Introduction
Since the prehistoric period, human beings have
treated various diseases with natural products, from plants, marine
materials, and animals. The earliest recorded medical text, which
came from ancient Egypt, described several plant-derived medicinal
substances. Today, plants are the main resource for modern
pharmacological research and the development of new drugs,
including anticancer agents. The first plant-derived anticancer
drugs were the vinca alkaloids, vinblastine and vincristine. They
come from the Madagascar periwinkle, Catharanthus roseus G.
Don, which was traditionally used to treat diabetes (1).
Podophyllotoxin was isolated as an active ingredient
of a plant used for the traditional treatment of skin cancer and
warts. Podophyllotoxin acetate (PA), which is a naturally occurring
derivative of podophyllotoxin, is obtained as an abundant lignan
from podophyllin, which is a type of resin produced by
Podophyllum peltatum Linnaeus. The lignans are a family of
abundant natural products and secondary metabolites that are
manufactured through the shikimic acid pathway, and consist of two
bound phenylpropane units. Podophyllotoxin exhibits the
aryltetralin structure of a cyclolignan, which is a lignin in which
the two phenylpropane units are joined by a carbocycle that
consists of two single carbon-carbon bonds that occur between the
side chains (one at the β-β′ positions). In terms of biological
effects, podophyllotoxin is known to have immunosuppressive
activity and antiviral effects against herpes, measles, influenza
and venereal warts (2).
It is also considered to be a candidate anticancer
agent, as it reversibly binds tubulin and interrupts its
polymerization, thereby preventing the formation of mitotic
spindles to trigger cell cycle arrest and inhibit cell
proliferation (2). Many
investigators have synthesized various derivatives in an effort to
improve the antitumor effects of podophyllotoxin. Three kinds of
representative semi-synthetic epipodophyllotoxin derivatives have
been developed: etoposide, teniposide and etopophos. These drugs do
not inhibit microtubule polymerization due to the presence of a
bulky glucoside moiety in their chemical structure. Instead, their
anticancer activity arises from their ability to bind DNA
topoisomerases, which are ubiquitous enzymes that control the
topological state of DNA in cells. There are two forms of DNA
topoisomerase: type I enzymes cleave a single strand of DNA, while
type II enzymes cleave both strands. Together, they decide the
topology of DNA in actively proliferating cancer cells. Thus, DNA
topoisomerases are among the main targets of anticancer drug
development. The three semi-synthetic epipodophyllotoxin
derivatives act on type II DNA topoisomerases, preventing the
re-ligation of DNA. Treatment of cells with these drugs leads to
the formation of a DNA-drug-enzyme complex, the breakage of one or
both of the DNA strands, and eventual cell death or apoptosis
(3).
In a previous study, we isolated PA from a library
of natural compounds and showed that it could induce
radio-sensitization of NCI-H460 cells (one of NSCLC cell line) and
inhibit their proliferation at a very low concentration (4). Here, we tested the effect of PA on
various NSCLS cell lines and sought to detail the molecular
mechanisms underlying PA-induced cell death.
Materials and methods
Cell culture and chemical reagents
The A549 and NCI-H1299 human NSCLC cell lines were
purchased from the American Type Culture Collection (Rockville, MD,
USA). All the cells were incubated at 37°C with 5% CO2
incubator. Propidium iodide (PI) was obtained from Sigma-Aldrich
(St. Louis, MO, USA). The Natural Product Collection, which
included PA, was obtained from MicroSource Discovery Systems, Inc.
(Gaylordsville, CT, USA).
MTT assay and IC50
determination
A549 and NCI-H1299 cells (4×103
cells/well) were exposed to different concentrations of PA for 72
h, and then treated with 50 μl of
3-(4,5-dimethylthiazol-2-yle)-2,5-diphenyltetrazolium bromide (MTT)
solution (2 mg/ml) for 2 h. All incubations were performed at 37°C.
The formazan crystals generated in living cells were dissolved in
200 μl/well of dimethyl sulfoxide (DMSO), and the absorbance of
individual wells was read at 545 nm using a microplate reader
(Original Multiscan; Thermo Scientific Co., Waltham, MA, USA). The
50% inhibitory concentration (IC50) was calculated from
a concentration-response analysis performed using Softmax Pro
software (Molecular Devices, Sunnyvale, CA, USA).
Microtubule assembly assay
The microtubule assembly assay was performed as
described in a previous study (5).
Briefly, A549 and NCI-H1299 cells were seeded (1×106
cells per 100-mm culture dish) and treated with 20 or 10 nM PA,
respectively, for 24–48 h. Cells were lyses, and the supernatant
fractions were collected as containing soluble αβ-tubulin dimers,
while the pellets were collected as containing polymerized
microtubules.
Immunocytochemical staining
A549 and NCI-H1299 cells (1×104) were
seeded in chamber slides and treated with 20 or 10 nM PA,
respectively, for 48 h. The treated cells were fixed with 1%
paraformaldehyde and then stained with an anti-α-tubulin antibody
and DAPI. Images of stained cells were acquired with a 710 confocal
microscope (Carl-Zeiss, Germany).
Immunoblot analysis
Immunoblot analyses were performed as described
previously (6). Membranes were
probed with antibodies against BiP, CHOP, phospho-PERK, IRE1-α,
beclin-1, Atg3, Atg5, Atg7, LC 3, caspase-3, -8 and -9,
phospho-JNK, Aurora B (all from Cell Signaling Technology, Beverly,
MA, USA), α-tubulin, Cdc2, cyclin B1, p21, survivin (all from Santa
Cruz Biotechnology, Santa Cruz, CA, USA). An anti-β-actin antibody
(Sigma-Aldrich) was used as the loading control. The relative band
densities were determined by densitometry and normalized with
respect to that of β-actin, using ImageJ software (NIH, Bethesda,
MD, USA).
Cell cycle analysis
A549 and NCI-H1299 cells (3×105 cells per
60-mm dish) were incubated with or without 20 or 10 nM PA for 24 or
48 h. The cells were then trypsinized, washed twice with ice-cold
phosphate-buffered saline (PBS), and fixed with ice-cold 70%
ethanol. Fixed cells were incubated with 50 μg/ml PI and 40 μg/ml
RNase at 37°C for 30 min, and then analyzed with a FACSort flow
cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA). The
percentages of cells in each phase of the cell cycle were
determined using Cell Quest software (Becton-Dickinson).
PI-Annexin V staining
PI-Annexin V staining was performed using a FITC
Annexin V Apoptosis Detection kit I (BD Pharmingen, San Jose, CA,
USA). Briefly, A549 and NCI-H1299 cells (3×105 cells)
were incubated with or without 15 or 7.5 nM PA, respectively, for
24 or 48 h. Treated cells were harvested by trypsinization, washed
twice with cold PBS, and resuspended in 500 μl of a solution
containing 1 μg/ml each of PI and Annexin V. The apoptotic fraction
was evaluated using a FACSort flow cytometer (Becton-Dickinson) as
described by the manufacturer.
Statistical analysis
Data were analyzed using GraphPad Prism software
(GraphPad Software, La Jolla, CA, USA), and the significance of
differences between experimental groups was determined using the
Student's t-test. P-values <0.05 were considered significant,
and the individual P-values in the figures are denoted by asterisks
(**P<0.01; ***P<0.001). The number
above each point or bar represents the mean results from three
independent experiments, and the error bars signify the standard
deviations (SDs).
Results
PA induces cell death of NSCLCs
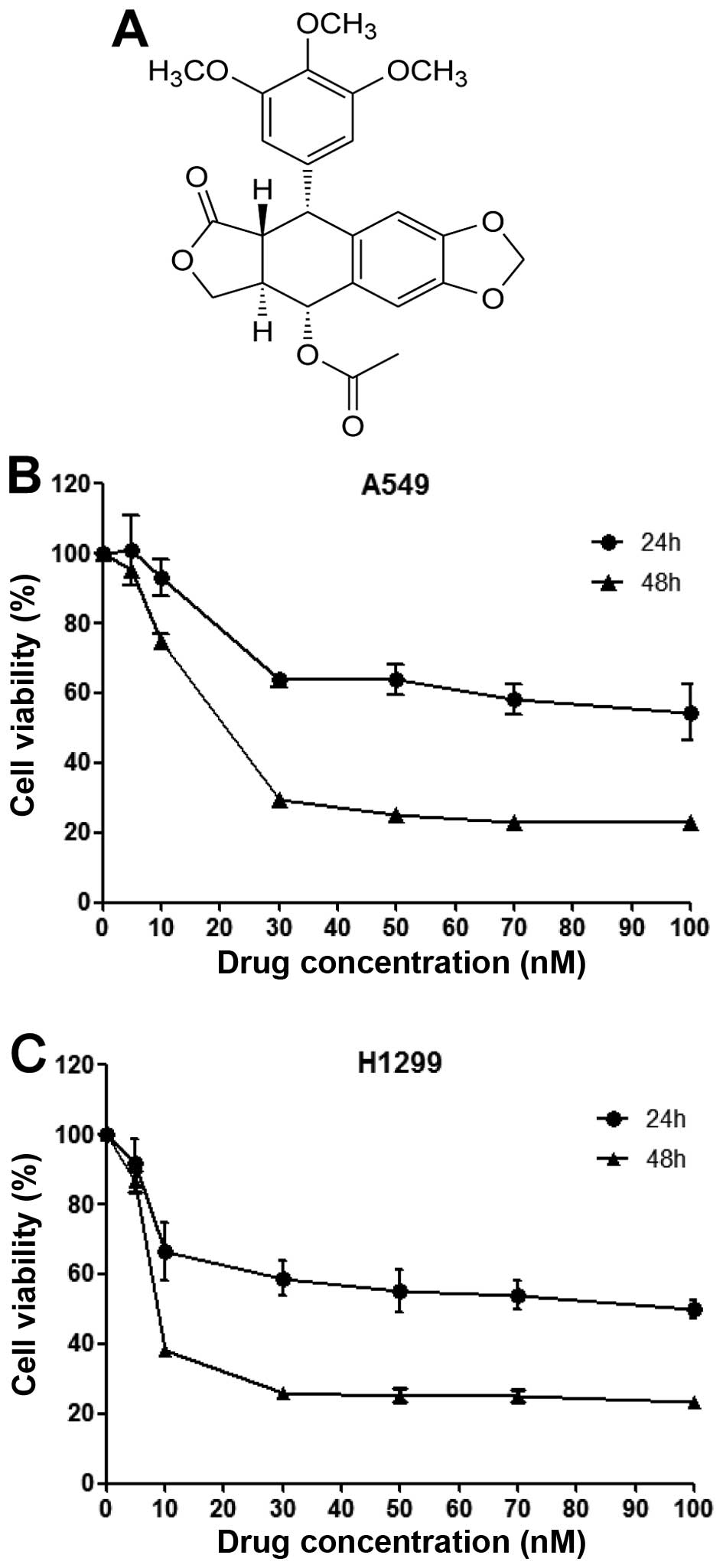
PA (Fig. 1A) was
previously screened from a natural product library as having
radiosensitizing effects against the NCI-H460 cell line in
vitro and in vivo. Here, we tested whether PA could also
induce cell death against two other NSCLC cell lines (A549 and
NCI-H1299 cells), and calculated its IC50 values against
these cells over 24 or 48 h. After 48 h of PA treatment, the
IC50 values of PA against and A549 and NCI-H1299 cells
were found to be 16.08 and 7.53 nM, respectively (Fig. 1B and C). We used these
IC50 values as standard doses in the following
experiments.
PA inhibits microtubule polymerization
and induces cell cycle arrest at G2/M phase
One of the anticancer activities of podophyllotoxin,
the precursor of PA, is the ability to interrupt of tubulin
polymerization and thereby trigger cell cycle arrest. To test
whether the anticancer potential of PA involves a similar
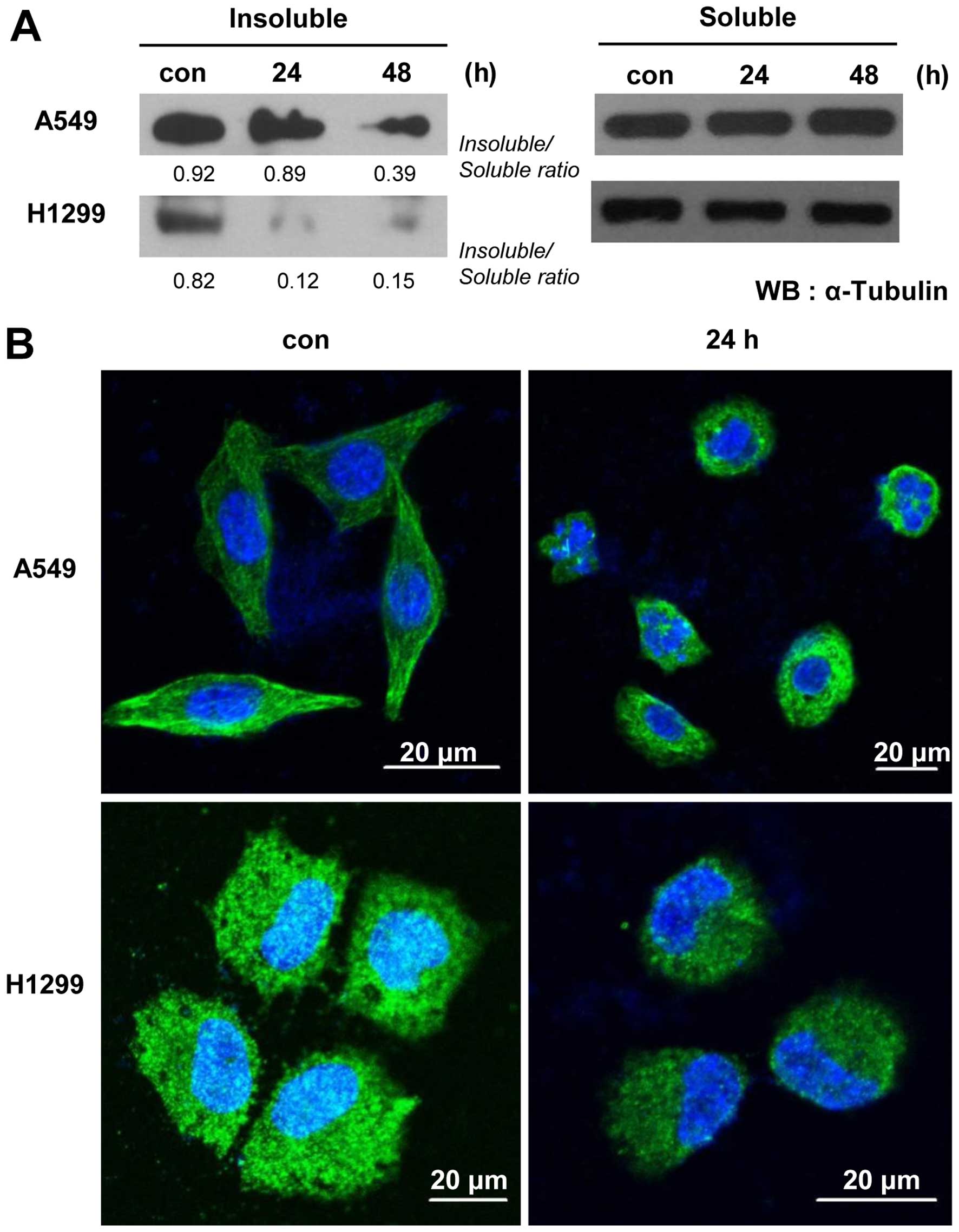
mechanism, we examined microtubule organization in PA-treated
cells. Our microtubule assembly assays and immunocytochemical
staining using an anti-α-tubulin antibody revealed that PA
treatment time-dependently decreased microtubule polymerization and
disrupted the organization of microtubules in A549 and NCI-H1299
cells (Fig. 2). To examine whether
the observed microtubule damage was followed by blockade of the
cell cycle, as is seen with various microtubule-targeting
anticancer reagents, we treated A549 cells with 5, 10 or 20 nM PA
for 8 or 16 h, and treated NCI-H1299 cells with 5 or 10 nM PA for 8
or 16 h, and assessed their cell cycle distributions. Our results
showed that PA-treated cells exhibited time- and dose-dependent
increases inG2/M phase arrest (Fig. 3A
and B, and Table I). The G2/M
arrest of A549 cells was maximal following the 20 nM/16-h
treatment, while that of NCI-H1299 cells was maximal following the
10 nM/8-h treatment dose- and time-dependently. Because intact cell
cycle patterns of NCI-H1299 cells were not detected after 16 h of
PA treatment (data not shown), cell cycle analysis of NCI-H1299
were performed until 16 h. We also observed G2/M phase
arrest-related molecules in A549 or NCI-H1299 cells treated with 20
nM or 10 nM PA, respectively, for 8, 16, or 24 h. Immunoblot
analyses (Fig. 3C and D) revealed
that the levels of cyclin B1 and Cdc2 were decreased, while those
of p21, Aurora B and survivin increased in a time-dependent manner.
These results were consistent with the observed increase in G2/M
phase arrest, suggesting that PA might block the proliferation of
NSCLC cells by triggering microtubule disorganization and
subsequent cell cycle arrest.
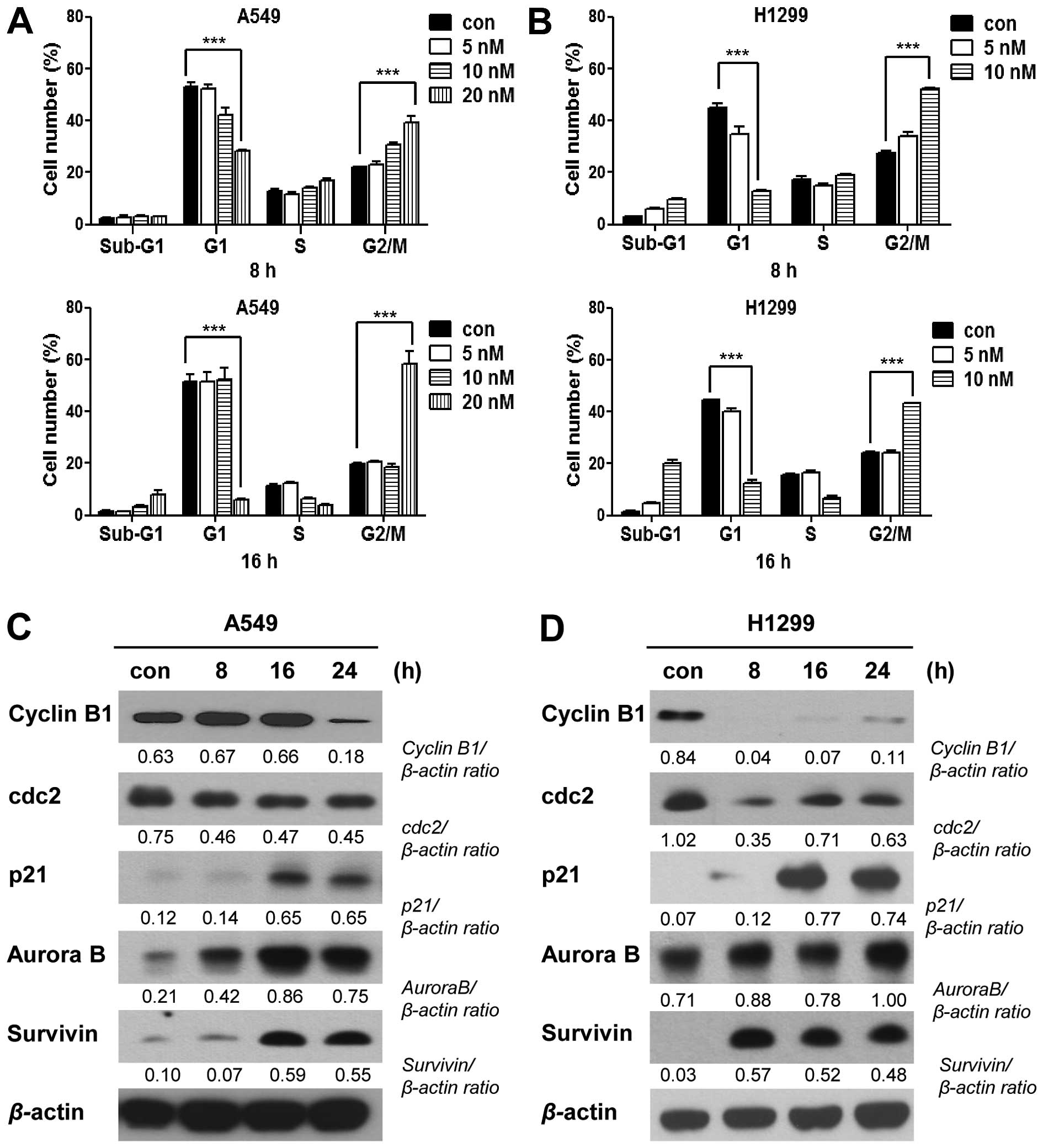 | Figure 3PA induces cell cycle arrest. (A and
B) Cell cycle analysis of A549 and NCI-H1299 cells treated with PA
for 8 and 16 h. Con, the mock-treated control, and 5, 10 and 20 nM
the groups treated with 5, 10 and 20 nM PA, respectively. (C and D)
Immunoblot analyses of the G2/M arrest-related proteins, cyclin B1,
Cdc2, p21, Aurora B, survivin, and β-actin. Con, the mock-treated
control, and 8, 16 or 24 h, the times at which cells were harvested
after treatment with PA (20 and 10 nM for A549 and NCI-H1299 cells,
respectively). |
 | Table IQuantitative analysis of cell cycle
arrest in A549 and NCI-H1299 cells treated with PA for 8 and 16
h. |
Table I
Quantitative analysis of cell cycle
arrest in A549 and NCI-H1299 cells treated with PA for 8 and 16
h.
| Con | 5 nM | 10 nM | 20 nM |
|---|
|
|
|
|
|
|---|
| A549 | 8 h | 16 h | 8 h | 16 h | 8 h | 16 h | 8 h | 16 h |
|---|
| Sub-G1 | 2.1 | 1.4 | 2.8 | 1.4 | 3.2 | 3.5 | 3.1 | 8.1 |
| G1 | 53.1 | 51.5 | 52.1 | 51.6 | 42.1 | 52.2 | 28.2 | 5.9 |
| S | 12.8 | 11.4 | 11.6 | 12.4 | 13.9 | 6.5 | 17.1 | 4.1 |
| G2/M | 22.0 | 19.7 | 23.2 | 20.4 | 30.6 | 18.7 | 39.3 | 58.3 |
|
| Con | 5 nM | 10 nM | 20 nM |
|
|
|
|
|
| NCI-H1299 | 8 h | 16 h | 8 h | 16 h | 8 h | 16 h | 8 h | 16 h |
|
| Sub-G1 | 2.9 | 1.6 | 5.8 | 4.6 | 9.6 | 20.0 | 15.1 | 31.2 |
| G1 | 45.0 | 44.4 | 34.9 | 40.2 | 12.8 | 12.3 | 8.2 | 2.2 |
| S | 7.3 | 15.8 | 14.7 | 16.4 | 19.0 | 6.7 | 17.6 | 4.1 |
| G2/M | 27.5 | 24.3 | 33.9 | 24.3 | 52.1 | 43.2 | 49.6 | 46.0 |
PA induces DNA damage and cell death via
G2/M phase arrest
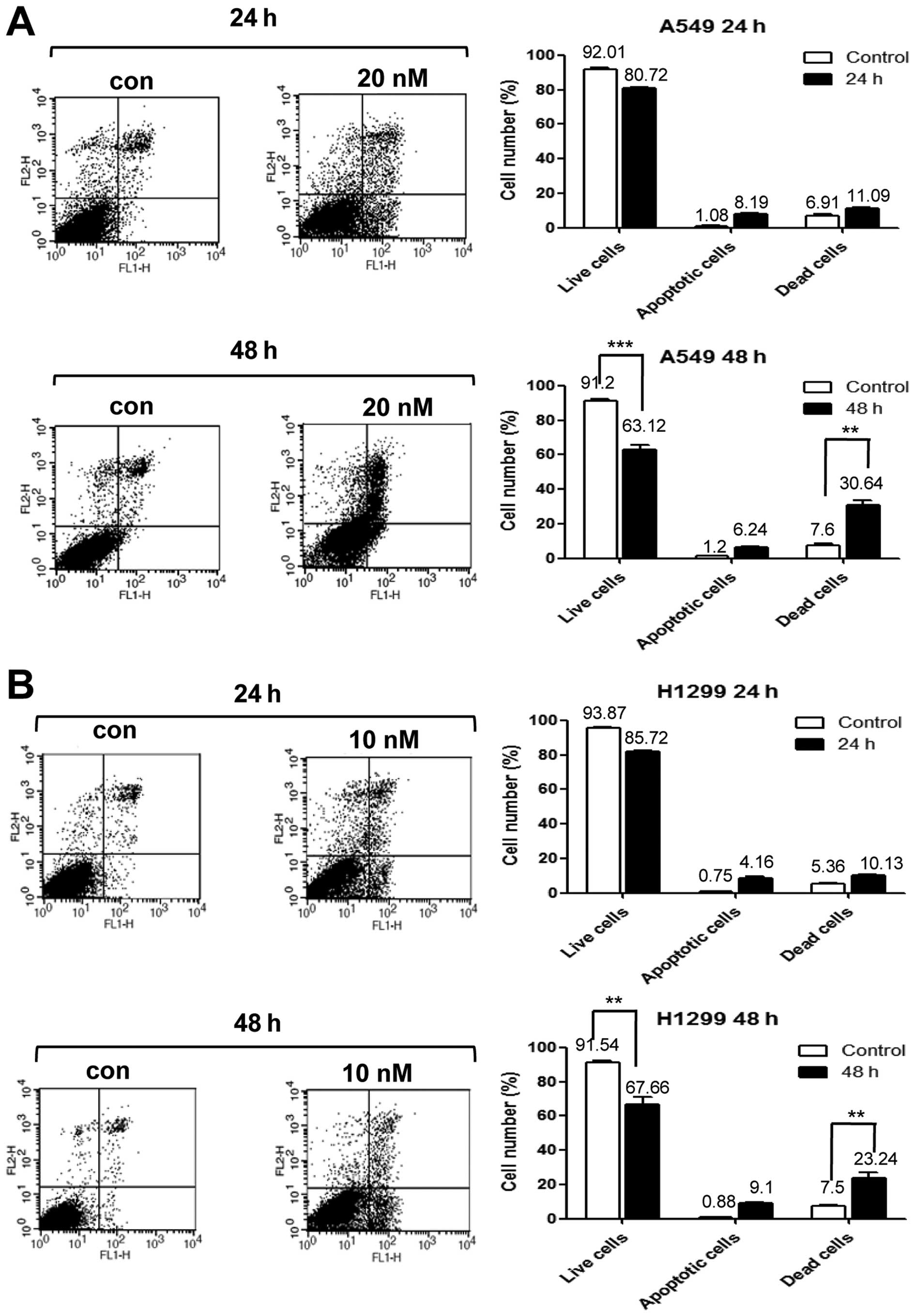
Next, we performed PI/Annexin V staining to detect
cell death in A549 cells treated with 20 nM PA for 24 or 48 h, and
in NCI-H1299 cells treated with 10 nM PA for 24 or 48 h. Our
results revealed that the cell death of A549 and NCI-H1299 cells
increased time-dependently, with maximal cell death seen at 48 h
(Fig. 4A and B). Immunoblot
analyses against caspase-3, -8 and 9 were performed on A549 and
NCI-H1299 cells treated with PA (20 and 10 nM, respectively) for 8,
16, 24 or 48 h (Fig. 4D).
Increases in the levels of cleaved caspase-3, -8 and -9 were
detected beginning at 16 h of PA treatment. Thus, we concluded that
the PA-induced cell cycle arrest triggered cell death. We also
observed expression of γ-H2AX under the same treatment conditions
in both cell lines, as a means to examine the DNA damage effects of
PA. Our results revealed that the expression of γ-H2AX increased
beginning at 16 h of PA treatment (Fig. 4C). Taken together, these results
suggest that PA induces the cell death of NSCLS cells via
microtubule disorganization accompanied by cell cycle G2/M arrest
and DNA damage.
PA might induce cell death via ER stress
and autophagic signaling
To examine whether other cell death machineries (in
addition to apoptosis) are involved in the PA-induced elimination
of NSCLC cells, we performed various immunoblot analyses. To
examine the potential involvement of endoplasmic reticulum (ER)
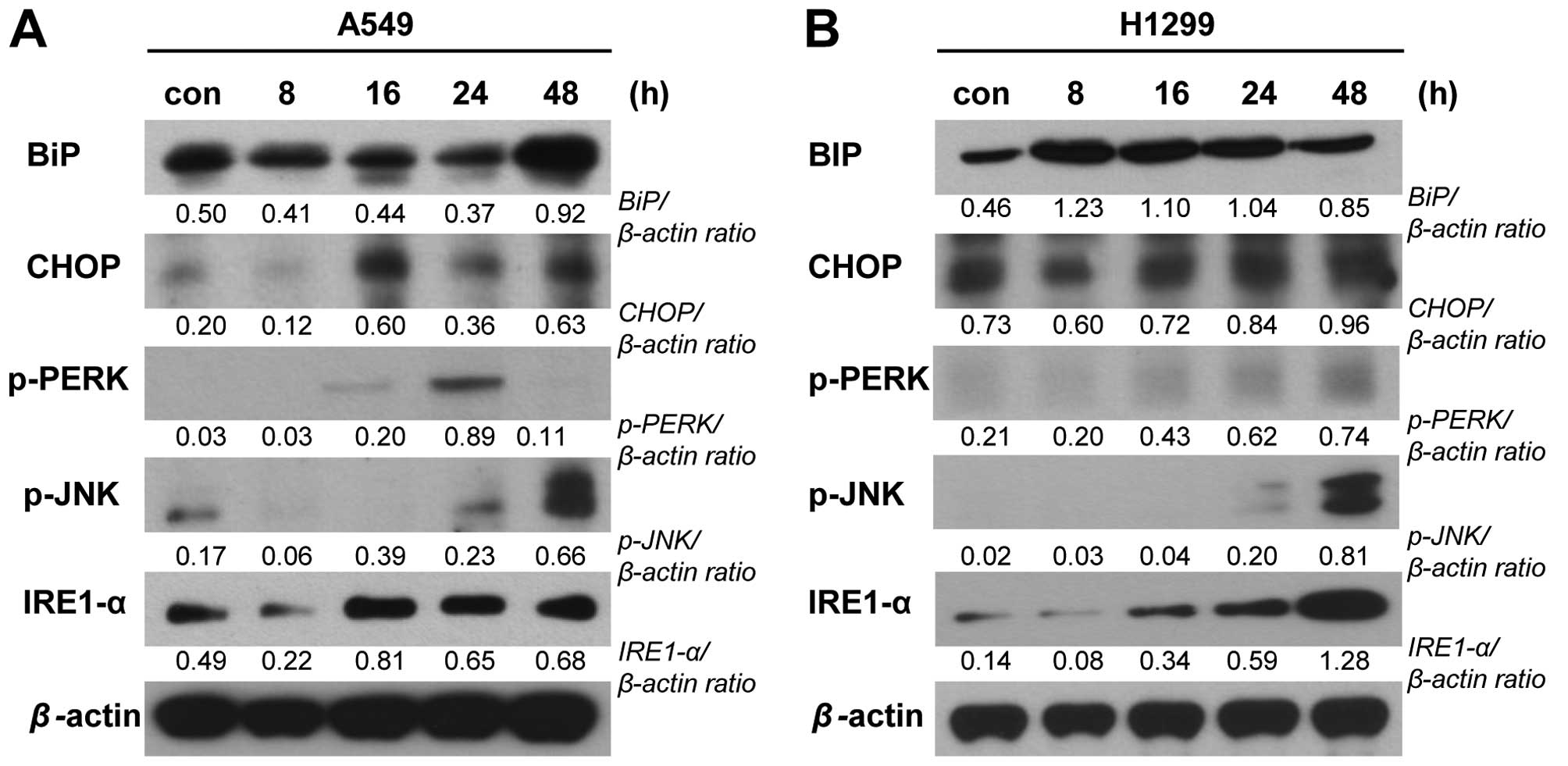
stress, we examined the expression levels of the ER stress-related
proteins, binding immunoglobulin protein (BiP),
CCAAT-enhancer-binding protein homologous protein (CHOP), protein
kinase RNA-like endoplasmic reticulum kinase (PERK),
inositol-requiring enzyme-1 (IRE1)-α and phospho-JNK were detected
in A549 and NCI-H1299 cells treated with PA (20 and 10 nM,
respectively) for 8, 16, 24 or 48 h. Our results revealed that the
levels of CHOP, phospho-PERK, IRE1-α and phospho-JNK increased
time-dependently in both cell lines following PA treatment, whereas
BiP time-dependently increased in A549 cells but not in NCI-H1299
cells (Fig. 5). These results are
consistent with a previous report that pro-apoptotic ER stress was
involved in the cell death induced by another podophyllotoxin
derivative. We also used immunoblot analyses to test the possible
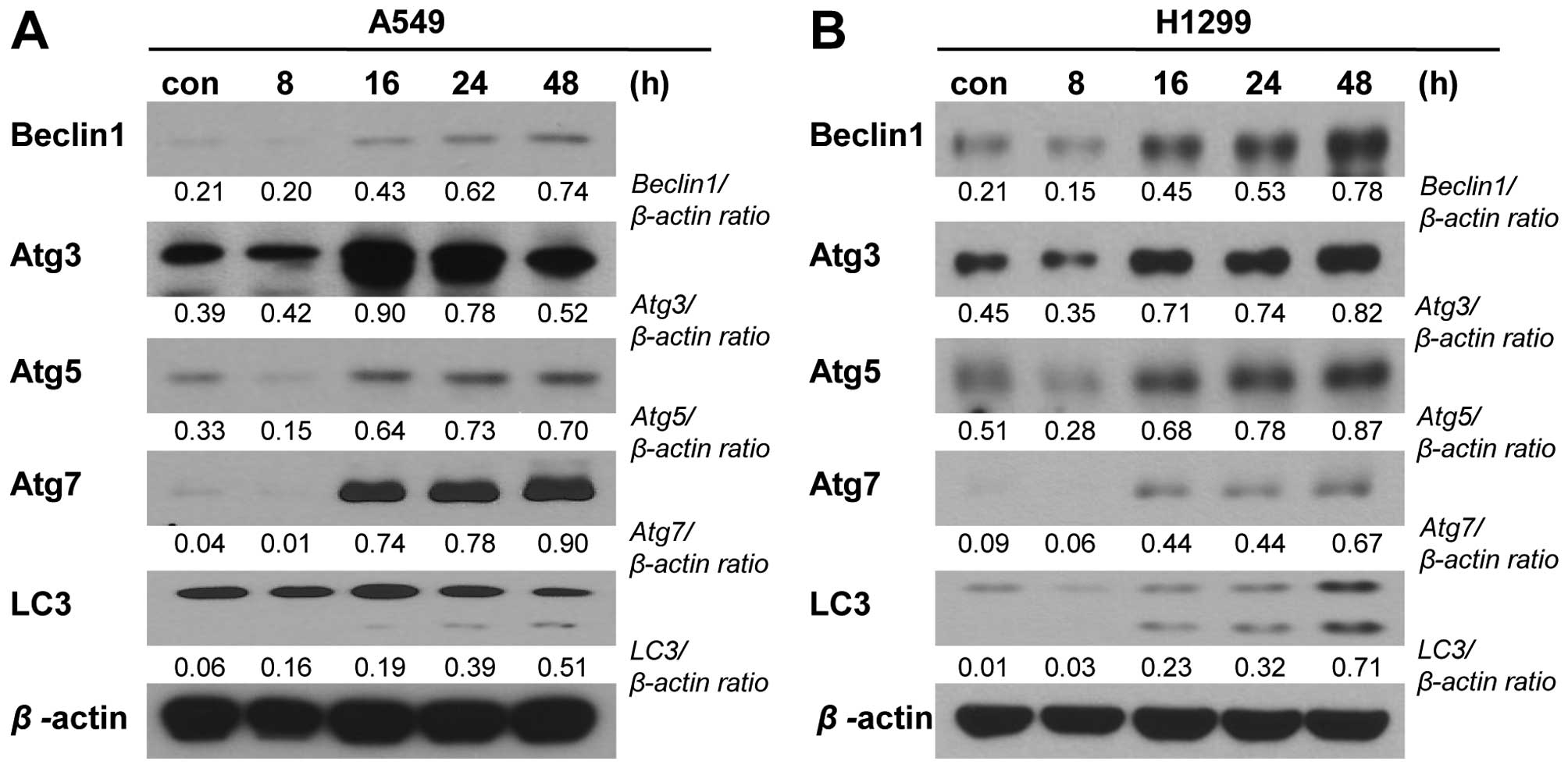
involvement of autophagic cell death in our experimental system.
Indeed, we detected time-dependent PA-induced increases in the
expression levels of autophagy-related gene (Atg)-3, -5 and -7 and
beclin-1, and in the cleavage of microtubule-associated protein
1A/1B-light chain 3 (LC3) (Fig.
6).
Discussion
Non-small cell lung cancer (NSCLC), which is a main
type of lung cancer, has a particularly low 5-year survival rate
(7). NSCLC therapy typically
involves surgery and/or chemo- or radiotherapy. Such therapies are
not fully effective, however, and chemo- or radiotherapy is often
associated with side effects and/or therapeutic resistance
(8). Efforts to develop new drugs
that have increased treatment efficiency and minimal side effects
are a key to the field of cancer treatment. Numerous candidate
anticancer reagents have been developed from natural plant products
and their secondary metabolites, which are proven sources for many
pharmaceutical reagents (1).
In this study, we tested whether PA has anticancer
effects against two NSCLC cell lines, and sought to reveal the
underlying molecular mechanisms. As shown in Fig. 1B, PA inhibited NSCLC proliferation
more effectively than etoposide, which is a representative
anticancer reagent and semi-synthetic derivative of
podophyllotoxin. The IC50 values of PA against NCI-H1299
and A549 cells were 7.53 and 16.08 nM, respectively, whereas those
reported for etoposide were 0.448 and 21.3 μM, respectively
(9,10). This indicates that the cell growth
inhibition mediated by PA is much stronger (~70–1,000-fold) than
that of etoposide. Our results suggested that the inhibitory effect
of PA was independent of the p53 or PTEN status, as A549 cells
contain wild-type p53 and PTEN, whereas NCI-H1299 cells are null
for both p53 and PTEN (6,11,12).
We also found that PA could damage the soluble
fraction of intracellular tubulin and destroy tubulin structures
(Fig. 2), suggesting that PA might
target the polymerization of tubulin, which is closely related to
cell cycle progression and cellular proliferation, and is a main
target of several anti-cancer reagents (13). On tubulin, the paclitaxel site, the
vinca domain and the colchicine domain are the main binding sites
for representative microtubule-targeting drugs. Among them, the
colchicine domain has attracted recent research interest from
groups seeking to develop vessel-targeting reagents for cancer
therapy (13). The PA-mediated
destruction of tubulins is consistent with the effects of other
podophyllotoxin derivatives, including etoposide (14).
Many tubulin-targeting drugs also reportedly inhibit
the cell cycle, due to their ability to disturb the rapid
microtubule dynamics of mitotic spindles (13). As shown in Fig. 3 and Table I, PA-treated cells showed G2/M
arrest at 8 or 16 h post-treatment, as confirmed at the protein
level by decreases in cyclin B1 and Cdc2, with corresponding
increases in p21, Aurora B and survivin. Moreover, PA treatment
induced DNA damage, as shown by time-dependent increases in the
levels of the DNA damage marker, γ-H2AX. Similar DNA-damaging
effects have been reported for other podophyllotoxin analogs,
particularly those known to act as DNA topoisomerase inhibitors
(14,15).
PA was found to enhance cell death via apoptosis, as
shown by an increase in the sub-G1 population (Fig. 3A and Table I) and by our PI-Annexin V staining
analysis (Fig. 4A). Apoptotic
signaling involves intrinsic and extrinsic pathways. The former is
activated by external stress and triggers mitochondrial
permeability changes and caspase-9 activation, while the latter
begins with death receptor/ligand binding and proceeds through
caspase-8 activation. Both apoptotic pathways feed to the
activation of caspase-3 as a common ‘executioner’. Consistent with
previous reports that these pathways are triggered by derivatives
of podophyllotoxin, we found that PA time-dependently increased the
activities of caspase-3, -8 and -9 (16).
In terms of other cell death pathways, Bicknell
et al (17) reported that
the inhibition of nuclear migration and cell division could induce
ER stress, and a report showed that the anticancer effects of
podophyllotoxin derivatives involved activation of the ER stress
pathway (18). Therefore, we
tested whether PA could induce ER stress and its related molecular
changes. Indeed, our results showed that PA treatment increased
various markers of ER stress, including the expression levels of
BiP, CHOP, and IRE1-α and the phosphorylation levels of PERK and
JNK (Fig. 5). The IRE1-α
serine/threonine kinase regulates the unfolded protein response
(UPR) that is part of the ER stress response, while PERK (an eIF2α
kinase) is an ER transmembrane protein that, upon activation by ER
stress, phosphorylates eIF2α to inhibit protein translation.
Phosphorylation of PERK at Thr980 is used as a marker of ER stress.
Elevated expression of CHOP, which mediates apoptosis, is also used
as an ER stress marker (19).
Therefore, our results suggest that the relationship between
apoptosis and ER stress pathways might be a main target of PA. The
elevated levels of ER stress markers by PA treatment did not
coincidence with patterns in our cell lines, however. We estimated
these differences of protein expression due to cell line specific
differences for ER stress pathway.
As another cell death-inducing pathway, we examined
the possible involvement of PA in autophagic cell death. A number
of anticancer regents reportedly induce cell death via the
autophagic pathway (20,21), which is generally activated by
conditions of nutrient deprivation, but has also been associated
with pathologies, including cancer (22,23).
The main function of the autophagic pathway is to provide the
building blocks (e.g., amino acids) for new cellular components via
the autophagosome-mediated degradation of abnormal or excess
cytoplasmic contents. Several Atg proteins are known to control
autophagosome formation, including the Atg12-Atg5 and LC3-II
(Atg8-II) complexes. The Atg4 protease cleaves LC3/Atg8 at its
C-terminus to yield the cytosolic LC3-I, which is then conjugated
to phosphatidylethanolamine (PE) via the actions of Atg7 and Atg3.
The other lipidated form of LC3, known as LC3-II, is also attached
to sequestosome 1 (SQSTM1, p62) on autophagosome, and it is a
ubiquitin binding protein. SQSTM1-containing protein aggregates to
the autophagosome and then promotes autophagy. In this study, we
observed increases in the expression levels of Atg-3, -5 and -7 and
beclin-1, and the cleavage of LC3 (24). These changes suggest that the
autophagic cell death pathway is also involved in the effects of PA
on the tested NSCLC cell lines. Some investigators have suggested
that the functional status of caspase-3 might control the switch
between apoptosis and autophagy in MCF-7 (a breast cancer cell
line) cells exposed to chemo-therapeutics (25,26).
This may suggest the existence of crossroads connecting the
apoptotic and autophagic pathways.
This study did not examine whether the low dose of
PA that is capable of eliminating NSCLC cells might spare normal
tissues. However, our present and previous results collectively
show that PA enhances apoptotic death of NSCLC cell lines (e.g.,
NCI-H460, A549 and NCI-H1299) via multiple cell death routes. This
suggests the novel possibility that PA could be a candidate
anticancer drug and/or could prove useful for other clinical
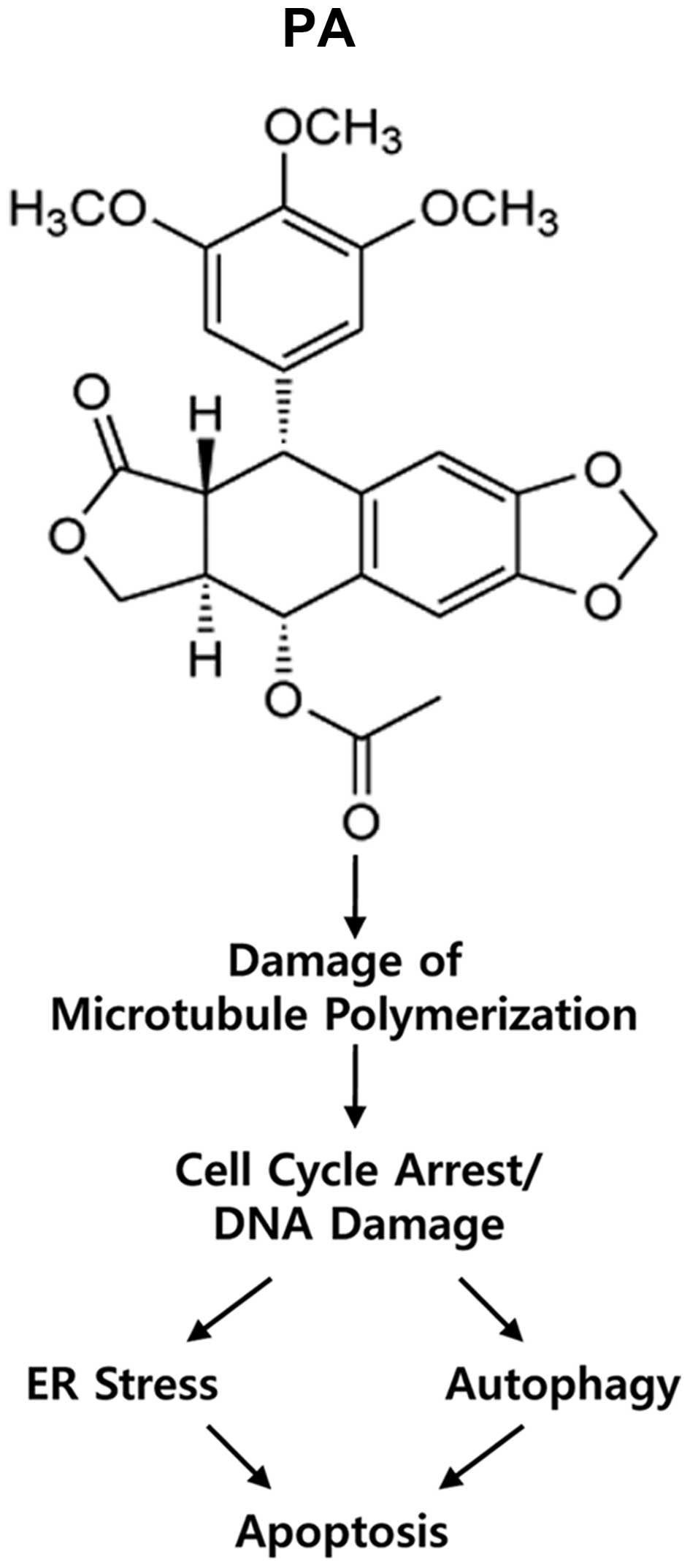
applications. Our results also suggest a model in which PA induces
the following sequence: damage of microtubule polymerization;
induction of cell cycle arrest/DNA damage; ER stress/autophagy; and
finally apoptotic cell death (Fig.
7). Our findings may facilitate the development of
podophyllotoxin derivatives as anticancer reagents. Finally, the
various molecules involved in the cytotoxic effects of PA could be
useful targets for the development of novel therapeutic agents
against cancer.
Acknowledgements
This study was supported by the Nuclear Research and
Development Program of the National Research Foundation of Korea
(NRF) grant funded by the Korean government (MEST)
(2012M2A2A7010422) and, in part, by the Basic Science Research
Program through the NRF (NRF-2014R1A1A2054985).
References
|
1
|
Cragg GM, Grothaus PG and Newman DJ:
Impact of natural products on developing new anti-cancer agents.
Chem Rev. 109:3012–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gordaliza M, García PA, del Corral JM,
Castro MA and Gómez-Zurita MA: Podophyllotoxin: Distribution,
sources, applications and new cytotoxic derivatives. Toxicon.
44:441–459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guerram M, Jiang Z-Z and Zhang L-Y:
Podophyllotoxin, a medicinal agent of plant origin: Past, present
and future. Chin J Nat Med. 10:161–169. 2012. View Article : Google Scholar
|
|
4
|
Choi JY, Cho HJ, Hwang SG, Kim WJ, Kim JI,
Um HD and Park JK: Podophyllotoxin acetate enhances γ-ionizing
radiation-induced apoptotic cell death by stimulating the
ROS/p38/caspase pathway. Biomed Pharmacother. 70:111–118. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuo CC, Hsieh HP, Pan WY, Chen CP, Liou
JP, Lee SJ, Chang YL, Chen LT, Chen CT and Chang JY: BPR0L075, a
novel synthetic indole compound with antimitotic activity in human
cancer cells, exerts effective antitumoral activity in vivo. Cancer
Res. 64:4621–4628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Park JK, Jung HY, Park SH, Kang SY, Yi MR,
Um HD and Hong SH: Combination of PTEN and gamma-ionizing radiation
enhances cell death and G(2)/M arrest through regulation of AKT
activity and p21 induction in non-small-cell lung cancer cells. Int
J Radiat Oncol Biol Phys. 70:1552–1560. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radio-therapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsai CM, Chang KT, Perng RP, Mitsudomi T,
Chen MH, Kadoyama C and Gazdar AF: Correlation of intrinsic
chemoresistance of non-small-cell lung cancer cell lines with
HER-2/neu gene expression but not with ras gene mutations. J Natl
Cancer Inst. 85:897–901. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xin Y, Yin F, Qi S, Shen L, Xu Y, Luo L,
Lan L and Yin Z: Parthenolide reverses doxorubicin resistance in
human lung carcinoma A549 cells by attenuating NF-κB activation and
HSP70 up-regulation. Toxicol Lett. 221:73–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Breen L, Heenan M, Amberger-Murphy V and
Clynes M: Investigation of the role of p53 in chemotherapy
resistance of lung cancer cell lines. Anticancer Res.
27A:1361–1364. 2007.
|
|
12
|
Jung IL, Kang HJ, Kim KC and Kim IG:
PTEN/pAkt/p53 signaling pathway correlates with the radioresponse
of non-small cell lung cancer. Int J Mol Med. 25:517–523.
2010.PubMed/NCBI
|
|
13
|
Jordan MA and Wilson L: Microtubules as a
target for anticancer drugs. Nat Rev Cancer. 4:253–265. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Montecucco A and Biamonti G: Cellular
response to etoposide treatment. Cancer Lett. 252:9–18. 2007.
View Article : Google Scholar
|
|
15
|
Kaufmann SH: Cell death induced by
topoisomerase-targeted drugs: More questions than answers. Biochim
Biophys Acta. 1400:195–211. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bicknell AA, Babour A, Federovitch CM and
Niwa M: A novel role in cytokinesis reveals a housekeeping function
for the unfolded protein response. J Cell Biol. 177:1017–1027.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen JY, Tang YA, Li WS, Chiou YC, Shieh
JM and Wang YC: A synthetic podophyllotoxin derivative exerts
anti-cancer effects by inducing mitotic arrest and pro-apoptotic ER
stress in lung cancer preclinical models. PLoS One. 8:e620822013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schröder M and Kaufman RJ: ER stress and
the unfolded protein response. Mutat Res. 569:29–63. 2005.
View Article : Google Scholar
|
|
20
|
Gills JJ, Lopiccolo J and Dennis PA:
Nelfinavir, a new anti-cancer drug with pleiotropic effects and
many paths to autophagy. Autophagy. 4:107–109. 2008. View Article : Google Scholar
|
|
21
|
Terés S, Lladó V, Higuera M,
Barceló-Coblijn G, Martin ML, Noguera-Salvà MA, Marcilla-Etxenike
A, García-Verdugo JM, Soriano-Navarro M, Saus C, et al:
2-Hydroxyoleate, a nontoxic membrane binding anticancer drug,
induces glioma cell differentiation and autophagy. Proc Natl Acad
Sci USA. 109:8489–8494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galluzzi L, Pietrocola F, Levine B and
Kroemer G: Metabolic control of autophagy. Cell. 159:1263–1276.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang ZJ, Chee CE, Huang S and Sinicrope
FA: The role of autophagy in cancer: Therapeutic implications. Mol
Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Klionsky DJ, Abdalla FC, Abeliovich H,
Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M,
Agostinis P, Aguirre-Ghiso JA, et al: Guidelines for the use and
interpretation of assays for monitoring autophagy. Autophagy.
8:445–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan CH, Yang YP, Qin ZH, Gu ZL, Reid P and
Liang ZQ: Autophagy is involved in cytotoxic effects of crotoxin in
human breast cancer cell line MCF-7 cells. Acta Pharmacol Sin.
28:540–548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Abedin MJ, Wang D, McDonnell MA, Lehmann U
and Kelekar A: Autophagy delays apoptotic death in breast cancer
cells following DNA damage. Cell Death Differ. 14:500–510. 2007.
View Article : Google Scholar
|