Introduction
The high mobility group box 1 (HMGB1) is an abundant
and ubiquitous chromatin associated protein in mammals, which acts
as a DNA chaperone in transcription, replication, recombination and
repair (1). HMGB1 bends DNA and
promotes the access of transcriptional protein assemblies to
specific DNA targets. HMGB1 plays an important role in
non-homologous end-joining, mismatch repair, and nucleotide
excision repair pathways (2,3) and
enhances ligation reactions of DNA double-strand breaks (DSBs)
(4,5).
HMGB1 is involved in tumor development,
proliferation, invasion, and metastasis, and its high levels are
associated with a poor clinical prognosis. However, HMGB1 may play
a conflicting dual role in tumors (6), and one study suggested that HMGB1
levels may correlate with radiosensitivity (7), although the underlying mechanism is
unclear.
Telomeres are specialized DNA-protein complexes
found at the ends of eukaryotic chromosomes. Telomeres are composed
of a variable number of TTAGGG sequences repeated in tandem and
associated proteins (8).
Telomerase is detected in ~90% of all malignant tumors (9) and is a highly attractive target for
cancer therapeutics. Our previous research suggested
radiosensitivity of Hep-2 cells (human laryngeal squamous carcinoma
cells) negatively correlated with telomere length, and positively
correlated with telomerase activity (10). Thus, telomere homeostasis is
closely related to radiosensitivity, and increased radiosensitivity
can help control the rate of tumor growth.
A couple of studies have focused on the roles of
HMGB1 in telomere biology. One study showed that HMGB1 had no
effect on telomerase activity in the plant Arabidopsis
thaliana, and that varying the expression of HMGB1 did not
cause any obvious changes in chromatin structure (11). However, Polanska et al
(12) showed that knockout of the
HMGB1 gene in mouse embryonic fibroblasts (MEFs) resulted in a
decline in telomerase activity and telomere dysfunction, while
overexpression of HMGB1 enhanced telomerase activity. Together,
these findings indicate that HMGB1 is indispensable for telomere
homeostasis, but that the relationship between HMGB1 and telomere
biology remains unclear in mammalian cells.
The present study was designed to determine the
effect of changing the expression of HMGB1 on telomere in human
cancer cells. Specifically, we investigated the role of HMGB1 on
telomere homeostasis and radiosensitivity in MCF-7 human breast
cancer cells.
Materials and methods
Cell lines, transfection, plasmids and
reagents
MCF-7 cells were obtained from the Key Laboratory of
Tumor Biological Behavior of Hubei Province and incubated under 5%
CO2 at 37°C in RPMI-1640 medium containing 10% fetal
bovine serum. Transfections were carried out using Lipofectamine
2000 (Invitrogen, Carlsbad, CA, USA) for plasmids and shRNA. The
Cell Counting Kit-8 (CCK-8) was purchased from Boster (Wuhan,
China).
Construction of stably transfected cell
lines
For the construction of HMGB1 knockdown vectors, a
human shRNA 5′-GCT CAAGGAGAATTTGTAA-3′ targeted to HMGB1 was
selected, the sequence had the strongest interference effect from
the reference; and then a scrambled human shRNA sequence
5′-GCTCTGGAGCAGTTCCGATATC-3′, possessing limited homology to human
genes, served as the negative control (13). The shRNAs were synthesized and
subcloned into the pGPU6/GFP/Neo shRNA vector (GenePharma,
Shanghai, China). The resultant vectors were named as
pGPU6/GFP/Neo-HMGB1 and pGPU6/GFP/Neo-shNC.
HMGB1 underexpressing cells and negative control
cells were selected using 600 μg/ml G418 (Merck) for 5 weeks. The
resultant stably transfected cell lines were named as MCF-7-shHMGB1
and MCF-7-NC, respectively.
Real-time PCR to detect the expression of
HMGB1 mRNA
Total RNA was extracted from the stably transfected
cells, and cDNA was synthesized using a reverse transcriptase kit
(Fermentas, Canada) at 42°C for 10 min, followed by 95°C for 2 min.
Duplicate PCR reactions were performed using the Takara real-time
PCR kit (Takara Bio, Otsu, Shiga, Japan) according to the
manufacturer’s instructions. Samples were preincubated at 95°C for
30 sec followed by 40 cycle of 95°C for 5 sec, 60°C for 15 sec and
72°C for 30 sec. HMGB1 forward and reverse primers were
5′-ATATGGCAAAAGCGGAC AAG-3′ and 5′-GCAACATCACCAATGGACAG-3′
(13). The β-actin forward and
reverse primers were 5′-TGGCACCCA GCACAATGAA-3′ and
5′-CTAAGTCATAGTCCGCCTAG AAGCA-3′. All experiments were repeated at
least three times. Thermal amplification was carried out on an
Mx3000P qPCR system (Stratagene, La Jolla, CA, USA), and the
results were analyzed using the MXP3000P analysis program.
Clonogenic assay
Cells were plated in 6-well culture flasks. After 24
h, cells were irradiated with graded doses (0, 1, 2, 4, 6, 8 and 10
Gy) using an X-ray generator (Primus High-Energy Siemens) at a dose
rate of 2 Gy/min. Cells were then cultured under 5% CO2
at 37°C for 14 days. The colonies were fixed and stained with
crystal violet (1% in absolute ethanol). Cell survival was measured
by counting the colonies containing >50 cells. The data were
entered into the linear-quadratic model, and the survival curve of
each group was demonstrated using Graphpad Prism 5 software.
Radiobiological parameters were calculated according to the
survival curves.
Western blot analysis
The expression of HMGB1, hTERT, Cyclin D1, CDC25C
(Abcam, Cambridge, UK), ATM, ATR, phosphor-ATM, phosphor-ATR, TRF1,
TRF2, PTOP, rH2AX (Cell Signaling Technology, Danvers, MA, USA),
and GAPDH (Santa Cruz Bio, Dallas, TX, USA; as a loading control)
were determined by western blotting as described previously
(14). All experiments were
repeated three times. The results were analyzed by ImageJ
software.
DNA extraction and real-time PCR to
determine relative telomere length
Genomic DNA was extracted from cells by standard
procedures using the TIANamp Genomic DNA kit (Tiangen Bio, Beijing,
China) and stored at 4°C. Relative telomere length was determined
by using qRT-PCR as described by Cawthon (15). Duplicate PCR reactions were
performed using the Takara real-time PCR kit (Takara Bio, Japan)
according to the manufacturer’s instructions. The telomere and
single copy gene specific primers used were as follows: tel1,
5′-GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAG GGT-3′. tel2,
5′-TCCCGACTATCCCTATCCCTATCCCTA TCCCTATCCCTA-3′. 36B4u,
5′-CAGCAAGTGGGAAGG TGTAATCC-3′. 36B4d, 5′-CCCATTCTATCATCAACGGG
TACAA-3′. The primers were synthesized by Sangon Biotech (Shanghai,
China), The cycling conditions consisted of preincubation for 5 sec
at 95°C, followed by 35 cycles of 95°C for 15 sec and 54°C for 2
min. Thermal amplification was carried out using Mx3000P
(Stratagene), and the results were analyzed using the MXP3000
analysis program. All experiments were repeated three times.
PCR-ELISA assay
Protein concentrations were determined by the BSA
assay following cell lysis. The telomerase activity of each sample
was determined using the Telo-TAGGG Telomerase PCR-ELISA kit
(Roche, Basel, Switzerland). The absorbance of each sample was
determined at 450 nm using a microplate reader (Bio-Rad, Hercules,
CA, USA) (with a blank reference wavelength of ~690 nm) 30 min
after addition of the stop reagent. Data were normalized using the
Renilla luciferase assay. Each experiment was performed three times
in triplicate wells.
Cell proliferation assay
Cells diluted with RPMI-1640 medium containing 10%
fetal bovine serum, were seeded at 103 cells/well in
96-well plates and cultured in 100 μl culture medium, six identical
wells were used for each sample. After 24 h, 10 μl of CCK-8 was
added to each well, and the plates were incubated at 37°C for 2 h.
The absorbance of each well was then read at 450 nm using a 96-well
plate reader. Each experiment was performed at least three times in
triplicate wells.
Analysis of cell cycle and apoptosis by
flow cytometry
The cell cycle was assessed in cells without
irradiation and cells exposed to 6 Gy of ionizing radiation, and
then incubated for the indicated times. Cells were fixed in 70%
ethanol overnight, and then treated with RNase for 20 min before
addition of 5 mg/ml propidium iodide, and analysis by flow
cytometry (Beckman Coulter, Brea, CA, USA). Experiments were
performed in triplicate.
Apoptosis was performed using an Annexin V-PE
Apoptosis Analysis kit (Sungene Bio, Tianjin, China) according to
the manufacturer’s instructions. Fluorescence was measured using a
flow cytometer and the data were analyzed with CellQuest software.
All samples were assayed in triplicate.
Immunofluorescence
Cells were fixed with 4% formaldehyde for 15 min and
then permeabilized with 0.2% Triton X-100 in PBS for 10 min at room
temperature. After treatment with blocking solution, cells were
incubated with the primary antibody overnight at 4°C, washed, and
incubated with the secondary antibody. Nuclei were stained with
DAPI (Sigma, San Francisco, CA, USA) for 5 min at room temperature.
Fluorescence was observed using a confocal microscope (Carl Zeiss
LSM710, Germany).
Statistical analysis
All data are expressed as means ± SD. Student’s
t-test was used to determine statistical significance at p<0.05.
SPSS17.0 and Graphpad Prism 5 software were used for the
statistical analyses.
Results
Downregulation of HMGB1 increases the
radiosensitivity of MCF-7 cells
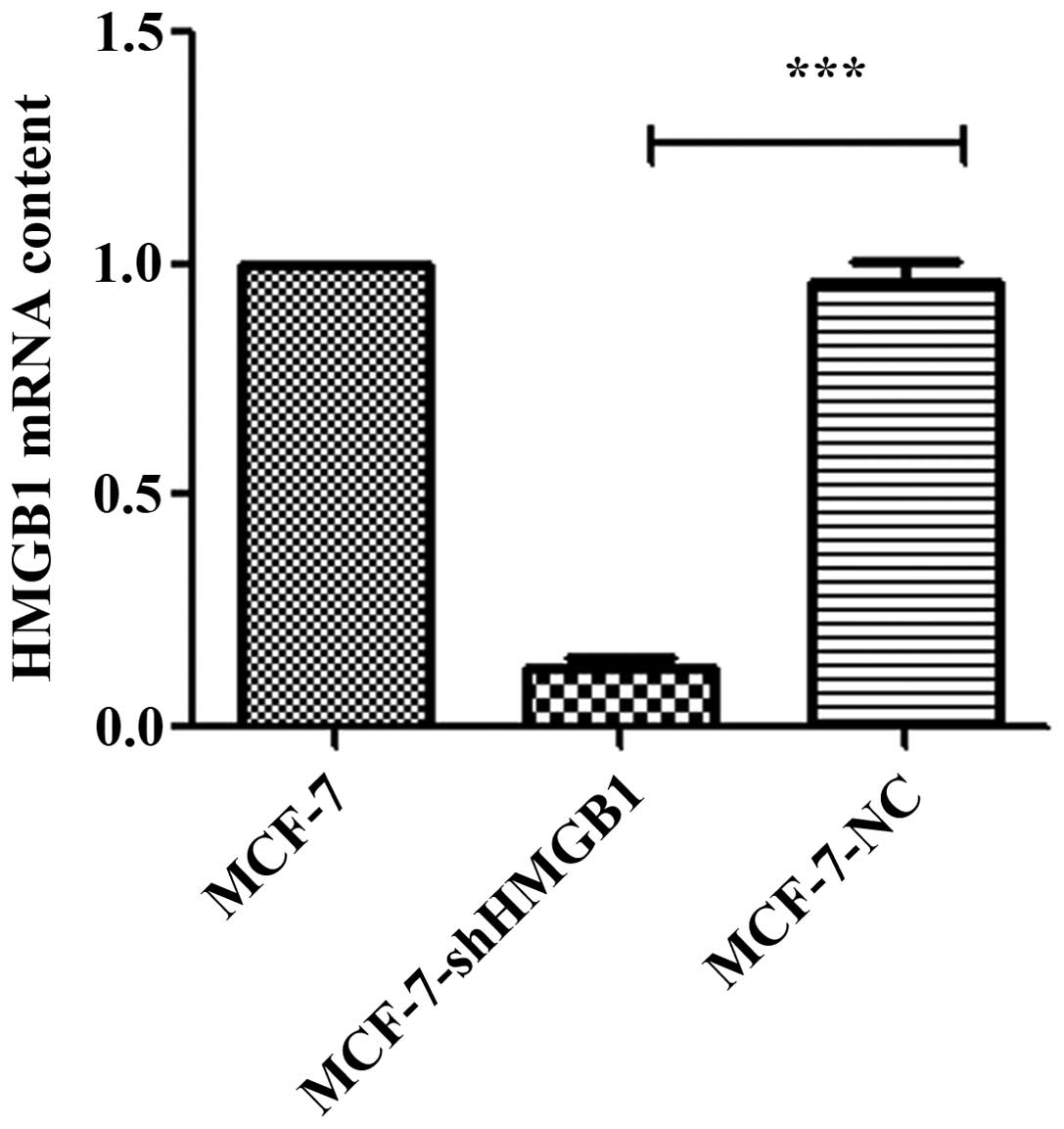
The effect of shRNA-HMGB1 on the expression of HMGB1
was determined in MCF-7 cells (Fig.
1). HMGB1 expression was not affected in MCF-7-NC cells
compared with the parental MCF-7 cells. However, there was a
significant inhibition of HMGB1 expression in MCF-7 cells stably
expressing shRNA-HMGB1.
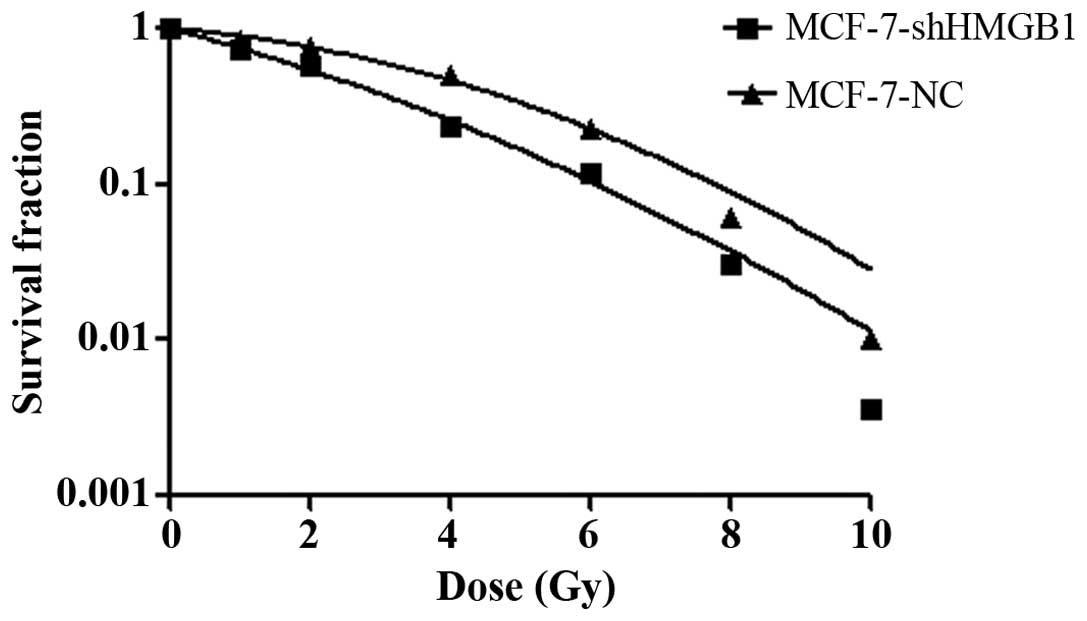
Control and shRNA-HMGB1 transfected MCF-7 cells were
exposed to different doses of radiation. After cell clones were
counted, the survival curves were plotted to evaluate the
radiobiological parameters of each group. Compared to the negative
MCF-7 cell control group, the survival fractions of the shRNA-HMGB1
group were much lower at each dose of radiation (Fig. 2). Plating efficiency and survival
fraction was calculated. Surviving fraction of cells after
irradiation in 2 Gy (SF2) was 0.7756±0.0016 and
0.5732±0.0031 (p<0.01), suggesting that HMGB1 downregulation
induced radiosensitive of MCF-7 cells.
Downregulation of HMGB1 leads to telomere
dysfunction, inhibits DNA damage repair and modulates the cell
cycle
To evaluate whether the level of HMGB1 correlates
with telomere homeostasis and the repair of DSBs in MCF-7 cells, we
constructed cell lines with stable downregulation of HMGB1.
Fig. 3A indicates that the
expressions of hTERT, PTOP, TRF1 and TRF2 were attenuated by
downregulating HMGB1. These data suggest that telomere homeostasis
was disrupted.
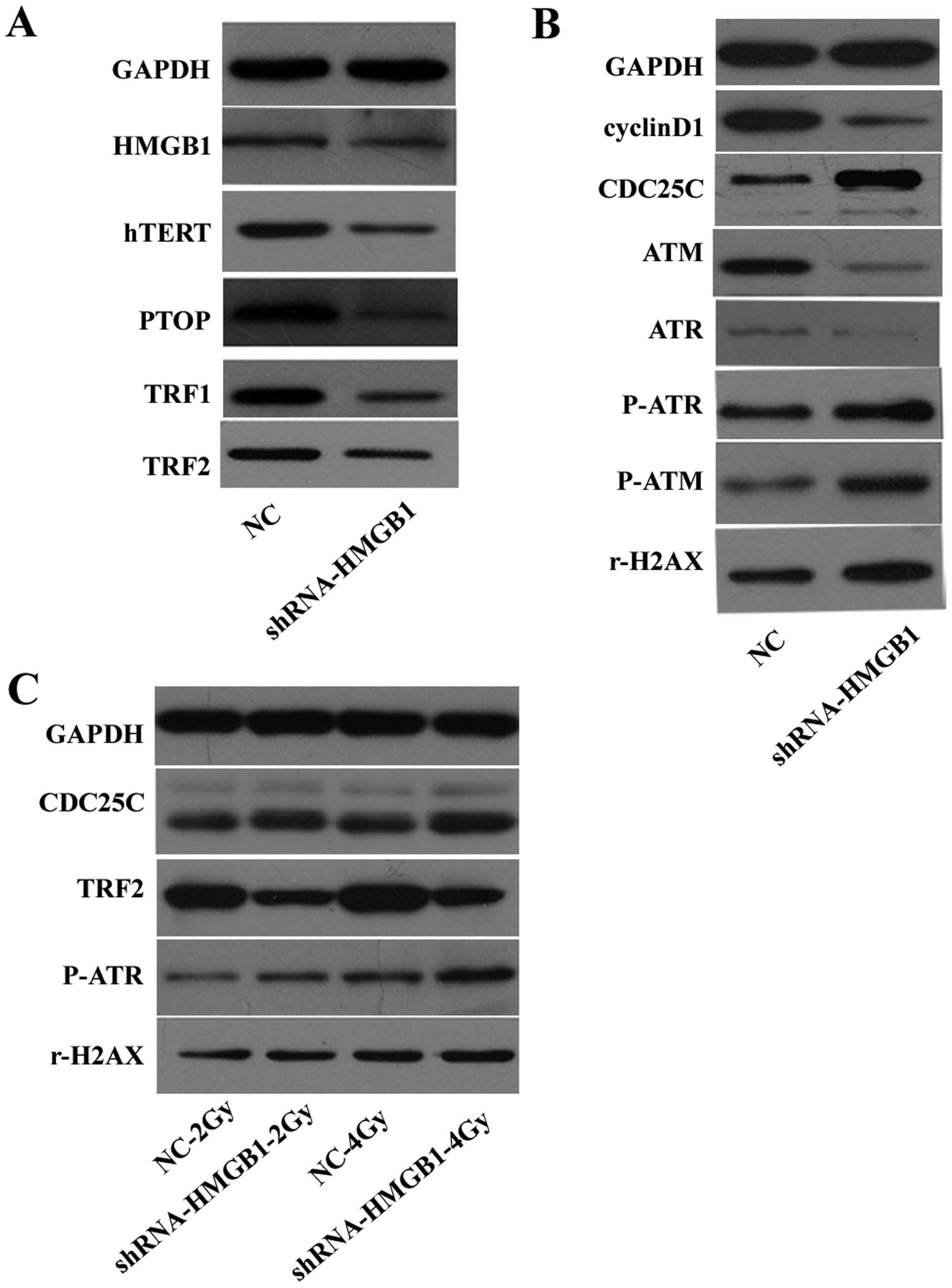 | Figure 3Protein levels of (HMGB1, hTERT, TPP1,
TRF1, TRF2, cyclin D1, ATM, ATR, phospho-ATR, phospho-ATM, rH2AX
and GAPDH) were examined by western blotting. (A) The effect of
downregulating HMGB1 on telomere homeostasis. (B) The effect of
downregulating HMGB1 on the repair of double-strand breaks and cell
cycle proteins. (C) The effect of downregulating HMGB1 on the
expression of CDC25C, TRF2, phospho-ATR and rH2AX after
irradiation. |
The expression of ataxia telangiectasia mutated
(ATM), ataxia telangiectasia rad3-related (ATR), and cyclin D1 were
reduced, while the expression of phosphor-ATM, phosphor-ATR, rH2AX
and CDC25C were increased in MCF-7-shHMGB1 cells compared to
MCF-7-NC cells (Fig. 3B). When
exposed to radiation, rH2AX, phosphor-ATR and CDC25C were further
increased when HMGB1 was downregulated. The increase in rH2AX and
phosphor-ATR protein levels was greater after 4 Gy exposure than
after 2 Gy radiation exposure. However, TRF2 decreased when HMGB1
was downregulated after irradiation (Fig. 3C).
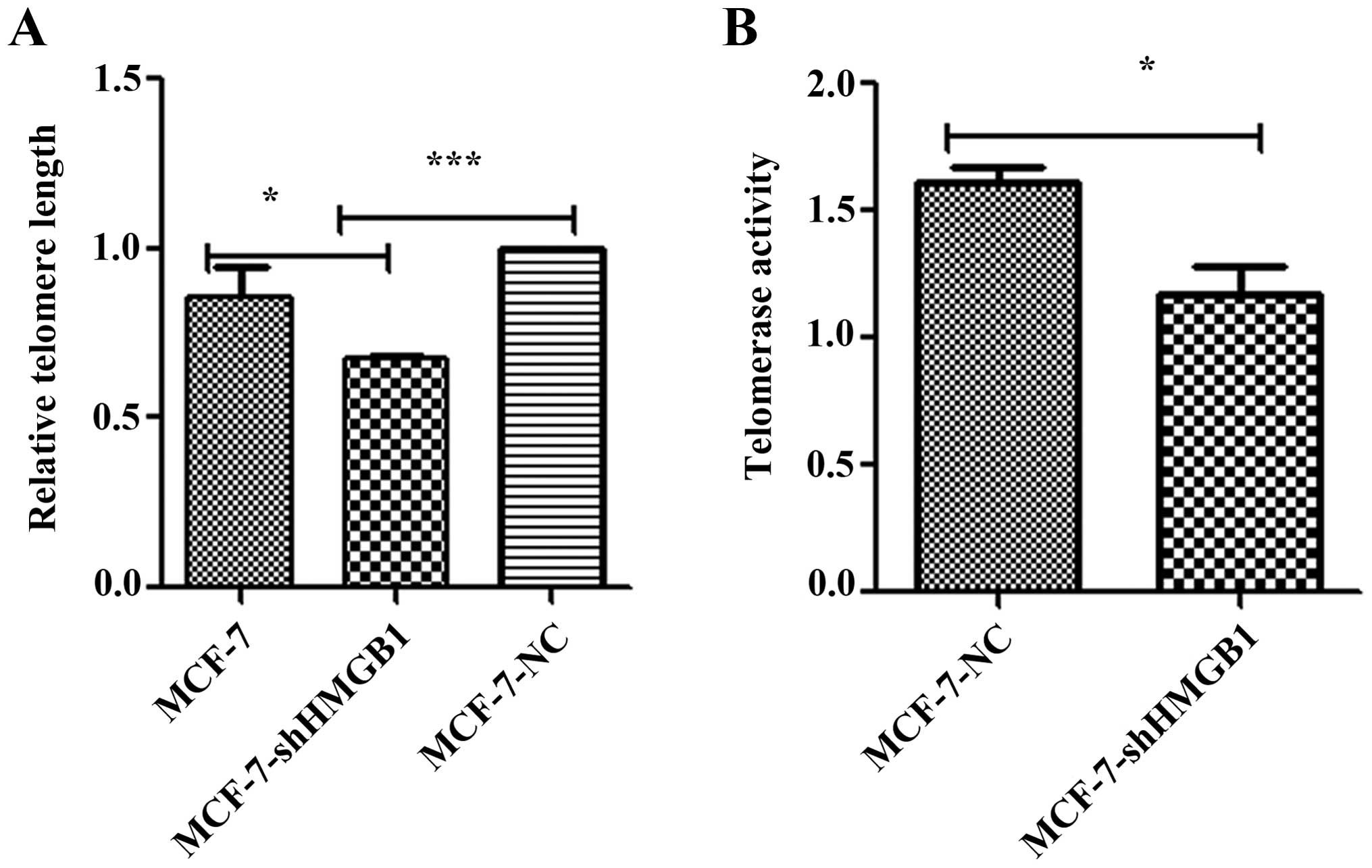
Downregulation of HMGB1 decreases
telomere length and telomerase activity
To investigate the role of HMGB1 in modulating
telomere length, MCF-7-shHMGB1 and MCF-7-NC cells were cultured for
15 population doublings, and telomere length was measured by
real-time PCR. The average telomere length of MCF-7-shHMGB1 cells
was shorter than that in control cells (Fig. 4A). These results suggest that HMGB1
is important in maintaining telomere length in MCF-7 cells.
Telomerase activity is regarded as the primary
determinant of tumor cell radiosensitivity. The telomerase
PCR-ELISA technique was used to determine the effect of HMGB1 on
telomerase activity. The activity of telomerase in MCF-7-shHMGB1
cells was lower than that measured in MCF-7-NC cells (p=0.012)
(Fig. 4B).
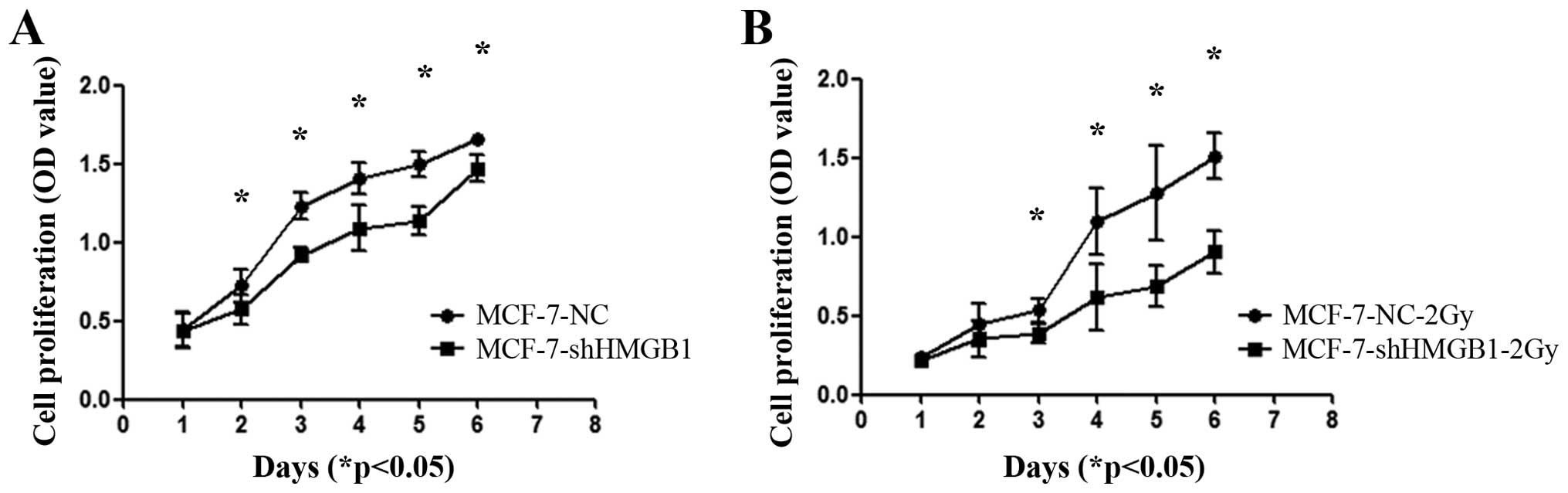
HMGB1 is involved in controlling the
proliferation and cell cycle of MCF-7 cells
Downregulation of HMGB1 inhibited the proliferation
of MCF-7 cells (Fig. 5A). Exposing
MCF-7-shHMGB1 cells to 2 Gy of radiation further decreased the
proliferation of these cells compared to MCF-7-NC cells (Fig. 5B) (p<0.05).
Downregulating HMGB1 decreases the
proportion of MCF-7 cells in the S phase
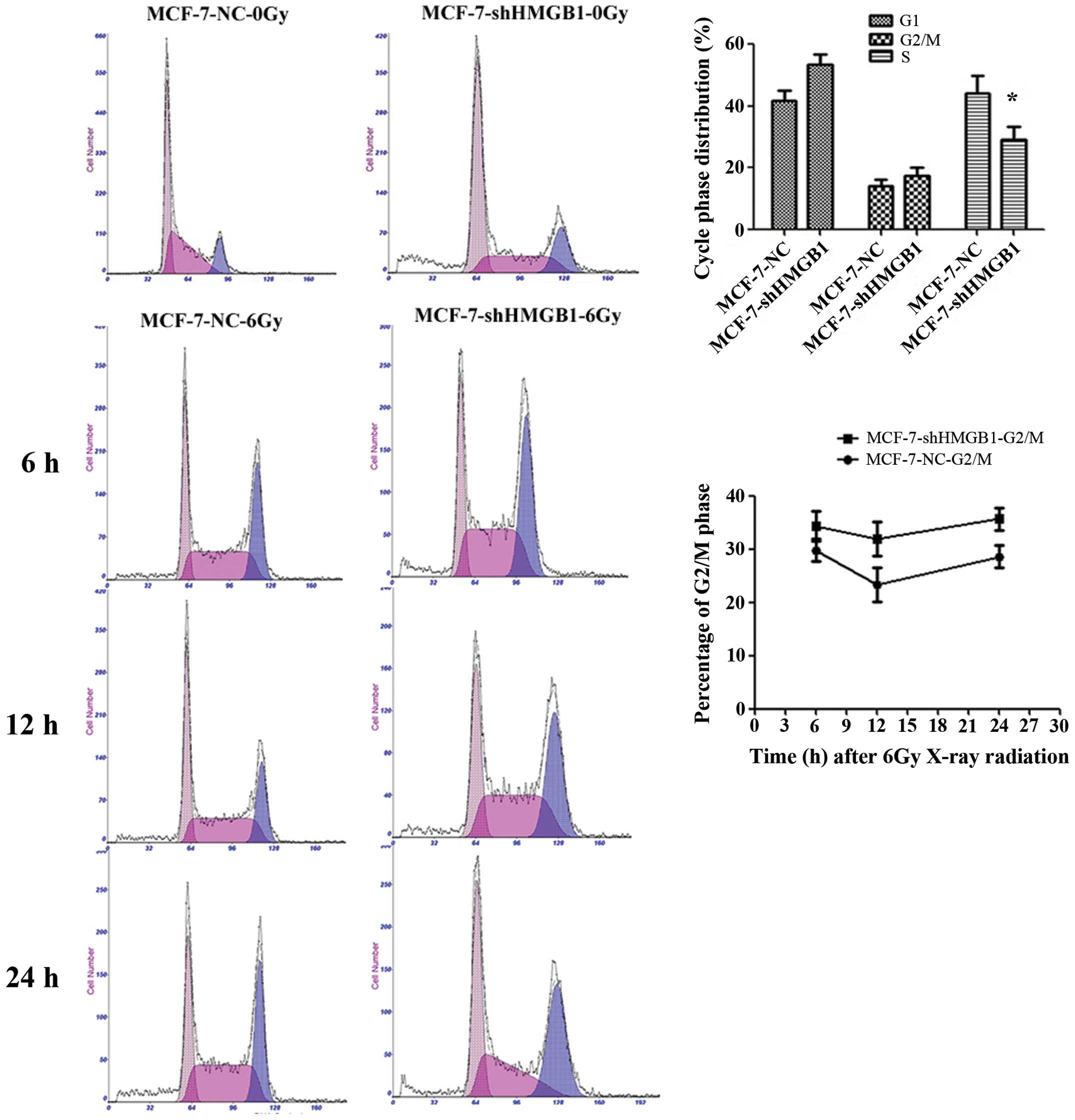
When exposed to radiation, there was an increase in
the percentage of MCF-7-shHMGB1 cells in the G2/M phase compared to
MCF-7-NC cells (p=0.0305). There were no significant differences in
the number cells in the G1 phase between MCF-7-NC and MCF-7-shHMGB1
cells (Fig. 6). Downregulation of
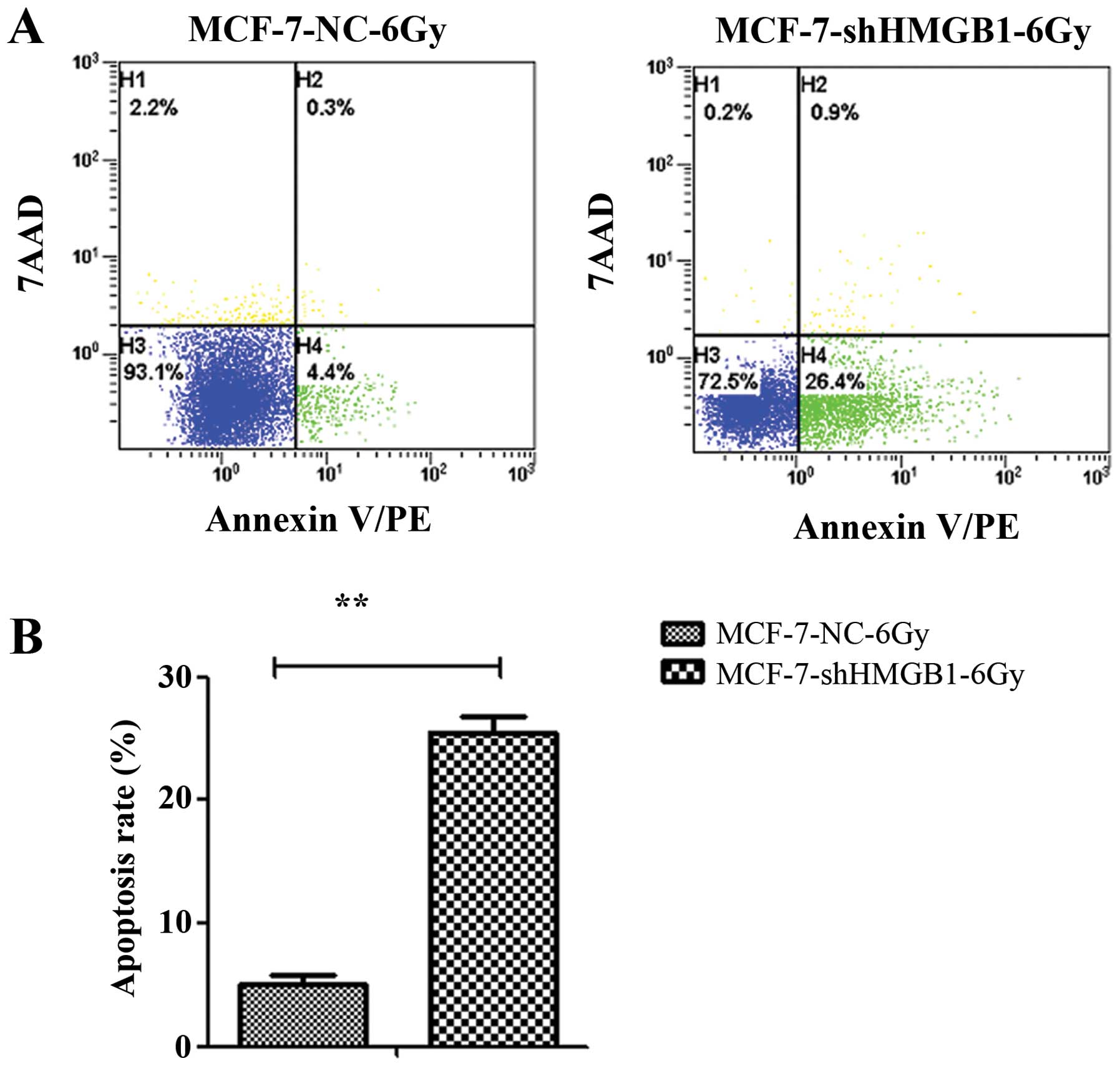
HMGB1 promotes apoptosis of MCF-7 cells. MCF-7-NC and MCF-7-shHMGB1
cells were irradiated with 6 Gy and incubated for 24 h. The
percentage of cells undergoing apoptosis was measured by flow
cytometry. Downregulating HMGB1 enhanced apoptosis in irradiated
MCF-7 cells (p=0.003) (Fig.
7).
Decreasing the expression of HMGB1
inhibits the repair kinetics of DNA damage induced by ionizing
radiation
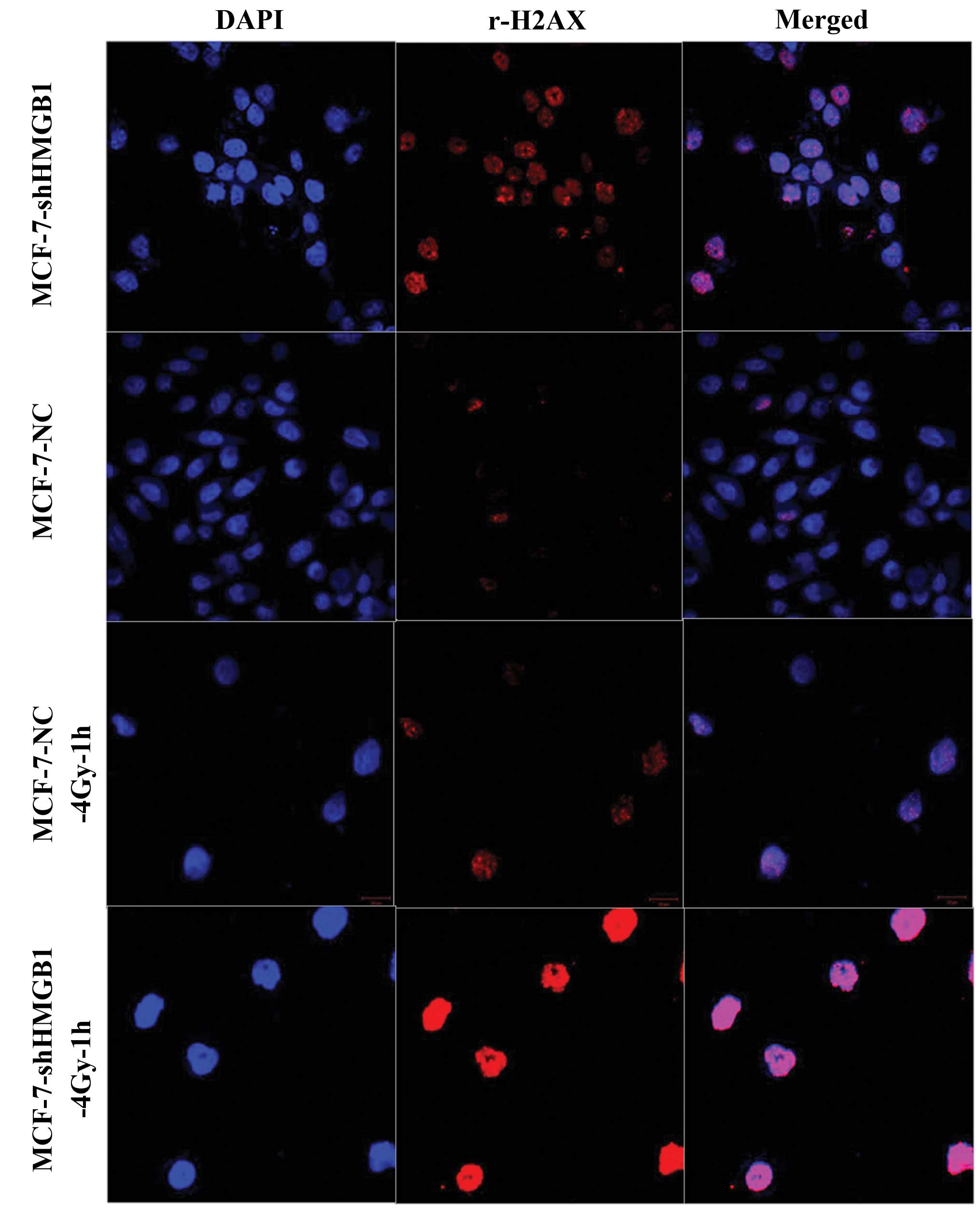
An immunofluorescence assay was used to establish
whether decreasing the expression of HMGB1 affected the repair
kinetics of DNA damage. There were significantly more foci
indicating spontaneous damage in the MCF-7-shHMGB1 cells compared
to the control cells (p<0.05). When exposed to 4 Gy of ionizing
radiation and stained 1 h later to identify the damaged foci. HMGB1
underexpression was found to attenuate the ability of MCF-7 cells
to repair these sites (Fig.
8).
Discussion
The results of the present study show that
decreasing the expression of HMGB1 was associated with damage to
telomeres and increased radiosensitivity of breast cancer cells.
This suggests that HMGB1 plays crucial roles in the regulation of
telomeres and the response of cells to DNA damage.
One previous study suggested that increasing the
expression of HMGB1 suppressed cell growth by initiating G1 arrest
and apoptosis in MCF-7 cells, furthermore showing that HMGB1
suppressed the growth of MCF-7 tumor xenografts in nude mice,
suggesting that HMGB1 functions as a tumor suppressor and
radio-sensitizer in breast cancer cells (16).
Previous research indicated that radiosensitivity
negatively correlates with telomere length (17). In our study, the expression of
HMGB1 was correlated with telomere length and radiosensitivity in
breast cancer cells. Furthermore, we found that downregulating
HMGB1 enhanced the radiosensitivity of these cells, decreasing both
telomere length and telomerase activity.
Lower telomerase activity correlated with higher
radiosensitivity, and could lead to a decrease in telomere length.
Both the hTERT and hTR subunits are required for telomerase
activity. In our research, we found that the downregulation of
HMGB1 decreased the level of hTERT, an effect that may explain the
decrease in telomerase activity. In contrast, another report
indicated that there was no change in RNA and protein level of TERT
in HMGB1 knockout MEFs (12). The
difference between these results may be due to differences in the
cell lines that were studied. Additional research in other cell
lines is required to determine the mechanism by which HMGB1
influences the telomere homeostasis.
The main role of cyclin D1 is to regulate the shift
from the G1 to the S phase of the cell cycle. The accumulation of
hTERT and cyclin D1 was able to attenuate the radiosensitivity of
MCF-7 cells (18), and there was a
direct positive correlation between the levels of cyclin D1
expression and resistance to radiation in tumor cells (19). We found that decreasing the
expression of HMGB1 led to a significantly decrease in cyclin D1.
Thus, the enhanced radiosensitivity seen in cells with diminished
HMGB1 may occur through the modulation of cyclin D1 expression.
Furthermore, because a decrease in cyclin D1 can inhibit the G1/S
transition, this may explain the significant inhibition of
proliferation that was seen in MCF-7-shHMGB1 cells. In addition,
the increased levels of CDC25C protein that were seen could promote
the G2 to M phase transition. Because the M phase is the most
radiosensitive phase of the cell cycle, this may also have enhanced
the radiosensitivity of these cells.
ATM and ATR protein kinases are major upstream
checkpoint kinases for the DNA damage response, at the G1/S
transition, ATR promotes progression and prevents stasis (20). In our research, downregulation of
HMGB1 inhibited the expression of ATM and ATR in MCF-7 cells. Other
studies have demonstrated that the inhibition of ATM or ATR results
in increased radiosensitivity (21,22),
and that the activation of ATR kinase during the G1 phase
facilitates the repair of ionizing radiation-induced DNA damage
(23).
Our results showed that the downregulation of HMGB1
enhanced the expression of phosphor-ATM and phosphor-ATR which
could manifest the level of DSB damage accumulation. The protein
level of γH2AX (marker of DSBs) also increased significantly when
HMGB1 was downregulated. Furthermore, when exposed to ionizing
radiation, the levels of phosphor-ATR and rH2AX increased
significantly in HMGB1 knockdown cells, and the protein levels of
phosphor-ATR and rH2AX were more enhanced when exposed to 4 Gy than
to 2 Gy of radiation.
Many studies have shown that the telomere serves as
a target in cancer treatment, especially in radiotherapy. The
stability of telomeres is maintained by telomerase as well as
associated proteins. Previous research suggested that high levels
of TPP1 and POT1 are directly associated with poor radiosensitivity
in LSCC cells, while low levels of TPP1 and POT1 have the opposite
effect (14). In addition,
suppression of TPP1 expression resulted in telomere dysfunction and
enhanced radiation sensitivity in telomerase-negative osteosarcoma
cell line (24). TPP1 helped to
stabilize the TRF1-TIN2-TRF2 interaction and promoted the formation
of the six-protein complex. Overexpression of TPP1 enhanced the
association between TIN2 and TRF2, while decreasing the expression
of TPP1 reduced the ability of endogenous TRF1 to associate with
the TRF2 complex (25).
Tumors with a high abundance of the TRF1 protein
exhibited greater telomerase activity and longer telomeres than
tumors with lower TRF1 protein levels. This indicated that telomere
length was significantly associated with TRF1 protein levels
(26). The present study showed
that TPP1 and TRF1 were downregulated when HMGB1 expression was
decreased. This was accompanied by an increased radiosensitivity in
breast cancer cells, suggesting the involvement of TPP1 and TRF1.
Another study suggested that TPP1 and TRF1 helped enhance the radio
resistance of breast cancer cells. Specifically, the expression of
TPP1 and TRF1 was significantly increased in radio resistant cell
lines than the parent cell lines, and in addition, after silencing
the TPP1 gene, the radio resistant cell lines significantly
decreased their radio resistance and telomerase activities
(27).
TRF2 is recruited to sites of DNA damage and plays a
critical role in the DNA damage response (28). Radiosensitization in U2OS cells may
be related to shortening of the telomeres and decreases in TRF2
(29). Upon removal of TRF2 from
TRF2F/− p53−/− MEFs, damage to telomeres was
observed at most chromosome ends, the telomeres lose the 3′
overhang and are processed by the non-homologous end-joining
pathway (30).
In our study, TRF2 also decreased after HMGB1
knockdown in MCF-7 cells, and the same results occurred when these
cells were irradiated. Thus, TRF2 may play an important role in the
radiosensitivity of MCF-7 cells.
Previous research investigating the effect of HMGB1
on telomere-binding proteins has been limited. In our study, there
was an obvious correlation between the expression of HMGB1 and
telomere dysfunction, which could explain the observed enhanced
radiosensitivity. HMGB1 protects cells against apoptosis by
influencing the stability of telomeres (31). The results from our study showed
that downregulation of HMGB1 increased apoptosis in MCF-7 cells
exposed to ionizing radiation. This might have resulted from the
downregulation of HMGB1, leading to telomeres being shortened to a
critical length, thereby initiating apoptosis. The increased
apoptosis may in turn be involved in the increased
radiosensitivity.
The results of this study demonstrate that the
decreasing HMGB1 levels promote telomere dysfunction and DNA
damage, and confer radiosensitivity in human breast cancer cells.
In addition, we provide evidence of correlation among HMGB1
expression, telomere homeostasis and intrinsic radiosensitivity,
suggesting that HMGB1 is a potential target in the radiotherapy of
breast cancer.
Acknowledgements
The authors would like to thank Dr Zhengkai Liao for
the advice during the study. This study was supported by the
National Natural Science Foundation of China (nos. 30800278 and
81071825), the Doctoral Fund of the Ministry of Education of China
(no. 20120141130010), the National Natural Science Foundation of
China (no. 81201755) and the Fundamental Research Funds for the
Central Universities.
Abbreviations:
|
HMGB1
|
high mobility group box 1
|
|
hTERT
|
human telomerase reverse
transcriptase
|
|
hTR
|
human telomerase RNA
|
|
MEFs
|
mouse embryonic fibroblasts
|
References
|
1
|
Agresti A and Bianchi ME: HMGB proteins
and gene expression. Curr Opin Genet Dev. 13:170–178. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muller S, Scaffidi P, Degryse B, et al:
New EMBO members’ review: the double life of HMGB1 chromatin
protein: architectural factor and extracellular signal. EMBO J.
20:4337–4340. 2001. View Article : Google Scholar
|
|
3
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamanaka S, Katayama E, Yoshioka K, Nagaki
S, Yoshida M and Teraoka H: Nucleosome linker proteins HMGB1 and
histone H1 differentially enhance DNA ligation reactions. Biochem
Biophys Res Commun. 292:268–273. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagaki S, Yamamoto M, Yumoto Y, Shirakawa
H, Yoshida M and Teraoka H: Non-histone chromosomal proteins HMG1
and 2 enhance ligation reaction of DNA double-strand breaks.
Biochem Biophys Res Commun. 246:137–141. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Campana L, Bosurgi L and Rovere-Querini P:
HMGB1: a two-headed signal regulating tumor progression and
immunity. Curr Opin Immunol. 20:518–523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Gao XS, Zhao J, et al:
Differential gene expression profiles of DNA repair genes in
esophageal cancer cells after X-ray irradiation. Chin J Cancer.
29:865–872. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blackburn EH: Switching and signaling at
the telomere. Cell. 106:661–673. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim NW, Piatyszek MA, Prowse KR, et al:
Specific association of human telomerase activity with immortal
cells and cancer. Science. 266:2011–2015. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiao CY, Zhou FX, Liu SQ, Xie CH, Dai J
and Zhou YF: Correlations of telomere length and telomerase
activity to radiosensitivity of human laryngeal squamous carcinoma
cells. Ai Zheng. 24:653–656. 2005.(In Chinese). PubMed/NCBI
|
|
11
|
Schrumpfova PP, Fojtova M, Mokros P,
Grasser KD and Fajkus J: Role of HMGB proteins in chromatin
dynamics and telomere maintenance in Arabidopsis thaliana. Curr
Protein Pept Sci. 12:105–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Polanska E, Dobsakova Z, Dvorackova M,
Fajkus J and Stros M: HMGB1 gene knockout in mouse embryonic
fibroblasts results in reduced telomerase activity and telomere
dysfunction. Chromosoma. 121:419–431. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yao X, Zhao G, Yang H, Hong X, Bie L and
Liu G: Overexpression of high-mobility group box 1 correlates with
tumor progression and poor prognosis in human colorectal carcinoma.
J Cancer Res Clin Oncol. 136:677–684. 2010. View Article : Google Scholar
|
|
14
|
Tang T, Zhou FX, Lei H, et al: Increased
expression of telomere-related proteins correlates with resistance
to radiation in human laryngeal cancer cell lines. Oncol Rep.
21:1505–1509. 2009.PubMed/NCBI
|
|
15
|
Cawthon RM: Telomere measurement by
quantitative PCR. Nucleic Acids Res. 30:e472002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiao Y, Wang HC and Fan SJ: Growth
suppression and radiosensitivity increase by HMGB1 in breast
cancer. Acta Pharmacol Sin. 28:1957–1967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong YH, Liao ZK, Zhou FX, et al:
Telomere length inversely correlates with radiosensitivity in human
carcinoma cells with the same tissue background. Biochem Biophys
Res Commun. 367:84–89. 2008. View Article : Google Scholar
|
|
18
|
Wang W, Yang L, Hu L, et al: Inhibition of
UBE2D3 expression attenuates radiosensitivity of MCF-7 human breast
cancer cells by increasing hTERT expression and activity. PLoS One.
8:e646602013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimura T, Kakuda S, Ochiai Y, et al:
Acquired radioresistance of human tumor cells by
DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene.
29:4826–4837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hurley PJ and Bunz F: ATM and ATR:
components of an integrated circuit. Cell Cycle. 6:414–417. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rainey MD, Charlton ME, Stanton RV and
Kastan MB: Transient inhibition of ATM kinase is sufficient to
enhance cellular sensitivity to ionizing radiation. Cancer Res.
68:7466–7474. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alao JP and Sunnerhagen P: The ATM and ATR
inhibitors CGK733 and caffeine suppress cyclin D1 levels and
inhibit cell proliferation. Radiat Oncol. 4:512009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gamper AM, Rofougaran R, Watkins SC,
Greenberger JS, Beumer JH and Bakkenist CJ: ATR kinase activation
in G1 phase facilitates the repair of ionizing radiation-induced
DNA damage. Nucleic Acids Res. 41:10334–10344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiang W, Wu Q, Zhou F, Xie C, Wu C and
Zhou Y: Suppression of telomere-binding protein TPP1 resulted in
telomere dysfunction and enhanced radiation sensitivity in
telomerase-negative osteosarcoma cell line. Biochem Biophys Res
Commun. 445:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
O’Connor MS, Safari A, Xin H, Liu D and
Songyang Z: A critical role for TPP1 and TIN2 interaction in
high-order telomeric complex assembly. Proc Natl Acad Sci USA.
103:11874–11879. 2006. View Article : Google Scholar
|
|
26
|
Valls-Bautista C, Pinol-Felis C,
Rene-Espinet JM, Buenestado-Garcia J and Vinas-Salas J: Telomeric
repeat factor 1 protein levels correlates with telomere length in
colorectal cancer. Rev Esp Enferm Dig. 104:530–536. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Z, Yang X, Xia N, et al: PTOP and TRF1
help enhance the radio resistance in breast cancer cell. Cancer
Cell Int. 14:72014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huda N, Abe S, Gu L, Mendonca MS, Mohanty
S and Gilley D: Recruitment of TRF2 to laser-induced DNA damage
sites. Free Radic Biol Med. 53:1192–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu L, Wu QQ, Wang WB, et al: Suppression
of Ku80 correlates with radiosensitivity and telomere shortening in
the U2OS telomerase-negative osteosarcoma cell line. Asian Pac J
Cancer Prev. 14:795–799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Celli GB and de Lange T: DNA processing is
not required for ATM-mediated telomere damage response after TRF2
deletion. Nat Cell Biol. 7:712–718. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Smolarczyk R, Cichon T, Jarosz M and Szala
S: HMGB1 - its role in tumor progression and anticancer therapy.
Postepy Hig Med Dosw (Online). 66:913–920. 2012.(In Polish).
View Article : Google Scholar
|