Introduction
Ovarian carcinoma is the most lethal gynecological
malignancy in American and Japanese women (1). Despite aggressive treatment, most
patients eventually experience a recurrence of a chemoresistant
form of the disease. Ovarian carcinoma is classified into four
molecularly distinct histological types: serous, mucinous,
endometrioid, and clear cell (2).
Ovarian clear cell carcinoma (OCCC), which constitutes
approximately 25% of ovarian carcinoma in Japan, is highly
resistant to conventional platinum based chemotherapy (3). As such, OCCC carries a poor
prognosis, despite early-stage diagnosis in 60% of cases. The
evasion of apoptosis is a hallmark of cancer cells (4), and the failure of anticancer
treatments to induce apoptosis leads to chemotherapeutic failure
and tumor progression. However, the role of autophagy, an
alternative caspase-independent cell death program (5,6), and
its underlying molecular mechanism in OCCC chemoresistance and
tumor progression remain unclear. Autophagy is a process of
degradation and recycling of cytoplasmic components for energy
utilization. Autophagy also occurs in response to certain forms of
therapeutic stress, including cytotoxic chemotherapy (7,8).
Approximately 30 yeast genes and 16 human homologues have been
identified as autophagy-related genes. Among these genes, Beclin 1
plays key roles in mammalian autophagy (9,10).
To the best of our knowledge, the role of autophagy
in chemoresistance and the clinocopahological siginificance of
Beclin 1, a key molecule for autophagy induction in OCCCs, have not
been examined. The goal of the present study was to clarify the
role of autophagy in cisplatin sensitivity in OCCCs and the role of
Beclin 1 in OCCC progression.
Materials and methods
Tissue samples
Formalin-fixed, paraffin-embedded tissue samples of
60 ovarian clear cell carcinomas were used in the present study.
Samples were obtained from the Department of Obstetrics and
Gynecology at the Shimane University Hospital and the Department of
Obstetrics and Gynecology at Seirei Hamamatsu General Hospital.
Diagnosis was based on conventional morphological examination of
sections stained with hematoxylin and eosin (H&E), and tumors
were classified according to the WHO guidelines. Tumor staging was
performed using the International Federation of Gynecology and
Obstetrics (FIGO) classification system. All patients were
primarily treated with cytoreductive surgery and adjuvant platinum
and taxane or CPT-11 chemotherapy (CBDCA AUC 5 with paclitaxel 175
mg/m2 or docetaxel 70 mg/m2 or CDDP 60
mg/m2 with CPT-11 180 mg/m2). All patients
received 6–12 courses of this combination regimen. Acquisition of
tissue specimens and clinical information was approved by the
institutional review board of Shimane University & Serrei
Hamamatsu General Hospital. The paraffin tissue blocks were
organized into tissue microarrays, each made by removing 3-mm
diameter cores of tumor from the block. Selection of the area to
core was made by a gynecologic oncologist (K.N) and pathology
technician (K.I) and was based on review of the H&E slides.
Immunohistochemistry
For immunohistochemistry, paraffin sections were
deparaffinized and incubated with a primary mouse Beclin 1 antibody
(Cell Signaling Technology, Danvers, MA, USA) at a dilution of
1:100 in a 4°C moist chamber overnight. Two independent observers
scored Beclin 1 immunoreactivity using a categorical scoring system
from 0 (not detectable) to 3 (intense) with the mean score recorded
from triplicate samples. Immunostaining and evaluation of ARID1A (a
dilution 1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was
previously performed (11).
Mutation analysis
Of the 60 OCCCs, 37 or 56 samples were available for
Kras or PIK3CA DNA sequencing respectively, and had
been previously evaluated (12).
Exons 9 and 20 of the PIK3CA gene and exon 1 of the
KRAS gene (including codons 12 and 13) were amplified by
polymerase chain reaction (PCR) using primer sets previously
described (12). Polymerase chain
reaction products were purified using the Qiagen PCR purification
kit (Qiagen, Valencia, CA, USA) and used for direct sequencing.
Gene amplification analysis
ZNF217 gene amplification analysis was
performed and evaluated as previously described (13). Briefly, BAC clones (RP5-823G15 and
RP4-724E16) containing the genomic sequences of the 20q13.2
amplicon for ZNF217 locus were purchased from BACPAC
Resource Center (Children's Hospital, Oakland, CA, USA) and
Invitrogen (Carlsbad, CA, USA). BAC clones corresponding to the
Ch20P centromere (RP5-1025A1 and RP4-738P15) were used to generate
reference probes. The method used for fluorescence in situ
hybridization (FISH) was described by Nakayama et al
(14).
Cell culture and cell lines
ES2 (clear cell carcinoma) and TOV-21G (clear cell
carcinoma) human ovarian cancer cell lines were obtained from the
American Type Culture Collection (ATCC; Rockville, MD, USA).
Western blot analysis
Western blot analysis was performed on ovarian
cancer cell lines ES2 and TOV-21G. Cell lysates were prepared by
dissolving cell pellets in Laemmli sample buffer (Bio-Rad
Laboratories, Hercules, CA, USA) supplemented with 5%
beta-mercaptoethanol (Sigma, St. Louis, MO, USA). Similar amounts
of total protein from each lysate were loaded and separated on 10%
Tris-glycine SDS-polyacrylamide gels (Novex, San Diego, CA, USA)
and electroblotted to Millipore Immobilon-P polyvinylidene
difluoride membranes. The membranes were probed with Beclin 1
antibody (1:100; Cell Signaling Technology), LC-III (1:1,000; Santa
Cruz Biotechnology), or p62 (1:100; Enzo Life Sciences, Inc.,
Plymouth Meeting, PA, USA) followed by peroxidase conjugated
anti-mouse or anti-rabbit immunoglobulin (1:20,000). The same
membrane was probed with a GAPDH antibody (1:10,000) (Cell
Signaling Technology) for loading controls. Western blots were
developed using a chemiluminescence kit (Pierce, Rockford, IL,
USA).
Silencing RNA knockdown of Beclin 1 gene
expression
Beclin 1 and control siRNA (luciferase siRNA) were
purchased from Cell Signaling Technology. Cells were seeded into
96-well plates and transfected with siRNAs using Oligofectamine
(Invitrogen). Following transfection, cells were collected at 48 h
for western blotting of Beclin 1 protein.
Cell proliferation assay
CDDP was purchased from Enzo Life Sciences.
Chloroquine disphosphate was purchased from Sigma. Cytotoxicity of
CDDP was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay (Sigma). Cells were seeded into 96-well plates
at a density of 3,000 cells/well. Cell number was determined
indirectly with an MTT assay (15). Data were expressed as the mean ± 1
SD of triplicate determinations. An MTT cell growth assay was
performed 96 h after treating the cells with Beclin 1 siRNA or
control siRNA. The data were expressed as a percentage of the DMSO
control. The mean and standard deviation were obtained from three
experiments.
Autophagy assays
Autophagy was measured using: i) western blot
analysis of LC3 and p62 and ii) microscopic observation of GFP-LC3
puncta (16,17).
Statistical methods for clinical
correlation
Progression-free and overall survivals were
calculated from the date of diagnosis to the date of first relapse
or the last follow-up. Age and performance status distributions
were similar between patients who did and did not express Beclin 1.
The data were plotted as Kaplan-Meier curves, and the statistical
significance was determined by the log-rank test. Data were
censored when patients were lost to follow-up. Student's t-test
(comparison of two groups) or one-way analysis of variance (ANOVA;
comparison of more than two groups) were used to evaluate numerical
data.
Results
CDDP induces cytoprotective autophagy in
OCCC cell lines
To determine the effect of CDDP on autophagy and the
role of autophagy in determining the sensitivity of cancer cells to
the drug, we first examined the activity of autophagy in OCCC cell
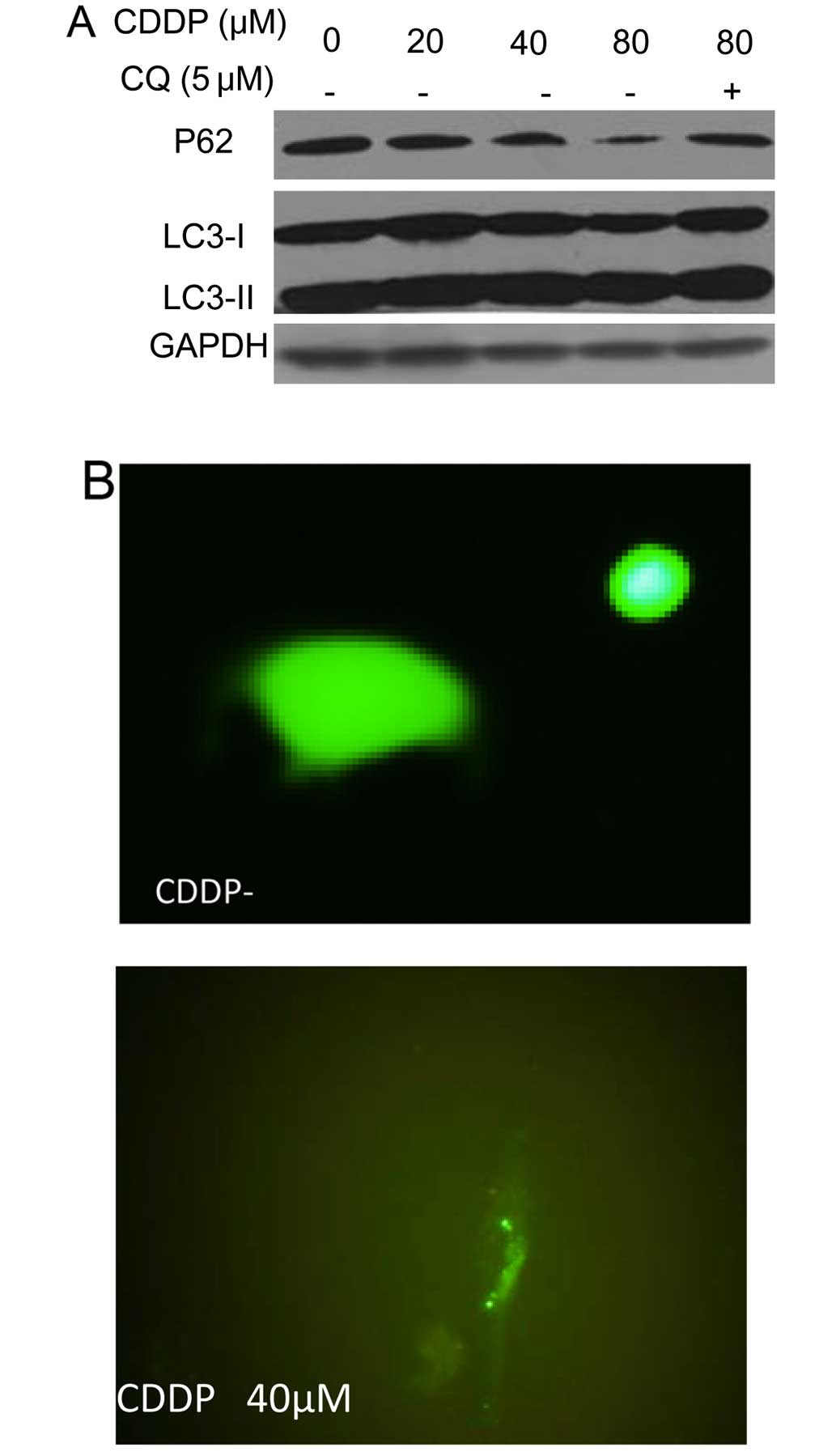
lines. As shown in Fig. 1A,
treatment of the human OCCC cell lines ES-2 and TOV-21G with CDDP
produced a dose-dependent activation of autophagy, as evidenced by
increases in the amount of LC3 II and decreases in the amount of
p62, two selective markers of autophagy. Fig. 1A also shows that LC3 II levels were
further elevated in the presence of chloroquine, an inhibitor of
autophagosome-lysosome fusion and LC3 II degradation, which is
indicative of increased autophagic flux in the CDDP-treated cells.
The stimulative effect of CDDP on autophagy was verified using a
green fluorescent protein (GFP)-LC3 puncta-formation assay, which
revealed an increase in the number of GFP-LC3 puncta in tumor cells
treated with CDDP (Fig. 1B and
C).
Inhibition of autophagy by chloroquine or
Beclin 1 siRNA does not enhance sensitivity of OCCC cell lines to
CDDP
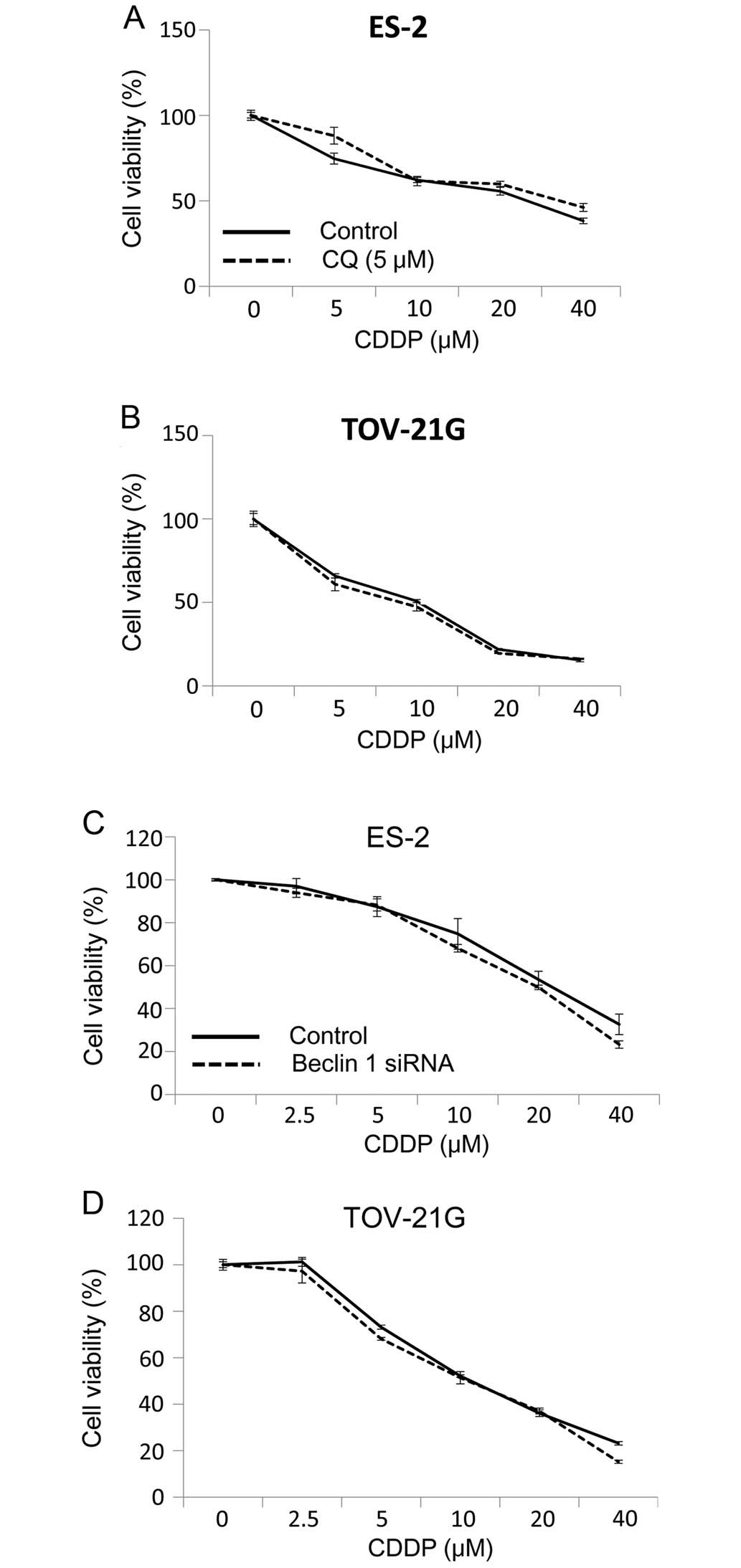
To address the question of whether CDDP-stimulated
autophagy has a protective or sensitizing role in CDDP-treated
tumors, we compared cytotoxicity under a variety of drug treatment
conditions. Concomitant treatment of OCCC cells with CDDP and
inhibitors of autophagy, chloroquine did not enhance the
cytotoxicity (Fig. 2A and B)
induced by CDDP. The effect of autophagy on the CDDP-sensitivity of
tumor cells was further explored by suppressing the expression of
autophagy-related gene Beclin 1 and measuring the effect on tumor
cell clonogenicity. Fig. 2C and D
shows that knockdown of Beclin 1 in ES-2 and TOV-21G cells did not
enhance CDDP-induced cytotoxicity. These results suggest that CDDP
induces canonical autophagy. However, induction of CDDP-induced
autophagy does not have a protective role in OCCC cell lines
subjected to CDDP cytotoxicity.
Relationship between Beclin 1 protein
expression and clinicopathological factors in OCCCs
Because Beclin 1 is a key regulator of autophagy, we
focused on the relationship between Beclin 1 expression and
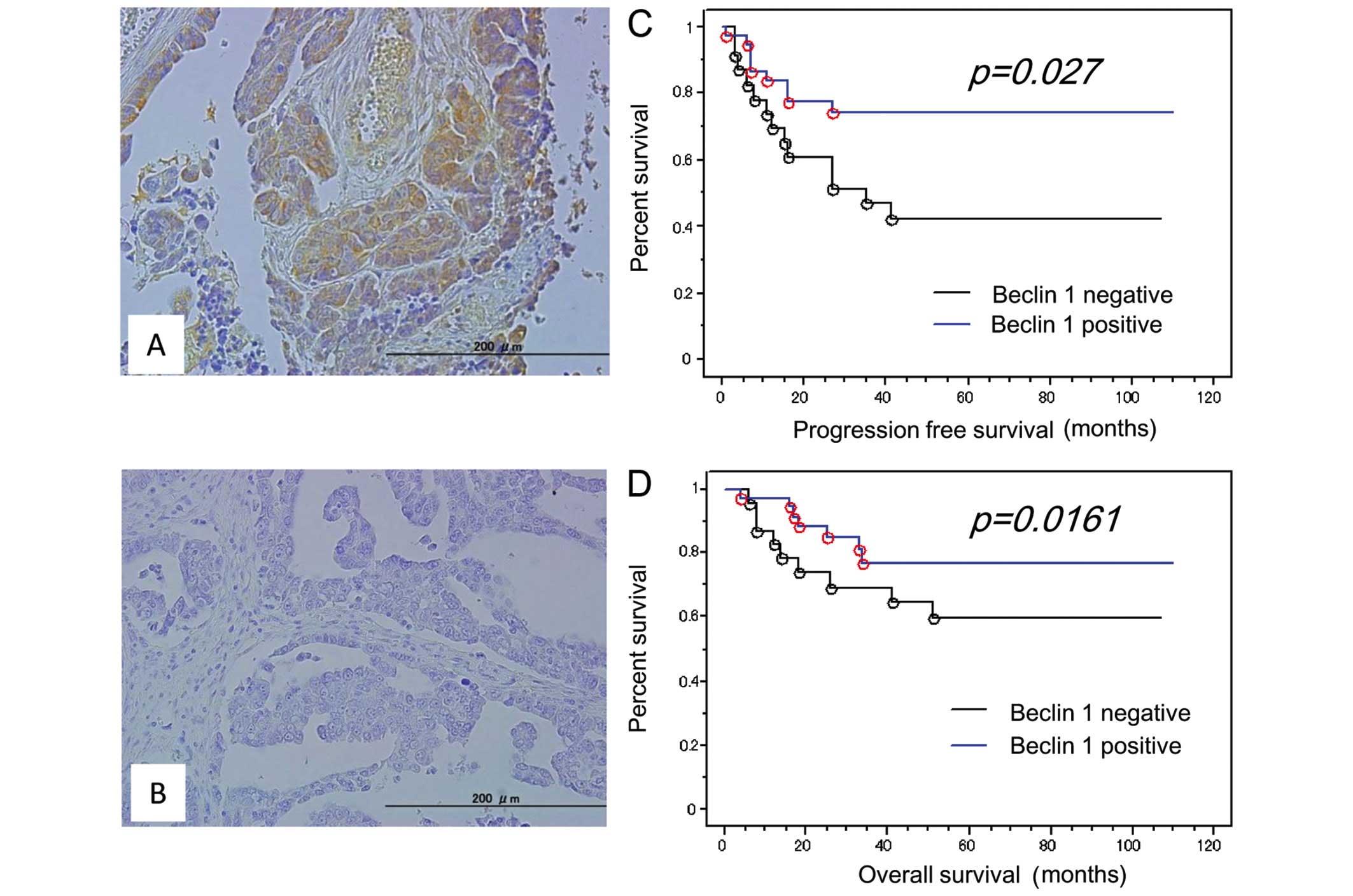
clinicopathological factors in OCCCs. Loss of Beclin 1 expression
(Beclin 1 immunointensity 0+) was observed in 38.3% (23/60) of
analyzed tumors (Fig. 3A and B).
Patients were stratified into one of two groups depending on their
status as determined by Beclin 1 immunostaining. The relationships
between Beclin 1 protein expression and clinicopathological factors
are shown in Table I. There were
no significant correlations between loss of Beclin 1 expression and
FIGO stage, CA125 levels, patient age, status of endometriosis,
Ki-67 labeling index, chemotherapy regimen, or status of residual
tumor. There was a marginally significant correlation between loss
of Beclin 1 expression and lymph node metastasis (P=0.0579).
 | Table IAssociation between Beclin 1
expression and clinicopathological factors in patients with ovarian
clear cell carcinoma. |
Table I
Association between Beclin 1
expression and clinicopathological factors in patients with ovarian
clear cell carcinoma.
| | Beclin 1
immunostaining | |
|---|
| |
| |
|---|
| Factors | Patients | Negative | Positive | P-value |
|---|
| FIGO stage |
| I, II | 45 | 14 | 30 | 0.0852 |
| III, IV | 15 | 9 | 7 | |
| CA125 U/ml |
| <90 | 30 | 9 | 21 | 0.1843 |
| ≥90 | 30 | 14 | 16 | |
| Age (years) |
| <54 | 30 | 12 | 18 | 0.7906 |
| ≥54 | 30 | 11 | 19 | |
| Endometriosis |
| Without | 32 | 15 | 17 | 0.8848 |
| With | 28 | 8 | 20 | |
| Ki-67 |
| Low | 30 | 14 | 16 | 0.1843 |
| High | 30 | 9 | 21 | |
| Residual tumor
(cm) |
| <2 | 48 | 6 | 42 | 0.2781 |
| ≥2 | 12 | 3 | 9 | |
| Lymph node
metastasis |
| Negative | 51 | 17 | 34 | 0.0579 |
| Positive | 9 | 6 | 3 | |
Relationship between loss of Beclin 1
expression and status of Kras and PIK3CA mutation, status of ZNF217
amplification, and status of ARID1A expression in OCCCs
The 60 tumor samples in the present study had been
characterized previously to determine mutational (Kras and
PIK3CA) amplification (ZNF217) and
immunohistochemical (ARID1A) status (11–13).
Statistical analysis showed no correlations between loss of Beclin
1 expression and Kras and PIK3CA mutation status in
OCCCs (Table II). In contrast,
loss of Beclin 1 expression was significantly correlated with
ZNF217 amplification (P=0.024), and tended to correlate with
loss of ARID1A expression in OCCCs (P=0.057) (Table II).
 | Table IIAssociation between Beclin 1
expression and status of ARID1A, K-ras, PIK3CA and
ZNF217 in patients with ovarian clear cell carcinoma. |
Table II
Association between Beclin 1
expression and status of ARID1A, K-ras, PIK3CA and
ZNF217 in patients with ovarian clear cell carcinoma.
| | Beclin 1
expression | |
|---|
| |
| |
|---|
| Factors | Patients | Negative | Positive | P-value |
|---|
| ARID1A |
| Negative | 9 | 6 | 3 | 0.0579 |
| Positive | 51 | 17 | 34 | |
| K-ras |
| Wild-type | 35 | 11 | 24 | 0.3443 |
| Mutant | 2 | 0 | 2 | |
| PIK3CA |
| Wild-type | 40 | 14 | 26 | 0.5412 |
| Mutant | 16 | 7 | 9 | |
| ZNF217 |
| Normal | 48 | 15 | 33 | 0.024 |
| Amplification | 12 | 8 | 4 | |
Effect of loss of Beclin 1 protein
expression on progression-free survival
We examined the effect of loss of Beclin 1 protein
expression on the prognosis for progression-free survival.
Kaplan-Meier estimates of progression-free/overall survival are
plotted in Fig. 2. Of the 60
patients diagnosed at stages I–IV, 23 patients with loss of Beclin
1 expression had a shorter progression-free survival than those
with positive Beclin 1 expression (P=0.027; log-rank test)
(Fig. 3C). Univariate analysis
demonstrated that FIGO stage III, IV (P<0.01; log-rank test),
CA125 levels (P=0.01; log-rank test), residual tumor (≥2 cm)
(P<0.01; log-rank test), and loss of Beclin 1 expression
(P=0.027; log-rank test) correlated with shorter progression-free
survival. When the data were stratified for multivariate analysis,
only residual tumor (≥2 cm) remained a significant (P=0.03) factor
for shorter disease-free survival (data not shown).
Effect of loss of Beclin 1 protein
expression on overall survival
Loss of Beclin 1 expression also tended to correlate
with shorter overall survival in OCCC patients treated with
platinum-based chemotherapy (P=0.161; log-rank test) (Fig. 3D). Univariate analysis demonstrated
that FIGO stage III, IV (P<0.01; log-rank test), CA125 levels
(P=0.01; log-rank test), and residual tumor (≥2 cm) (P<0.01;
log-rank test) significantly correlated with shorter overall
survival. When these data were stratified for multivariate
analysis, only residual tumor (≥2 cm) remained a significant
(P=0.04) predictor for shorter overall survival (data not
shown).
Relationship between Beclin 1 expression
and chemotherapeutic response
Of the 60 OCCC patients, 14 had measurable residual
disease following primary cytoreductive surgery. Of these 14
patients, 6 (42.8%) responded to chemotherapy and 8 (57.2%) did
not. Beclin 1-negative tumors were not more resistant to primary
adjuvant chemotherapy than the Beclin 1-positive tumors (50.0 vs.
66.7%, P=0.937) (Table III).
 | Table IIIThe relationship between Becin 1
expression and platinum-based chemotherapeutic response. |
Table III
The relationship between Becin 1
expression and platinum-based chemotherapeutic response.
| Responder n
(%) | Non-responder n
(%) | P-value |
|---|
| Negative | 4 (50.0) | 4 (50.0) | 0.937 |
| Positive | 2 (33.3) | 4 (66.7) | |
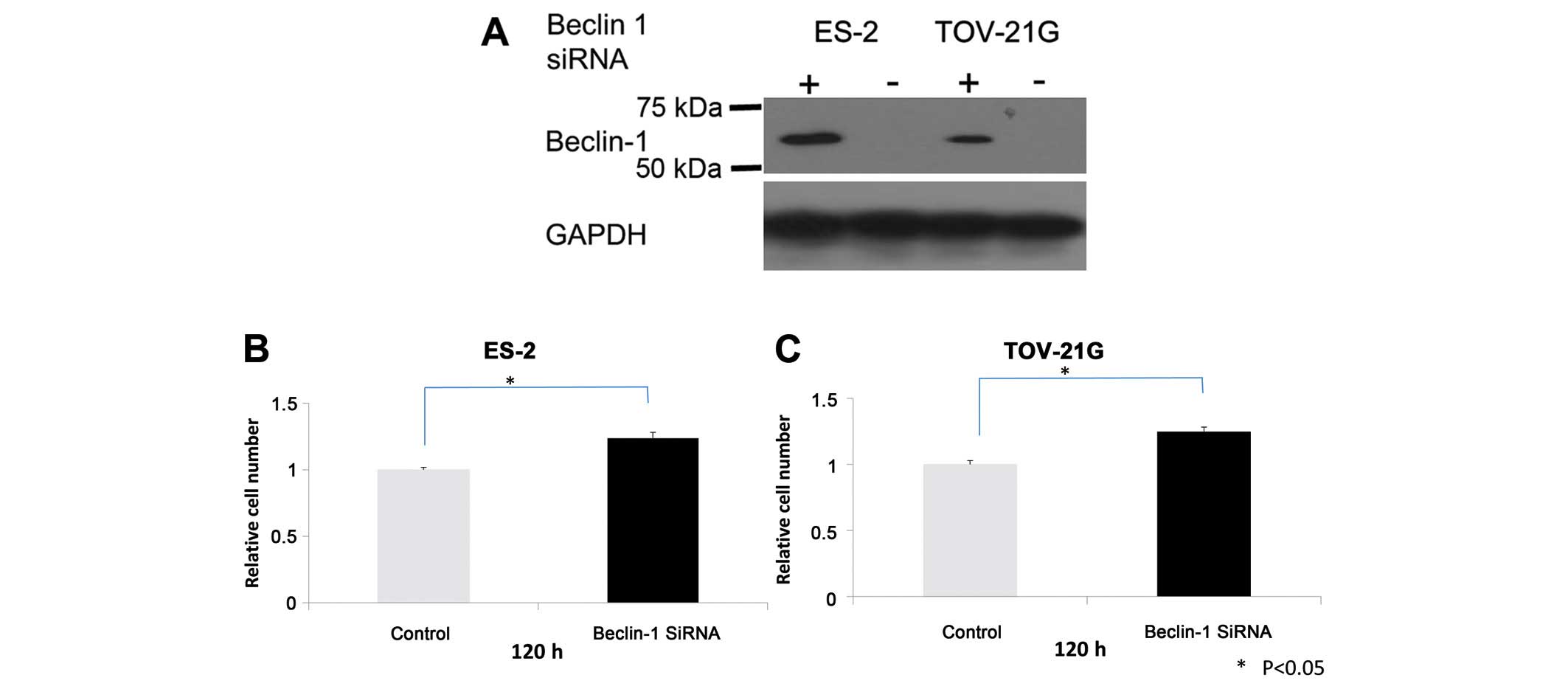
Beclin 1 knockdown increases growth, but
not cell migration and invasion in OCCC cells
The findings of the present study suggest that
Beclin 1 is a potential tumor suppressor in OCCCs. To assess the
contribution of Beclin 1 expression to OCCC cell growth and
survival, OCCC cell lines were treated with Beclin 1 siRNA and
Beclin 1 levels and cell growth were assessed. Following Beclin 1
knockdown (Fig. 4A), cell growth
increased in ES-2 and TOV-21G OCCC cell lines with positive Beclin
1 expression (Fig. 4B and C).
However, there was no profound inhibition or activation of cell
migration and invasion observed in Beclin 1 siRNA-treated OCCC
cells (data not shown).
Discussion
OCCCs, which comprise approximately 25% of all
ovarian carcinomas in Japan, display a distinct gene expression
profile relative to other histological types (18,19).
They are more aggressive and carry a worse prognosis than
stage-matched serous adenocarcinomas (20,21),
possibly because OCCC is frequently refractory to platinum-based
chemotherapy (20,21). The mechanism of CDDP resistance in
OCCC is still unclear. Therefore, in the present study, we first
investigated whether autophagy is related to CDDP sensitivity in
OCCC cell lines using autophagy inhibitors chloroquine or Beclin 1
siRNA. However, we did not find a relationship between autophagy
and CDDP sensitivity, although several recent reports state that
autophagy is related to chemoresistance in several types of cancer
(7,8). This discrepancy may be due to
differences in organ-specific oncogenic pathways. Consequently,
autophagy may not be an important factor in the OCCC carcinogenesis
pathway.
We next focused on the relationship between Beclin 1
expression and clinicopathological, prognostic significance in
OCCCs. Our most notable finding is that loss of Beclin 1 in OCCCs
predicted a shorter progression-free interval. To date, there are
only few molecular markers that predict the risk of early tumor
recurrence in OCCCs (22,23). Therefore, loss of Beclin 1
expression may be useful, alone or in combination with other
markers, in identifying OCCC patients who are more susceptible to
early recurrence. This is important because at least 60% of
advanced-stage OCCC patients with a complete response to primary
therapy ultimately develop recurrent disease (24). These observations could have an
impact on clinical management. Patients with recurrent OCCCs derive
the most benefit from secondary cytoreduction if recurrent tumors
are small and localized (24–28).
Therefore, patients who lack Beclin 1 expression could then be
followed more frequently in order to detect recurrences early
enough to benefit from either secondary cytoreductive surgery or
second line chemotherapy. This indicates that the
immunohistochemical analysis of Beclin 1 may be a useful predictor
to find OCCC patients who tend to recur in the clinical
setting.
In the present study, all patients were treated with
either platinum and taxane combination or platinum and CPT-11
combination as the primary adjuvant regimen. Patients whose disease
recurred after primary platinum-based chemotherapy were then
treated with various second line chemotherapy agents, including
liposomal doxorubicin, gemcitabine and docetaxel. Differing
responses to these agents may have masked the effect of loss of
Beclin 1 expression on overall survival in OCCC patients. In the
present study, patients with Beclin 1-negative tumors did not have
a significantly inferior response to chemotherapy when compared
with patients who had Beclin 1-positive tumors. This clinical
finding is consistent with current in vitro results showing
that autophagy is not related to CDDP resistance. Furthermore, our
in vitro Beclin 1 knockdown experiment also showed that loss
of Beclin 1 expression was not related to OCCC metastasis.
Therefore, the loss of Beclin 1 expression may be masking another
mechanism related to shorter progression-free survival in OCCC.
Our in vitro data showed that silencing
Beclin 1 using siRNA enhances cell growth in OCCC cell lines.
Previous reports showed that autophagy also plays a role in
tumorigenesis. For example, the essential autophagy regulator
Beclin 1 is monoallelically deleted in human ovarian, breast and
prostate cancers (29,30). In addition, Beclin 1+/−
or Atg4C−/− mice are prone to tumors (31–33).
Paradoxically, these findings suggest that the loss of a survival
pathway enhances tumor growth. Recent studies have shown that
simultaneous defects in autophagy and apoptosis activate the DNA
damage response in vitro, promote gene amplification and
aneuploidy, and accelerate mammary tumorigenesis (34,35).
Thus, loss of the prosurvival role of autophagy caused by a defect
in Beclin 1 expression is likely to contribute to tumor progression
by promoting genome damage and instability in OCCC development.
Molecular genetic evidence suggests that OCCCs
develop as the result of a multistep process of oncogenic
activation and tumor suppressor inactivation (36,37).
In this study, we focused on some of the important genetic events
of OCCCs: inactivation of the tumor suppressor gene ARID1A,
gene amplification of the potential oncogene ZNF217, and
oncogenic mutation of Kras and PIK3CA (11–13).
In addition, we also analyzed the association between these genetic
events and loss of Beclin 1 expression. No significant relationship
was observed between oncogenic mutation of
Kras/PIK3CA and loss of Beclin 1 expression, which
indicates that the loss of autophagy caused by loss of Beclin 1
expression is independent of these carcinogenesis events in OCCCs.
Also, loss of Beclin 1 expression was significantly correlated with
ZNF217 amplification and tended to be correlated with loss
of ARID1A expression. However, the significance of these
relationships during OCCC development remains unclear. Thus,
further studies are required to fully explore the relationship
between the loss of Beclin 1 expression and these genetic events
in vitro.
To the best of our knowledge, this is the first
report suggesting that loss of Beclin 1 expression is a marker of
poor progression-free survival in OCCCs. This study is limited by
its small size given the relative rarity of OCCC. Larger
prospective trials are needed to confirm our findings and to more
fully explore the role of Beclin 1 in OCCC behavior.
In conclusion, the present study shows that
autophagy defects caused by loss of Beclin 1 may be associated with
malignant phenotype and poor prognosis of OCCC.
Acknowledgements
The present study was supported by grants from the
Ministry of Education, Culture, Sports, Science and Technology in
Japan.
Abbreviations:
|
CDDP
|
cisplatin
|
|
OCCCs
|
ovarian clear cell carcinomas
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for Hispanics/Latinos, 2012. CA Cancer J Clin.
62:283–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar :
|
|
3
|
Mandai M, Matsumura N, Baba T, Yamaguchi
K, Hamanishi J and Konishi I: Ovarian clear cell carcinoma as a
stress-responsive cancer: Influence of the microenvironment on the
carcinogenesis and cancer phenotype. Cancer Lett. 310:129–133.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimizu S, Kanaseki T, Mizushima N, Mizuta
T, Arakawa-Kobayashi S, Thompson CB and Tsujimoto Y: Role of Bcl-2
family proteins in a non-apoptotic programmed cell death dependent
on autophagy genes. Nat Cell Biol. 6:1221–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rashmi R, Pillai SG, Vijayalingam S,
Ryerse J and Chinnadurai G: BH3-only protein BIK induces
caspase-independent cell death with autophagic features in Bcl-2
null cells. Oncogene. 27:1366–1375. 2008. View Article : Google Scholar
|
|
7
|
Furuya D, Tsuji N, Yagihashi A and
Watanabe N: Beclin 1 augmented cis-diamminedichloroplatinum induced
apoptosis via enhancing caspase-9 activity. Exp Cell Res.
307:26–40. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Y, Liu JH, Jin L, Pan L, Sui YX, Yang
Y and Shi H: Beclin 1 influences cisplatin-induced apoptosis in
cervical cancer CaSki cells by mitochondrial dependent pathway. Int
J Gynecol Cancer. 22:1118–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J: Beclin 1 bridges autophagy,
apoptosis and differentiation. Autophagy. 4:947–948. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eskelinen EL and Saftig P: Autophagy: A
lysosomal degradation pathway with a central role in health and
disease. Biochim Biophys Acta. 1793:664–673. 2009. View Article : Google Scholar
|
|
11
|
Katagiri A, Nakayama K, Rahman MT, Rahman
M, Katagiri H, Nakayama N, Ishikawa M, Ishibashi T, Iida K,
Kobayashi H, et al: Loss of ARID1A expression is related to shorter
progression-free survival and chemoresistance in ovarian clear cell
carcinoma. Mod Pathol. 25:282–288. 2012.
|
|
12
|
Rahman M, Nakayama K, Rahman MT, Nakayama
N, Ishikawa M, Katagiri A, Iida K, Nakayama S, Otsuki Y, Shih IeM,
et al: Clinicopathologic and biological analysis of PIK3CA mutation
in ovarian clear cell carcinoma. Hum Pathol. 43:2197–2206. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rahman MT, Nakayama K, Rahman M, Nakayama
N, Ishikawa M, Katagiri A, Iida K, Nakayama S, Otsuki Y, Shih IeM,
et al: Prognostic and therapeutic impact of the chromosome 20q13.2
ZNF217 locus amplification in ovarian clear cell carcinoma. Cancer.
118:2846–2857. 2012. View Article : Google Scholar
|
|
14
|
Nakayama K, Nakayama N, Jinawath N, Salani
R, Kurman RJ, Shih IeM and Wang TL: Amplicon profiles in ovarian
serous carcinomas. Int J Cancer. 120:2613–2617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakayama K, Miyazaki K, Kanzaki A,
Fukumoto M and Takebayashi Y: Expression and cisplatin sensitivity
of copper-transporting P-type adenosine triphosphatase (ATP7B) in
human solid carcinoma cell lines. Oncol Rep. 8:1285–1287.
2001.PubMed/NCBI
|
|
16
|
Wu H, Zhu H, Liu DX, Niu TK, Ren X, Patel
R, Hait WN and Yang JM: Silencing of elongation factor-2 kinase
potentiates the effect of 2-deoxy-D-glucose against human glioma
cells through blunting of autophagy. Cancer Res. 69:2453–2460.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng Y, Li H, Ren X, Niu T, Hait WN and
Yang J: Cytoprotective effect of the elongation factor-2
kinase-mediated autophagy in breast cancer cells subjected to
growth factor inhibition. PLoS One. 5:e97152010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schwartz DR, Kardia SL, Shedden KA, Kuick
R, Michailidis G, Taylor JM, Misek DE, Wu R, Zhai Y, Darrah DM, et
al: Gene expression in ovarian cancer reflects both morphology and
biological behavior, distinguishing clear cell from other
poor-prognosis ovarian carcinomas. Cancer Res. 62:4722–4729.
2002.PubMed/NCBI
|
|
19
|
Yamaguchi K, Mandai M, Oura T, Matsumura
N, Hamanishi J, Baba T, Matsui S, Murphy SK and Konishi I:
Identification of an ovarian clear cell carcinoma gene signature
that reflects inherent disease biology and the carcinogenic
processes. Oncogene. 29:1741–1752. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sugiyama T, Kamura T, Kigawa J, Terakawa
N, Kikuchi Y, Kita T, Suzuki M, Sato I and Taguchi K: Clinical
characteristics of clear cell carcinoma of the ovary: A distinct
histologic type with poor prognosis and resistance to
platinum-based chemotherapy. Cancer. 88:2584–2589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goff BA, Sainz de la Cuesta R, Muntz HG,
Fleischhacker D, Ek M, Rice LW, Nikrui N, Tamimi HK, Cain JM, Greer
BE, et al: Clear cell carcinoma of the ovary: A distinct histologic
type with poor prognosis and resistance to platinum-based
chemotherapy in stage III disease. Gynecol Oncol. 60:412–417. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Köbel M, Xu H, Bourne PA, Spaulding BO,
Shih IeM, Mao TL, Soslow RA, Ewanowich CA, Kalloger SE, Mehl E, et
al: IGF2BP3 (IMP3) expression is a marker of unfavorable prognosis
in ovarian carcinoma of clear cell subtype. Mod Pathol. 22:469–475.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho CM, Cheng WF, Lin MC, Chen TC, Huang
SH, Liu FS, Chien CC, Yu MH, Wang TY and Hsieh CY: Prognostic and
predictive values of E-cadherin for patients of ovarian clear cell
adenocarcinoma. Int J Gynecol Cancer. 20:1490–1497. 2010.PubMed/NCBI
|
|
24
|
Díaz-Montes TP and Bristow RE: Secondary
cytoreduction for patients with recurrent ovarian cancer. Curr
Oncol Rep. 7:451–458. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harter P and du Bois A: The role of
surgery in ovarian cancer with special emphasis on cytoreductive
surgery for recurrence. Curr Opin Oncol. 17:505–514. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gadducci A, Iacconi P, Cosio S, Fanucchi
A, Cristofani R and Genazzani AR: Complete salvage surgical
cytoreduction improves further survival of patients with late
recurrent ovarian cancer. Gynecol Oncol. 79:344–349. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gadducci A, Iacconi P, Fanucchi A, Cosio
S, Teti G and Genazzani AR: Surgical cytoreduction during
second-look laparotomy in patients with advanced ovarian cancer.
Anticancer Res. 20:1959–1964. 2000.PubMed/NCBI
|
|
28
|
Zang RY, Li ZT, Tang J, Cheng X, Cai SM,
Zhang ZY and Teng NN: Secondary cytoreductive surgery for patients
with relapsed epithelial ovarian carcinoma: Who benefits? Cancer.
100:1152–1161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aita VM, Liang XH, Murty VV, Pincus DL, Yu
W, Cayanis E, Kalachikov S, Gilliam TC and Levine B: Cloning and
genomic organization of beclin 1, a candidate tumor suppressor gene
on chromosome 17q21. Genomics. 59:59–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh
H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al:
Promotion of tumorigenesis by heterozygous disruption of the beclin
1 autophagy gene. J Clin Invest. 112:1809–1820. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yue Z, Jin S, Yang C, Levine AJ and Heintz
N: Beclin 1, an autophagy gene essential for early embryonic
development, is a haploinsufficient tumor suppressor. Proc Natl
Acad Sci USA. 100:15077–15082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mariño G, Salvador-Montoliu N, Fueyo A,
Knecht E, Mizushima N and López-Otín C: Tissue-specific autophagy
alterations and increased tumorigenesis in mice deficient in
Atg4C/autophagin-3. J Biol Chem. 282:18573–18583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Karantza-Wadsworth V, Patel S, Kravchuk O,
Chen G, Mathew R, Jin S and White E: Autophagy mitigates metabolic
stress and genome damage in mammary tumorigenesis. Genes Dev.
21:1621–1635. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mathew R, Kongara S, Beaudoin B, Karp CM,
Bray K, Degenhardt K, Chen G, Jin S and White E: Autophagy
suppresses tumor progression by limiting chromosomal instability.
Genes Dev. 21:1367–1381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuo KT, Mao TL, Jones S, Veras E, Ayhan A,
Wang TL, Glas R, Slamon D, Velculescu VE, Kuman RJ, et al: Frequent
activating mutations of PIK3CA in ovarian clear cell carcinoma. Am
J Pathol. 174:1597–1601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jones S, Wang TL, Shih IeM, Mao TL,
Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, et
al: Frequent mutations of chromatin remodeling gene ARID1A in
ovarian clear cell carcinoma. Science. 330:228–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|