Introduction
Lung cancer is one of the most frequent causes for
cancer-related death all over the world (1) and non-small cell lung cancer (NSCLC)
accounts for ~85% of lung cancer cases, which can be classified as
squamous cell carcinoma (SC), adenocarcinoma (AC), large cell
carcinoma, and bronchoalveolar carcinoma (BAC) (2). First-line therapeutic adoptions for
all the cell types of lung cancers consist of surgery and
chemotherapy, and platinum based combined chemotherapy remains the
most frequently adopted therapy for patients with advanced NSCLC
(3). Although there are other
targeted therapy strategies, such as monoclonal antibodies and
small molecule tyrosine kinase inhibitors, only a minimal survival
advantage has been observed and ~90% of NSCLC patients die within 5
years of diagnosis (4). Therefore,
novel therapeutic agents and measures with high antitumor
efficiency need continuous investigation.
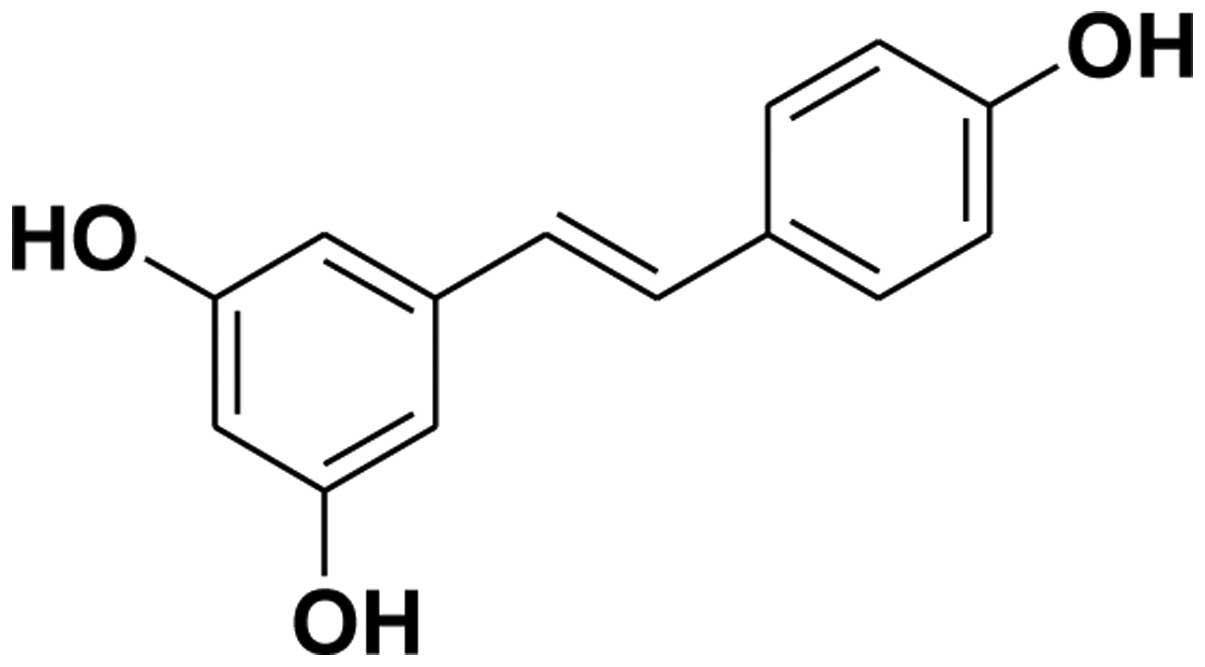
Resveratrol (3,4′,5-trihydroxystilbene) is a
phytochemical which abundantly exists in natural foods, such as
grapes, red wine, berries and peanuts, and Chinese herbal medicine,
such as Polygonum cuspidatum, Veratrum nigrum and
Cassia obtusifolia (5).
Basic research and clinical application have suggested that
resveratrol possesses a wide spectrum of biological and
pharmacological effects due to its multiple hydroxyls (structure
shown in Fig. 1). It has also been
considered that resveratrol would be an ideal alternative drug in
the therapy of cancers and cardiovascular diseases (6,7).
Research on the anticancer effects revealed that resveratrol
induces cell cycle arrest by deregulating expression of cyclin D1,
increasing cancer suppressors, such as p53 and cdk inhibitor p21
(8), and modulating expression of
protein kinase C (PKC) (9). It has
been confirmed that resveratrol induced cell apoptosis by
interfering with expression and function of Bcl-2 phosphorylation
(10), Bax mitochondrial
translocation (11) and inhibition
of DNA synthesis (12). However,
anticancer effects of resveratrol on NSCLC are complicated and
further investigations are needed in order to illustrate how
resveratrol exhibited its anticancer effects in NSCLC.
Various agents exhibit their anticancer effects
mainly through cell cycle arrest and cell apoptosis induction, and
apoptosis was induced by cell death receptor-mediated extrinsic
pathway and mitochondrial mediated intrinsic pathway which can be
triggered by various stimuli, such as reactive nitrogen species
(RNS), reactive oxygen species (ROS), cell-cell interaction,
hormones, growth factor withdrawal, antigens and chemotherapeutics.
It has been widely reported that natural products and agents could
induce cell apoptosis by disturbing the balance between the
production of ROS and the depletion of glutathione (GSH). The
imbalanced redox status could further open the mitochondrial
permeability transition pore (PTP) which may lead to depolarization
of mitochondrial membrane (ΔΨm). Then, cytochrome c is
released into cytosol followed by activation of caspase-3 and
apoptotic cell death (13,14).
Taking the above into account, we hypothesized that
resveratrol treatment could facilitate the anticancer effects of
cisplatin on depolarization of mitochondrial membrane and release
of cytochrome c followed by abnormal activation of apoptotic
regulators and cell death. In order to evaluate this hypothesis, we
investigated the effects of resveratrol combined with or without
cisplatin on mitochondrial membrane potential as well as following
signaling pathways in H838 and H520 cells. Results from the present
study showed that resveratrol induced cell apoptosis in NSCLC cell
lines and enhanced the anticancer effects of cisplatin via
activation of mitochondrial intrinsic apoptosis cascade.
Materials and methods
Cell lines and culture
Human lung cancer cell lines, H838 and H520, were
purchased from the American Type Culture Collection (ATCC, USA).
the two cell lines were maintained in RPMI-1640 containing 10%
fetal calf serum (Gibco BRL, Grand Island, NY, USA) in a humidified
atmosphere containing 5% CO2 at 37°C. All experiments
were carried out with cells in logarithmic phase.
Cell growth inhibition assay
The effects of resveratrol on cell viability were
determined by microculture tetrazolium (MTT) assay. Briefly, H838
and H520 cells were seeded in 96-well plates at a density of
1×104 cell/well and treated with various doses of
resveratrol or cisplatin for 24 h. After that selected dosage of
resveratrol and cisplatin were used in H838 and H520 cells for 24,
48 and 72 h. Finally, the two cell lines were treated with combined
usage of resveratrol and cisplatin. Following these treatments,
cells were further incubated with MTT (5 mg/ml, dissolved in PBS
and filtered through a 0.22-mm membrane) at 37°C for 4 h before
DMSO was added into each well to dissolve farmazan crystals, and
the absorbance of each well was determined at 492 nm on an
automated Bio-Rad 550 micro-plate reader (Bio-Rad Laboratories
Ltd., Shanghai, China).
Examination of morphological changes of
cells
H838 and H520 cells were seeded in a 24-well plate
and then stimulated with resveratrol and/or cisplatin for 24 h. The
images of the cells were captured with an inverted microscope at
10×10 magnification (DMI6000B, Leica, Germany). Changes of cell
morphology indicated cytotoxicity of resveratrol on H838 and H520
cells.
Measurement of mitochondrial membrane
potential (MMP)
Tetrechloro-tetraethylbenzimidazol carbocyanine
iodide (JC-1, Beyotime Institute of Biotechnology, China) is a
mitochondrial-specific cationic dye, it is a monomer when the
mitochondrial membrane potential is <120 mV and emit a green
light (540 nm) following excitation by blue light (490 nm). JC-1
was used, in the present study, to evaluate the changes of MMP in
H838 and H520 cells with or without stimulation of resveratrol,
cisplatin or combined usage of the two agents. Briefly, cells were
seeded in a 24-well plate at the density of 2×105 cell
per well, and incubated with 5 μM JC-1 for 30 min after challenge
by resveratrol and cisplatin. Finally, fluorescence was captured
with the inverted fluorescence microscope and changes in
fluorescence intensity ratio between the measurements at
wavelengths of 590 (red) and 540 nm (green) were used to evaluate
the mitochondrial membrane potential.
Cell apoptosis analysis by flow
cytometry
The apoptotic rate of H838 and H520 cells challenged
with or without resveratrol and cisplatin was detected by flow
cytometry using Annexin V-FITC/PI staining (KeyGen Biotech,
Jiangsu, China). Briefly, cancer cells were seeded and incubated in
6-well plates and treated with resveratrol and/or cisplatin for 24
h. After that, cells were collected, washed with PBS and
resuspended in binding buffer containing PI and Annexin V-FITC and
incubated at room temperature in the dark for 15 min according to
the manufacturer's instructions. Finally, cells were analyzed by
flow cytometer (Becton-Dickinson, San Jose, CA, USA).
Western blot analysis
The extraction of cytosolic and total proteins of
the cells from different groups was carried out according to
instructions of protein extraction kit (Beyotime Institute of
Biotechnology, Jiangsu, China). Protein concentrations were
determined by BCA method with an assay kit (Beyotime). Equal amount
of proteins from each group were electrophoresed on 12% SDS-PAGE
before transferring to PVDF membrane (Millipore, MA, USA). The
membrane was then blocked with 5% (w/v) non-fat milk and washed
with Tris-buffered saline-Tween solution (TBST). Then, the
membranes were incubated overnight at 4°C with primary antibodies
according to the manufacturer's instructions. After being washed
with TBST, membranes were further incubated with secondary antibody
at room temperature for 2 h. Finally, immune-reactive signals were
detected by ECL detection system (Amersham Pharmacia Biotech).
Statistical analysis
Statistical analysis was performed with SPSS 17.0.
Numeric variables are expressed as means ± SD. Statistical
differences among experimental conditions were performed by one-way
analysis of variance (ANOVA) followed by Dunnett's test. P<0.05
was considered to be statistically significant.
Results
Effects of resveratrol on lung cancer
cell proliferation
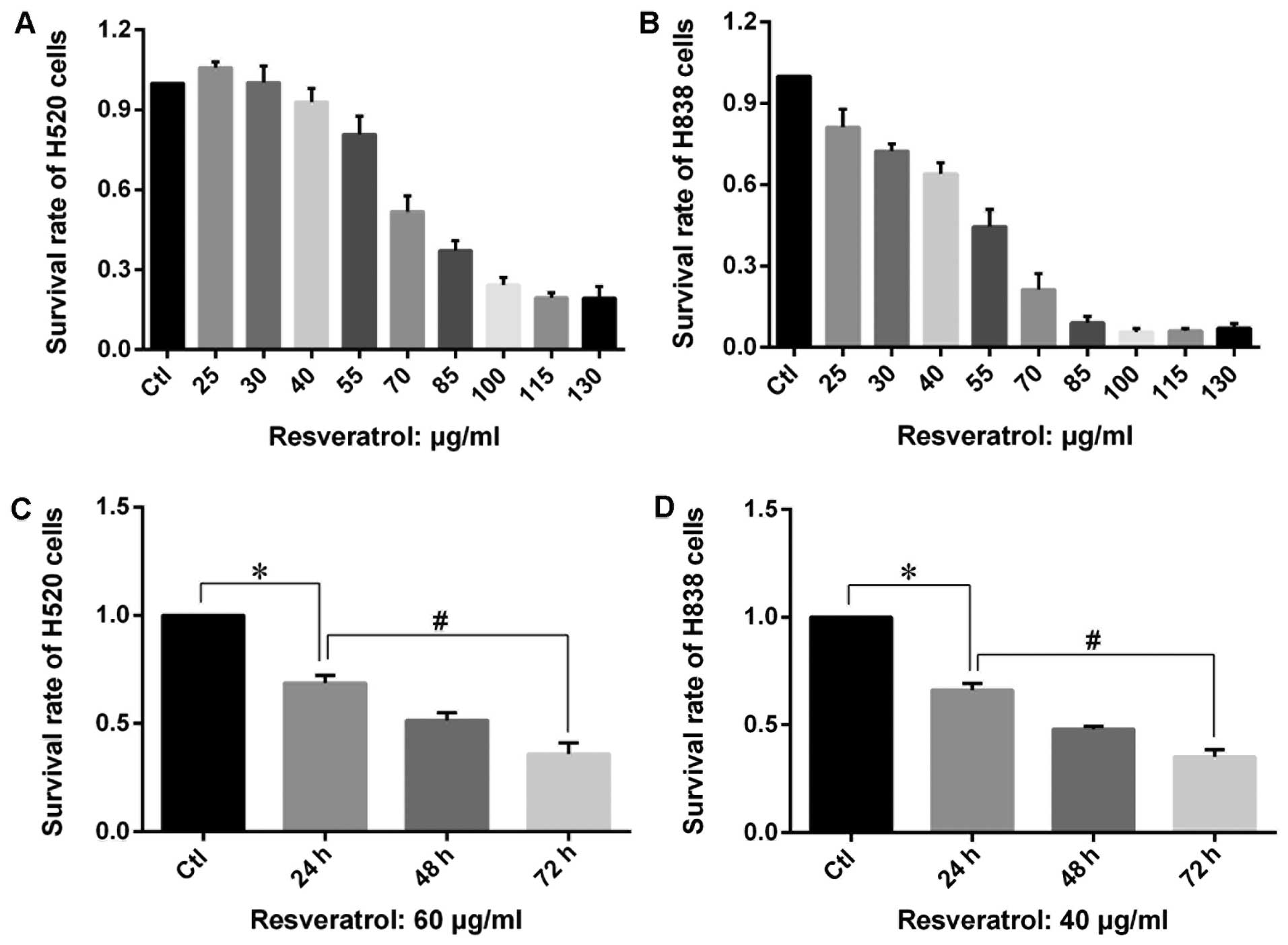
MTT assay was carried out in order to assess the
growth inhibitory effects of resveratrol on proliferation of H838
and H520 cells. The results showed that an extremely low dose of
resveratrol (<40 μg/ml) exhibited a mild enhancing effect on the
proliferation of H520 cells, while higher dose of resveratrol
(>50 μg/ml) inhibited the proliferation of H838 and H520 cells
in a dose- and time-dependent manner (Fig. 2). The MTT results also revealed
that the same dose of resveratrol leads to a much stronger
inhibition on growth of H838 cells compared with that of H520
cells.
Influence of resveratrol on morphology of
lung cancer cells
As shown in Fig. 3A and
E, cell colonies were found in normal H838 and H520 cells, and
most of the normal cells were scattered in the whole field of
microscope. The cell bodies of normal H838 and H520 cells were
stretched in different directions, and nucleus of those normal
cells stayed in the center and formed a relative dark area. The
cells stimulated by resveratrol at 40 μg/ml in H838 cells and 55
μg/ml in H520 cells (shown in Fig. 3B
and F) were sparse with decreased cell number. On the other
hand, single cells challenged by resveratrol exhibited cell
shrinkage, condensed cytoplasm and increased percentage of the
nucleus.
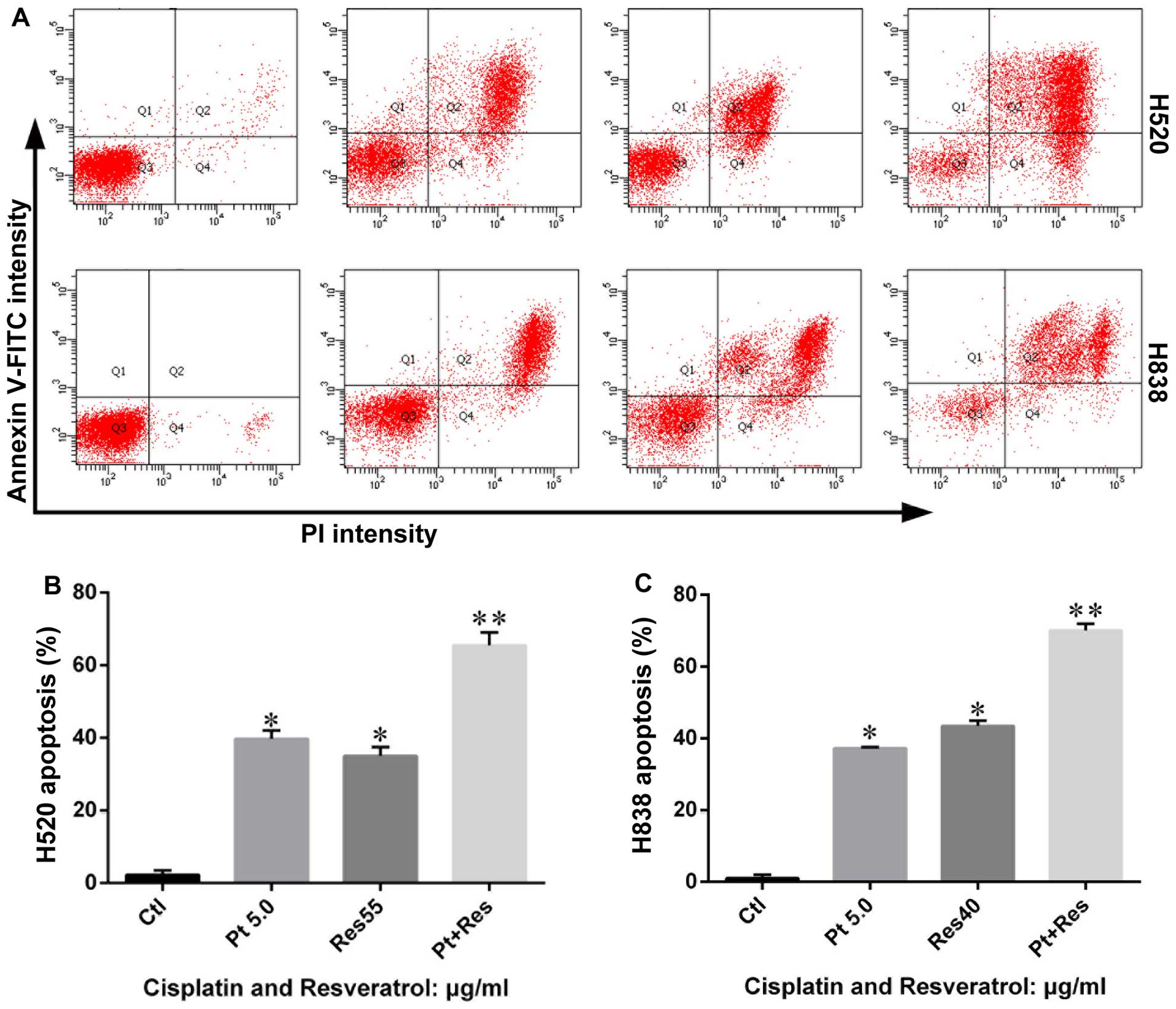
Resveratrol induces apoptosis in H838 and
H520 cells
In order to investigate anticancer effects of
resveratrol on NSCLC, H838 and H520 cells were treated with
resveratrol as described in Materials and methods and the
percentage of cells undergoing apoptosis were determined by flow
cytometric analysis after being stained with Annexin V-FITC and PI.
The results showed that resveratrol (40 μg/ml for H838 cells and 55
μg/ml for H520 cells) caused apoptosis in H838 and H520 cells.
Early apoptotic cells in each experimental group significantly
increased compared with that of control group (Fig. 4). Although there was some
difference in data from H838 and H520 cells, the results from the
two cell types indicated a similar trend after being challenged by
resveratrol.
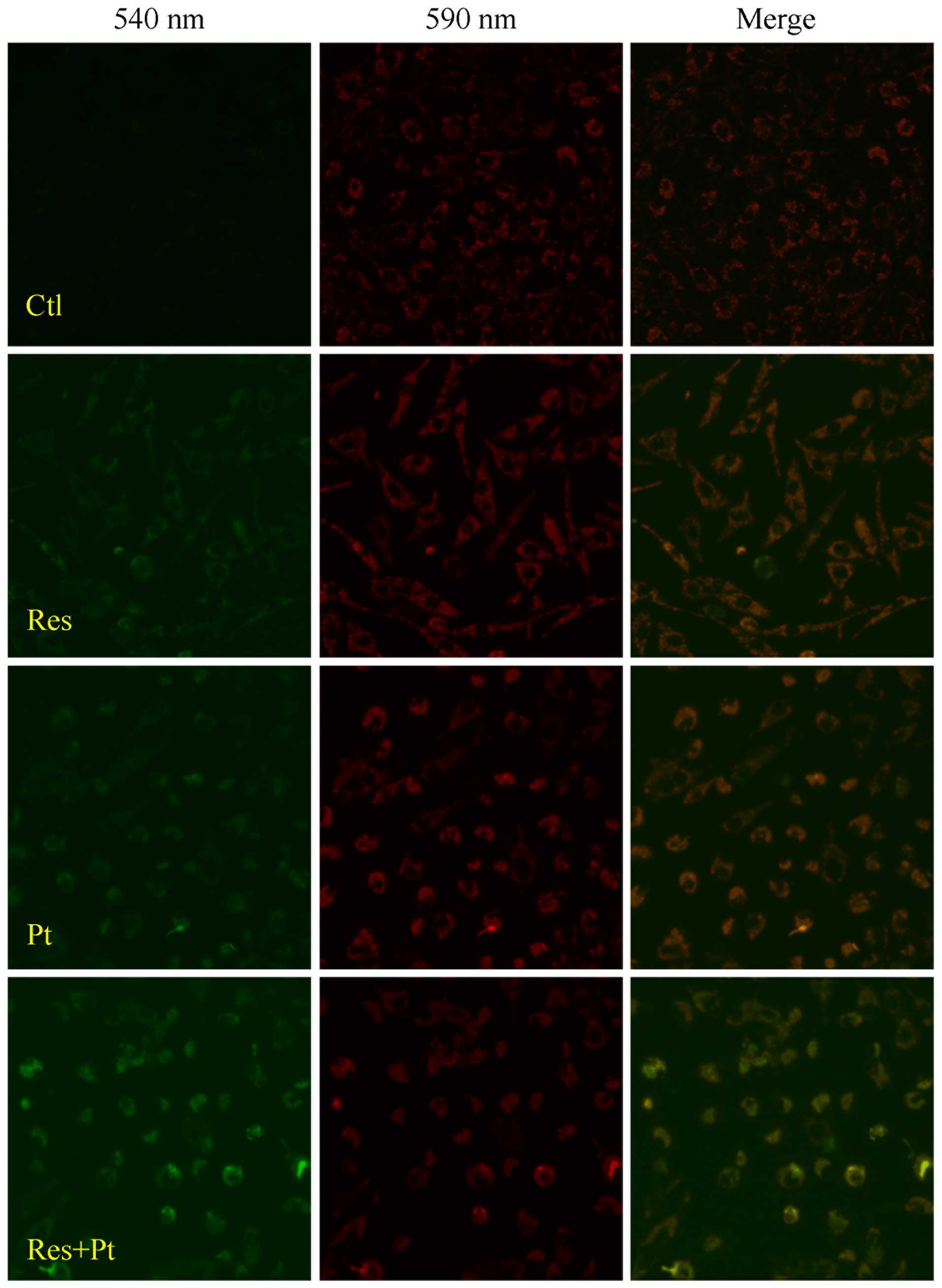
Effects of resveratrol on MMP
According to the above findings, we hypothesized
that the anticancer effects of resveratrol was associated with
function of mitochondria. We evaluated the MMP of cells from each
group stimulated by resveratrol (55 μg/ml) or not. As shown in
Fig. 5, green light in H520 cells
became stronger compared with that of control when stimulated by
resveratrol, which meant a decreased MMP in the cells when
challenged by resveratrol.
Effects of resveratrol on release of
cytochrome c
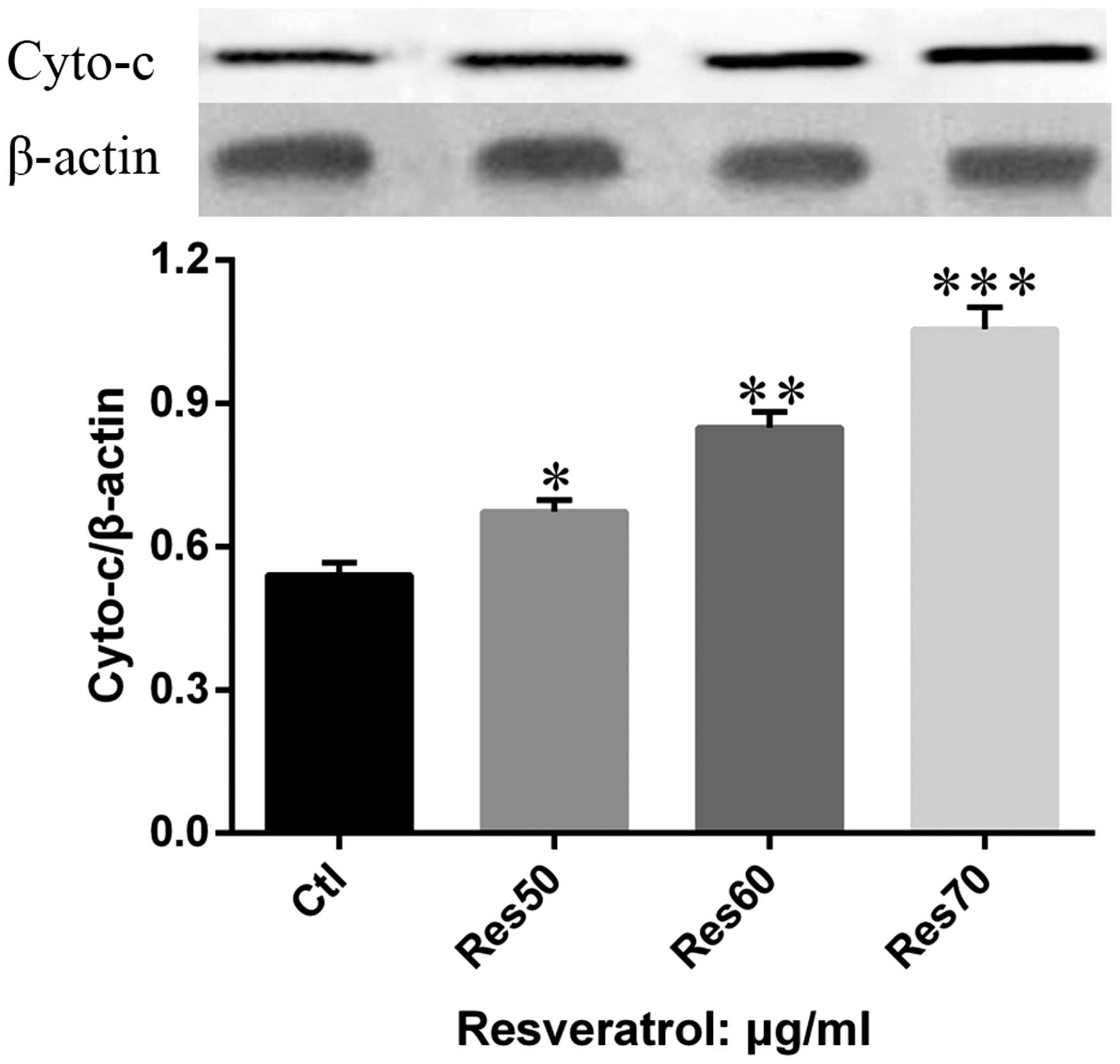
In order to further confirm the role of mitochondria
in resveratrol induced apoptosis in H838 and H520 cells, the
release of cytochrome c from mitochondria to cytosol was
examined in H520 cells treated with different dose of resveratrol
(50, 60 and 70 μg/ml). The results show (Fig. 6) that the content of cytosol
cytochrome c increased in a dose-dependent manner.
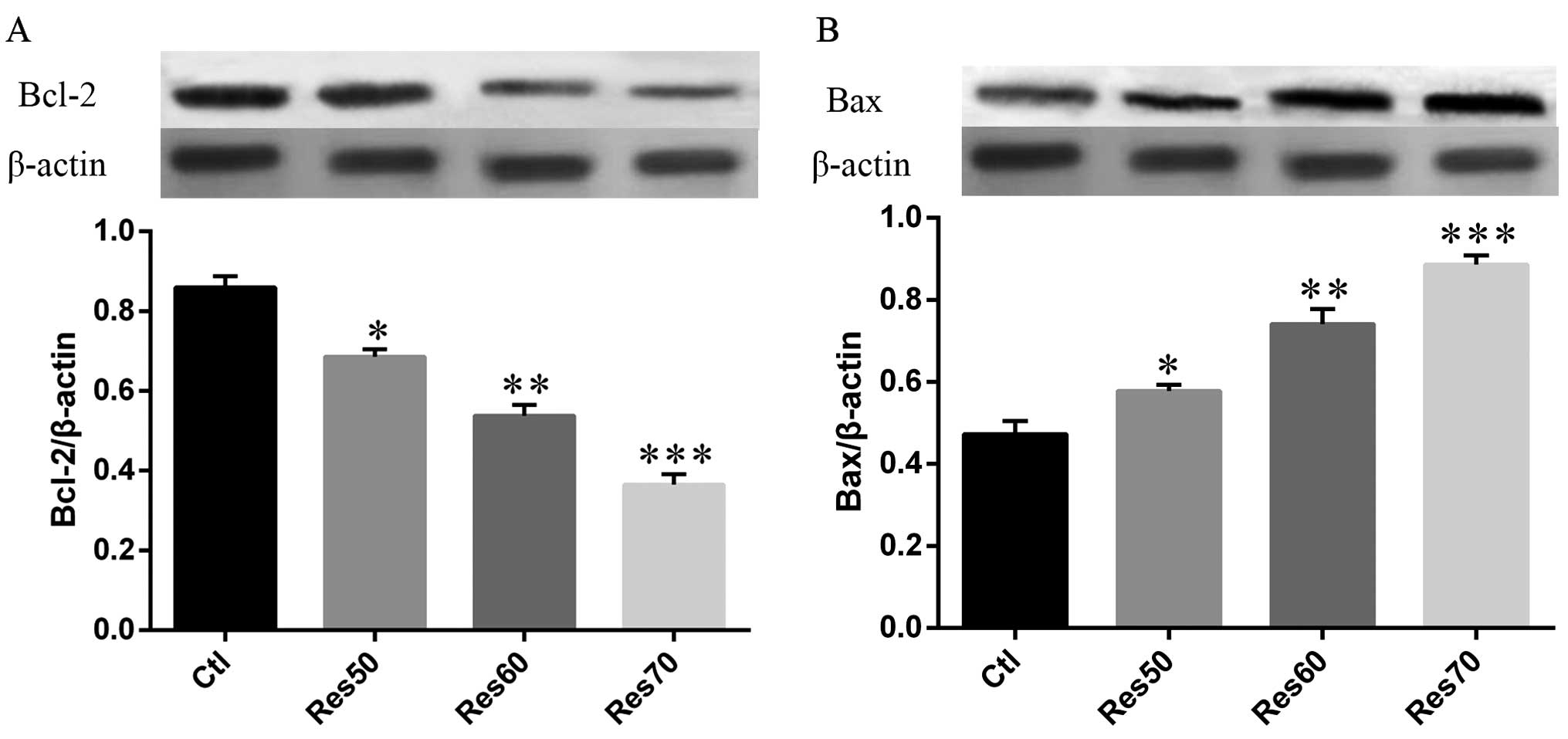
Effects of resveratrol on the expression
of apoptosis regulators
Our data showed that resveratrol-induced apoptosis
was closely related to MMP depolarization. It has been broadly
accepted that Bcl-2 protein family participated in the
mitochondrial apoptotic pathway (14). In order to further illustrate the
mechanism of resveratrol in cell apoptotic effects, we evaluated
the expression of Bcl-2 family proteins in H520 cells treated with
different dose of resveratrol (50, 60 and 70 μg/ml). As shown in
Fig. 7. the expression of Bax was
markedly increased by resveratrol compared with that of control. On
the other hand, the expression of Bcl-2 was significantly decreased
by resveratrol. More importantly, the expression of Bcl-2 and Bax
changed in a dose-dependent manner.
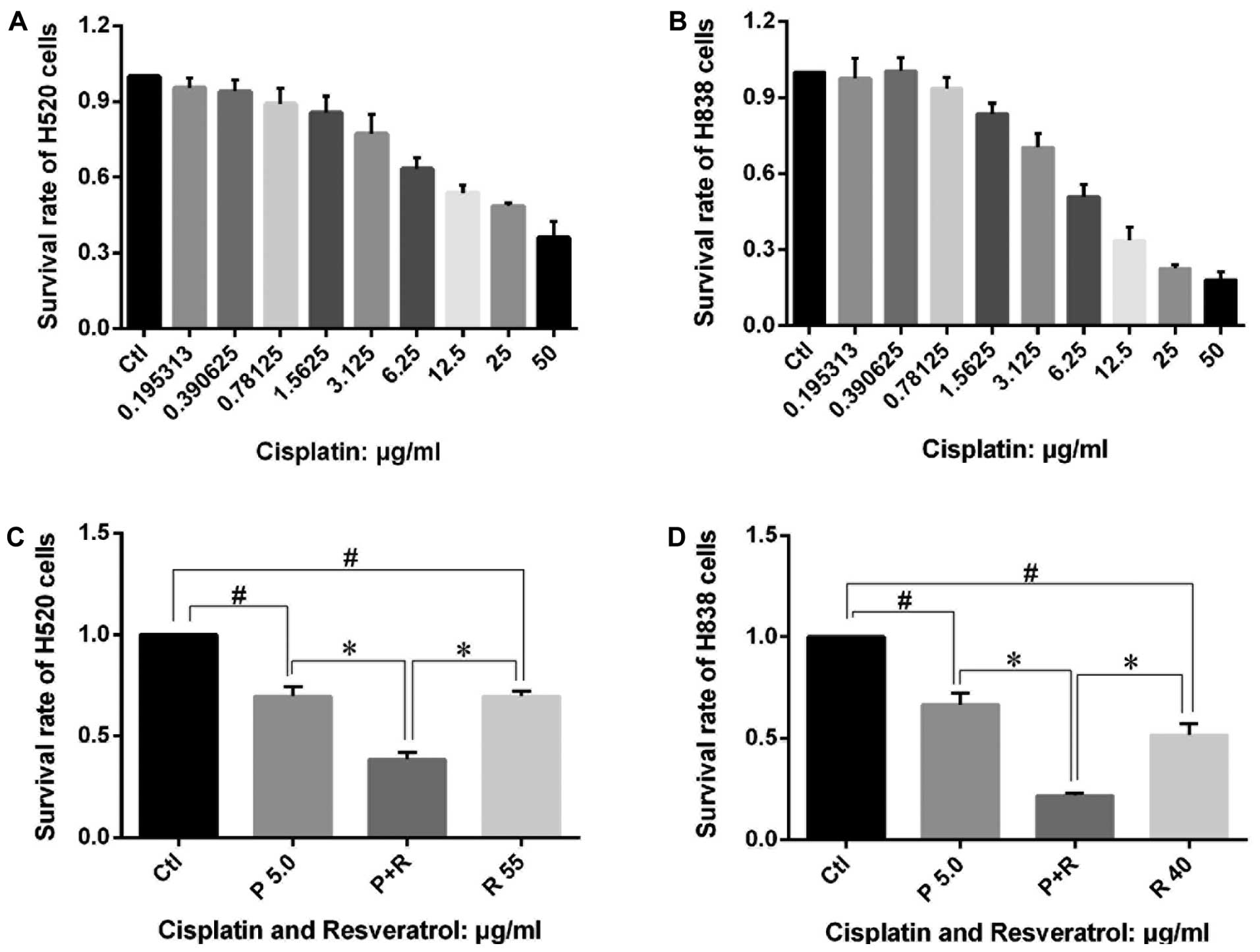
Resveratrol enhances the effects of
cisplatin on NSCLC cell proliferation morphological changes
After confirmation that resveratrol exhibited
anticancer effects by interfering with the mitochondria-related
apoptotic pathway, we evaluated whether resveratrol could enhance
the anticancer effects of cisplatin. MTT assay was carried in H838
and H520 cells stimulated by cisplatin combined with or without
resveratrol. As shown in Fig. 8A and
B, cisplatin inhibited the proliferation of H838 and H520 cells
in a dose-dependent manner, besides, low dose of cisplatin also
suppressed H838 and H520 cell proliferation which was different
from the effects of resveratrol. The combined use of cisplatin (5
μg/ml) and resveratrol (at 40 μg/ml in H838 cells and 55 μg/ml in
H520 cells) exhibited a much better inhibition effect on the
proliferation of the cells than single usage of the two agents
(Fig. 8C and D). The joint
application of cisplatin and resveratrol also resulted in much more
apparent morphological changes in H838 and H520 cells (Fig. 3D and H) compared with that of
cisplatin alone (Fig. 3C and
G).
Resveratrol enhances the effects of
cisplatin on MMP and cell apoptosis
We further examined the MMP and cell apoptosis in
the cells treated with cisplatin combined with or without
resveratrol. Results showed that (Fig.
5) cisplatin could decrease the MMP in H520 cells and there was
severe decrease in MMP in cells stimulated by cisplatin (5 μg/ml)
combined with resveratrol (55 μg/ml). Cell apoptotic results also
showed that resveratrol accelerated cisplatin induced apoptosis in
H838 and H520 cells (Fig. 4).
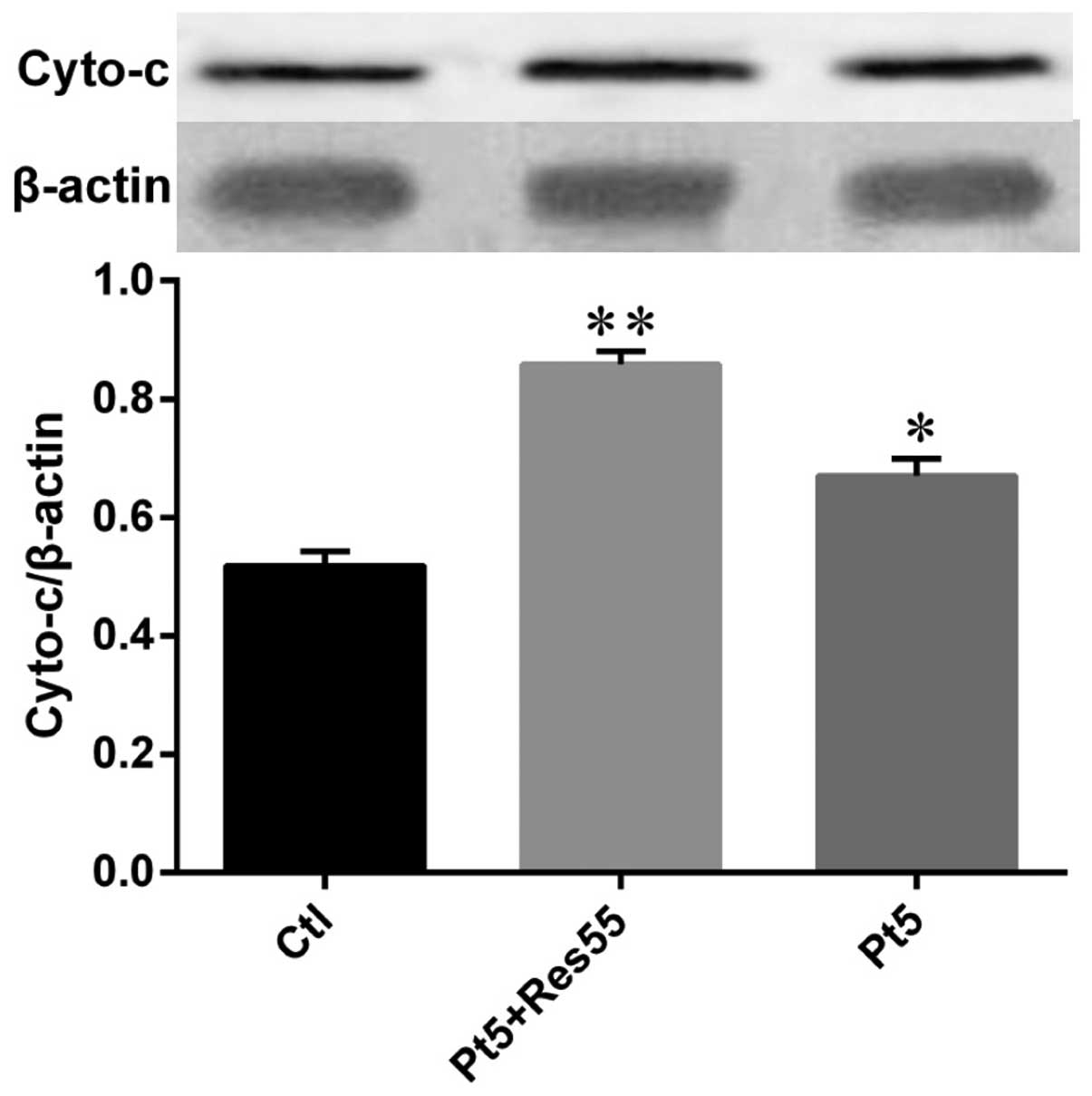
Resveratrol promotes cisplatin-induced
cytochrome c release
In order to validate the enhancement on the
anticancer effects of resveratrol, we examined the cytosol
cytochrome c release in H520 cells treated with cisplatin
combined with or without resveratrol. The results (Fig. 9) showed that cisplatin (5 μg/ml)
increased the content of cytochrome c in cytosol while much
more cytochrome c was released into the cytosol once cells
were stimulated by cisplatin combined with resveratrol (55
μg/ml).
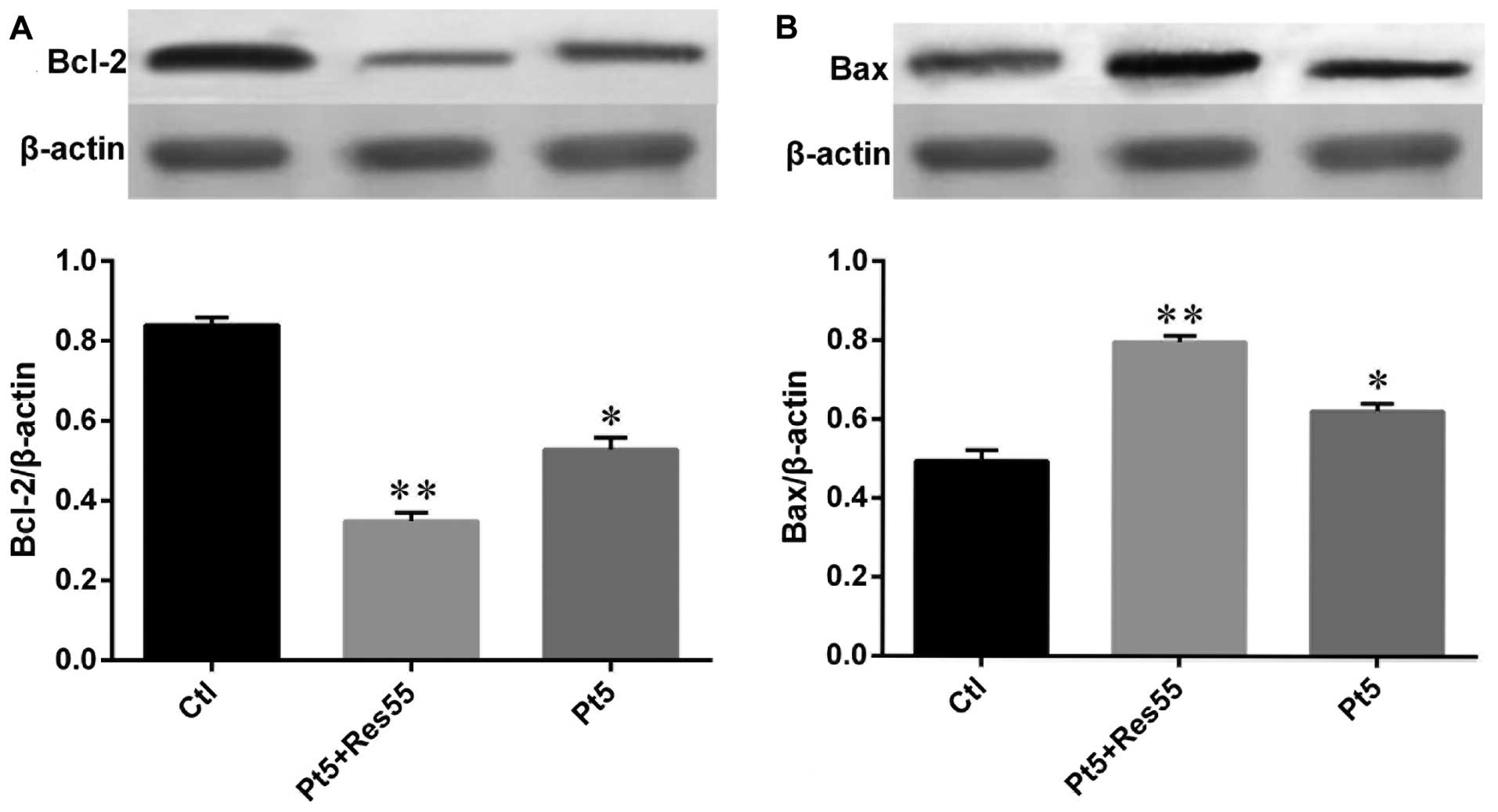
Resveratrol promotes the effects of
cisplatin on apoptosis regulators
The expression of Bcl-2 protein family in H520 cells
stimulated by cisplatin, resveratrol and joint application of the
two agents were also evaluated by western blot analysis. The
results (Fig. 10) showed that
both cisplatin (5 μg/ml) and resveratrol (55 μg/ml) decreased Bcl-2
expression and increased the expression of Bax, while combined
usage of cisplatin and resveratrol led to more marked decrease in
Bcl-2 expression and resulted in more expression of Bax compared
with that in cells challenged by a single agent.
Discussion
The high toxicity of anticancer drugs adopted in
clinical first line therapy to normal tissues and cells is a near
impassable barrier for cancer therapy. However, agents derived from
various plants with few or no side effects have been recognized as
potential alternative or auxiliary cure for cancer patients.
Resveratrol, extracted from grape or polygonum, is such a natural
compound and has been confirmed to possess anti-cancer potentials,
reduce blood viscosity, maintaining blood flow, and inhibiting
platelet aggregation (15,16). Previous studies have also indicated
that resveratrol enhanced the sensitivity of tumor cells to
chemotherapeutic agents, which was one of the major goals in the
development of auxiliary chemotherapeutic drugs (17–19).
However, the mechanisms how resveratrol sensitized tumor cells to
the drugs were not clearly understood.
In the present study, we investigated the inhibitory
effect of resveratrol on the proliferation of non-small cell lines
H838 and H520 in vitro. The results revealed that
resveratrol decreased the cell viability in a dose- and
time-dependent manner, besides; resveratrol treatment also induced
apoptosis in the cell lines. Further examinations indicated that
resveratrol could lead to depolarization of MMP, and pathway
analysis showed that resveratrol increased the release of
cytochrome c from mitochondria to cytosol, upregulated the
expression of Bax, deregulated Bcl-2 expression and finally
resulted in cell apoptosis. Moreover, the results from combined use
of resveratrol and cisplatin showed that resveratrol enhanced the
effects of cisplatin on inhibition of cancer cell proliferation and
induction of apoptosis in H838 and H520 cells. Those findings
indicated that resveratrol exerted anticancer effects and made it
easier for cisplatin to play its role in proliferation inhibition
and apoptosis induction on non-small cell lung cancer H838 and H520
cells through mitochondrial apoptotic pathway.
Mitochondria are intracellular dynamic organelles
and provide 95% of adenosine triphosphate (ATP) needed in human
body which means mitochondria are essential for cells to maintain
their normal functions (20).
During the process of oxidative phosphorylation, energy is stored
as asymmetric distribution of protons and other ions between inner
and outer mitochondrial membranes which is known as mitochondrial
membrane potential (MMP) (21,22).
Normal MMP is essential for oxidative phosphorylation and
generation of ATP, and decreased MMP is the hallmark of early cell
apoptosis induced by various stimuli. It has been confirmed, in the
present study, that individual use of resveratrol or cisplatin
decreased the MMP in H520 cells, however, combined application of
the two agents dramatically upgraded the effects of cisplatin on
depolarization of MMP in H520 cells. All these results indicated
that resveratrol affected the biological functions of cancer cells
and enhanced the effects of cisplatin in mitochondria related
pathway.
Depolarization of MMP is associated with the opening
of mitochondrial PTP which may lead to release of cytochrome
c from mitochondria to the cytosol. Cytochrome c is a
kind of electron transfer in oxidative phosphorylation and
participates in a variety of enzymatic reaction (23,24).
Cytochrome c released from mitochondria activated caspases
and the degradation products of cleaved caspases could further
increase the permeability of mitochondrial membrane, which may form
a positive feedback and finally leads to cell apoptosis. Based on
these clues, we evaluated the content of cytochrome c in
cytosol by western blot analysis. The results showed that the
content of the cytochrome c increased in a dose-dependent
manner in resveratrol treated H520 cells and it was also revealed
that resveratrol exaggerated the effects of cisplatin on the
release of cytochrome c.
Previous studies have confirmed that the release of
cytochrome c is at least partially controlled by Bcl-2
proteins (25). There are also
studies that showed that Bcl-2 could modulate cell apoptosis even
after cytochrome c was released into cytosol because Bcl-2
may regulate cell apoptosis up- and downstream of cytochrome
c related apoptotic pathway (26). Bcl-2 family proteins are pivotal
regulators for cell survival and apoptosis (27). On the one hand, Bcl-2 was the first
discovered death regulator which could enhance cell survival and
proliferation (28). On the other
hand, Bax is an important pro-apoptosis protein which activated
executors of apoptosis promoting apoptotic cell death (29). Besides, there are studies
indicating that Bcl-2 protein family regulated cell apoptosis by
interfering with the permeability of mitochondrial outer membrane.
Moreover, the expression balance of pro-apoptosis and
anti-apoptosis proteins was also essential for cell survival
because apoptosis would be triggered if Bcl-2 could not restrain
the expression of Bax (30). In
the present study, we evaluated the expression of Bcl-2 and Bax in
the H520 cells treated with resveratrol, cisplatin or combined use
of the two agents. The results revealed that resveratrol
upregulated Bax expression in a dose-dependent manner and decreased
expression of Bcl-2 dose-dependently, resveratrol enhanced the
effects of cisplatin on the upregulation of Bax and de-regulation
of Bcl-2 expression.
In conclusion, results from the present study
demonstrated for the first time that resveratrol inhibited H838 and
H520 cell proliferation, and induced apoptosis in NSCLC cells
through mitochondrial apoptotic pathway. Resveratrol enhanced the
proliferation inhibition and apoptosis inducing effects of
cisplatin at least partially through this pathway. However, further
studies are still needed to fully evaluate anticancer effects of
cisplatin and phloretin combination in vivo and to assess
whether resveratrol could be adopted as a novel auxiliary
therapeutic in cancer treatment.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81071933).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lerouge D, Riviere A, Dansin E, Chouaid C,
Dujon C, Schott R, Lavole A, Le Pennec V, Fabre E, Crequit J, et
al: A phase II study of cisplatin with intravenous and oral
vinorelbine as induction chemotherapy followed by concomitant
chemoradiotherapy with oral vinorelbine and cisplatin for locally
advanced non-small cell lung cancer. BMC Cancer. 14:2312014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jakopovic M, Thomas A and Lopez-Chavez A:
From platinum compounds to targeted therapies in advanced thoracic
malignancies. Anticancer Res. 34:477–482. 2014.PubMed/NCBI
|
|
4
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I: Recent cancer survival
in Europe: a 2000–02 period analysis of EUROCARE-4 data. Lancet
Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chi YC, Lin SP and Hou YC: A new herb-drug
interaction of Polygonum cuspidatum, a resveratrol-rich
nutraceutical, with carbamazepine in rats. Toxicol Appl Pharmacol.
263:315–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Das S and Das DK: Resveratrol: a
therapeutic promise for cardiovascular diseases. Recent Pat
Cardiovasc Drug Discov. 2:133–138. 2007. View Article : Google Scholar
|
|
7
|
Demoulin B, Hermant M, Castrogiovanni C,
Staudt C and Dumont P: Resveratrol induces DNA damage in colon
cancer cells by poisoning topoisomerase II and activates the ATM
kinase to trigger p53-dependent apoptosis. Toxicol In Vitro.
29:1156–1165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Narayanan BA, Narayanan NK, Re GG and
Nixon DW: Differential expression of genes induced by resveratrol
in LNCaP cells: P53-mediated molecular targets. Int J Cancer.
104:204–212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stewart JR, Ward NE, Ioannides CG and
O'Brian CA: Resveratrol preferentially inhibits protein kinase
C-catalyzed phosphorylation of a cofactor-independent,
arginine-rich protein substrate by a novel mechanism. Biochemistry.
38:13244–13251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tinhofer I, Bernhard D, Senfter M, Anether
G, Loeffler M, Kroemer G, Kofler R, Csordas A and Greil R:
Resveratrol, a tumor-suppressive compound from grapes, induces
apoptosis via a novel mitochondrial pathway controlled by Bcl-2.
FASEB J. 15:1613–1615. 2001.PubMed/NCBI
|
|
11
|
Mahyar-Roemer M, Kohler H and Roemer K:
Role of Bax in resveratrol-induced apoptosis of colorectal
carcinoma cells. BMC Cancer. 2:272002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fontecave M, Lepoivre M, Elleingand E,
Gerez C and Guittet O: Resveratrol, a remarkable inhibitor of
ribonucleotide reductase. FEBS Lett. 421:277–279. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; an
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wightman EL, Reay JL, Haskell CF,
Williamson G, Dew TP and Kennedy DO: Effects of resveratrol alone
or in combination with piperine on cerebral blood flow parameters
and cognitive performance in human subjects: a randomised,
double-blind, placebo-controlled, cross-over investigation. Br J
Nutr. 112:203–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kennedy DO, Wightman EL, Reay JL, Lietz G,
Okello EJ, Wilde A and Haskell CF: Effects of resveratrol on
cerebral blood flow variables and cognitive performance in humans:
a double-blind, placebo-controlled, crossover investigation. Am J
Clin Nutr. 91:1590–1597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aires V, Limagne E, Cotte AK, Latruffe N,
Ghiringhelli F and Delmas D: Resveratrol metabolites inhibit human
metastatic colon cancer cells progression and synergize with
chemo-therapeutic drugs to induce cell death. Mol Nutr Food Res.
57:1170–1181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mikstacka R and Ignatowicz E:
Chemopreventive and chemotherapeutic effect of trans-resveratrol
and its analogues in cancer. Pol Merkur Lekarski. 168:496–500.
2010.(In Polish).
|
|
19
|
Frampton GA, Lazcano EA, Li H, Mohamad A
and DeMorrow S: Resveratrol enhances the sensitivity of
cholangiocarcinoma to chemotherapeutic agents. Lab Invest.
90:1325–1338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hamada M, Sumida M, Okuda H, Watanabe T,
Nojima M and Kuby SA: Adenosine
triphosphate-adenosine-5′-monophosphate phosphotransferase from
normal human liver mitochondria. Isolation, chemical properties,
and immunochemical comparison with Duchenne dystrophic serum
aberrant adenylate kinase. J Biol Chem. 257:13120–13128.
1982.PubMed/NCBI
|
|
21
|
Lei T, Guo N, Tan MH and Li YF: Effect of
mouse oocyte vitrification on mitochondrial membrane potential and
distribution. J Huazhong Univ Sci Technolog Med Sci. 34:99–102.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diaz G, Setzu MD, Zucca A, Isola R, Diana
A, Murru R, Sogos V and Gremo F: Subcellular heterogeneity of
mitochondrial membrane potential: relationship with organelle
distribution and intercellular contacts in normal, hypoxic and
apoptotic cells. J Cell Sci. 112:1077–1084. 1999.PubMed/NCBI
|
|
23
|
Kosekova G, Mitovska M, Minkov I, Dancheva
K and Atanasov B: Effect of di-substituted cytochrome C pyridoxal
phosphate on oxidative phosphorylation in cytochrome C-deficient
liver mitochondria. Eksp Med Morfol. 20:12–17. 1981.(In
Bulgarian).
|
|
24
|
Wilson DF and Vinogradov SA: Mitochondrial
cytochrome c oxidase: mechanism of action and role in regulating
oxidative phosphorylation. J Appl Physiol. 117:1431–1439. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gleichmann M, Beinroth S, Reed JC,
Krajewski S, Schulz JB, Wullner U, Klockgether T and Weller M:
Potassium deprivation-induced apoptosis of cerebellar granule
neurons: cytochrome c release in the absence of altered expression
of Bcl-2 family proteins. Cell Physiol Biochem. 8:194–201. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karabay AZ, Aktan F, Sunguroglu A and
Buyukbingol Z: Methylsulfonylmethane modulates apoptosis of
LPS/IFN-gamma-activated RAW 264.7 macrophage-like cells by
targeting p53, Bax, Bcl-2, and PARP proteins. Immunopharmacol
Immunotoxicol. 36:379–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rong Y and Distelhorst CW: Bcl-2 protein
family members: versatile regulators of calcium signaling in cell
survival and apoptosis. Annu Rev Physiol. 70:73–91. 2008.
View Article : Google Scholar
|
|
28
|
Anvekar RA, Asciolla JJ, Missert DJ and
Chipuk JE: Born to be alive: a role for the BCL-2 family in
melanoma tumor cell survival, apoptosis, and treatment. Front
Oncol. 1:342011. View Article : Google Scholar
|
|
29
|
Yan W, Suominen J, Samson M, Jegou B and
Toppari J: Involvement of Bcl-2 family proteins in germ cell
apoptosis during testicular development in the rat and pro-survival
effect of stem cell factor on germ cells in vitro. Mol Cell
Endocrinol. 165:115–129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fletcher JI, Meusburger S, Hawkins CJ,
Riglar DT, Lee EF, Fairlie WD, Huang DC and Adams JM: Apoptosis is
triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc
Natl Acad Sci USA. 105:18081–18087. 2008. View Article : Google Scholar : PubMed/NCBI
|