Introduction
Liver cancer is the sixth most frequent cancer and
the second leading cause of cancer-related deaths worldwide.
Hepatocellular carcinoma (HCC) represents 90% of all primary liver
cancers (1–3). The 5-year survival rate for HCC is
<30% and the recurrence rate is ~70% (4,5).
Current HCC treatments, including surgical resection, liver
transplantation, chemotherapy, or immuno-biological cancer
therapies, are typically not very effective.
Cantharidin is a terpenoid isolated from Chinese
blister beetles, and norcantharidin (NCTD) is a demethylated analog
of cantharidin that has anticancer activity in breast cancer, lung
cancer, leukemia, colon and liver cancer. One possible explanation
for the anticancer actions of NCTD is its inhibition of protein
phosphatases, through which G0/G1 or G2/M arrest is triggered.
Thus, apoptosis is subsequently induced via ROS generation and the
mitochondrial pathway (6,7).
Autophagy, an important pathophysiological process,
is crucial for cell development, differentiation, survival and
homeostasis. Additionally, autophagy has an important role in liver
cancer. Previous studies have shown that autophagy is induced in
liver cancer. Inhibition of autophagy was able to inhibit tumor
cell growth and enhanced HCC cell apoptosis, whereas increased
autophagy induced resistance to apoptosis in HCC cells (8,9).
NCTD and decreased autophagy could have an anticancer role in liver
cancer; however, the mechanisms of autophagy inhibition in liver
cancer, as well as the link between NCTD and autophagy, are not
well elucidated. Whether NCTD and autophagy coordinate an increase
in apoptosis in liver cancer, and elucidation of the downstream
apoptotic pathways are not well defined. Because decreased
autophagy may induce liver cancer cell apoptosis, NCTD may have an
important anticancer role in the liver.
Therefore, the objective of the present study was to
examine the relationship between NCTD and autophagy in anticancer
activities in the liver. Based on previous data, we hypothesized
that autophagy inhibition may enhance NCTD-induced apoptosis in
HCC.
Materials and methods
Cell lines and treatment
The human hepatoma cell line HepG2 was obtained from
the Key Laboratory of Living Donor Liver Transplantation, the First
Affiliated Hospital of Nanjing Medical University (Nanjing, China).
Cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
plus 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100
U/ml streptomycin at 37°C with 5% CO2 (Life Technologies
Carlsbad, CA, USA).
Cells were divided into eight groups: i) Sham group:
cells were cultured in DMEM without any additional treatment; ii)
NCTD group: cells were cultured in DMEM containing NCTD (2.5, 5,
10, 20 or 40 μg/ml; Sigma-Chemical, St. Louis, MO, USA) for 24 h
(10); iii) S group: cells were
cultured in HBSS media with Ca2+ and Mg2+
supplemented with 10 mM HEPES (1 ml/well; Sigma-Aldrich) for 0.5 h
to induce autophagy (11), washed
twice with PBS, and cultured for 24 h in DMEM. iv) SN group: cells
were cultured in HBSS media with Ca2+ and
Mg2+ supplemented with 10 mM HEPES (1 ml/well) for 0.5 h
to induce autophagy, and washed with PBS before culture for 24 h in
DMEM containing NCTD (10 μg/ml); v) MA group: cells were cultured
in DMEM containing 3-MA (10 mmol/l; Sigma-Aldrich) (12); vi) MAN group: cells were cultured
in DMEM containing 3-MA (10 mmol/l), washed with PBS before culture
for 24 h in DMEM containing NCTD (10 μg/ml); vii) SS group: cells
were transfected with scrambled siRNA (Ambion, Austin, TX, USA) and
cultured in DMEM; viii) SSN group: cells were transfected with
scrambled siRNA, cultured in DMEM, washed twice with PBS and
cultured for 24 h in DMEM containing NCTD (10 μg/ml).
Small-interfering RNA (siRNA)
transfection
Small-interfering RNA (siRNA) against Atg5
and a non-specific scrambled siRNA were purchased from Ambion. All
siRNAs were synthesized by Qiagen (Chatsworth, CA, USA). HepG2
cells were cultured in 6-well plates. Lipofectamine 2000
(Invitrogen, Rockville, MD, USA) was mixed into serum-free DMEM
containing siRNA1, siRNA2 or scrambled RNA. Additionally, mock
controls were transfected with Lipofectamine 2000 alone, incubated
at room temperature for 20 min and distributed into duplicate
wells. Transfections were performed at 37°C in 5% CO2.
Media were replaced with DMEM containing 10% heat-inactivated FBS
for 4–6 h. After 2 h, cells were treated as indicated.
Reverse transcription-quantitative
real-time polymerase chain reaction (RT-qPCR)
We isolated and purified total RNA from cells by
using TRIzol reagent (Invitrogen) and synthesized cDNA using the
PrimeScript RT Master Mix. cDNA (2 μl) was used as a template in a
20-μl reaction. Primers were synthesized by Invitrogen (Shanghai,
China). The primers for Atg5 were:
5′-TTCTCAAAATATACTGTTTC-3′ (sense) and 5′-TATTATGTATCACAAATGG-3′
(antisense). β-actin was used as a control. The primers for
β-actin were: 5′-TCACCCACACTGTGCCCATCTACGA-3′ (sense) and
5′-CAGCGGAACCGCTCATTGCCAATGG-3′ (antisense). For RT-PCR, we used
the following cycles: 95°C for 30 sec, 40 cycles of 95°C for 5 sec
and 60°C for 31 sec. The dissociation stage was 95°C for 15 sec,
60°C for 1 min and 95°C for 15 sec.
Western blot analyses
HepG2 cells were washed with cold PBS and
homogenized in RIPA lysis buffer in the presence of 1% (v/w)
protease inhibitor (Pierce Biotechnology, Rockford, IL, USA). Cell
lysates were then shaken at 4°C for 1 h and centrifuged for 1 h at
40,000 × g at 4°C. For cytochrome c analyses, cell pellets
were cultured in HEPES buffer containing 250 mM sucrose and
homogenized. Homogenates were centrifuged for 15 min at 800 × g at
4°C and supernatants were centrifuged for 15 min at 10,000 × g at
4°C. Finally, the mitochondrial pellets and cytosolic fractions
were collected (13). Protein
concentrations were determined using BCA protein assays. Protein
lysates (50 μg) were separated by 12% (w/v) SDS-PAGE, and proteins
were transferred to polyvinylidene difluoride (PVDF) membranes
(Millipore, Bedford, MA, USA). Membranes were incubated with the
primary antibodies (caspase antibodies were obtained from Abcam,
Cambridge, UK; the LC3-II antibody was from Novus Biologicals,
Littleton, CO, USA) overnight at 4°C, incubated for 1 h with the
secondary antibody (1:4,000, β-actin; Abcam), and visualized using
an enhanced chemiluminesence (ECL) detection kit (Pierce
Biotechnology). For statistical analyses, the gray value of each
protein was measured using Image-Pro Plus 6.0.
Immunofluorescence
HepG2 cells on coverslips were fixed in 4%
paraformaldehyde for 20 min. Cells were then blocked in 10% BSA for
1 h, followed by overnight incubation with the primary antibody
(LC3II in 10% BSA). Cells were incubated with the secondary
antibody (1:100) for 1 h at 37°C and then mounted using
4′,6-diamidino-2-phenylindole (DAPI) mounting media. We evaluated
the cells for autophagy by randomly choosing ten visual fields and
measuring the optical density. Semi-quantitative analyses were
performed using Image-Pro Plus 6.0.
Annexin V/propidium iodide (PI) staining
assays
We quantified apoptosis in HepG2 cells using the
Annexin V-FITC kit. Cells were washed twice with ice-cold PBS and
resuspended in binding buffer (400 μl) at a concentration of
5×105 cells/ml. After incubation, 5 μl Annexin V-FITC
and 5 μl PI were added. Tubes were gently mixed on a vortex and
incubated for 15 min in the dark at 4°C. Samples were analyzed
using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA,
USA) and CellQuest software (BD Biosciences).
ROS production analyses
Intracellular ROS levels were detected by flow
cytometry using 2′,7′-dichlorodihydro-fluorescein diacetate
(DCFH-DA; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). HepG2
cells were washed three times with PBS solution, and cultured in
serum-free DMEM containing DCFH-DA (100 μM) for 30 min in the dark
at 37°C. Cells were then washed in serum-free DMEM and treated with
pancreatic enzymes. DCF fluorescence was detected at an excitation
wavelength of 488 nm and an emission wavelength of 525 nm.
Mitochondrial membrane potential (ΔΦm)
assays
The loss of mitochondrial membrane potential was
monitored using the membrane permeable dye JC-1 (Beyotime Institute
of Biotechnology, Haimen, China). At high ΔΦm, JC-1 shows red
fluorescence, and the ratio between green and red fluorescence
provides an estimate of ΔΦm. Briefly, JC-1 dyeing liquid was added
to each well (1 ml/well) and cells were incubated at 37°C for 20
min. Cells were then washed twice with dyeing buffer (1X) and
cultured in DMEM. Finally, cells were observed using fluorescence
microscopy.
Statistical analyses
Data are expressed as the mean ± standard deviation.
Differences between groups were evaluated using variance, q-tests
and Student's t-tests. SPSS 11.0 software was used for all
statistical analyses, and P<0.05 was considered statistically
significant.
Results
NCTD induces apoptosis in HepG2
cells
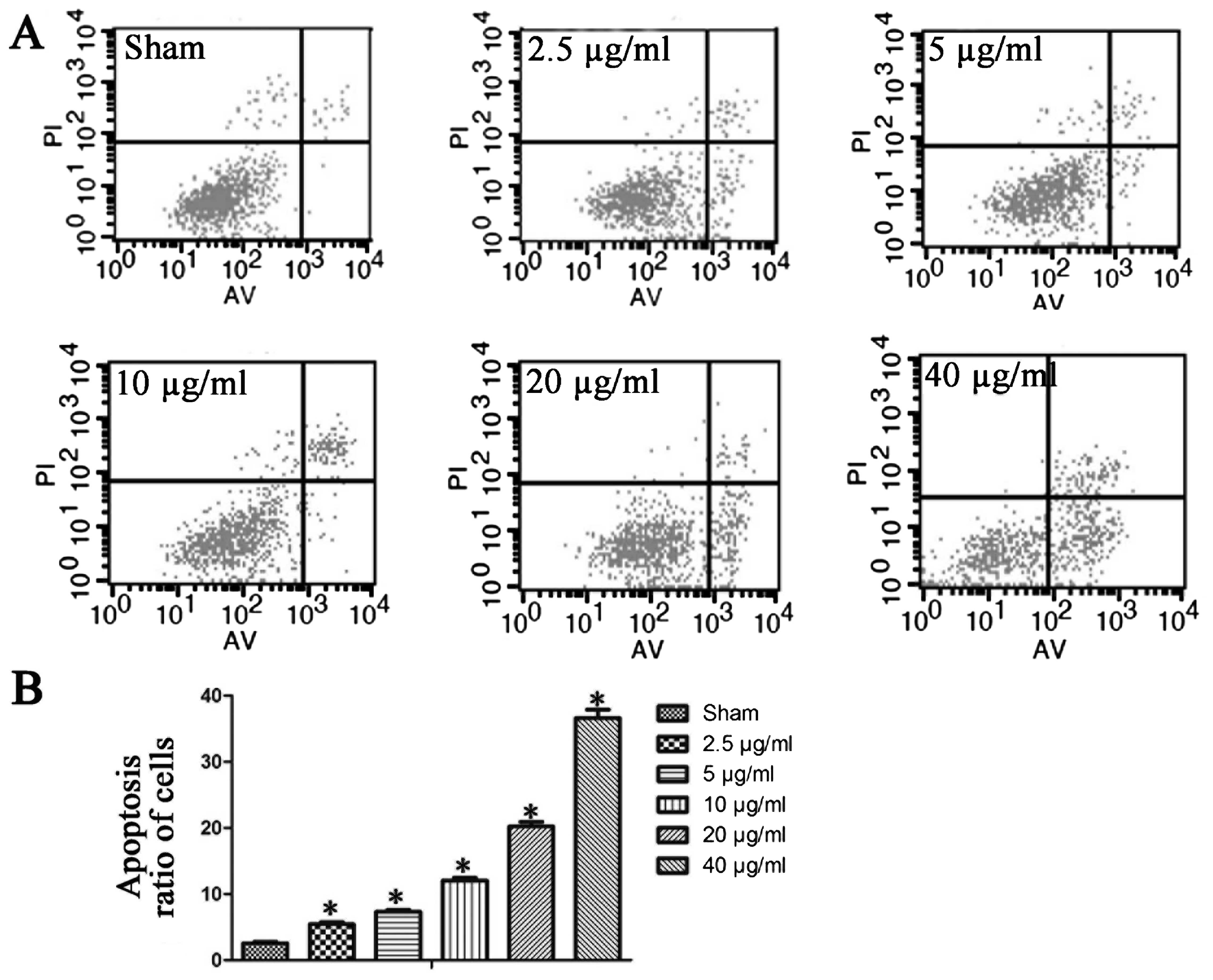
First, we examined apoptosis in HepG2 cells. We
found that NCTD dose-dependently increased the apoptosis ratios in
HepG2 cells. The basal apoptotic population of the sham group was
2.6±0.5%. After treatment for 24 h with 2.5, 5, 10, 20 and 40 μg/ml
NCTD, the apoptotic rates increased to 5.5±0.7, 7.4±0.6, 12.1±0.8,
20.2±1.5 and 36.6±2.8%, respectively (Fig. 1). Based on these data, 10 μg/ml
NCTD was used in all subsequent experiments.
Autophagy counteracts NCTD-induced
apoptosis
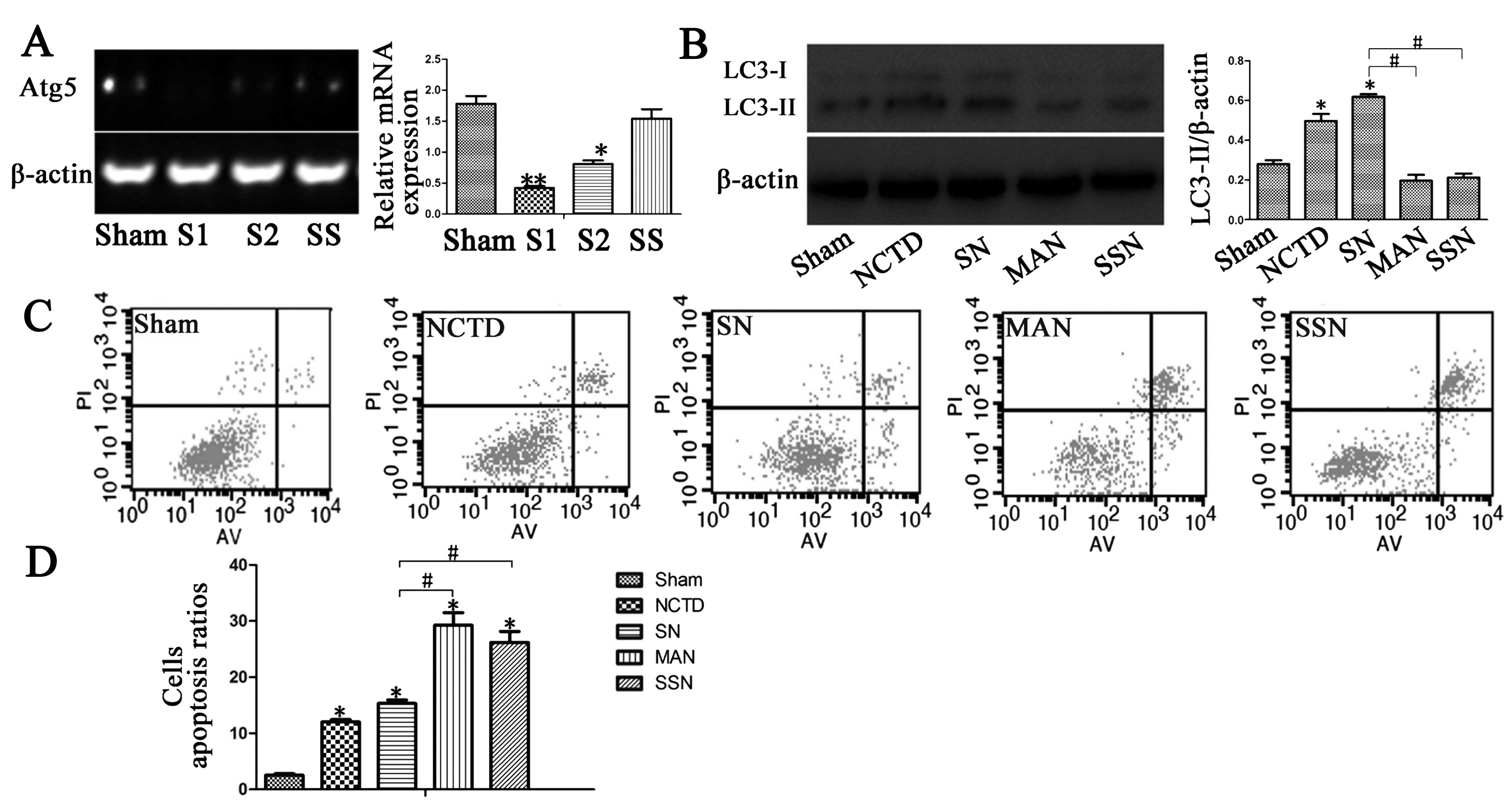
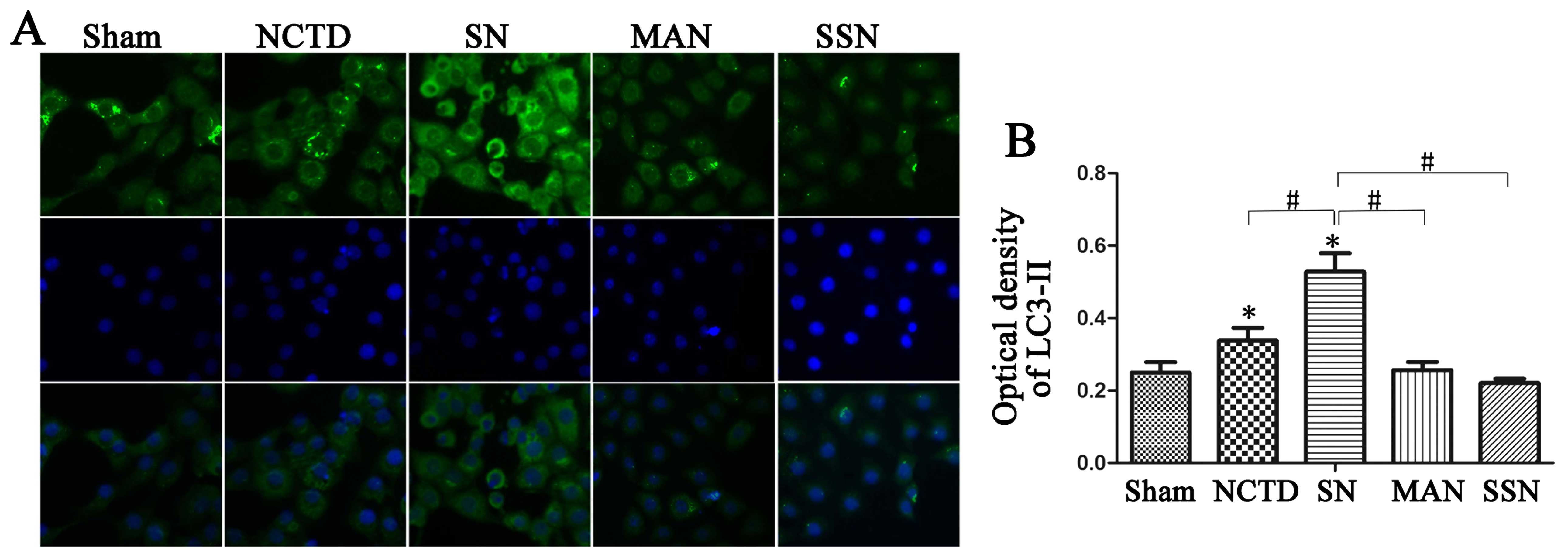
Next, we examined LC3-II expression and found that
HBSS pre-treatment increased LC3-II protein expression. Decreased
apoptotic rates were observed in the HBSS and NCTD (SN) group
compared with the sham group. HBSS-treated HepG2 cells showed
increased LC3-II protein expression in western blotting and
immunostaining analyses (Figs. 2B
and 3; P<0.05). Moreover,
Annexin V/PI staining indicated that the induction of autophagy in
the HBSS and NCTD (SN) group decreased the rates of early and late
apoptosis in HepG2 cells compared with the sham group (Fig. 2C; P<0.05).
Inhibition of autophagy induces HepG2
cell apoptosis
3-MA and Atg5 siRNA were used in HepG2 cells
to inhibit LC3-II expression. We observed decreased Atg5
gene expression in the Atg5 siRNA and NCTD (SSN) group
compared with the sham group (Fig.
2A; P<0.05). In addition, LC3-II expression was inhibited in
the 3-MA and NCTD (MAN) or Atg5 siRNA and NCTD (SSN) groups
compared with the sham group as shown by western blotting and
immunostaining assays (Figs. 2B
and 3; P<0.05). Finally, we
evaluated the apoptotic ratios and found that autophagy inhibition
increased the rate of apoptosis in HepG2 cells (Fig. 2C; P<0.05).
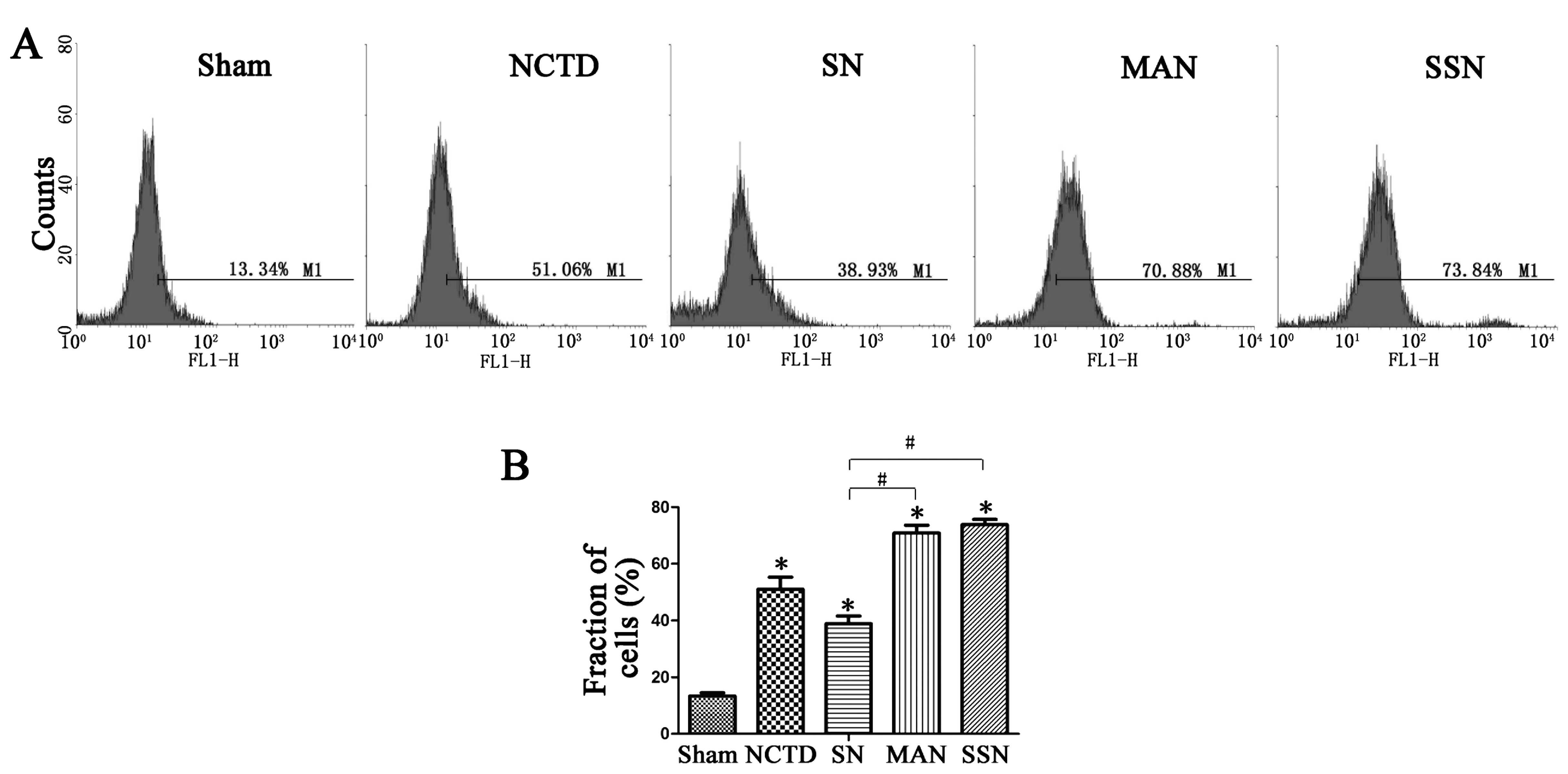
NCTD induces ROS generation and autophagy
inhibition enhances this activity
To further examine the role of NCTD and autophagy,
we evaluated ROS levels in cells after the various treatments. We
found increased intracellular ROS generation in cells treated with
NCTD. Moreover, autophagy inhibition further enhanced ROS
generation compared with the NCTD group. However, when autophagy
was induced, ROS levels were downregulated (Fig. 4).
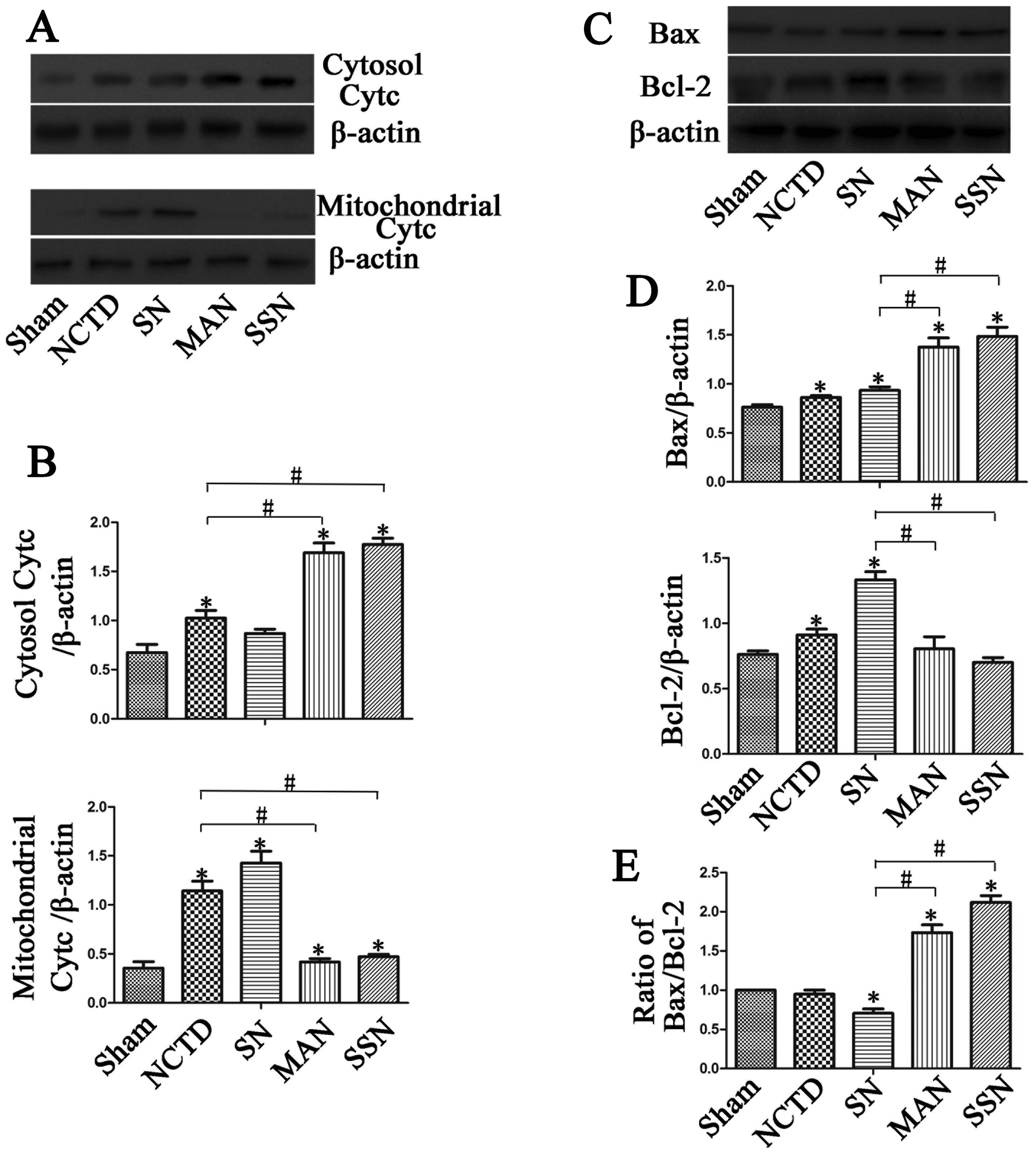
Autophagy inhibition increases Bax,
cytochrome c, caspase-3, caspase-9 and PARP protein expression and
decreases Bcl-2 expression
To further elucidate the mechanisms of apoptosis, we
examined the expression of apoptotic proteins. As shown in Fig. 5, downregulation of autophagy
increased Bax and cytosolic cytochrome c protein expression
compared with the sham group. However, the increase was abrogated
when autophagy was induced in the HBSS and NCTD (SN) group
(P<0.05). Additionally, mitochondrial cytochrome c and
Bcl-2 protein expression was downregulated in the HBSS and NCTD
(SN) group compared with the sham, 3-MA and NCTD (MAN) or
Atg5 siRNA and NCTD (SSN) groups (P<0.05).
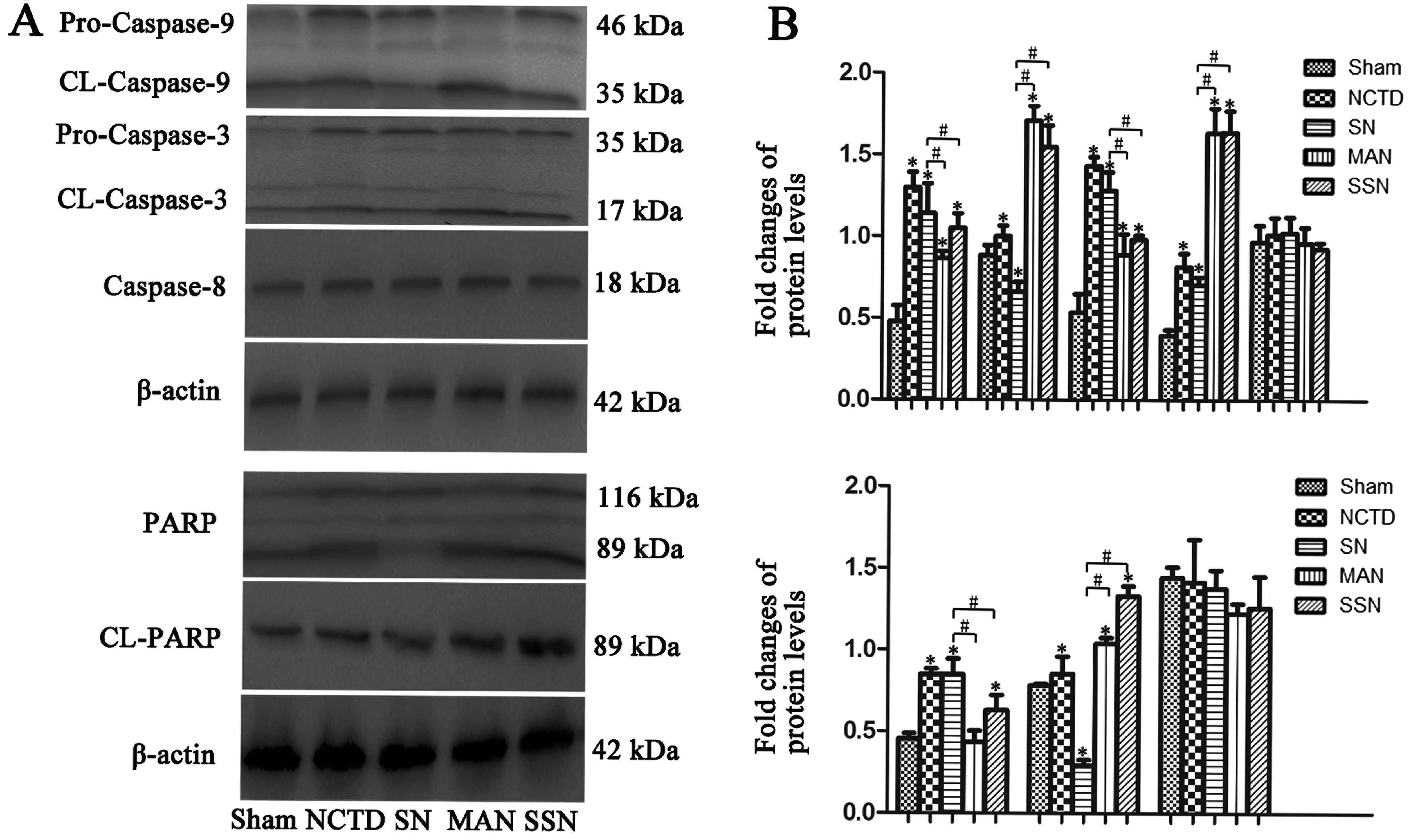
Next, we evaluated the cleavage of the caspase-3,
caspase-8 and caspase-9. Expression of cleaved caspase-3 and
caspase-9 was rapidly increased in the 3-MA and NCTD (MAN) or
Atg5 siRNA and NCTD (SSN) groups compared with the sham
group; however, the increase was abrogated in the HBSS and NCTD
(SN) group. We observed no significant changes in the cleaved
caspase-8 expression in any of the groups (Fig. 6; P<0.05). Inhibition of
autophagy in HepG2 cells also promoted PARP cleavage (Fig. 6; P<0.05).
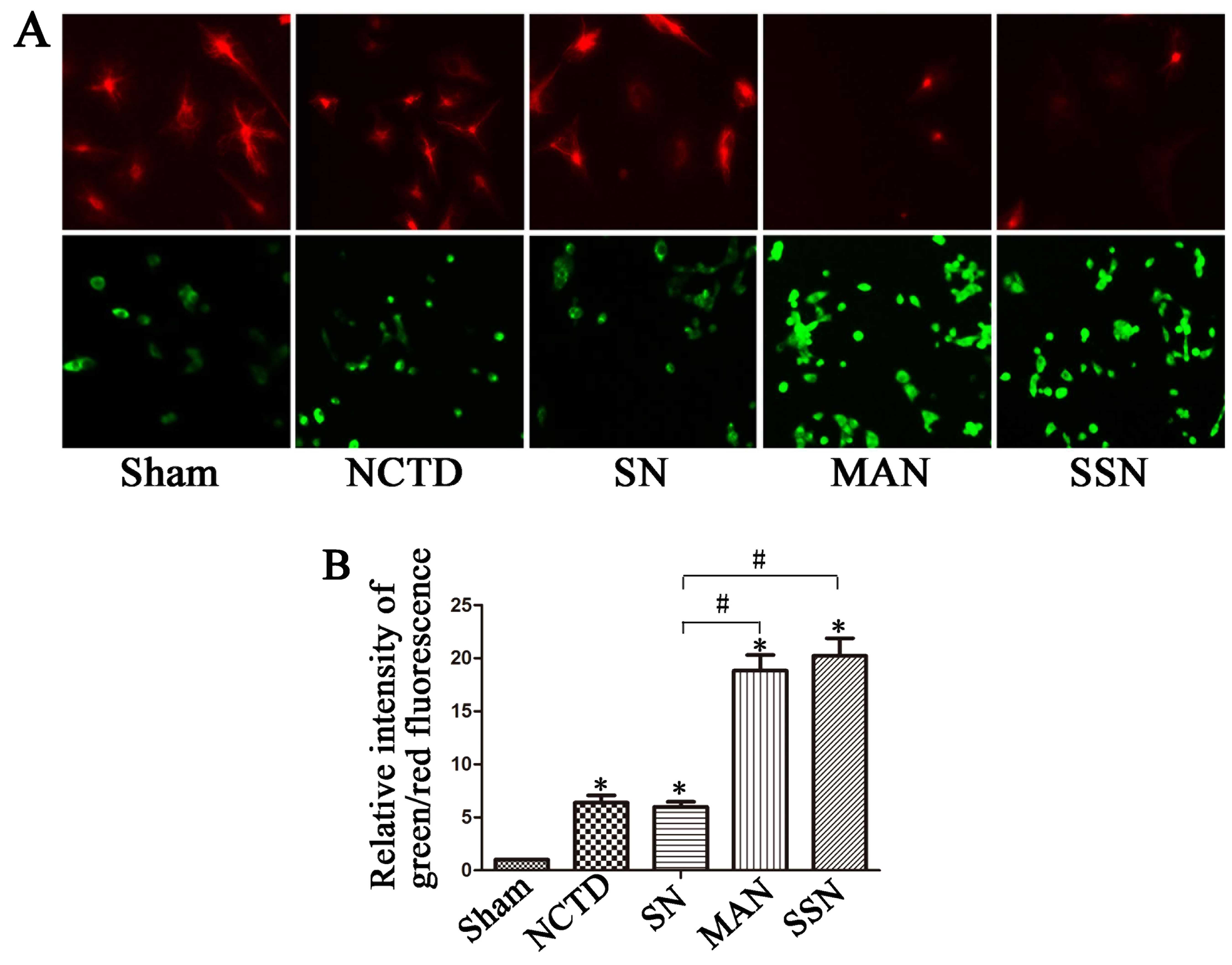
The mitochondrial membrane potential is
disturbed in cells treated with NCTD
Increased green fluorescence in cells indicates
increased mitochondrial membrane potential disturbance. In the
present study, we observed more red fluorescence in the sham and
HBSS and NCTD (SN) cells, with more green fluorescence in the 3-MA
and NCTD (MAN) or Atg5 siRNA and NCTD (SSN) groups (Fig. 7; P<0.05). These data indicate
that there is increased mitochondrial membrane potential
disturbance in cells treated with NCTD, and inhibition of autophagy
enhanced this effect.
Discussion
In the present study, we found that NCTD induced
HepG2 cell apoptosis. Decreased apoptotic ratios were detected when
autophagy was induced in these cells. Moreover, inhibition of
autophagy in HepG2 cells induced HepG2 cell apoptosis.
To further determine the mechanism of apoptosis, we
examined expression of cytochrome c, Bax, Bcl-2, caspase-3,
caspase-8 and caspase-9 protein. We found that inhibition of LC3-II
expression using 3-MA and Atg5 siRNA increased HepG2 cell
apoptosis ratios as shown by increased expression of cytosolic
cytochrome c and Bax. In addition, there was increased
cleavage of caspase-3 and -9. However, when autophagy was induced,
less caspase-3 and -9 was cleaved, and mitochondrial cytochrome
c protein and Bcl-2 protein expression increased. Finally,
enhanced ROS generation was observed in cells where autophagy was
downregulated. When autophagy was induced, ROS levels were
downregulated. Based on our current data and data from previous
reports, we hypothesize that decreasing autophagy increases HepG2
cell apoptosis partly through ROS generation and activation of the
mitochondrial apoptotic pathway.
Hepatocellular carcinoma (HCC) is the most common
type of liver cancer and is one of the leading causes of cancer
deaths worldwide. Because of the complex mechanisms of HCC,
effective therapies toward HCC have not significantly improved
(1,2). As a heterogeneous tumor, HCC involves
multiple pathways and molecular alterations (14). With medical technology advances,
there are increasing number of new treatments towards HCC,
including surgical resection, liver transplantation and
chemotherapy or immuno-biological cancer therapy. Cantharidin,
which is extracted from the Chinese blister beetle, has been used
for many years in Chinese medicine to treat tumors. However,
because of nephrotoxic and other side effects, norcantharidin
(NCTD) was synthesized in an effort to reduce toxicity. Currently,
NCTD is widely used in the treatment of tumors (7,15).
It has been shown that NCTD has antitumor roles in
various experimental animal models and extends the life of some of
these animals. The mechanism of action of NCTD involves inhibition
of protein and nucleic acid synthesis. N-hydroxycantharidimide,
which is synthesized by cantharidin, has certain curative effects
in liver cancer (16). Previous
data showed that NCTD could induce cytotoxicity in HepG2 cells by
inducing apoptosis (10);
therefore, in the present study, we evaluated HepG2 cell apoptosis.
The basal apoptotic population of the sham group was 0.3±0.1%.
After treatment with 2.5, 5, 10, 20 and 40 μg/ml NCTD, the
apoptotic rate increased to 5.5±0.7, 7.4±0.6, 12.1±0.8, 20.2±1.5
and 36.6±2.8%, respectively. These data indicate that NCTD
increases apoptosis in HepG2 cells, and further demonstrate the
dose-dependence of NCTD on the apoptotic ratios in these cells.
Our previous studies showed that miR-101 may enhance
cisplatin-induced apoptosis in HCC cells, and the possible
mechanism involved inhibition of autophagy via targets including
RAB5A, STMN1 and ATG4D (17).
Other studies have reported that autophagy affects liver cancer.
Autophagy is a process whereby cytoplasmic macromolecules or
organelles are degraded in lysosomes to maintain the cellular
balance. Previous data indicate that autophagy functions as a
survival mechanism and elicits protective effects against tissue
and cell injury (18). In general,
it has been noted that autophagy has dual functions, it can lead to
autophagic cell death under certain conditions but also can keep
cells alive under stressful, ‘life-threatening’ conditions. It has
been shown that inhibition of autophagy induced apoptosis of tumor
cells, and knockdown of TP53-induced glycolysis regulator (TIGAR)
expression could enhance the antitumor effects of epirubicin by
increasing cellular ROS levels, activating apoptotic pathways, and
inhibiting autophagy-enhanced epirubicin-induced apoptosis
(19). Like the above
chemotherapeutic drug, in the present study, we found that NCTD was
able to induce HepG2 cell apoptosis, and that inhibition of
autophagy in HepG2 cells induced apoptosis. Taken together, these
data suggest that autophagy leads to autophagic cell death in HepG2
cells.
Apoptosis can be initiated via alternative signaling
pathways: the intrinsic pathway, which is also called the
mitochondria-mediated pathway and the extrinsic or death
receptor-mediated pathway. Mitochondria play a critical role in
apoptotic processes, including drug-induced apoptosis (20,21).
Reports have shown that NCTD could be used to prevent and treat
cancer, likely due to increased apoptosis via ROS generation and
the mitochondrial pathway (22).
Mitophagy is a process that refers to mitochondria degradation via
autophagy. Selective degradation of mitochondria is highly
regulated by various molecules, and is crucial in cell apoptosis
(23).
Therefore, to further dissect the mechanism of NCTD
action, we focused on the apoptotic pathways. It was reported that
the anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax
are crucial for initiation of the mitochondrial death cascade. We
observed downregulation of Bax and upregulation of Bcl-2 in the
autophagy-induced group. However, inhibition of LC3-II expression
using 3-MA and Atg5 siRNA may prevent changes in Bax and
Bcl-2 expression. Moreover, cytosolic cytochrome c protein
expression was induced and mitochondrial cytochrome c
protein expression decreased when autophagy was inhibited. Cleaved
caspase-3 and caspase-9 were also detected, indicating that
activation of caspase-3 and caspase-9 are important in the
mitochondrial apoptotic pathway. The present study found increased
cleavage of caspase-3 and caspase-9 in the 3-MA and NCTD (MAN) or
Atg5 siRNA and NCTD (SSN) groups in which autophagy was
decreased. However, the cleavage of caspase-3 and caspase-9 was
decreased in the sham and HBSS and NCTD (SN) cells in which
autophagy was increased.
We also evaluated PARP protein levels. The PARP
protein is an important apoptosis protein, which is always cleaved
by activated caspase-3. In the 3-MA and NCTD (MAN) or Atg5
siRNA and NCTD (SSN) groups, we observed increased PARP protein
cleavage from the full-length 116 kDa form to its cleaved 89 kDa
form. However, PARP was not cleaved in the SN group. Finally, we
examined the mitochondrial membrane potential. We detected more red
fluorescence in the control and SN groups, but more green
fluorescence in the 3-MA and NCTD (MAN) or Atg5 siRNA and
NCTD (SSN) groups, indicating disturbed mitochondrial membrane
potential. Our data suggest that the inhibition of autophagy
increased NCTD-induced apoptosis, which may occur via a
mitochondria-mediated pathway.
ROS are a series of reactive oxygen species produced
during metabolic processes that are important in tumor occurrence,
development and recurrence. Recent studies have shown that ROS can
accelerate tumor cell death and have antitumorigenic roles
(24). Drugs that increase ROS
levels have been produced and used in clinical trials (24,25).
Some studies have reported that ROS act as a second messenger in
apoptosis. The generation of ROS can induce mitochondrial membrane
potential disturbance, thereby contributing to mitochondrial damage
and leading to cell death by acting as an apoptotic signaling
molecule (26). We found that NCTD
induced ROS production in HepG2 cells. The inhibition of autophagy
caused an NCTD-induced increase in ROS in HepG2 cells, and the
increase in ROS was abolished or attenuated by increasing
autophagy.
In conclusion, we demonstrate that NCTD induced
HepG2 cell apoptosis. The inhibition of autophagy in these cells
also induced apoptosis. Inhibiting LC3-II expression by 3-MA and
Atg5 siRNA also has pro-apoptotic effects via the
mitochondrial apoptotic pathway, and enhanced ROS generation was
observed in cells where autophagy was downregulated. Therefore, we
propose that decreased autophagy increases HepG2 cell apoptosis,
partly via ROS generation and activation of the mitochondrial
apoptotic pathway. However, the exact relationship between
autophagy and the mitochondrial apoptotic pathway, as well as the
function of autophagy in apoptosis, require further study.
Acknowledgements
The present study was supported by grants from the
City Subject Foundation of Xuzhou (KC14SH020) and the Xuzhou City
Central Hospital (XZS2013018).
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
LC3-II
|
light chain 3-II
|
|
NCTD
|
norcantharidin
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Dubois-Pot-Schneider H, Fekir K, Coulouarn
C, Glaise D, Aninat C, Jarnouen K, Le Guével R, Kubo T, Ishida S,
Morel F, et al: Inflammatory cytokines promote the
retrodifferentiation of tumor-derived hepatocyte-like cells to
progenitor cells. Hepatology. 60:2077–2090. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su YH, Lin SY, Song W and Jain S: DNA
markers in molecular diagnostics for hepatocellular carcinoma.
Expert Rev Mol Diagn. 14:803–817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
White JA, Redden DT, Bryant MK, Dorn D,
Saddekni S, Aal AKA, Zarzour J, Bolus D, Smith JK, Gray S, et al:
Predictors of repeat transarterial chemoembolization in the
treatment of hepatocellular carcinoma. HPB (Oxford). 16:1095–1101.
2014. View Article : Google Scholar
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
5
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeh CH, Yang YY, Huang YF, Chow KC and
Chen MF: Induction of apoptosis in human Hep3B hepatoma cells by
norcantharidin through a p53 independent pathway via TRAIL/DR5
signal transduction. Chin J Integr Med. 18:676–682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han W, Wang S, Liang R, Wang L, Chen M, Li
H and Wang Y: Non-ionic surfactant vesicles simultaneously enhance
antitumor activity and reduce the toxicity of cantharidin. Int J
Nanomed. 8:2187–2196. 2013.
|
|
8
|
Rautou PE, Mansouri A, Lebrec D, Durand F,
Valla D and Moreau R: Autophagy in liver diseases. J Hepatol.
53:1123–1134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Longo L, Platini F, Scardino A, Alabiso O,
Vasapollo G and Tessitore L: Autophagy inhibition enhances
anthocyanin-induced apoptosis in hepatocellular carcinoma. Mol
Cancer Ther. 7:2476–2485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chang C, Zhu YQ, Mei JJ, Liu SQ and Luo J:
Involvement of mitochondrial pathway in NCTD-induced cytotoxicity
in human hepG2 cells. J Exp Clin Cancer Res. 29:1452010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Settembre C, Di Malta C, Polito VA, Garcia
Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D,
Colella P, et al: TFEB links autophagy to lysosomal biogenesis.
Science. 332:1429–1433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carchman EH, Rao J, Loughran PA, Rosengart
MR and Zuckerbraun BS: Heme oxygenase-1-mediated autophagy protects
against hepatocyte cell death and hepatic injury from
infection/sepsis in mice. Hepatology. 53:2053–2062. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lewis JS, Meeke K, Osipo C, Ross EA,
Kidawi N, Li T, Bell E, Chandel NS and Jordan VC: Intrinsic
mechanism of estradiol-induced apoptosis in breast cancer cells
resistant to estrogen deprivation. J Natl Cancer Inst.
97:1746–1759. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jhunjhunwala S, Jiang Z, Stawiski EW, Gnad
F, Liu J, Mayba O, Du P, Diao J, Johnson S, Wong KF, et al: Diverse
modes of genomic alteration in hepatocellular carcinoma. Genome
Biol. 15:4362014.PubMed/NCBI
|
|
15
|
Yeh CB, Su CJ, Hwang JM and Chou MC:
Therapeutic effects of cantharidin analogues without bridging ether
oxygen on human hepatocellular carcinoma cells. Eur J Med Chem.
45:3981–3985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Williams LA, Möller W, Merisor E, Kraus W
and Rösner H: In vitro anti-proliferation/cytotoxic activity of
cantharidin (Spanish Fly) and related derivatives. West Indian Med
J. 52:10–13. 2003.PubMed/NCBI
|
|
17
|
Xu Y, An Y, Wang Y, Zhang C, Zhang H,
Huang C, Jiang H, Wang X and Li X: miR-101 inhibits autophagy and
enhances cisplatin-induced apoptosis in hepatocellular carcinoma
cells. Oncol Rep. 29:2019–2024. 2013.PubMed/NCBI
|
|
18
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie JM, Li B, Yu HP, Gao QG, Li W, Wu HR
and Qin ZH: TIGAR has a dual role in cancer cell survival through
regulating apoptosis and autophagy. Cancer Res. 74:5127–5138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie SQ, Zhang YH, Li Q, Xu FH, Miao JW,
Zhao J and Wang CJ: 3-Nitro-naphthalimide and nitrogen mustard
conjugate NNM-25 induces hepatocellular carcinoma apoptosis via
PARP-1/p53 pathway. Apoptosis. 17:725–734. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qi F, Inagaki Y, Gao B, Cui X, Xu H,
Kokudo N, Li A and Tang W: Bufalin and cinobufagin induce apoptosis
of human hepatocellular carcinoma cells via Fas- and
mitochondria-mediated pathways. Cancer Sci. 102:951–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen B, He PJ and Shao CL: Norcantharidin
induced DU145 cell apoptosis through ROS-mediated mitochondrial
dysfunction and energy depletion. PLoS One. 8:e846102013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim I and Lemasters JJ: Mitophagy
selectively degrades individual damaged mitochondria after
photoirradiation. Antioxid Redox Signal. 14:1919–1928. 2011.
View Article : Google Scholar :
|
|
24
|
Zhang R, Humphreys I, Sahu RP, Shi Y and
Srivastava SK: In vitro and in vivo induction of apoptosis by
capsaicin in pancreatic cancer cells is mediated through ROS
generation and mitochondrial death pathway. Apoptosis.
13:1465–1478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang JY, Eggert M, Mouli S, Aljuffali I,
Fu X, Nie B, Sheil A, Waddey K, Oldham CD, May SW, et al:
Pharmacokinetics, antitumor and cardioprotective effects of
liposome-encapsulated phenylaminoethyl selenide in human prostate
cancer rodent models. Pharm Res. 32:852–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ott M, Gogvadze V, Orrenius S and
Zhivotovsky B: Mitochondria, oxidative stress and cell death.
Apoptosis. 12:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|