Introduction
Pancreatic cancer, a biologically aggressive tumor
with early metastasis and a dismal prognosis, has increased in
global incidence in recent decades and is a major cause of death
worldwide (1,2). However, most patients are locally
advanced or metastatic cases when diagnosed and have to rely on
chemotherapy, radiation or even palliative care (3). Less than 20% of patients are suitable
for a radical surgical procedure; therefore, it is critical for
surgeons to establish a standard surgical procedure that can be
widely accepted, especially with regard to the optimal
lymphadenectomy protocol during the resection (4–6).
As for determining the optimal lymphadenectomy
during pancreatoduodenectomy for pancreatic head cancer, whether an
extended lymphadenectomy should be performed during a radical
resection remained controversial. However, a recent multicenter
randomised controlled trial (RCT) with 244 enrolled cases showed
that extended lymphadenectomy including the dissection of lymph
node stations 8, 9, 12, 13, 14, 16 and 17 did not result in a
survival benefit for pancreatic head cancer patients compared with
a standard procedure that only includes lymph nodes 12c, 13 and 17
(7). Likewise, a consensus
statement from the ISGPS (International Study Group on Pancreatic
Surgery) declared that a standard lymph node resection of LN
stations 5, 6, 8a, 12b, 12c, 13, 14a, 14b and 17 should be
recommended rather than an extended dissection (8). Within both authoritative reports,
there still remained disputes regarding the dissection of LN
stations 8, 12 and 14 as a standard procedure. However, it was
recommended that the resection of para-aortic lymph nodes (LN16),
which consistently fell into the category of an extended
lymphadenectomy, not be routinely performed during Whipple
surgery.
Based on the Japanese Pancreas Society (JPS) staging
systems for pancreatic cancer, the para-aortic lymph nodes (LN16)
were categorized into a Group 3 lymph node station, the involvement
of which was generally regarded as indicative of distant metastasis
for pancreatic head cancer (9).
Nevertheless, the situation of LN16 positivity in pancreatic head
cancer is relatively common. In some cases, it is even more common
than the involvement of the Group 1 or 2 lymph node stations
(10–12). Despite the lack of level I
evidence, some cohorts of patients with metastatic LN16 could still
benefit from surgery, and a multicenter study that included 822
cases even declared that metastasis to LN16 was not an independent
prognostic factor for pancreatic cancer (13). Consequently, it still remains
embarrassing for a surgeon to encounter a confirmed or suspected
metastatic LN16 in surgical procedure with a resectable pancreatic
cancer. We are left with the question of whether the curative
resection should be abandoned regardless of whether the primary
tumor is resectable, only because the positive para-aortic lymph
nodes are pathologically confirmed.
To address the issue of whether para-aortic lymph
nodes should be excluded from standard lymphadenectomy for all
cases of pancreatic head cancer, an expert panel from high volume
centers in China participated in a consensus conference hosted by
the Chinese Study Group for Pancreatic Cancer (CSPAC) in April 2015
to review the published literature and discuss the indication of
LN16 resection in a Whipple procedure for pancreatic cancer. This
first Chinese expert consensus from the CSPAC on pancreatic cancer
formulated a personalized proposal on the extent of lymph node
dissection for different subgroups of resectable pancreatic head
cancer, which may add to the armamentarium available to pancreatic
surgeons.
Materials and methods
For the consensus statement, a PubMed literature
search was performed in Feb 2015 by entering the terms including
‘pancreatic cancer’, ‘pancreatic head cancer’, ‘carcinoma of the
head of the pancreas’, ‘lymph node metastasis’, ‘extended
lymphadenectomy’, ‘para-aortic lymph node’,
‘pancreatoduodenectomy’, ‘metastasis’, ‘dorsal pancreas’, ‘ventral
pancreas’, ‘mesopancreas’, ‘total mesopancreas excision’, ‘R0
resection’, ‘R1 resection’, ‘prognosis’ and ‘lymph node sampling’.
According to the evidence level recommended by the Oxford Centre
for Evidence-based Medicine, the reports were evaluated and rated;
all case reports and non-English papers were excluded. A draft of
the consensus statement was prepared by two CSPAC members (CL and
XJY), then discussed, followed by an agreement by CSPAC members at
a conference held in April 2015 in Shanghai, China.
Results and consensus statements
i) The strategy for resectable pancreatic
head cancer cases in which LN16 is not suspected to be involved by
pre- or intra-operative judgment
Although the LN16 nodes were defined as Group 3
lymph node stations by JPS, the involvement of para-aortic lymph
nodes (LN16) was quite common in pancreatic head cancer cases.
Nakao et al reported that the incidence of LN16 positivity
in pancreatic head cancer was as high as 26% (12). Several other studies also found a
high rate of LN16 metastasis that was close to or even higher than
the incidence of some Group 2 lymph node stations such as LN8, LN12
(10–12,14).
In a more recent study, metastasis to LN16 was surprisingly found
to be as common as Group 1 lymph node stations (15). A Japanese study of the lymphatic
drainage pathway for the pancreatic head area also inferred that
the lymph node stations around the superior mesenteric artery (SMA)
that belong to Group 2 were not necessarily superior to LN16
stations (16). Based on embryonic
development, the head of the pancreas can be anatomically divided
into two segments, the ventral and dorsal pancreas (17). Accordingly, we wondered if there is
any possibility that there are two or more different patterns of
lymphatic metastasis for pancreatic head cancer. Although several
studies in Asian centers have confirmed that there are different
lymphatic flow characteristics for pancreatic head cancer between
the ventral and dorsal pancreas (18,19),
level I evidence is still lacking to demonstrate that para-aortic
lymph nodes should belong to some group of lymph node stations that
are superior to Group 3 in certain parts of the head of the
pancreas based on tumor location.
For pancreatic head ductal carcinoma, achieving a R0
resection, which is widely acknowledged as a critical prognostic
factor for this disease, or minimizing the rate of R1 resection is
the ultimate goal for a pancreatic surgeon (7,20,21).
Consequently, avoiding the involvement of a retroperitoneal margin
in pancreatic surgery, and an en bloc resection of specimen
including the primary tumor, peripancreatic lymph nodes, and
mesopancreas becomes increasingly appreciated and commended by
experts from several high volume centers (21–24).
The mesopancreas is a virtual anatomic concept that consists of
soft tissues, lymph nodes, vessels, and nerve fibers between the
uncinate process of the pancreas and SMA, which is anatomically
continuous with the para-aortic area. During the
pancreaticoduodenectomy, a total mesopancreas excision will be
smoothly performed when an extensive Kocher's maneuver has been
accomplished, and then para-aortic lymph nodes are easily
incorporated into the resection plane (24,25).
We questioned whether a surgeon should abandon the resection of a
group of lymph nodes that will be easily removed and subsequently
R0 resection could be achieved, just because these lymph nodes are
probably imprecisely categorized into Group 3 stations.
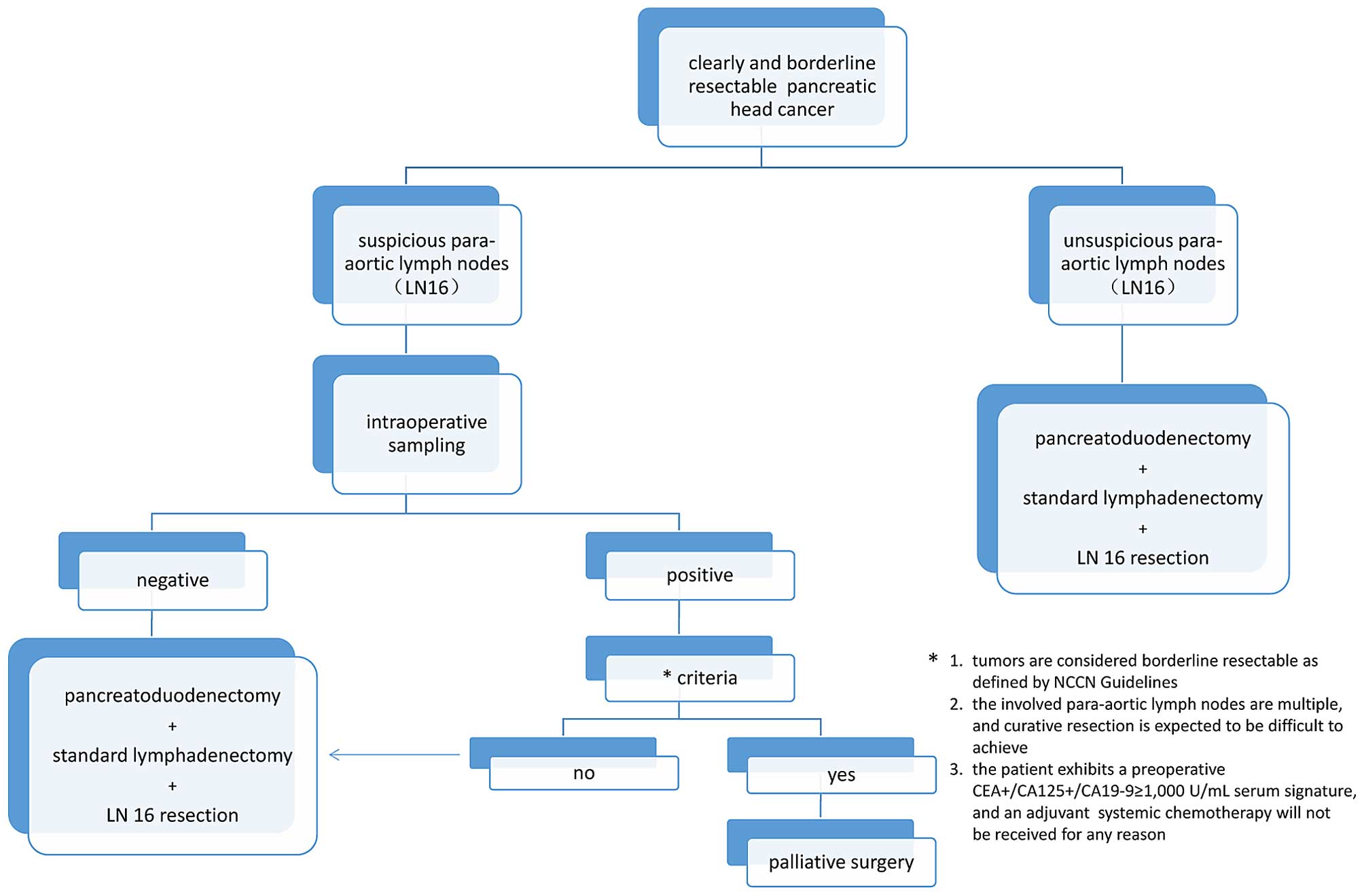
Consensus statements
There is a relatively high incidence of LN16
involvement in pancreatic head ductal carcinoma, along with
uncertainty about the precise lymphatic drainage pathway and
category of para-aortic lymph nodes stations in the dorsal, ventral
or both locations of the head of the pancreas. Therefore, in cases
where pre-operative imaging signs or intra-operative detection
suggest that LN16 is not involved, surgeons are suggested to
dissect the para-aortic lymph nodes including 16a2 and 16b1, which
should be easily incorporated into a resection plane when an
excision with curative intent has performed in addition to a
standard lymphadenectomy (Fig.
1).
ii) The strategy for resectable
pancreatic head cancer cases in which pre- or intra-operative
judgments suspect LN16 involvement
Indeed, for pancreatic head ductal carcinoma,
metastasis to LN16 is perceived as a factor of poor prognosis, as
demonstrated by most reports (15,26).
Based on these reports, a pancreatic cancer patient with LN16
positivity is expected to live <1 year, and disease-free
survival (DFS) is also estimated to be only ~6 months, even in
cases who received curative resection. These survival rates are
basically the same as those for cases who received chemotherapy or
palliative care (26–28). However, a recent multicenter study
of 822 cases reported that metastasis to para-aortic lymph nodes
had no significant value in predicting survival (13), in addition, the presence of
micro-metastasis to LN16 was also found to have no affect on
overall survival (OS) and DFS (27–29).
As reported previously, there could exist some unique cohorts of
patients with pancreatic carcinomas that cannot achieve any
survival benefit from a surgery with curative intent. For instance,
a preoperative high metabolic tumor burden measured by
18F-FDGPET/CT was significantly correlated with poor OS
and recurrence-free survival (RFS) of resected pancreatic cancer
cases (30), and a subsequent
sizeable study demonstrated that a preoperative
CEA+/CA125+/CA19-9 ≥ 1,000 U/ml serum
signature in resected pancreatic cancer patients can distinguish a
subgroup of patients with dismal survival outcomes (31). Despite a similar probability of
poor surgical outcome, some subgroups of LN16 positive cases have
been shown to achieve long-term survival instead of an overall poor
prognosis (32). With or without
adjuvant or neoadjuvant chemotherapy, the numbers of involved LN16,
primary tumor size, the age of the patient, and the presence of
pathological portal vein invasion were all considered as critical
impact factors that altered the prognosis after a curative
resection of pancreatic cancer, even with LN16 metastasis (13,15,32–34).
A recent prospective study claimed the presence of LN16 involvement
should be regarded as a contra-indication to curative resection for
pancreatic cancer (28); however,
due to lack of a subgroup analysis, level I evidence on the
significance of metastasis to para-aortic lymph nodes in pancreatic
head carcinoma still remains elusive. Thus, should the single
indicator of LN16 positivity alone determine the destiny of
patients to be provided or deprived of an opportunity to access a
curative treatment?
It was reported that an intraoperative pathological
sampling of para-aortic lymph nodes was necessary before a curative
resection will be performed, and sensitivity and specificity to
diagnose the involvements of LN16 by frozen sections was 70 and
100%, respectively (28,35). Para-aortic lymph nodes, which are
supposed to be submitted for a frozen section assessment
intraoperatively, reside between the celiac axis and the inferior
mesenteric artery and are divided into 16a2 and 16b1 by the left
renal vein (28,35). In the light of its retroperitoneal
location, it is technically difficult to acquire histopathological
evidence by sampling that can completely reveal the profile of the
para-aortic lymph nodes as involved, ‘micro-involved’ or not
involved. Although several promising techniques such as
intra-operative ultrasound localization and a transperitoneal
laparoscopic approach were introduced into clinical practice
(36), it is still uncommon for
LN16 to be sampled before performing a curative resection for all
the resectable pancreatic cancer cases due to an issue of
sensitivity.
Consensus statements
According to the aforementioned facts, a careful
intraoperative sampling is recommended before a curative intent
surgery is performed only when para-aortic lymph nodes are highly
suspicious to be involved as judged pre- or intra-operatively by
qualified surgeons. If the histopathological results are positive,
a palliative surgery such as by-pass procedure will be performed,
provided that the patients meet any of the following criteria: i)
tumors are considered borderline resectable as defined by NCCN
Guidelines (version 1.2014); ii) the metastatic para-aortic lymph
nodes are multiple, and curative resection is expected to be
difficult to achieve; and iii) the patient exhibits a preoperative
CEA+/CA125+/CA19-9 ≥ 1,000 U/ml serum
signature, and an adjuvant systemic chemotherapy will not be
received for any reason. Otherwise, a curative surgery combined
with LN16 resection is still recommended (Fig. 1).
Discussion
Unlike other digestive organs such as the stomach,
small intestine, and colon, the pancreas does not have a real
anatomic mesopancreas that usually accompanies the peripancreas
lymphatic drainage pathway. Particularly, the head of pancreas
consists of two parts of embryological segments of which ventral
pancreas is bound to dorsal pancreas by a connecting line between
common bile duct and superior mesenteric vein (17). Due to the close adjacency to the
main venous system, including the renal vein and inferior vena
cava, LN16 involvement could develop into a systemic metastasis in
early disease stage even if LN16 should have been categorized as
belonging to a proximal lymph node station rather than a distant
station. This possibility is especially likely for a primary tumor
in the dorsal pancreas such as the uncinate process. Accordingly,
the lymphatic drainage pathway in pancreatic head ductal carcinoma
could be more complicated depending on tumor location, instead of a
single grouping of lymph node stations for every single case.
Pancreatic cancer is a biologically unique tumor,
and there could be one or more cohorts of patients who cannot
benefit from all available therapeutic regimens even with a radical
resection (31). Although the
involvement of a para-aortic lymph node is indicated as a poor
prognostic factor by most studies (26,28,32,33),
level I evidence or sizeable, multiple center, prospective,
controlled trials, especially those with a subgroup analysis of
LN16 status based on different tumor locations for pancreatic head
cancer, still appear insufficient. Several retrospective studies
declared that not all the cases with LN16 positivity always exhibit
poor prognostic signatures (13,15,32–34),
and anatomically, the para-aortic area is continuous with the
mesopancreas and LN16 can technically be incorporated into a
surgical resection plane when R0 intent resection is performed
(24,25). Consequently, LN16 (16a2, 16b1)
dissection is suggested to be routinely included in a standard
lymphadenectomy during pancreatoduodenectomy for pancreatic head
cancer by CSPAC, unless any contraindication mentioned above
mentioned is present.
The current consensus concerning the issue of
para-aortic lymph nodes in the setting of resectable pancreatic
head cancer (including clearly and borderline resectable cases
according to NCCN Guidelines version 1.2014) is supported and
unanimously approved by CSPAC members from several high volume
pancreas centers in China and suggests that an optimal
lymphadenectomy should be performed during the Whipple procedure
for experienced pancreatic surgeons in specialized centers.
Although the dissection of para-aortic lymph nodes is recommended
by the CSPAC in most cases, a multimodal therapy particularly
including systemic chemotherapy should be underlined in the cases
in which LN16 nodes are pathologically involved
postoperatively.
References
|
1
|
Li D, Xie K, Wolff R and Abbruzzese JL:
Pancreatic cancer. Lancet. 363:1049–1057. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi S, Yao W, Xu J, Long J, Liu C and Yu
X: Combinational therapy: New hope for pancreatic cancer? Cancer
Lett. 317:127–135. 2012. View Article : Google Scholar
|
|
3
|
Long J, Luo GP, Xiao ZW, Liu ZQ, Guo M,
Liu L, Liu C, Xu J, Gao YT, Zheng Y, et al: Cancer statistics:
Current diagnosis and treatment of pancreatic cancer in Shanghai,
China. Cancer Lett. 346:273–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu C, Long J, Liu L, Xu J, Zhang B, Yu X
and Ni Q: Pancreatic stump-closed pancreaticojejunostomy can be
performed safely in normal soft pancreas cases. J Surg Res.
172:e11–e17. 2012. View Article : Google Scholar
|
|
5
|
Pedrazzoli S, DiCarlo V, Dionigi R, Mosca
F, Pederzoli P, Pasquali C, Klöppel G, Dhaene K and Michelassi F;
Lymphadenectomy Study Group. Standard versus extended
lymphadenectomy associated with pancreatoduodenectomy in the
surgical treatment of adenocarcinoma of the head of the pancreas: A
multicenter, prospective, randomized study. Ann Surg. 228:508–517.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Farnell MB, Pearson RK, Sarr MG, DiMagno
EP, Burgart LJ, Dahl TR, Foster N and Sargent DJ; Pancreas Cancer
Working Group. A prospective randomized trial comparing standard
pancreatoduodenectomy with pancreatoduodenectomy with extended
lymphadenectomy in resectable pancreatic head adenocarcinoma.
Surgery. 138:618–630. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jang JY, Kang MJ, Heo JS, Choi SH, Choi
DW, Park SJ, Han SS, Yoon DS, Yu HC, Kang KJ, et al: A prospective
randomized controlled study comparing outcomes of standard
resection and extended resection, including dissection of the nerve
plexus and various lymph nodes, in patients with pancreatic head
cancer. Ann Surg. 259:656–664. 2014. View Article : Google Scholar
|
|
8
|
Tol JA, Gouma DJ, Bassi C, Dervenis C,
Montorsi M, Adham M, Andrén-Sandberg A, Asbun HJ, Bockhorn M,
Büchler MW, et al; International Study Group on Pancreatic Surgery.
Definition of a standard lymphadenectomy in surgery for pancreatic
ductal adenocarcinoma: A consensus statement by the International
Study Group on Pancreatic Surgery (ISGPS). Surgery. 156:591–600.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo S: Japanese Pancreas Society staging
systems for pancreatic cancer. Pancreatic Cancer. Neoptolemos JP,
Urrutia R, Abbruzzese JL and Bücher MW: Springer; New York, NY: pp.
1035–1050. 2010, View Article : Google Scholar
|
|
10
|
Kayahara M, Nagakawa T, Ueno K, Ohta T,
Tsukioka Y and Miyazaki I: Surgical strategy for carcinoma of the
pancreas head area based on clinicopathologic analysis of nodal
involvement and plexus invasion. Surgery. 117:616–623. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kayahara M, Nagakawa T, Kobayashi H, Mori
K, Nakano T, Kadoya N, Ohta T, Ueno K and Miyazaki I: Lymphatic
flow in carcinoma of the head of the pancreas. Cancer.
70:2061–2066. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakao A, Harada A, Nonami T, Kaneko T,
Murakami H, Inoue S, Takeuchi Y and Takagi H: Lymph node metastases
in carcinoma of the head of the pancreas region. Br J Surg.
82:399–402. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sho M, Murakami Y, Motoi F, Satoi S,
Matsumoto I, Kawai M, Honda G, Uemura K, Yanagimoto H, Kurata M, et
al: Postoperative prognosis of pancreatic cancer with para-aortic
lymph node metastasis: A multicenter study on 822 patients. J
Gastroenterol. 50:694–702. 2015. View Article : Google Scholar
|
|
14
|
Sakai M, Nakao A, Kaneko T, Takeda S,
Inoue S, Kodera Y, Nomoto S, Kanazumi N and Sugimoto H: Para-aortic
lymph node metastasis in carcinoma of the head of the pancreas.
Surgery. 137:606–611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamada S, Nakao A, Fujii T, Sugimoto H,
Kanazumi N, Nomoto S, Kodera Y and Takeda S: Pancreatic cancer with
paraaortic lymph node metastasis: A contraindication for radical
surgery? Pancreas. 38:e13–e17. 2009. View Article : Google Scholar
|
|
16
|
Hirono S, Tani M, Kawai M, Okada K,
Miyazawa M, Shimizu A, Uchiyama K and Yamaue H: Identification of
the lymphatic drainage pathways from the pancreatic head guided by
indocyanine green fluorescence imaging during
pancreaticoduo-denectomy. Dig Surg. 29:132–139. 2012. View Article : Google Scholar
|
|
17
|
Okamura Y, Fujii T, Kanzaki A, Yamada S,
Sugimoto H, Nomoto S, Takeda S and Nakao A: Clinicopathologic
assessment of pancreatic ductal carcinoma located at the head of
the pancreas, in relation to embryonic development. Pancreas.
41:582–588. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Makino I, Kitagawa H, Ohta T, Nakagawara
H, Tajima H, Ohnishi I, Takamura H, Tani T and Kayahara M: Nerve
plexus invasion in pancreatic cancer: Spread patterns on
histopathologic and embryological analyses. Pancreas. 37:358–365.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kitagawa H, Ohta T, Makino I, Tani T,
Tajima H, Nakagawara H, Ohnishi I, Takamura H, Kayahara M, Watanabe
H, et al: Carcinomas of the ventral and dorsal pancreas exhibit
different patterns of lymphatic spread. Front Biosci. 13:2728–2735.
2008. View Article : Google Scholar
|
|
20
|
Hartwig W, Vollmer CM, Fingerhut A, Yeo
CJ, Neoptolemos JP, Adham M, Andrén-Sandberg A, Asbun HJ, Bassi C,
Bockhorn M, et al; International Study Group on Pancreatic Surgery.
Extended pancreatectomy in pancreatic ductal adenocarcinoma:
Definition and consensus of the International Study Group for
Pancreatic Surgery (ISGPS). Surgery. 156:1–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gaedcke J, Gunawan B, Grade M, Szöke R,
Liersch T, Becker H and Ghadimi BM: The mesopancreas is the primary
site for R1 resection in pancreatic head cancer: Relevance for
clinical trials. Langenbecks Arch Surg. 395:451–458. 2010.
View Article : Google Scholar :
|
|
22
|
Gockel I, Domeyer M, Wolloscheck T,
Konerding MA and Junginger T: Resection of the mesopancreas (RMP):
A new surgical classification of a known anatomical space. World J
Surg Oncol. 5:442007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Inoue Y, Saiura A, Yoshioka R, Ono Y,
Takahashi M, Arita J, Takahashi Y and Koga R: Pancreatoduodenectomy
with systematic Mesopancreas dissection using a Supracolic anterior
artery-first approach. Ann Surg. Jan 13–2015.(Epub ahead of print).
View Article : Google Scholar
|
|
24
|
Adham M and Singhirunnusorn J: Surgical
technique and results of total mesopancreas excision (TMpE) in
pancreatic tumors. Eur J Surg Oncol. 38:340–345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peparini N and Chirletti P: Mesopancreas:
A boundless structure, namely R1 risk in pancreaticoduodenectomy
for pancreatic head carcinoma. Eur J Surg Oncol. 39:1303–1308.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Doi R, Kami K, Ito D, Fujimoto K,
Kawaguchi Y, Wada M, Kogire M, Hosotani R, Imamura M and Uemoto S:
Prognostic implication of para-aortic lymph node metastasis in
resectable pancreatic cancer. World J Surg. 31:147–154. 2007.
View Article : Google Scholar
|
|
27
|
Choi SH, Kim SH, Choi JJ, Kang CM, Hwang
HK and Lee WJ: Clinical necessity of the immunohistochemical
reassessment of para-aortic lymph nodes in resected pancreatic
ductal adenocarcinoma. Oncol Lett. 6:1189–1194. 2013.PubMed/NCBI
|
|
28
|
Schwarz L, Lupinacci RM, Svrcek M,
Lesurtel M, Bubenheim M, Vuarnesson H, Balladur P and Paye F:
Para-aortic lymph node sampling in pancreatic head adenocarcinoma.
Br J Surg. 101:530–538. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kayahara M, Funaki K, Tajima H, Takamura
H, Ninomiya I, Kitagawa H and Ohta T: Surgical implication of
micrometastasis for pancreatic cancer. Pancreas. 39:884–888. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu HX, Chen T, Wang WQ, Wu CT, Liu C, Long
J, Xu J, Zhang YJ, Chen RH, Liu L, et al: Metabolic tumour burden
assessed by 18F-FDG PET/CT associated with serum CA19-9
predicts pancreatic cancer outcome after resection. Eur J Nucl Med
Mol Imaging. 41:1093–1102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Xu H, Wang W, Wu C, Chen Y, Yang J,
Cen P, Xu J, Liu C, Long J, et al: A preoperative serum signature
of CEA+/CA125+/CA19-9 ≥ 1000 U/ml indicates
poor outcome to pancreatectomy for pancreatic cancer. Int J Cancer.
136:2216–2227. 2015. View Article : Google Scholar
|
|
32
|
Yamada S, Fujii T, Sugimoto H, Kanazumi N,
Kasuya H, Nomoto S, Takeda S, Kodera Y and Nakao A: Pancreatic
cancer with distant metastases: A contraindication for radical
surgery? Hepatogastroenterology. 56:881–885. 2009.PubMed/NCBI
|
|
33
|
Murakami Y, Uemura K, Sudo T, Hashimoto Y,
Yuasa Y and Sueda T: Prognostic impact of para-aortic lymph node
metastasis in pancreatic ductal adenocarcinoma. World J Surg.
34:1900–1907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kanda M, Fujii T, Nagai S, Kodera Y,
Kanzaki A, Sahin TT, Hayashi M, Yamada S, Sugimoto H, Nomoto S, et
al: Pattern of lymph node metastasis spread in pancreatic cancer.
Pancreas. 40:951–955. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Imai H, Doi R, Kanazawa H, Kamo N, Koizumi
M, Masui T, Iwanaga Y, Kawaguchi Y, Takada Y, Isoda H, et al:
Preoperative assessment of para-aortic lymph node metastasis in
patients with pancreatic cancer. Int J Clin Oncol. 15:294–300.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim H, Hyung WJ, Lim JS, Park MS, Choi JY,
Chung YE, Kim MJ and Kim KW: Laparoscopic ultrasonography-assisted
retroperitoneal lymph node sampling in patients evaluated for
stomach cancer recurrence. J Ultrasound Med. 27:1229–1233.
2008.PubMed/NCBI
|