Introduction
Apoptosis is one of the major mechanisms of cell
death in response to cancer therapy (1). The regulatory balance of apoptosis is
set by interactions on the outer mitochondrial membrane between
members of three distinct subgroups of the BCL-2 (B-cell
CLL/lymphoma 2) protein family: i) the BH3-only proteins, which
mediate signals to initiate apoptosis, ii) the pro-apoptotic
effector proteins BAX (BCL-2-associated X protein) and BAK
(BCL-2 antagonist/killer) and iii) the pro-survival family
members such as BCL-2 and Bcl-xL (BCL2-like 1) (2). Bcl-xL is upregulated in a broad range
of human cancers including bladder cancer (BCa) (3,4). An
overexpression of anti-apoptotic proteins such as Bcl-xL in BCa and
other tumor entities is associated with disease maintenance and
progression, resistance to chemotherapy, and poor clinical outcome
(5).
BCa is the most common malignancy of the urinary
tract and the seventh most prevalent cancer worldwide (6). At first diagnosis, 75% of the BCa are
non-muscle invasive bladder cancers (NMIBC), which can be managed
with transuretheral resection (TUR) by removing all visible lesions
followed by an intravesical therapy (7). Recurrence rates in patients with
NMIBC range from 31 to 78% within 5 years from diagnosis in the
low-risk and high-risk subgroups, respectively (8). The remaining 25% of the BCa grow
invasively and are grouped as muscle-invasive bladder cancers
(MIBC). The standard treatment for locally advanced tumors is a
radical cystectomy combined with a cisplatin-based chemotherapy
where necessary (7). However, the
risk of recurrence and progression remains considerable and
requires additional and improved therapy strategies.
Resistance against chemotherapeutics such as
cisplatin (cis-diamminedichloroplatinum (II); CDDP) is a
major problem in the treatment of BCa and is caused by the
upregulation of anti-apoptotic genes such as Bcl-xL (9,10).
Therefore, the knockdown of such genes by nucleic acid-based
expression inhibitors could potentially sensitize BCa cells towards
chemotherapy (10–12). Antisense oligodeoxynucleotides
(AS-ODNs), which depend on ribonuclease H-mediated cleavage of the
mRNA, as well as RNA interference triggered by small interfering
RNA molecules (siRNAs) act in a sequence-specific manner and can
cause efficient knockdown of specific target genes (12,13).
It has previously been shown that AS-ODNs (14,15)
or siRNAs (16) directed at Bcl-xL
can cause an efficient downregulation of Bcl-xL expression in
different tumor models. Furthermore, Lebedeva et al
demonstrated that a stable overexpression of Bcl-xL in T24 BCa
cells desensitized these cells to different cytotoxic agents and a
subsequent AS-ODN-mediated Bcl-xL inhibition improved chemotherapy
effectiveness (11).
However, the described Bcl-xL-directed AS-ODNs alone
did not promote a significant inhibition of viability of BCa cells
(11,17,18).
For this reason, alternative AS-ODNs with a potentially higher
inhibitory efficiency would be preferable. Their therapeutic
potential should be evaluated in comparison to siRNAs, which
represent an expression inhibitor type with assumed higher and more
specific inhibitory effects (12).
Therefore, the aim of this study was to sensitize BCa cells to the
commonly used chemotherapeutic CDDP in order to increase its
cytotoxic efficacy by a specific pretreatment with AS-ODNs or siRNA
constructs directed at the Bcl-xL mRNA. For this purpose we
systematically designed new AS-ODNs for Bcl-xL knock-down in the
BCa cell line UM-UC-3 representing an NMBIC cellular model and in
the BCa cell line EJ28 originating from a MIBC and compared their
effects to a previously characterized siRNA (16). Analyses of target expression, cell
viability and apoptosis should reveal the effectiveness of the
Bcl-xL downregulation by these expression inhibitors with regard to
a chemosensitization of BCa cells.
Materials and methods
Design and selection of AS-ODN and siRNA
sequences
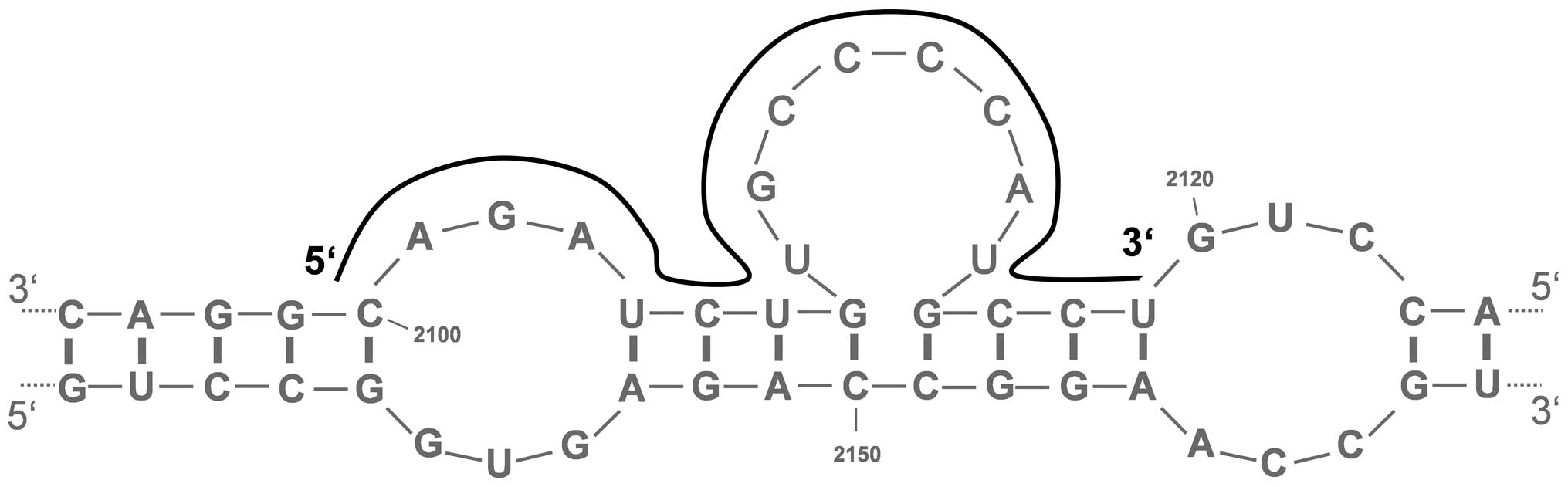
The secondary structure of the Bcl-xL mRNA
(accession no. NM_138578) was predicted using the mfold 3.6
software (http://mfold.rna.albany.edu/?q=mfold). The ten most
probable and stable structures with the lowest free energies (ΔG)
were calculated and screened for potential single-stranded (ss)
regions with at least six unpaired nucleotides. The conservation of
the ss-motifs was calculated as percentage. Putative sites with a
conservation of ≥40% were used as target sequences for the AS-ODN
design (Table I). In addition, the
software sfold 2.2 (http://sfold.wadsworth.org/cgi-bin/index.pl) generated
a probability profile with predicted accessible sites for AS-ODN
hybridization on the target mRNA. Compared to the sequences
predicted with mfold the best conserved motifs were selected.
Furthermore, AS-ODNs were selected based on a low binding energy, a
GC content between 40 and 60% and the avoidance of GGGG motifs. A
BLAST database search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was performed
to exclude homologies to human coding RNA sequences. The eight
finally selected nucleotide sequences of the AS-ODNs are shown in
Table I.
 | Table IDesignations and target sequences of
AS-ODNs against Bcl xL. |
Table I
Designations and target sequences of
AS-ODNs against Bcl xL.
| mfold | sfold | AS-ODN
design |
|---|
|
|
|
|---|
| Ma | Predicted
single-stranded mRNA-regiona | Cb (%) | Mc | Target
sequencec (5′-3′) | AS-ODNd | AS-ODN sequence
(5′-3′) | GC Content (%) |
|---|
| 172–177 | ACCUGU | 40 | 168–187 |
CCCGACCUGUGAUACAAAAG | BX168 |
CTTTTGTATCACAGGTCGGG | 50 |
| 723–730 |
AUAUCAGA | 70 | 720–739 |
AGCAUAUCAGAGCUUUGAAC | BX720 |
GTTCAAAGCTCTGATATGCT | 40 |
| 994–1000 | UUCAACC | 100 | 995–1014 |
UCAACCGCUGGUUCCUGACG | BX995 |
CGTCAGGAACCAGCGGTTGA | 60 |
| 1469–1481 |
CUUUGUUUUGAU | 70 | 1472–1491 |
UGUUUUGAUGUUUGUGGCCU | BX1472 |
AGGCCACAAACATCAAAACA | 40 |
| 1678–1685 |
AAUGUCCU | 60 | 1675–1694 |
CCAAAUGUCCUCCAGAAGCC | BX1675 |
GGCTTCTGGAGGACATTTGG | 55 |
| 1843–1851 |
GGCCCAAGA | 70 | 1845–1864 |
CCCAAGACAGAUGCCCCAGA | BX1845 |
TCTGGGGCATCTGTCTTGGG | 60 |
| 2040–2049 |
AGAGCCUGCU | 70 | 2034–2053 |
GGAAGGAGAGCCUGCUGCAU | BX2034 |
ATGCAGCAGGCTCTCCTTCC | 57 |
| 2108–2115 |
UGCCCCAU | 70 | 2100–2119 |
CAGAUCUGUGCCCCAUGCCU | BX2100 |
AGGCATGGGGCACAGATCTG | 57 |
AS-ODNs were synthesized as unmodified 20mers by
biomers.net (Ulm, Germany). Two additional Bcl-xL-directed AS-ODNs
(20mers) were taken from the literature: 5′-AAAGTATCCCAGCCGCCGTT-3′
(17) and
5′-TCCCGGTTGCTCTGAGACAT-3′ (ASO 15999) (15). The nonsense-ODN NS-K1
(5′-TAAGCTGTTCTATGTGTT-3′) served as control-ODN for normalization
(19). For siRNA treatment the
previously characterized construct si-BX713
(5′-GGGACAGCAUAUCAGAGCU-3′) was selected (16). The non-silencing-siRNA construct
ns-si (reference: SR-CL000-005) from Eurogentec (Liège, Belgium)
served as control.
Cell culture and treatment
procedures
The human BCa cell line UM-UC-3 (ATCC, Rockville,
MD, USA) was cultured in minimum essential medium with 10% fetal
calf serum and 1% non-essential amino acids (all from Life
Technologies, Karlsruhe, Germany). The human BCa cell line EJ28
(University of Frankfurt, Frankfurt, Germany) was cultured in
Dulbecco's modified Eagle's medium with 4.5 g/l glucose, 10% fetal
calf serum and 1% non-essential amino acids (all from Life
Technologies). Cells were cultured at 37°C in humidified atmosphere
containing 5% CO2.
For viability assays, 2,000 UM-UC-3 cells and 1,500
EJ28 cells were seeded per well into 96-well plates. For gene
expression analysis, western blotting and apoptosis detection
150,000 UM-UC-3 cells and 15,000 EJ28 cells were seeded per well
into 6-well plates. Seventy-two hours after seeding, cells were
washed with phosphate-buffered saline (PBS) and transfected with
the AS-ODNs (250 or 500 nM) and siRNAs (40 nM) as well as with the
corresponding controls using the liposomal transfection reagent
DOTAP (Roche, Mannheim, Germany) at a 1:3 (w/w) ratio. The
transfections were performed for 4 h in serum-free OptiMEM medium
(Life Technologies). After transfection the medium was replaced by
fresh culture medium. Cells were harvested from 6-well plates by
trypsin treatment (0.05% trypsin/0.02% EDTA, 5 min, 37°C) 24 h
after start of transfection. For further analyses, detached and
adherent cells were pooled, counted and analyzed together.
Furthermore, a CDDP concentration treatment series
on UM-UC-3 and EJ28 cells should reveal the IC50 values
and optimal CDDP concentrations for the chemosensitization
experiments. According to the dose response curves CDDP
concentrations of 0.25 and 0.5 μg/ml were used for UM-UC-3 cells
and of 0.75 and 1.00 μg/ml for EJ28 cells, respectively. For
chemosensitization experiments, BCa cells were first transfected
with AS-ODNs (500 nM) or siRNAs (40 nM). Twenty-four hours after
transfection start, BCa cells were treated with CDDP for another 24
h. Subsequently, cells were washed with PBS and cultivated with
fresh culture medium for another 24 h. Analyses were performed 72 h
after initiation of the transfection.
Expression analysis at RNA and protein
level
Total RNA was isolated from ≤5×106 cells
using the InviTrap Spin Cell RNA Mini kit according to the
manufacturer's instructions (Invitek, Berlin, Germany). SuperScript
II Reverse Transcriptase (Life Technologies) and random hexamer
primers (Amersham Biosciences, Freiburg, Germany) were used for the
reverse transcription of 500 ng total RNA into first-strand cDNA
according to the manufacturer's instructions. Transcript amounts of
Bcl-xL and the reference gene TATA box binding protein (TBP) were
determined by quantitative PCR (qPCR) using the LightCycler system
(Roche). For Bcl-xL mRNA quantitation a target-specific real-time
reagent mix from AJ Roboscreen (Leipzig, Germany) containing the
appropriate primers and a TaqMan probe was applied. For TBP the
forward primer 5′-GAATATAATCCCAAGCGGTTTG-3′, the reverse primer
5′-TTCACATCACAGCTCCCC-3′ and hybridization probes labeled with
fluorescein (5′-TTTCCCAGAACTGAAAATCAGTGCC-FL-3′) and LC Red640
(5′-LC-TGGTTCGTGGCTCTCTTATCCTCATG-PH-3′) were used. The relative
transcript levels of Bcl-xL normalized to TBP were used for
statistical calculations.
For western blot analysis
5×104–1×105 cells per sample were lysed in
loading buffer, incubated at 95°C for 5 min and separated on a
4–12% sodium dodecyl sulfate-polyacrylamide gel. Western blot
analysis was performed according to a standard protocol using a
monoclonal mouse anti-human Bcl-xL antibody (clone 2D1, OriGene
Technologies, Rockville, MD, USA) at a 1:100 dilution. β-actin
detected by a monoclonal mouse anti-human β-actin antibody (AC-74,
Sigma-Aldrich, Steinheim, Germany) at a 1:50,000 dilution served as
a loading control. As secondary antibody a polyclonal rabbit
anti-mouse antibody conjugated with horseradish peroxidase (Dako,
Glostrup, Denmark) was used and an enhanced chemiluminescence kit
(Life Technologies) was employed for visualization.
Viability assays
Viability of UM-UC-3 cells was examined by using
crystal violet, which stained the DNA of intact cells. For this
test cells were fixed with 100 μl methanol (Merck KGaA, Darmstadt,
Germany) per well for 10 min. Cells were washed with 100 μl water
and were subsequently stained with 100 μl of a 0.1% crystal violet
solution (AppliChem GmbH, Darmstadt, Germany) in 100% ethanol (VWR,
Darmstadt, Germany) for 10 min. Afterwards, crystal violet was
solubilized in 100 μl 0.1 M sodium citrate (Promega, Mannheim,
Germany) and the absorbance was measured at 590 nm by using the
Mithras LB 940 Multimode Microplate Reader (Berthold Technologies,
Bad Wildbad, Germany). For the BCa cell line EJ28, cellular
viability was analyzed using the cell proliferation reagent WST-1
(Roche). WST-1 reagent (10 μl per well) was added to the cells 72 h
after start of transfection. Color development was monitored for up
to 2 h by measuring the absorbance at 450 and 620 nm (reference).
Furthermore, an alternative assay system based on the activity of a
dead cell protease (Promega) was used according to the
manufacturer's instructions for the determination of viability of
both cell lines. All viability assays were performed in duplicates
or triplicates. The results were normalized to the appropriate
controls NS-K1 and ns-si.
Apoptosis detection
Apoptosis was assessed by Annexin V/propidium iodide
(PI) staining (Annexin V-FITC Apoptosis Detection Kit I; BD
Biosciences, Heidelberg, Germany) 72 h after transfection start
using flow cytometry (FACScan; BD Biosciences) according to the
manufacturer's instructions. Annexin V staining and PI
counterstaining allows the discrimination and quantification of
early apoptotic cells (Annexin V-positive and PI-negative), late
apoptotic cells (double-positive) and necrotic cells (Annexin
V-negative and PI-positive). Annexin V-FITC/PI (FL1/FL2) plots of
2×104 cells were examined by quadrant analysis using the
Flowing software 2.5.1 (http://www.flowingsoftware.com/).
Statistics
All statistical calculations were performed using
GraphPad Prism 5.03 (GraphPad Software, Inc., La Jolla, CA, USA).
All results were presented as mean ± standard error of the mean
(SEM). Data from the treatment groups AS-ODN vs NS-K1, siRNA vs
ns-si, CDDP vs untreated, AS-ODN+CDDP vs NS-K1+CDDP and siRNA+CDDP
vs ns-si+CDDP were compared using ANOVA followed by Bonferroni's
correction. A p-value <0.05 was considered as statistically
significant.
Synergistic effects were calculated by the formula
X=[AB]/([A]+[B]), where [A] is the single treatment with AS-ODN or
siRNA and [B] the single treatment with CDDP. [AB] represents the
combination treatment with AS-ODN or siRNA and CDDP. Synergy is
defined as X >1, additivity is when X=1, and antagonism is when
X <1.
Results
Selection of AS-ODN and siRNA constructs
in UM-UC-3 cells
The prediction of the secondary structure of the
Bcl-xL mRNA using the mfold 3.6 software revealed the existence of
several putative single-stranded regions. From ten theoretical and
most stable mRNA structures, eight promising regions with a degree
of conservation of ≥40% were selected for AS-ODN design. These
target sequences in the Bcl-xL mRNA, which were also predicted by
the sfold 2.2 software, were located at nucleotide positions
168–187 (BX168), 720–739 (BX720), 995–1014 (BX995), 1472–1491
(BX1472), 1675–1694 (BX1675), 1845–1864 (BX1845), 2034–2053
(BX2034) and 2100–2119 (BX2100) (Fig.
1 and Table I). BLAST database
search revealed no homologies to other human mRNAs.
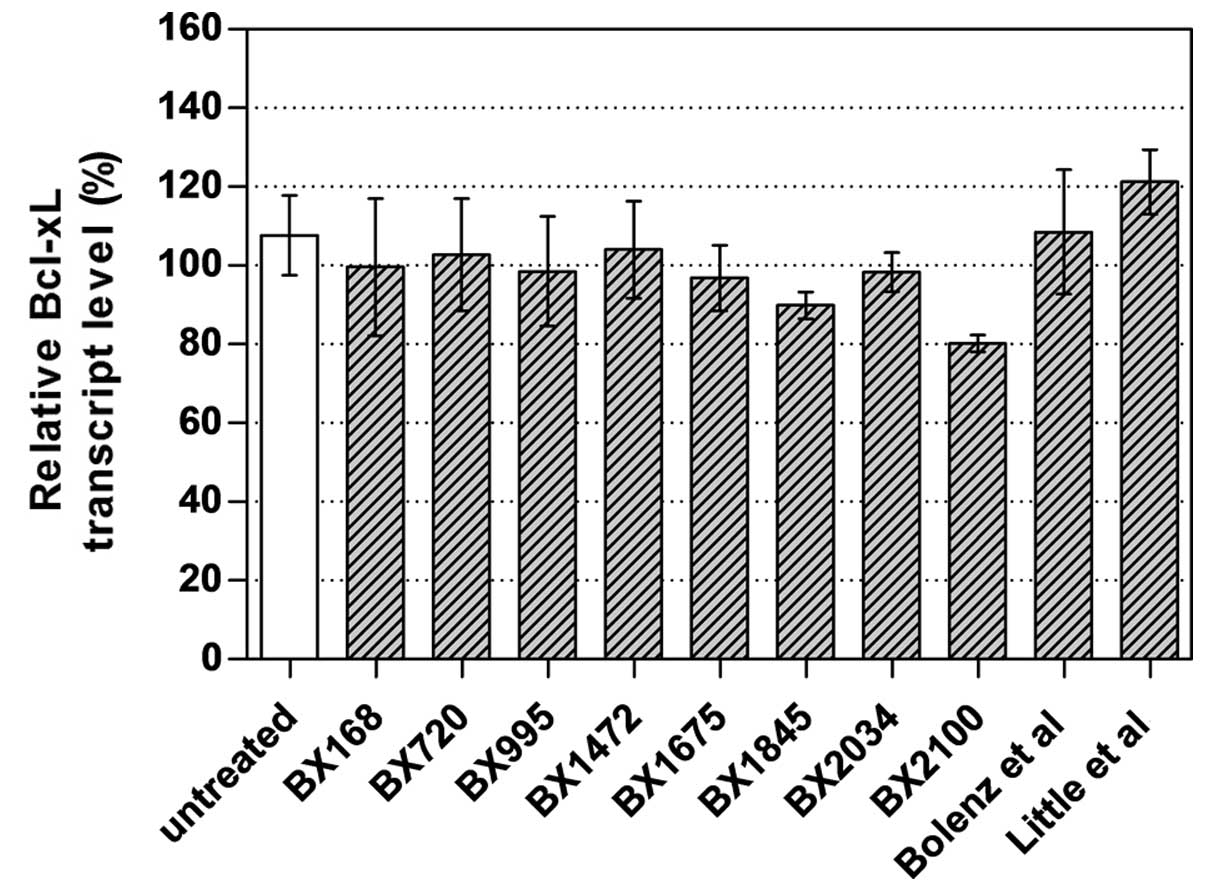
To select the best AS-ODN constructs for further
studies, UM-UC-3 cells were transfected with 250 nM of the newly
designed AS-ODNs and two AS-ODNs from literature. AS-BX2100 showed
the strongest inhibitory effect with a reduction of 19.8% of Bcl-xL
mRNA expression in UM-UC-3 cells (Fig.
2). Therefore, this and another newly designed AS-ODN
(AS-BX2034) were selected for further chemosensitization
experiments. The si-BX713 construct caused a clear reduction of the
Bcl-xL transcript levels by 76% in UM-UC-3 cells (data not
shown).
Selection of CDDP concentrations for
combined treatment
On the basis of the dose-response curves CDDP
concentrations inducing only a moderate inhibition of cellular
viability were selected. Ultimately, 0.25 μg/ml (CDDP1) and 0.5
μg/ml CDDP (CDDP2) where used for UM-UC-3 cells, whereas 0.75 μg/ml
(CDDP1) and 1.00 μg/ml (CDDP2) were applied for EJ28 cells.
Additionally, IC50 values of 1.49 and 3.23 μg/ml for
CDDP were determined in UM-UC-3 and EJ28 cells, respectively (data
not shown).
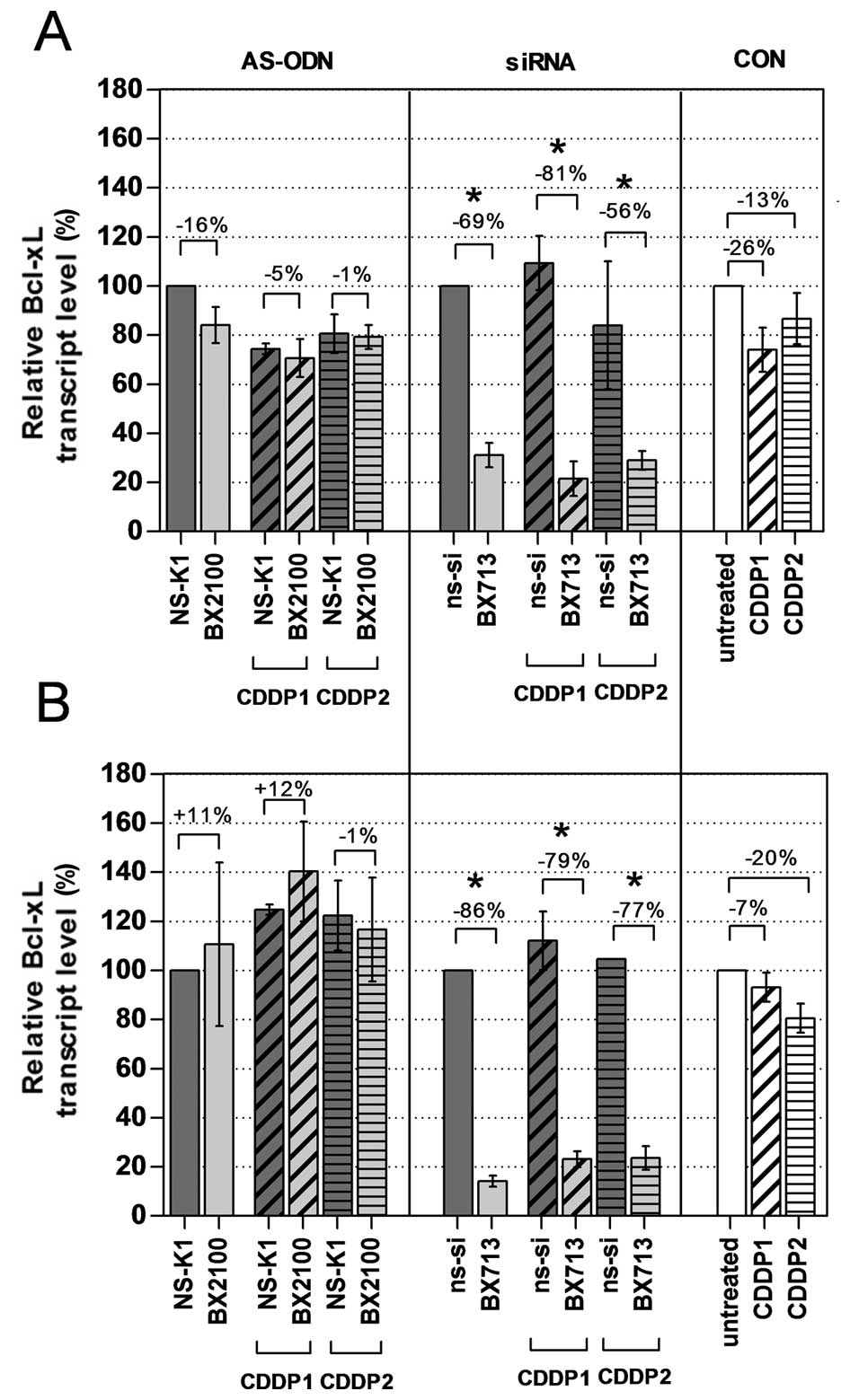
Effects on Bcl-xL mRNA and protein
expression by single and combined treatment
The mRNA and protein expression levels of Bcl-xL in
the BCa cell lines UM-UC-3 and EJ28 were evaluated 72 h after
treatment with AS-ODNs or siRNAs with and without CDDP. A single
treatment with the AS-BX2100 construct only reduced the mRNA
expression level by 16% in UM-UC-3 cells and had no inhibitory
effect on the Bcl-xL transcript level in EJ28 cells compared to
NS-K1 (Fig. 3). In contrast, after
treatment with si-BX713 a clear reduction of the Bcl-xL transcript
levels by 69% in UM-UC-3 cells and by 86% in EJ28 cells, normalized
to the ns-si control, was measured. Specific inhibition rates were
detected by normalizing the combination therapies of AS-BX2100+CDDP
or si-BX713+CDDP to the appropriate control treatments NS-K1+CDDP
or ns-si+CDDP, respectively. Treatments combining AS-BX2100 with
CDDP1 or CDDP2 caused only little, or no effect on Bcl-xL
expression in UM-UC-3 and EJ28 cells (Fig. 3). In contrast, a combined treatment
with si-BX713 and CDDP caused a remarkable reduction of Bcl-xL
transcript levels in both BCa cell lines. Inhibition rates were 81
and 56% (CDDP1/2) in UM-UC-3 cells and 79 and 77% (CDDP1/2) in EJ28
cells.
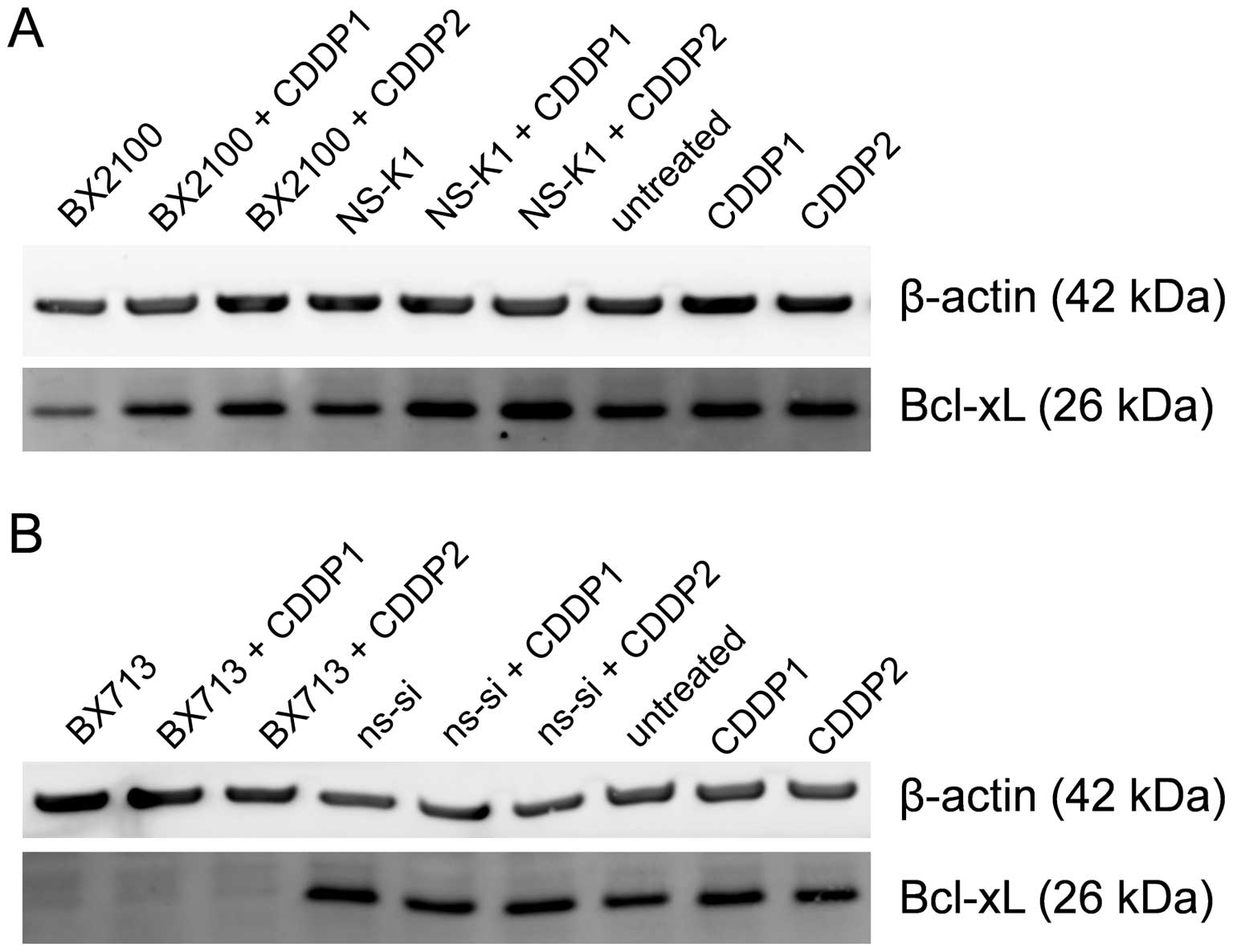
Western blot analysis showed that AS-BX2100 alone
and in combination with CDDP only marginally reduced the Bcl-xL
protein level in EJ28 cells compared to the respective control
treatments (Fig. 4A). In contrast,
a single treatment with si-BX713 as well as the combination with
CDDP caused a complete inhibition of Bcl-xL protein expression
compared to ns-si alone and ns-si+CDDP, respectively (Fig. 4B). In UM-UC-3 cells, no reduction
at the Bcl-xL protein level following treatment with AS-BX2100
alone or in combination with CDDP was detectable (data not shown).
However, the Bcl-xL protein level was moderately diminished after
si-BX713 mono-treatment as well as combined treatment with CDDP
(data not shown).
Effects on cellular viability by single
and combined treatment
The inhibition of viability of the BCa cell lines
was assessed by using crystal violet (UM-UC-3) and the WST-1 assay
(EJ28) 72 h after start of transfection with AS-ODNs or siRNA with
and without CDDP. Compared to the corresponding controls, a
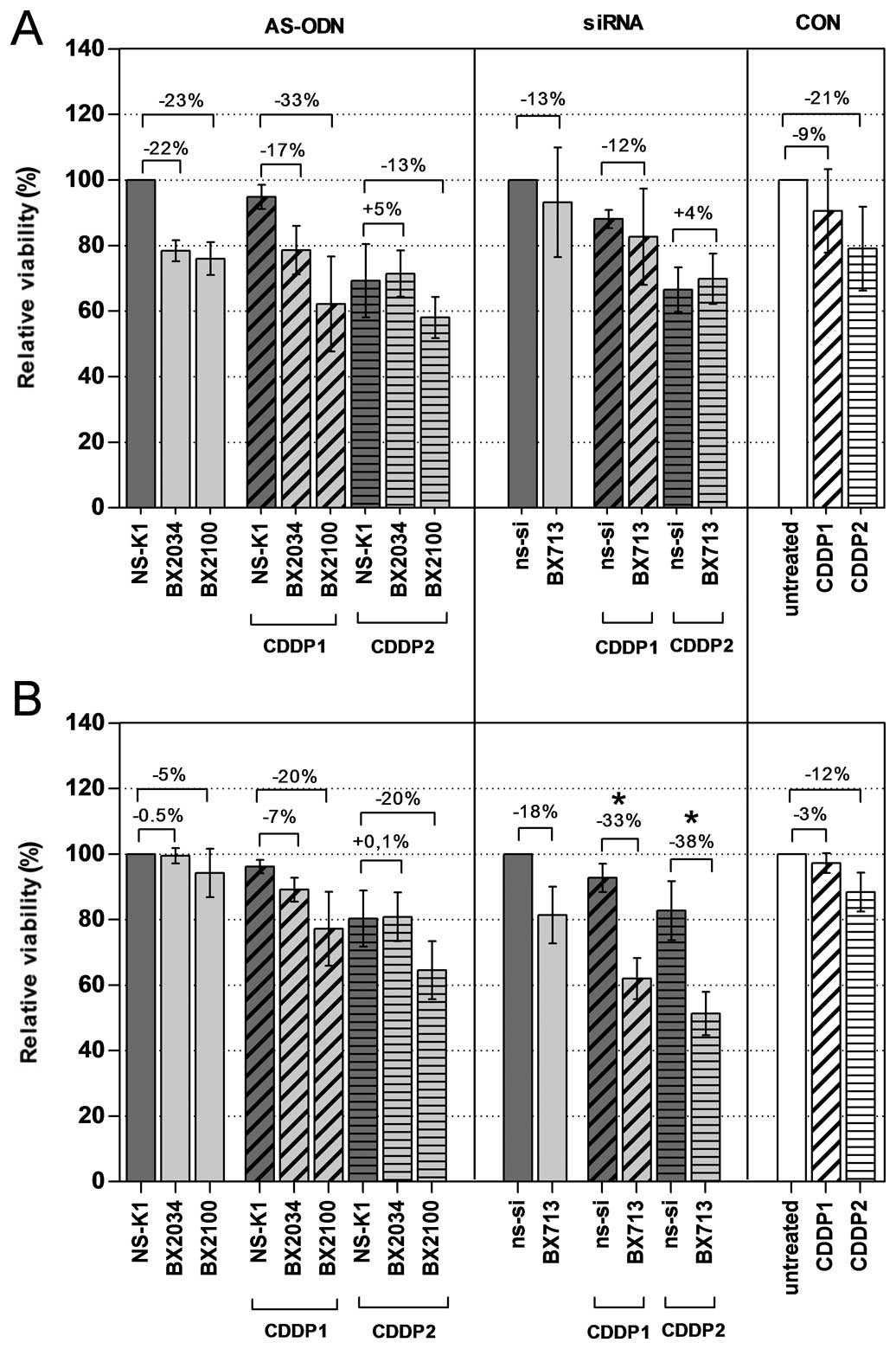
reduction of viability by 22, 23 and 13% in UM-UC-3 cells and by
0.5, 5 and 18% in EJ28 cells by single treatments with AS-BX2034,
AS-BX2100 and si-BX713, respectively, were observed (Fig. 5 and Table II). Treatments combining AS-ODNs
with CDDP caused a moderate inhibition of cellular viability
compared to NS-K1+CDDP in both cell lines. For example, a reduction
of 33 and 13% (CDDP1/2) in UM-UC-3 cells and 20 and 20% (CDDP1/2)
in EJ28 cells was observed after combinatory treatment with
AS-BX2100. A combined treatment of si-BX713 and CDDP had only
little or no effects in UM-UC-3 cells compared to ns-si+CDDP. In
contrast, in EJ28 cells significant differences were observed for
the treatment groups si-BX713+CDDP1 vs ns-si+CDDP1 and
si-BX713+CDDP2 vs ns-si+CDDP2 with an additional inhibition of
cellular viability of 33 and 38%, respectively (Fig. 5 and Table II).
 | Table IICalculation of potential synergistic
effects on cellular function of UM-UC-3 and EJ28 cells after
AS-ODN/CDDP or siRNA/CDDP treatment.a |
Table II
Calculation of potential synergistic
effects on cellular function of UM-UC-3 and EJ28 cells after
AS-ODN/CDDP or siRNA/CDDP treatment.a
| Inhibition of
viabilityb (%) | Inhibition of
viabilityc (%) | Induction of
apoptosis (%) |
|---|
|
|
|
|
|---|
| UM-UC-3 | EJ28 | UM-UC-3 | EJ28 | UM-UC-3 | EJ28 |
|---|
| CDDP1 + NS-K1 | 5.2±4.9 | 3.8±2.8 | 0.4±5.2 | 28.3±0.2 | 29.3±23.3 | −21.0±3.1 |
| CDDP2 + NS-K1 | 30.7±13.0 | 19.7±10.8 | 26.5±1.7 | 44.8±1.0 | 37.7±53 | 20.5±4.6 |
| AS-BX2034
alone | 21.6±3.8 | 0.5±2.9 | −5.1±8.7 | 2.6±0.5 | ND | ND |
| EE for CDDP1 | 26.7 | 4.4 | −4.7 | 30.9 | ND | ND |
| ME for CDDP1 | 21.4±9.1 | 10.9±4.3 | 3.4±0.8 | 31.5±4.0 | ND | ND |
|
n-fold increase | 0.8 | 2.5 | −0.7 | 1.0 | ND | ND |
| EE for CDDP2 | 52.3 | 20.2 | 24.1 | 47.4 | ND | ND |
| ME for CDDP2 | 28.5±9.2 | 19.2±8.7 | 27.4±2.5 | 46.0±5.9 | ND | ND |
|
n-fold increase | 0.5 | 1.0 | 1.4 | 1.0 | ND | ND |
| AS-BX2100
alone | 24.0±6.0 | 5.7±9.7 | 4.0±23.7 | 32.6±1.1 | 32.3±13.8 | 26.1±0.4 |
| EE for CDDP1 | 29.1 | 9.6 | 21.4 | 60.8 | 61.5 | 5.1 |
| ME for CDDP1 | 37.8±19.2 | 22.8±14.7 | 32.7±3.7 | 57.2±3.1 | 78.8±52.2 | 13.7±34.8 |
|
n-fold increase | 1.3 | 2.4 | 1.3 | 0.9 | 1.3 | 2.7 |
| EE for CDDP2 | 54.7 | 25.4 | 50.2 | 77.3 | 69.9 | 64.7 |
| ME for CDDP2 | 41.9±8.3 | 35.5±11.8 | 51.4±2.3 | 72.4±1.8 | 109.7±51.4 | 59.7±32.5 |
|
n-fold increase | 0.8 | 1.4 | 1.0 | 0.9 | 1.6 | 1.3 |
| CDDP1 + ns-si | 11.9±3.7 | 7.3±5.7 | 15.4±1.8 | 37.3±3.4 | 58.2±50.1 | −15.8±2.4 |
| CDDP2 + ns-si | 33.4±8.8 | 17.3±10.5 | 32.2±0.1 | 40.9±5.1 | 45.3±31.3 | −6.2±15.8 |
| si-BX713 alone | 6.8±21.8 | 18.6±11.3 | 10.9±0.2 | 29.9±6.3 | −8.7±17.8 | 36.2±14.4 |
| EE for CDDP1 | 18.7 | 25.9 | 26.3 | 67.3 | 49.5 | 20.3 |
| ME for CDDP1 | 17.3±18.7 | 38.0±8.0 | 35.8±1.7 | 71.8±1.1 | 52.1±55.9 | 79.8±3.4 |
|
n-fold increase | 0.9 | 1.5 | 1.4 | 1.1 | 1.1 | 3.9 |
| EE for CDDP2 | 40.3 | 35.9 | 43.1 | 70.9 | 36.6 | 30.0 |
| ME for CDDP2 | 30.1±10.2 | 48.7±7.7 | 62.8±1.3 | 82.1±0.7 | 94.1±37.2 | 104.7±6.5 |
|
n-fold increase | 0.7 | 1.4 | 1.5 | 1.2 | 2.6 | 3.5 |
The combination of AS-BX2034, AS-BX2100 or si-BX713
with CDDP led to synergistic effects on viability mainly in EJ28
cells measured by WST-1 assay (Table
II). Compared to the expected additive effects calculated from
individual treatments the actual inhibition of cellular viability
of EJ28 cells was increased by 2.5-fold for the combination
AS-BX2034+CDDP1, by 2.4-fold for the combination of AS-BX2100+CDDP1
and by 1.5-fold for the combination of si-BX713+CDDP1.
Interestingly, the lower CDDP concentration led to stronger
synergistic effects. In UM-UC-3 cells treatments combining AS-ODNs
or siRNAs with CDDP caused no further increasing effect on
viability measured by crystal violet (Table II).
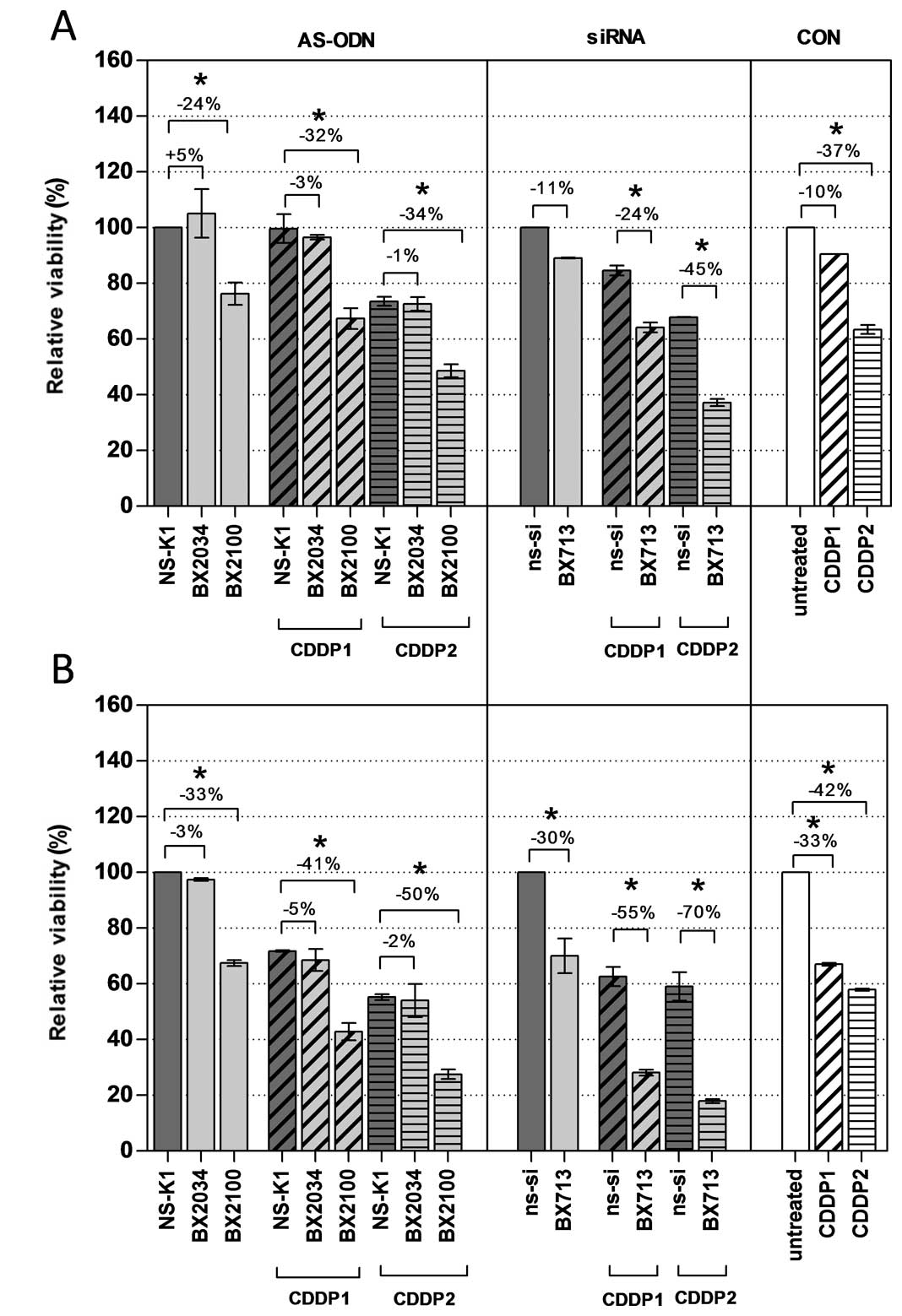
Additional analysis of cellular viability based on
the measurement of the dead cell protease activity should
independently reveal the effectiveness of the AS-ODN and siRNA
constructs 72 h after initiation of the transfection (Fig. 6). A single treatment with AS-BX2034
had no effect on cell viability in UM-UC-3 and EJ28 cells compared
to NS-K1. In contrast, AS-BX2100 alone significantly reduced cell
viability by 24 and 33% in UM-UC-3 and EJ28 cells, respectively. A
combined treatment of AS-BX2034 and CDDP caused no clear reduction
of viability in the cell lines. In contrast, all combinations of
AS-BX2100 with CDDP significantly reduced cell viability in both
BCa cell lines whereupon the inhibition was generally stronger in
EJ28 cells (Fig. 6). A single
treatment with si-BX713 inhibited cell viability by 11 and 30% in
UM-UC-3 and EJ28 cells, respectively. Inhibition rates of si-BX713
combined with CDDP were 24 and 45% (CDDP1/2) in UM-UC-3 cells and
55 and 70% (CDDP1/2) in EJ28 cells (all p<0.5). Compared to the
added single effects the actual inhibition of cellular viability in
UM-UC-3 and EJ28 cells was synergistically increased by 1.5- and
1.2-fold for the combination of si-BX713+CDDP2 in UM-UC-3 and EJ28
cells, respectively (Table
II).
Effects on apoptosis by single and
combined treatment
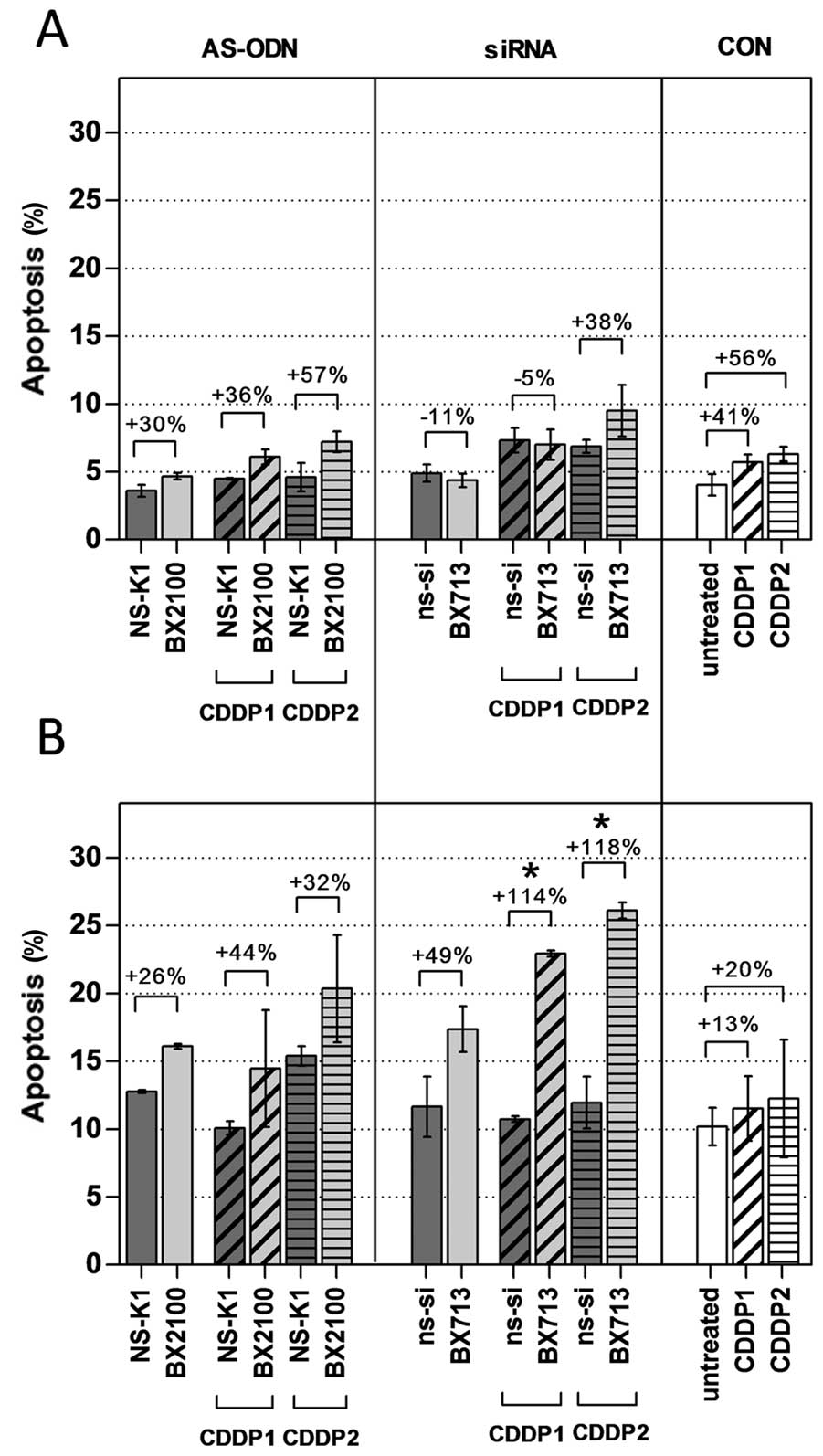
Induction of apoptosis was measured 72 h after the
start of the transfection in UM-UC-3 and EJ28 cells by an Annexin
V-FITC/PI double staining. Rates of apoptosis were generally higher
in EJ28 cells than in UM-UC-3 cells (Fig. 7). Treatment with AS-BX2100 with and
without CDDP induced a moderate, but no significant increase in
apoptosis by 26–57% normalized to the corresponding controls in
both cell lines. This was also seen for siRNA treatment in UM-UC-3
cells. In contrast, a combined therapy with si-BX713 and CDDP
produced a significant effect on apoptosis in EJ28 cells. A single
treatment with si-BX713 induced an increase of apoptosis by 49%,
whereas the combination with CDDP1 and CDDP2 led to a significant
enhancement by 114 and 115%, respectively, compared to the controls
(Fig. 7). This observation was in
agreement with the synergistic enhancement of 3.5- to 3.9-fold
calculated for the combined treatments in EJ28 cells (Table II).
Discussion
One of the major clinical challenges in the
successful treatment of tumors is the frequently occurring
resistance to commonly applied therapies such as radiation or
chemotherapy. One mechanism by which tumor cells develop resistance
to cytotoxic agents is related to resistance to apoptosis (5). This is often a consequence of the
increased expression of anti-apoptotic proteins such as Bcl-xL
(11,18,20).
Strategies to decrease the cellular expression of such proteins
would enhance chemotherapy effectiveness (9). Therefore, the specific downregulation
of these genes by nucleic acid-based inhibitors like AS-ODNs or
siRNAs represents an attractive approach for molecular based
sensitization (11,21).
Lebedeva et al were able to show in
vitro that a stable, forced overexpression of the
anti-apoptotic protein Bcl-xL in T24 BCa cells led to significant
chemodesensitization (11). In
contrast, the treatment of the BCa cell lines T24 and 5637 by
AS-ODNs caused a clear downregulation of Bcl-xL mRNA and protein
expression and increased their sensitivity to cytotoxic agents.
Interestingly, an AS-ODN treatment in T24 and 5637 cells alone did
not decrease cellular viability, indicating that Bcl-xL might not
be essential for the survival of these tumor cells but for the
regulation of programmed cell death, which is a consequence of
apoptotic stimuli (11).
Furthermore, the inhibitory efficiency of AS-ODNs depended on the
sequences and modifications of the constructs as well as on the
type of delivery agent (11).
However, it was reported in several studies that a treatment of BCa
cells with Bcl-xL-directed AS-ODNs alone did not induce significant
inhibition of viability, whereas a combination with
chemotherapeutics promoted a significant increase of apoptosis in
tumor cell lines as evidence for chemosensitization (11,14,17).
To improve the efficiency of Bcl-xL-targeted AS-ODNs we
systematically designed and characterized new AS-ODNs.
For the design of AS-ODNs a combination of the
prediction tools mfold and sfold was used, which can determine the
most probable occurring secondary structure of target mRNAs with
minimal overall free energy as a potential AS-ODN target site
(22). Furthermore, the
conservation of the ss-motifs, the achievement of an optimal CG
content and a BLAST search for avoidance of unspecific
hybridization was considered in contrast to former studies
(11,23). The AS-ODNs designed by this
strategy were compared to the therapeutic potential of a
Bcl-xL-directed siRNA which was reported to be efficient in
previous studies (16).
The constructs AS-BX2100, AS-BX2034 and si-BX713
were selected for detailed analyses in the BCa cell lines UM-UC-3
and EJ28 to compare their efficacy in enhancing the cytotoxic
effects of the chemotherapeutic agent CDDP. Analysis of changes in
Bcl-xL expression in UM-UC-3 and EJ28 cells treated with si-BX713
with or without CDDP revealed a similar distinct downregulation of
Bcl-xL mRNA expression by 69–86% 72 h after the start of the
transfection. A single and combined treatment of both BCa cell
lines with si-BX713 or si-BX713+CDDP caused a considerably higher
reduction of Bcl-xL mRNA and protein expression (Figs. 3 and 4) compared to AS-BX2100 or
AS-BX2100+CDDP, respectively. Moreover, the treatment of UM-UC-3
and EJ28 cells with si-BX713 caused a stronger reduction of cell
viability as well as a more potent induction of apoptosis compared
to an AS-ODN treatment in nearly all cases. In similar studies,
Fuessel et al also demonstrated a higher efficiency of
siRNAs compared to AS-ODNs directed at the anti-apoptotic survivin,
which clearly reduced target expression and cell viability of EJ28
and 5637 BCa cells (24).
Numerous studies revealed siRNA constructs as
superior tools with significantly lower IC50 values and prolonged
effects in comparison to AS-compounds (13,25,26).
In our studies, si-BX713 showed better inhibition of Bcl-xL mRNA
expression at a lower dosis (40 nM) compared to AS-BX2100 (500 nM)
(Fig. 3). Nevertheless, in our
studies we used an even lower dose of AS-ODNs (500 nM) compared to
other studies that applied higher doses of AS-ODNs of up to 5 μM
(14,18) which in turn could increase the risk
of unspecific effects. Therefore, AS-ODNs and siRNAs must be used
at the lowest effective concentration to minimize unwanted side
activities. In general, there are several advantages of using siRNA
constructs as inhibitors of gene expression (13,27).
The higher stability of siRNA constructs as well as their possible
recycling after target mRNA degradation and thus, the reuse as
catalytic compounds may explain the longer duration of action in
comparison to AS-ODNs (13,26).
Off-target effects are the consequence of non-sequence-specific
downregulation of gene expression. For example, the construct
AS-BX2034 had no influence on the Bcl-xL mRNA expression level but
on the viability of UM-UC-3 cells (Figs. 2 and 6). This might be caused by off-target
effects. Nevertheless, for effective application of nucleic
acid-based inhibitors some of the problems are similar: efficient
delivery, enhanced stability, minimization of off-target effects
and the identification of degradation-sensitive sites in the target
RNAs (13).
In most of the tested combinations, the enhancement
of CDDP effects with regard to inhibition of viability and
induction of apoptosis by a pretreatment with AS-ODNs or siRNAs
were stronger in EJ28 cells compared to UM-UC-3 cells. This
difference might be caused by different basic Bcl-xL expression
levels in these cell lines. The MIBC-derived cell line EJ28
displayed a 10-fold higher basic Bcl-xL mRNA expression level
compared to the NMIBC-derived cell line UM-UC-3 (data not shown).
Hence, it could be possible that an increased expression of Bcl-xL
is associated with advanced BCa stages and more important for cell
survival compared to those cell lines which express Bcl-xL at a
lower level.
Interestingly, a stronger inhibition of viability
was observed for lower CDDP doses (CDDP1) in combination with
Bcl-xL downregulation by AS-BX2100 and AS-BX2034 in EJ28 cells
(Fig. 5 and Table II). It is possible that a
treatment with higher CDDP concentrations (CDDP2) alone could
already reach strong effects on BCa cells and thus, no further
inhibition of cell viability with an AS-ODN pretreatment could be
achieved. An obvious advantage of lower doses of chemo-therapeutic
agents is a lower rate of adverse effects caused by drug toxicity
in patients. In contrast, the pretreatment with the Bcl-xL-directed
siRNA always induced synergistic effects compared to the single
effects of both CDDP doses, possibly originating from its superior
effectiveness and specificity.
In this study, a number of new AS-ODNs was
systematically designed and characterized with regard to the
inhibition of Bcl-xL expression in two different BCa cell lines.
The potential for chemosensitization of the most promising
AS-constructs was compared to a specific siRNA, which induced a
significantly stronger reduction of Bcl-xL expression at mRNA and
protein levels. A combined treatment with siRNA and CDDP led to a
more potent inhibition of cell viability as well as to a stronger
induction of apoptosis compared to AS-BX2100 and CDDP in both cell
lines. Therefore, Bcl-xL might serve as a suitable target depending
on the basic expression levels in the tumor. The application of
siRNAs as gene expression inhibitors and chemosensitizers appears
more promising than AS-ODNs for the treatment of BCa.
Acknowledgements
This study was supported by the German Cancer Aid
(grant no. 109616). Furthermore, the authors are grateful to Kati
Erdmann for her useful advice.
Abbreviations:
|
AS-ODN
|
antisense oligodeoxynucleotides
|
|
BAX
|
BCL-2-associated X protein
|
|
BAK
|
BCL-2 antagonist/killer
|
|
BCa
|
bladder cancer
|
|
Bcl-xL
|
BCL2-like 1
|
|
BCL-2
|
B-cell CLL/lymphoma 2
|
|
CDDP
|
cisplatin
|
|
MIBC
|
muscle invasive bladder cancer
|
|
NMIBC
|
non-muscle invasive bladder cancer
|
|
PBS
|
phosphate-buffered saline
|
|
PI
|
propidium iodide
|
|
qPCR
|
quantitative PCR
|
|
SEM
|
standard error of the mean
|
|
siRNA
|
small interfering RNA
|
|
ss
|
single-stranded
|
|
TBP
|
TATA box binding protein
|
|
TUR
|
transurethral resection
|
References
|
1
|
Luo J, Solimini NL and Elledge SJ:
Principles of cancer therapy: Oncogene and non-oncogene addiction.
Cell. 136:823–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar
|
|
3
|
Yu X, Yang L, Cairns MJ, Dass C, Saravolac
E, Li X and Sun LQ: Chemosensitization of solid tumors by
inhibition of Bcl-xL expression using DNAzyme. Oncotarget.
5:9039–9048. 2014.PubMed/NCBI
|
|
4
|
Gazzaniga P, Gradilone A, Silvestri I,
Gandini O, Giuliani L, Vincenzoni A, Gallucci M, Frati L and
Agliano AM: Variable levels of bcl-2, bcl-x and bax mRNA in bladder
cancer progression. Oncol Rep. 5:901–904. 1998.PubMed/NCBI
|
|
5
|
Giménez-Bonafé P, Tortosa A and
Pérez-Tomás R: Overcoming drug resistance by enhancing apoptosis of
tumor cells. Curr Cancer Drug Targets. 9:320–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Youssef RF and Lotan Y: Predictors of
outcome of non-muscle-invasive and muscle-invasive bladder cancer.
Sci World J. 11:369–381. 2011. View Article : Google Scholar
|
|
7
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A:
European Association of Urology: EAU guidelines on muscle-invasive
and metastatic bladder cancer: Summary of the 2013 guidelines. Eur
Urol. 65:778–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Palou Redorta J, et al: European Association of Urology: EAU
guidelines on non-muscle-invasive urothelial carcinoma of the
bladder: Update 2013. Eur Urol. 64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duggan BJ, Gray S, Johnston SR, Williamson
K, Miyaki H and Gleave M: The role of antisense oligonucleotides in
the treatment of bladder cancer. Urol Res. 30:137–147. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshimine S, Kikuchi E, Kosaka T, Mikami
S, Miyajima A, Okada Y and Oya M: Prognostic significance of Bcl-xL
expression and efficacy of Bcl-xL targeting therapy in urothelial
carcinoma. Br J Cancer. 108:2312–2320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lebedeva I, Raffo A, Rando R, Ojwang J,
Cossum P and Stein CA: Chemosensitization of bladder carcinoma
cells by bcl-xL antisense oligonucleotides. J Urol. 166:461–469.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Achenbach TV, Brunner B and Heermeier K:
Oligonucleotide-based knockdown technologies: Antisense versus RNA
interference. Chem Biochem. 4:928–935. 2003.
|
|
13
|
Scherer LJ and Rossi JJ: Approaches for
the sequence-specific knockdown of mRNA. Nat Biotechnol.
21:1457–1465. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bolenz C, Becker A, Trojan L, Schaaf A,
Cao Y, Weiss C, Alken P and Michel MS: Optimizing chemotherapy for
transitional cell carcinoma by application of bcl-2 and bcl-xL
antisense oligodeoxynucleotides. Urol Oncol. 25:476–482. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Littlejohn JE, Cao X, Miller SD, Ozvaran
MK, Jupiter D, Zhang L, Rodarte C and Smythe WR: Bcl-xL antisense
oligo-nucleotide and cisplatin combination therapy extends survival
in SCID mice with established mesothelioma xenografts. Int J
Cancer. 123:202–208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kunze D, Erdmann K, Froehner M, Wirth MP
and Fuessel S: siRNA-mediated inhibition of antiapoptotic genes
enhances chemotherapy efficacy in bladder cancer cells. Anticancer
Res. 32:4313–4318. 2012.PubMed/NCBI
|
|
17
|
Bolenz C, Weiss C, Wenzel M, Gabriel U,
Steidler A, Becker A, Herrmann E, Trojan L and Michel MS: In vivo
evaluation of intravesical paclitaxel and combined bcl-xL antisense
oligodeoxynucleotide treatment for orthotopic urothelial carcinoma.
J Cancer Res Clin Oncol. 135:679–686. 2009. View Article : Google Scholar
|
|
18
|
Gabriel U, Bolenz C, Becker A, Schaaf A,
Steidler A, Trojan L, Weiss C and Michel MS: Evaluation of
cytotoxic effects induced by bcl-2 and bcl-xL
antisense-oligodeoxynucleotides in normal urothelium and
transitional cell carcinoma. Oncol Rep. 20:1419–1423.
2008.PubMed/NCBI
|
|
19
|
Kraemer K, Fuessel S, Schmidt U, Kotzsch
M, Schwenzer B, Wirth MP and Meye A: Antisense-mediated hTERT
inhibition specifically reduces the growth of human bladder cancer
cells. Clin Cancer Res. 9:3794–3800. 2003.PubMed/NCBI
|
|
20
|
Kirsh EJ, Baunoch DA and Stadler WM:
Expression of bcl-2 and bcl-X in bladder cancer. J Urol.
159:1348–1353. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kunze D, Wuttig D, Fuessel S, Kraemer K,
Kotzsch M, Meye A, Grimm MO, Hakenberg OW and Wirth MP: Multitarget
siRNA inhibition of antiapoptotic genes (XIAP, BCL2, BCL-X(L)) in
bladder cancer cells. Anticancer Res. 28B:2259–2263. 2008.
|
|
22
|
Chan JH, Lim S and Wong WS: Antisense
oligonucleotides: From design to therapeutic application. Clin Exp
Pharmacol Physiol. 33:533–540. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lebedeva I, Rando R, Ojwang J, Cossum P
and Stein CA: Bcl-xL in prostate cancer cells: Effects of
overexpression and down-regulation on chemosensitivity. Cancer Res.
60:6052–6060. 2000.PubMed/NCBI
|
|
24
|
Fuessel S, Herrmann J, Ning S, Kotzsch M,
Kraemer K, Schmidt U, Hakenberg OW, Wirth MP and Meye A:
Chemosensitization of bladder cancer cells by survivin-directed
antisense oligodeoxy-nucleotides and siRNA. Cancer Lett.
232:243–254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyagishi M, Hayashi M and Taira K:
Comparison of the suppressive effects of antisense oligonucleotides
and siRNAs directed against the same targets in mammalian cells.
Antisense Nucleic Acid Drug Dev. 13:1–7. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bertrand JR, Pottier M, Vekris A, Opolon
P, Maksimenko A and Malvy C: Comparison of antisense
oligonucleotides and siRNAs in cell culture and in vivo. Biochem
Biophys Res Commun. 296:1000–1004. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kretschmer-Kazemi Far R and Sczakiel G:
The activity of siRNA in mammalian cells is related to structural
target accessibility: A comparison with antisense oligonucleotides.
Nucleic Acids Res. 31:4417–4424. 2003. View Article : Google Scholar : PubMed/NCBI
|