Introduction
Gastric cancer is one of the most common malignant
tumors, >70% of new cases and deaths occur in developing
countries each year (1). In China,
the incidence and mortality rate of gastric cancer are increasing.
Although there are distinct discrepancies in diagnosis, prognosis,
and treatment efficacy for gastric cancer patients, the 5-year
survival rate of gastric cancer is generally <20% (2). At present, the management of gastric
cancer includes surgery, radiotherapy, conventional chemotherapy,
molecular targeted therapy and biological therapy, but these
methods have not reached expected clinical efficacy, which mainly
focus on mass cell killing without high specificity and often
causes severe side-effects and toxicities. Moreover, recurrences
and metastasis frequently occur in the majority of patients
(2). Hence, it is urgent to
explore safe and effective therapeutic agents for gastric cancer to
improve the survival rate and quality of life.
Antimicrobial peptides (AMPs) are
evolutionarily-conserved components of the innate immune system
(3), which exert their activities
against a broad range of microbial pathogens. Compared to
conventional treatments, AMPs have many advantages, such as
specific cytotoxicity for cancer cells, ability to bypass the
multidrug-resistance mechanism, and additive effects in combination
therapy (4), suggesting that AMPs
has potential as a novel antitumor agent for the treatment of
cancer.
AMPs can directly kill cancer cells by a cell
membranelytic effect (5), or
trigger apoptosis in cancer cells via the Fas
death-receptor-induced extrinsic pathway (6) and the
mitochondria-apoptosome-mediated apoptotic intrinsic pathway
(7). AMPs with cationic and
amphipathic amino acid composition can penetrate anionic cell
membrane and subsequently bind to mitochondrial membrane, which
destroy membrane structure, increase the level of reactive oxygen
species (ROS) and decrease mitochondrial membrane potential
(Δψm) (8).
Mitochondrial dysfunction elicits the release of cytochrome
c from mitochondria to cytosol, which activates the
caspase-cascade system and induces cell apoptosis (9). It has been reported that AMPs
inhibited the growth or proliferation of cancer cells through
regulation of numerous proteins and pathways, including the caspase
family and the Bcl-2 family (10,11),
the mitogen-activated protein kinase (MAPK) family (12), the nuclear factor-kappa B (NF-κB)
(13), phosphoinositide 3-kinase
(PI3K)/protein kinase B (Akt) signal transduction pathways
(14,15) and ER stress-mediated apoptosis
pathway (16,17). Furthermore, certain AMPs are potent
inhibitors of blood vessel development (angiogenesis) that is
associated with tumor progression (18).
We isolated a 37-AA cationic antimicrobial peptide
with specific amphipathic α-helices (named as cecropinXJ) from the
larvae of Bombyx mori (19), which has a broad effect spectrum
against bacteria (20). Our
previous studies have shown that cecropinXJ can inhibit the
proliferation of human gastric cancer in vitro such as AGS
cells (21), but have no hemolytic
activity against human erythrocytes and no toxicity to normal
mammalian cells (22,23). Due to difficulty of AGS cells to
form a solid tumor in vivo, we investigated the cytotoxicity
and the mechanism of cecropinXJ against the human gastric cancer
BGC823 cells in the present study. Our results showed that
cecropinXJ suppressed the proliferation and induced apoptosis of
BGC823 cells in vitro and in vivo through
mitochondrial-mediated apoptosis pathways and inhibition of
angiogenesis, which could provide experimental evidence for the
potential application of cecropinXJ as a new antitumor candidate
for treatment of gastric cancer.
Materials and methods
Preparation of antimicrobial peptide
cecropinXJ
CecropinXJ of B. mori was prepared through
the Saccharomyces cerevisiae eukaryotic expression system
and purified with Ni-NTA agarose column as reported (23). The concentration of purified
recombinant cecropinXJ protein was detected by a Bradford protein
assay kit (KeyGen Biotech, Nanjing, China). The amino acid sequence
is WKIFKKIEKMGRNIRDGIVKAGPAIEVLGSAKAIGK. Before use, the peptide
was dissolved in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone,
Logan, UT, USA) at a concentration of 1 mg/ml and sterilized by
filtration through a 0.2-mm filter.
Cell culture
The human gastric cancer BGC823 cells and normal
gastric epithelial GES-1 cells were kindly provided by Professor
Youyong Lv (Beijing Cancer Hospital, Beijing, China). Cells were
cultured in DMEM medium, supplemented with 10% fetal bovine serum
(FBS; Gibco-BRL, Grand Island, NY, USA), 100 μg/ml streptomycin and
100 U/ml penicillin (Beijing Solarbio, Beijing, China) in a
humidified atmosphere of 5% CO2 at 37°C.
Cell viability assay
To evaluate the effects of cecropinXJ on the
proliferation of BGC823 cells and GES-1 cells, cell viability was
measured by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium
bromide (MTT) assay (Sigma, St. Louis, MO, USA). The BGC823 cells
and GES-1 cells in the logarithmic phase of growth were collected
and seeded in 96-well plates at a density of 5×103
cells/well and cultured overnight. After 24 h, BGC823 cells and
GES-1 cells were treated with or without cecropinXJ at different
concentrations (0–1,000 μg/ml) for 24 h, respectively. To examine
the time-dependent effect of cecropinXJ, BGC823 cells and GES-1
cells were treated by different concentrations of cecropinXJ (20,
50, 80 and 100 μg/ml) for 24, 48, 72, 96 and 120 h and 10 μg/ml
doxorubicin (Dox) served as a positive control. To investigate the
effects of caspases on cell viability, cells were pre-treated with
100 μM PAN-caspase inhibitor (Ac-VAD-fmk), caspase-3 inhibitor
(Ac-DEVD-fmk), caspase-8 inhibitor (Ac-IETD-fmk) and caspase-9
inhibitor (Ac-LEHD-fmk) (Enzo Life Sciences, Lausen, Switzerland)
for 60 min, then treated with different concentrations of
cecropinXJ (0, 20, 50, 80 and 100 μg/ml) for 24 h. After treatment,
the culture medium was removed and 100 μl MTT solution (5 mg/ml)
was added into each well, then incubated at 37°C for 4 h. After 4
h, 150 μl of dimethyl sulfoxide (DMSO; Beijing Solarbio) was added
into each well and incubated at 37°C for 10 min. Absorbance was
measured at OD540/655nm using a 96-well microplate
reader (Bio-Rad Laboratories, Hercules, CA, USA). Cell viability
(%) = [(ODtreated -
ODblank)/(ODnon-treated -
ODblank)] × 100%.
Colony formation assay
One thousand BGC823 cells were resuspended in 4.4 ml
mixture of 0.5% agar solution containing cell culture medium (2×
DMEM and 20% FBS) at a final concentration of 20, 50, 80 and 100
μg/ml cecropinXJ and layered on the top of 0.7% agar layer in 60-mm
petri-dish (Corning). The mixtures without cecropinXJ or with 10
μg/ml Dox served as negative and positive controls, respectively.
Dishes were incubated for three weeks at 37°C in a humidified
atmosphere of 5% CO2. Cell colonies were observed by
light microscopy (Leica Microsystems, Wetzlar, Germany) and
visualized by the treatment of 0.5 ml p-iodonitrotetrazolium violet
(Sigma) for 16 h, then photographed and the number of cell clonies
was counted. Colonies (%) = the colony number of treatment
group/the colony number of control group × 100%.
LDH detection
BGC823 and GES-1 cells at 4×105
cells/well were inoculated into 24-well plates and treated with 0,
20, 50, 80 and 100 μg/ml of cecropinXJ for 24 or 48 h. Medium was
collected and the ratio of LDH release was detected according to
the manufacturer’s instructions.
Cell cycle analysis of cecropinXJ on
BGC823 cells by flow cytometry
BGC823 cells at 5×106 cells/ml were
inoculated into 100-mm dishes and incubated at 37°C for 24 h. After
the medium was removed, fresh medium with different concentrations
of cecropinXJ was added and cultured. Medium without cecropinXJ was
served as the control. After culture for 24 or 48 h, the cells were
collected and washed with cold phosphate-buffered saline (PBS, pH
7.4) and fixed with 75% ethanol overnight at −20°C. After washing,
DNA staining solution (Multisciences, Hangzhou, China) was added
and incubated at 37°C in darkness for 30 min. The samples were
analyzed with a flow cytometry (BD Biosciences, San Jose, CA,
USA).
Apoptosis rate determined by flow
cytometry
BGC823 cells at 5×105 cells/ml were
inoculated into 60-mm dishes and incubated at 37°C for 24 h. After
the medium was removed, 2.0 ml of DMEM medium with the final
concentrations of cecropinXJ at 20, 50, 80 and 100 μg/ml was added
to each dish. No cecropinXJ served as the control. After cultured
for 24 or 48 h, cells were collected after digestion with 0.25%
trypsin, washed with PBS two times, and suspended in 400 μl binding
buffer. Cell suspensions were stained with 5 μl of Annexin V-FITC
and 10 μl of propidium iodide (PI) (BestBio Biotechnologies,
Shanghai, China) according to the manufacturer’s instructions, and
the samples were analyzed with a flow cytometry.
Morphological observation by Hoechst
33258 staining and transmission electron microscopy
The BGC823 cells were treated with different
concentrations of cecropinXJ for 24 h. For Hoechst 33258 staining,
cells were washed with PBS and fixed with 95% ethanol at 4°C for 15
min. After washing with PBS, cells were stained with 0.5 μg/ml
Hoechst 33258 (Sigma) for 10 min at 4°C in the dark. Samples were
observed under a fluorescence microscope (Leica Microsystems). For
transmission electron microscope, cells were fixed with 2.5%
glutaraldehyde in 0.1 M PBS at 4°C overnight, and fixed with 1%
osmium tetraoxide after washing with PBS. The cells were then
dehydrated using graded alcohol and embedded in araldite. Ultrathin
sections were stained with uranyl acetate and lead citrate and
examined with a Philips CM10 transmission electron microscope.
Detection of reactive oxygen species
(ROS) and mitochondrial membrane potential (Δψm)
ROS were detected by non-fluorescent
2,7-dichlorodihydrofluorescein (H2DCF-DA) (Sigma) (a
fluorogenic freely permeable tracer), which is specific for ROS
assessment. Changes in the mitochondrial membrane potential
(Δψm) were measured using a lipophilic cationic dye
3,3-dihexyloxacarbocyanine iodide [DiOC6(3)] (Sigma).
Briefly, BGC823 cells (2×106 cells/ml) were treated with
different concentrations of cecropinXJ for 24 h or treated with 100
μg/ml cecropinXJ for different times in 100-mm dishes. After
treatment, cells were incubated with 10 μM H2DCF-DA for
30 min or 40 nM DiOC6(3) for 15 min at 37°C, and washed
with PBS. The samples were analyzed by flow cytometry.
Quantitative real-time
reverse-transcription PCR (qRT-PCR)
Gene expression was analyzed by qRT-PCR. Total RNA
was extracted from BGC823 cells and tumors using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
protocol. cDNA was synthesized from total RNA by M-MLV reverse
transcriptase according to the instruction. Gene expression was
detected by SYBR® Premix Ex Taq™ II (Tli RNaseH Plus)
(Takara, Tokyo, Japan) using the 7500™ Real-time system (Applied
Biosystems, Waltham, MA, USA). GAPDH was used as the
reference gene. The primers are shown in Table I. For qRT-PCR, each reaction was
run in triplicate and contained the following: 12.5 μl of Premix Ex
Taq™II, 2 μl of forward primer and reverse primer (2.5 μM), 0.5 μl
of ROX Reference Dye II (50X) and 100 ng cDNA template in a final
reaction volume of 25 μl. After a pre-incubation step at 95°C for
30 sec to activate DNA polymerase, 40 cycles of PCR were done
following the conditions: 95°C for 30 sec, 56°C for 30 sec and 72°C
for 45 sec. The expression levels were quantified using the cycle
threshold value (Ct). Data were normalized to GAPDH and the
relative expression levels of the genes were calculated using the
2−ΔΔCt method.
 | Table IPrimers were designed for
qRT-PCR. |
Table I
Primers were designed for
qRT-PCR.
| Primers | Sequences
(5′-3′) | Length (bp) |
|---|
| Caspase-3 | F:
AGACATACTCCTTCCATCA
R: TCATAGCACAGCATCACT A | 182 |
| Caspase-8 | F:
CAGAAGAAGTGAGCAGAT
R: GAGTCCGAGATTGTCATT | 262 |
| Caspase-9 | F:
GCCTGGAGTCTTAGTTG
R: CTGCTTGCCTGTTAGTT | 313 |
| Bcl-2 | F:
GAACTGGGGGAGGATTGTGG
R: GGATCCAGGTGTGCAGGTGC | 143 |
| Bax | F:
GATGATTGCCGCCGTGGAC
R: GGGTGAGGAGGCTTGAGGAG | 267 |
| Cytochrome
c | F:
TGGATACTCTTACACAGCCG
R: AAGTCTGCCCTTTCTTCCTT | 150 |
| CD31 | F:
GAGTCCTGCTGACCCTTCTG
R: ATCTGGTGCTGAGGCTTGAC | 188 |
| CD34 | F:
TCAGGCATCAGAGAAGTG
R: CAAGACCAGCAGTAGACA | 210 |
| bFGF | F:
AGCGACCCTCACATCAAG
R: AAGAAACACTCATCCGTAACAC | 143 |
| VEGF | F:
AACTTTCTGCTGTCTTGGG
R: ACTTCGTGATGATTCTGCC | 116 |
| VWF | F:
CGCATCCAGCATACAGTGAC
R: GAGGGGGTAAGGAAGTCGTC | 176 |
| GAPDH | F:
ACACCCACTCCTCCACCTTT
R: ACCCTGTTGCTGTAGCCAA | 106 |
Western blot analysis
BGC823 cells were treated with cecropinXJ (0, 20,
50, 80 and 100 μg/ml) for 24 h and washing with ice-cold PBS twice,
cells were collected and lysed using 100 μl CytoBuster™ protein
extraction reagent (Novage, Madison, WI, USA) on ice for 15 min.
After centrifugation at 14,000 rpm 4°C for 10 min, the protein
concentration in supernatant was determined by the bicinchoninic
acid (BCA) assay kit. Proteins (40 μg/lane) were separated by 12%
SDS-PAGE and transferred onto a nitrocellulose membranes
(Millipore, Billerica, MA, USA). After washing with TBS-T buffer
(20 mM Tris-HCl, 150 mM NaCl, 0.05% Tween-20), membrane was blocked
with 5% skim milk at room temperature for 1 h, and then incubated
with the primary antibodies (1:2,000; Cell Signaling Technology,
Danvers, MA, USA) overnight at 4°C. After washing with TBS-T,
membrane was incubated with the corresponding HRP-conjugated
secondary antibodies (Cell Signaling Technology) for 2 h at room
temperature. Membrane was washed with TBS-T and exposed using
enhanced chemiluminescent (ECL) detection kit (CWBio, Inc.,
Beijing, China).
Caspase activity
BGC823 cells (5×107 cells/dish) were
seeded in 100-mm dishes and treated with 0, 20, 50, 80 and 100
μg/ml of cecropinXJ for 24 h. After treatment, BGC823 cells were
lysed and supernatant was collected. The protein concentration was
determined by Bradford protein assay kit. Cellular extracts (30 μg)
were incubated with 20 ng Ac-DEVD-pNA, Ac-IETD-pNA
and Ac-LEHD-pNA for overnight at 37°C and caspase activity
was measured by cleavage of the substrates to pNA, the absorbance
was measured at 405 nm. Relative caspase activity was calculated as
a ratio ODtreated/ODuntreated.
Xenograft tumor model
Four-week-old athymic nude female mice (BALB/c
nu/nu) were purchased from the Beijing Laboratory Animal Research
Center (Beijing, China) and received pathogen-free water and food.
The mice were inoculated subcutaneously with BGC823 cells
(5×106) in 0.1 ml of PBS. All the mice developed tumors
at day 5 and were randomized into two groups (n=6). The experiment
group was peritumorally injected with 0.1 ml cecropinXJ (100 μg)
five times per week (from Monday to Friday) during a 2-week period
and the control group was injected with 0.1 ml of BSA (100 μg).
Mouse weight and tumor size were measured every day. After two
weeks, mice were sacrificed and tumors were dissected. After
weighing, tumors were fixed in 10% formalin and embedded in
paraffin. Sections were stained with hematoxylin and eosin.
TUNEL staining
The TUNEL staining assay was performed according to
the manufacturer’s instructions (Biobox, Nanjing, China).
Paraffin-embedded tissue slides were dewaxed and rehydrated by
heating at 60°C, followed by washing in xylene and rehydration
through a graded series of ethanol and double-distilled water.
Tissue sections were incubated with proteinase K (20 μg/ml) at room
temperature for 15 min and with 3% H2O2 for
10 min. Then sections were incubated with the TdT enzyme at 37°C
for 60 min. After incubation with streptavidin-HRP solution in
darkness for 30 min, the sections were stained with
3,3-diaminobenzidine (DAB) substrate for 2 min and then
counterstained with hematoxylin.
Immunohistochemistry
Paraffin-embedded tissue were cut
four-micrometer-thick in consecutive sections and processed for
immunohistochemistry for CD31, CD34, basic fibroblast growth factor
(bFGF), vascular endothelial growth factor (VEGF) and von
Willebrand factor (VWF) (Biosynthesis, Beijing, China). Tissue
sections were deparaffinized, dewaxed and rehydrated by heating at
60°C, followed by washing with PBS/T. Antigen retrieval was carried
out in a microwave oven for 10 min. The sections were blocked with
10% goat serum (formulated with 3% BSA) at 37°C for 15 min and
washed thrice with PBS. The primary antibodies against
angiogenesis-associated proteins with 1:200 dilution were incubated
for 1 h. After washing with TBS-T, slides were incubated with the
HRP-conjugated secondary antibodies for 1 h at room temperature.
Sections were stained with DAB, counterstained with hematoxylin and
mounted. The immunohistochemical staining was observed under light
microscope.
Statistical analysis
Data are expressed as the mean ± SD. Statistical
analyses were performed using Student’s t-test. Values of P<0.05
or 0.01 was considered statistically significant and indicated in
the figures with * and **, respectively.
Results
CecropinXJ effectively suppress the
proliferation of gastric cancer BGC823 cells
We first examined whether cecropinXJ could suppress
the proliferation of gastric adenocarcinoma BGC823 cells via MTT
assay. Normal gastric epithelial cell GES-1 was used as control.
Compared to GES-1 cells, the proliferation of BGC823 cells was
significantly inhibited by cecropinXJ in dose- and time-dependent
manner (Fig. 1A and B). Dox
suppressed the growth of both types of cells. The inhibitory
effects of 100 μg/ml cecropinXJ on BGC823 cells were similar to 10
μg/ml Dox (Fig. 1B). When the
concentration of cecropinXJ was <500 μg/ml, the growth of GES-1
cells was not impaired by the cecropinXJ treatment compared to
without cecropinXJ treatment (Fig.
1A).
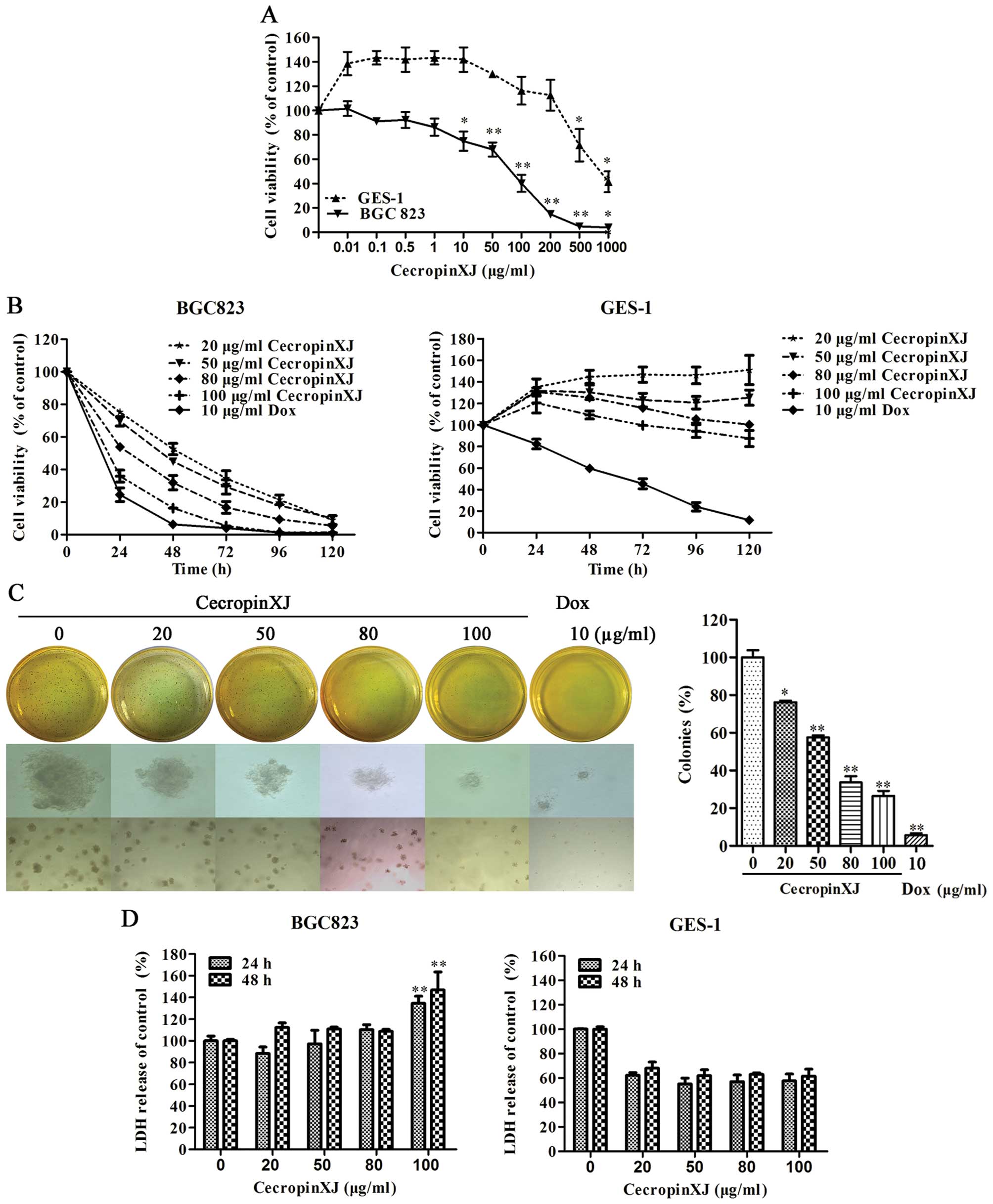 | Figure 1Effects of cecropinXJ on the
proliferation of BGC823 cells and GES-1 cells. The proliferation of
cells was detected by MTT assay. (A) BGC823 and GES-1 cells were
treated with different concentrations (0–1,000 μg/ml) of cecropinXJ
for 24 h. (B) BGC823 and GES-1 cells were treated with different
concentrations (20, 50, 80 and 100 μg/ml) of cecropinXJ for 24, 48,
72, 96 and 120 h. Dox (10 μg/ml) was used as positive control. (C)
The colony formation of BGC823 cells. BGC823 cells were treated
with different concentrations (0, 20, 50, 80 and 100 μg/ml) of
cecropinXJ and the colonies were detected by soft agar colony
formation assay (magnification, upper panel ×40 and lower panel
×10). (D) The analysis of LDH release. BGC823 cells and GES-1 cells
were treated with different concentrations (0, 20, 50, 80 and 100
μg/ml) of cecropinXJ for 24 and 48 h. The LDH release was detected
after treatment. Data are from three independent experiments.
*P<0.05, **P<0.01. |
The soft agar colony formation assay showed that
cecropinXJ treatment dose-dependently inhibited the colony
formation and colony growth of BGC823 cells. The colony formation
ratio was only (26.46±4.65%) under 100 μg/ml cecropinXJ treatment
(Fig. 1C). These results suggest
that cecropinXJ might have a long-term effect on the proliferation
of BGC823 cells.
Many AMPs can destroy the lipid membrane and lead to
cell lysis and death (24). We
investigated whether cecropinXJ could destroy cell membrane through
measuring the released cytoplasmic enzyme LDH into the cell culture
supernatant. Compared with untreated group, cecropinXJ treatment
did not significantly increase LDH release of BGC823 cells either
for 24 or 48 h until the concentration of cecropinXJ reached up to
100 μg/ml, suggesting that high concentration of cecropinXJ
treatment destroyed the integrity of plasma membrane in BGC823
cells but low concentration of cecropinXJ did not. CecropinXJ did
not affect the plasma membrane in GES-1 cells.
CecropinXJ arrests the cell cycle of
BGC823 cells at S phase
To determine whether cecropinXJ affects the cell
cycle of BGC823 cells, the cell cycle distribution of
cecropinXJ-treated BGC823 cells was analyzed by flow cytometry.
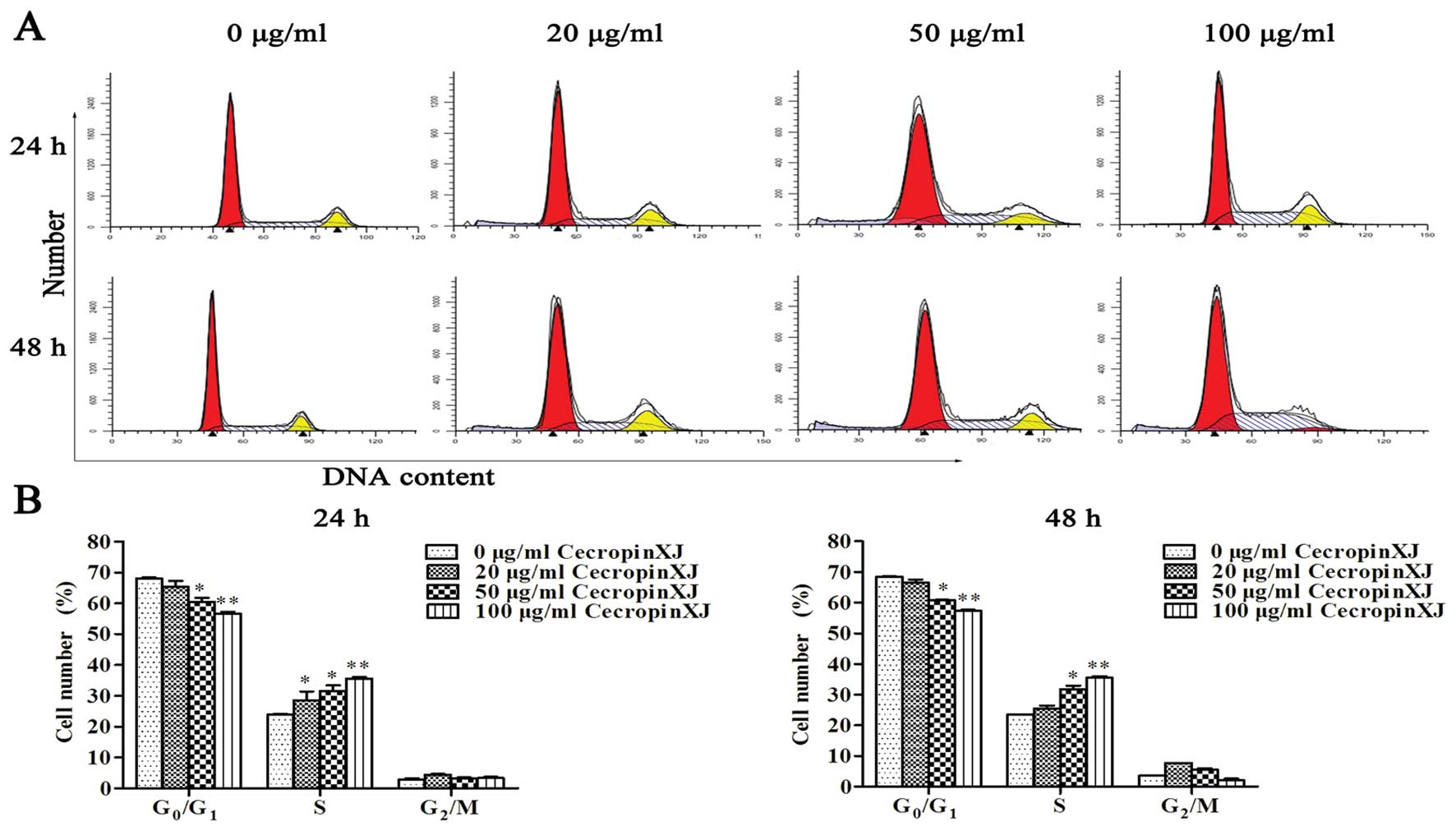
Upon cecropinXJ treatment, the percentage of cells at
G0/G1-phase significantly decreased (56.67
and 57.28% in the 100 μg/ml cecropinXJ treatment for 24 and 48 h,
respectively, vs. 68.14 and 68.52% in the control group), whereas
the percentage of cells at S-phase significantly increased (35.65
and 35.75% in the 100 μg/ml cecropinXJ treatment for 24 and 48 h,
respectively, vs. 23.87 and 23.48% in the control group) (Fig. 2). BGC823 cells with cecropinXJ
treatment achieved peak apoptosis. These results indicated that
cecropinXJ induced S-phase arrest and apoptosis in a dose-dependent
manner.
CecropinXJ dose-dependently induces
apoptosis of BGC823 cells
The above results showed that both low (<80
μg/ml) and high concentration of cecropinXJ could inhibit BGC823
cell proliferation with or without damage of plasma membrane. To
investigate the mechanism of cecropinXJ-mediated proliferation
inhibition of BGC823 cells, BGC823 cells were treated with
different concentrations of cecropinXJ for 24 or 48 h and stained
with Annexin V-FITC and PI. The samples were analyzed by flow
cytometry. CecropinXJ treatment dose-dependently increased
apoptosis of BGC823 cells at both early and late stages. The
apoptosis of BGC823 cells was increased from (4.88±2.53%)
(untreated) to (39.56±4.36%) (100 μg/ml cecropinXJ treated) for 24
h. Compared to 24 h, the apoptosis of BGC823 cells was also
increased 48 h after cecropinXJ treatment. Furthermore, 100 μg/ml
cecropinXJ treatment induced necrosis of BGC823 cells compared to
other groups for both 24 and 48 h (Fig. 3A and B).
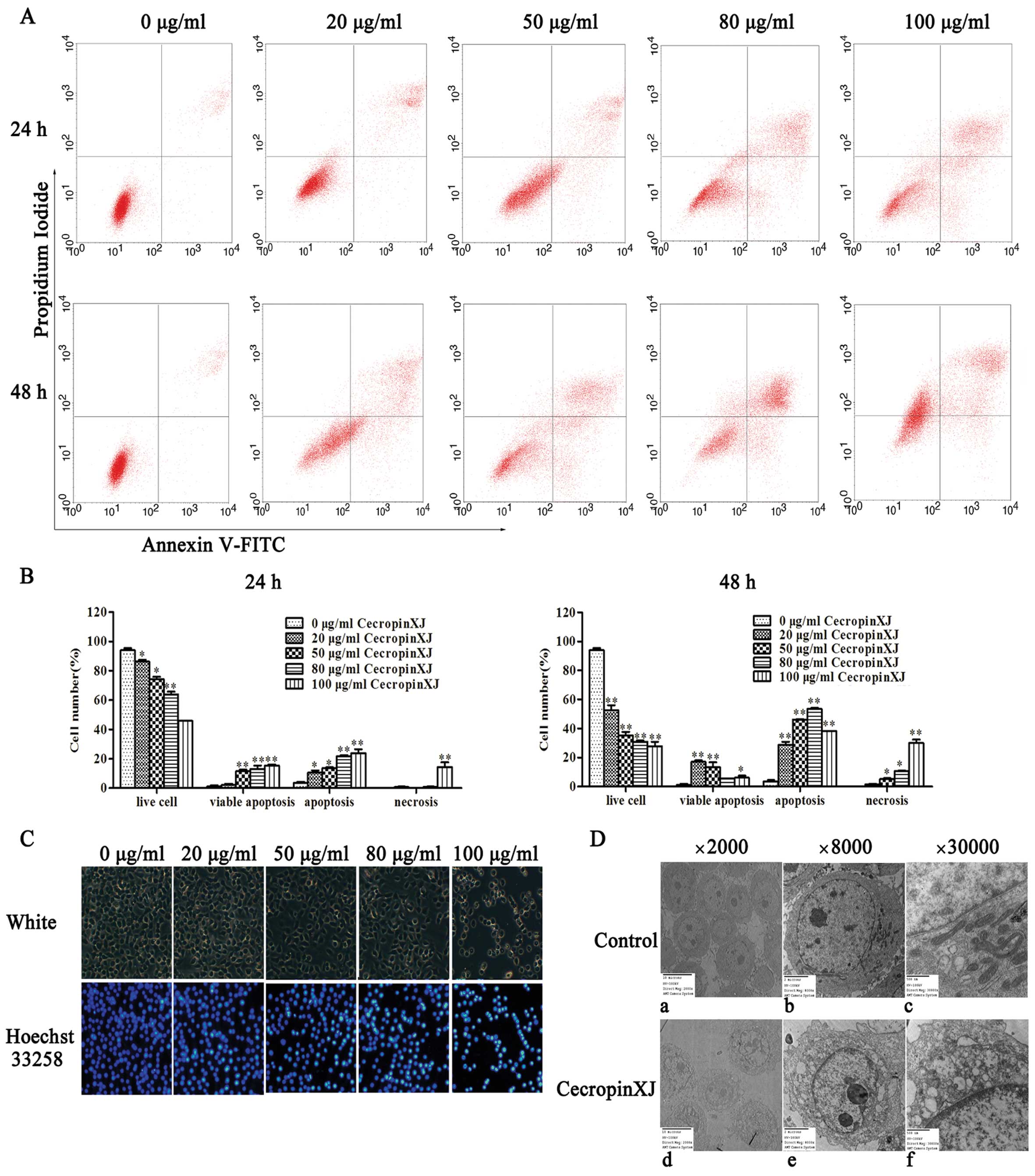 | Figure 3CecropinXJ induces apoptosis in
BGC823 cells. (A) BGC823 cells were treated with cecropinXJ (0, 20,
50, 80 and 100 μg/ml) for 24 and 48 h. After treatment, cells were
stained with Annexin V-FITC/PI and analyzed by flow cytometry. The
percentage (mean ± SD) of apoptotic cells is shown. Data are from
three independent experiments. (C) The nuclear morphology of BGC823
cells. BGC823 cells were treated with cecropinXJ (0, 20, 50, 80 and
100 μg/ml) for 24 h and stained with Hoechst 33258. Changes in
apoptotic nuclear morphology were observed by fluorescent
microscopy (magnification, ×10). (D) BGC823 cells were treated with
0 μg/ml (a, b and c) and 100 μg/ml CecropinXJ (d, e and f) for 24 h
and the morphology was observed by a transmission electron
microscope (TEM) under different magnifications (a and d, ×2,000; b
and e ×8,000; c and f, ×30,000). Representative data are shown from
one of three independent experiments. *P<0.05,
**P<0.01. |
Morphological characteristics of apoptosis include
chromatin condensation, DNA fragmentation and membrane blebbing
(25). Nuclear morphology was
observed by Hoechst 33258 staining. As shown in Fig. 3C, the untreated cells were round
and homogeneously stained, whereas cecropinXJ-treated cells showed
obvious chromatin condensation and fragmentation. Moreover, the
numbers of apoptotic nuclei containing condensed chromatin
increased significantly with the increased concentration of
cecropinXJ.
Ultrastructure of BGC823 cells was observed using
transmission electron microscopy and revealed that cecropinXJ
induced significantly morphological changes. The untreated BGC823
cells showed intact membrane, normal nucleus and integrated
mitochondria. However, cecropinXJ-treated cells showed nuclear
heterochromatin and condensed chromatin distribution around the
nuclear membrane. Moreover, mitochondria was osteoporosized and
swollen and cristae disappeared (Fig.
3D). Taken together, these results suggested that cecropinXJ
induced apoptosis of BGC823 cells.
CecropinXJ promotes ROS production and
decreases mitochondrial membrane potential (Δψm)
It has been reported that cancer cell apoptosis
induced by cecropins may be due to mitochondrial dysfunction and
overproduction of ROS (26).
Intracellular ROS production and loss of Δψm cause
mitochondrial membrane depolarization and trigger a cascade of
mitochondrion-dependent apoptotic signaling (27). The ROS level and the Δψm
were measured by flow cytometry using H2DCF-DA and
DiOC6(3), respectively. In this assay, cecropinXJ
increased ROS generation in dose- and time-dependent manner.
Moreover, cecropinXJ-treated BGC823 cells significantly decreased
their Δψm (Fig. 4).
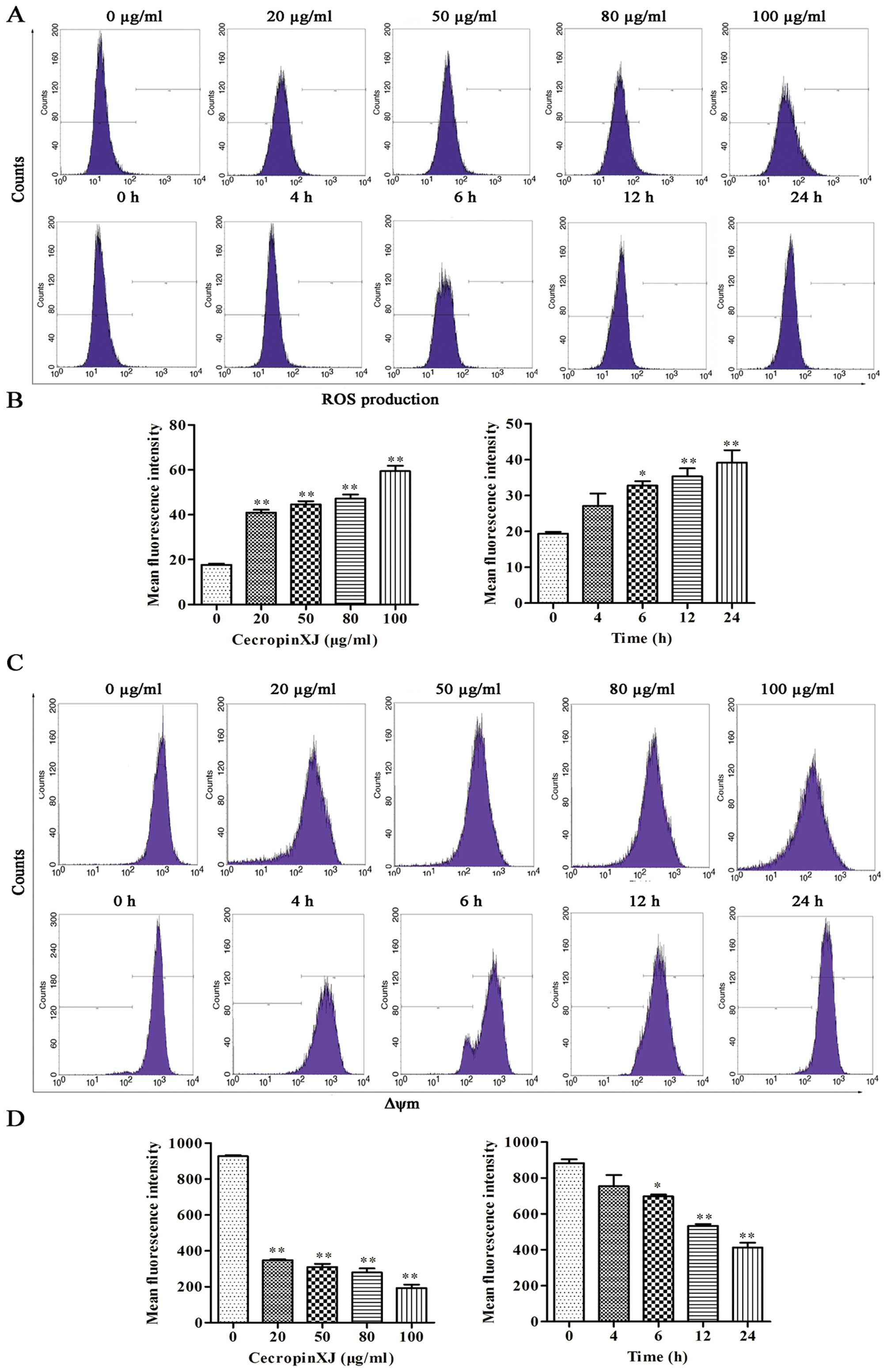 | Figure 4CecropinXJ promotes ROS production
and decreases mitochondrial membrane potential (Δψm) in
BGC823 cells. BGC823 cells were treated with different
concentrations (0, 20, 50, 80 and 100 μg/ml) of cecropinXJ for 24 h
or 100 μg/ml cecropinXJ for 0, 4, 6, 12 and 24 h. ROS production
(A) and Δψm (C) were detected by DCFH2-DA and
DiOC6(3), respectively, and analyzed by flow cytometry.
(B and D) Mean fluorescence intensity (mean ± SD) of ROS and
Δψm is shown in B and D, respectively. Data are from
three independent experiments. *P<0.05,
**P<0.01. |
CecropinXJ induces apoptosis through
activation of caspase pathway
It is well known that proteins in the caspase-family
and Bcl-2-family play critical roles in the apoptosis process
(28). To investigate whether
caspase-family and Bcl-2-family proteins were involved in
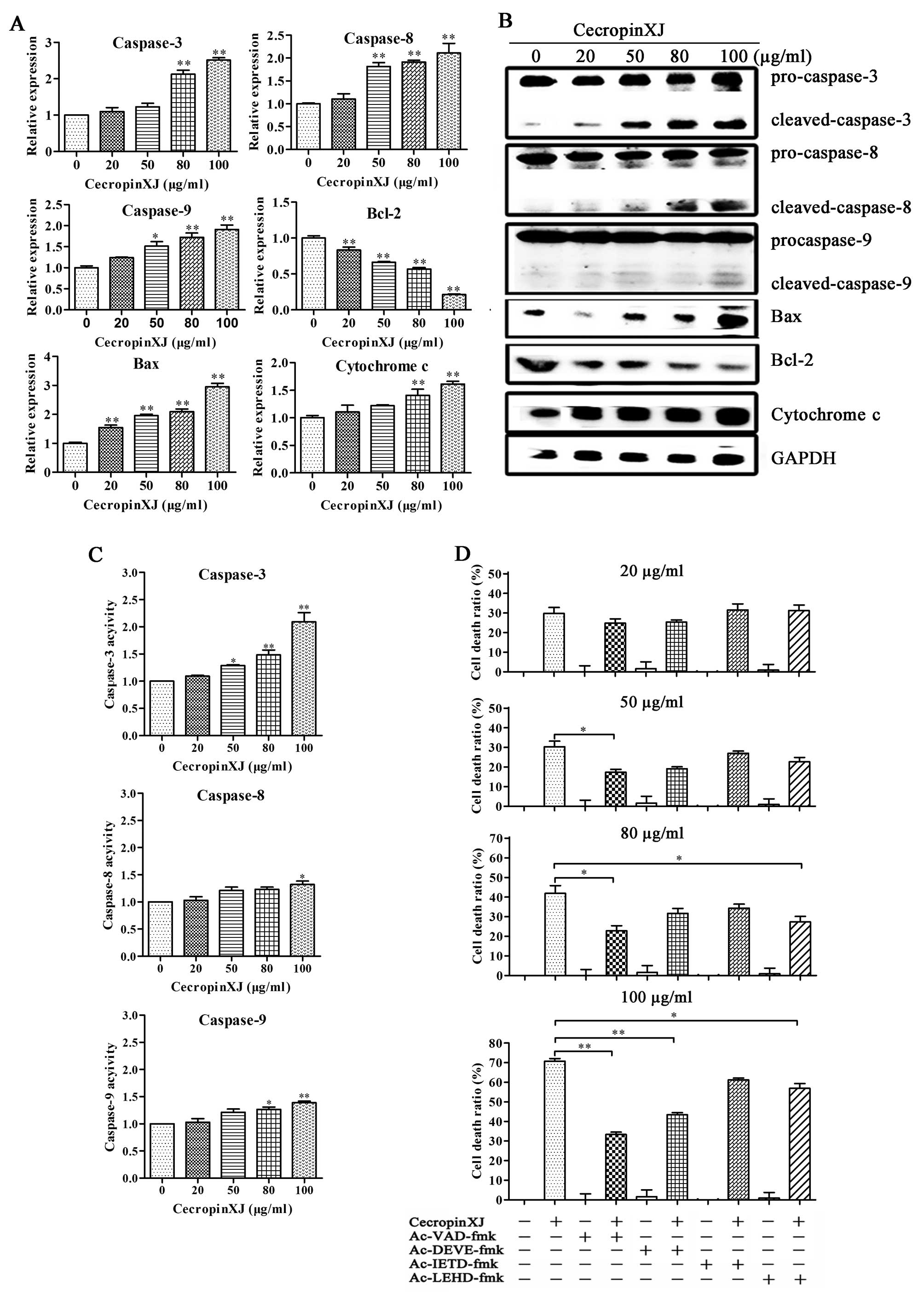
cecropinXJ-induced apoptosis, we measured the expression and
activation of these apoptosis-associated proteins by qRT-PCR and
western blot analysis. According to the results of the western blot
analysis, these enzymes including caspase-3 (effector caspase), -8
and -9 (initiator caspase) were all cleaved. Moreover, cecropinXJ
treatment dose-dependently increased the expression of Bax and
cytochrome c at both RNA and protein levels but decreased
the expression of anti-apoptotic molecule Bcl-2 (Fig. 5A and B). The Bax/Bcl-2 ratio
increased in BGC823 cells, suggesting that these proteins were
involved in cecropinXJ-induced apoptosis.
The activities of caspase-3, -8 and -9 were further
detected in cecropinXJ-treated cells using the colorimetric
tetrapeptide substrates Ac-DEVD-pNA, Ac-IETD-pNA and
Ac-LEHD-pNA, which have been shown to be specific for
caspase-3-, caspase-8-and caspase-9-like enzymatic activity,
respectively (29). After 100
μg/ml cecropinXJ treatment for 24 h, the activities of caspase-3,
-8 and -9 were increased ~2.3-, 1.3- and 1.3-fold compared to
untreated control (Fig. 5C).
Before cecropinXJ treatment, adding the caspase inhibitors
(Ac-VAD-fmk for pan-caspase, Ac-DEVE-fmk for caspase-3 and
Ac-LEHD-fmk for caspase-9) significantly reduced cecropinXJ-induced
cell death (P<0.01), but caspase-8 inhibitor had no effect
(Fig. 5D). These results suggest
that cecropinXJ might induce apoptosis of BGC823 cells via
activation of caspase-3 and -9.
CecropinXJ inhibits tumor growth in
vivo
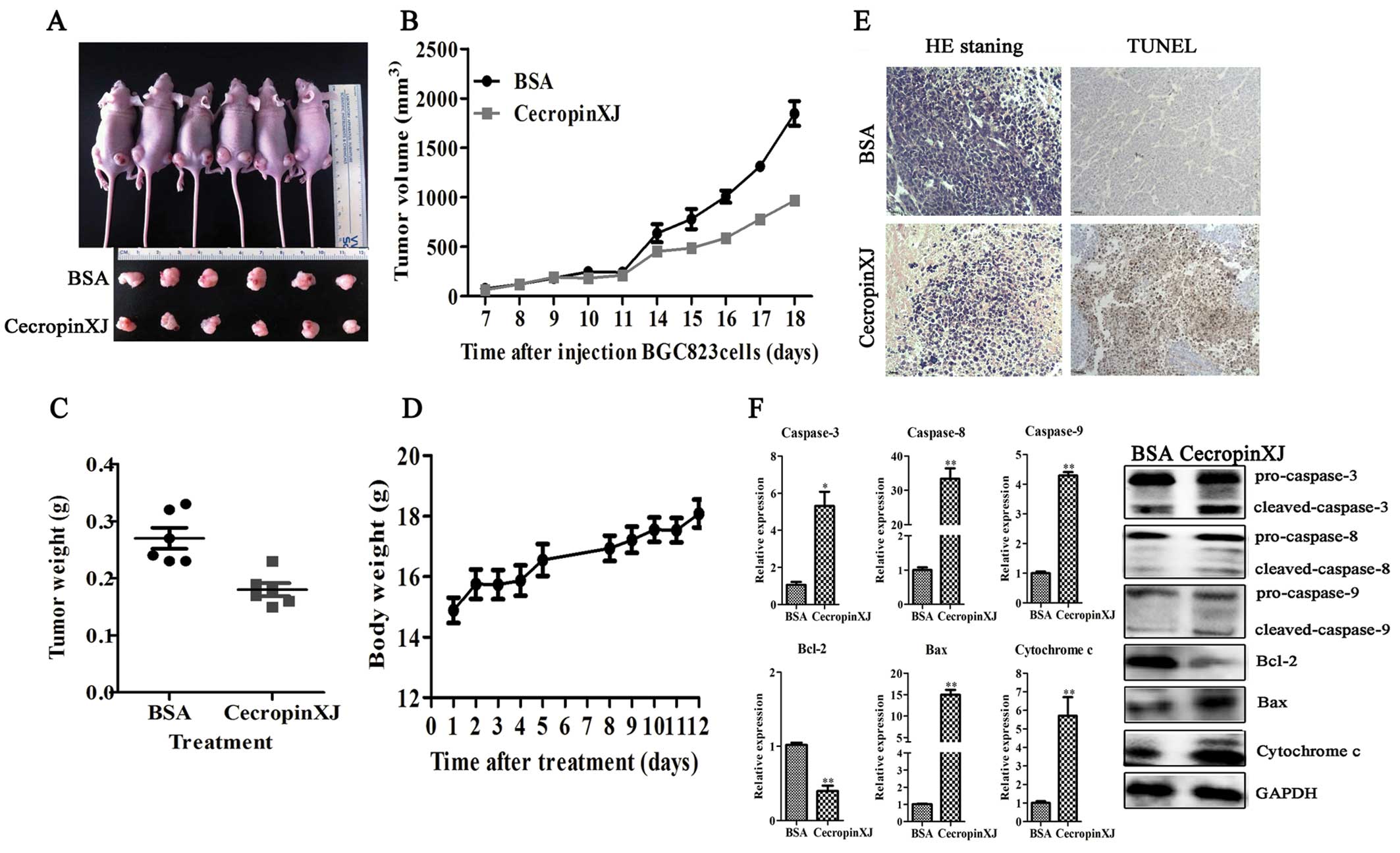
To investigate whether cecropinXJ could suppress the
growth of BGC823 cells in vivo, mice were injected with
BGC823 cells and treated with BSA (control group) or cecropinXJ on
day 7. Twelve days after treatment, the tumors were removed from
these animals and their volumes and weights were measured (Fig. 6). The tumor volume and tumor weight
in mice treated with cecropinXJ were markedly reduced compared with
BSA. CecropinXJ appeared to be non-toxic to normal cells, and there
were no notable side-effects on the body weight of mice (the mice
had tumors) at the end of the experiment (Fig. 6D). Moreover, we observed abundant
apoptotic cells in tumors from cecropinXJ-treated group via H&E
and TUNEL staining (Fig. 6E).
The expression of caspase-3, -8, -9, Bax, Bcl-2 and
cytochrome c in tumors was further detected. We found that
cecropinXJ treatment increased the expression of caspase-3, -8, -9,
Bax and cytochrome c but decreased the expression of Bcl-2,
which was consistent with the in vitro data.
CecropinXJ inhibits the expression of
angiogenesis-associated proteins in vitro and in vivo
Tumor angiogenesis is initiated by production of
angiogenic growth factors from tumor, stromal and infiltrating
inflammatory cells (30). Upon
cecropinXJ treatment, we examined the expressions of
angiogenesis-associated proteins including CD31, CD34, bFGF, VEGF
and VWF in BGC823 cells and tumors by qRT-PCR and western blot
analysis. As shown in Fig. 7, the
expression of all these proteins in BGC823 cells treated with
cecropinXJ was downregulated at both RNA and protein levels.
Importantly, the expression of all these proteins in tumors treated
with cecropinXJ was also decreased by immunohistochemical staining.
The results suggest that cecropinXJ could inhibit tumor
angiogenesis.
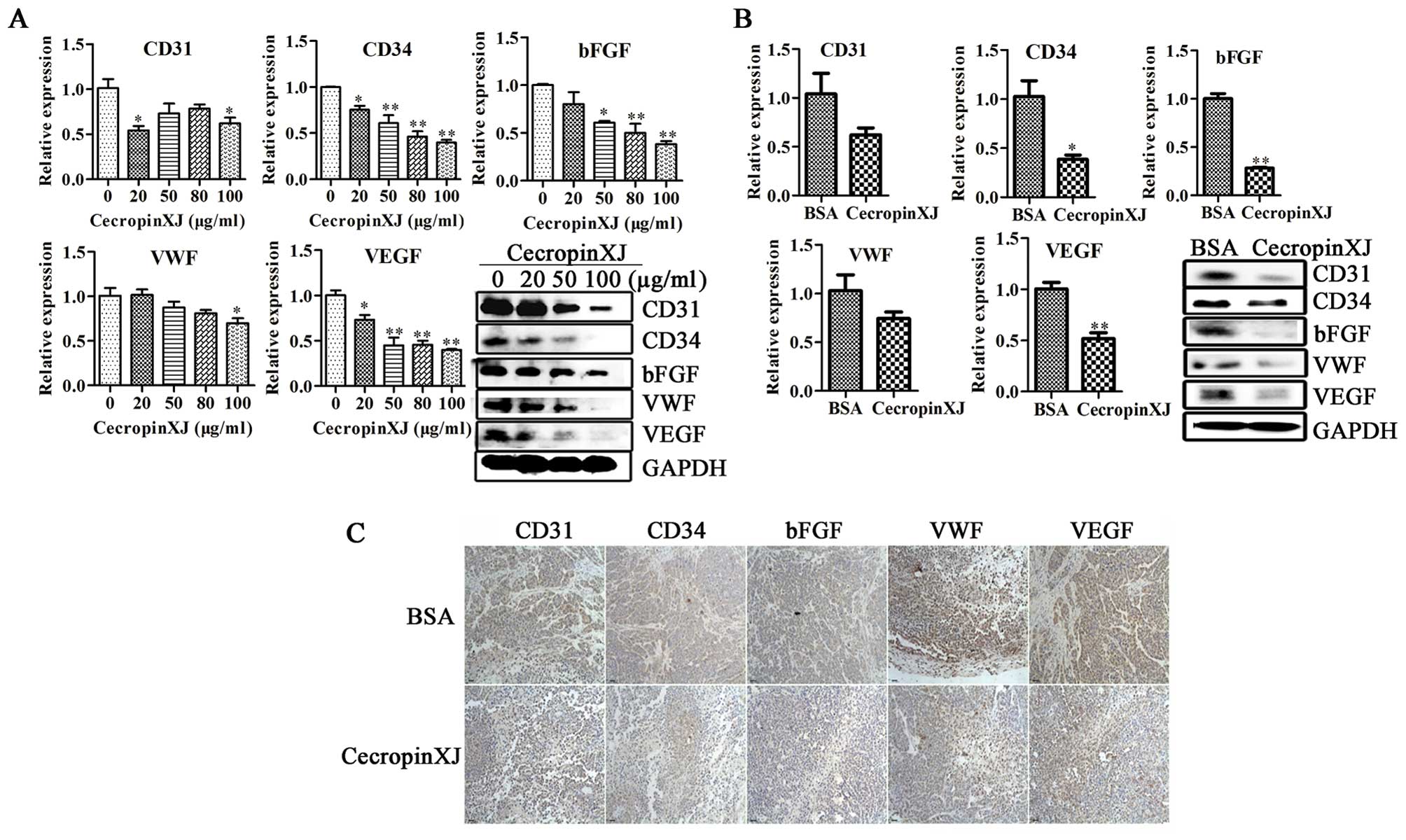 | Figure 7Effects of cecropinXJ on the
angiogenesis of BGC823 cells in vitro and in vivo.
BGC823 cells were treated with various concentrations of cecropinXJ
(0, 20, 50, 80 and 100 μg/ml) for 24 h and tumors were treated with
cecropinXJ and BSA. (A) The expression of CD31, CD34, bFGF, VEGF
and VWF in BGC823 cells were analyzed by qRT-PCR and western blot
analysis. (B and C) qRT-PCR, western blot analysis and
immunohistochemical staning were used to detection the expression
of CD31, CD34, bFGF, VEGF and VWF in tumors. |
Discussion
Chemotherapy alone shows low efficacy that may be
due to drug resistance, and severe side-effects that affect normal
mammalian cells (31,32). Since Moore et al (33) first found that Cecropin B possessed
potent anticancer activity in mammalian cancer cells, a large body
of evidence shows that cecropin families exerted cytotoxic effects
on a number of tumor derived cell lines in vitro and
exhibited inhibitory effects on tumor growth in vivo, such
as human breast adenocarcinoma (34) and hepatocellular carcinoma
(35). Cecropins contained
amphipathic α-helices that allowed them to target non-polar lipid
cell membranes and formed ion-permeable channels to result in cell
depolarization, irreversible cytolysis and final cell death
(36). The characteristic of
cecropins may avoid drug resistance.
Previous study has determined that the cecropins
from Chinese oak silkworm possess effective antitumor activity
against the human colon adenocarcinoma cells but without
cytotoxicity against normal human gastric epithelial cells (GES-1),
suggesting cecropins could specifically kill tumor cells (37). Other evidence also showed that
cecropins could suppress the growth of tumor cells such as leukemia
cells, but have no hemolytic activity against human erythrocytes
and no or little toxicity to normal mammalian cells (38). Consistently, we observed that
cecropinXJ inhibited the proliferation of BGC823 cells but did not
affect normal GES-1 cells. The inhibitory effects of 100 μg/ml
cecropinXJ on the proliferation of BGC823 cells was similar to 10
μg/ml Dox. The specific effects on cancer cells might be attributed
to the nature of cecropins contained cationic sequence to interact
with non-polar anionic plasma membranes of cancer cells (39). Wang et al (40) reported that cecropins could disrupt
plasma membrane integrity of cancer cells when the concentration
was >60.9 or 81.2 μg/ml. Lee et al (41) observed that CopA3 mainly caused
necrotic cell death of the gastric cancer cells. However, in the
present study, the LDH release of BGC823 cells was not increased
after 80 μg/ml cecropinXJ treatment, and cecropinXJ mainly induced
apoptosis of BGC823 cells. Consistent with our results, cecropin of
Musca domestica induced DNA fragmentation to induce
apoptosis (6). Therefore,
cecropins may inhibit tumor cells through different mechanisms via
inducing necrosis or apoptosis.
Cerón et al (26) reported that cecropin A was able to
induce apoptosis in HL-60 cells through the production of ROS but
independent of caspase activation. Huang et al (42) showed that pardxin, an antimicrobial
peptide was first isolated from secretions of the Red Sea Moses
sole, exerted antitumor effects on human fibrosarcoma HT-1080 cells
by increasing intracellular ROS and activating caspase-3/7
activities. Our results showed that cecropinXJ increased ROS level
and activated caspase-3/8/9 activity in BGC823 cells. Using caspase
inhibitors, we further found that caspase-3/9 were involved in the
induced apoptosis, but caspase-8 had no effect.
Bcl-2 family proteins including anti-apoptotic
(Bcl-2 and Bcl-xL) and pro-apoptotic members (Bax and Bad) play
critical roles in the regulation of mitochondrial-mediated
apoptosis or cell survival (43).
Bcl-2 protein can bind to the outer membrane of the mitochondrion
and prevent apoptosis, while Bax is responsible for permeabilizing
the membrane (44,45). It has been reported that melittin
could decrease the expression of Bcl-2 and induce the translocation
of Bax to the mitochondria with the release of cytochrome c
into the cytosol, suggesting that mitochondria are involved in
melittin-induced apoptosis (46).
In the present study, cecropinXJ treatment increased the release of
cytochrome c into the cytoplasm, decreased mitochondria
Δψm, upregulated the expression of Bax and downregulated
the expression of Bcl-2 in vitro, suggesting that cecropinXJ
triggered mitochondrion-dependent apoptosis. We further found that
cecropinXJ inhibited growth of gastric cancer in mice and induced
cancer cell apoptosis in tumors. Consistent with the in
vitro results, cecropinXJ treatment could upregulate the
expression of Bax and downregulate the expression of Bcl-2. Hence,
cecropinXJ were able to induce the BGC823 cells apoptosis both
in vitro and in vivo.
Angiogenesis is the formation of new blood vessels
by the sprouting of endothelial cells from pre-existing vessels
(47) and is essential for tumor
growth and metastasis (48). It
has been reported that suppression of angiogenesis might be an
effective therapeutic approach against gastric cancer (49). A number of growth factors related
to tumor angiogenesis have been identified which includes bFGF
(50), VWF (51) and members of VEGF family (52). We found the expression of the CD31,
CD34, VEGF, bFGF and VWF gene in BGC823 cells and BGC823-xenograft
tumors was significantly decreased by cecropinXJ treatment,
suggesting that cecropinXJ might inhibit tumor angiogenesis in
vivo.
In summary, our results showed that cecropinXJ
inhibits the growth of human BGC823 cells in vitro and in
vivo through inducing apoptosis and preventing tumor
angiogenesis. These results suggested that cecropinXJ has potential
as a novel antitumor therapeutic strategy for the treatment of
gastric cancer.
Acknowledgements
We thank Professor Youyong Lv for providing the
gastric cancer BGC823 cells and normal gastric epithelial GES-1
cells. The present study was supported by a grant from the
High-Tech Research and Development Program of Xinjiang (no.
201110101).
Abbreviations:
|
AMPs
|
antimicrobial peptides
|
|
ROS
|
reactive oxygen species
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor-kappa B
|
|
PI3K/Akt
|
phosphoinositide 3-kinase/protein
kinase B
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagini S: Carcinoma of the stomach: a
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zasloff M: Antimicrobial peptides of
multicellular organisms. Nature. 415:389–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Papo N and Shai Y: Host defense peptides
as new weapons in cancer treatment. Cell Mol Life Sci. 62:784–790.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paredes-Gamero EJ, Martins MN, Cappabianco
FA, Ide JS and Miranda A: Characterization of dual effects induced
by antimicrobial peptides: regulated cell death or membrane
disruption. Biochim Biophys Acta. 1820:1062–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin X, Mei H, Li X, Ma Y, Zeng A, Wang Y,
Lu X, Chu F, Wu Q and Zhu J: Apoptosis-inducing activity of the
antimicrobial peptide cecropin of Musca domestica in human
hepatocellular carcinoma cell line BEL-7402 and the possible
mechanism. Acta Biochim Biophys Sin. 42:259–265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang C, Zhou Y, Li S, Li HB, Tian LL, Wang
H and Shang DJ: Anticancer mechanisms of temporin-1CEa, an
amphipathic α-helical antimicrobial peptide, in Bcap-37 human
breast cancer cells. Life Sci. 92:1004–1014. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Risso A, Braidot E, Sordan MC, Vianello A,
Macrì F, Skerlavaj B, Zanetti M, Gennaro R and Bernardi P: BMAP-28,
an antibiotic peptide of innate immunity, induces cell death
through opening of the mitochondrial permeability transition pore.
Mol Cell Biol. 22:1926–1935. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun KW, Ma YY, Guan TP, Xia YJ, Shao CM,
Chen LG, Ren YJ, Yao HB, Yang Q and He XJ: Oridonin induces
apoptosis in gastric cancer through Apaf-1, cytochrome c and
caspase-3 signaling pathway. World J Gastroenterol. 18:7166–7174.
2012. View Article : Google Scholar
|
|
10
|
Furlong SJ, Mader JS and Hoskin DW: Bovine
lactoferricin induces caspase-independent apoptosis in human
B-lymphoma cells and extends the survival of immune-deficient mice
bearing B-lymphoma xenografts. Exp Mol Pathol. 88:371–375. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen YL, Li JH, Yu CY, Lin CJ, Chiu PH,
Chen PW, Lin CC and Chen WJ: Novel cationic antimicrobial peptide
GW-H1 induced caspase-dependent apoptosis of hepatocellular
carcinoma cell lines. Peptides. 36:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huh JE, Kang JW, Nam D, Baek YH, Choi DY,
Park DS and Lee JD: Melittin suppresses VEGF-A-induced tumor growth
by blocking VEGFR-2 and the COX-2-mediated MAPK signaling pathway.
J Nat Prod. 75:1922–1929. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang C, Chen T, Zhang N, Yang M, Li B, Lü
X, Cao X and Ling C: Melittin, a major component of bee venom,
sensitizes human hepatocellular carcinoma cells to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha
kinase-NFkappaB. J Biol Chem. 284:3804–3813. 2009. View Article : Google Scholar
|
|
14
|
Xu XX, Jiang HR, Li HB, Zhang TN, Zhou Q
and Liu N: Apoptosis of stomach cancer cell SGC-7901 and regulation
of Akt signaling way induced by bovine lactoferrin. J Dairy Sci.
93:2344–2350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jeong YJ, Choi Y, Shin JM, et al: Melittin
suppresses EGF-induced cell motility and invasion by inhibiting
PI3K/Akt/mTOR signaling pathway in breast cancer cells. Food Chem
Toxicol. 68:218–225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan Q, Hu Y, Pang H, Sun J, Wang Z and Li
J: Melittin protein inhibits the proliferation of MG63 cells by
activating inositol-requiring protein-1α and X-box binding protein
1-mediated apoptosis. Mol Med Rep. 9:1365–1370. 2014.PubMed/NCBI
|
|
17
|
Ting CH, Huang HN, Huang TC, Wu CJ and
Chen JY: The mechanisms by which pardaxin, a natural cationic
antimicrobial peptide, targets the endoplasmic reticulum and
induces c-FOS. Biomaterials. 35:3627–3640. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song CC, Lu X, Cheng BB, DU J, Li B and
Ling CQ: Effects of melittin on growth and angiogenesis of human
hepatocellular carcinoma BEL-7402 cell xenografts in nude mice. Ai
Zheng. 26:1315–1322. 2007.(In Chinese). PubMed/NCBI
|
|
19
|
Li J, Zhang F and Ma Z: Prokaryotic
expression of cecropin gene isolated from the silk worm Bombyx mori
Xinjiang race and antibacterial activity of fusion cecropin. Acta
Entomol Sin. 47:407–411. 2004.(In Chinese).
|
|
20
|
Liu Z, Zhang F, Cai L, Zhao G and Wang B:
Studies on the properties of cecropin-XJ expressed in yeast from
Xinjiang silkworm. Wei Sheng Wu Xue Bao. 43:635–641. 2003.(In
Chinese).
|
|
21
|
Wu Y, Xia L and Zhang F: Inhibition of
CecropinXJ on proliferation of human gastric cancer AGS cells.
Chinese J Cell Biol. 36:1355–1361. 2014.(In Chinese).
|
|
22
|
Xia L, Zhang F, Liu Z, Ma J and Yang J:
Expression and characterization of cecropinXJ, a bioactive
antimicrobial peptide from Bombyx mori (Bombycidae, Lepidoptera) in
Escherichia coli. Exp Ther Med. 5:1745–1751. 2013.PubMed/NCBI
|
|
23
|
Xia L, Liu Z, Ma J, Sun S, Yang J and
Zhang F: Expression, purification and characterization of cecropin
antibacterial peptide from Bombyx mori in Saccharomyces cerevisiae.
Protein Expr Purif. 90:47–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shai Y: Mechanism of the binding,
insertion and destabilization of phospholipid bilayer membranes by
alpha-helical antimicrobial and cell non-selective membrane-lytic
peptides. Biochim Biophys Acta. 1462:55–70. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mahassni SH and Al-Reemi RM: Apoptosis and
necrosis of human breast cancer cells by an aqueous extract of
garden cress (Lepidium sativum) seeds. Saudi J Biol Sci.
20:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cerón JM, Contreras-Moreno J, Puertollano
E, de Cienfuegos GÁ, Puertollano MA and de Pablo MA: The
antimicrobial peptide cecropin A induces caspase-independent cell
death in human promyelocytic leukemia cells. Peptides.
31:1494–1503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeong JC, Jang SW, Kim TH, Kwon CH and Kim
YK: Mulberry fruit (Moris fructus) extracts induce human glioma
cell death in vitro through ROS-dependent mitochondrial pathway and
inhibits glioma tumor growth in vivo. Nutr Cancer. 62:402–412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
She EX and Hao Z: A novel piperazine
derivative potently induces caspase-dependent apoptosis of cancer
cells via inhibition of multiple cancer signaling pathways. Am J
Transl Res. 5:622–633. 2013.PubMed/NCBI
|
|
29
|
Bao R, Shu Y, Wu X, et al: Oridonin
induces apoptosis and cell cycle arrest of gallbladder cancer cells
via the mitochondrial pathway. BMC Cancer. 14:2172014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Folkman J: What is the evidence that
tumors are angiogenesis dependent? J Natl Cancer Inst. 82:4–6.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orditura M, Galizia G, Sforza V, et al:
Treatment of gastric cancer. World J Gastroenterol. 20:1635–1649.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garnier-Suillerot A, Marbeuf-Gueye C,
Salerno M, Loetchutinat C, Fokt I, Krawczyk M, Kowalczyk T and
Priebe W: Analysis of drug transport kinetics in
multidrug-resistant cells: implications for drug action. Curr Med
Chem. 8:51–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moore AJ, Devine DA and Bibby MC:
Preliminary experimental anticancer activity of cecropins. Pept
Res. 7:265–269. 1994.PubMed/NCBI
|
|
34
|
Suttmann H, Retz M, Paulsen F, Harder J,
Zwergel U, Kamradt J, Wullich B, Unteregger G, Stöckle M and
Lehmann J: Antimicrobial peptides of the Cecropin-family show
potent antitumor activity against bladder cancer cells. BMC Urol.
8:52008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin XB, Wang YJ, Liang LL, Pu QH, Shen J,
Lu XM, Chu FJ and Zhu JY: Cecropin suppresses human hepatocellular
carcinoma BEL-7402 cell growth and survival in vivo without
side-toxicity. Asian Pac J Cancer Prev. 15:5433–5436. 2014.
View Article : Google Scholar
|
|
36
|
Boman HG: Antibacterial peptides: basic
facts and emerging concepts. J Intern Med. 254:197–215. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang WM, Lai ZS, He MR, Xu G, Huang W and
Zhou DY: Effects of the antibacterial peptide cecropins from
Chinese oak silkworm, antheraea pernyi on 1,
2-dimethylhydrazine-induced colon carcinogenesis in rats. Di Yi Jun
Yi Da Xue Xue Bao. 23:1066–1068. 2003.(In Chinese). PubMed/NCBI
|
|
38
|
Hui L, Leung K and Chen HM: The combined
effects of antibacterial peptide cecropin A and anti-cancer agents
on leukemia cells. Anticancer Res. 22:2811–2816. 2002.
|
|
39
|
Harris F, Dennison SR, Singh J and Phoenix
DA: On the selectivity and efficacy of defense peptides with
respect to cancer cells. Med Res Rev. 33:190–234. 2013. View Article : Google Scholar
|
|
40
|
Wang C, Tian LL, Li S, Li HB, Zhou Y, Wang
H, Yang QZ, Ma LJ and Shang DJ: Rapid cytotoxicity of antimicrobial
peptide Tempoprin-1CEa in breast cancer cells through membrane
destruction and intracellular calcium mechanism. PLoS One.
8:e604622013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JH, Kim IW, Kim SH, Yun EY, Nam SH,
Ahn MY, Kang DC and Hwang JS: Anticancer activity of CopA3 dimer
peptide in human gastric cancer cells. BMB Rep. Jul 22–2014.(Epub
ahead of print).
|
|
42
|
Huang TC, Lee JF and Chen JY: Pardaxin, an
antimicrobial peptide, triggers caspase-dependent and ROS-mediated
apoptosis in HT-1080 cells. Mar Drugs. 9:1995–2009. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsujimoto Y: Bcl-2 family of proteins:
life-or-death switch in mitochondria. Biosci Rep. 22:47–58. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Du L, Mei HF, Yin X and Xing YQ: Delayed
growth of glioma by a polysaccharide from Aster tataricus involve
upregulation of Bax/Bcl-2 ratio, activation of caspase-3/8/9, and
downregulation of the Akt. Tumour Biol. 35:1819–1825. 2014.
View Article : Google Scholar
|
|
45
|
Das A, Banik NL, Patel SJ and Ray SK:
Dexamethasone protected human glioblastoma U87MG cells from
temozolomide induced apoptosis by maintaining Bax:Bcl-2 ratio and
preventing proteolytic activities. Mol Cancer. 3:362004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gajski G and Garaj-Vrhovac V: Melittin: a
lytic peptide with anticancer properties. Environ Toxicol
Pharmacol. 36:697–705. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Emmett MS, Dewing D and Pritchard-Jones
RO: Angiogenesis and melanoma: from basic science to clinical
trials. Am J Cancer Res. 1:852–868. 2011.
|
|
48
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:15–18. 2002. View Article : Google Scholar
|
|
49
|
Javle M, Smyth EC and Chau I: Ramucirumab:
successfully targeting angiogenesis in gastric cancer. Clin Cancer
Res. 20:5875–5881. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Presta M, Dell’Era P, Mitola S, Moroni E,
Ronca R and Rusnati M: Fibroblast growth factor/fibroblast growth
factor receptor system in angiogenesis. Cytokine Growth Factor Rev.
16:159–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Starke RD, Ferraro F, Paschalaki KE, et
al: Endothelial von Willebrand factor regulates angiogenesis.
Blood. 117:1071–1080. 2011. View Article : Google Scholar :
|
|
52
|
Aoyagi K, Kouhuji K, Kizaki J, Isobe T,
Hashimoto K and Shirouzu K: Molecular targeting to treat gastric
cancer. World J Gastroenterol. 20:13741–13755. 2014. View Article : Google Scholar : PubMed/NCBI
|