Introduction
Estrogens serve various physiological functions and
are critical for the development and maintenance of diverse
tissues, including reproductive tissues, adipose tissues, and
skeletal tissues though their interactions with estrogen receptors
(ERs). In turn, estrogens and their receptors have been implicated
in various pathophysiologic conditions, including cancers,
cognitive diseases, and postmenopausal syndrome. Accordingly, there
have been numerous efforts to develop compounds capable of
modulating ER-mediated signaling pathways, and several selective
estrogen receptor modulators (SERMs) and aromatase inhibitors have
been approved. To date, the major clinical applications of SERMs
are in the treatment of ER-positive breast cancer and the
prevention and treatment of postmenopausal symptoms caused by the
low levels of estrogens that accompany osteoporosis (1–3). In
addition, recent studies suggest the possible use of SERMs as
neuroprotective agents (4,5).
Anti-estrogenic ER-modulating agents such as
tamoxifen and toremifene, which directly interact with ERs and
suppress their function, have been commonly used to treat patients
with ER-positive breast cancer (6–8).
However, a majority of patients treated with tamoxifen eventually
develop resistance and experience metastases (9,10).
Moreover, tamoxifen is known to increase the risk of
thromboembolism and endometrial changes, including endometrial
cancer (11–13).
On the other hand, estrogen and estrogenic compounds
have been used to treat estrogen deficiency-related postmenopausal
symptoms. It has been reported that ~75% of perimenopausal and
postmenopausal women experience various symptoms owing to estrogen
withdrawal, including vasomotor menopausal syndrome (VMS) and hip,
spine or wrist fracture due to osteoporosis (1,14,15).
Hormone replacement therapy (HRP) was once widely used to
ameliorate and prevent postmenopausal symptoms, including VMS and
osteoporosis. However, its use has drastically declined since a
report by the Women’s Health Initiative (WHI) showed that HRP
increases the risk of strokes, breast cancer, and pulmonary
embolism (16–19). To overcome these limitations,
several next generation SERMs have been developed or are under
development (3). For example,
raloxifene, a SERM that exerts estrogenic effects on bones and
anti-estrogenic effects on the uterus and breast, has been used
clinically to prevent osteoporosis in postmenopausal women
(2,20). Recently, another estrogenic SERM,
ospemifene, was approved by the US Food and Drug Administration for
dyspareunia associated with vulvar and vaginal atrophy and
menopause (21). Considering the
increased risks and adverse effects of using SERMs, there are still
unmet needs for alternative, safer SERMs with an improved
risk-benefit ratio. In an effort to identify new SERMs, we
generated a chemical library composed of synthetic compounds
structurally related to bisphenol A, which is well known to exert
hormone-like actions. In the present study, we identified a
synthetic compound that acts as an ER-signaling agonist and
characterized its anticancer effects.
Materials and methods
Synthesis of NJK13054 and NJK14013
NJK13054
[(E)-1-(4-hydroxyphenyl)ethanone-O-butyl oxime]
To a solution of 4-hydroxyacetophenone (30 mg, 0.22
mmol) in ethanol (1 ml) was added O-butylhydroxylamine
hydrochloride (33 mg, 0.26 mmol). The reaction mixture was stirred
at ambient temperature for 4 h, quenched with H2O, and
diluted with ethyl acetate (EtOAc). The combined organic layer was
washed with H2O, dried over MgSO4, and
concentrated in vacuo. Purification of the residue via flash
column chromatography on silica (EtOAc:n-hexane = 1:6-1:5)
yielded 36 mg of NJK13054 (79%); 1H-NMR (400 MHz,
CDCl3) δ 7.46 (2H, d, J=8.7 Hz), 6.71 (2H,
d, J=8.7 Hz), 6.53 (1H, s), 4.17 (2H, t, J=6.6 Hz),
2.21 (3H, s), 1.71-1.64 (2H, m), 1.44-1.38 (2H, m), 0.94 (3H, t,
J=7.4 Hz).
NJK14013
[bis(4-hydroxyphenyl)methanone-O-isopentyl oxime]
To a solution of 4,4′-dihydroxybenzophenone (50 mg,
0.23 mmol) in ethanol (2 ml) was added
O-isopentyl-hydroxylamine hydrochloride (33 mg, 0.32 mmol).
The reaction mixture was stirred at 70°C for 6 h, quenched with
H2O, and diluted with EtOAc. The combined organic layer
was washed with H2O, dried over MgSO4, and
concentrated in vacuo. Purification of the residue via flash
column chromatography on silica (EtOAc:n-hexane = 1:6-1:5)
yielded 36 mg of NJK14013 (53%); 1H-NMR (400 MHz,
CDCl3) δ 7.31 (2H, d, J=8.6 Hz), 7.25 (2H,
d, J=8.6 Hz), 6.80 (2H, d, J=8.6 Hz), 6.70 (2H, d,
J=8.6 Hz), 6.00 (1H, s), 4.19 (2H, t, J=6.9 Hz), 1.65
(1H, m, J=6.8 Hz), 1.60-1.55 (2H, m), 1.26 (1H, m), 0.89
(6H, d, J=6.5 Hz).
Cells
MCF7 and MDA-MB-231 human breast cancer cell lines
and the LNCaP (CRL-1740) human prostate cancer cell line were
maintained in RPMI-1640 medium containing 10% fetal bovine serum
(FBS) and penicillin/streptomycin (100 U/ml). The Ishikawa human
endometrial adenocarcinoma cell line and A549 human lung
adenocarcinoma epithelial cell line were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) containing 10% FBS and
penicillin/streptomycin (100 U/ml). Human umbilical vein
endothelial cells (HUVECs; ATCC CRL-1730) were propagated in
endothelial cell growth media (Lonza). For luciferase assays and
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR) assays using 17β-estradiol (E2) or test compounds, MCF7
cells were maintained in phenol red-free RPMI containing 10%
charcoal-stripped FBS for 1 day before treatment.
Luciferase assays
Stimulation of ER transcriptional activity by test
compounds was assessed by dual-luciferase assays as previously
described, with slight modifications (22). Briefly, MCF7 cells were
co-transfected with estrogen response element (ERE)-Luc and
thymidine kinase-Renilla (pRL-TK) expression plasmids and
maintained in phenol-red-free RPMI containing 10% charcoal-stripped
(CS) FBS for 1 day. Cells were treated with the indicated compounds
for 24 h and analyzed using dual-luciferase assays (Promega,
Madison, WI, USA) according to the manufacturer’s instructions.
Activation of the androgen receptor (AR) was assessed in a similar
manner by transfecting LNCaP cells with a prostate-specific antigen
(PSA)-enhancer/promoter-luciferase reporter construct or mouse
mammary tumor virus (MMTV) enhancer/promoter-luciferase reporter
plasmid together with a pRL-TK expression plasmid.
Immunoblotting
Effects of NJK14013 on the phosphorylation status of
ERα were analyzed by immunoblotting. MCF7 cells were treated with
different concentrations of NJK14013 for 24 h. After lysing cells
with RIPA buffer containing phosphatase inhibitors (Sigma-Aldrich,
St. Louis, MO, USA), proteins were resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
immunoblotted using anti-phospho-ER (Cell Signaling Technology,
#8644, Beverly, MA, USA), anti-ERα (Cell Signaling Technology,
#8644), and anti-β-actin (Santa Cruz, sc47778, Dallas, TX, USA)
antibodies. Cell proliferation was assessed by treating cells with
E2 or NJK14013 for 24 h, then lysing cells and analyzing cell
lysates by immunoblotting using an anti-PCNA antibody (Santa Cruz,
sc7907).
Molecular modeling of interactions
between ER and NJK14013
Molecular docking studies were performed based on
the human-ERα crystal structure (PDB code: 2QXS) using Autodock 4.2
(Molecular Graphic Laboratory) (23). Results of docking studies were
visualized using Chimera 1.10 software (24).
RNA preparation and RT-qPCR
The transcriptional activity of ER was analyzed by
determining transcript levels of the ER target, growth regulation
by estrogen in breast cancer 1 (GREB1), by RT-qPCR, as
described previously (22). MCF7
cells were seeded and maintained in phenol-red-free RPMI containing
10% CS FBS for 1 day prior to treatment with E2 or NJK14013. After
incubating cells for an additional 24 h, RNA was extracted and
cDNA, used as a template for quantitative PCR, was synthesized from
total RNA using Superscript III reverse transcriptase (Invitrogen,
Carlsbad, CA, USA) and oligo20(dT) primers. β-actin was
used as a reference gene for normalization. The following primer
pairs were used for qPCR: GREB1, 5′-gtggtagccgagtggacaat-3′ (sense)
and 5′-aaacccgtctgtggtacagc-3′ (antisense); and β-actin,
5′-gggaaatcgtgcgtgacatt-3′ (sense) and 5′-ggagttgaaggtagtttcgt-3′
(antisense).
Cell proliferation and cell cytotoxicity
assay
The effects of compounds on tumor cell growth were
determined by seeding MCF7, Ishikawa, MDA-MB-232, and A549 cells
(1×104 cells/well) onto 96-well tissue culture plates
and treating with different concentrations of E2 or NJK14013 for
24, 48 or 72 h. Cell proliferation was determined using an MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay. After incubation with the compound, the culture medium was
replaced with fresh medium containing 20 μl of MTT (5 mg/ml), and
cells were incubated for an additional 4 h. Thereafter, the medium
was removed, and cells were lysed with dimethyl sulfoxide. The
absorbance of lysates was determined at 570 nm using a microplate
reader. The cytotoxicity of compounds toward tumor cells was
analyzed by seeding Ishikawa cells and HUVEC cells
(4×104 cells/well) onto 96-well plates and treating as
indicated in the text. Cell viability was determined by MTT assays
in a similar manner.
Apoptosis assay
Apoptotic cell death was assessed by Annexin V/7-AAD
staining. For Annexin V assays, cells were treated with vehicle or
NJK14013 (10 μM) as indicated. Cells were stained with Annexin V-PE
and 7-AAD using a Guava Nexin kit (Millipore, Billerica, MA, USA)
and analyzed with a Guava easyCyte flow cytometer according to the
manufacturer’s instructions.
Results
NJK14013 is a novel synthetic ER agonist
that stimulates ER transcriptional activity
With the aim of developing new ER-modulating agents,
we screened an in-house synthetic library containing
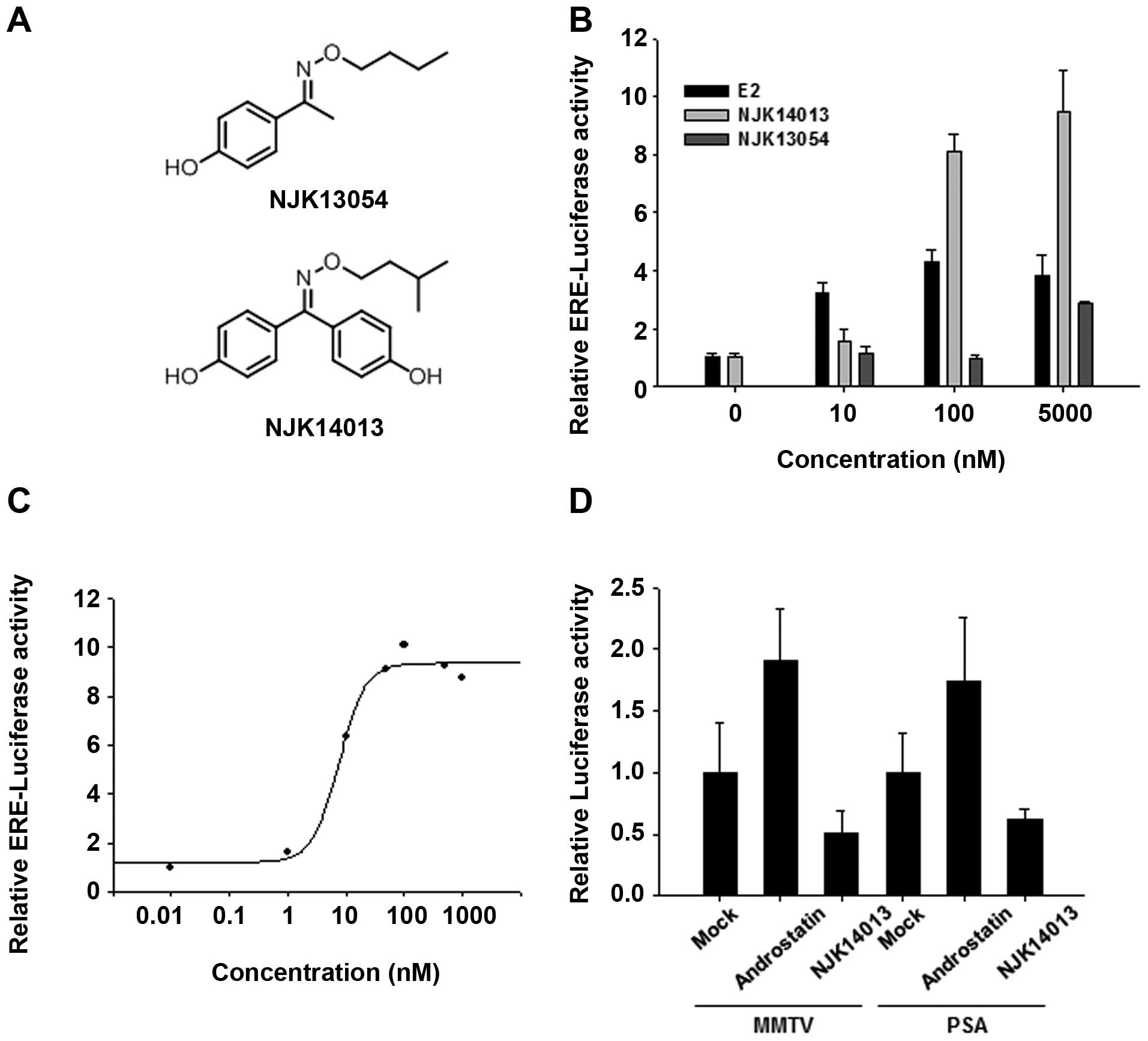
bisphenol-A-inspired compounds. These screens identified two
compounds, NJK14013 and NJK13054, that upregulated the activity of
an estrogen response element (ERE)-containing luciferase reporter
construct (ERE-luciferase) in MCF7 cells (Fig. 1A and B). Notably, NJK14013 showed
higher activity than E2, the endogenous ligand, at concentrations
of 100 nM and 1 μM, but displayed weaker activity at 10 nM
(Fig. 1B). Since the agonistic
effect of NJK14013 was greater than that of NJK13054, NJK14013 was
used for subsequent studies. NJK14013 induced
concentration-dependent stimulation of ERE-mediated transcriptional
activity with a median effective concentration (EC50)
value of 46.3±12 nM (Fig. 1C). To
determine whether this activity was specific for ER-mediated
transcription, we analyzed the effect of NJK14013 on AR-mediated
transcription using MMTV promoter- and PSA promoter-driven
luciferase reporters. Unlike ER-driven transcription, AR-driven PSA
promoter- and MMTV enhancer/promoter-reporter activity in LNCaP
cells were not activated by NJK14013 (Fig. 1D). These results suggest that
NJK14013 differentially interacts with and regulates the ER and AR,
although the molecular basis for these differential effects is not
clear.
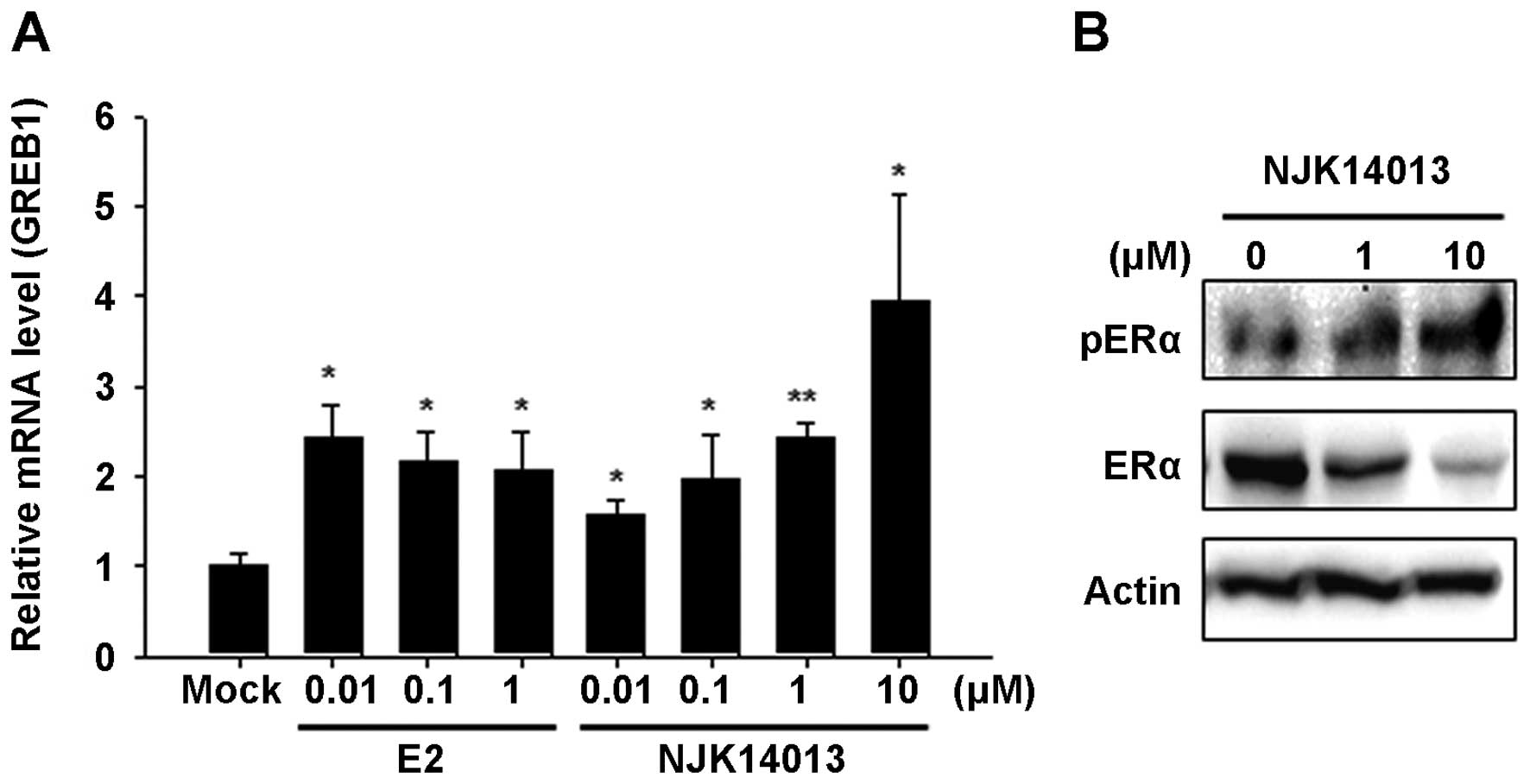
To further confirm the agonistic activity of
NJK14013 on ER transcriptional activity, we analyzed the effects of
NJK14013 on the transcription of GREB1, an ER-responsive
gene. As depicted in Fig. 2A,
treatment with E2 or NJK14013 induced a significant increase in
GREB1 mRNA. Since interaction with estrogen causes the ER to
undergo phosphorylation, which results in ER dimerization and
nuclear translocation, we examined whether NJK14013 affected the
phosphorylation status of the ER. As shown in Fig. 2B, cells treated with NJK14013
showed increased levels of phosphorylated ER (Ser104/106) and
decreased levels of total ER protein.
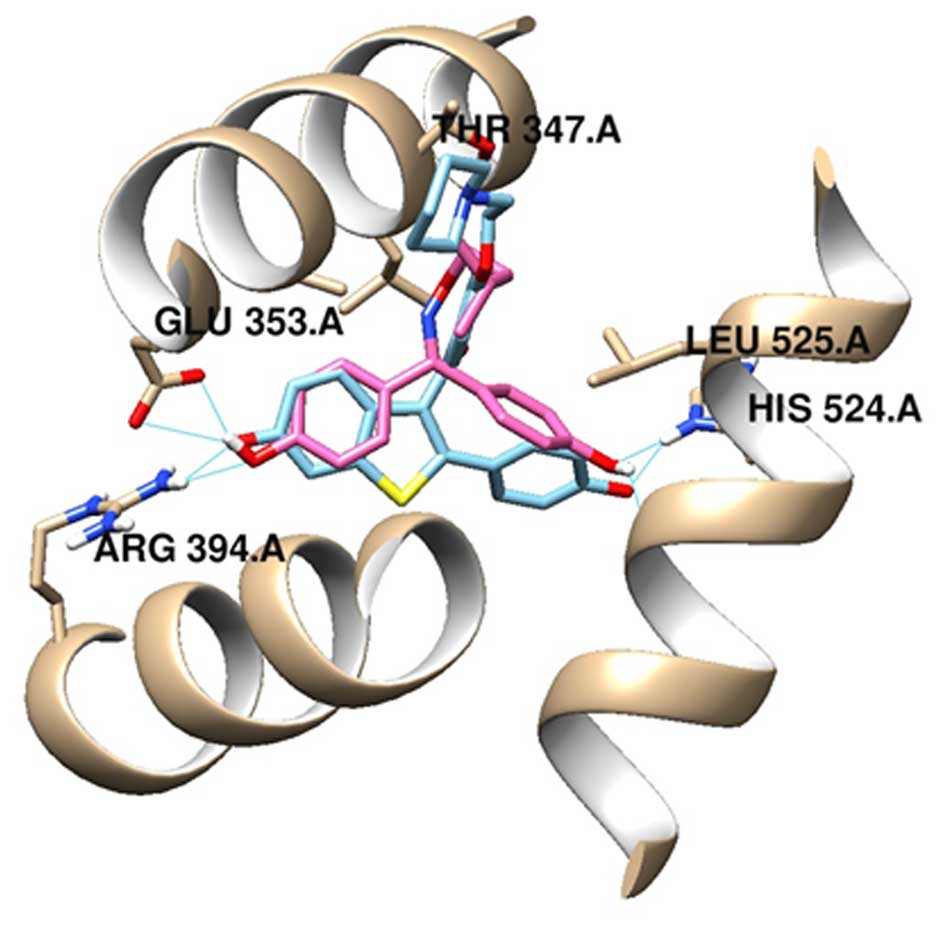
These results suggest that NJK14013 may interact
with ER and modulate its activity. To investigate the binding modes
of NJK14013 in the active site of the ER ligand-binding domain(LBD)
active site, we performed molecular docking studies based on the
human ERα crystal structure (PDB code: 2QXS). As expected, the
compound fit well into the active site, with an estimated free
energy of binding of −8.08 kcal/mol. One of the phenols in NJK14013
interacted with Glu353 of the protein, whereas the other phenol
formed a hydrogen bond with His524. In addition, an isopentyl
tether occupied the hydrophobic region of the pocket. The key
hydrogen bonding interaction and overall binding mode with ERα were
similar to those of raloxifene (Fig.
3).
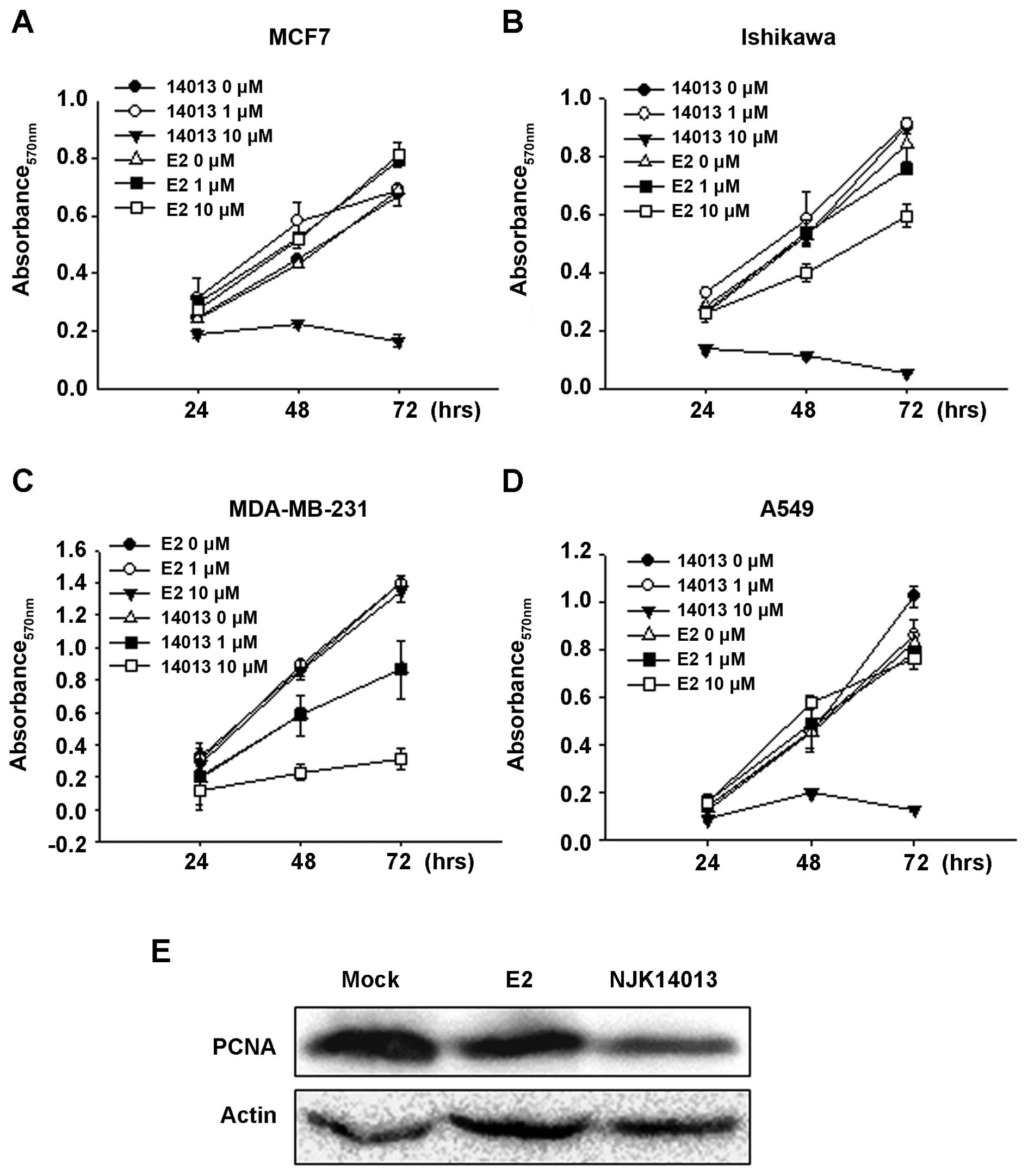
NJK14013 suppresses proliferation of
tumor cells in an ER-independent manner
Next, we tested the effect of NJK14013 on the
proliferation of various cancer cell lines. To our surprise,
treatment with 10 μM NJK14013 clearly suppressed the proliferation
of MCF7 cells; in contrast, the same concentration of E2 did not
significantly affect MCF7 cell proliferation (Fig. 4A). NJK14013 also suppressed the
proliferation of Ishikawa endometrial cancer cells, MDA-MB-231
breast cancer cells, and A549 lung adenocarcinoma cells, indicating
that it is capable of suppressing diverse types of tumor cells
(Fig. 4B–D). Consistent with MTT
assay results, the protein level of proliferating cell nuclear
antigen (PCNA), a well-known proliferation marker, was
significantly decreased by NJK14013 treatment in MCF7 cells
(Fig. 4E). Considering that
MDA-MB-231 is an ER-negative breast cancer cell line, these results
imply that the tumor-suppressive effect of NJK14013 is not
ER-dependent.
NJK14013 induces apoptotic death of tumor
cells
Finally, we tested whether NJK14013 induced tumor
cell death in addition to suppressing tumor cell proliferation
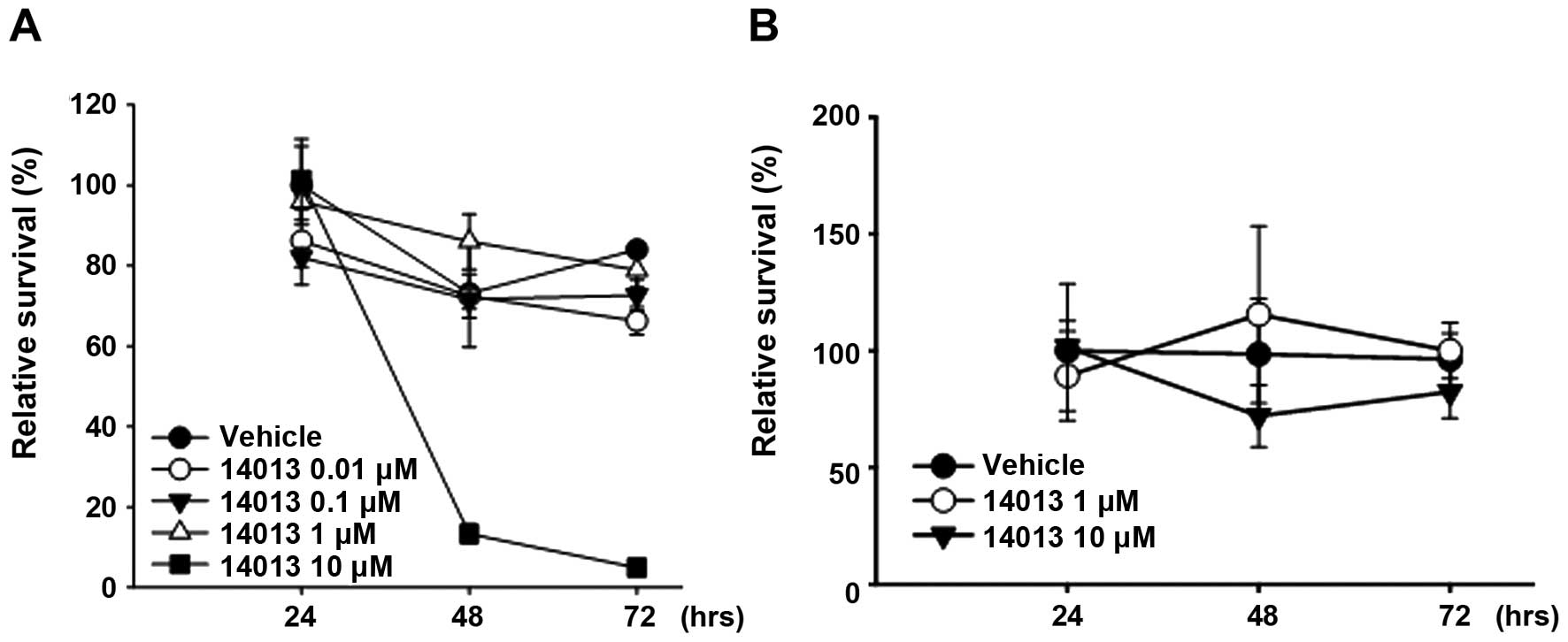
using Ishikawa cells. Unlike E2, treatment with NJK14013 (10 μM)
resulted in 87 and 95% cell death after 48 and 73 h, respectively
(Fig. 5A). In stark contrast, the
same concentrations caused no significant change in the viability
of HUVECs within 72 h after treatment (Fig. 5B).
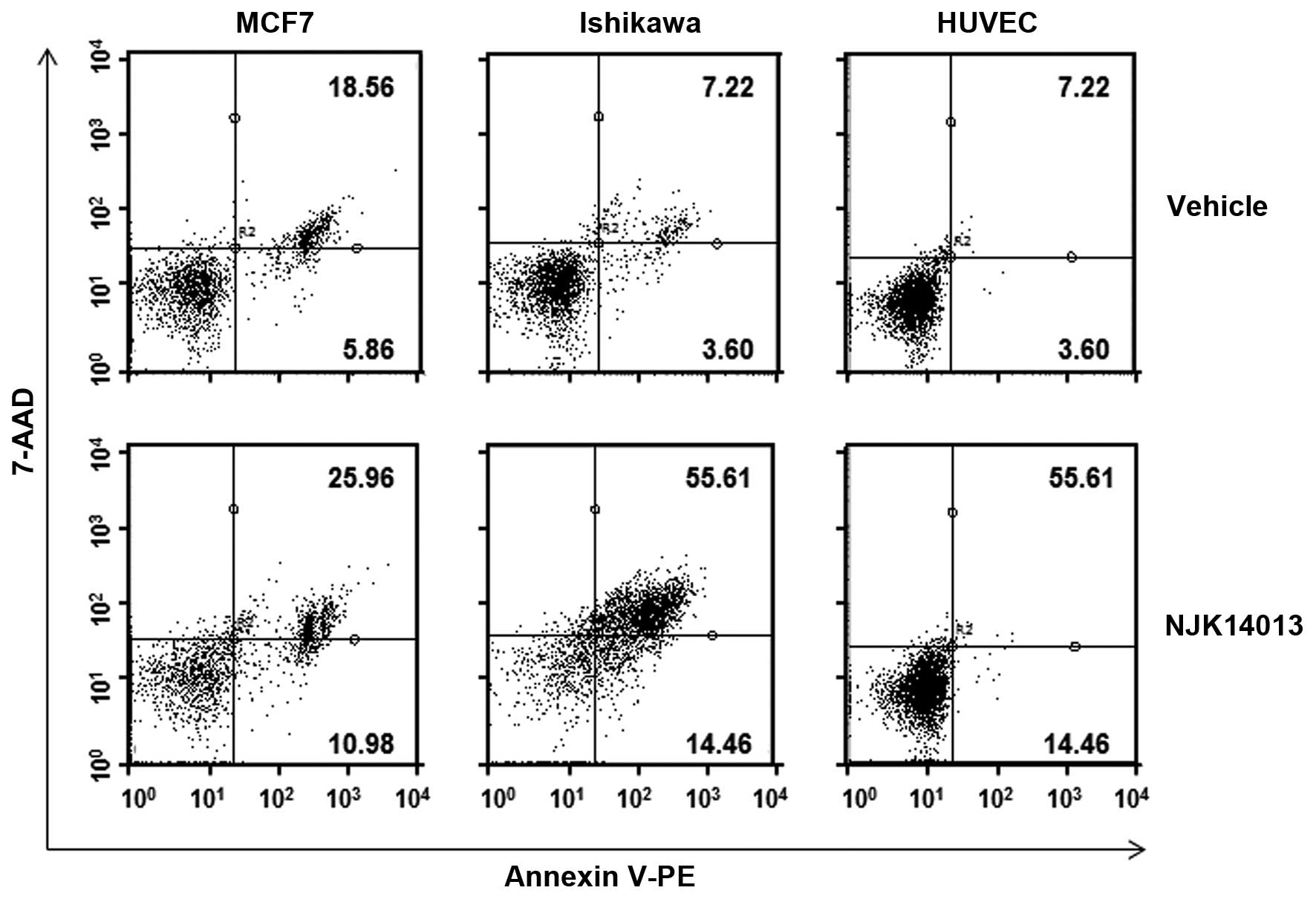
To examine whether NJK14013 is able to cause
apoptotic death of cancer cells, we analyzed MCF7, Ishikawa, and
HUVEC cells by Annexin V/7-AAD assays after treatment with NJK14013
for 48 h. As shown in Fig. 6,
treatment with NJK14013 caused apoptotic cell death in 25.96 and
55.61% of MCF7 and Ishikawa cells, respectively. In contrast,
NJK14013 treatment did not significantly increase apoptosis of
HUVECs, further supporting cell viability data (Fig. 6).
Discussion
Estrogens and SERMs exert various effects on
different types of tumor cells. For instance, tamoxifen displays an
ER antagonistic effect in MCF7 cells and suppresses breast cancer
cell proliferation, whereas it increases the risk of endometrial
cancer. Hormone replacement therapy using estrogen to treat
postmenopausal syndrome and osteoporosis has been associated with
an increased risk of breast cancer. In this study, we sought to
identify biologically active compounds that are capable of
stimulating ER activity while suppressing proliferation of various
tumor cells. We identified NJK14013 as a novel compound which
enhances the transcriptional activity of ERα. The agonistic effect
of NJK14013 on the ER suggests its possible use for
estrogen-deficiency-related conditions, such as osteoporosis.
However, the ER agonistic effect in MCF7 also raises concerns that
NJK14013 might increase the risk of breast cancer or facilitate the
proliferation of cancer cells.
Our results demonstrate that NJK14013 is a novel
SERM capable of inducing apoptotic death in various cancer cell
types. An ER-agonistic role of NJK14013 in MCF7 cells indicates
that NJK14013 may act through a different mechanism than tamoxifen
and raloxifene, which exert ER-antagonistic activity in MCF7 cells.
The SERM actions of NJK14013 in various other tissues, including
bone and endometrium, remain to be determined. Although the
mechanism underlying the anticancer effect of NJK14013 is not
clear, it does not appear to be dependent on its ER-modulating
activity since NJK14013 showed similar effects on ER-negative
cancer cells. Interestingly, a recent study identified ospemifene
derivatives that were cytotoxic to both MCF7 and MDA-MB-231,
despite the fact that ospemifene itself is selective for
ER-positive MCF7; moreover, these compounds were not toxic to
normal mouse embryonic fibroblasts (25). Thus, it is conceivable that some
SERMs may be able to acquire ER-independent, cancer cell-specific
cytotoxicity while retaining ER-modulating activity. Elucidating
the molecular target and underlying cytotoxicity mechanism of
NJK14013 will require further studies.
Collectively, our results suggest the potential use
of NJK14013 as a novel SERM for hormone-replacement therapy with
reduced risk of carcinogenesis or tumor progression. In addition,
its tumor-suppressive effects on various cancer cell types at
concentrations that do not affect the viability of non-cancer cells
(HUVECs) indicate its potential use as an anticancer
therapeutic.
Acknowledgements
This study was supported by Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Science, ICT & Future Planning
(NRF-2012R1A1A1015130) and in part a grant from Kyung Hee
University in 2013 (KHU-20130981).
References
|
1
|
Mirkin S, Archer DF, Pickar JH and Komm
BS: Recent advances help understand and improve the safety of
menopausal therapies. Menopause. 22:351–360. 2015. View Article : Google Scholar
|
|
2
|
Martinkovich S, Shah D, Planey SL and
Arnott JA: Selective estrogen receptor modulators: Tissue
specificity and clinical utility. Clin Interv Aging. 9:1437–1452.
2014.PubMed/NCBI
|
|
3
|
Chedraui P, Pérez-López FR, Hidalgo L,
Villacreses D, Domínguez A, Escobar GS, Genazzani AR and Simoncini
T; Research Group for the Omega Women’s Health Project. Evaluation
of the presence and severity of menopausal symptoms among
postmenopausal women screened for the metabolic syndrome. Gynecol
Endocrinol. Oct 27;2014 (Epub ahead of print). 1–7. 2014.PubMed/NCBI
|
|
4
|
Ismailoğlu O, Oral B, Gorgulu A, Sutcu R
and Demir N: Neuroprotective effects of tamoxifen on experimental
spinal cord injury in rats. J Clin Neurosci. 17:1306–1310. 2010.
View Article : Google Scholar
|
|
5
|
Ishihara Y, Itoh K, Ishida A and Yamazaki
T: Selective estrogen-receptor modulators suppress microglial
activation and neuronal cell death via an estrogen
receptor-dependent pathway. J Steroid Biochem Mol Biol. 145:85–93.
2015. View Article : Google Scholar
|
|
6
|
Pietras RJ: Biologic basis of sequential
and combination therapies for hormone-responsive breast cancer.
Oncologist. 11:704–717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuss JT, Muss HB, Hoen H and Case LD:
Tamoxifen as initial endocrine therapy for metastatic breast
cancer: Long term follow-up of two Piedmont Oncology Association
(POA) trials. Breast Cancer Res Treat. 42:265–274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vogel CL, Johnston MA, Capers C and
Braccia D: Toremifene for breast cancer: A review of 20 years of
data. Clin Breast Cancer. 14:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Viedma-Rodríguez R, Baiza-Gutman L,
Salamanca-Gómez F, Diaz-Zaragoza M, Martínez-Hernández G, Ruiz
Esparza-Garrido R, Velázquez-Flores MA and Arenas-Aranda D:
Mechanisms associated with resistance to tamoxifen in estrogen
receptor-positive breast cancer (review). Oncol Rep. 32:3–15.
2014.PubMed/NCBI
|
|
10
|
Kurebayashi J: Endocrine-resistant breast
cancer: Underlying mechanisms and strategies for overcoming
resistance. Breast Cancer. 10:112–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Decensi A, Maisonneuve P, Rotmensz N,
Bettega D, Costa A, Sacchini V, Salvioni A, Travaglini R, Oliviero
P, D’Aiuto G, et al; Italian Tamoxifen Study Group. Effect of
tamoxifen on venous thromboembolic events in a breast cancer
prevention trial. Circulation. 111:650–656. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonneterre J, Thürlimann B, Robertson JF,
Krzakowski M, Mauriac L, Koralewski P, Vergote I, Webster A,
Steinberg M and von Euler M: Anastrozole versus tamoxifen as
first-line therapy for advanced breast cancer in 668 postmenopausal
women: Results of the Tamoxifen or Arimidex Randomized Group
Efficacy and Tolerability study. J Clin Oncol. 18:3748–3757.
2000.PubMed/NCBI
|
|
13
|
Nagy E, Gajjar KB, Patel II, Taylor S,
Martin-Hirsch PL, Stringfellow HF, Martin FL and Phillips DH: MGMT
promoter hypermethylation and K-RAS, PTEN and TP53 mutations in
tamoxifen-exposed and non-exposed endometrial cancer cases. Br J
Cancer. 110:2874–2880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Avis NE, Crawford SL and McKinlay SM:
Psychosocial, behavioral, and health factors related to menopause
symptomatology. Womens Health. 3:103–120. 1997.PubMed/NCBI
|
|
15
|
Buster JE: Transdermal menopausal hormone
therapy: Delivery through skin changes the rules. Expert Opin
Pharmacother. 11:1489–1499. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jordan VC: The new biology of
estrogen-induced apoptosis applied to treat and prevent breast
cancer. Endocr Relat Cancer. 22:R1–R31. 2015. View Article : Google Scholar
|
|
17
|
Kelly JP, Kaufman DW, Rosenberg L, Kelley
K, Cooper SG and Mitchell AA: Use of postmenopausal hormone therapy
since the Women’s Health Initiative findings. Pharmacoepidemiol
Drug Saf. 14:837–842. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al; Writing Group for the Women’s Health
Initiative Investigators. Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results From
the Women’s Health Initiative randomized controlled trial. JAMA.
288:321–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chlebowski RT, Hendrix SL, Langer RD,
Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG,
Thomson CA, et al: Influence of estrogen plus progestin on breast
cancer and mammography in healthy postmenopausal women: The Women’s
Health Initiative Randomized Trial. JAMA. 289:3243–3253. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Craig Jordan V, McDaniel R, Agboke F and
Maximov PY: The evolution of nonsteroidal antiestrogens to become
selective estrogen receptor modulators. Steroids. 90:3–12. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
DeGregorio MW, Zerbe RL and Wurz GT:
Ospemifene: A first-in-class, non-hormonal selective estrogen
receptor modulator approved for the treatment of dyspareunia
associated with vulvar and vaginal atrophy. Steroids. 90:82–93.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim HI, Quan FS, Kim JE, Lee NR, Kim HJ,
Jo SJ, Lee CM, Jang DS and Inn KS: Inhibition of estrogen signaling
through depletion of estrogen receptor alpha by ursolic acid and
betulinic acid from Prunella vulgaris var. lilacina. Biochem
Biophys Res Commun. 451:282–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Morris GM, Huey R, Lindstrom W, Sanner MF,
Belew RK, Goodsell DS and Olson AJ: AutoDock4 and AutoDockTools4:
Automated docking with selective receptor flexibility. J Comput
Chem. 30:2785–2791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pettersen EF, Goddard TD, Huang CC, Couch
GS, Greenblatt DM, Meng EC and Ferrin TE: UCSF Chimera - a
visualization system for exploratory research and analysis. J
Comput Chem. 25:1605–1612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaur G, Mahajan MP, Pandey MK, Singh P,
Ramisetti SR and Sharma AK: Design, synthesis and evaluation of
Ospemifene analogs as anti-breast cancer agents. Eur J Med Chem.
86:211–218. 2014. View Article : Google Scholar : PubMed/NCBI
|