Introduction
GC is a type of malignant tumor with high incidence
and mortality (1). Due to the
complex pathogenesis of gastric cancer, it lacks more effective
treatments. Therefore, clarifying the pathogenesis of gastric
cancer is a priority.
miRNAs are a class of small non-coding
single-stranded RNAs. They combine with 3′- or 5′-untranslated
region (UTR) of mRNAs in specific target genes to inhibit mRNAs
translation or directly degrade their specific mRNAs, involving in
regulating a variety of physiological and pathological processes
such as cell proliferation, differentiation, apoptosis and
metabolism (2–5). miR-125a is located in 19q13, which is
frequently downregulated in several human cancers, including breast
cancer (6), ovarian cancer
(7), lung cancer (8) and medulloblastoma (9). Low expression of miR-125a was
associated with the malignant potential indicators of enhanced GC
such as tumor size and tumor invasion (10). Survival analysis indicated that low
expression of miR-125a was an independent prognostic factor for GC
patients, which may be related to the target gene Her-2 (11). However, the mechanism of miR-125a
underlying GC is still unclear.
VEGF-A is considered to be the most potent
angiogenic factors, which exerts its effect by activating vascular
endothelial growth factor receptor 2 (VEGFR-2). During
angiogenesis, VEGF-A binds to VEGFR-2 and activates multiple
downstream pathways via signaling intermediates, such as PI3K/Akt
(12–16). As a result, VEGF signaling may
promote endothelial cell (EC) proliferation, survival, migration,
filopodial extension and chemotaxis (17–19).
VEGF-A plays an important role in gastric cancer, not only
enhancing the malignant potential of tumor cells but also promoting
the angiogenesis in the tumors (20–22).
Moreover, expression level of VEGF-A in gastric cancer can directly
affect the survival of patients (23).
The aim of the present study was to investigate the
role of miR-125a in gastric cancer and the mechanism underlying it.
We confirmed that miR-125a can function as a crucial tumor
suppressor in human gastric cancer. The results showed that
miR-125a decreased significantly in gastric cancer and was
negatively correlated with the expression of VEGF-A, suggesting
that miR-125a may inhibit angiogenesis of gastric cancer by
downregulating VEGF-A.
Materials and methods
Patients
Consecutive series of 73 cases of gastric cancer and
20 cases of normal gastric tissues specimens were collected from
Institute of Pathology of Tongji Hospital, Huazhong University of
Science and Technology in 2009. Patients with a previous history of
another primary tumor, or those who had previously received
chemotherapy and/or radiotherapy were excluded from the study. As
to the diagnosis of gastric cancer, tumors were analyzed and
discussed by two pathologists, and then a definite diagnosis was
made. The age, sex, tumor size, lymph node (LN) metastasis and
clinical stage of the patients were recorded.
Cell culture
Cell lines GES-1, AGS, SGC7901, BCG-823 and HUVEC
were purchased from China Center for type culture collection in
Wuhan. All cells were cultured with RPMI-1640 medium (Gibco, USA)
containing 10% fetal bovine serum (FBS) (Gibco), while instructions
were followed additionally under special circumstances.
Cell transfection
AGS cells were grown in 6-well plates to reach a
cell density of 60% the next day. Lipofectamine 2000 (Invitrogen,
USA) was mixed with miR-125a mimics and miR-125a inhibitors
(GenePharma, China) respectively for 10 min. After washing cells
twice with serum-free opti-MEM medium, appropriate amount of
opti-MEM was added to each well, then the above mixture was added
to the medium dropwise to reach a concentration of 40 nM. The cells
were incubated at 37°C for 6 h, then the mixture was replaced with
medium containing 10% FBS.
QRT-PCR
For miRNAs, in vivo, formalin-fixed
paraffin-embedded tissue (FFPET) samples were cut into 10-μm thin
slices, with a total thickness of 40 μm. miRNAs from FFPET was
extracted in accordance with the manufacturer's protocol (Aidlab,
RN3101, China). In vitro, total RNA was extracted from AGS
cells using TRIzol (Invitrogen). U6 snRNA was used as internal
reference for qRT-PCR. Hsa-miR-125a-5p and U6 specific primers as
well as the miRNA qRT-PCR Detection kit were purchased from
GeneCopoeia. For mRNAs, total cellular RNA was extracted with
TRIzol (Invitrogen). With random primer dNTP (Fermentas, R0191) and
revertAid reverse transcriptase (Fermentas, EP0442), mRNA was
reversely transcribed to cDNA. The PCR primers were as follows:
VEGF-A forward, 5′-CGAAGTGGTGGTCATGGATG-3′; reverse,
5′-TTCTGTATCAGTCTTTCCTGGTGA-G-3′; GAPDH forward,
5′-AGGTCGGAGTCAACGGATTTG-3′; reverse, 5′-GTGATGGCA-TGGACTGTGGT-3′.
Real-time PCR was performed using the ABI7500 real-time by Applied
Biosystems.
Western blotting
Total cellular protein was collected using RIPA
lysis buffer (Beyotime, China). Bicinchoninic acid (BCA) Protein
Assay kit (Pierce, USA) was used to quantify the protein
concentration. Equal amounts of protein were loaded into
SDS-polyacrylamide gels and transferred onto polyvinylidene
difluoride filter (PVDF) membrane. After blocked with 5% skim milk
for 1 h, the membranes were incubated with VEGF-A antibody (Santa
Cruz, USA), Akt antibody and p-Akt antibody (Cell Signaling
Technology, USA) respectively, at 4°C overnight. The next day, the
membranes were incubated with HRP-conjugated secondary antibody
(Santa Cruz) for 1 h at 37°C and then detected by chemiluminescence
system (Beyotime).
ELISA
Forty-eight hours after transfection, the old medium
was replaced with serum-free medium and cells were incubated for
another 24 h. Then the medium was collected to detect the VEGF-A
concentration by ELISA assay (Abcam, USA) according to the
manufacturer's protocol. Thereafter, the serum-free medium
containing different concentrations of VEGF-A (AGS-VEGF-A-medium)
was placed on standby at −80°C for EC proliferation and tube
formation assays (24).
Luciferase reporter assay
First, we predicted that human miR-125a was able to
regulate the expression of VEGF-A, and we aimed to identify the
possible site (http://www.microrna.org). The 3′-UTR of human VEGF-A
was amplified by PCR (25). The
primers for PCR amplification are: forward,
5′-ATCTCAGCATGCCTGGTCAGTTACCTACTAATAGC GGGCCTG-3′ and reverse:
5′-GCCCTGAGTGCTGAGC GATCAAGTGTCATTTGACGTATCGCT-3′. Then the
amplified sequence was cloned into the XbaI site of the pGL3
control vector (Promega, USA). The mutated putative miR-125a-5p
binding site in the 3′-UTR of VEGF-A was generated using the
QuickChange Site-directed Mutagenesis kit (Stratagene, Cedar Creek)
according to the manufacturer's protocol. The day before
transfection, AGS cells were seeded in 24-well plates
(5×104 cells/well), then we transfected 500 ng
VEGF-A-3′-UTR-pGL3 and 500 ng miR-125a mimics or control miRNA
mimics (GenePharma, China) into cells using Lipofectamine 2000.
Forty-eight hours after transfection, luciferase activity was
determined using dual-luciferase reporter assay system (Promega)
according to the manufacturer's protocol.
Cell proliferation assay
Cell proliferation assays were performed using HUVEC
essentially as described (26–28).
HUVECs were resuspended to a density of 2×104/ml. For
CCK-8 proliferation assay, 100 μl cell suspension was planted into
each well of 96-well plates. The medium was replaced after cell
adherence. The AGS-VEGF-A-medium or VEGF-A-medium (VEGF-A
recombinant protein, 3 ng/ml, Sino Biological Inc., China) was
formulated as conditioned medium containing 2% FBS, and Akt
activity inhibitor (MK-2206 2HCI, 10 μM, Selleck, USA) and used to
conduct control experiment. The cell proliferation activity was
detected using cell counting kit-8 (CCK8) (Beyotime). After
cultured for a few days, 10 μl CCK8 was added to each well and
incubated for 1 h in the cell incubator. The optical density (OD)
value was read at 450 nm wavelength. For plate colony formation
assay, 1×103 cells were seeded into 6-well plates and
cultured for 2 weeks. The colonies were fixed with 4%
paraformaldehyde for 10 min and stained with 0.1% crystal violet
(Beyotime) for 5 min. Clones with >50 cells were counted
(29,30). Experiments were repeated at least 3
times.
Cell migration assay
HUVEC migration was evaluated with a transwell
system (Corning Costar, USA) that comprised 8-μm inserts in 24-well
plates. Cells were co-cultured as described (31,32).
Firstly, genetically modified AGS cells or VEGF-A-medium (3 ng/ml)
were added into the lower chamber. When AGS cell fusion reached to
100%, the growth medium was replaced with serum-free medium. HUVECs
were resuspended in serum-free medium with 0.1% bovine serum
albumin (BSA) to a density of 5×105/ml. Then 100 μl
medium containing HUVECs were added in each upper chamber (presence
or absence of Akt inhibitor). After incubation for 24 h, cells that
did not migrate in the upper chamber were removed with a cotton
swab. Then the migrated cells were fixed with 4% paraformaldehyde
for 20 min. After that, the cells were stained with 0.1% crystal
violet (Beyotime) for 5 min and counted and photographed with a
fluorescence microscope on ×100 magnification. Experiments were
repeated at least 3 times.
In vitro tube formation assay
In vitro tube formation assays were performed
using HUVEC essentially as described (33,34).
Briefly, 50 μl growth factor-reduced Matrigel (BD, USA), which was
thawed on ice in advance, were plated in 96-well plates and
incubated at 37°C for 45 min. In order to investigate the effect of
AGS-VEGF-A-medium or VEGF-A-medium (absence of FBS, presence or
absence of Akt inhibitor) on angiogenesis in HUVECs, the cells (100
μl, 2×105 cells/ml) were seeded on the matrigel and
incubated at 37°C with 5% CO2 for 4 h. After the culture
media were removed, the cells were washed twice with PBS. Then
stained with 0.1% crystal violet for 5 min and washed with PBS
twice again. Images were captured using a digital camera (×100
magnification). Tubes and nodal structures were counted by two
independent researchers. Experiments were repeated at least 3
times.
Immunohistochemistry (IHC)
The tumor specimens were fixed, dehydrated and
embedded in paraffin, then cut into 3-μm thin slices. After dewaxed
and rehydrated, they were autoclaved for 2 min and then incubated
with 3% hydrogen peroxide for 10 min at room temperature to remove
endogenous peroxidase activity. The slices were treated with 5% BSA
for 30 min, followed by incubating with Her-2 antibody (ZSGB-BIO,
China) and VEGF-A antibody (Santa Cruz) at 4°C overnight,
respectively, then washed in phosphate-buffered saline (PBS) for 2
min and incubate with secondary antibodies (Dako, USA) at 37°C for
1 h. Then staining was with diaminobenzidine (DAB) substrate
chromogen solution for 5 min at room temperature. Her-2 was
evaluated by two pathologists independently. Her-2(0), Her-2(1+)
and Her-2(2+) were defined as Her-2(−), while Her-2(3+) was defined
as Her-2(+). The evaluation of VEGF-A was analyzed using ImageJ
software (National Institutes of Health, USA). The raw data was
adjusted appropriately to gain normalized score, and the normalized
score greater than 2 was designated as VEGF-A positive, while the
other negative.
MVD analysis
CD31 (Santa Cruz) was detected using IHC staining
methods in 73 cases of FFPET to observe MVD in gastric cancer.
Evaluation of MVD was performed by two authors independently. MVD
in the tumor was determined as described (35,36).
In brief, areas rich in tumor tissue and blood vessels were
selected at low magnification (×40). Then the blood vessels were
counted in the region at high magnification (×100), or even a
higher magnification (×200) for some individual vessels. A single
independent CD31 staining cell, or microvessel that had formed
tubular structure was considered as a countable microvessel. In
order to reflect the MVD more accurately, we kept count of five
horizons (×100) accumulatively.
Statistical analysis
The experimental data were presented as mean ± SD.
The differences among variables were assessed by χ2
analysis or 2-tailed Student's t-test. Survival curves were plotted
by the Kaplan-Meier method, and differences were assessed by the
log-rank test. Correlation parameters were submitted to Pearson's
correlation. Statistical analyses were conducted using GraphPad
Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). P<0.05
was considered to have a significant statistical difference.
Results
miR-125a regulates the expression of
VEGF-A in GC cells
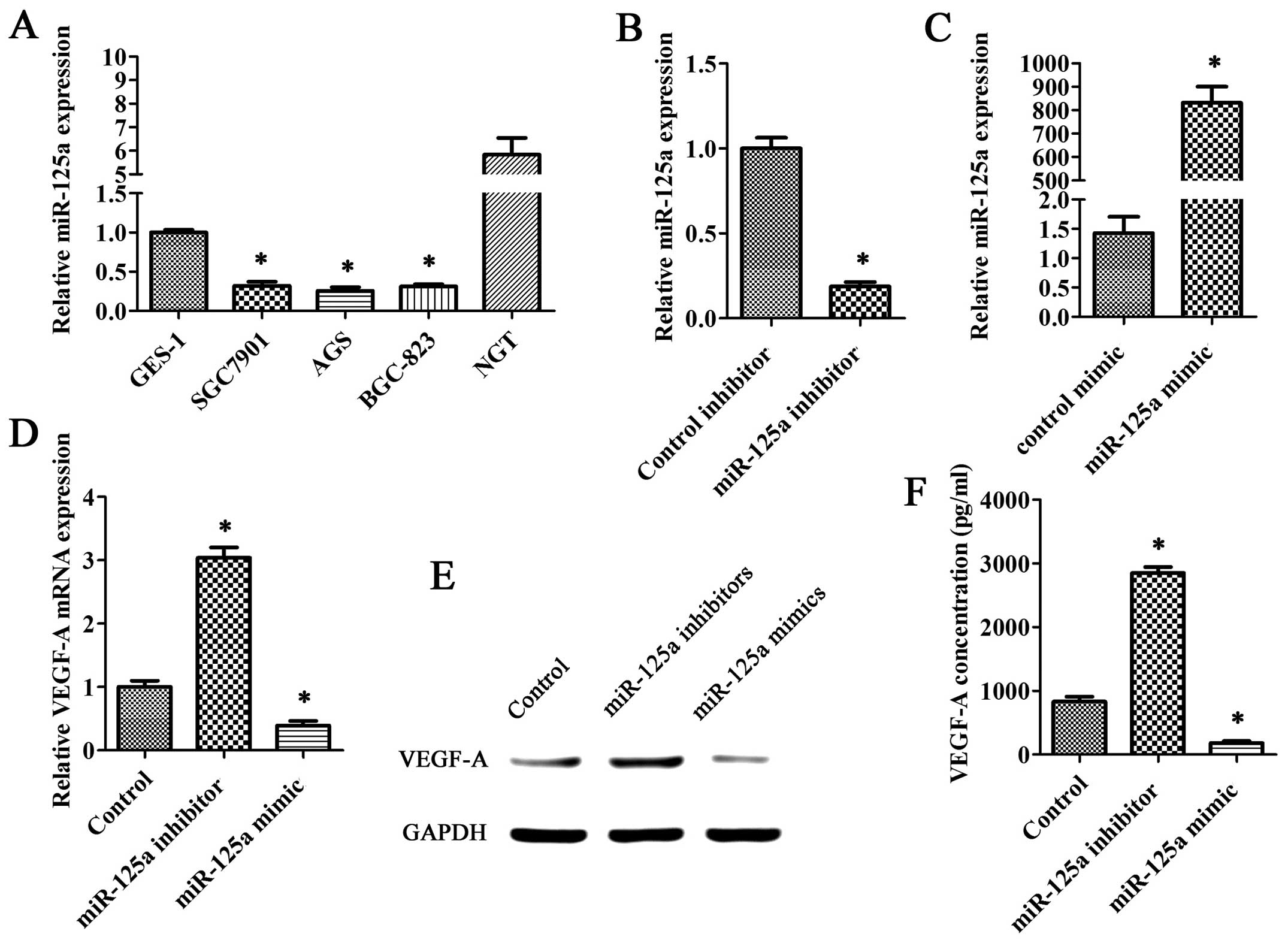
QRT-PCR analysis showed that miR-125a expression in
gastric cancer cells (SGC7901, AGS and BGC-823) were lower than
that in GES-1 cells or normal gastric tissue (NGT) (Fig. 1A). We transfected miR-125a
inhibitors or miR-125a mimics into AGS cells (Fig. 1B and C). Our investigations
revealed that the VEGF-A mRNA levels in groups transfected with
miR-125a mimics were significantly lower than the control group; in
contrast, the VEGF-A mRNA levels were significantly higher in
groups transfected with miR-125a inhibitors (Fig. 1D). On the other hand, VEGF-A
protein levels detected by western blotting were also reduced in
groups that transfected with miR-125a mimics and increased in
groups transfected with miR-125a inhibitors (Fig. 1E). Moreover, we obtained a similar
result when compared VEGF-A protein levels in the medium of the
transfected groups with that in control groups using ELISA
technique (Fig. 1F).
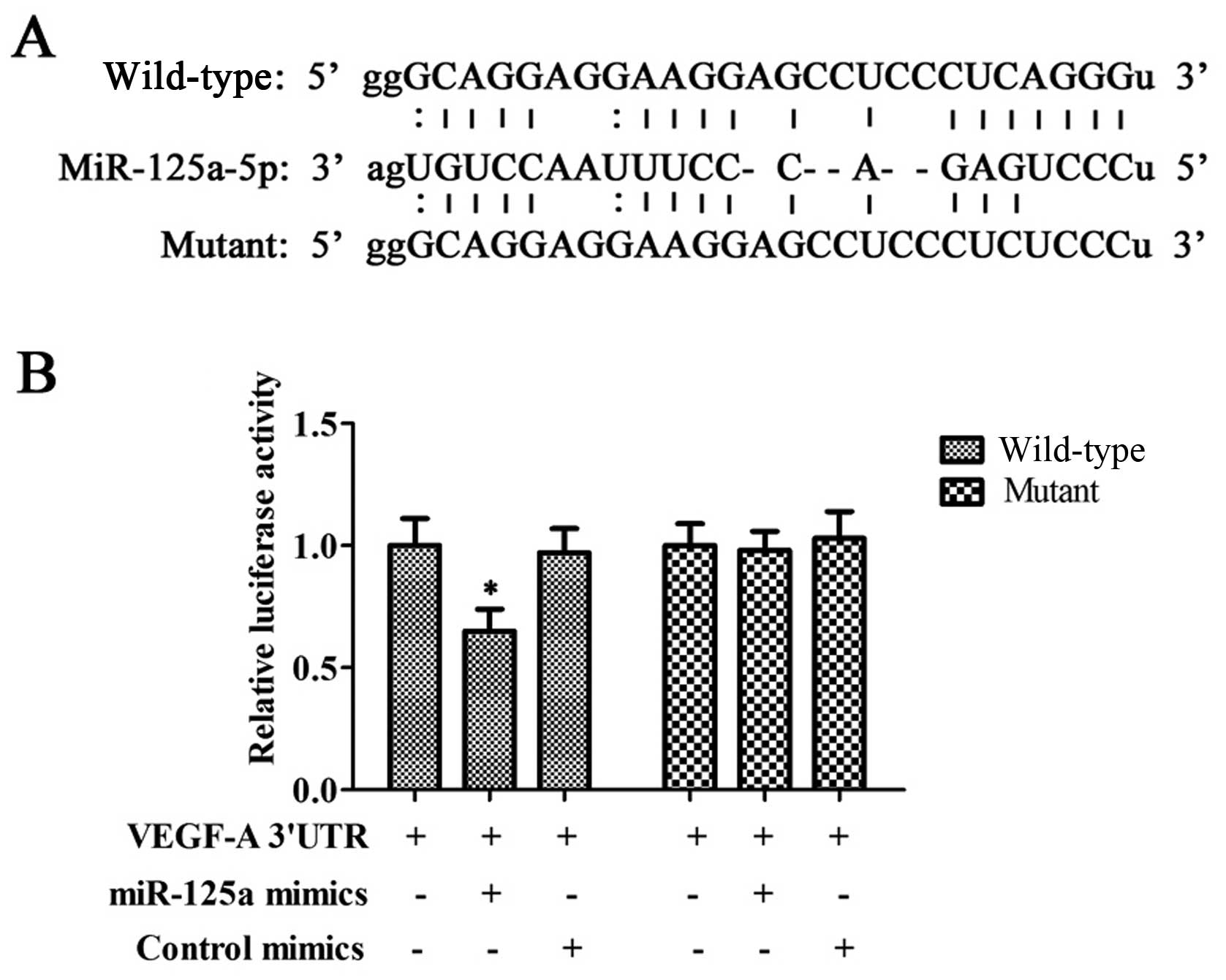
VEGF-A is a target gene of miR-125a
Via predictive analysis, we found that miR-125a had
binding sites in the 3′-UTR region of VEGF-A mRNA (Fig. 2A). To verify whether miR-125a was
capable of regulating the expression of VEGF-A, we performed a
luciferase reporter assay. The luciferase reporter assay revealed
that miR-125a can result in a significant reduction of the relative
luciferase activity while mutating the binding sites abolished this
effect (Fig. 2B).
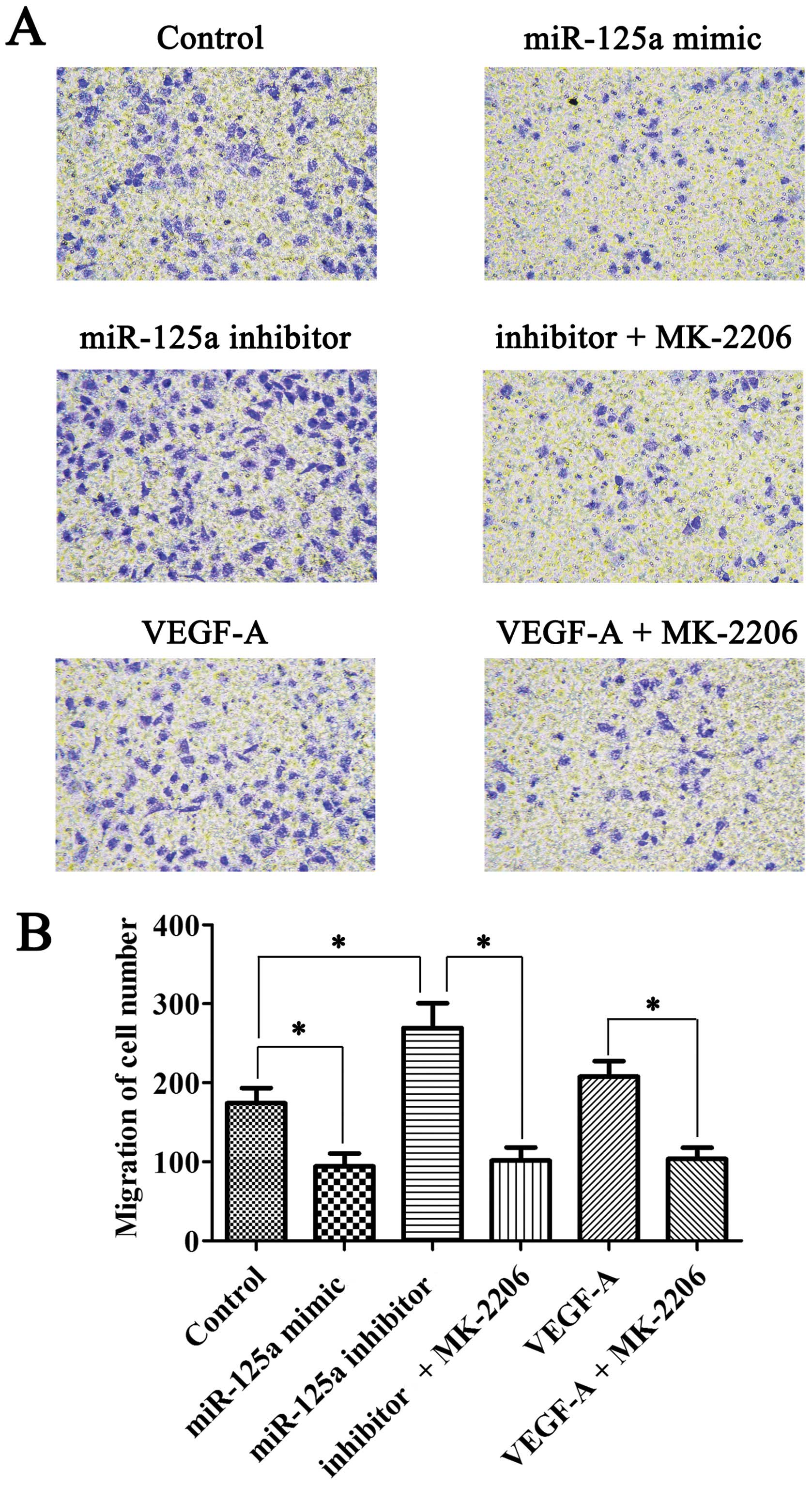
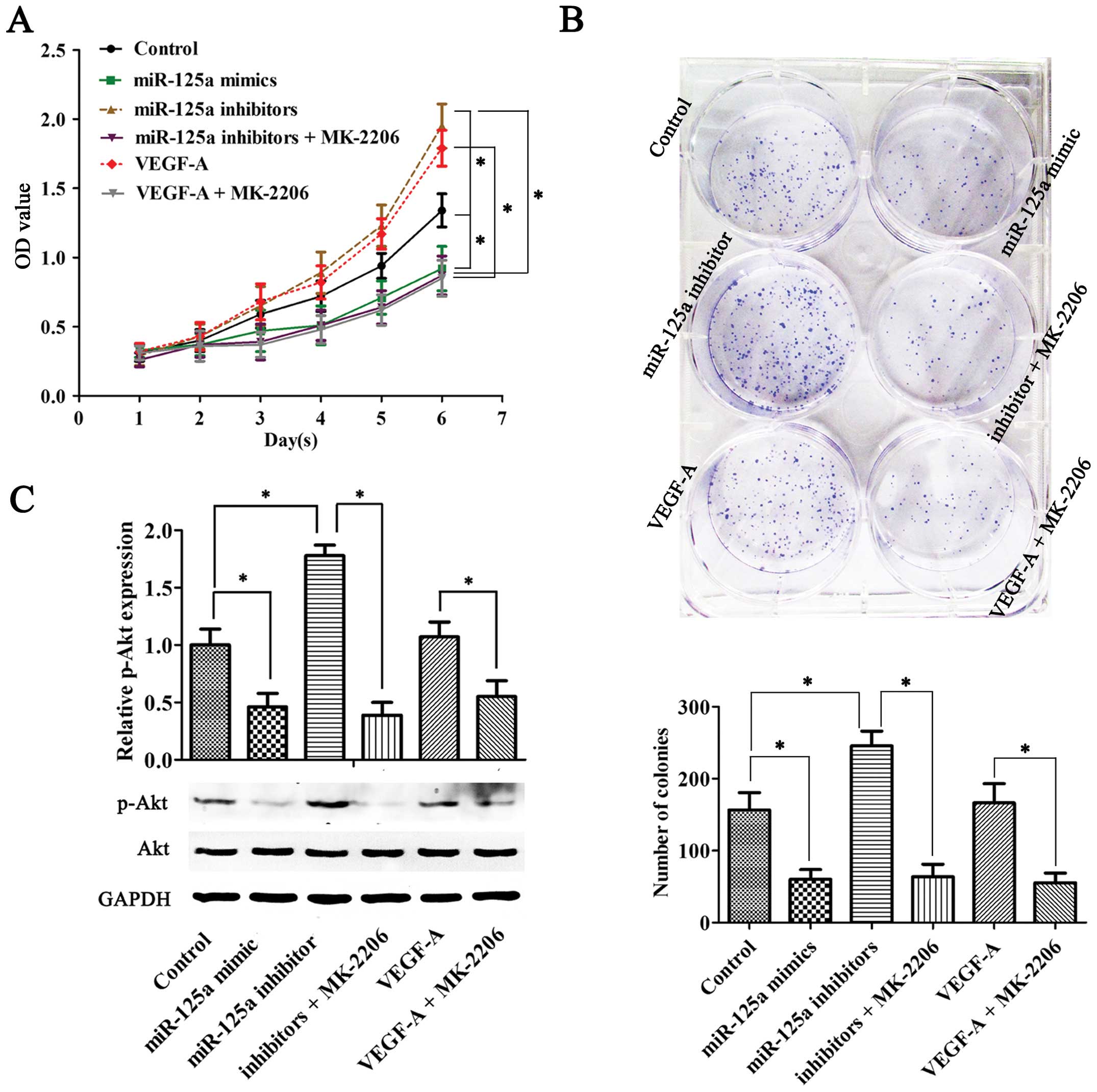
Enhanced migration potential of HUVECs
due to increased secretion of VEGF-A in gastric cancer cells
Co-culture transwell assay was used to detect the
migration ability of HUVECs. The migration ability of HUVECs
declined when HUVECs were co-cultured with miR-125a mimic
transfected AGS cells, while the capacity was enhanced in miR-125a
inhibitor transfection system. However, this effect can be repealed
by Akt inhibitor MK-2206. Moreover, VEGF-A recombinant protein may
contribute to the migration of HUVECs. Similarly, this effect can
be abolished by MK-2206 (Fig.
3).
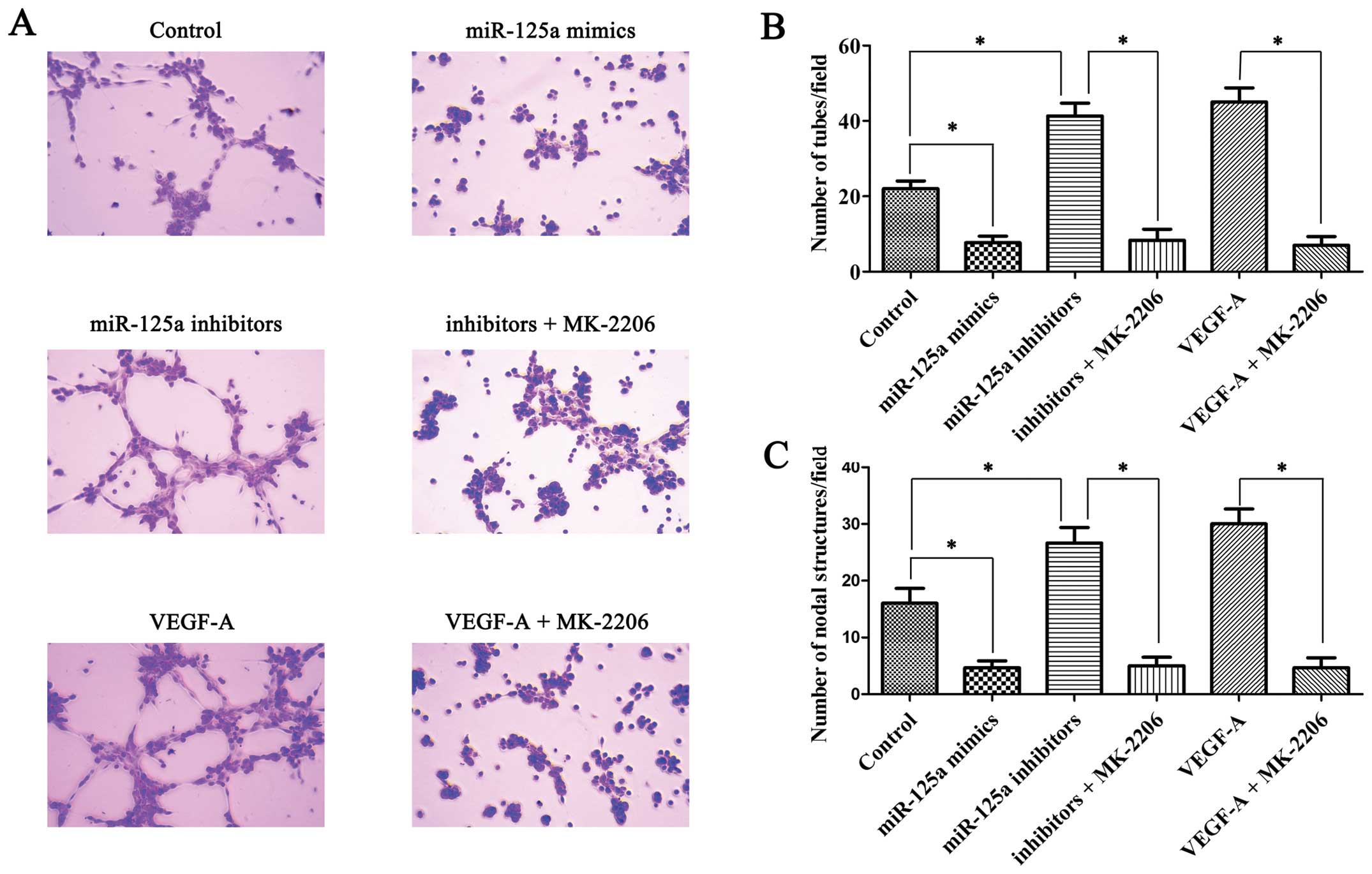
Increased secretion of VEGF-A in gastric
cancer cells may enhance the angiogenesis potential of HUVECs
In vitro tube formation assay was peformed to
detect the angiogenesis potential of HUVECs in different
conditioned media. Comparing conditioned medium of control AGS
cells with that of miR-125a mimic-transfected AGS cells, the latter
contained lower VEGF-A and decreased tube formation (Fig. 4A and B) and nodal structure
(Fig. 4A and C); however,
conditioned medium of miR-125a inhibitor transfected AGS cells
improved the ability of HUVECs to form tube and nodal structures
and this effect can be inhibited by Akt inhibitor MK-2206 (Fig. 4A and B). Moreover, exogenous
recombinant VEGF-A protein enhanced the tube and nodal structure
forming ability of HUVECs, similarly to that shown by MK-2206
(Fig. 4A and C).
Increased secretion of VEGF-A in gastric
cancer cells may enhance the proliferation potential of HUVECs
The proliferation potential of endothelial cells
directly affected angiogenesis; therefore, it was also necessary to
detect changes in the proliferation ability of endothelial cells in
conditioned medium. We cultured HUVECs with AGS-VEGF-A-medium or
VEGF-A-medium containing different concentrations of VEGF-A. The
colony formation assay and CCK-8 proliferation assay revealed
significant changes in the proliferation capacity of HUVECs.
Increased VEGF-A in media can promote the proliferation of HUVECs,
similarly, this effect can be abolished by the Akt inhibitor
(Fig. 5A and B). The change in
biological function of HUVECs may be associated with the VEGF-A
concentration in ambient environment. Increased VEGF-A may
contribute to the migration, proliferation and angiogenesis through
activation of Akt signaling pathway in endothelial cells, while
reduced VEGF-A or inhibition of Akt activity decreased the ability
(Fig. 5C).
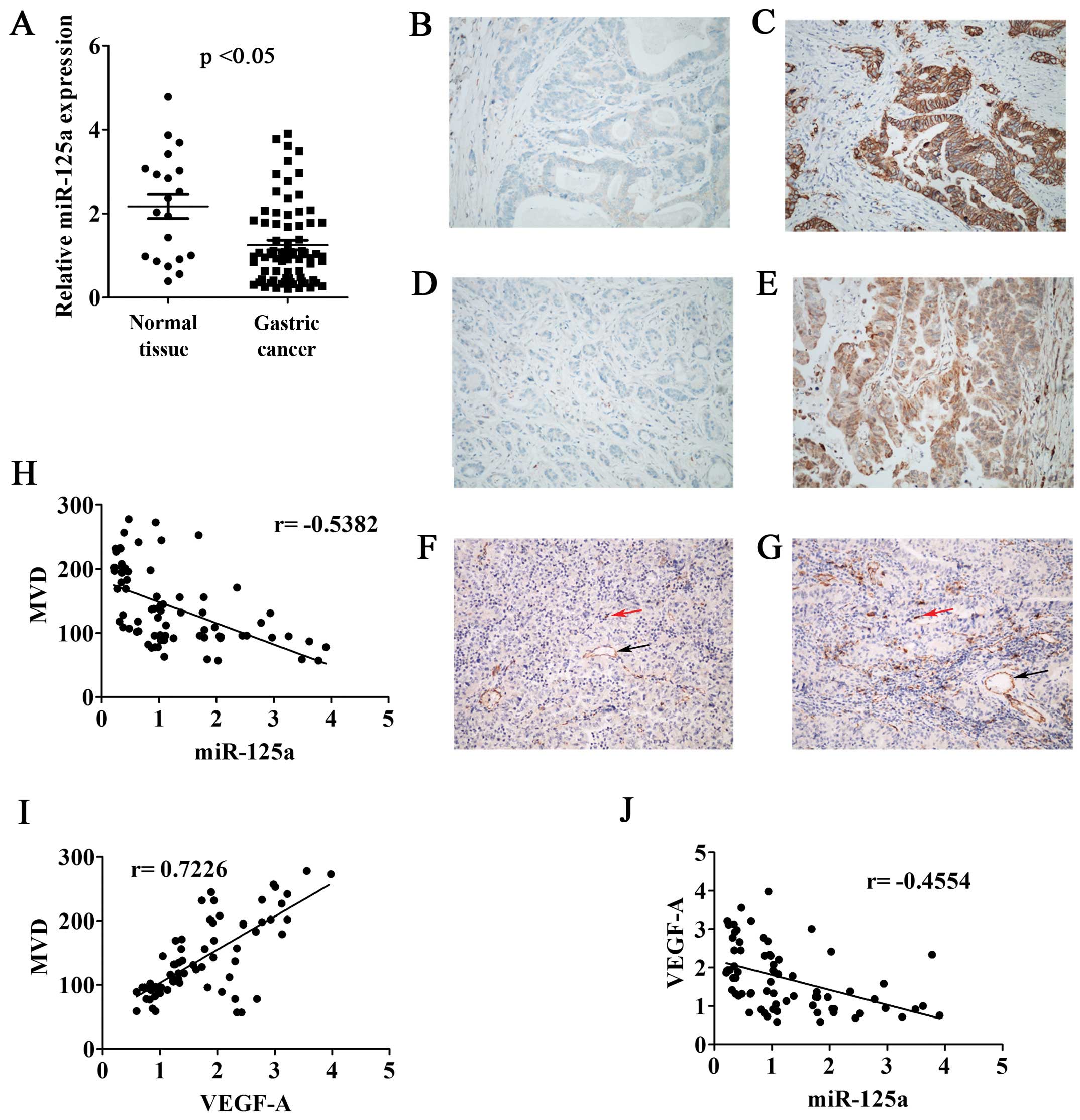
Relationship between the expression of
miR-125a, VEGF-A and MVD in gastric cancer tissues
QRT-PCR was performed to detect the relative
expression of miR-125a in GCT and NGT. We found that the expression
levels of miR-125a in GCT were lower than that in NGT, which showed
a significant statistical difference (p<0.05) (Fig. 6A). The relative expression of
miR-125a in tumors scored <1.00 were classified as low
expression, while the other cases as high expression. According to
this artificial demarcation, the low expression ratio of miR-125a
in these 73 cases of gastric cancer tissues was 50.7%. Furthermore,
we analyzed the expression of Her-2 and VEGF-A in 73 cases of
gastric cancer tissues by IHC. Her-2 was expressed on the membrane
of the tumor cells, with a positive rate of 17.8% (Fig. 6B and C). VEGF-A cytoplasmic
expression showed positive rate of 31.5% (Fig. 6D and E). In order to reveal the
relationship between the expression of miR-125a, VEGF-A and the
numbers of tumor microvessels in gastric cancer, we performed IHC
staining of vascular marker CD31 in 73 cases of gastric cancer
tissues. Single independent endothelial cells (red arrow) or
microvessels that had formed a complete tubular structure (black
arrow) was considered as a countable microvessel (Fig. 6F and G). Correlation analysis
suggested that the expression of miR-125a was inversely
proportional to MVD (r=−0.5382) (Fig.
6H), while VEGF-A expression was positively related to MVD
(r=0.7226) (Fig. 6I). Moreover,
the expression of miR-125a and VEGF-A also showed an inverse
correlation (r=−0.4554) (Fig.
6J).
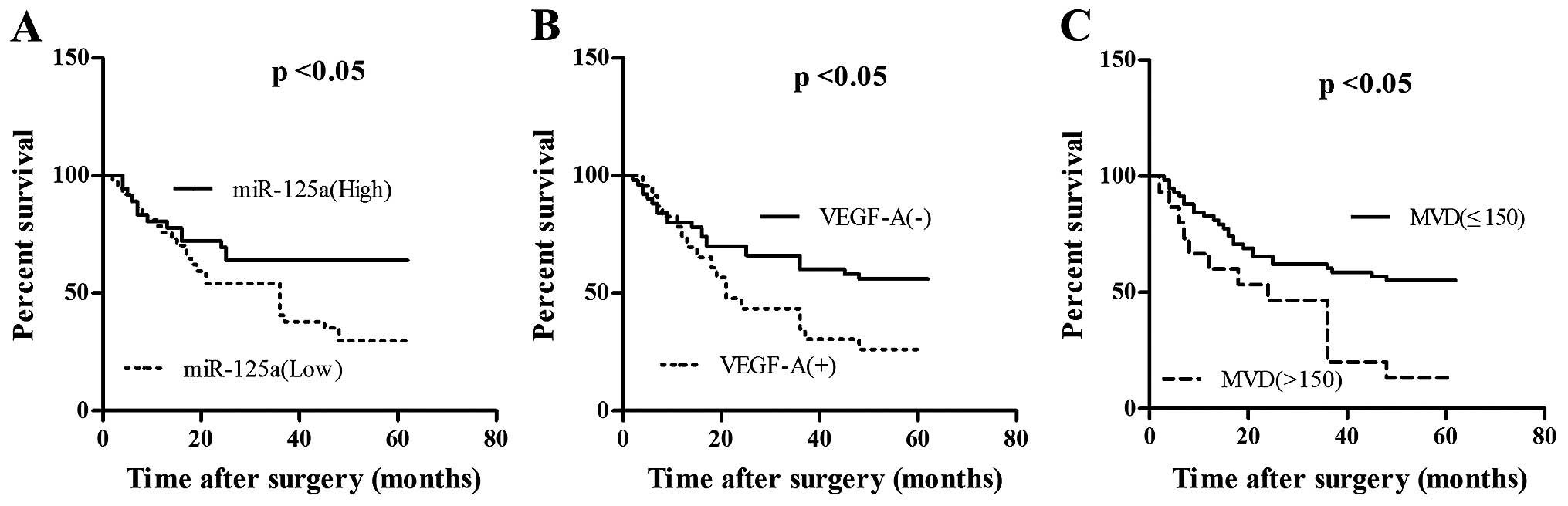
Correlation analysis between miR-125a and
clinicopathologic parameters in gastric cancer
We analyzed the correlation between miR-125a and
clinicopathological parameters in gastric cancer (Table I). The expression of miR-125a was
correlated with lymph node status, the MVD of GC tissues, the
clinical stage of GC and the expression of VEGF-A and Her-2
(p<0.05). The clinical significance of miR-125a and VEGF-A in GC
was further analyzed based on follow-up of the patients. The low
expression of miR-125a indicated a worse prognosis in patients
compared to the high expression of miR-125a (Fig. 7A), while low expression of VEGF-A
indicated a better prognosis compared to the high expression of
VEGF-A (Fig. 7B). MVD was divided
into low-density group and high-density group when 150 was set as
the critical value. The results indicate that high-density group
had a poorer prognosis (Fig.
7C).
 | Table ICorrelation of miR-125a and the
clinicopathological parameters. |
Table I
Correlation of miR-125a and the
clinicopathological parameters.
| miR-125a |
|---|
|
|
|---|
| Clinicopathological
parameters | Low | High | P-value |
|---|
| Age (years) |
| ≤50 | 16 | 18 | 0.563 |
| >50 | 21 | 18 | |
| Gender |
| Male | 18 | 16 | 0.719 |
| Female | 19 | 20 | |
| Size (cm) |
| ≤5 | 20 | 23 | 0.393 |
| >5 | 17 | 13 | |
| LN status |
| Negative | 7 | 16 |
0.019a |
| Positive | 30 | 20 | |
| MVD |
| ≤150 | 24 | 34 |
0.002a |
| >150 | 13 | 2 | |
| TNM |
| I, II | 9 | 22 |
0.001a |
| III, IV | 28 | 14 | |
| Her-2 |
| 0–2+ | 26 | 34 |
0.007a |
| 3+ | 11 | 2 | |
| VEGF-A |
| Negative | 19 | 31 |
0.001a |
| Positive | 18 | 5 | |
Discussion
miRNAs have been shown to play important roles in
the development of tumors by participating in regulation of gene
expression (37–40). miRNAs have been reported as
potential new tumor markers in recent years. Therefore, exploring
the clinical roles and molecular mechanisms of miRNAs in the
malignant development is an effective way to delay the development
of GC and improve the prognosis of GC patients. In this study, we
mainly investigated the role of miR-125a in angiogenesis in GC.
Substantial evidence has suggested that higher
density of tumor blood vessels predict worse prognosis of cancer
patients (36,41,42).
Rich blood vessels are the basis for abnormal growth of the tumor,
while the paracrine amount of pro-angiogenic factors by tumor cells
is an important link to decide the angiogenesis potential of tumor
(43–46). VEGF is a class of
endothelium-specific growth factor, which plays an important role
in the development of tumor angiogenesis. There are seven members
in VEGF families, including VEGF-A, -B, -C, -D, -E, -F and
placental growth factor (47). The
activation of VEGF-A/VEGFR-2 pathway is sufficient to promote the
EC proliferation, migration and tube formation. VEGF-A/VEGFR-2 can
promote the tumor growth of both primary and metastatic cancer,
indicating that VEGF-A plays a key role in tumor angiogenesis
(48). This study demonstrated
that the growth of GC was attributable to the overexpression of
VEGF-A by facilitating angiogenesis, as the expression of VEGF-A
was positively related to microvessel quantity in tumor tissues.
In vivo, MVD analysis showed that the number of microvessels
in VEGF-A-positive GC had a statistically significant difference
compared to VEGF-A-negative GC. In vitro, gastric cancer
cells were transfected with miR-125a mimics or miR-125a inhibitors,
resulting in the reduction or increase of VEGF-A secretion. Then
the medium containing different concentrations of VEGF-A were
co-cultured with HUVECs, which may change the proliferation,
migration and angiogenesis ability of HUVECs at least partly by
VEGF-A/VEGFR2/PI3K/Akt signaling pathway (12,16,49,50).
miR-125a was an independent prognostic factor in
gastric cancer, which may be related to the target gene Her-2
(10,11). Ectopic expression of miR-125a-5p
substantially inhibited the proliferation, migration and invasion
by targeting E3F3 in gastric cancer cells (51). It has also been reported that
miR-125a may inhibit the proliferation and metastasis of
hepatocellular carcinoma partly by downregulating MMP11 and VEGF-A
(52). However, the relative
contribution of miR-125a in regulating VEGF-A in gastric cancer
remained unknown. This study demonstrated that miR-125a decreased
significantly in gastric cancer. Moreover, the expression of
miR-125a was associated with the clinical stage of gastric cancer
and the expression of VEGF-A, suggesting that it may become a new
biological marker. Further study showed that the expression levels
of VEGF-A in the groups transfected with miR-125a mimics were
significantly lower than the control groups, which indicated that
miR-125a may downregulate the expression of VEGF-A in gastric
cancer. This conclusion was corroborated by our luciferase reporter
assay, which showed that VEGF-A was a direct target gene of
miR-125a in gastric cancer. VEGF-A secreted by gastric cancer cells
acted on vascular endothelial cells around the tumor, promoting the
proliferation, and migration of the endothelial cells, and thereby
enhancing angiogenesis. Combining correlation analysis between
miR-125a, VEGF-A and MVD with in vitro results, we
hypothesized that miR-125a/VEGF-A signaling pathway may be a new
approach to regulate angiogenesis in GC. Gastric cancer metabolize
vigorously. High-density microvascularity can provide a wealth of
nutrients for tumor cells, transport the metabolites and thereby
promote the development of the tumor. The follow-up study showed
that miR-125a, VEGF-A, and MVD in tumor tissues can all predict the
prognosis in patients. In fact, anti-angiogenesis approach has
already been used to treat cancer in recent years. Although it has
certain effects, many deficiencies exist (43,53,54).
Theoretically, any molecule in miR-125a/VEGF-A/VEGFR2/Akt signaling
pathway may become a therapeutic target. However, anti-angiogenic
drugs targeting VEGF or its receptors, which aim at blocking the
blood supply to tumors, may also cause hypoxia of normal body and
thereby fuel tumor progression and treatment resistance. Perhaps
targeting the upstream molecules of VEGF-A signaling, such as
miR-125a, can achieve a therapeutic effect; however, there is a
long way to go yet.
In conclusion, this study provided evidence that
miR-125a inhibited the growth of gastric cancer by targeting
VEGF-A. Furthermore, increased levels of miR-125a resulted in
inhibition of angiogenesis in gastric cancer. Taken together, these
findings may help to understand the pathogenesis of gastric cancer
in depth and bring new ideas to the treatment of gastric cancer in
the future.
Acknowledgements
This study was supported by grants from Natural
Science Foundation of China (no. 30570725).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lujambio A and Lowe SW: The microcosmos of
cancer. Nature. 482:347–355. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Day E and Lal A: MicroRNAs and their
target gene networks in breast cancer. Breast Cancer Res.
12:2012010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim
JH, Kim JW and Kim S: MicroRNA expression profiles in serous
ovarian carcinoma. Clin Cancer Res. 14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang L, Huang Q, Zhang S, Zhang Q, Chang
J, Qiu X and Wang E: Hsa-miR-125a-3p and hsa-miR-125a-5p are
downregulated in non-small cell lung cancer and have inverse
effects on invasion and migration of lung cancer cells. BMC Cancer.
10:3182010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferretti E, De Smaele E, Po A, Di
Marcotullio L, Tosi E, Espinola MS, Di Rocco C, Riccardi R,
Giangaspero F, Farcomeni A, et al: MicroRNA profiling in human
medulloblastoma. Int J Cancer. 124:568–577. 2009. View Article : Google Scholar
|
|
10
|
Hashiguchi Y, Nishida N, Mimori K, Sudo T,
Tanaka F, Shibata K, Ishii H, Mochizuki H, Hase K, Doki Y, et al:
Down-regulation of miR-125a-3p in human gastric cancer and its
clinicopathological significance. Int J Oncol. 40:1477–1482.
2012.PubMed/NCBI
|
|
11
|
Nishida N, Mimori K, Fabbri M, Yokobori T,
Sudo T, Tanaka F, Shibata K, Ishii H, Doki Y and Mori M:
MicroRNA-125a-5p is an independent prognostic factor in gastric
cancer and inhibits the proliferation of human gastric cancer cells
in combination with trastuzumab. Clin Cancer Res. 17:2725–2733.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerber HP, McMurtrey A, Kowalski J, Yan M,
Keyt BA, Dixit V and Ferrara N: Vascular endothelial growth factor
regulates endothelial cell survival through the
phosphatidylinositol 3′-kinase/Akt signal transduction pathway.
Requirement for Flk-1/KDR activation. J Biol Chem. 273:30336–30343.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coultas L, Chawengsaksophak K and Rossant
J: Endothelial cells and VEGF in vascular development. Nature.
438:937–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caron C, Spring K, Laramée M, Chabot C,
Cloutier M, Gu H and Royal I: Non-redundant roles of the Gab1 and
Gab2 scaffolding adapters in VEGF-mediated signalling, migration,
and survival of endothelial cells. Cell Signal. 21:943–953. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elayappan B, Ravinarayannan H, Pasha SPBS,
Lee KJ and Gurunathan S: PEDF inhibits VEGF- and EPO- induced
angiogenesis in retinal endothelial cells through interruption of
PI3K/Akt phosphorylation. Angiogenesis. 12:313–324. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Radisavljevic Z, Avraham H and Avraham S:
Vascular endothelial growth factor up-regulates ICAM-1 expression
via the phosphatidylinositol 3 OH-kinase/AKT/Nitric oxide pathway
and modulates migration of brain microvascular endothelial cells. J
Biol Chem. 275:20770–20774. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gerhardt H, Golding M, Fruttiger M,
Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C,
Alitalo K, Shima D, et al: VEGF guides angiogenic sprouting
utilizing endothelial tip cell filopodia. J Cell Biol.
161:1163–1177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adams RH and Alitalo K: Molecular
regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell
Biol. 8:464–478. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herbert SP and Stainier DYR: Molecular
control of endothelial cell behaviour during blood vessel
morphogenesis. Nat Rev Mol Cell Biol. 12:551–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Chen X, Fang J and Yang C:
Overexpression of both VEGF-A and VEGF-C in gastric cancer
correlates with prognosis, and silencing of both is effective to
inhibit cancer growth. Int J Clin Exp Pathol. 6:586–597.
2013.PubMed/NCBI
|
|
21
|
Zhao R, Liu XQ, Wu XP, Liu YF, Zhang ZY,
Yang GY, Guo S, Niu J, Wang JY and Xu KS: Vascular endothelial
growth factor (VEGF) enhances gastric carcinoma invasiveness via
integrin alpha(v)beta6. Cancer Lett. 287:150–156. 2010. View Article : Google Scholar
|
|
22
|
Peng Z, Wei D, Wang L, Tang H, Zhang J, Le
X, Jia Z, Li Q and Xie K: RUNX3 inhibits the expression of vascular
endothelial growth factor and reduces the angiogenesis, growth, and
metastasis of human gastric cancer. Clin Cancer Res. 12:6386–6394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ji YN, Wang Q, Li Y and Wang Z: Prognostic
value of vascular endothelial growth factor A expression in gastric
cancer: A meta-analysis. Tumour Biol. 35:2787–2793. 2014.
View Article : Google Scholar
|
|
24
|
De Luca A, Lamura L, Gallo M, Maffia V and
Normanno N: Mesenchymal stem cell-derived interleukin-6 and
vascular endothelial growth factor promote breast cancer cell
migration. J Cell Biochem. 113:3363–3370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yue X, Wang P, Xu J, Zhu Y, Sun G, Pang Q
and Tao R: MicroRNA-205 functions as a tumor suppressor in human
glioblastoma cells by targeting VEGF-A. Oncol Rep. 27:1200–1206.
2012.
|
|
26
|
Wen J, Zhao Y, Li J, Weng C, Cai J, Yang
K, Yuan H, Imperato-McGinley J and Zhu YS: Suppression of
DHT-induced paracrine stimulation of endothelial cell growth by
estrogens via prostate cancer cells. Prostate. 73:1069–1081. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vacca A, Ria R, Ribatti D, Semeraro F,
Djonov V, Di Raimondo F and Dammacco F: A paracrine loop in the
vascular endothelial growth factor pathway triggers tumor
angiogenesis and growth in multiple myeloma. Haematologica.
88:176–185. 2003.PubMed/NCBI
|
|
28
|
Kinnaird T, Stabile E, Burnett MS, Lee CW,
Barr S, Fuchs S and Epstein SE: Marrow-derived stromal cells
express genes encoding a broad spectrum of arteriogenic cytokines
and promote in vitro and in vivo arteriogenesis through paracrine
mechanisms. Circ Res. 94:678–685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neviani P, Santhanam R, Oaks JJ, Eiring
AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N,
Gambacorti-Passerini C, et al: FTY720, a new alternative for
treating blast crisis chronic myelogenous leukemia and Philadelphia
chromosome-positive acute lymphocytic leukemia. J Clin Invest.
117:2408–2421. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qu J, Lu W, Li B, Lu C and Wan X: WWOX
induces apoptosis and inhibits proliferation in cervical cancer and
cell lines. Int J Mol Med. 31:1139–1147. 2013.PubMed/NCBI
|
|
31
|
Shao R, Xia W and Hung MC: Inhibition of
angiogenesis and induction of apoptosis are involved in
E1A-mediated bystander effect and tumor suppression. Cancer Res.
60:3123–3126. 2000.PubMed/NCBI
|
|
32
|
Su SJ, Yeh TM, Chuang WJ, Ho CL, Chang KL,
Cheng HL, Liu HS, Cheng HL, Hsu PY and Chow NH: The novel targets
for anti-angiogenesis of genistein on human cancer cells. Biochem
Pharmacol. 69:307–318. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dallaglio K, Bruno A, Cantelmo AR,
Esposito AI, Ruggiero L, Orecchioni S, Calleri A, Bertolini F,
Pfeffer U, Noonan DM, et al: Paradoxic effects of metformin on
endothelial cells and angiogenesis. Carcinogenesis. 35:1055–1066.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zaytseva YY, Elliott VA, Rychahou P,
Mustain WC, Kim JT, Valentino J, Gao T, O'Connor KL, Neltner JM,
Lee EY, et al: Cancer cell-associated fatty acid synthase activates
endothelial cells and promotes angiogenesis in colorectal cancer.
Carcinogenesis. 35:1341–1351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu C and Tanigawa N: Spontaneous apoptosis
is inversely related to intratumoral microvessel density in gastric
carcinoma. Cancer Res. 57:221–224. 1997.PubMed/NCBI
|
|
36
|
Hillen HF, Hak LE, Joosten-Achjanie SR and
Arends JW: Microvessel density in unknown primary tumors. Int J
Cancer. 74:81–85. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li XJ, Ren ZJ and Tang JH: MicroRNA-34a: A
potential therapeutic target in human cancer. Cell Death Dis.
5:e13272014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Blandino G, Fazi F, Donzelli S, Kedmi M,
Sas-Chen A, Muti P, Strano S and Yarden Y: Tumor suppressor
microRNAs: A novel non-coding alliance against cancer. FEBS Lett.
588:2639–2652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maroof H, Salajegheh A, Smith RA and Lam
AKY: Role of microRNA-34 family in cancer with particular reference
to cancer angiogenesis. Exp Mol Pathol. 97:298–304. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yan JW, Lin JS and He XX: The emerging
role of miR-375 in cancer. Int J Cancer. 135:1011–1018. 2014.
View Article : Google Scholar
|
|
41
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, Gatter KC and Harris AL: High DLL4 expression in
tumour-associated vessels predicts for favorable radiotherapy
outcome in locally advanced squamous cell head-neck cancer (HNSCC).
Angiogenesis. 16:343–351. 2013. View Article : Google Scholar
|
|
42
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
A new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Epstein RJ: VEGF signaling inhibitors:
More pro-apoptotic than anti-angiogenic. Cancer Metastasis Rev.
26:443–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu F, You X, Wang Y, Liu Q, Liu Y, Zhang
S, Chen L, Zhang X and Ye L: The oncoprotein HBXIP enhances
angiogenesis and growth of breast cancer through modulating FGF8
and VEGF. Carcinogenesis. 35:1144–1153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shih YP, Liao YC, Lin Y and Lo SH: DLC1
negatively regulates angiogenesis in a paracrine fashion. Cancer
Res. 70:8270–8275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kryza T, Achard C, Parent C, Marchand-Adam
S, Guillon-Munos A, Iochmann S, Korkmaz B, Respaud R, Courty Y and
Heuzé-Vourc'h N: Angiogenesis stimulated by human
kallikrein-related peptidase 12 acting via a platelet-derived
growth factor B-dependent paracrine pathway. FASEB J. 28:740–751.
2014. View Article : Google Scholar
|
|
47
|
Otrock ZK, Makarem JA and Shamseddine AI:
Vascular endothelial growth factor family of ligands and receptors
(review). Blood Cells Mol Dis. 38:258–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar
|
|
49
|
Dayanir V, Meyer RD, Lashkari K and Rahimi
N: Identification of tyrosine residues in vascular endothelial
growth factor receptor-2/FLK-1 involved in activation of
phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem.
276:17686–17692. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pan Y, Wu Q, Qin L, Cai J and Du B: Gold
nanoparticles inhibit VEGF165-induced migration and tube formation
of endothelial cells via the Akt pathway. BioMed Res Int.
2014:4186242014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu Y, Huang Z and Liu Y: Reduced
miR-125a-5p expression is associated with gastric carcinogenesis
through the targeting of E2F3. Mol Med Rep. 10:2601–2608.
2014.PubMed/NCBI
|
|
52
|
Bi Q, Tang S, Xia L, Du R, Fan R, Gao L,
Jin J, Liang S, Chen Z, Xu G, et al: Ectopic expression of MiR-125a
inhibits the proliferation and metastasis of hepatocellular
carcinoma by targeting MMP11 and VEGF. PLoS One. 7:e401692012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cao Y: VEGF-targeted cancer
therapeutics-paradoxical effects in endocrine organs. Nat Rev
Endocrinol. 10:530–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jain RK: Antiangiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014. View Article : Google Scholar : PubMed/NCBI
|