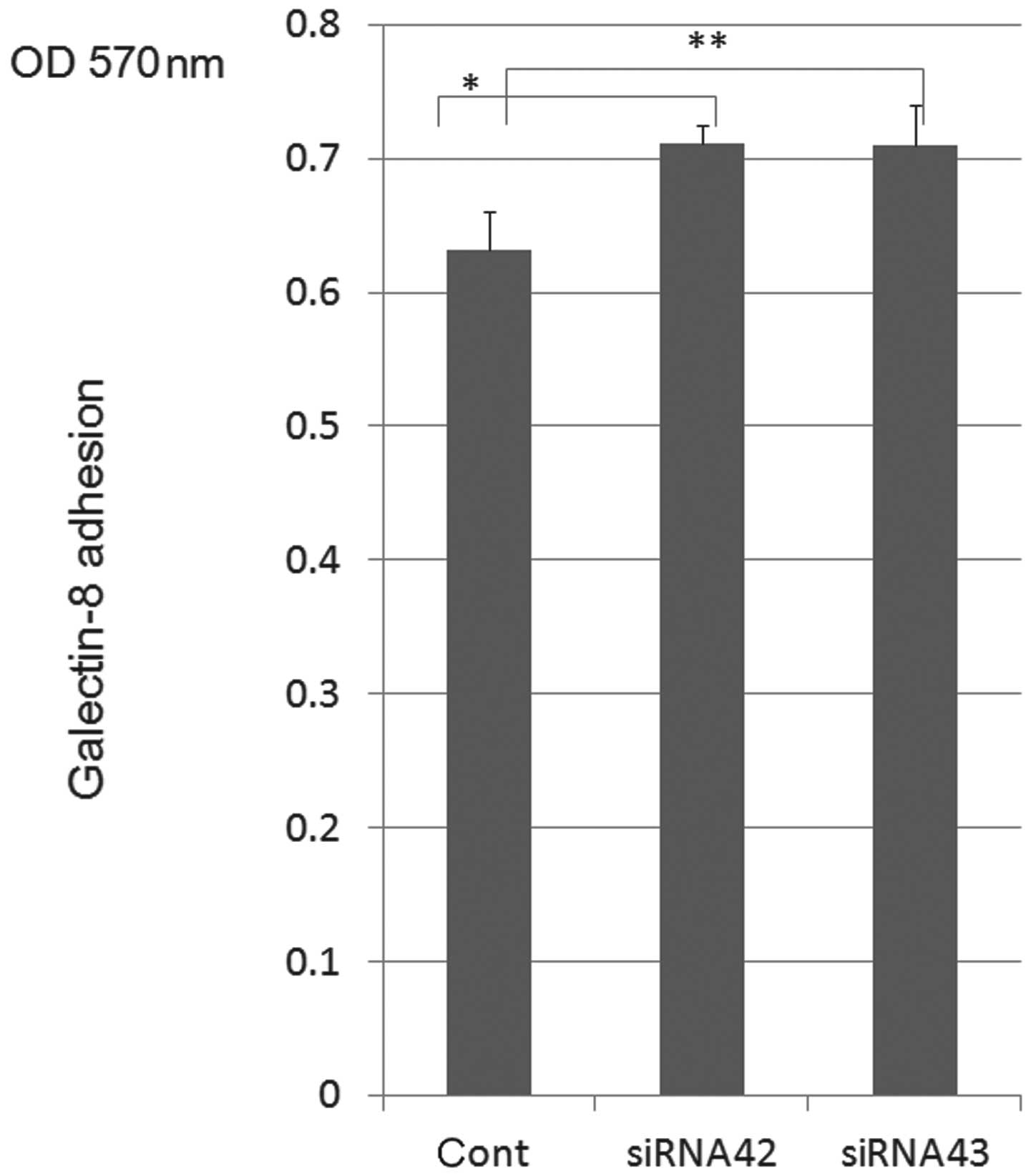
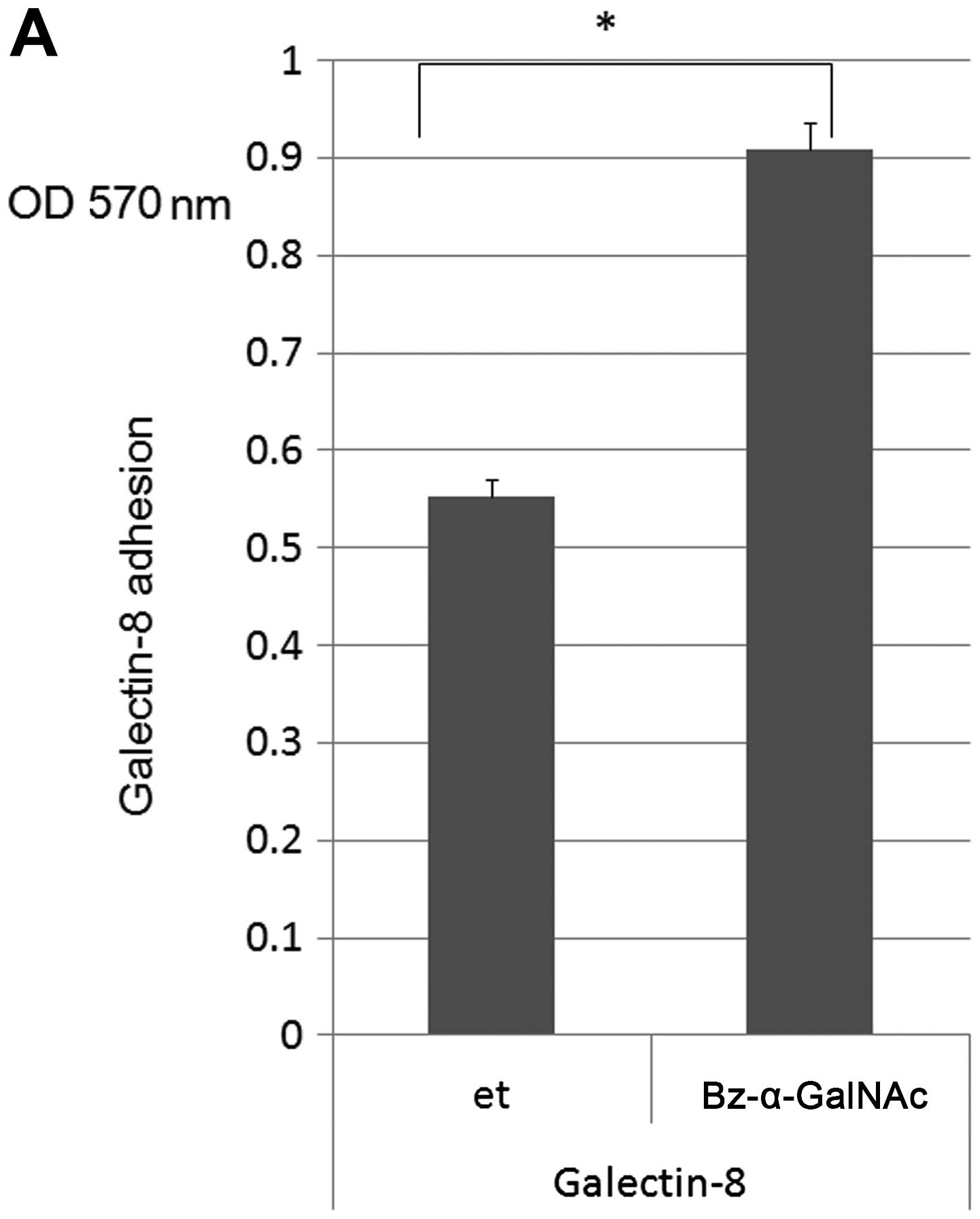
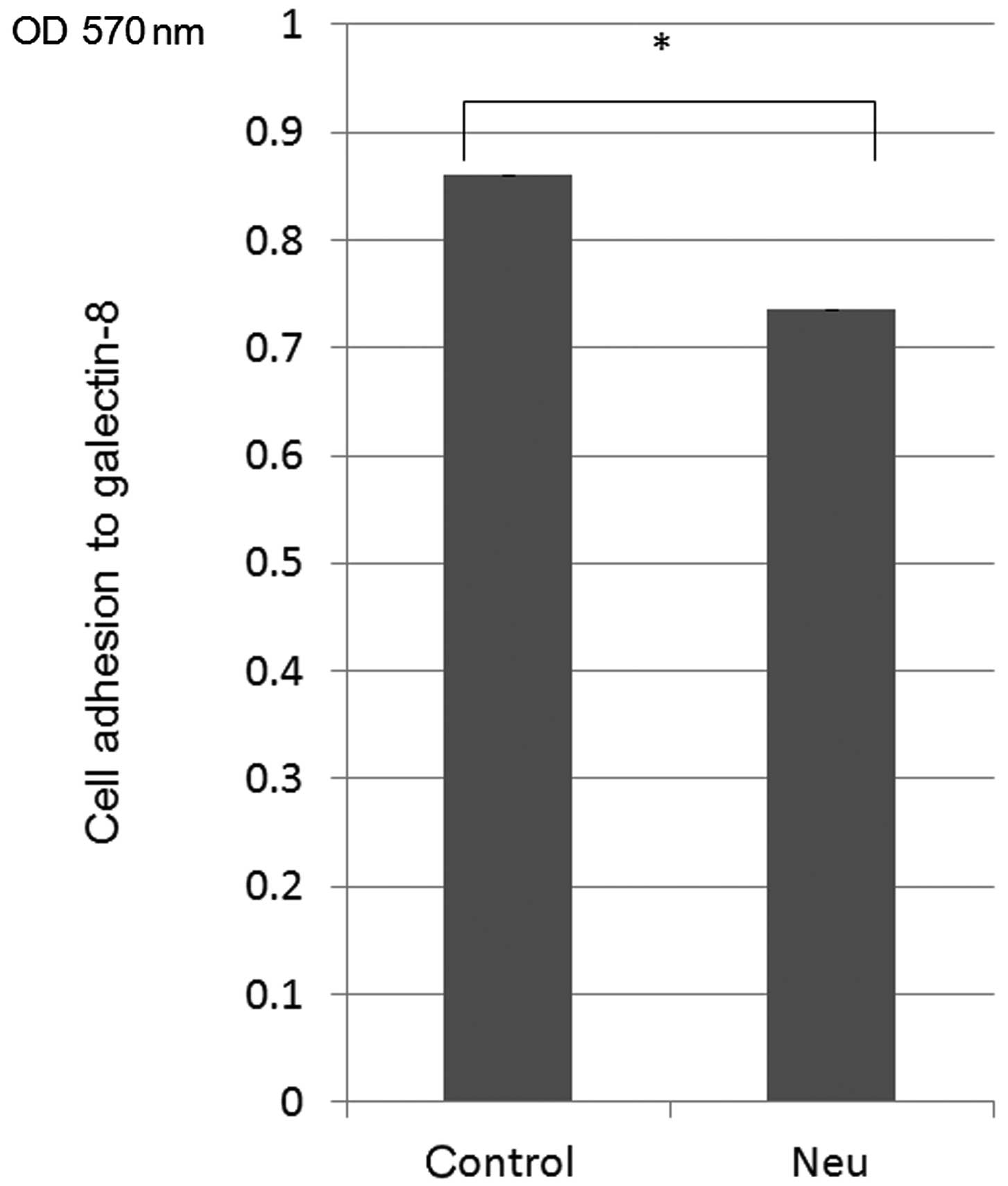
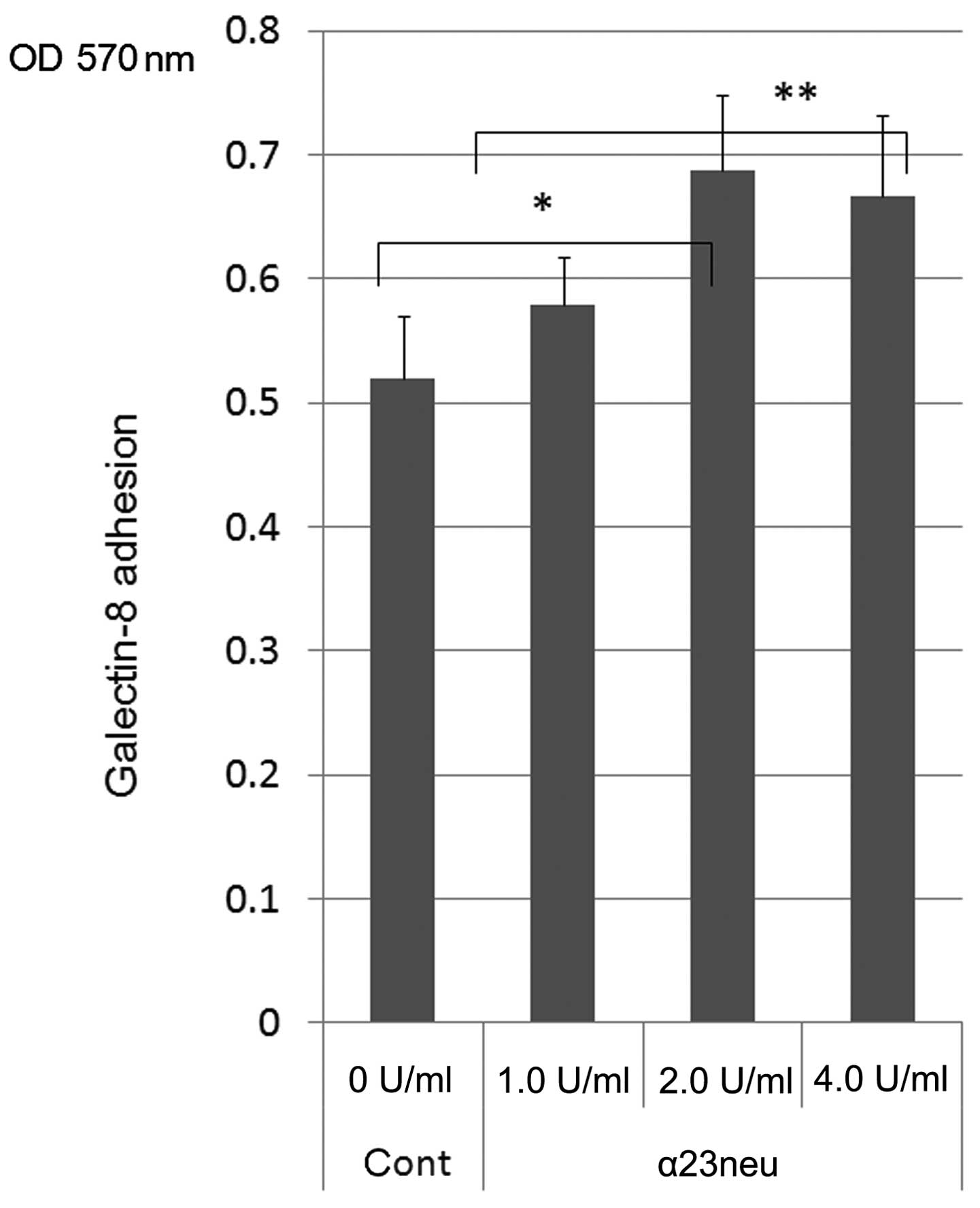
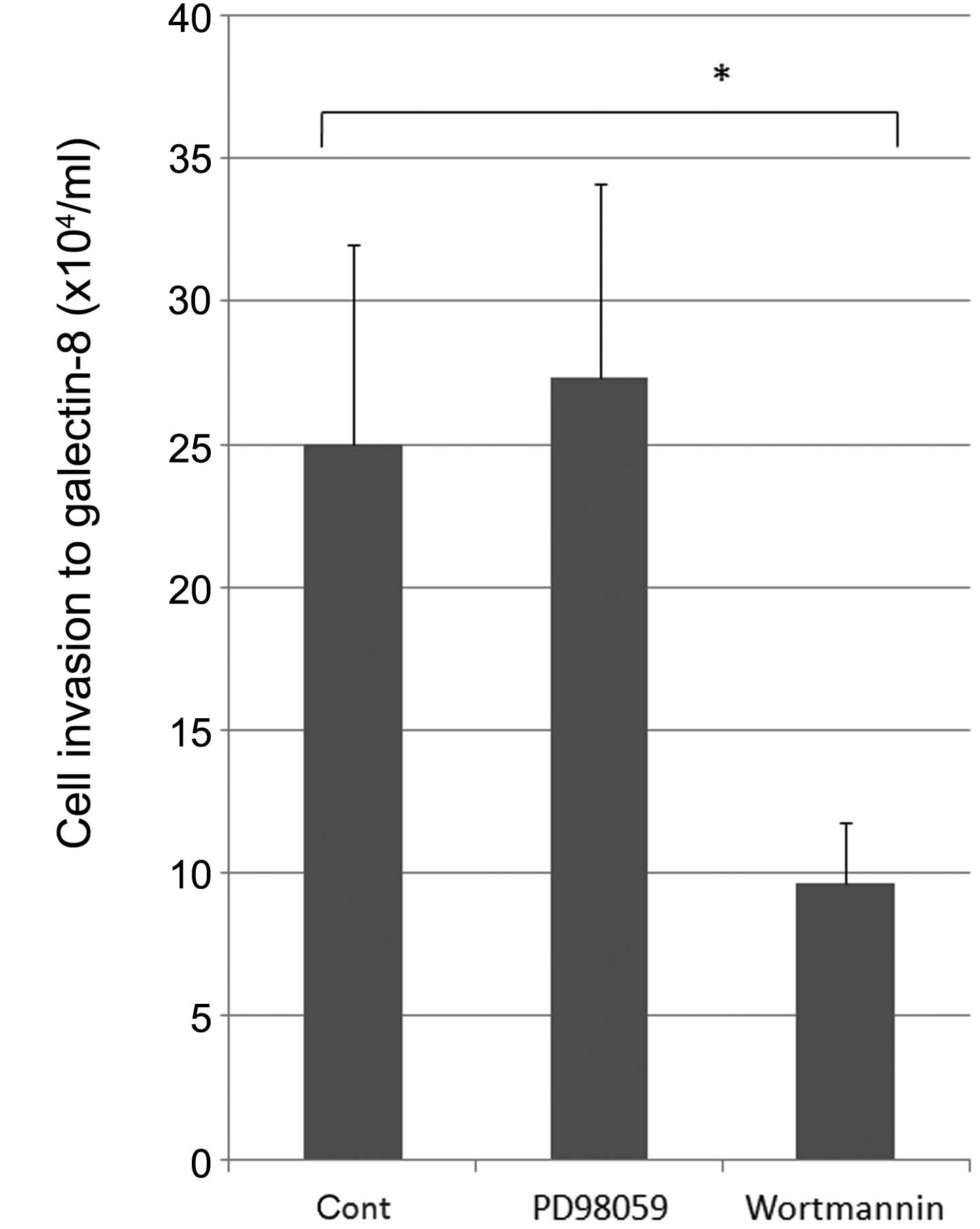
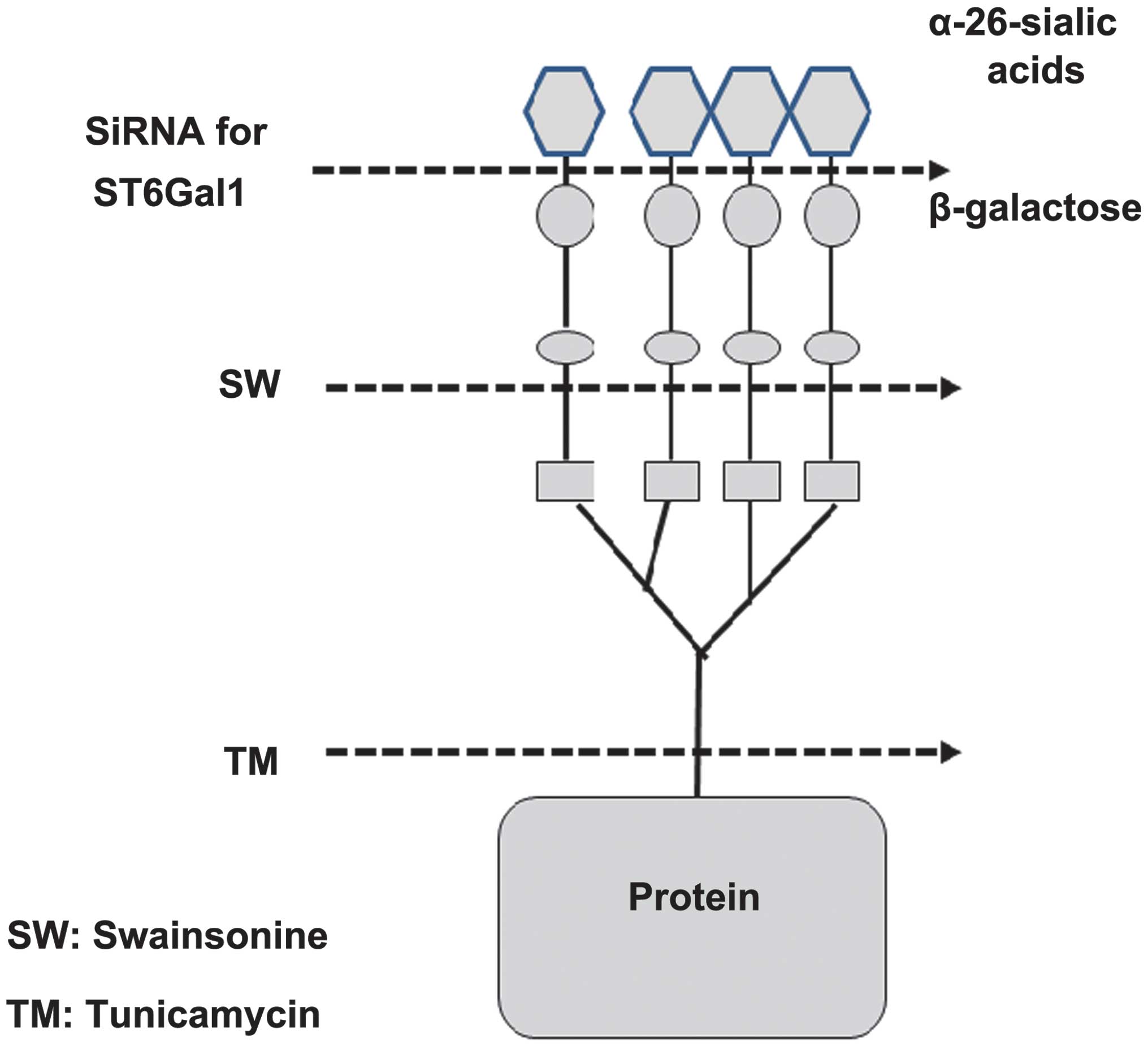
|
1
|
Suzuki O and Abe M: Galectin-1-mediated
cell adhesion, invasion and cell death in human anaplastic large
cell lymphoma: Regulatory roles of cell surface glycans. Int J
Oncol. 44:1433–1442. 2014.PubMed/NCBI
|
|
2
|
Zhuo Y and Bellis SL: Emerging role of
alpha2,6-sialic acid as a negative regulator of galectin binding
and function. J Biol Chem. 286:5935–5941. 2011. View Article : Google Scholar :
|
|
3
|
Dall’Olio F, Chiricolo M, Ceccarelli C,
Minni F, Marrano D and Santini D: Beta-galactoside alpha2,6
sialyltransferase in human colon cancer: contribution of multiple
transcripts to regulation of enzyme activity and reactivity with
Sambucus nigra agglutinin. Int J Cancer. 88:58–65. 2000. View Article : Google Scholar
|
|
4
|
Dall’Olio F, Chiricolo M and Lau JT:
Differential expression of the hepatic transcript of
beta-galactoside alpha2,6-sialyltransferase in human colon cancer
cell lines. Int J Cancer. 81:243–247. 1999. View Article : Google Scholar
|
|
5
|
Levy Y, Arbel-Goren R, Hadari YR, Eshhar
S, Ronen D, Elhanany E, Geiger B and Zick Y: Galectin-8 functions
as a matricellular modulator of cell adhesion. J Biol Chem.
276:31285–31295. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zick Y, Eisenstein M, Goren RA, Hadari YR,
Levy Y and Ronen D: Role of galectin-8 as a modulator of cell
adhesion and cell growth. Glycoconj J. 19:517–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamamoto H, Nishi N, Shoji H, Itoh A, Lu
L, Hirashima M and Nakamura T: Induction of cell adhesion by
galectin-8 and its target molecules in Jurkat T-cells. J Biochem.
143:311–324. 2008. View Article : Google Scholar
|
|
8
|
Diskin S, Chen WS, Cao Z, Gyawali S, Gong
H, Soza A, González A and Panjwani N: Galectin-8 promotes
cytoskeletal rearrangement in trabecular meshwork cells through
activation of Rho signaling. PLoS One. 7:e444002012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Landemarre L, Cancellieri P and Duverger
E: Cell surface lectin array: parameters affecting cell glycan
signature. Glycoconj J. 30:195–203. 2013. View Article : Google Scholar
|
|
10
|
Suzuki O, Tasaki K, Kusakabe T and Abe M:
UDP-GlcNAc2-epimerase regulates cell surface sialylation and
ceramide-induced cell death in human malignant lymphoma. Int J Mol
Med. 22:339–348. 2008.PubMed/NCBI
|
|
11
|
Suzuki O, Nozawa Y, Kawaguchi T and Abe M:
Phaseolus vulgaris leukoagglutinationg lectin-binding reactivity in
human diffuse large B cell lymphoma and its relevance to the
patient’s clinical outcome: lectin histochemistry and lectin blot
analysis. Pathol Int. 49:874–880. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suzuki O, Nozawa Y and Abe M: The
regulatory roles of cell surface sialylation and N-glycans in human
B cell lymphoma cell adhesion to galectin-1. Int J Oncol.
28:155–160. 2006.
|
|
13
|
Suzuki O, Nozawa Y, Kawaguchi T and Abe M:
UDP-GlcNAc2-epimerase regulates cell surface sialylation and cell
adhesion to extracellular matrix in Burkitt’s lymphoma. Int J
Oncol. 20:1005–1011. 2002.PubMed/NCBI
|
|
14
|
Amano M, Galvan M, He J and Baum LG: The
ST6Gal I sialyltransferase selectively modifies N-glycans on CD45
to negatively regulate galectin-1-induced CD45 clustering,
phosphatase modulation, and T cell death. J Biol Chem.
278:7469–7475. 2003. View Article : Google Scholar
|
|
15
|
Suzuki O, Nozawa Y and Abe M: Regulatory
roles of altered N- and O-glycosylation of CD45 in
galectin-1-induced cell death in human diffuse large B cell
lymphoma. Int J Oncol. 26:1063–1068. 2005.PubMed/NCBI
|
|
16
|
Ideo H, Matsuzaka T, Nonaka T, Seko A and
Yamashita K: Galectin-8-N-domain recognition mechanism for
sialylated and sulfated glycans. J Biol Chem. 286:11346–11355.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Perillo NL, Pace KE, Seilhamer JJ and Baum
LG: Apoptosis of T cells mediated by galectin-1. Nature.
378:736–739. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rodig SJ, Ouyang J, Juszczynski P, Currie
T, Law K, Neuberg DS, Rabinovich GA, Shipp MA and Kutok JL:
AP1-dependent galectin-1 expression delineates classical Hodgkin
and anaplastic large cell lymphomas from other lymphoid
malignancies with shared molecular features. Clin Cancer Res.
14:3338–3344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Juszczynski P, Ouyang J, Monti S, Rodig
SJ, Takeyama K, Abramson J, Chen W, Kutok JL, Rabinovich GA and
Shipp MA: The AP1-dependent secretion of galectin-1 by Reed
Sternberg cells fosters immune privilege in classical Hodgkin
lymphoma. Proc Natl Acad Sci USA. 104:13134–13139. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fouillit M, Joubert-Caron R, Poirier F,
Bourin P, Monostori E, Levi-Strauss M, Raphael M, Bladier D and
Caron M: Regulation of CD45-induced signaling by galectin-1 in
Burkitt lymphoma B cells. Glycobiology. 10:413–419. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suzuki O and Abe M: Cell surface
N-glycosylation and sialylation regulates galectin-3-induced
apoptosis in human diffuse large B cell lymphoma. Oncol Rep.
19:743–748. 2008.PubMed/NCBI
|
|
22
|
Hadari YR, Arbel-Goren R, Levy Y,
Amsterdam A, Alon R, Zakut R and Zick Y: Galectin-8 binding to
integrins inhibits cell adhesion and induces apoptosis. J Cell Sci.
113:2385–2397. 2000.PubMed/NCBI
|