Introduction
Breast cancer is the most common cancer in women
worldwide and the leading cause of death from cancer among women
globally. According to the GLOBOCAN 2012 data (1), World Health Organization
International Agency for Research on Cancer, there were nearly 1.7
million new cases diagnosed in 2012 which is ~12% of all new cancer
cases and 25% of all cancers in women. Fortunately, the increasing
application of effective therapies such as adjuvant medical therapy
made it possible for the mortality rate to decline over the last
decades. However, it also poses a challenge for clinicians to avoid
over-treatment, insufficient treatment or incorrect treatment due
to lack of useful prognostic, diagnostic and monitoring biomarkers
(2).
Routine clinical therapies for breast cancer are
based on the classical clinical and pathological features from
immunohistochemistry evaluations, radiologic evaluations and serum
tumor markers. Unfortunately, these features are incapable of
providing enough information on the ongoing metastasis as early as
possible for predicting the clinical outcome with high accuracy and
reproducibility. As an emerging tumor biomarker, circulating tumor
cell (CTC) analysis is a promising new diagnostic field for
micrometastasis, although it depends on the emergence of
increasingly advanced and sensitive technologies to isolate and
characterize human CTCs (3).
Multiple independent studies have demonstrated that CTCs can be
recognized as novel tumor biomarkers for prognostic and predictive
purposes in metastatic breast cancer (4–8).
Various detection technologies and devices have been
developed to enumerate and characterize CTCs (9–13),
but no gold standard could define the absolute accuracy,
sensitivity and specificity in detecting CTCs. For the isolation of
CTCs, a variety of currently used methods were based on various
properties of CTCs compared with leukocytes and broadly divided
into two isolation/enrichment strategies (positive or negative
strategy). The positive strategy was based on epithelial cell
adhesion molecule (EpCAM) or cytokeratin expression profile or cell
size, while the negative strategy was developed by targeting and
removing the normal blood cells. For the detection of CTCs,
immunocytological approaches and molecular techniques such as
RT-PCR are the most widely used. To date, the
CellSearch® system is the only approved assay by FDA for
prognosis in metastatic breast, prostate and colon cancer which
captures the CTCs by immunomagnetic beads coated with antibodies
targeting EpCAM and identify CTCs by fluorescent probes for
intracellular protein cytokeratin 8, 18 and 19. However, the
CellSearch® system was shown to have a lower sensitivity
in early breast cancer (14).
In the present study, we developed a new CTC
detection approach based on negative enrichment strategy and
real-time quantitative RT-PCR technique. The approach applied in
this study for breast cancer CTC detection showed both high
sensitivity and specificity, especially in early breast cancer.
Materials and methods
Ethical statement
Human blood samples were obtained from the
Affiliated Hospital of Academy of Military Medical Sciences
(Beijing, China). All patients and healthy donors enrolled in the
present study signed the Consent Forms approved by the Ethics
Review Committee of the Academy of Military Medical Sciences.
Materials
Fetal bovine serum (FBS), RPMI-1640 media, DMEM
media, TRIzol reagent, glycogen, Ambion TUBRO DNA-free kit,
SuperScript™ III First-Strand Synthesis system for RT-PCR,
TaqMan® Universal Master Mix II kit, rabbit
anti-cytokeratin (Pan) polyclonal antibody, Alexa Fluor 594 labeled
goat anti-rabbit IgG antibody, Dynabeads CD45, Hoechst 33342 and
Mitotracker® Red CMXRos were obtained from Life
Technologies (Grand Island, NY, USA). T-25 cell culture flasks, 50-
and 15-ml tubes were purchased from Corning Inc. (Corning, NY,
USA). Ficoll-Paque Plus was purchased from GE Healthcare (Uppsala,
Sweden). BD Vacutainer® evacuated blood collection tube
with acid citrate dextrose anticoagulant were from Becton-Dickinson
(Franklin Lakes, NJ, USA).
Cell lines and culture conditions
Human breast cancer cell line SK-BR-3, MCF-7,
MDA-MB-453, ZR 75-1, human promyelocytic leukemia cell line HL-60,
human Brukitt’s lymphoma cell line Raji and human T-cell leukemia
cell line Jurkat were obtained from the Cell Resource Center (IBMS,
CAMS/PUMC, Beijing, China). The SK-BR-3, ZR 75-1, HL-60, Raji and
Jurkat cell lines were maintained in RPMI-1640 medium supplemented
with 10% FBS, the MCF-7 and MDA-MB-453 cell lines were maintained
in DMEM medium supplemented with 10% FBS. All cell cultures were
maintained at 37°C in a humidified atmosphere with 5%
CO2.
Tumor cell enrichment
We developed the tumor cell enrichment method based
on negative enrichment and density gradient centrifuge strategy
that updated our previously published method (15). Briefly, 1 ml of peripheral blood
collected in BD Vacutainer tube was diluted with PBS to 15 ml and
subsequently incubated with 100 μl Dynabeads CD45 for 30 min at
room temperature with gentle tilting and rotation. Then, the whole
blood sample was carefully layered on 7.5 ml Ficoll-Paque Plus in a
50-ml centrifuge tube, followed by spinning at 350 × g for 5 min at
4°C. Supernatants were transferred into a centrifuge tube followed
by spinning at 650 × g for 5 min. Cell pellet was stained in glass
slide and subsequently subjected to fluorescent microscope
observation, or extracted mRNA and performed real-time RT-PCR
detection.
Primers and probes design
Primers and probes (Table I) were designed with AlleleID 6.0
(Premier Biosoft) and synthesized by Life Technologies Corp.
(Beijing, China). Nucleotide sequences used for design of
probe-primers were retrieved from NCBI database and the designed
probe-primers were aligned by BLAST to confirm gene
specificity.
 | Table IOligonucleotide primer and probe
sequences used in the present study. |
Table I
Oligonucleotide primer and probe
sequences used in the present study.
| Gene symbol | Accession number | Primer-probe | Sequence (5′-3′) | Tm (°C) | Amplicon size
(bp) |
Fluorophore-quencher |
|---|
| ERBB2 | NM_004448 | Primer_F |
CCTGGCCGTGCTAGACAATGG | 58.5 | 138 | |
| | Primer_R |
GGGTTCCGCTGGATCAAGACC | 58.2 | | |
| | Probe | CGCTGAACAATACCA | 69 | | VIC-MGB-NFQ |
| KRT19 | NM_002276 | Primer_F |
CAGATCGACAATGCCCGTCTGG | 59 | 149 | |
| | Primer_R |
TGCATCTCCAGGTCGGTCCTG | 59 | | |
| | Probe | AGATGACTTCCGAAC | 69 | | FAM-MGB-NFQ |
| EPCAM | Nm_002354 | Primer_F |
GCTGGCCGTAAACTGCTTTGTG | 58.5 | 115 | |
| | Primer_R |
TGCCTTCATCACCAAACATTTGGC | 58.6 | | |
| | Probe | AATCGTCAATGCCAG | 69 | | VIC-MGB-NFQ |
| MUC1 | NM_002456 | Primer_F |
GGTGCTGGTCTGTGTTCTGG | 58.9 | 136 | |
| | Primer_R |
GTACTCGCTCATAGGATGGTAGG | 58.3 | | |
| | Probe |
CCATTGTCTATCTCATTGC | 69 | | NED-MGB-NFQ |
RNA isolation and complementary DNA
synthesis
Total RNA from enriched cells was extracted using
TRIzol reagent according to the manufacturer’s manual. To enhance
the precipitation of RNA from small quantity of cell samples,
glycogen (final concentration 250 μg/ml) was added to the cell
lysate before phase separation. The contaminating DNA from RNA
preparation was removed using Ambion® TURBO DNA-free kit
according to the manufacturer’s protocol.
Complementary DNA (cDNA) synthesis was performed
using the SuperScript™ III First-Strand Synthesis system for
RT-PCR. Briefly, the following conditions were performed in a total
volume of 20 μl: 1 μl oligo(dT) primer and 1 μl 10 mM dNTP mix were
mixed with 8 μl mRNA, incubated for 5 min at 65°C for 5 min, and
then placed on ice for at least 1 min. Then, 10 μl of cDNA
synthesis mix was added [2 μl 10X RT buffer, 4 μl 25 mM
MgCl2, 2 μl 0.1 M DTT, 1 μl RNaseOUT™(40 U/μl),
SuperScript™ III RT (200 U/μl)] to each RNA/primer mixture and
incubated for 50 min at 50°C and the reactions at 85°C for 5 min
was terminated. The sample was chilled on ice and 1 μl of RNase H
was added to the tube and incubated for 20 min at 37°C. The product
of cDNA synthesis reaction was stored at −20°C or used for
real-time PCR immediately.
Multiplex real-time PCR
The real-time PCR was performed using the
TaqMan® Universal Master Mix II kit with the
StepOnePlus™ real-time PCR instrument. The PCR experiments were
performed according to the protocol and cycling conditions outlined
in the manual. The final concentrations of each primer and probe in
the real-time PCR reaction were 0.4 and 0.2 μM, respectively if not
otherwise indicated.
Immunostaining and identification of
enriched CTCs
Immunostaining of CTCs were performed as previously
described with some modifications (15). Briefly, enriched cells were fixed
by 2% paraformaldehyde on glass slides and then permeabilized with
0.1% Triton X-100, followed by incubation with rabbit
anti-cytokeratin (Pan) polyclonal antibody for 1 h. Slides were
washed three times with PBS, followed by incubated with Alexa Fluor
594 labeled goat anti-rabbit IgG antibody for 1 h. After being
washed three times with PBS, slides were applied with mounting
media containing DAPI (Vector Laboratories) and subsequently
subjected to image analysis using laser confocal scanning
microscope FV1000 (Olympus). For identification of CTC, each
positive CTC had to meet the following criteria: cell size >4
μm, cells were intact with round to oval morphology with visible
DAPI stained nucleus, and positive for cytokeratin staining.
Spiking study
Several validation experiments by spiking study were
performed to establish the accuracy of the method to detect cancer
cells in blood. To validate the recovery rate of the enrichment
method, liver human breast cancer cells were labeled with Hoechst
33342 and Mitotracker® Red CMXRos for 1 h. Different
numbers of cells were counted with a fluorescent microscope (BX53;
Olympus, Tokyo, Japan) and spiked into 1 ml blood from healthy
donor. The samples were treated by the enrichment isolation
procedure and recovered cancer cells were enumerated using a
fluorescent microscope (BX53; Olympus) by an observer in a blinded
manner. To validate the accuracy of real-time RT-PCR, liver breast
cancer cells were spiked into different blood cells or cell lines
(Raji, HL-60, Jurkat and isolated leukocytes) respectively, and the
mixed samples were detected the expression of tumor marker genes by
real-time RT-PCR assay.
Patients and specimens
Fifteen healthy donors and sixteen breast cancer
patients were enrolled in the present study. Peripheral blood (2
ml) was drawn from the median cubital vein into a BD Vacutainer
tube (with acid citrate dextrose anticoagulant), and to avoid
potential epithelial cell contamination, the first 2 ml of blood
were discarded before each collection of blood samples. The blood
sample was equally divided into two aliquots and processed
immediately. Then, the enriched CTCs from two aliquots were
identified by immunostaining or real-time RT-PCR analysis,
respectively.
Results
Validation of recovery efficiency of the
new enrichment strategy by spiking study
A series of blinded spiking studies were performed
to validate the developed enrichment method of detecting breast
cancer cells in blood. Live human breast cancer cell lines
MDA-MB-453, MCF-7 and SK-BR-3 were labeled with
Mitotracker® Red CMXRos and Hoechst 33342 as described
in Materials and methods. After counting under a microscope, the
labeled cells were spiked into 1 ml fresh peripheral blood
collected from healthy donors to simulate the blood of tumor
patients. The blood samples were treated with the enrichment
procedure and the recovered tumor cells were enumerated using a
fluorescent microscope (BX53; Olympus) by an observer in a blinded
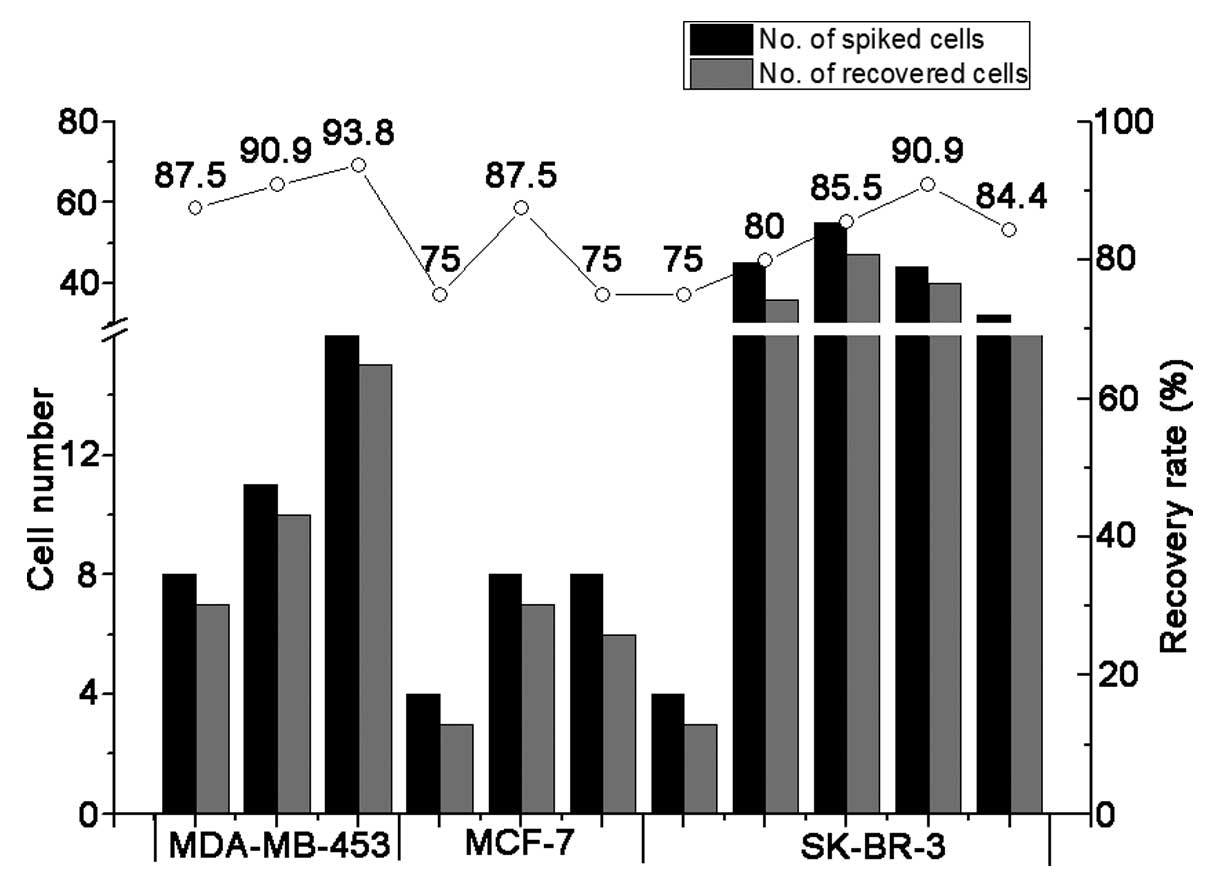
manner. As shown in Fig. 1,
>75% cells (range from 75 to 93.8%, mean 84.1%) could be
recovered from spiked MDA-MB-453, MCF-7 and SK-BR-3 cells. These
results indicated that our enrichment method was able to
efficiently enrich and recover spiked breast cancer cells from
peripheral blood, with a high degree of accuracy.
Development of multiplex real-time RT-PCR
detection of breast CTCs
For the real-time RT-PCR detection of breast CTCs,
MUC1, EpCAM, ERBB2 and KRT19 were selected as candidate marker
genes. To evaluate if the selected genes were appropriate marker
genes for RT-PCR assay, we first detected the expression of these
genes in different breast tumor cells and human blood cells. The
results showed that all these selected genes were expressed highly
in breast tumor cell lines SK-BR-3, MCF-7 and ZR 75-1 (data not
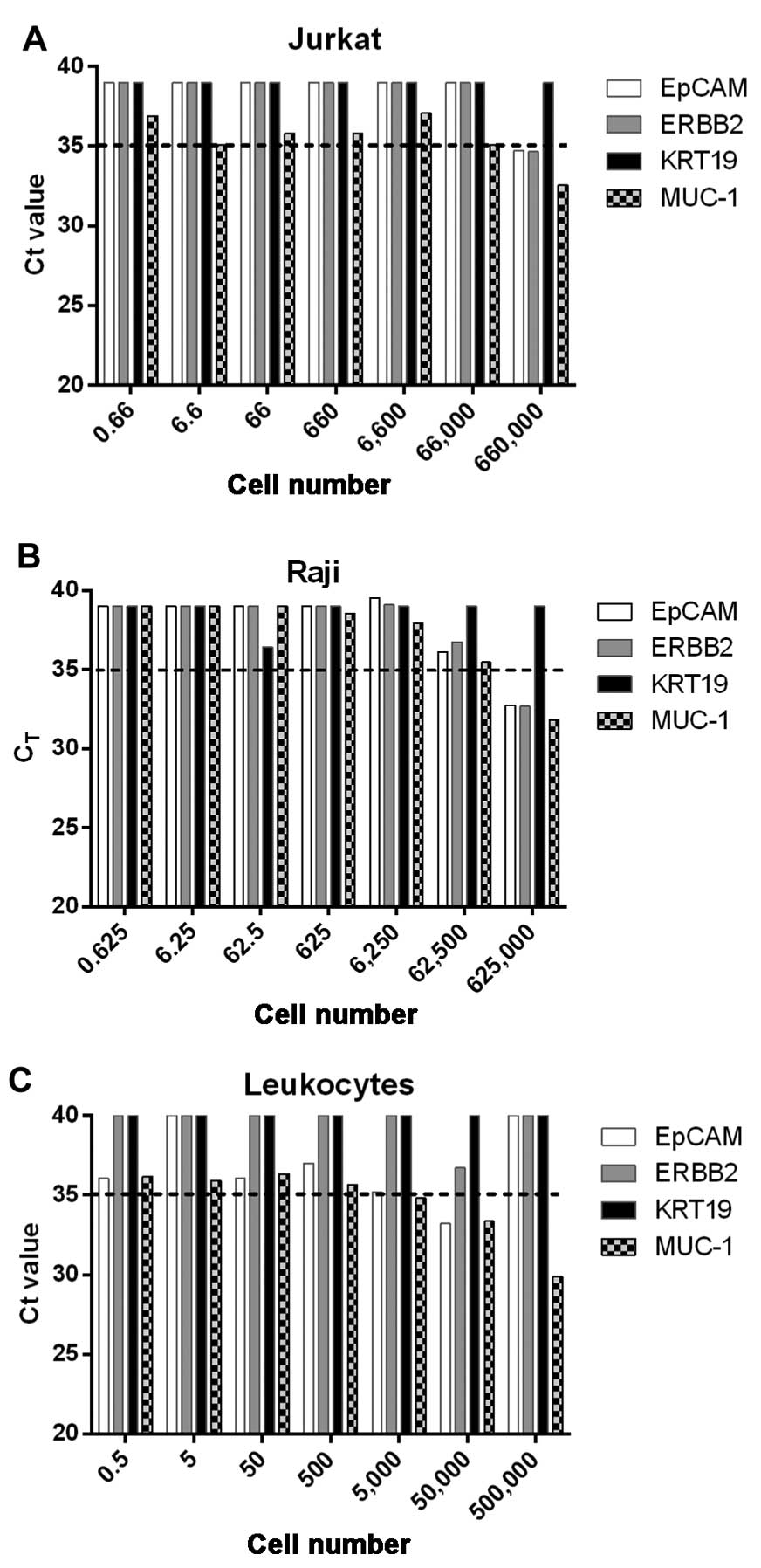
shown). However, the expression of MUC1 gene could also be detected
in Raji, Jurkat and isolated leukocytes when the cell number was
>50,000 (Fig. 2). These results
indicated that MUC1 may not be a suitable marker gene for detection
of CTCs in peripheral blood.
Then, we tried to determine the sensitivity of the
real-time RT-PCR detection of breast CTCs. For this, serial
dilutions of total RNA from breast cancer cell line SK-BR-3, MCF-7
and ZR 75-1 were performed to detect ERBB2, KRT19 and EpCAM gene,
respectively. The detection limit using ERBB2 or KRT19 (Table II) as targeted gene was
consistently one tumor cell RNA of SK-BR-3, MCF-7 or ZR 75-1, which
indicated that ERBB2 and KRT19 could potentially detect a single
breast cancer cell. But, the sensitivity of real-time RT-PCR
detection of CTCs using EpCAM gene as targeted gene was not
consistent in all tested cell lines (Table II). Based on these results, we
chose the ERBB2 and KRT19 as the marker genes to develop the
real-time RT-PCR detection of breast CTCs.
 | Table IICt values of ERBB2, KRT19 and EpCAM
transcripts in serial dilutions of RNA extracted from the cell line
SK-BR-3, MCF-7 and ZR 75-1. |
Table II
Ct values of ERBB2, KRT19 and EpCAM
transcripts in serial dilutions of RNA extracted from the cell line
SK-BR-3, MCF-7 and ZR 75-1.
| | SK-BR-3 | MCF-7 | ZR 75-1 |
|---|
| |
|
|
|
|---|
| Gene | Cell no. | Ct (mean ± SD) | % positive
replicates | Ct (mean ± SD) | % positive
replicates | Ct (mean ± SD) | % positive
replicates |
|---|
| ERBB2 | 10,000 | 18.9±1.28 | 100 | 25.58±0.14 | 100 | 20.96±1.02 | 100 |
| 1,000 | 22.99±1.83 | 100 | 29.28±0.14 | 100 | 24.54±1.28 | 100 |
| 100 | 26.91±1.96 | 100 | 32.30±0.08 | 100 | 28.01±1.54 | 100 |
| 10 | 29.96±1.92 | 100 | 33.77±0.09 | 100 | 30.97±1.74 | 100 |
| 1 | 32.98±1.57 | 100 | 34.29±0.01 | 100 | 32.96±1.94 | 100 |
| 0.1 | 34.59±1.40 | 100 | 34.24±0.10 | 100 | 33.37±2.04 | 100 |
| KRT19 | 10,000 | 17.98±0.88 | 100 | 22.28±1.80 | 100 | 17.59±3.62 | 100 |
| 1,000 | 22.64±1.08 | 100 | 27.33±2.33 | 100 | 22.18±4.91 | 100 |
| 100 | 26.75±1.34 | 100 | 30.99±2.01 | 100 | 26.12±5.07 | 100 |
| 10 | 30.08±1.23 | 100 | 33.65±2.00 | 100 | 29.72±4.82 | 100 |
| 1 | 33.64±1.09 | 100 | 34.27±2.25 | 100 | 32.52±3.18 | 100 |
| 0.1 | 37.74±2.34 | 86 | 35.96±0.00 | 50 | 34.26±2.29 | 67 |
| EpCAM | 10,000 | 20.94±1.78 | 100 | 19.84±0.40 | 100 | 16.63±1.10 | 100 |
| 1,000 | 25.18±1.49 | 100 | 23.53±0.29 | 100 | 20.35±1.52 | 100 |
| 100 | 28.30±0.38 | 93 | 27.11±0.04 | 100 | 23.90±1.84 | 100 |
| 10 | 30.71±0.85 | 86 | 30.87±0.29 | 100 | 27.51±2.09 | 100 |
| 1 | 32.05±1.38 | 86 | 34.05±0.37 | 100 | 31.53±1.94 | 100 |
| 0.1 | 33.29±2.06 | 71 | 34.30±0.17 | 100 | 32.88±2.67 | 83 |
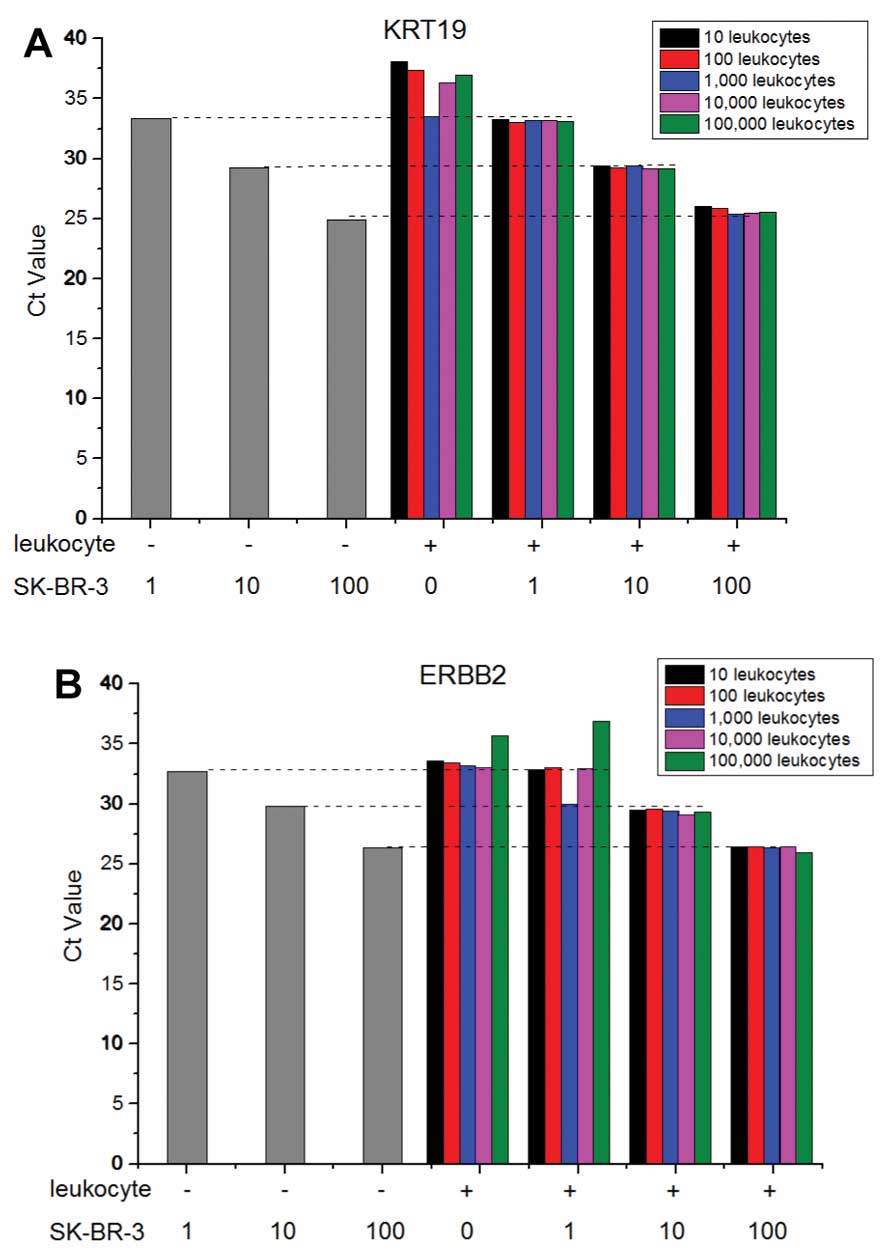
To test if the contaminated leukocytes after
negative enrichment affect the sensitivity and specificity of
real-time RT-PCR detection of CTCs, various numbers of SK-BR-3
cells were spiked into a serial diluted leukocytes and detected by
real-time RT-PCR assay. As shown in Fig. 3, the Ct number of breast CTCs
without contaminated leukocytes was exactly equal to that of equal
breast CTCs with the different numbers of contaminated leukocytes,
which indicated the contaminating leukocytes did not affect the
amplification of ERBB2 and KRT19 genes in breast CTCs.
Based on the above results, we decided to develop
the duplex real-time RT-PCR detection of breast CTCs using KRT19
and ERBB2 primer-probe sets. Next, we compared the sensitivity of
the duplex and singleplex assays by detecting total RNA of 10-fold
serial dilutions of SK-BR-3 (from 105 to
10−1). For the duplex reactions, the primers and probes
were each used at 0.2 μM, and for the singleplex reactions, the
probe concentration was set at 0.2 μM, while the primer
concentration was set at 0.4 μM. The amplification plots achieved
with duplex assay overlapped with those from the singleplex assays
(data not shown), and equivalent Ct value, slope and R2
were achieved for each gene regardless of whether a duplex or a
singleplex assay was performed (Table III). These results indicated that
the developed duplex assay had equivalent sensitivity with
singleplex assays.
 | Table IIIComparation of amplification
efficiency of KRT19 and ERBB2 in a duplex assay and corresponding
singleplex assays. |
Table III
Comparation of amplification
efficiency of KRT19 and ERBB2 in a duplex assay and corresponding
singleplex assays.
| Gene | Assay | Slope | R2 |
|---|
| KRT19 | Duplex | −3.54 | 0.973 |
| Singleplex | −3.88 | 0.977 |
| ERBB2 | Duplex | −3.51 | 0.98 |
| Singleplex | −3.61 | 0.925 |
Enrichment and detection of CTCs from
breast cancer patients
Having proved that the breast tumor cells could be
recovered efficiently by negative enrichment and detected
quantitatively by duplex real-time RT-PCR assay, we applied the
approaches to the detection of CTCs in the whole blood samples. In
the present study, 15 healthy donors (Table IV) and 16 breast cancer patients
(Table V) including 2 stage I, 6
stage II, 3 stage III and 5 stage IV were enrolled.
Tumor-node-metastasis (TNM) staging of breast cancer patients was
performed according to seventh edition of cancer staging manual by
the American Joint Committee on Cancer (AJCC). Blood samples from
breast cancer patients were collected before drug therapy and
subsequently subjected to enrichment and CTC counting. To evaluate
the results of real-time RT-PCR assay, enriched CTCs from another
aliquot of those blood samples were also identified by
immunostaining with anti-cytokeratin (pan) antibody and counted
under fluorescence microscopy (Table
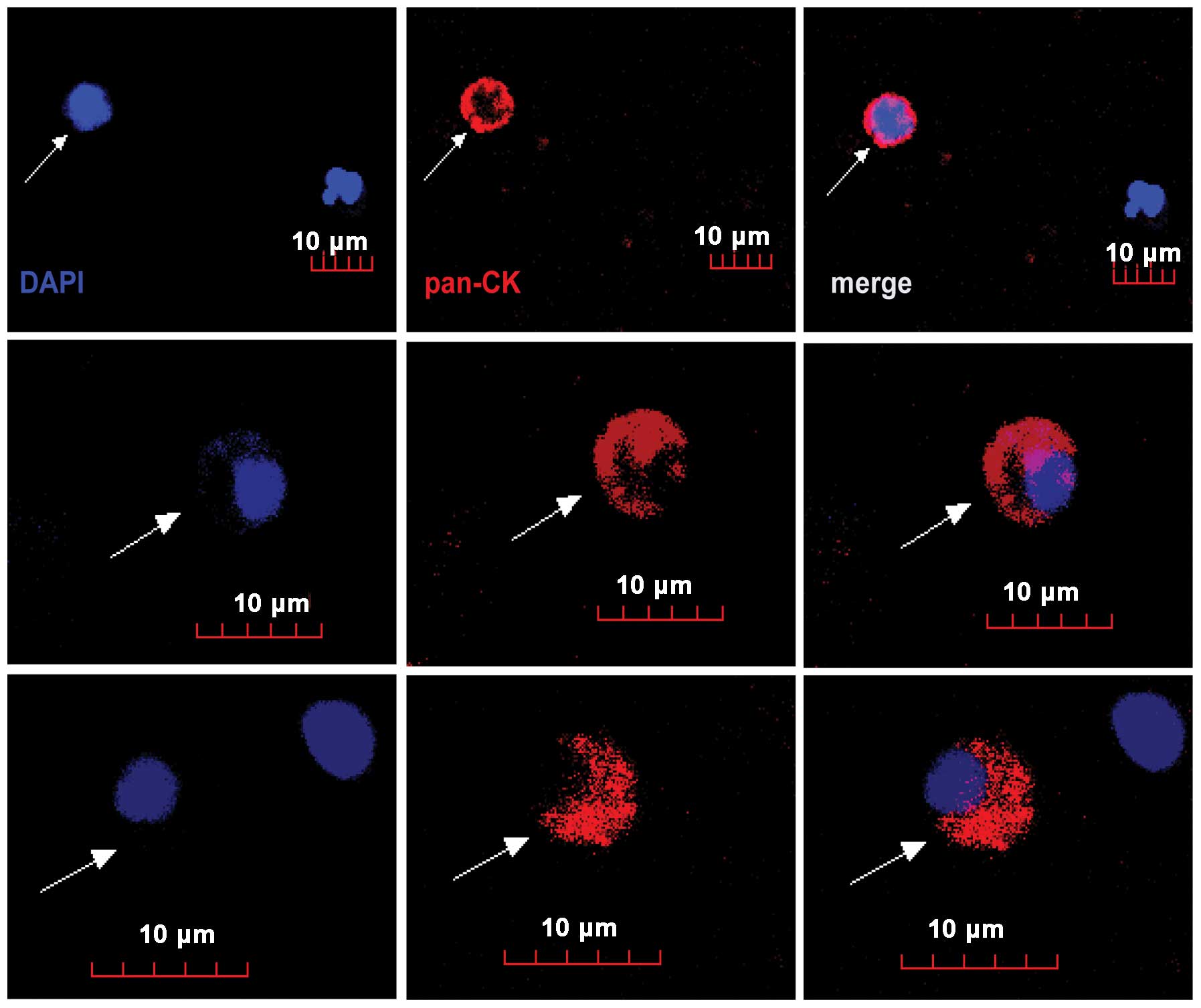
V). Fig. 4 shows some
representative enriched breast circulating tumor cells which were
intact with round to oval morphology with visible DAPI stained
nucleus and positive for anti-cytokeratin (pan) staining.
 | Table IVQuantification of CTCs of blood
samples from healthy people. |
Table IV
Quantification of CTCs of blood
samples from healthy people.
| Healthy sample
no. | CTCs identified by
ICC | CTCs idenfied by
KRT19 | CTCs identified by
ERBB2 |
|---|
| HD01 | 0 | 0 | 0 |
| HD02 | 0 | 0 | 0 |
| HD03 | 0 | 0 | 1 |
| HD04 | 0 | 0 | 0 |
| HD05 | 0 | 0 | 0 |
| HD06 | 0 | 0 | 0 |
| HD07 | 0 | 2 | 0 |
| HD08 | 0 | 1 | 0 |
| HD09 | 0 | 1 | 0 |
| HD10 | 0 | 0 | 1 |
| HD11 | 0 | 0 | 0 |
| HD12 | 0 | 0 | 1 |
| HD13 | 0 | 2 | 1 |
| HD14 | 0 | 0 | 0 |
| HD15 | 0 | 0 | 0 |
 | Table VQuantification of CTCs of blood
samples from patients with breast cancer. |
Table V
Quantification of CTCs of blood
samples from patients with breast cancer.
| Sample no. | Type of breast
cancer | TNM stage | Age (years) | Surgery status | Recurrence | IHC index | CTCs identified by
ICC | CTCs identified by
KRT19 | CTCs identified by
ERBB2 |
|---|
|
|---|
| ER | PR | HER-2 |
|---|
| BCA21 | Invasive breast
carcinoma | T2N1M0, IIB | 70 | Post | | +++ | +++ | ++ | 1 | 17.5 | 36.8 |
| BCA22 | Invasive ductal
carcinoma | T2N2M0, IIIA | 39 | Post | | +++ | + | + | 7 | 99.6 | 65.8 |
| BCA23 | Invasive breast
carcinoma | T4N2M0, IIIB | 62 | No | Yes | − | − | +++ | 9 | 55 | 18.6 |
| BCA24 | Invasive ductal
carcinoma | T1N2M1, IV | 46 | Post | | ++ | − | +++ | 1 | 50.4 | 22.4 |
| BCA25 | Ductal carcinoma
in situ | T2N0M0, IIA | 31 | No | | − | + | ++ | 0 | 29.8 | 18.4 |
| BCA26 | Invasive ductal
carcinoma | T2N2M0, IIIA | 60 | Post | | − | − | +++ | 3 | 42.6 | 19.2 |
| BCA27 | Invasive ductal
carcinoma | T1N2M1, IV | 56 | No | | − | + | − | 11 | 21.2 | 11 |
| BCA28 | Invasive lobular
carcinoma | T1N1M0, IIA | 57 | No | | + | + | ++ | 3 | 2.4 | 4.8 |
| BCA29 | Invasive ductal
carcinoma | T4N2M1, IV | 59 | Post | Yes | − | − | +++ | 23 | 2.6 | 15.2 |
| BCA30 | Invasive ductal
carcinoma | T1N0M0, I | 32 | Post | | + | + | +++ | 1 | 11.2 | 28.6 |
| BCA31 | Invasive ductal
carcinoma | T2N0M0, IIA | 48 | No | | − | − | − | 0 | 10.2 | 0.2 |
| BCA32 | Invasive breast
carcinoma | T1N1M0, IIA | 60 | No | | +++ | + | ++ | 1 | 10.9 | 8.2 |
| BCA33 | Invasive ductal
carcinoma | T1N0M0, I | 36 | Post | | + | − | + | 0 | 7 | 0 |
| BCA34 | Invasive ductal
carcinoma | T4N2M1, IV | 59 | Post | Yes | +++ | +++ | ++ | 19 | 20.6 | 11.6 |
| BCA35 | Invasive ductal
carcinoma | T3N1M1, IV | 40 | No | | + | + | +++ | 21 | 70.6 | 13.86 |
| BCA36 | Invasive lobular
carcinoma | T2N1M0, IIA | 44 | No | | N/A | N/A | N/A | 3 | 3.1 | 20.78 |
For the quantification of enriched CTCs by real-time
RT-PCR assay, total RNAs extracted from a serial of concentrations
of SK-BR-3 cells served as a template for an external calibration
curve to calculate the quantity of breast CTCs. Among the 15
healthy donors, 8 people were detected with 1 or 2 CTCs by KRT19
and/or ERBB2 genes. For this reason, we set 5 as a cut-off value of
positive CTCs for all real-time RT-PCR assay. For the
immunocytochemistry staining, no healthy donors could be detected
with CTC. Thus, a positive patient was defined as one whose CTC
count in 1 ml blood was >2 for immunocytochemistry staining.
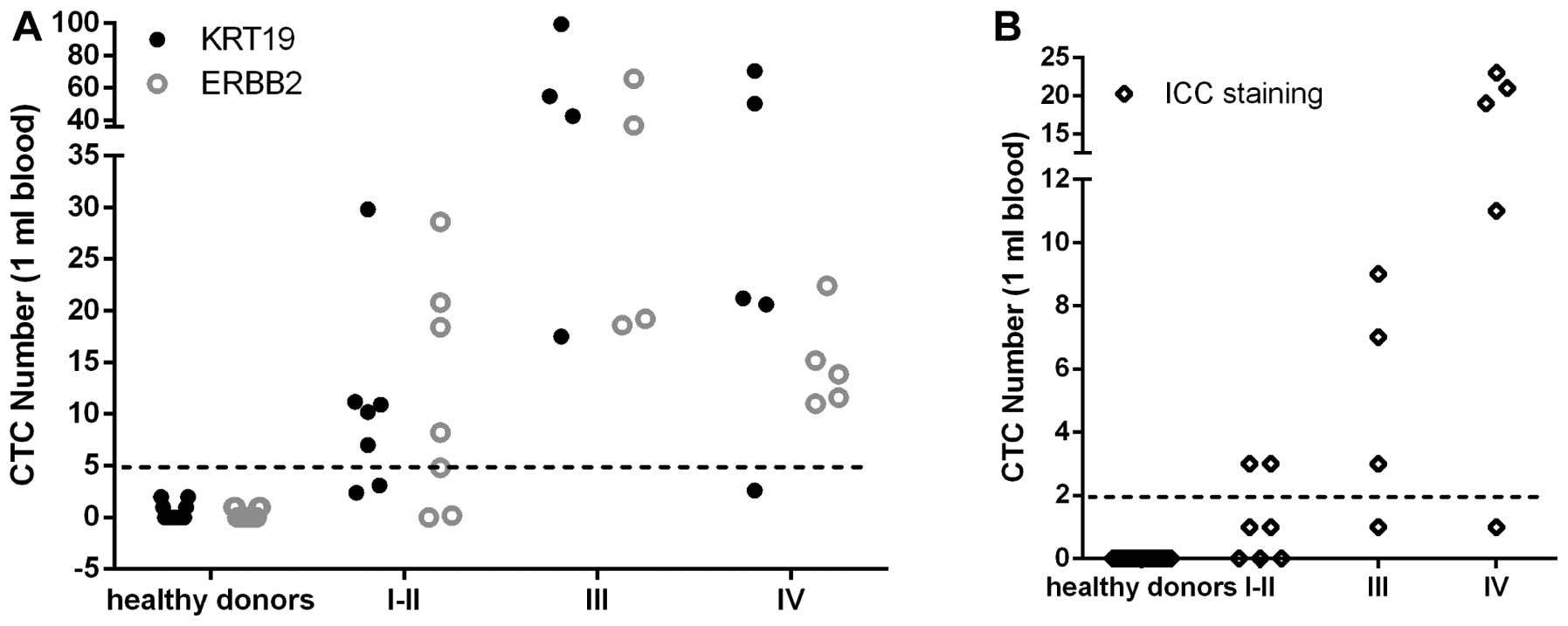
Distributions of positive CTC cells among healthy
donors and patients are demonstrated in Fig. 5 and summarized in Table VI. According to the cut-off value,
the positive CTC detection rate was zero in healthy donors by
real-time RT-PCR assay or immunocytochemistry staining. For
immunocytochemistry staining, the positive detection rates for all
histologic cancers according to stage I–II, III and IV were 28.5,
75 and 80%, respectively. For real-time RT-PCR assay by KRT19 gene,
the positive detection rates for stage I–II, III and IV were 71,
100 and 80%, respectively. For real-time RT-PCR assay by ERBB2
gene, the positive detection rates for stage I–II, III and IV were
57, 100 and 100%. Combining the results of real-time RT-PCR assay
by KRT19 and ERBB2 gene together, the positive detection rate for
stage I–II, III and IV reached 85.7, 100 and 100%, respectively.
These results indicated that breast CTCs detection by duplex
real-time RT-PCR assay had higher detection sensitivity than that
by immunostaining.
 | Table VIAnalysis of CTC detection by
real-time PCR and immunostaining. |
Table VI
Analysis of CTC detection by
real-time PCR and immunostaining.
| | CTCs >5 | CTCs >2 |
|---|
| |
|
|
|---|
| Pathological
type | Number | Identified by KRT19
Positive (%) | Identified by ERBB2
Positive (%) | Identified by ICC
staining Positive (%) |
|---|
| Healthy donors | 15 | 0 (0) | 0 (0) | 0 (0) |
| I–II | 7 | 5 (71) | 4 (57) | 2 (28.5) |
| III | 4 | 4 (100) | 4 (100) | 3 (75) |
| IV | 5 | 4 (80) | 5 (100) | 4 (80) |
Discussion
In the present study, we developed a novel
circulating breast tumor cell detection approach using duplex
real-time RT-PCR technique in combination with negative enrichment
strategy. Our results showed that the developed negative enrichment
approach could efficiently enrich and recover breast tumor cells
from peripheral blood with a high degree of accuracy, and the
duplex real-time RT-PCR assay using KRT19 and ERBB2 as targeted
genes could consistently detect one breast tumor cell even in the
environment containing relative large quantities of contaminating
leukocytes.
Since CTCs are rare cells in peripheral blood,
isolation of CTCs represents a major technological challenge and
enrichment prior to the actual detection procedure could improve
the sensitivity of CTCs detection. The most widely used CTC
isolation technologies rely on positive enrichment by
antibody-based capture of CTCs which express epithelial cell
surface markers such as the epithelial cell adhesion molecule
(EpCAM) that are absent from normal leukocytes. But, not all CTCs
express the EpCAM antigen. Moreover, EpCAM expression tends to
change dynamically during the epithelial-mesenchymal transition
(EMT) process in the metastatic cascade (16–18).
To avoid the bias of selecting cells by virtue of EpCAM expression,
negative enrichment by removing leukocytes has been advocated which
has the potential to purify CTCs irrespective of presumed cell
surface markers and the advantages such as keeping the CTCs in an
intact/untargeted form over positive enrichment in isolating rare
cells (18–21).
To determine the performance of the system in the
negative enrichment process, the overall level of enrichment,
recovery rate and sensitivity were the common quantitative
measures. Different negative enrichment approaches based on
immunomagnetic separation demonstrated different overall level of
enrichment, ranging from 2.7 to 5.66 log for fresh peripheral
blood, and different recovery rate by spiking experiment, range
from 36 to 85% (22). Although a
completely negative selection methodology might achieve a higher
enrichment performance, it also might reduce the recovery rate
(22). In the present study, we
tried to perform the negative enrichment process with as few steps
as possible to balance between enrichment level and recovery rate.
We used a density gradient centrifugation step instead of the red
blood cell lysis step to reduce the possible harm and loss to CTCs
(23,24). Here, we achieved an average 3 log
enrichment (data not shown), and such concentration is sufficient
to perform further RT-PCR analysis of CTCs with less background
contamination. Furthermore, we achieved a high recovery rate (range
from 75 to 93.8%, mean 84.1%) to increase the overall sensitivity
of the CTCs detection.
Another major challenge for CTC identification was
the prevailing difficulty of finding an mRNA marker which could
distinguish normal expression in blood from that due to the
presence of CTCs. Several mRNA markers have been used for
RT-PCR-based detection of CTCs and evaluated for their sensitivity,
specificity and clinical potential in breast cancer, such as CK19
(25), mammaglobin (26), HER2 (27), MUC1 (28). The fact that few markers provide
adequate sensitivity individually makes combination of markers a
good choice for CTCs detection (29,30).
In the present study, we screened the sensitivity and specificity
of CK19, HER2, EpCAM and MUC1 for breast CTCs detection, and found
that the real-time RT-PCR assay using KRT19 and ERBB2 as targeted
genes could consistently detect a tumor cell in various breast
cancer cell lines. In addition, duplexing assay using KRT19 and
ERBB2 improved the sensitivity of CTCs detection in breast cancer,
especially in early breast cancer compared to that of
immunocytochemistry staining.
In summary, our CTC detection approach that combines
negative enrichment with real-time quantitative RT-PCR assay using
KRT19 and ERBB2 as targeted genes demonstrated high sensitivity,
specificity and potential clinical utility in breast cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81071434).
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M, et al:
GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC
CancerBase No. 11. International Agency for Research on Cancer;
Lyon: 2013 Last accessed April, 2014
|
|
2
|
Weigel MT and Dowsett M: Current and
emerging biomarkers in breast cancer: prognosis and prediction.
Endocr Relat Cancer. 17:R245–R262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu M, Stott S, Toner M, Maheswaran S and
Haber DA: Circulating tumor cells: approaches to isolation and
characterization. J Cell Biol. 192:373–382. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wallwiener M, Hartkopf AD, Baccelli I, et
al: The prognostic impact of circulating tumor cells in subtypes of
metastatic breast cancer. Breast Cancer Res Treat. 137:503–510.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayes DF, Cristofanilli M, Budd GT, et al:
Circulating tumor cells at each follow-up time point during therapy
of metastatic breast cancer patients predict progression-free and
overall survival. Clin Cancer Res. 12:4218–4224. 2006. View Article : Google Scholar
|
|
6
|
Cristofanilli M, Budd GT, Ellis MJ, et al:
Circulating tumor cells, disease progression, and survival in
metastatic breast cancer. N Engl J Med. 351:781–791. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Liu Q, Wang T, et al: Circulating
tumor cells in HER2-positive metastatic breast cancer patients: a
valuable prognostic and predictive biomarker. BMC Cancer.
13:2022013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang ZF, Cristofanilli M, Shao ZM, et al:
Circulating tumor cells predict progression-free and overall
survival in Chinese patients with metastatic breast cancer,
HER2-positive or triple-negative (CBCSG004): a multicenter,
double-blind, prospective trial. Ann Oncol. 24:2766–2772. 2013.
View Article : Google Scholar
|
|
9
|
Yusa A, Toneri M, Masuda T, et al:
Development of a new rapid isolation device for circulating tumor
cells (CTCs) using 3D palladium filter and its application for
genetic analysis. PLoS One. 9:e888212014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu S, Liu Z, Liu S, Lin L, Yang W and Xu
J: Enrichment and enumeration of circulating tumor cells by
efficient depletion of leukocyte fractions. Clin Chem Lab Med.
52:243–251. 2014.PubMed/NCBI
|
|
11
|
Zhao M, Schiro PG, Kuo JS, et al: An
automated high-throughput counting method for screening circulating
tumor cells in peripheral blood. Anal Chem. 85:2465–2471. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HJ, Cho HY, Oh JH, et al: Simultaneous
capture and in situ analysis of circulating tumor cells using
multiple hybrid nanoparticles. Biosens Bioelectron. 47:508–514.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagrath S, Sequist LV, Maheswaran S, et
al: Isolation of rare circulating tumour cells in cancer patients
by microchip technology. Nature. 450:1235–1239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krishnamurthy S, Cristofanilli M, Singh B,
et al: Detection of minimal residual disease in blood and bone
marrow in early stage breast cancer. Cancer. 116:3330–3337. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu C, Hao H, Li L, et al: Preliminary
investigation of the clinical significance of detecting circulating
tumor cells enriched from lung cancer patients. J Thorac Oncol.
4:30–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gorges TM, Tinhofer I, Drosch M, et al:
Circulating tumour cells escape from EpCAM-based detection due to
epithelial-to-mesenchymal transition. BMC Cancer. 12:1782012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Armstrong AJ, Marengo MS, Oltean S, et al:
Circulating tumor cells from patients with advanced prostate and
breast cancer display both epithelial and mesenchymal markers. Mol
Cancer Res. 9:997–1007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu M, Bardia A, Wittner BS, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Z, Fusi A, Klopocki E, et al: Negative
enrichment by immunomagnetic nanobeads for unbiased
characterization of circulating tumor cells from peripheral blood
of cancer patients. J Transl Med. 9:702011. View Article : Google Scholar
|
|
20
|
Hyun KA, Lee TY and Jung HI: Negative
enrichment of circulating tumor cells using a geometrically
activated surface interaction chip. Anal Chem. 85:4439–4445. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sajay BN, Chang CP, Ahmad H, et al:
Microfluidic platform for negative enrichment of circulating tumor
cells. Biomed Microdevices. 6:537–548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang L, Lang JC, Balasubramanian P, et al:
Optimization of an enrichment process for circulating tumor cells
from the blood of head and neck cancer patients through depletion
of normal cells. Biotechnol Bioeng. 102:521–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lara O, Tong X, Zborowski M and Chalmers
JJ: Enrichment of rare cancer cells through depletion of normal
cells using density and flow-through, immunomagnetic cell
separation. Exp Hematol. 32:891–904. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tong X, Yang L, Lang JC, Zborowski M and
Chalmers JJ: Application of immunomagnetic cell enrichment in
combination with RT-PCR for the detection of rare circulating head
and neck tumor cells in human peripheral blood. Cytometry B Clin
Cytom. 72:310–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stathopoulou A, Gizi A, Perraki M, et al:
Real-time quantification of CK-19 mRNA-positive cells in peripheral
blood of breast cancer patients using the lightcycler system. Clin
Cancer Res. 9:5145–5151. 2003.PubMed/NCBI
|
|
26
|
Ntoulia M, Stathopoulou A, Ignatiadis M,
et al: Detection of Mammaglobin A-mRNA-positive circulating tumor
cells in peripheral blood of patients with operable breast cancer
with nested RT-PCR. Clin Biochem. 39:879–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ignatiadis M, Kallergi G, Ntoulia M, et
al: Prognostic value of the molecular detection of circulating
tumor cells using a multimarker reverse transcription-PCR assay for
cytokeratin 19, mammaglobin A, and HER2 in early breast cancer.
Clin Cancer Res. 14:2593–2600. 2008. View Article : Google Scholar
|
|
28
|
de Cremoux P, Extra JM, Denis MG, et al:
Detection of MUC1-expressing mammary carcinoma cells in the
peripheral blood of breast cancer patients by real-time polymerase
chain reaction. Clin Cancer Res. 6:3117–3122. 2000.PubMed/NCBI
|
|
29
|
Varangot M, Barrios E, Sonora C, et al:
Clinical evaluation of a panel of mRNA markers in the detection of
disseminated tumor cells in patients with operable breast cancer.
Oncol Rep. 14:537–545. 2005.PubMed/NCBI
|
|
30
|
Reinholz MM, Nibbe A, Jonart LM, et al:
Evaluation of a panel of tumor markers for molecular detection of
circulating cancer cells in women with suspected breast cancer.
Clin Cancer Res. 11:3722–3732. 2005. View Article : Google Scholar : PubMed/NCBI
|