Introduction
Malignant gliomas, especially glioblastomas, are the
most frequently occurring primary tumors of the central nervous
system, and represent one of the most lethal malignancies.
Glioblastomas have been reported to be heterogeneous bulk tumors
comprising differentiated and undifferentiated cells with a
self-renewal ability, pluripotency, and tumorigenicity (1). Such heterogeneity may contribute to
tumor expansion, invasion, metastasis, and drug resistance. The
undifferentiated cells, a distinct subpopulation, within the tumors
may derive from a limited source of glioblastoma cells and are
termed glioblastoma stem-like cells (GSCs) (2–4).
These cells are considered to be capable of aberrantly
differentiating into diverse cell types, differentiated glioma
cells (non-glioma stem-like cells: non-GSCs), in response to their
microenvironment (5–7). Furthermore, there may be
interconversion between GSCs and non-GSCs (7).
The efficacy of postoperative radiotherapy with
concomitant and adjuvant temozolomide (TMZ) as the first-line
treatment for glioblastomas was reported to be 9.8% in terms of the
5-year survival rate versus 1.9% with radiotherapy alone in a
recent EORTC/NCIC randomized phase III trial (8,9).
Concomitant radiotherapy with TMZ followed by adjuvant TMZ
chemotherapy has thus become a current standard postoperative
treatment for glioblastomas. Among the factors that may contribute
to TMZ resistance, O6-methylguanine-DNA
methyltransferase (MGMT, a protein that removes drug-induced
alkylguanine adducts from DNA created by TMZ) is thought to be
involved in its crucial mechanisms (9,10).
Human interferon-beta (IFN-β), a type I interferon,
was first discovered on the basis of its antiviral activities.
Subsequently, it was found to exhibit pleiotropic biological
activities including immunomodulatory activity, anti-angiogenetic
activity and direct antitumor effects: e.g., growth inhibition, and
apoptosis (11–13). Recently, a synergistic antitumor
effect between TMZ and IFN-β was reported in malignant glioma cells
in vitro (14,15). Natsume et al suggested that
a sensitizing effect between IFN-β and TMZ in TMZ-resistant glioma
cells was possibly due to attenuation of MGMT expression via
induction of the protein p53 (14). More recently, the INTEGRA clinical
study (integrated Japanese multicenter clinical trial: a phase II
study on IFN-β and TMZ for glioma in combination with radiotherapy)
was undertaken to evaluate the clinical effectiveness in
glioblastomas (16,17).
Concerning the treatment of glioblastomas, it is
important to elucidate the detailed features of GSCs as well as the
underlying mechanisms of interconversion between GSCs and non-GSCs.
To this end, we examined whether IFN-β could exert some effect on
the interconversion between GSCs and non-GSCs, especially the
conversion process of non-GSCs into GSCs.
Materials and methods
Cell culture
As GSCs, we employed 0222-GSC provided by Nagoya
University School of Medicine (Nagoya, Japan) (7,8). The
0222-GSC satisfied the following criteria: i) the cell lines could
be maintained in serum-free-media for 3 months (minimum) and ii)
103 cells formed tumors in the brain of nonobese
diabetic mice with severe combined immunodeficiency disease
(18). 0222-GSC culture was
undertaken in serum-free neurobasal (NBE) media (Invitrogen,
Carlsbad, CA, USA) comprising N2 and B27 supplements (Invitrogen),
human recombinant basic fibroblast growth factor (bFGF; R&D
Systems, Minneapolis, MN, USA), and epidermal growth factor (EGF;
R&D Systems).
Human malignant glioma cell lines A-172, AM-38,
T98G, U-251MG, YH-13 (purchased from Health Science Research
Resources Bank, Sennan, Osaka, Japan), U-87MG, and U-138MG
(purchased from American Type Culture Collection, Manassas, VA,
USA) were also used in the present study. These human malignant
glioma cell lines were cultured in Dulbecco's modified Eagle's
medium (Nissui Pharmaceutical, Tokyo, Japan) containing 10% fetal
bovine serum (FBS) (Life Technologies, Grand Island, NY, USA)
(18,19).
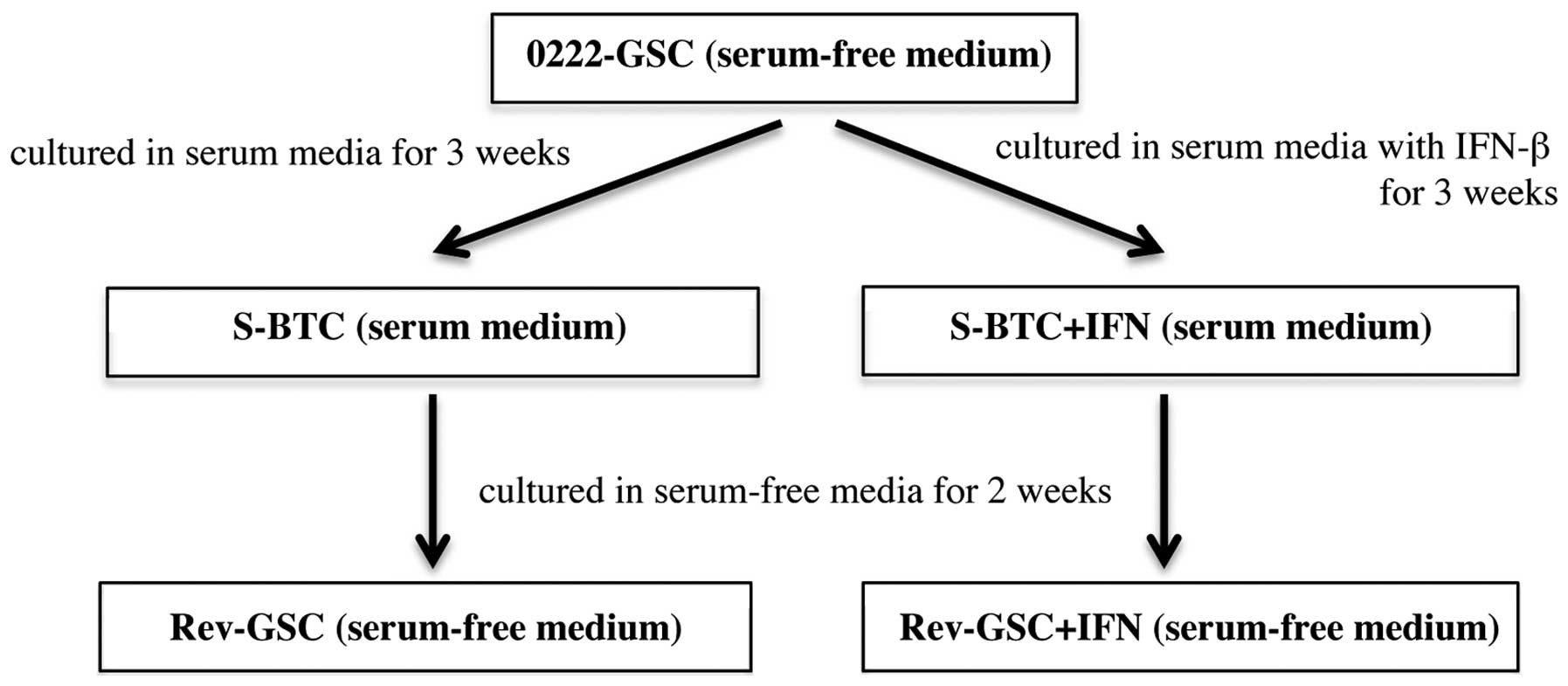
Populations of serum-induced brain tumor cells
(S-BTC) were established by culturing 0222-GSC in serum medium for
3 weeks. Moreover, populations of revertant-glioma stem-like cells
(Rev-GSC) were established by additional culturing of S-BTC in
serum-free medium for 2 weeks. On the other hand, populations of
S-BTC+IFN were established by culturing 0222-GSC in serum medium
with 10 IU/ml IFN-β (Toray Industries, Tokyo, Japan) twice a week
for 3 weeks (the total number of administrations was 6).
Populations of Rev-GSC+IFN were then established by additional
culturing of S-BTC+IFN in serum-free medium for 2 weeks (Fig. 1). Additionally, populations of
GSC+IFN were established by culturing 0222-GSC in serum-free medium
with 10 IU/ml IFN-β for one week.
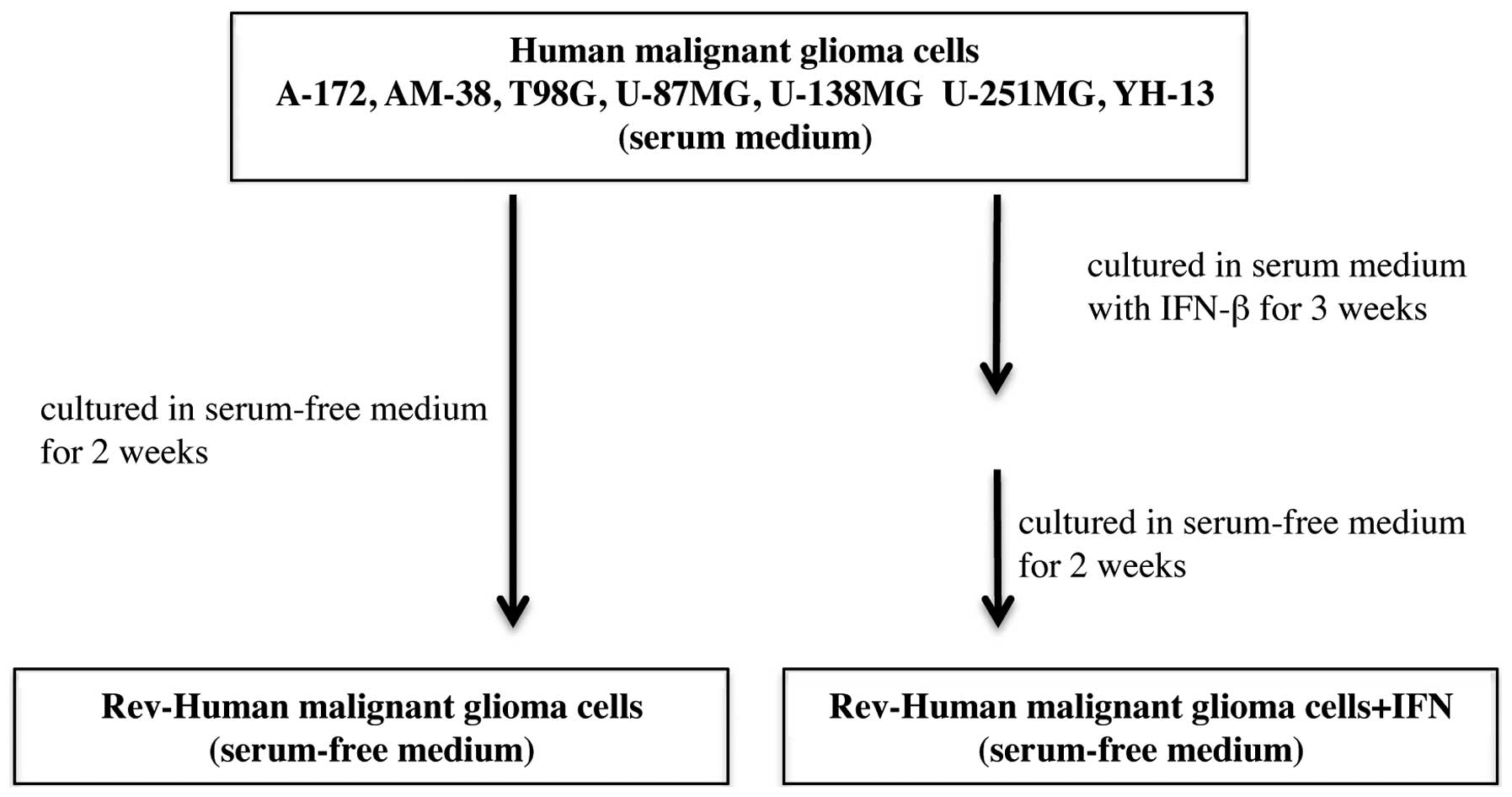
Rev-A-172, Rev-AM-38, Rev-T98G, Rev-U-87MG,
Rev-U-138MG, Rev-U-251MG, and Rev-YH-13 were established by
culturing the respective cells in serum-free medium for 2 weeks.
Moreover, Rev-A-172+IFN, Rev-AM-38+IFN, Rev-T98G+IFN,
Rev-U-87MG+IFN, Rev-U-138MG+IFN, Rev-U-251MG+IFN, and Rev-YH-13+IFN
were established by culturing the respective cells in serum-free
medium for 2 weeks after culture in serum supplemented medium with
10 IU/ml IFN-β twice a week for 3 weeks (the total number of
administrations was 6) (Fig.
2).
Flow cytometric analysis
The neural stem cell marker CD133 was employed as a
marker of GSCs. Furthermore, glial fibrillary acidic protein (GFAP)
was used as a marker of astrocytes, and galactocerebroside C (GalC)
was used as a marker of oligodendrocytes (2,18,20–22).
We employed the following fluorescence conjugated monoclonal
antibodies: anti-CD133 (CD133-PE, 130-080-801; Miltenyi Biotec,
Auburn, CA, USA), anti-GFAP (anti-GFAP-Alexa Fluor 488, 561449;
Becton-Dickinson, NJ, USA), and anti-GalC (antiGalC-Alexa Fluor
488, MAB342A4; Millipore, Temecula, CA, USA).
The expressions of CD133, GFAP and GalC in 0222-GSC,
S-BTC, Rev-GSC, Rev-GSC+IFN, and the 7 human malignant glioma cell
lines were analyzed with a fluorescence-activated cell sorter
(FACS). The fluorescence was measured using FACSCalibur flow
cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA), and the DNA
histograms were analyzed with Flowjo software (BioLegend, San
Diego, CA, USA).
The FACS analyses were repeated at least 3 times in
each experiment, and we confirmed that similar tendencies were
obtained.
mRNA expressions of Nanog
We analyzed the mRNA expression of pluripotency
markers, Nanog, in 0222-GSC, S-BTC, Rev-GSC, Rev-GSC+IFN,
Rev-U-87MG, and Rev-U-87MG+IFN by the real-time polymerase chain
reaction (real-time PCR) (23,24).
An RNeasy Mini kit (Qiagen Inc., Valencia, CA, USA) was employed
for the extraction of mRNA. A SepOne Real-time PCR System (Applied
Biosystems, Foster City, CA, USA) was used for the RT-PCR reaction.
The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was employed for the control. The following primers,
synthesized by Opero (Tokyo, Japan), were used in the real-time PCR
as described previously (7):
Nanog (forward, 5′-GTC CCG GTC AAG AAA CAG AA; reverse,
5′-TGC GTC ACA CCA TTG CTA TT) and GAPDH (forward, 5′-TCG
GTG CGT GCC CAG TTG AAC C; reverse: 5′-ATG CGG CTG ACT GTC GAA CAG
GAG). The real-time PCR was carried out with a final volume of 50
μl containing 10 pmol of each sense and antisense primer, 2.5 μl of
50 mM Mn(OAc)2, 25 μl of RNA-direct™ SYBR Green
Real-time PCR Master Mix (Toyobo, Osaka, Japan), 2 μg of extracted
mRNA, and RNA-free water. Amplification was carried out by initial
denaturing at 90°C for 30 sec, reverse transcription at 61°C for 20
min, second denaturing at 95°C for 1 min, followed by 40 cycles of
extension at 95°C for 15 sec, 55°C for 15 sec, and 74°C for 45 sec.
The expression levels were calculated using the following equations
by comparing the threshold cycles (CT): ΔCT = CT of Nanog - CT of
GAPDH, ΔΔCT = ΔCT (target cell line) - ΔCT (reference cell line),
and ratio = 2−ΔΔCT (25).
Growth inhibitory effect of IFN-β on
0222-GSC
The growth inhibitory effect of IFN-β was evaluated
by counting the number of cells using a Coulter Counter (Coulter
Counter ZI, Beckman coulter, Fullerton, CA, USA). Briefly, cells
were plated at 2×104 cells per well in 24-well,
flat-bottomed plates (Iwaki, Chiba, Japan) and incubated in the
medium with or without 1.0–100 IU/ml of IFN-β. After 5 days of
exposure to various concentrations of IFN-β, the cells were counted
with the cell counter.
Sphere formation assay
0222-GSC cells were placed into 96-well plates (50
cells/well) in serum-free medium. IFN-β (10 IU/ml) was administered
on one side (48-wells). At day 7 after seeding, the spheres
containing >10 cells were counted.
Statistical evaluations
Statistical analyses were performed using the
unpaired, Mann-Whitney U test. If the samples comprised more than
three groups, the significance of the overall samples was evaluated
by the Kruskal-Wallis test before evaluating the significant
differences between pairs of groups by the Mann-Whitney U test. All
quantitative data are presented as the means ± SE from at least six
samples per data point. Statistical software IBM SPSS Statistics
version 21.0 (International Business Machines Corp., Armonk, NY,
USA) was employed for the data analysis.
Results
Characteristics of GSCs and effects of
IFN-β
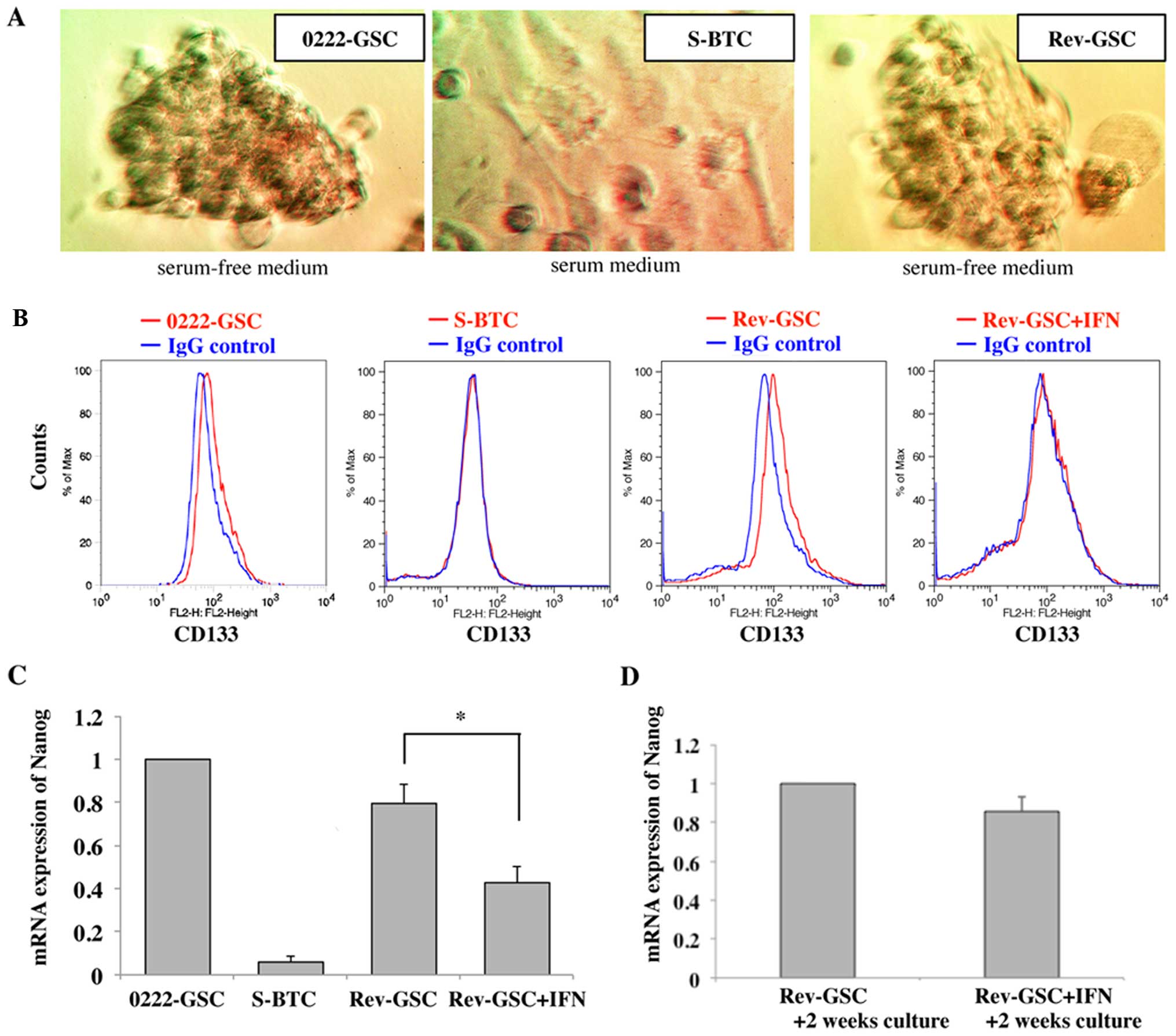
0222-GSC formed tumor spheres in serum-free medium.
However, the cells did not show tumor spheres in response to a
change to serum medium for 3 weeks (S-BTC). In addition, S-BTC
reformed tumor spheres again after culturing in serum-free medium
for 2 weeks (Rev-GSC) (Fig.
3A).
In the FACS analysis, 0222-GSC expressed a positive
reaction for CD133, but the expressions became negatively converted
in S-BTC. Further, the expression of CD133 again positively
converted in Rev-GSC (expression was not observed when S-BTC were
cultured in serum-free medium for only a week: data not shown). On
the other hand, the expression of CD133 was suppressed in
Rev-GSC+IFN (Fig. 3B).
The mRNA expression of Nanog in 0222-GSC,
S-BTC, Rev-GSC, and Rev-GSC+IFN were analyzed by the real-time PCR.
The expression in S-BTC was reduced to 0.06±0.03-fold as compared
to the expressions in 0222-GSC. However, the expression in Rev-GSC
increased again to 0.79±0.09-fold as compared to the expressions in
0222-GSC. On the other hand, the expression of mRNA Nanog in
Rev-GSC+IFN was also increased to 0.42±0.07-fold as compared to the
expressions in 0222-GSC, although the level was significantly lower
than that for Rev-GSC (p<0.01; Fig.
3C).
Furthermore, Rev-GSC and Rev-GSC+IFN were cultured
continuously for an additional 2 weeks in serum-free media (total
of 4 weeks culture in serum-free media). Although the expression of
mRNA Nanog in Rev-GSC+IFN was reduced to 0.86±0.08-fold as
compared to those in Rev-GSC, there was no significant difference
between Rev-GSC+IFN and Rev-GSC (p=0.09; Fig. 3D).
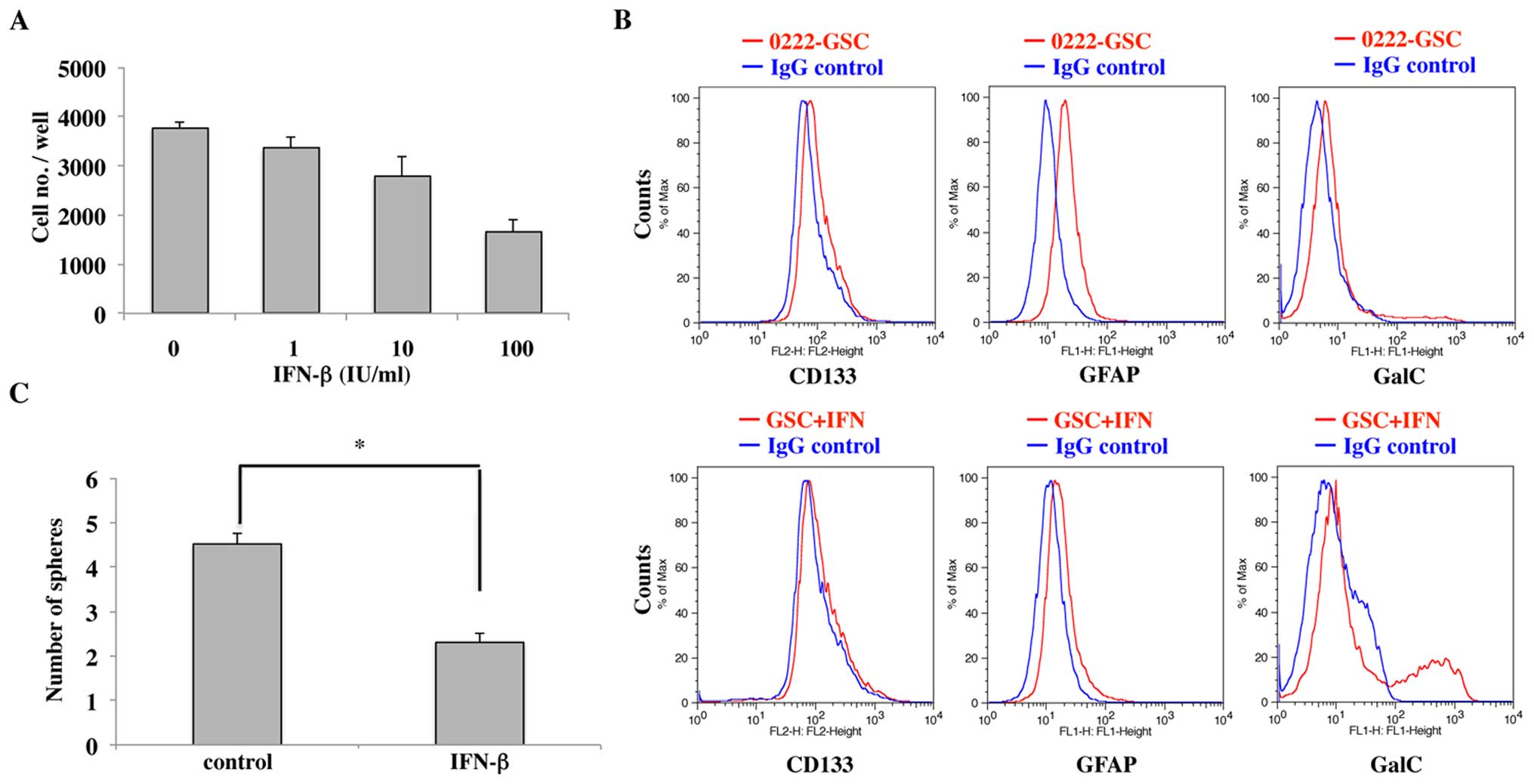
Effect of IFN-β on GSC
As shown in Fig.
4A, cell growth inhibitory effects of IFN-β on 0222-GSC were
observed in a dose-dependent manner.
We next examined the expression of CD133, GFAP and
GalC in 0222-GSC and GSC+IFN by FACS analysis. The expression of
CD133 and GFAP was suppressed, but the expression of GalC was
enhanced in GSC+IFN as compared to those in 0222-GSC (Fig. 4B). The data obtained indicated
IFN-β induced oligodendrogenesis in 0222-GSCs, as reported
previously (18).
The numbers of tumor spheres were counted after 7
days of culture in serum-free medium and compared between IFN-β
treatment, but not in 0222-GSC. As shown in Fig. 4C, the number of tumor spheres in
the IFN-β-treated cells were significantly lower than the number of
tumor spheres in the non-treated cells (p<0.01). We found that
the sphere formation ability was attenuated by IFN-β treatment,
although both groups could form tumor spheres.
Effect of IFN-β on malignant glioma cell
lines
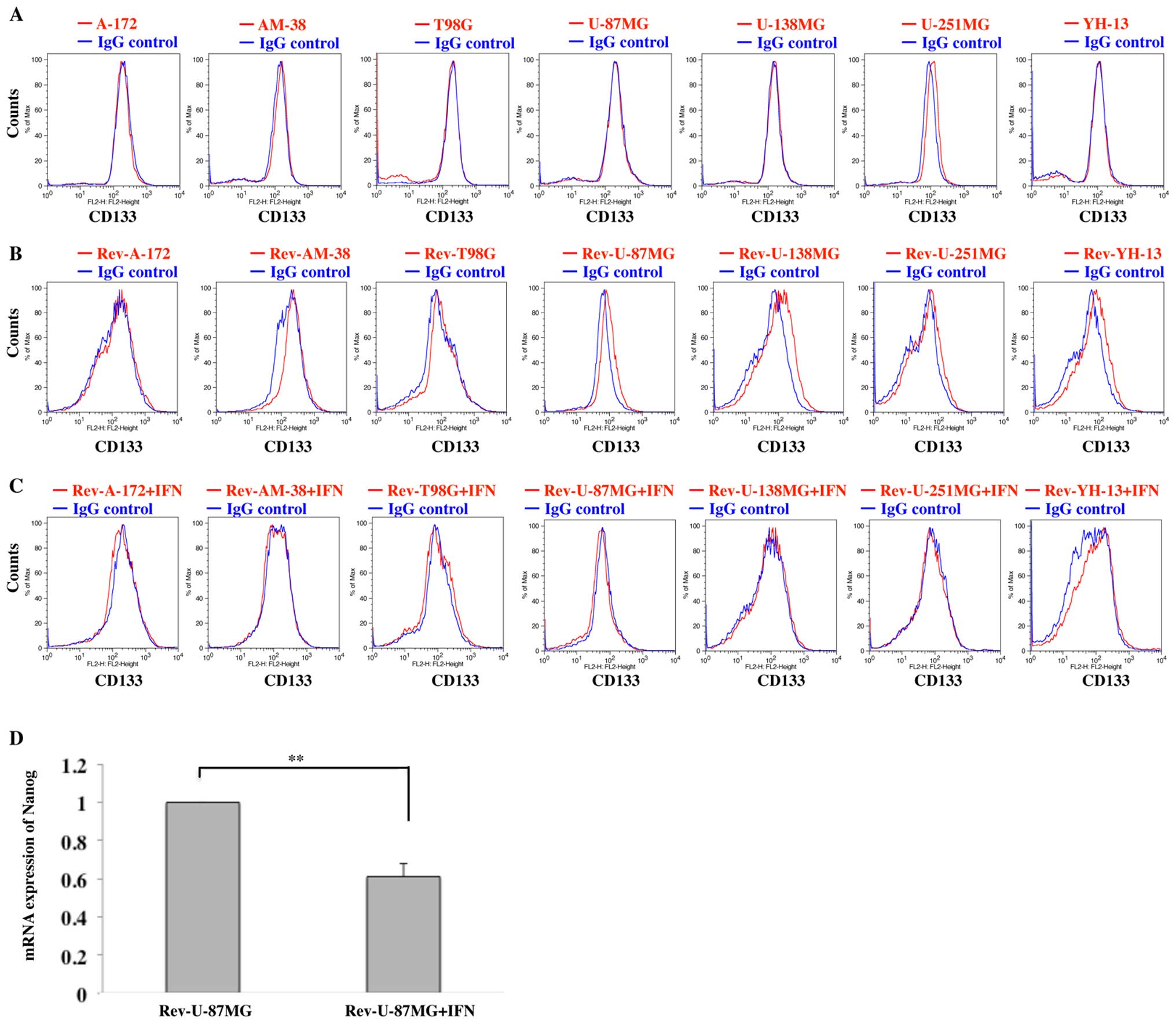
The human malignant glioma cell lines did not form
tumor spheres. On FACS analysis, U-251MG expressed CD133, even
though the other 6 cell lines did not (Fig. 5A). Subsequently, each of the cell
lines was able to form tumor spheres when cultured in serum-free
medium for 2 weeks (viz., Rev-A-172, Rev-AM-38, Rev-T98G,
Rev-U-87MG, Rev-U-138MG, Rev-U-251MG and Rev-YH-13). Expressions of
CD133 was newly observed in U-87MG, U-138MG and YH-13 on FACS
analysis (Fig. 5B). Each of the
cell lines, which had been administered IFN-β previously under
serum medium, also formed tumor spheres when cultured in serum-free
medium for 2 weeks (viz., Rev-A-172+IFN, Rev-AM-38+IFN,
Rev-T98G+IFN, Rev-U-87MG+IFN, Rev-U-138MG+IFN, Rev-U-251MG+IFN and
Rev-YH-13+IFN), but none of these cell lines expressed CD133
(Fig. 5C).
Moreover, we analyzed the mRNA expressions of
Nanog in Rev-U-87MG and Rev-U-87MG+IFN by the real-time PCR.
The expressions in Rev-U-87MG+IFN was significantly reduced to
0.61±0.07-fold as compared to the expression in Rev-U-87MG
(p<0.01; Fig. 5D).
Discussion
GSCs share many properties with normal stem cells
including self-renewal and pluripotency, and exhibit tumorigenetic
ability. Furthermore, GSCs may contribute to tumor development,
invasion, recurrence and chemo/radiation resistance in
glioblastomas (7,18,26,27).
It is important therefore to take GSCs fully into accound when
deciding treatment strategies for glioblastomas.
The undifferentiated state of GSCs displays
characteristics such as tumor sphere formation, CD133 expression,
and mRNA Nanog expression (7,18,20–22).
The GSC cell line, 0222-GSC, demonstrated tumor sphere formation,
CD133 expression, and a high expression of mRNA Nanog.
However, it lost such characteristics on exposure to serum medium
(S-BTC). This may indicate that GSCs can change/differentiate to
non-GSCs in response to signals from their microenvironment
(7,18). On the other hand, S-BTC
re-exhibited the characteristics of tumor sphere formation, CD133
expression, and a high expression of mRNA Nanog upon removal
of the serum from the medium. Such changes suggested that the
non-GSCs had regained an undifferentiated state in response to
signals from their microenvironment. These findings are in keeping
with those described in previous reports (7).
Human malignant glioma cell lines cultured in
conventional medium did not form tumor spheres, nor did they
express CD133 (except for U-251MG) in the present study. All cell
lines formed tumor spheres when cultured in serum-free medium, and
furthermore U-87MG, U-138MG, and YH-13 expressed CD133. Qiang et
al, investigated the percentage of CD133-positive cells in
A-172, U-87MG, and U-251MG by FACS analysis, and found that the
existence of CD133-positive cells was common in U-251MG (28). They also reported that human
malignant glioma cell lines formed tumor spheres and showed
increased expression of CD133 when cultured in serum-free medium
(28). We obtained similar
results, and confirmed that human malignant glioma cells (non-GSCs)
could acquire an undifferentiated state (return to GSCs) in
response to signals from their microenvironment.
It might be possible to enhance the effects of
radiotherapy/chemotherapy for glioblastomas, if we could promote
the differentiation of GSCs and/or suppress the return process of
non-GSCs to GSCs. Not only GSCs themselves but also the
interconversion between GSCs and non-GSCs could be new targets in
glioblastoma treatment. In the present study, IFN-β revealed a cell
growth inhibitory effect and a cell differentiation effect with
suppression of tumor sphere formation and CD133 expression in
0222-GSC. In addition, IFN-β suppressed the acquisition process of
undifferentiated features in S-BTC and some of the human malignant
glioma cell lines investigated. Thus, IFN-β might represent an
effective agent not only through its cell growth inhibitory effect
on GSCs but also as a means of targeting the interconversion
between GSCs and non-GSCs.
0222-GSC cells were induced to differentiate into
oligodendrogenetic cells in the present study, as reported
previously (8). The
oligodendrogliomas display a better prognosis than the
astrocytomas, through their high sensitivity to adjuvant therapy
including radiotherapy and chemotherapy (29). Such a differentiation effect is
therefore considered to offer a new treatment strategy for
glioblastomas, and IFN-β may represent an effective drug for the
treatment of glioblastomas, not only when used alone but also in
combination with TMZ. Further, in the present study, IFN-β also
induced suppression of acquisition processes involved in the
undifferentiation of non-GSCs at 2 weeks, although the suppression
of mRNA Nanog was not significant at 4 weeks after IFN-β treatment.
The interval of IFN-β a dministration employed during maintenance
therapy in the INTEGRA study was once every 4 weeks (17), so that a higher effect could be
expected if the intervals were shorter.
Finally, the effects of IFN-β on the tumor cells
were considered as not only temporary, but also genetic or
epigenetic changes, since the effects of IFN-β lasted >2 weeks.
DNA methylation, and histone modification (acetylation,
methylation, and phosphorylation) are known to represent epigenetic
changes of the cells. Among them, histone methylation has been
reported to play an important role in the control of normal stem
cell differentiation. In particular, histone H3 lysine 27
trimethylation (H3K27me3) in the promoter region of the
differentiation-associated genes is known to inhibit the
transcription of these genes (30,31).
H3K27me3 is catalyzed by enhancer of zeste homolog 2 (EZH2; histone
methyltransferase). EZH2 has been reported to contribute to
undifferentiated features in normal stem cells (32–36).
Enhanced gene expressions of EZH2 have been observed in
various cancers (32,37–39),
and inhibitors of EZH2 have been found to exhibit antitumor effects
(40,41). While EZH2 has been described
as an oncogene, it has also been reported as a tumor-suppressor
gene since mutations of EZH2 have been observed extinguished
in several cancers (42). Although
the detailed relationships between EZH2 and cancer remain to be
elucidated, there is a report suggesting that EZH2 may contribute
to the interconversion between GSCs and non-GSCs (7). Further research is clearly necessary,
but an association could exist between EZH2 and the effects of
IFN-β.
Acknowledgements
This study was supported in part by a grant from the
Health Sciences Research Institute, Inc., Yokohama, Japan, to the
Division of Companion Diagnostics, Department of Pathology of
Microbiology, Nihon University School of Medicine. The authors are
grateful to Hiroyuki Satake and Nobuo Miyazaki, Toray Industries
Inc. (Tokyo, Japan), for their invaluable discussions and supply of
natural type IFN-β. Some parts of this study have been submitted
for the Japanese-language thesis of Shun Yamamuro's Ph.D. degree at
Nihon University School of Medicine. Toshihiko Wakabayashi received
funds for other research projects not related to this study from
Eisai Co., Ltd, Chugai Pharmaceutical Co., Ltd., MSD K.K., Mizuho
Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Takeda Pharmaceutical
Co., Ltd., and Toray Industries Inc. Takuya Ueda received funds for
other research projects not related to this study from the Ministry
of Education, Culture, Sports, Science and Technology, Japan,
Minister of Economy, Trade and Industry, Japan and Human Frontier
Science Program. Yoichi Katayama received research funds for
another research project from Medtronic Japan Co., Ltd.
References
|
1
|
Lee J, Kotliarova S, Kotliarov Y, Li A, Su
Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al:
Tumor stem cells derived from glioblastomas cultured in bFGF and
EGF more closely mirror the phenotype and genotype of primary
tumors than do serum-cultured cell lines. Cancer Cell. 9:391–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson RJ, Thomas CD, Fox R, Roy DB and
Kunin WE: Spatial patterns in species distributions reveal
biodiversity change. Nature. 432:393–396. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee J, Son MJ, Woolard K, Donin NM, Li A,
Cheng CH, Kotliarova S, Kotliarov Y, Walling J, Ahn S, et al:
Epigenetic-mediated dysfunction of the bone morphogenetic protein
pathway inhibits differentiation of glioblastoma-initiating cells.
Cancer Cell. 13:69–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peñuelas S, Anido J, Prieto-Sánchez RM,
Folch G, Barba I, Cuartas I, García-Dorado D, Poca MA, Sahuquillo
J, Baselga J, et al: TGF-beta increases glioma-initiating cell
self-renewal through the induction of LIF in human glioblastoma.
Cancer Cell. 15:315–327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta PB, Chaffer CL and Weinberg RA:
Cancer stem cells: Mirage or reality? Nat Med. 15:1010–1012. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gupta PB, Fillmore CM, Jiang G, Shapira
SD, Tao K, Kuperwasser C and Lander ES: Stochastic state
transitions give rise to phenotypic equilibrium in populations of
cancer cells. Cell. 146:633–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Natsume A, Ito M, Katsushima K, Ohka F,
Hatanaka A, Shinjo K, Sato S, Takahashi S, Ishikawa Y, Takeuchi I,
et al: Chromatin regulator PRC2 is a key regulator of epigenetic
plasticity in glioblastoma. Cancer Res. 73:4559–4570. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al; European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group. Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al; European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group. Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pegg AE: Mammalian
O6-alkylguanine-DNA alkyltransferase: Regulation and
importance in response to alkylating carcinogenic and therapeutic
agents. Cancer Res. 50:6119–6129. 1990.PubMed/NCBI
|
|
11
|
Saito R, Mizuno M, Hatano M, Kumabe T,
Yoshimoto T and Yoshida J: Two different mechanisms of apoptosis
resistance observed in interferon-beta induced apoptosis of human
glioma cells. J Neurooncol. 67:273–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vannucchi S, Chiantore MV, Mangino G,
Percario ZA, Affabris E, Fiorucci G and Romeo G: Perspectives in
biomolecular therapeutic intervention in cancer: From the early to
the new strategies with type I interferons. Curr Med Chem.
14:667–679. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshino A, Katayama Y, Yokoyama T,
Watanabe T, Ogino A, Ota T, Komine C, Fukushima T and Kusama K:
Therapeutic implications of interferon regulatory factor (IRF)-1
and IRF-2 in diffusely infiltrating astrocytomas (DIA): Response to
interferon (IFN)-beta in glioblastoma cells and prognostic value
for DIA. J Neurooncol. 74:249–260. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Natsume A, Ishii D, Wakabayashi T, Tsuno
T, Hatano H, Mizuno M and Yoshida J: IFN-beta down-regulates the
expression of DNA repair gene MGMT and sensitizes resistant glioma
cells to temozolomide. Cancer Res. 65:7573–7579. 2005.PubMed/NCBI
|
|
15
|
Park JA, Joe YA, Kim TG and Hong YK:
Potentiation of anti-glioma effect with combined temozolomide and
interferon-beta. Oncol Rep. 16:1253–1260. 2006.PubMed/NCBI
|
|
16
|
Wakabayashi T, Kayama T, Nishikawa R,
Takahashi H, Yoshimine T, Hashimoto N, Aoki T, Kurisu K, Natsume A,
Ogura M, et al: A multicenter phase I trial of interferon-beta and
temozolomide combination therapy for high-grade gliomas (INTEGRA
Study). Jpn J Clin Oncol. 38:715–718. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wakabayashi T, Kayama T, Nishikawa R,
Takahashi H, Hashimoto N, Takahashi J, Aoki T, Sugiyama K, Ogura M,
Natsume A, et al: A multicenter phase I trial of combination
therapy with interferon-β and temozolomide for high-grade gliomas
(INTEGRA study): The final report. J Neurooncol. 104:573–577. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuki K, Natsume A, Yokoyama H, Kondo Y,
Ohno M, Kato T, Chansakul P, Ito M, Kim SU and Wakabayashi T:
Induction of oligodendrogenesis in glioblastoma-initiating cells by
IFN-mediated activation of STAT3 signaling. Cancer Lett. 284:71–79.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshino A, Tashiro S, Ogino A, Yachi K,
Ohta T, Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Sano E, et
al: Gene expression profiles predicting the response to IFN-β and a
combination of temozolomide and IFN-β in malignant gliomas. Int J
Oncol. 39:529–542. 2011.PubMed/NCBI
|
|
20
|
Griguer CE, Oliva CR, Gobin E, Marcorelles
P, Benos DJ, Lancaster JR Jr and Gillespie GY: CD133 is a marker of
bioenergetic stress in human glioma. PLoS One. 3:e36552008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Poltavtseva RA, Marey MV, Aleksandrova MA,
Revishchin AV, Korochkin LI and Sukhikh GT: Evaluation of
progenitor cell cultures from human embryos for
neurotransplantation. Brain Res Dev Brain Res. 134:149–154. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
23
|
Hatano SY, Tada M, Kimura H, Yamaguchi S,
Kono T, Nakano T, Suemori H, Nakatsuji N and Tada T: Pluripotential
competence of cells associated with Nanog activity. Mech Dev.
122:67–79. 2005. View Article : Google Scholar
|
|
24
|
Mitsui K, Tokuzawa Y, Itoh H, Segawa K,
Murakami M, Takahashi K, Maruyama M, Maeda M and Yamanaka S: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
26
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiang L, Yang Y, Ma YJ, Chen FH, Zhang LB,
Liu W, Qi Q, Lu N, Tao L, Wang XT, et al: Isolation and
characterization of cancer stem like cells in human glioblastoma
cell lines. Cancer Lett. 279:13–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smith TJ: The art of oncology: When the
tumor is not the target. Tell it like it is. J Clin Oncol.
18:3441–3445. 2000.PubMed/NCBI
|
|
30
|
Kondo Y, Shen L, Cheng AS, Ahmed S,
Boumber Y, Charo C, Yamochi T, Urano T, Furukawa K, Kwabi-Addo B,
et al: Gene silencing in cancer by histone H3 lysine 27
trimethylation independent of promoter DNA methylation. Nat Genet.
40:741–750. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sparmann A and van Lohuizen M: Polycomb
silencers control cell fate, development and cancer. Nat Rev
Cancer. 6:846–856. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chi P, Allis CD and Wang GG: Covalent
histone modifications--miswritten, misinterpreted and mis-erased in
human cancers. Nat Rev Cancer. 10:457–469. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ougolkov AV, Bilim VN and Billadeau DD:
Regulation of pancreatic tumor cell proliferation and
chemoresistance by the histone methyltransferase enhancer of zeste
homologue 2. Clin Cancer Res. 14:6790–6796. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raaphorst FM, Meijer CJ, Fieret E,
Blokzijl T, Mommers E, Buerger H, Packeisen J, Sewalt RA, Otte AP
and van Diest PJ: Poorly differentiated breast carcinoma is
associated with increased expression of the human polycomb group
EZH2 gene. Neoplasia. 5:481–488. 2003. View Article : Google Scholar
|
|
35
|
Sauvageau M and Sauvageau G: Polycomb
group proteins: Multifaceted regulators of somatic stem cells and
cancer. Cell Stem Cell. 7:299–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schwartz YB and Pirrotta V: Polycomb
silencing mechanisms and the management of genomic programmes. Nat
Rev Genet. 8:9–22. 2007. View Article : Google Scholar
|
|
37
|
Bracken AP and Helin K: Polycomb group
proteins: Navigators of lineage pathways led astray in cancer. Nat
Rev Cancer. 9:773–784. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kleer CG, Cao Q, Varambally S, Shen R, Ota
I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al: EZH2
is a marker of aggressive breast cancer and promotes neoplastic
transformation of breast epithelial cells. Proc Natl Acad Sci USA.
100:11606–11611. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cebrià F, Kobayashi C, Umesono Y, Nakazawa
M, Mineta K, Ikeo K, Gojobori T, Itoh M, Taira M, Sánchez Alvarado
A, et al: FGFR-related gene nou-darake restricts brain tissues to
the head region of planarians. Nature. 419:620–624. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fiskus W, Wang Y, Sreekumar A, Buckley KM,
Shi H, Jillella A, Ustun C, Rao R, Fernandez P, Chen J, et al:
Combined epigenetic therapy with the histone methyltransferase EZH2
inhibitor 3-deazaneplanocin A and the histone deacetylase inhibitor
panobinostat against human AML cells. Blood. 114:2733–2743. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan J, Yang X, Zhuang L, Jiang X, Chen W,
Lee PL, Karuturi RK, Tan PB, Liu ET and Yu Q: Pharmacologic
disruption of Polycomb-repressive complex 2-mediated gene
repression selectively induces apoptosis in cancer cells. Genes
Dev. 21:1050–1063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ernst T, Chase AJ, Score J, Hidalgo-Curtis
CE, Bryant C, Jones AV, Waghorn K, Zoi K, Ross FM, Reiter A, et al:
Inactivating mutations of the histone methyltransferase gene EZH2
in myeloid disorders. Nat Genet. 42:722–726. 2010. View Article : Google Scholar : PubMed/NCBI
|