tokyo, Japan) with the following specific primer
sets: Gal-3 (upstream primer, 5′-CCA AAG AGG GAA TGA TGT TGC C and
downstream primer, 5′-TGA TTG TAC TGC AAC AAG TGA GC); IL-6
(upstream primer, 5′-GTG TTG CCT GCT GCC TTC CCT G and downstream
primer, 5′-CTC TAG GTA TAC CTC AAA CTC CAA); IL-8 (upstream primer,
5′-ATG ACT TCC AAG CTG GCC GTG GCT and downstream primer, 5′-TCT
CAG CCC TCT TCA AAA ACT TCT C); VEGF (upstream primer, 5′-AGG AGG
GCA GAA TCA TCA CG and downstream primer, 5′-CAA GGC CCA CAG GGA
TTT TCT); MMP2 (upstream primer, 5′-TGG CAA GTA CGG CTT CTG TC and
downstream primer, 5′-TTC TTG TCG CGG TCG TAG TC); MMP9 (upstream
primer, 5′-TGC GCT ACC ACC TCG AAC TT and downstream primer, 5′-GAT
GCC ATT GAC GTC GTC CT); STAT3 (upstream primer, 5′-ACC TGC AGC AAT
ACC ATT GAC and downstream primer, 5′-AAG GTG AGG GAC TCA AAC TGC).
A specific primer set for β-actin (upstream primer, 5′-ATC CAC GAA
ACT ACC TTC AA and downstream primer, 5′-ATC CAC ACG GAG TAC TTG C)
was used as a control. The relative amount of mRNA was calculated
using the arithmetic formula 2−ΔΔ Cq, where ΔCq is the
difference between the threshold cycle of a given target cDNA and
an endogenous reference cDNA.
Immunoblotting
Cells were washed in PBS and lysed in NP-40 buffer
(Elpis Biotech, Daejeon, Korea) supplemented with a protease
inhibitor cocktail (Sigma-Aldrich). To address phosphorylation
events, an additional set of phosphatase inhibitors (Cocktail II;
Sigma-Aldrich) was added to the NP-40 buffer. Protein concentration
was determined using a BCA assay kit (Pierce, Rockford, IL, USA).
Proteins (10 μg/sample) were resolved by SDS-PAGE and transferred
to nitrocellulose (Millipore Corp., Billerica, MA, USA). The
membranes were blocked with 5% skim milk and standard western blot
analysis was performed. Chemiluminescence was detected using an ECL
kit (Advansta Corp., Menlo Park, CA, USA) and the multiple Gel DOC
system (Fujifilm). Primary antibodies (Abs) against the following
proteins were used: MMP2, MMP9, caspase-3, caspase-9, PARP,
β-actin, phospho-FAK (Tyr397), phospho-FAK
(Tyr925), FAK, phospho-Src (Tyr416), Src,
phospho-Lyn (Tyr507), Lyn, phospho-Stat3
(Tyr705), Stat3, phospho-PI3K p85 (Tyr458),
PI3K p85, phospho-Akt (Ser473), and Akt (Cell Signaling
Technology); Gal-3 and VEGF (Santa Cruz Biotechnology). Data were
analyzed using ImageJ 1.38 software.
Confocal microscopy
Cells were permeabilized with permeabilization
buffer (0.1% saponin in PBS) and incubated with primary Ab against
Gal-3, β-catenin, phospho-Src, phospho-Lyn and phospho-FAK for 30
min on ice, followed by incubation with FITC-conjugated secondary
Ab for 20 min. The nucleus was stained with PI (BD Biosciences) for
10 min. Cells were mounted in a Dako fluorescent mounting medium
and observed by confocal laser scanning microscope (Carl Zeiss) at
×400 magnification. Images were acquired using confocal microscopy
software release 3.0 (510META; Carl Zeiss).
Statistical analysis
Data were expressed as mean ± standard deviation
(SD). Statistical analysis was conducted using one-way analysis of
the variance (ANOVA). P-values <0.05 were considered
statistically significant.
Results
Effect of Gal-3 silencing on
proliferation and cell cycle distribution of human osteosarcoma
cells
Many previous studies have implied that Gal-3 is
involved in growth, angiogenesis, migration and invasion in various
cancers (3,4). To determine the potential role of
Gal-3 in OS progression, we inhibited Gal-3 expression in the HOS
cell line using a siRNA system, which is a powerful tool for
investigating gene function. We first confirmed that HOS cells
expressed high levels of Gal-3 by RT-PCR (data not shown) and
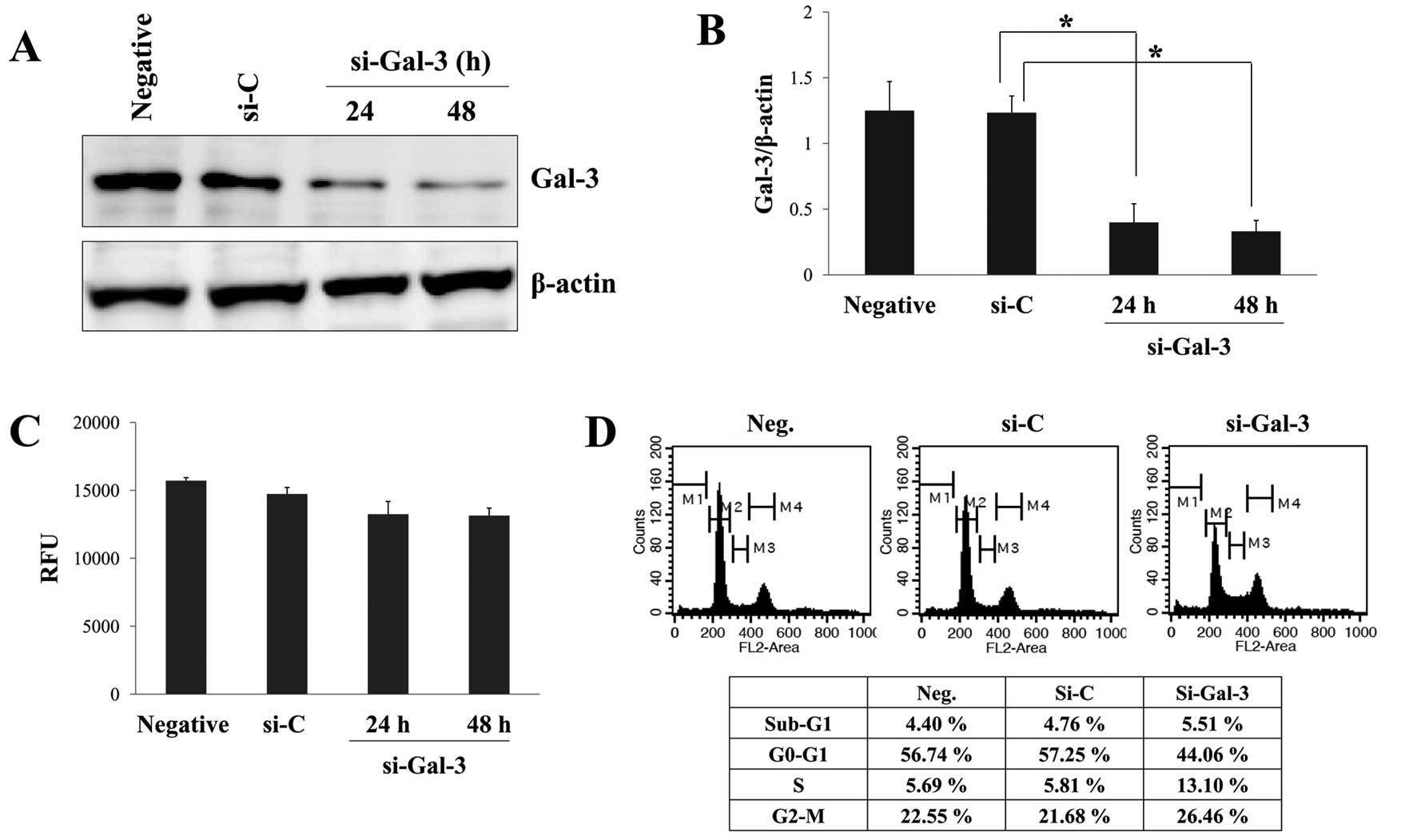
western blot analysis (Fig. 1A)
analyses. Significant inhibition of Gal-3 expression at the protein
level was detected 24 and 48 h after transient silencing of Gal-3
with specific Gal-3-siRNA, whereas control-siRNA had little effect
on Gal-3 expression and there was no noticeable difference between
untreated control cells and control-siRNA transfected cells
(Fig. 1A and B). At 48 h after
transfection, protein levels of Gal-3 were reduced by ~77% in cells
transfected with Gal-3-siRNA. We examined whether Gal-3-siRNA
affected proliferation and cell cycle distribution of HOS cells.
Contrary to our expectations, there was no significant difference
in proliferation between cells transfected with Gal-3-siRNA or
control-siRNA (Fig. 1C). Although
treatment with Gal-3-siRNA slightly affected cell cycle
distribution, Gal-3 silencing did not cause great changes in the
distribution compared with control-siRNA (Fig. 1D). These data suggested that Gal-3
silencing does not block the proliferation of HOS cells completely
but slightly contributes to the growth and cell cycle.
Gal-3 silencing suppresses migration and
invasion of osteosarcoma cells
In addition to proliferation, increased migration
and invasion are crucial features defining cancer progression and
inhibition of these functions may be a possible target for
anticancer therapy. Gal-3 expression in pancreatic cancer and
gastric cancer cells increase cell migration and invasion (5,6). We
therefore characterized the role of Gal-3 in the migration and
invasion of OS cells. Wound healing and migration assays were
performed to examine the effect of Gal-3 silencing on cell
motility. The time required for wound closure in HOS cells with
silenced Gal-3 was significantly longer than that required for
corresponding control cells (Fig.
2A), suggesting the involvement of Gal-3 in cell migration. As
shown in Fig. 2B, Transwell
migration assays showed that the migration capacity was reduced
~3.5-fold in Gal-3-siRNA transfected cells compared with the
control-siRNA group (Gal-3-siRNA, 479±60 RFU; control-siRNA,
1.701±56 RFU). Furthermore, we found that Gal-3 silencing also
suppressed invasiveness of the HOS cells; cell invasion was
decreased ~4-fold in Gal-3-silenced cells compared with
control-siRNA cells (Gal-3-siRNA, 9.027±0.466%; control-siRNA,
36.324±0.719%). These results indicate that Gal-3 mainly regulates
OS cell migration and invasion at least in vitro in our
experimental systems.
Gal-3 silencing decreases β-catenin
expression and suppresses activation of FAK/Src/Lyn and
Akt/ERK
Next, we investigated the molecular mechanism by
which Gal-3 mediates the motility of HOS cells. Recent studies have
shown that Gal-3 is closely associated with β-catenin/Wnt signaling
in several tumor types (8,9). The PI3K/Akt and ERK1/2 pathways are
key signaling pathways involved in the migratory behavior or
invasiveness of various tumors (21). ERK1/2 controls cytoskeletal
reorganization and focal adhesion turnover through the activation
of specific cytoskeletal and focal adhesion proteins, such as FAK
and paxillin (22). First, we
investigated the potential signaling molecules underlying
Gal-3-mediated β-catenin expression, focusing on FAK and Src family
tyrosine kinases (SFKs). Gal-3 silencing markedly reduced β-catenin
expression and the phosphorylation of FAK at Y397 and Y925 residues
in a time-dependent manner (Fig.
3A) and decreased phosphorylation of Src and Lyn, downstream
signaling molecules of FAK, time-dependently (Fig. 3B). To further investigate the
signaling pathway that mediates β-catenin expression, we assessed
the expression of PI3K/Akt and ERK1/2 by western blotting. We
observed that the phosphorylation levels of PI3K/Akt and ERK1/2
were strongly reduced in a time-dependent manner after Gal-3
silencing (Fig. 3C). In contrast,
cells transfected with control-siRNA exhibited little or no change
in phospho-PI3K/Akt or phospho-ERK1/2. Notably, after transfection
with Gal-3-siRNA, decreases in phospho-FAK/Src/Lyn and
phospho-Akt/ERK1/2 occurred within 24 h and a subsequent decrease
in β-catenin expression was detected at 36 h (Fig. 3). These results imply that the
reduction in phosphorylation of these tyrosine kinases occurs
before the downregulation of β-catenin after silencing Gal-3 in HOS
cells. We also observed changes in the subcellular distribution of
these molecules after depletion of Gal-3 using confocal microscopy.
As indicated in Fig. 4, the levels
of phospho-FAK, phospho-Src, phospho-Lyn, and β-catenin in
Gal-3-silenced cells were significantly attenuated compared with
control-siRNA cells. In particular, Gal-3 and β-catenin were
distributed in the cytoplasm and nuclei of the control-siRNA group
(Fig. 4), suggesting
co-localization of these proteins.
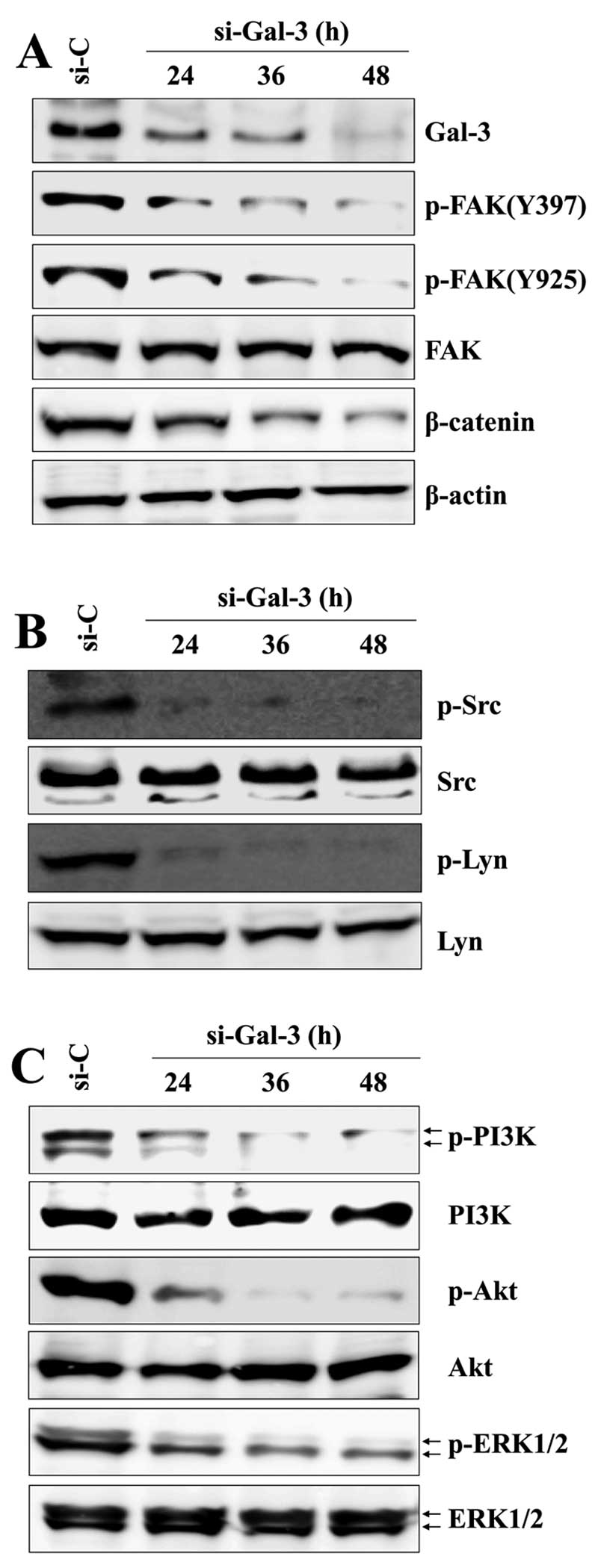 | Figure 3Gal-3 silencing decreases the
expression of β-catenin and suppresses the activation of FAK/Src
family and ERK/Akt. After transfection with Gal-3-siRNA or
control-siRNA for the indicated times, the levels of total and
phosphorylated proteins were detected by western blotting with Abs
to (A) Gal-3, p-FAK (Tyr397 and Tyr925), FAK, β-catenin, (B) p-Src,
Src, p-Lyn, Lyn, and (C) p-PI3K, PI3K, p-Akt, Akt, p-ERK1/2 and
ERK1/2. β-actin was used as a loading control. Results are
representative of three independent experiments. |
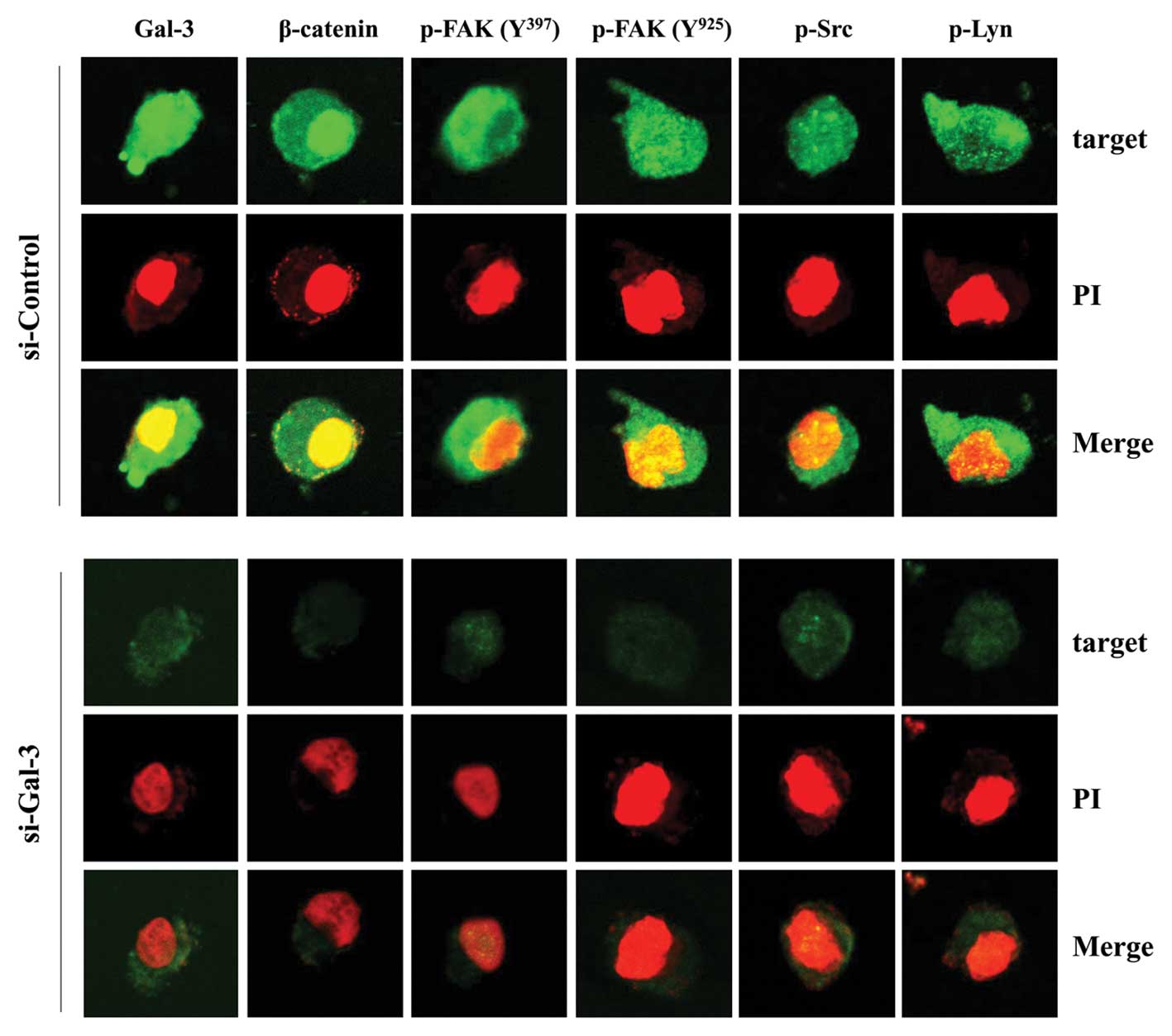 | Figure 4Subcellular distribution of p-FAK
(Tyr397 and Tyr925), p-Src, p-Lyn and β-catenin in HOS cells after
Gal-3 silencing. After transfection with either Gal-3- or
control-siRNA for 48 h, cells were permeabilized with 0.1% saponin
in PBS. Intracellular staining was performed using Abs against
p-FAK (Tyr397 and Tyr925), p-Src, p-Lyn, β-catenin and Gal-3. The
nucleus was stained with PI. Cells were observed under a confocal
microscope (magnification, ×400). Green fluorescence indicates
Gal-3, β-catenin, p-FAK (Tyr397 and Tyr925), p-Src or p-Lyn and red
fluorescence indicates the nucleus. |
The FAK/Src-Lyn/Akt-ERK axis induces
β-catenin and promotes migration and invasion of osteosarcoma
cells
To clarify the order of Gal-3-mediated events and
confirm whether these signals are essential for Gal-3-mediated
β-catenin regulation in HOS cells, we used β-catenin-siRNA,
FAK-siRNA, the PI3K/Akt inhibitor LY294002 and the ERK1/2 inhibitor
PD98059. After treatment of HOS cells with these inhibitors for 36
h, we analyzed protein expression by western blotting. As shown in
Fig. 5A and B, treatment with
FAK-siRNA inhibited the activation of Akt, ERK1/2, Src and Lyn and
expression of β-catenin, whereas LY294002 treatment suppressed only
β-catenin expression. However, inhibition of ERK1/2 or β-catenin
had no effect on these proteins. We next examined whether these
signals directly contribute to cell motility. As shown in Fig. 5C, there was a dramatic inhibition
of migration in cells with silenced β-catenin, FAK or Akt compared
with cells treated with control-siRNA or DMSO (459±25, 422±17,
507±19 RFU vs. 1569±18, 1472±16 RFU, respectively), whereas the
ERK1/2 inhibitor PD98059 (1148±39 RFU) had little effect on cell
migration. Invasion was also suppressed by treatment with
β-catenin-siRNA, FAK-siRNA or LY294002 (7.914±0.671, 9.767±0.794
and 11.539±0.473%, respectively), but not by PD98059
(26.243±1.764%), compared with control-siRNA or DMSO-treated cells
(35.108±0.849 and 34.051±0.543%). These results suggest that FAK
leads to the activation of Src and Lyn and then induces the
activation of Akt and ERK1/2.
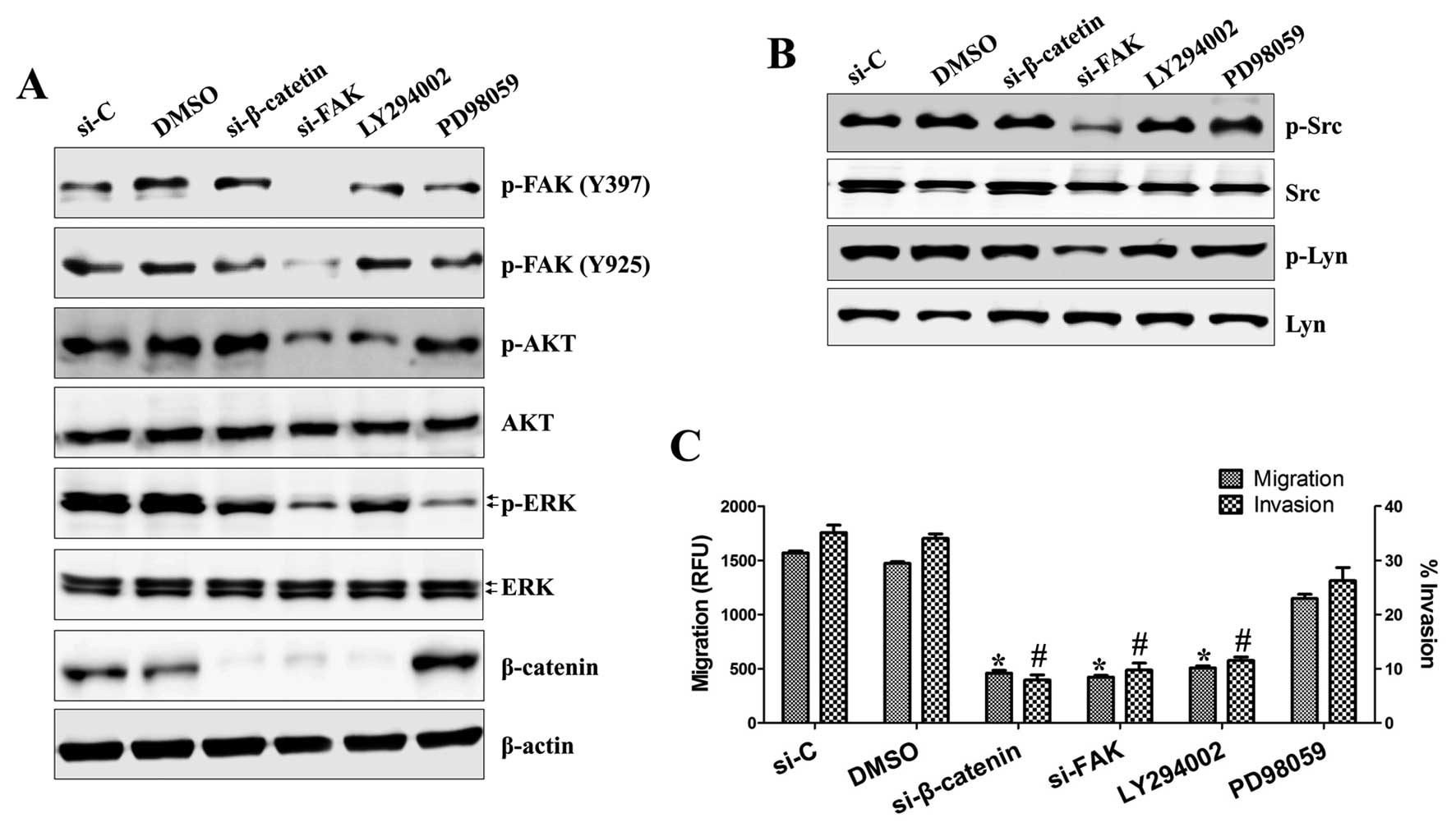 | Figure 5FAK, Src, Lyn, Akt and β-catenin, but
not ERK1/2, are key mediators of osteosarcoma cell migration and
invasion. Cells were transfected with siRNA specific for β-catenin,
FAK or control-siRNA for 36 h or treated with LY294002 (25 μM) or
PD98059 (20 μM) for 36 h as indicated. (A and B) Total protein was
extracted from cell lysates and subjected to western blotting for
phospho-FAK (Tyr397 and Tyr925), phospho-Akt, phospho-ERK1/2,
β-catenin, phospho-Src, phospho-Lyn and β-actin. (C) The migratory
capacity and invasiveness of HOS cells was inhibited by the
knockdown of β-catenin, FAK and Akt, but not ERK1/2, as detected by
Transwell migration assay kit and BME cell invasion assay kit. The
procedure is described in detail in Materials and methods.
*P<0.002. #P<0.001. Each value is the
mean ± SD of three determinations. |
Expression of matrix metalloproteinases
and proinflammatory cytokines is mediated by Gal-3 through the FAK
and Akt/ERK signaling pathway
Gal-3 has a proinflammatory function in lung and
liver fibrotic disease (23,24)
and mediates a number of important signaling molecules, including
IL-8, IL-6, MMPs and Stat3 (25).
FAK has been reported to regulate cell migration and invasion by
promoting proinflammatory cytokines, and MMP-mediated matrix
degradation has been implicated in FAK- and β-catenin-induced
migration and invasion (22). We
therefore examined whether proinflammatory expression of cytokines
and MMPs was controlled by Gal-3 signaling. We observed a
remarkable decrease in MMP2 and MMP9 expression in Gal-3-silenced
cells (Fig. 6A and B). Gal-3
silencing also suppressed the secretion and expression of VEGF,
MCP-1, IL-8 and IL-6 (Fig. 6A and
C) and abolished the phosphorylation of Stat3 compared with
control-siRNA (Fig. 6A and B). The
secretion levels of VEGF, MCP-1, IL-8 and IL-6 were suppressed in
cells treated with FAK-siRNA, β-catenin-siRNA, LY294002 or PD98059
(Fig. 6D). Expression of both MMP2
and MMP9 was blocked in cells treated with FAK-siRNA,
β-catenin-siRNA or LY294002, whereas PD98059 had little effect
(Fig. 6E). Stat3 activation was
inhibited in cells treated with FAK-siRNA, β-catenin-siRNA,
LY294002 or PD98059 (Fig. 6E).
These data suggest that Gal-3-mediated HOS cell migration and
invasion is achieved through the activation of FAK, which regulates
expression of β-catenin and MMP2/9 and the production of
proinflammatory cytokines through PI3K/Akt and ERK1/2
signaling.
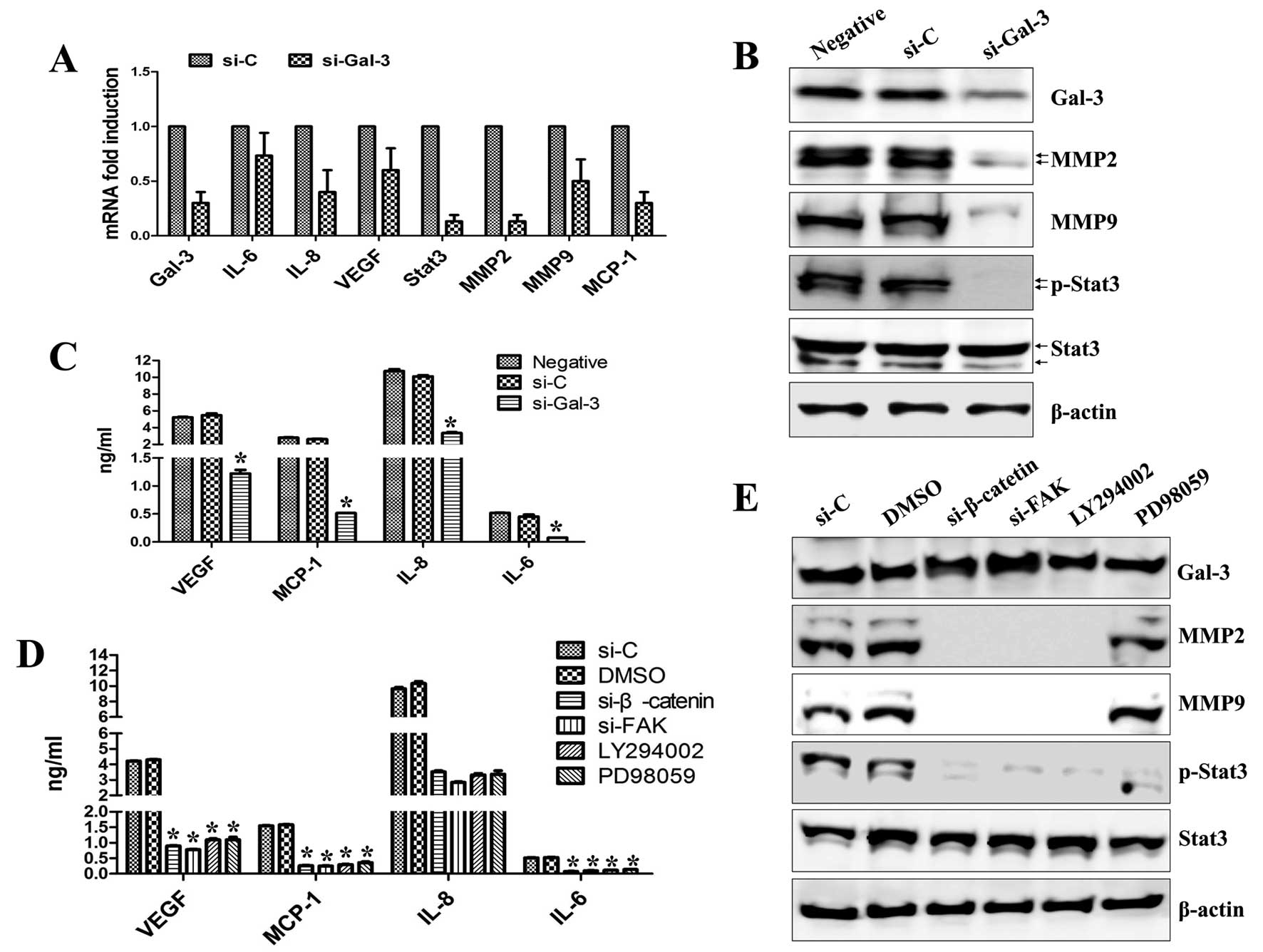 | Figure 6Gal-3 silencing inhibits MMP
expression and cytokine secretion in HOS cells. Cells were
transfected with siRNA specific for Gal-3, β-catenin, FAK or
control-siRNA for 36 h or treated with LY294002 (25 μM) or PD98059
(20 μM) for 36 h as indicated. (A) Total RNA was extracted from
cell lysates and subjected to real-time PCR for Gal-3, MMP2, MMP9,
Stat3, IL-6, IL-8, VEGF, MCP-1 and β-actin mRNA expression.
*P<0.05. Each value is the mean ± SD of three
determinations. (B) The effect of knockdown of Gal-3 on the
secretion of VEGF, MCP-1, IL-6 and IL-8. (C) The effect of
knockdown of Gal-3 on the expression of Gal-3, MMP2, MMP9 and
Stat3. (D) The effect of knockdown of β-catenin, FAK, Akt or ERK1/2
on the secretion of VEGF, MCP-1, IL-6 and IL-8. (E) The effect of
knockdown of β-catenin, FAK, Akt or ERK1/2 on the expression of
Gal-3, MMP2, MMP9 and Stat3. (C and E) The levels of total and
phosphorylated proteins were detected by western blotting. β-actin
was used as a loading control. (B and D) After transfection, VEGF,
MCP-1, IL-8 and IL-6 concentrations in the culture supernatants
were quantified by ELISA. *P<0.05. Each value is the
mean ± SD of three determinations. |
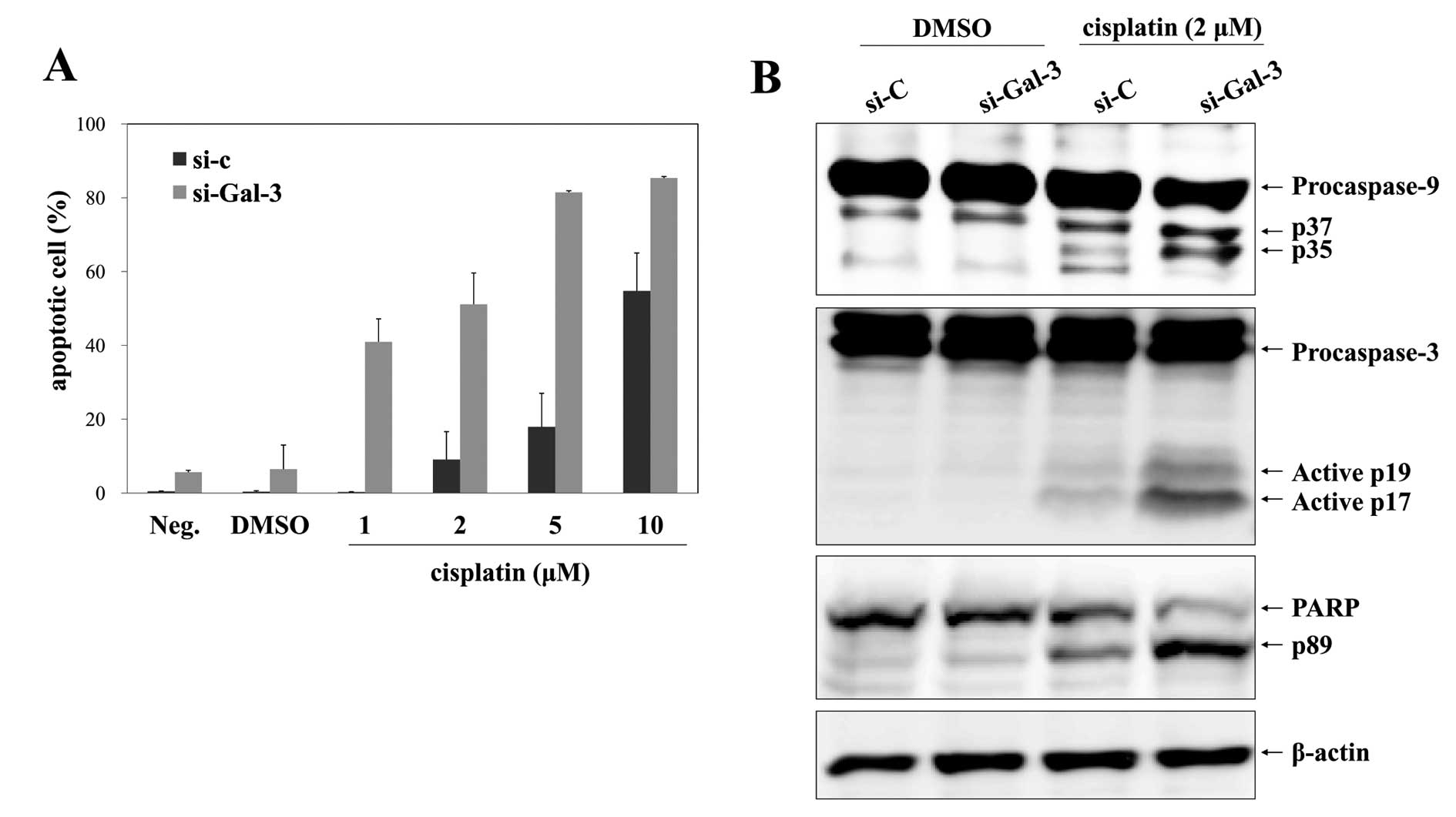
Gal-3 silencing sensitizes osteosarcoma
cells to cisplatin
The potential effect of Gal-3 silencing on
drug-induced apoptosis in HOS cells was analyzed by Annexin V/7-AAD
staining. After transfection for 24 h, cells were exposed to
cisplatin or DMSO for a further 24 h. Cisplatin treatment of cells
transfected with control-siRNA did not have a great effect on cell
apoptosis up to concentrations of 5 μM, and treatment with 2 μM
cisplatin induced apoptosis in only 9.06±7.55% of cells (Fig. 7A). Noatbly, transfection with
Gal-3-siRNA dramatically increased the extent of apoptosis induced
by 2 μM cisplatin to 51.15±8.46%, whereas treatment with
Gal-3-siRNA alone had little effect on apoptosis (Fig. 7A). Moreover, combination treatment
with Gal-3-siRNA and cisplatin clearly elicited the activation of
caspase-9 and caspase-3 and the cleavage of PARP, whereas these
signals were barely detectable in the control-siRNA group (Fig. 7B).
Discussion
The migration and invasion of cancer cells are
important processes in metastasis. Therefore, inhibition of the
invasion and metastasis of cancer cells has been considered a
feasible strategy for the treatment of malignancy. Upregulation of
Gal-3 has been shown to be a potential metastatic factor in a
variety of human cancers (3,4) and
rat OS (26). However, the role of
Gal-3 in OS in humans remains obscure. The results of the present
study implicate a role of Gal-3 in regulating the migration and
invasion of OS cells through the activation of FAK and Src/Lyn. In
particular, we provide the first evidence that Gal-3 regulates Lyn
kinase. To understand how Gal-3 regulates OS progression, we used a
siRNA system for Gal-3 silencing. Silencing of Gal-3 inhibited the
activation of PI3K/Akt, ERK1/2, FAK, Src and Lyn and suppressed
MMP2 and MMP9 expression. Our results suggest a novel mechanism by
which Gal-3 contributes to the acquisition of the OS metastatic
phenotype by increasing Lyn expression.
Overexpression of Gal-3 has been reported in
multiple types of human tumors, including thyroid, pancreatic
cancer, hepatocellular carcinoma, gastric, colon, breast, prostate
cancer, head and neck squamous cell carcinomas and glioma (27). However, the role of Gal-3 in
osteosarcoma progression is not clear. Recent studies have shown
that Gal-3 is closely associated with β-catenin in the invasion of
various cancer cells (8,9), although the relationship between
galectin-3 and Wnt signaling in OS remained unclear. We observed
that Gal-3 silencing significantly diminished the expression of
β-catenin in HOS cells (Figs. 3A
and 4), suggesting that Gal-3
expression activates β-catenin/Wnt signaling in OS.
Recent studies have shown that Gal-3 activates the
K-Ras/ RAF1/ERK pathway, resulting in migration of colon cancer
cells (28), and the functions of
Gal-3 include binding to cell adhesion molecules and suppression of
cell-cell and cell-matrix interactions (3). Diverse reports have shown that
targeting the PI3K/Akt signaling pathway results in downregulation
of tumor invasion and tumorigenesis in malignant cancer cells,
including OS (29,30). However, our results showed that
Gal-3-induced phospho-ERK1/2 did not influence the invasion and
migration of HOS cells (Fig. 5C and
D).
FAK and SFKs, including Src, Lyn, Fyn, Lck, Hck,
Fgr, Blk and Yes, are non-receptor tyrosine kinases that are
activated in response to stimulation by various cellular factors
(31,32). Expression of FAK and SFKs is
increased in a variety of tumors and contributes to the regulation
of tumor progression, adhesion, motility, angiogenesis, invasion
and metastasis (32,33). FAK activation is a central factor
in different signaling transduction cascades including the
PI3K/Akt, ERK1/2 and p38-MAPK pathways (22). Abnormal activation of Lyn has been
implicated in the progression and migration of various tumors,
including prostate and breast cancer (34,35).
Inhibition of Lyn using siRNA was recently shown to significantly
suppress tumor growth and decrease lung metastases in Ewing’s
sarcoma (36). Although some
studies have linked Src and Lyn to Ewing’s sarcoma, there are no
studies demonstrating the involvement of FAK, Src and ERK in
Gal-3-mediated invasion in OS.
Recent studies have reported a correlation between
Gal-3 and FAK expression; Gal-3 triggered FAK activation in HUVEC
cells (37) and was required for
stabilization of FAK in thyroid cancer (38). In the present study, we found that
depletion of FAK by specific siRNA reduced the phosphorylation
level of Src, Lyn, Akt and ERK1/2 (Fig. 5A and B). The data suggest that FAK
might be the upstream modulator of SFKs, Akt and MAPKs and directly
or indirectly interacts with Src, Lyn, Akt and ERK1/2 in OS. It is
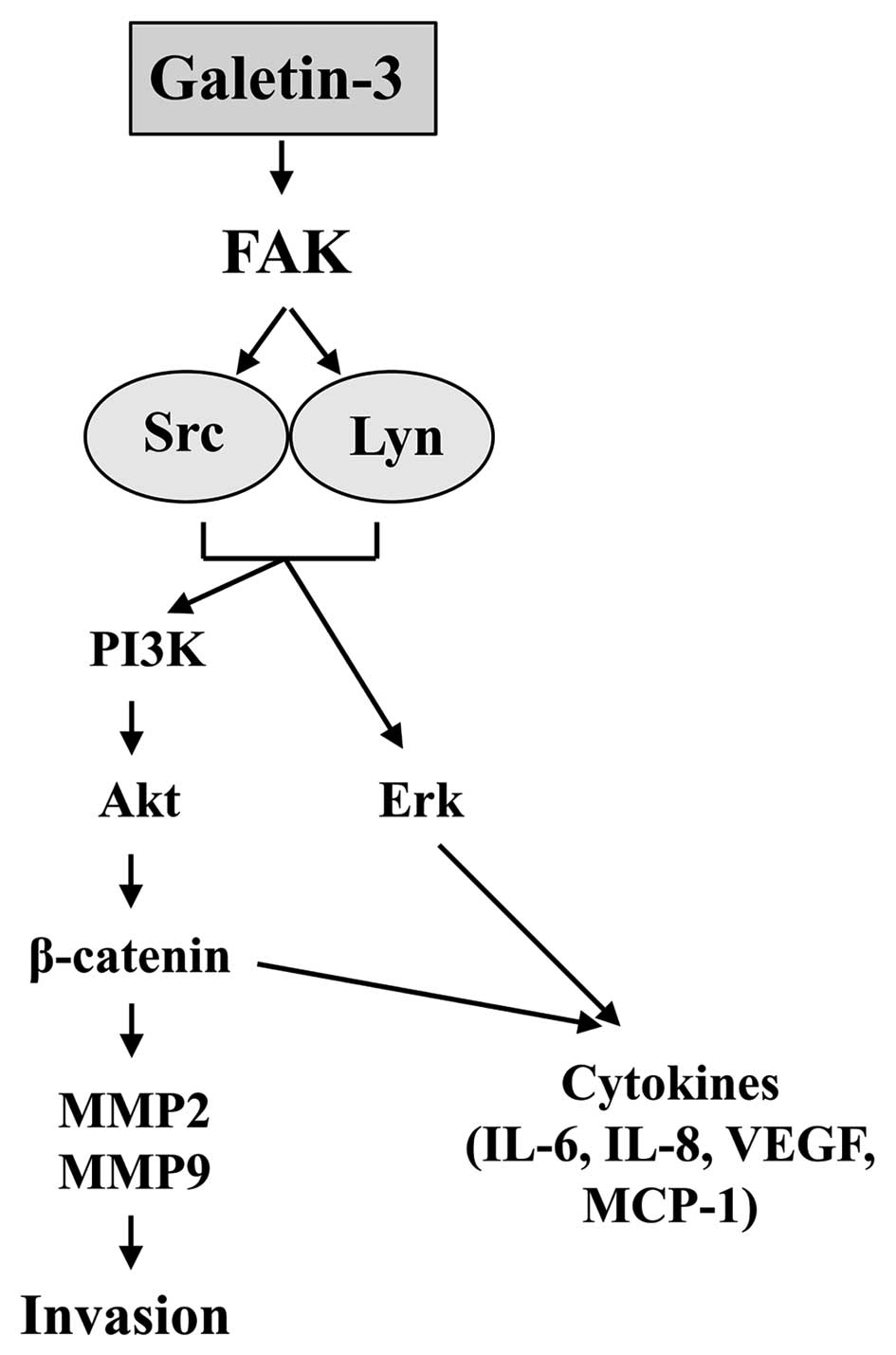
possible that Gal-3 directly or indirectly induces the activation
of FAK, Src, Lyn, Akt and ERK1/2, leading to cell invasion
(Fig. 8).
Activation of PI3K/Akt enhances MMP expression and
facilitates tumor metastasis (21). IL-8, IL-6 and VEGF are directly
regulated by Wnt signaling and influence cancer cell migration and
invasion (39). MMPs play a
crucial role in cancer cell invasion and metastasis by breaking
down the extracellular matrix to expedite the penetration of cancer
cells (40). MMP2 and MMP9 have
been proposed as significant downstream targets of the β-catenin
signaling pathway (41). MMP2 and
MMP9 are commonly overexpressed in OS tissue and their activation
has been implicated in tumor invasion and metastasis in OS
(42). We found that silencing of
Gal-3 or β-catenin suppressed the secretion of IL-8, IL-6, VEGF and
MCP-1 and reduced expression of MMP2, MMP9 and phospho-Stat3
compared with cells transfected with control-siRNA (Fig. 6). Therefore, we conclude that the
β-catenin signaling pathway regulates MMPs and proinflammatory
cytokines in HOS cells.
Although standard chemotherapeutic drugs such as
cisplatin, doxorubicin, cyclophosphamide, methotrexate, ifosfamide
and bleomycin have improved the efficiency of OS therapy,
uncontrolled tumor expansion and metastasis are key factors
responsible for the poor prognosis of OS (43). The knockdown of Gal-3 in HOS cells
also enhanced the efficacy of cisplatin to induce apoptosis through
activation of the caspase pathway. The PI3K/Akt pathway is well
known to be a major cell survival pathway in many cancers,
including OS (31,44). In the present study, we observed
that Gal-3 and FAK directly activate the downstream effectors Src,
Lyn, Akt and ERK1/2. These data suggest that combination treatment
with Gal-3 blocking and cisplatin might be a new treatment for
advanced or metastatic OS.
In conclusion, the present study demonstrates that
Gal-3-mediated migration and invasion of human OS cells is induced
by the activation of Akt and ERK1/2 through FAK and Src/Lyn
signaling pathways. We propose that Gal-3 might be a feasible
therapeutic target for OS.
Acknowledgements
This study was supported by Korea Drug Development
Fund (KDDF) funded by Ministry of Science, ICT and Future Planning,
Ministry of Trade, Industry & Energy and Ministry of Health
& Welfare (KDDF-201404-04, Republic of Korea), and the National
R&D Program for Cancer Control, Ministry for Health, Welfare
and Family Affairs, Republic of Korea (grant no. 0920040).
References
|
1
|
Leffler H, Carlsson S, Hedlund M, Qian Y
and Poirier F: Introduction to galectins. Glycoconj J. 19:433–440.
2004. View Article : Google Scholar
|
|
2
|
Krześlak A and Lipińska A: Galectin-3 as a
multifunctional protein. Cell Mol Biol Lett. 9:305–328. 2004.
|
|
3
|
Liu FT and Rabinovich GA: Galectins as
modulators of tumour progression. Nat Rev Cancer. 5:29–41. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukumori T, Kanayama HO and Raz A: The
role of galectin-3 in cancer drug resistance. Drug Resist Updat.
10:101–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song S, Ji B, Ramachandran V, Wang H,
Hafley M, Logsdon C and Bresalier RS: Overexpressed galectin-3 in
pancreatic cancer induces cell proliferation and invasion by
binding Ras and activating Ras signaling. PLoS One. 7:e426992012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SJ, Choi IJ, Cheong TC, Lee SJ, Lotan
R, Park SH and Chun KH: Galectin-3 increases gastric cancer cell
motility by up-regulating fascin-1 expression. Gastroenterology.
138:1035–1045. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khaldoyanidi SK, Glinsky VV, Sikora L,
Glinskii AB, Mossine VV, Quinn TP, Glinsky GV and Sriramarao P:
MDA-MB-435 human breast carcinoma cell homo- and heterotypic
adhesion under flow conditions is mediated in part by
Thomsen-Friedenreich antigengalectin-3 interactions. J Biol Chem.
273:4127–4134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang LP, Chen SW, Zhuang SM, Li H and Song
M: Galectin-3 accelerates the progression of oral tongue squamous
cell carcinoma via a Wnt/β-catenin-dependent pathway. Pathol Oncol
Res. 19:461–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimura T, Takenaka Y, Tsutsumi S, Hogan
V, Kikuchi A and Raz A: Galectin-3, a novel binding partner of
β-catenin. Cancer Res. 64:6363–6367. 2004. View Article : Google Scholar
|
|
10
|
Morin PJ: Beta-catenin signaling and
cancer. Bioessays. 21:1021–1030. 1999. View Article : Google Scholar
|
|
11
|
MacDonald BT and He X: Frizzled and LRP5/6
receptors for Wnt/β-catenin signaling. Cold Spring Harb Perspect
Biol. 4:2012.pii: a007880. View Article : Google Scholar
|
|
12
|
Bodine PV: Wnt signaling control of bone
cell apoptosis. Cell Res. 18:248–253. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gaur T, Lengner CJ, Hovhannisyan H, Bhat
RA, Bodine PV, Komm BS, Javed A, van Wijnen AJ, Stein JL, Stein GS
and Lian JB: Canonical WNT signaling promotes osteogenesis by
directly stimulating Runx2 gene expression. J Biol Chem.
280:33132–33140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Selvarajah GT, Kirpensteijn J, van
Wolferen ME, Rao NA, Fieten H and Mol JA: Gene expression profiling
of canine osteosarcoma reveals genes associated with short and long
survival times. Mol Cancer. 8:722009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: data
from the Surveillance, Epidemiology, and End Results Program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lamoureux F, Trichet V, Chipoy C,
Blanchard F, Gouin F and Redini F: Recent advances in the
management of osteosarcoma and forthcoming therapeutic strategies.
Expert Rev Anticancer Ther. 7:169–181. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim J and Hwan Kim S: CK2 inhibitor
CX-4945 blocks TGF-β1-induced epithelial-to-mesenchymal transition
in A549 human lung adenocarcinoma cells. PLoS One. 8:e743422013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Santos A, Bakker AD, Zandieh-Doulabi B, de
Blieck-Hogervorst JM and Klein-Nulend J: Early activation of the
beta-catenin pathway in osteocytes is mediated by nitric oxide,
phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase.
Biochem Biophys Res Commun. 391:364–369. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haydon RC, Deyrup A, Ishikawa A, Heck R,
Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, Peabody T,
Simon MA, Montag AG and He TC: Cytoplasmic and/or nuclear
accumulation of the beta-catenin protein is a frequent event in
human osteosarcoma. Int J Cancer. 102:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dieudonne FX, Marion A, Hay E, Marie PJ
and Modrowski D: High Wnt signaling represses the proapoptotic
proteoglycan syndecan-2 in osteosarcoma cells. Cancer Res.
70:5399–5408. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye Q, Cai W, Zheng Y, Evers BM and She QB:
ERK and AKT signaling cooperate to translationally regulate
survivin expression for metastatic progression of colorectal
cancer. Oncogene. 33:1828–1839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG,
Song GY, Kwon KI, Jeong TC and Jeong HG: Suppression of EGF-induced
tumor cell migration and matrix metalloproteinase-9 expression by
capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK,
p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 55:594–605. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Henderson NC, Mackinnon AC, Farnworth SL,
Poirier F, Russo FP, Iredale JP, Haslett C, Simpson KJ and Sethi T:
Galectin-3 regulates myofibroblast activation and hepatic fibrosis.
Proc Natl Acad Sci USA. 103:5060–5065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishi Y, Sano H, Kawashima T, Okada T,
Kuroda T, Kikkawa K, Kawashima S, Tanabe M, Goto T, Matsuzawa Y,
Matsumura R, Tomioka H, Liu FT and Shirai K: Role of galectin-3 in
human pulmonary fibrosis. Allergol Int. 56:57–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Filer A, Bik M, Parsonage GN, Fitton J,
Trebilcock E, Howlett K, Cook M, Raza K, Simmons DL, Thomas AM,
Salmon M, Scheel-Toellner D, Lord JM, Rabinovich GA and Buckley CD:
Galectin 3 induces a distinctive pattern of cytokine and chemokine
production in rheumatoid synovial fibroblasts via selective
signaling pathways. Arthritis Rheum. 60:1604–1614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aubin JE, Gupta AK, Bhargava U and Turksen
K: Expression and regulation of galectin 3 in rat osteoblastic
cells. J Cell Physiol. 169:468–480. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Radosavljevic G, Volarevic V, Jovanovic I,
Milovanovic M, Pejnovic N, Arsenijevic N, Hsu DK and Lukic ML: The
roles of Galectin-3 in autoimmunity and tumor progression. Immunol
Res. 52:100–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu KL, Huang EY, Jhu EW, Huang YH, Su WH,
Chuang PC and Yang KD: Overexpression of galectin-3 enhances
migration of colon cancer cells related to activation of the
K-Ras-Raf-Erk1/2 pathway. J Gastroenterol. 48:350–359. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu KH, Yang HW, Su CW, Lue KH, Yang SF and
Hsieh YS: Phyllanthus urinaria suppresses human osteosarcoma
cell invasion and migration by transcriptionally inhibiting u-PA
via ERK and Akt signaling pathways. Food Chem Toxicol. 52:193–199.
2013. View Article : Google Scholar
|
|
30
|
Bartholomeusz C and Gonzalez-Angulo AM:
Targeting the PI3K signaling pathway in cancer therapy. Expert Opin
Ther Targets. 16:121–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ishizawar R and Parsons SJ: c-Src and
cooperating partners in human cancer. Cancer Cell. 6:209–214. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
McLean GW, Carragher NO, Avizienyte E,
Evans J, Brunton VG and Frame MC: The role of focal-adhesion kinase
in cancer - a new therapeutic opportunity. Nat Rev Cancer.
5:505–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Summy JM and Gallick GE: Src family
kinases in tumor progression and metastasis. Cancer Metastasis Rev.
22:337–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai H, Smith DA, Memarzadeh S, Lowell CA,
Cooper JA and Witte ON: Differential transformation capacity of Src
family kinases during the initiation of prostate cancer. Proc Natl
Acad Sci USA. 108:6579–6584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi YL, Bocanegra M, Kwon MJ, Shin YK,
Nam SJ, Yang JH, Kao J, Godwin AK and Pollack JR: LYN is a mediator
of epithelial-mesenchymal transition and a target of dasatinib in
breast cancer. Cancer Res. 70:2296–2306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guan H, Zhou Z, Gallick GE, Jia SF,
Morales J, Sood AK, Corey SJ and Kleinerman ES: Targeting Lyn
inhibits tumor growth and metastasis in Ewing’s sarcoma. Mol Cancer
Ther. 7:1807–1816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Markowska AI, Liu FT and Panjwani N:
Galectin-3 is an important mediator of VEGF- and bFGF-mediated
angiogenic response. J Exp Med. 207:1981–1993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shankar J, Wiseman SM, Meng F, Kasaian K,
Strugnell S, Mofid A, Gown A, Jones SJ and Nabi IR: Coordinated
expression of galectin-3 and caveolin-1 in thyroid cancer. J
Pathol. 228:56–66. 2012.PubMed/NCBI
|
|
39
|
Heijink IH, de Bruin HG and van den Berge
M: Role of aberrant WNT signalling in the airway epithelial
response to cigarette smoke in chronic obstructive pulmonary
disease. Thorax. 68:709–716. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuniyasu H, Ellis LM, Evans DB, Abbruzzese
JL, Fenoglio CJ, Bucana CD, Cleary KR, Tahara E and Fidler IJ:
Relative expression of E-cadherin and type IV collagenase genes
predicts disease outcome in patients with resectable pancreatic
carcinoma. Clin Cancer Res. 5:25–33. 1999.PubMed/NCBI
|
|
41
|
Brabletz T, Jung A, Dag S, Reu S and
Kirchner T: Beta-catenin induces invasive growth by activating
matrix metalloproteinases in colorectal carcinoma. Verh Dtsch Ges
Pathol. 84:175–181. 2000.(In German).
|
|
42
|
Xin ZF, Kim YK and Jung ST: Risedronate
inhibits human osteosarcoma cell invasion. J Exp Clin Cancer Res.
28:1052009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Federman N, Bernthal N, Eilber FC and Tap
WD: The multi-disciplinary management of osteosarcoma. Curr Treat
Options Oncol. 10:82–93. 2009. View Article : Google Scholar
|
|
44
|
Zhang Y, Sun S, Chen J, Ren P, Hu Y, Cao
Z, Sun H and Ding Y: Oxymatrine induces mitochondria dependent
apoptosis in human osteosarcoma MNNG/HOS cells through inhibition
of PI3K/Akt pathway. Tumour Biol. 35:1619–1625. 2014. View Article : Google Scholar : PubMed/NCBI
|