Introduction
Hepatocellular carcinoma (HCC) is the sixth most
common cancer and the third most frequent cause of cancer death
worldwide (1). Although partial
liver resection and liver transplantation have significantly
improved survival in patients with small tumors, the prognosis for
HCC remains poor because of tumor invasiveness, frequent
intrahepatic spread and extrahepatic metastases (2). Thus, effective non-surgical
strategies and identification of the molecular mechanisms are
crucially responsible for control of the growth, invasive and
metastatic potential of HCC.
Therefore, a clearer understanding of the molecular
mechanisms underlying tumor invasiveness and migration is essential
for the development of new therapies for HCC.
Epithelial-mesenchymal transition (EMT) is defined as a process
during which epithelial cells lose their cell polarity, phenotypic
characteristics and acquire mesenchymal cell features (3). It has been suggested that EMT might
be closely associated with the acquisition of aggressive traits by
tumor cells, thus facilitating the early stages of metastasis and
the subsequent dissemination of carcinoma cells (4–6).
Increasing evidence indicates that aberrant regulation of EMT is a
determinant of the cancer cell invasive and metastatic behavior.
Therefore, targeting EMT may serve as an efficient strategy for the
treatment of malignant and metastatic tumors.
All-trans retinoic acid (ATRA), as a
predominant natural metabolite of vitamin A, is involved in many
important biological processes, including vision, morphogenesis,
differentiation, growth, metabolism, and cellular homeostasis
(7). ATRA is currently used to
induce remission in patients with acute promyelocytic leukemia and
has the potential for use in the treatment of solid tumors
including HCC (8–11). Many studies have reported that ATRA
has anti-HCC ability. Additionally, ATRA combined with other
chemotherapeutic agents could induce cancer cell differentiation,
increase the sensitivity of hepatocarcinomas to chemotherapy,
reduce cell migration in vitro and metastasis in vivo
(12,13). However, the mechanisms underlying
the effect of ATRA on HCC are largely unknown. It has been revealed
that ATRA can induce mesenchymal to epithelial transition of HCT116
cells (10), while ATRA modulates
epithelial-to-mesenchymal-transition of mammary tumor cells via the
TGFβ and NOTCH pathways (11).
Thus, we raised a hypothesis that the anti-HCC effect of ATRA might
be closely related to the reverse process of EMT.
In this study, we demonstrated that ATRA could
inhibit the proliferation, migration, invasion of mouse hepa1-6
hepatocarcinoma cell lines, as well as induce their differentiation
and hepatic function. Then, we found that the antitumor process was
correlated with the decreased expression of mesenchymal marker
genes and the increased expression of epithelial marker genes by
regulation of ATRA. Our study may support the understanding of
anti-HCC effect of ATRA and promote its clinical application to
this disease.
Materials and methods
Cell and chemicals
The mouse hepa1-6 hepatocarcinoma cell lines was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and maintained in complete Dulbecco's modified Eagle's medium
(DMEM, Gibco Life Technologies, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS, Gibco Life Technologies), 100
U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5%
CO2. Unless indicated otherwise, all chemicals were
purchased from Sigma-Aldrich (St. Louis, MO, USA), all antibodies
were purchased from Santa Cruz (USA). PCR primers were synthesized
by Huada Gene Inc (Shenzhen, China).
Treatment of ATRA
The Hepa1-6 cells were treated with complete DMEM
medium containing different concentrations of ATRA (0.1, 1.0 and
10.0 μmol/l). The culture was replaced every three days. The group
without ATRA treatment was set up as control group. Three
independent experiments were performed in duplicate.
Trypan blue staining
Cells were incubated in 24-well plates at
5.0×104 cells per well with different concentrations of
ATRA treatment. Cell viability was measured by trypan blue staining
after treatment at days 0, 2, 4 and 6. Both adherent and suspended
cells were collected and mixed with 0.4% 2X trypan blue buffer.
Cell mixture (10 μl) (~106 cells/ml) was counted using
hemocytometer under a microscope (Nikon Eclipse Ti, Japan). The
dead cells were stained blue. The mean and standard deviation were
calculated.
Colony formation assay
The colony formation rate indicates the independent
proliferation and viability of cells. Briefly, logarithmic phase
cells were planted in 12-well plates at 500 cells per well. Culture
medium was replaced when the generative cells attached to the wall.
After incubation for 20 days, the visible colonies were fixed with
4% paraformaldehyde for 30 min and then stained with 0.1% crystal
violet for 10 min. Cells were washed twice with PBS after each
step. The cell colonies were observed and counted under the
inverted microscope. Fifty cells are equivalent to one colony and
the cell colony formation rate was calculated as follows,
Rcf = Ncf/Nvc × 100%, where Rcf is
the rate of colony formation, Ncf is the number of
colonies formed, Nvc is the number of vaccinated
cells.
Wound-healing assay
To determine the cell migratory ability, the
classical scratch would-healing assay was performed as described
(14). Briefly, 5.0×104
cells per well were incubated in 6-well plates. A linear wound was
made by scraping a pipette tip across the cell layer. After washing
three times with PBS to remove floating cells and debris, cells
were incubated in culture medium with different concentrations of
ATRA. Then the scratched fields were photographed at 0, 24 and 48 h
to calculate the wound healing.
Transwell migration and invasion
assay
For Transwell invasion assay, logarithmic phase
cells were trypsinized and re-suspended in DMEM/ATRA without FBS.
Cell suspension (200 μl) was added to the Transwell upper chamber
coated with Matrigel membrane in triplicate. DMEM culture solution
containing 10% FBS was added to the lower chamber of each well and
incubated for 48 h at 37°C. The non-invasive cells on the upper
surface of the membrane were removed and the invasive cells were
stained in 100 μl crystal violet (Bioteke, Beijing, China). The
invasive cells in five random high power fields were counted. The
Transwell migration assay was the same as the invasion assay, with
the exception of the Matrigel coating. The cells were stained and
counted, as aforementioned.
RNA extraction and quantitative real-time
polymerase chain reaction (real-time qPCR)
Cells were incubated with different ATRA treatments
for 2, 4 and 7 days. As previously described (15), total RNA was extracted by using an
RNA Extraction kit (Bioteke) according to the manufacturer's
instructions and then reverse transcribed into cDNA by using
Superscript II reverse transcriptase (Thermo Fisher Scientific,
USA). The PCR templates were prepared with 5- to 10-fold dilution
of the first strand cDNA products. The cDNA samples at days 2 and 4
were used to detect the EMT genes (E-cadherin, N-cadherin,
vimentin, snail, twist and fibronectin). N-cadherin, vimentin,
snail, twist and fibronectin are mesenchymal markers, E-cadherin is
an epithelial marker (16,17). The cDNA samples at day 7 were
detected for the differentiation genes as follows ALB (albumin),
CK18 (cytokeratin 18), TAT (tyrosine aminotransferase), ApoB
(apolipoprotein B), and AFP (α fetoprotein). The genes of interest
were amplified with qPCR primers which were designed using the
Primer 3.0 program (Table I). A
real-time PCR protocol for real-time PCR amplification was
performed as follows: 72°C × 3 min, 94°C × 3 min, 92°C × 20 sec,
72°C × 20 sec, 9 cycles, with 1°C degree decrease per cycle,
followed by 94°C × 20 sec, 55°C × 20 sec, 72°C × 20 sec for 25–30
cycles and 72°C × 3 min. All samples were normalized by endogenous
levels of β-actin.
 | Table ISequences of primers for real-time
qPCR. |
Table I
Sequences of primers for real-time
qPCR.
| Name of genes | Sequences of
primers (5′-3′) |
|---|
|
|---|
| Forward | Reverse |
|---|
| β-actin |
AGGGAAATCGTGCGTGAC |
CGCTCGTTGCCAATAGTGA |
| ALB |
CCAGACATTCCCCAATGC |
CAAGTTCCGCCCTGTCAT |
| AFP |
ACGAGGAAAGCCCCTCAG |
GCCATTCCCTCACCACAG |
| CK18 |
CTGGGCTCTGTGCGAACT |
ACAGAGCCACCCCAGACA |
| TAT |
ACCTTCAATCCCATCCGA |
TCCCGACTGGATAGGTAG |
| ApoB |
CATGTGATCCCCACAGCA |
TCCCAGGACCATGGAAAA |
| E-cadherin |
CAAGGACAGCCTTCTTTTCG |
TGGACTTCAGCGTCACTTTG |
| N-cadherin |
CTGGGACGTATGTGATGACG |
TGATGATGTCCCCAGTCTCA |
| vimentin |
CAGATGCGTGAGATGGAAGA |
TCCAGCAGCTTCCTGTAGGT |
| snail |
AAACCCACTCGGATGTGAAG |
GAAGGAGTCCTGGCAGTGAG |
| twist |
CAGCGGGTCATGGCTAACG |
CTTGTCCGAGGGCAGCGT |
| fibronectin |
GCAGAACCAGAGGAGGCACA |
CAATGGCGTAATGGGAAACC |
Western blot analysis
After treatment of ATRA for 7 days, total proteins
of each group were extracted. Thereafter 20 μg of total protein per
group were separated on a 10% SDS-PAGE (Beyotime Institute of
Biotechnology, Shanghai, China) and electrophoretically transferred
to PVDF membrane (Milipore, Billerica, MA, USA). After blocked with
5% fat-free skim milk at room temperature for 2 h, membranes were
incubated at 4°C overnight with primary antibodies against AFP
(1:200; goat. no. sc-8108; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), ALB (1:200; goat. no. sc-46293; Santa Cruz Biotechnology,
Inc.), CK18 (1:200; mouse. no. sc-51582; Santa Cruz Biotechnology,
Inc.) and β-actin (1:200; mouse. no. sc-47718; Santa Cruz
Biotechnology, Inc.), followed by probing with the appropriate
second antibody at room temperature for 1 h. The presence of the
proteins of interest was detected by using the G-BOX iChemi XR gel
documentation system (Syngene, Cambridge, UK).
Immunofluorescence staining
Cells receiving the indicated treatments were
cultured in 24-well plates for 7 days; immunofluorescence staining
was carried out as previously reported (15). Cells were fixed with 4%
paraformaldehyde for 15 min and then perforated with 5% triton for
20 min. The fixed cells were blocked with 5% goat serum for 1 h,
followed by incubation with primary antibodies against AFP and CK18
at 4°C overnight, then incubated with DyLight® 594- or
488-conjugated secondary antibody (Jackson ImmunoResearch
Laboratories, Inc., West Grove, PA, USA) for 1 h. Nuclei were
stained with DAPI. Cells were washed twice with PBS after each
step. The presence of proteins was examined under a fluorescence
microscope (TE2000-S, Nikon).
Indocyanine green (ICG) uptake and
release
Cells in 24-well plates were washed twice with PBS
and incubated with DMEM supplemented with freshly prepared ICG at a
final concentration of 1 mg/ml for 1 h at 37°C 5% CO2.
Then DMEM was removed and cells were gently washed three times with
PBS, the green-stained cells were photographed under a microscope.
Complete medium was then added and the cells were incubated for
>6 h; the cells were then observed under a microscope to ensure
ICG release. At least 10 non-overlapping fields of vision were
recorded.
Periodic acid-Schiff (PAS) staining
Cells cultured as described above were fixed with 4%
paraformaldehyde at day 7. Subsequently, cells were stained by 0.5%
periodic acid for 5 min, and Schiff's solution for 15 min, in turn.
All steps were carried out at room temperature and cells were
washed with ddH2O gently after each step. The positive
cells stained purple. More than 10 non-overlapping fields of vision
in each group were recorded under a microscope.
Statistical analysis
The data are presented as mean ± standard deviation
(SD) and analyzed using the SPSS 15.0 software package. A
two-tailed Student's t-test assuming equal variances was performed
to measure significant differences between two groups and variance
analysis was performed to measure significant differences among at
least three groups. A p<0.05 was considered to be statistically
significant.
Results
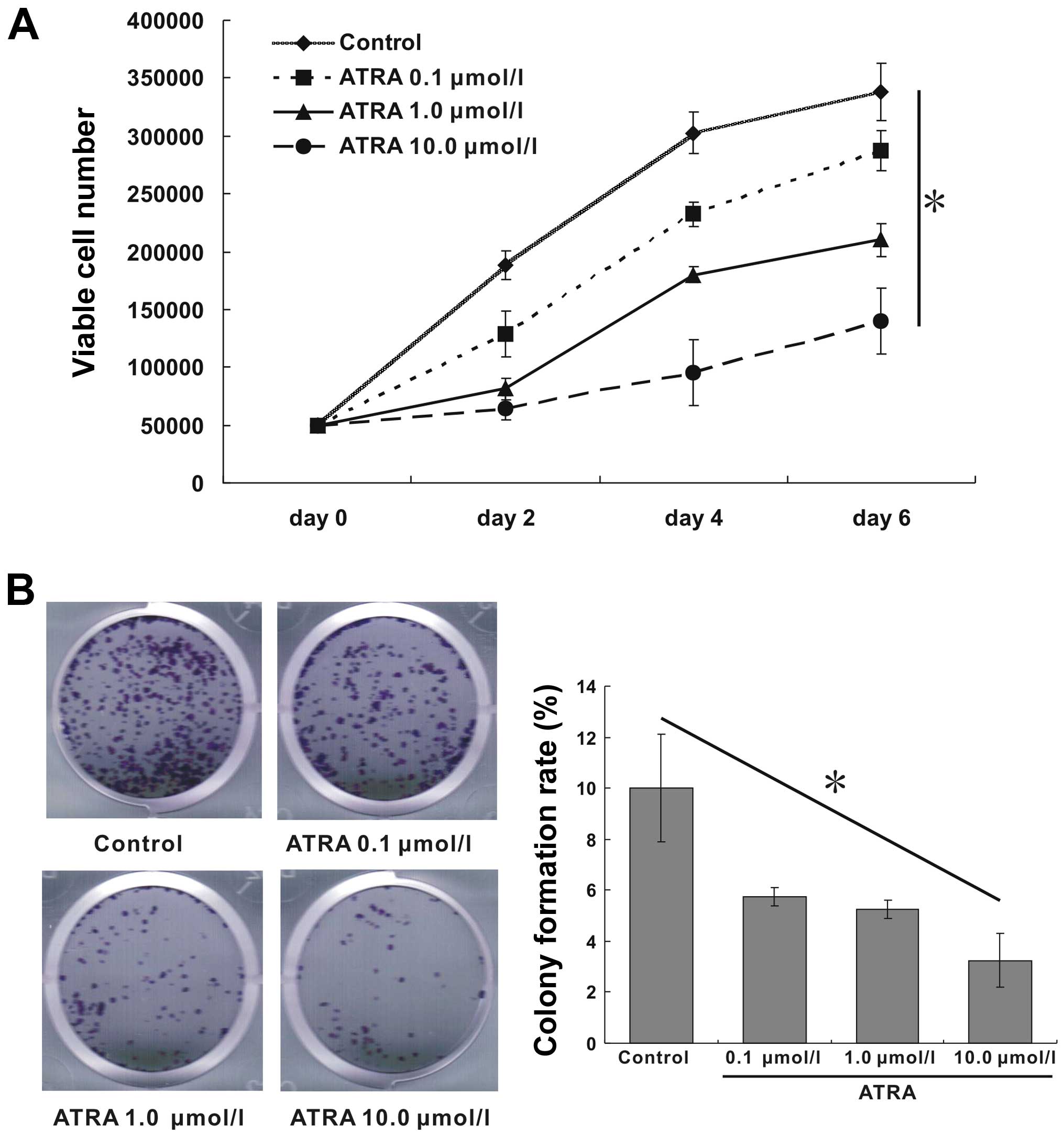
ATRA inhibits proliferation and colony
formation of hepa1-6 cells
As shown in Fig.
1A, ATRA effectively inhibited cell proliferation compared with
blank control. The inhibition efficiency of ATRA was enhanced
gradually with the increase of concentration, which was strongest
at day 6 with 10.0 μmol/l ATRA treatment. The colony formation rate
indicates the independent viability of cells. Treatment with ATRA
significantly exhibited inhibitory effects on cell colony
formation. Moreover, the rate of cell colony formation also
decreased gradually with the increase of ATRA concentration. The
colony formation rates of ATRA at concentration of 0, 0.1, 1.0,
10.0 μmol/l were 10, 5.75, 5.25 and 3.25%, respectively (Fig. 1B, p<0.05). Therefore, this
result suggested that ATRA inhibited cell proliferation in a
dose-dependent manner.
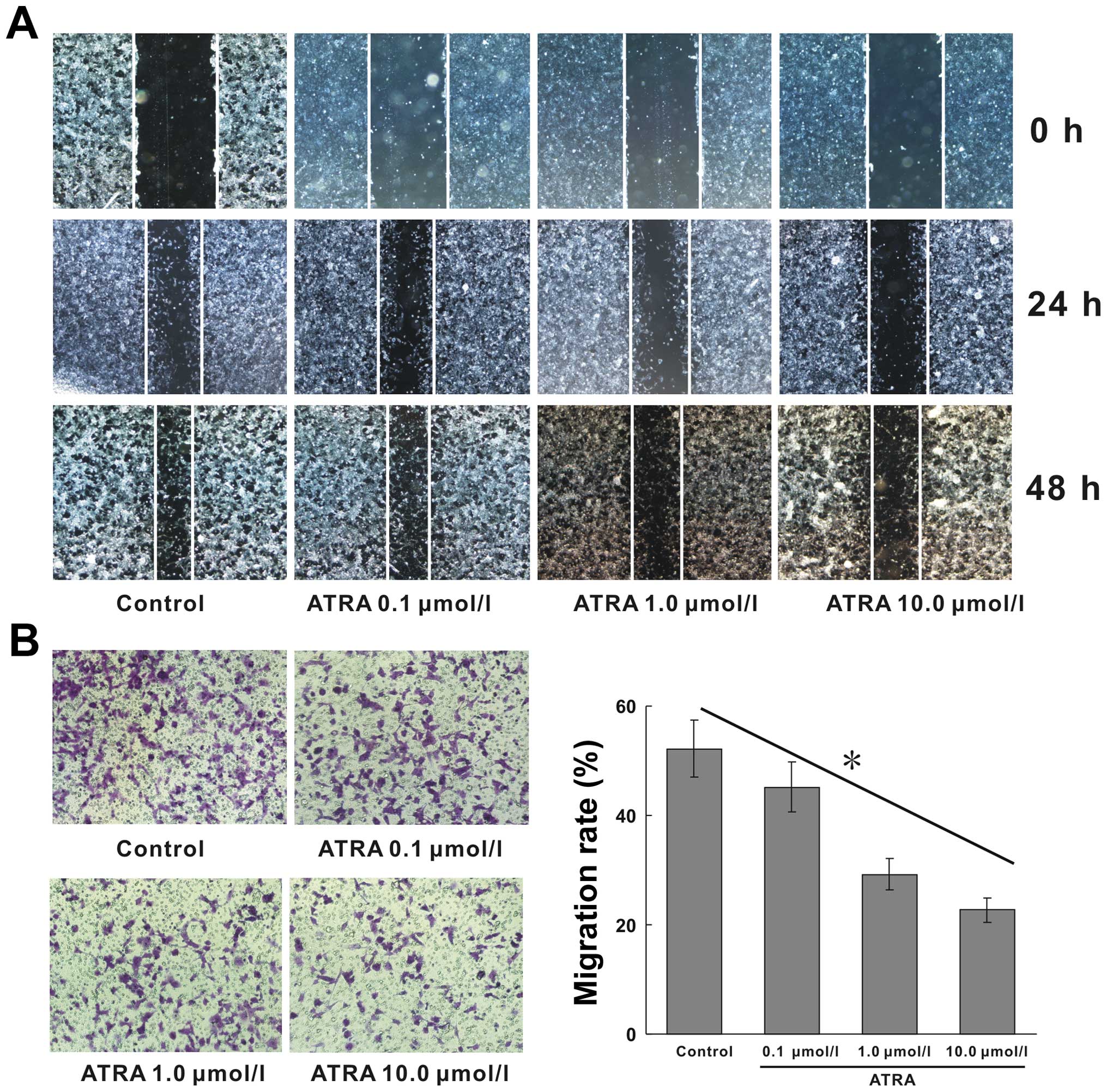
ATRA suppresses the migration and
invasion of hepa1-6 cells
HCC has highly metastatic capacity because of its
strong migratory ability. Wound-healing assay was used to detect
the horizontal migration capability of cells. We found that ATRA
inhibited the horizontal migration ability of hepa1-6 cells
(Fig. 2A). The similar results of
Transwell migration assay showed, that cell migration followed a
downward trend as the concentration of ATRA increased. The
migration rates in 0, 0.1, 1.0, 10.0 μmol/l ATRA treated groups
were 52.14, 45.17, 29.24 and 22.68%, respectively, the migration
rate of 10.0 μmol/l group declined by >56.50% compared with
blank control (Fig. 2B,
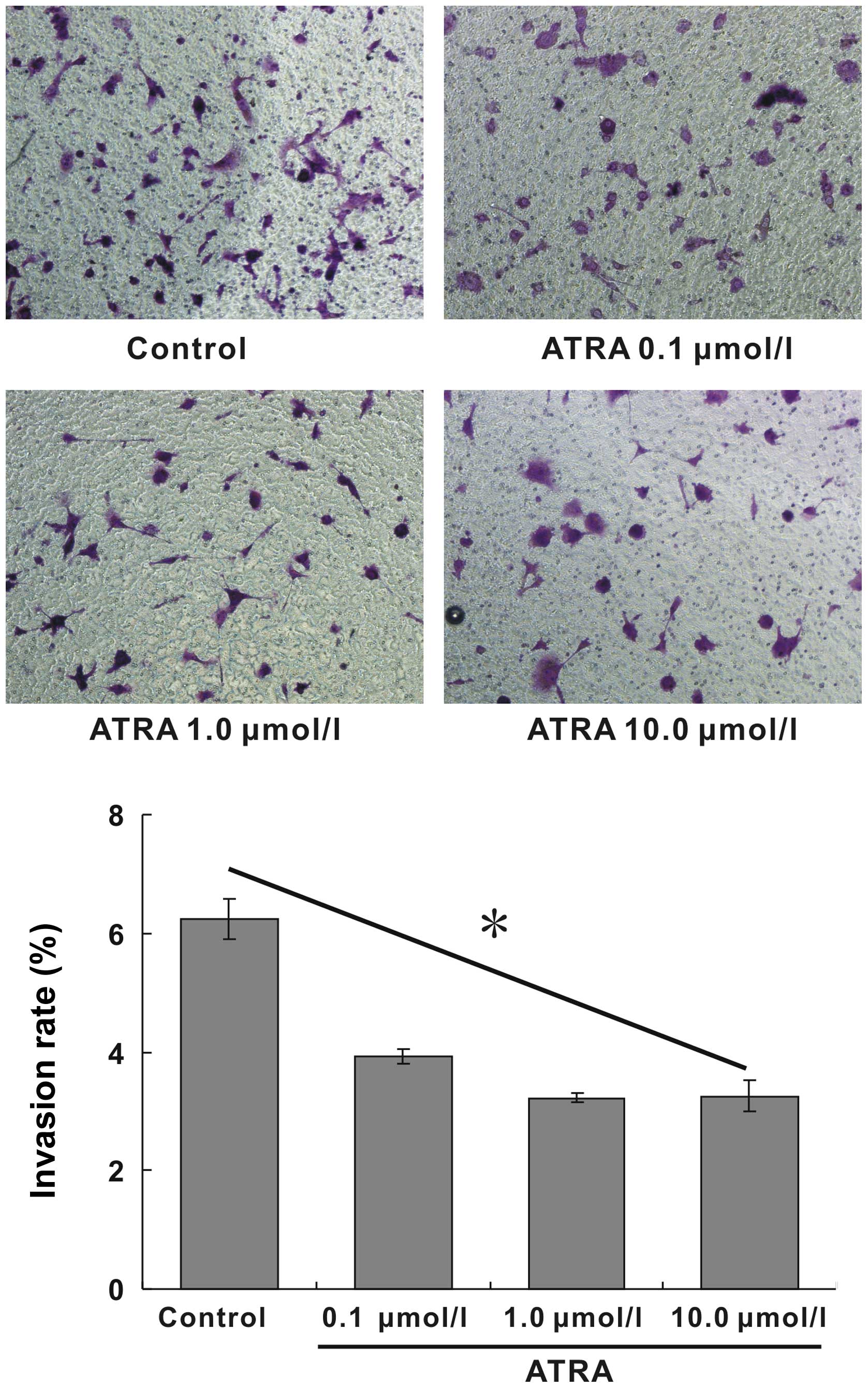
p<0.05). The invasive ability of the cells induced by ATRA was
detected by the Matrigel invasion assay. The invasive ability of
hepa1-6 cells after ATRA treatment was remarkably attenuated and
also exhibited a dose-dependent manner. The positive invasive rates
at corresponding ATRA concentration were 6.25, 3.92, 3.36 and
3.12%, respectively, the invasive rate of 10.0 μmol/l group reduced
by 50% compared with blank control (Fig. 3, p<0.05). These results
indicated that ATRA possessed excellent inhibitory effects on the
migration and invasion of hepa1-6 cells.
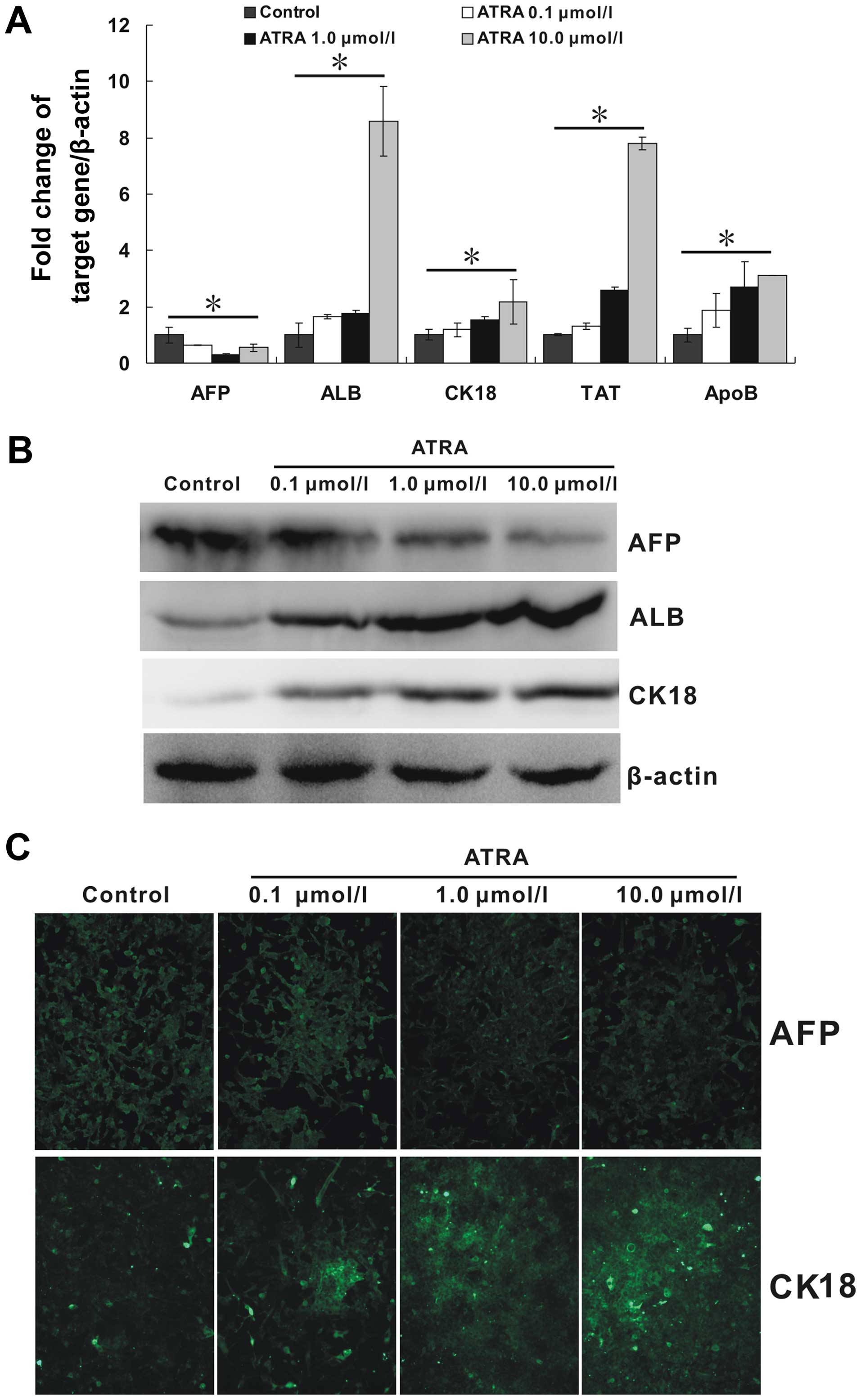
ATRA induces the differentiation of
hepa1-6 cells
Due to the inhibitory ability of ATRA on mesenchymal
phenotype in hepa1-6 cells, we tried to further detect the
regulation of ATRA on its epithelial phenotype. ALB, CK18, TAT, and
ApoB are important molecules associated with mature hepatocytes. As
shown in Fig. 4A, all the mRNA
expression of these markers were upregulated by ATRA. Moreover, the
regulation effect appeared to be strengthened with the increasing
concentration of ATRA (p<0.05). Additionally, AFP, a marker of
hepatocarcinoma, was downregulated (p<0.05). The patterns of the
protein expression of ALB, CK18 and AFP as detected by the western
blotting results (Fig. 4B),
largely corresponded to those observed by quantitative real-time
PCR. In addition, immunofluorescence staining carried out to detect
the protein expression of AFP and CK18 also showed a similar trend
(Fig. 4C). All these results
indicated that ATRA could effectively induce hepa1-6 cells to
mature hepatocytes at least in the mRNA and protein levels.
ATRA induces hepa1-6 cells to display
functions
To further substantiate the hypothesis that ATRA may
induce hepa1-6 cells to be functional cells; we performed ICG
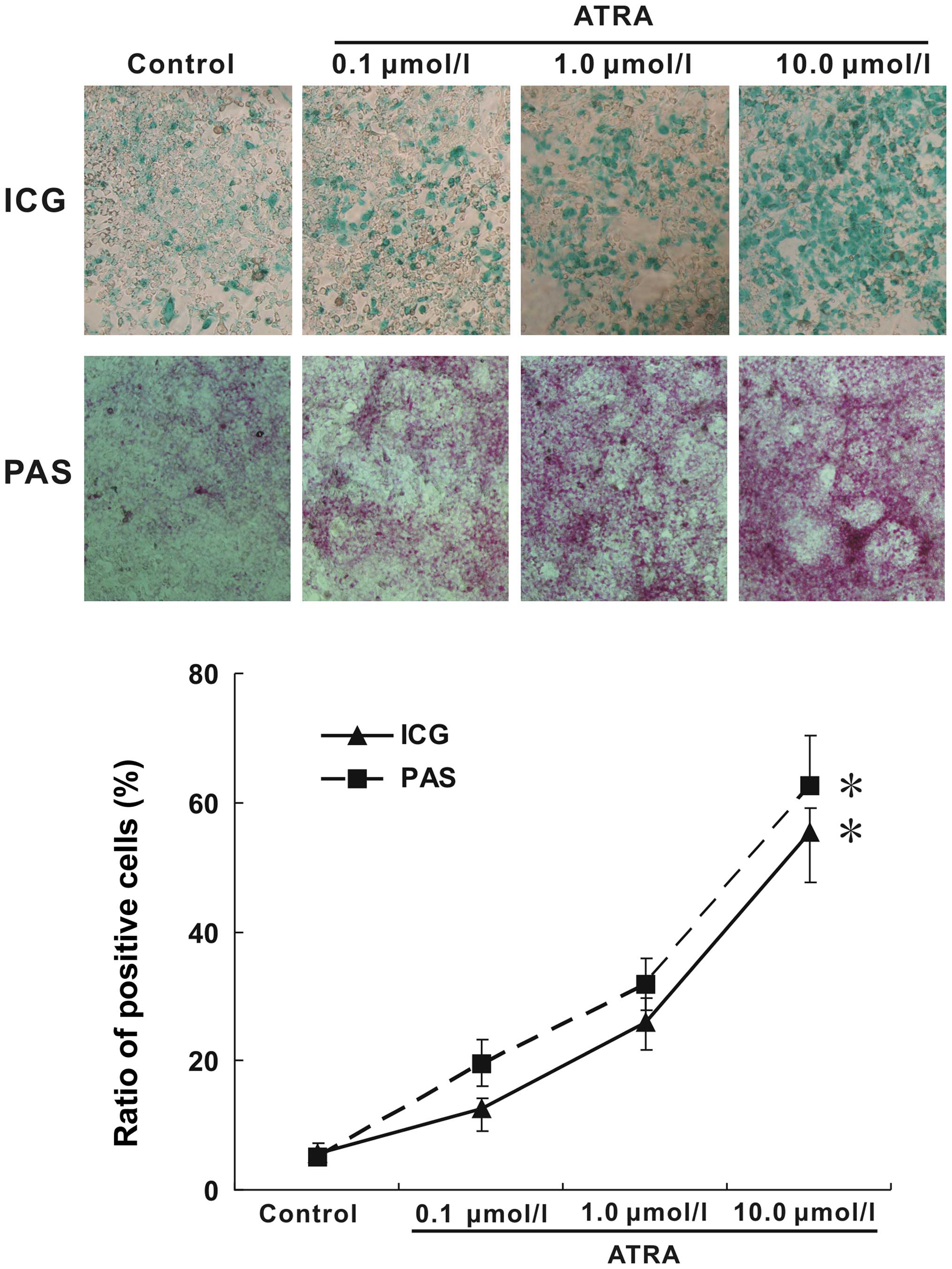
uptake and PAS staining. The evaluation of ICG uptake is a common
way to estimate liver function (18). As shown in Fig. 5, almost no the green-positive cells
were observed in blank control, while many areas of cells in the
treatment groups were stained green. On the other hand, mature
hepatocytes have the ability of glycogen synthesis and storage. PAS
staining method is used to detect glycogen which is displayed by a
purple color in the cytoplasm (19). Following ATRA treatment indicated
above, the purple color in cytoplasm was significantly increased.
Furthermore, with the increasing concentration of ATRA, the
positive rate in both ICG uptake and PAS staining were increasing.
Taken together, these results suggested that ATRA induction could
not only improve the expression of hepatic markers and inhibit
hepatocarcinoma markers, but also effectively induced hepa1-6 cells
to display functions similar to those of mature hepatocytes.
ATRA regulates the expression of EMT
markers
Finally, we investigated whether the regulation
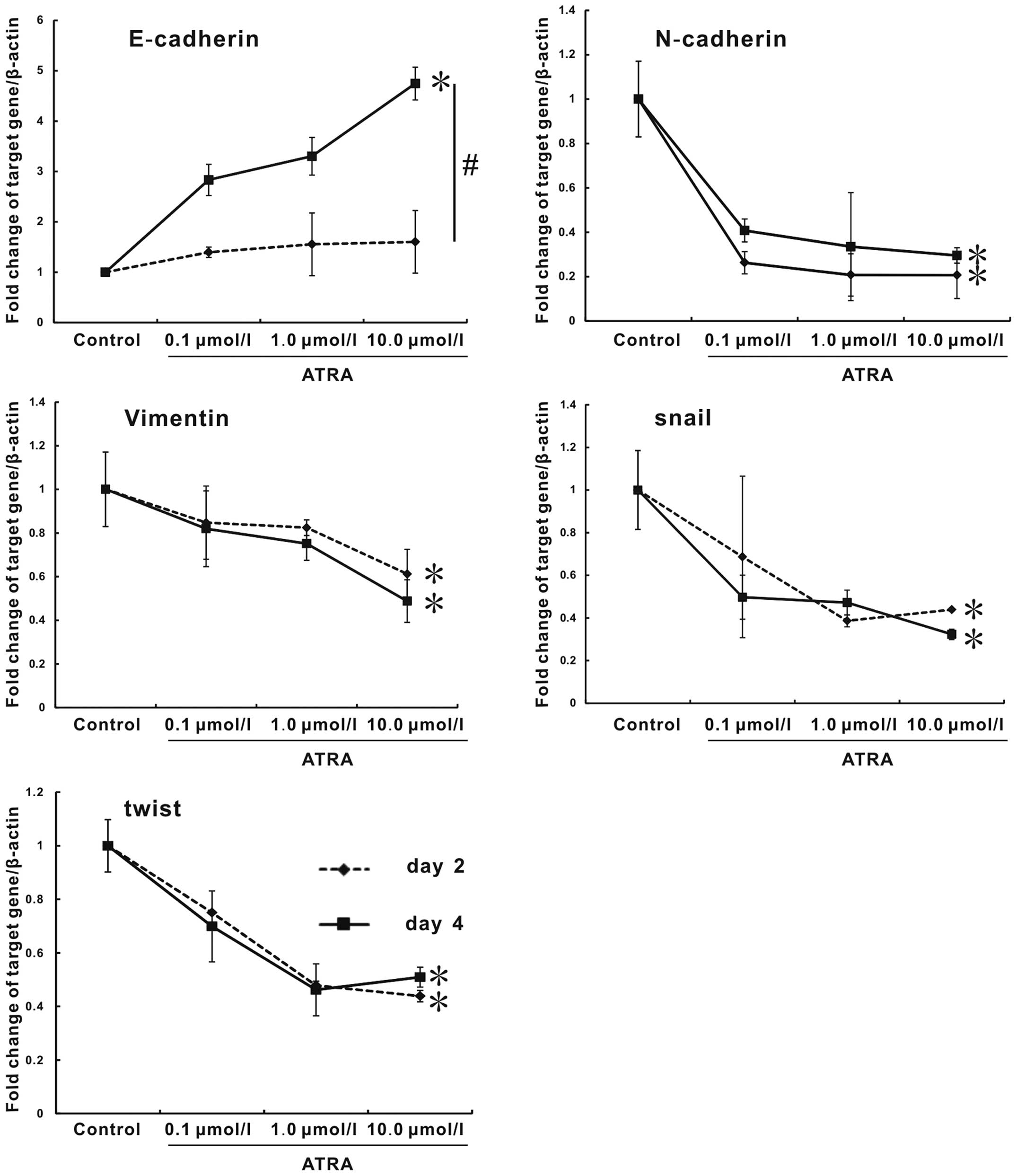
effect of ATRA on hepa1-6 cells had association with EMT. For this
purpose, mRNA expression of critical genes for EMT process
including E-cadherin, N-cadherin, vimentin, snail, twist, and
fibronectin were assessed by real-time PCR at days 2 and 4.
E-cadherin plays a central role in maintaining epithelial cell-cell
adhesion and polarity (20).
Compared to control, E-cadherin expression had a remarkable
increase in the ATRA groups, and its expression between days 2 and
4 had statistical significance. In contrast, ATRA treatment
resulted in a strong repression on the expression of mesenchamal
markers, including N-cadherin, vimentin, snail and twist. However,
there was no difference between days 2 and 4. Moreover, the
expression presented a dose-dependent trend. The expression of
fibronectin could not be regulated by ATRA (data not shown).
Especially, the expression of E-cadherin of 10.0 μmol/l group at
day 4 was remarkably enhanced, with a >3.7-fold increase, the
expressions of N-cadherin and twist at day 2, vimentin and snail at
day 4 in 10.0 μmol/l group were decreased by 75, 56.1, 51.2, and
67.7%, respectively, compared with blank control (Fig. 6, p<0.05). Therefore, we
demonstrated that ATRA was able to reverse EMT process of hepa1-6
cells by regulating EMT marker genes.
Discussion
Recurrence and metastasis are characteristic
features of HCC and the main factors related to poor prognosis in
patients with HCC. In recent years, an increasing number of
researchers have reported the role for EMT in tumor invasion and
metastatic spread. On the other hand, the transition from primary
CSCs (cancer stem cells) to EMT-positive CSCs is a significant step
in HCC progression. Studies suggest that CSCs having EMT phenotypes
are associated with sensitivity and resistance to chemotherapy in
various tumor models. Therefore, chemotherapeutics which can
inhibit or reverse EMT process would be effective for the treatment
of malignant and metastatic tumors including HCC. ATRA, the most
important active form of vitamin A, is especially required with
respect to embryonic development, growth, night vision, and the
maintenance of the immune system. ATRA also functions as a
hormone-like growth factor for epithelial and other cells, can
induce differentiation in many cell types and is the most widely
used differentiating therapeutic agent (21,22).
ATRA is the first example of a clinically useful
cyto-differentiating agent to be used in the treatment of acute
promyelocytic leukemia (23). Now
it is proposed as an antitumor agent not only in the context of
hematological malignancies but also the chemo-prevention and
treatment of many cancers including HCC (8–11).
In addition, it has been reported that ATRA could be used as a
chemopreventive agent to inhibit the progression of premalignant
lesions of the breast (24,25).
In the present study, we investigated the effect of ATRA on the
proliferation, migration, invasion and differentiation of
hepatocarcinoma cells and explored whether ATRA regulate EMT in the
antitumor process.
In this study, we investigated whether ATRA at
different concentrations influence the cell phenotype and
biological characteristics of hepatocarcinoma hepa1-6 cells. We
found that ATRA significantly inhibited the proliferation,
migration and invasion capability of cells in a dose-dependent
manner. It is worth mentioning, the trypan blue exclusion assay is
a very common assay for evaluating the cell viability (26,27),
the blue stained cell indicates incomplete cell membrane, whereas
live and apoptosis cells possess intact cell membranes that remain
unstained. In this study, proportions of unstained and stained
cells among control group and all the ATRA treatment groups were
not significantly different (data not shown). Thus, we suggested
ATRA at the concentration range of 0.1–10.0 μmol/l had almost no
cytotoxicity to hepa1-6 cells. In addition, the cell density of
each group in the wound-healing assay exhibited no difference after
2 days of ATRA treatment, indicating the reduced cell migration was
mainly attributed to the decreased migratory capability of hepa1-6
cells, rather than the decreased cell number caused by apoptosis.
Furthermore, it was reported that ATRA at low concentration (0.1
μmol/l) could generate these influences. In addition, with the
increasing of concentration, the inhibitory effects were enhanced.
It has been reported that ATRA at 10 μg/ml (=33.3 μmol/l) inhibited
lung adenocarcinoma A549 cell activity (28), 50.0 μmol/l ATRA treatment could
shorten the G2/M phase of A549 cell cycle and keep it at G0/G1
phase (29). ATRA (1 μmol/l) was
able to re-differentiate trMCF (transformed breast epithelial
cells) at their early stages by regulating breast cancer associated
genes. While, the invasive and tumorigenic breast cells did not
show any changes in morphology after ATRA treatment (24). Thus, hepatocarcinoma hepa1-6 cells
may be more sensitive to the low concentration of ATRA, supporting
a beneficial clinical application of ATRA on the therapy of HCC.
Next, we further evaluated the effects of ATRA on cell
differentiation. Studies have reported ATRA induced maturation of
certain kinds of cancer cells. For example, ATRA facilitates the
differentiation of acute promyelocytic leukemia cells toward mature
granulocytes (30). Oral
administration of ATRA induces differentiation of promyelocytic
leukemic cells to mature neutrophils (31). Our results also proved that ATRA
could effectively induce the expression of late hepatic markers of
hepa1-6 cells and improved their hepatic function of metabolism and
synthesis. Moreover, this regulation appeared to be strengthened
with the increasing concentration of ATRA. Migration and invasion
capability are indicators of mesenchymal phenotype, while matured
differentiation of hepa1-6 cells represents the enhancement of
their epithelial phenotype. Therefore, our results indicated that
ATRA might reverse EMT of hepa1-6 cells.
The downregulation of epithelial markers and the
upregulation of mesenchymal markers are both characteristic of EMT.
E-cadherin is a key protein in cell polarity and epithelial
organization. The reduction or loss of E-cadherin has become one of
the hallmarks of EMT, and was frequently associated with
dedifferentiation, metastasis and invasion in a variety of human
malignancies, including HCC. N-cadherin, vimentin, snail, twist,
and fibronectin are known as mesenchymal markers, which are closely
linked to several human malignancies (32–34).
Moreover, combined detection of these EMT markers has more
significance for the mechanism of cancers. Ye et al
(34) reported that the low
expression of E-cadherin and high expression of N-cadherin were
significantly related with local infiltration depth, tumor staging,
vascular invasion, and tumor differentiation level. The combined
detection of E-cadherin and vimentin has a prognostic value for the
patients with oral squamous cell carcinoma (35). Some anticancer drugs targeting
these EMT markers are currently used in clinic (36–38),
such as silibinin, which inhibits HCC cell proliferation,
migration, and invasion via the inhibition of vimentin expression
(36). Here we investigate whether
ATRA regulates the EMT process and related markers of hepa1-6
cells. We found that ATRA treatment significantly modulated these
markers including enhanced expression of E-cadherin and suppressed
the expression of N-cadherin, vimentin, snail and twist in HCC
cells. In addition, CK18 is one of the epithelial markers
associated with EMT and closely related to various cancers
(39,40). The expression of CK18 was
upregulated by ATRA and was dose-dependent, as was E-cadherin.
However, the expression of fibronectin could not be regulated by
ATRA (data not shown). Interestingly, the expression of E-cadherin
showed significant difference between days 2 and 4, but no
difference of all mesenchymal marker genes was obtained between
days 2 and 4, which suggested that the regulation of ATRA on
mesenchymal phenotype was triggered probably earlier than the
epithelial phenotype. These results indicated that ATRA could
inhibit EMT and promote the reverse process in HCC cells. In
conclusion, this study demonstrated that ATRA remarkably suppressed
hepatocarcinoma proliferation, migration and invasion, as well as
effectively induced hepatic tumor cells to restore mature function
in vitro through reversing EMT. Interestingly, these effects
of ATRA displayed a strengthened trend with the increasing
concentration. Our results will contribute to a better
understanding of the regulatory mechanisms of ATRA in HCC and
provide a strong foundation for the clinical application of ATRA
for HCC therapy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (no. 81100309 to Y.B).
Abbreviations:
|
ATRA
|
all-trans retinoic acid
|
|
HCC
|
hepatocellular carcinoma
|
|
EMT
|
epithelial-mesenchymal transition
|
|
ICG
|
indocyanine green
|
|
PAS
|
periodicacid-schiff
|
|
ALB
|
albumin
|
|
AFP
|
α fetoprotein
|
|
CK18
|
cytokeratin 18
|
|
TAT
|
tyrosine aminotransferase
|
|
ApoB
|
apolipoprotein B
|
References
|
1
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM and Bruix J: Molecular targeted
therapies in hepatocellular carcinoma. Hepatology. 48:1312–1327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bogachek MV, De Andrade JP and Weigel RJ:
Regulation of epithelial-mesenchymal transition through SUMOylation
of transcription factors. Cancer Res. 75:11–15. 2015. View Article : Google Scholar
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trimboli AJ, Fukino K, de Bruin A, Wei G,
Shen L, Tanner SM, Creasap N, Rosol TJ, Robinson ML, Eng C, et al:
Direct evidence for epithelial-mesenchymal transitions in breast
cancer. Cancer Res. 68:937–945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross SA, McCaffery PJ, Drager UC and De
Luca LM: Retinoids in embryonal development. Physiol Rev.
80:1021–1054. 2000.PubMed/NCBI
|
|
8
|
Feldman DR, Patil S, Trinos MJ, Carousso
M, Ginsberg MS, Sheinfeld J, Bajorin DF, Bosl GJ and Motzer RJ:
Progression-free and overall survival in patients with
relapsed/refractory germ cell tumors treated with single-agent
chemotherapy: Endpoints for clinical trial design. Cancer.
118:981–986. 2012. View Article : Google Scholar
|
|
9
|
Li M, Sun Y, Guan X, Shu X and Li C:
Advanced progress on the relationship between RA and its receptors
and malignant tumors. Crit Rev Oncol Hematol. 91:271–282. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Woo YJ and Jang KL: All-trans retinoic
acid activates E-cadherin expression via promoter hypomethylation
in the human colon carcinoma HCT116 cells. Biochem Biophys Res
Commun. 425:944–949. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zanetti A, Affatato R, Centritto F,
Fratelli M, Kurosaki M, Barzago MM, Bolis M, Terao M, Garattini E
and Paroni G: All-trans-retinoic acid modulates the plasticity and
inhibits the motility of breast cancer cells: Role of NOTCH1 and
transforming growth factor (TGFβ). J Biol Chem. 290:17690–17709.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chansri N, Kawakami S, Yamashita F and
Hashida M: Inhibition of liver metastasis by all-trans retinoic
acid incorporated into O/W emulsions in mice. Int J Pharm.
321:42–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Guan DX, Shi J, Gao H, Li JJ,
Zhao JS, Qiu L, Liu J, Li N, Guo WX, et al: All-trans retinoic acid
potentiates the chemotherapeutic effect of cisplatin by inducing
differentiation of tumor initiating cells in liver cancer. J
Hepatol. 59:1255–1263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodriguez LG, Wu X and Guan JL:
Wound-healing assay. Methods Mol Biol. 294:23–29. 2005.
|
|
15
|
Bi Y, Huang J, He Y, Zhu GH, Su Y, He BC,
Luo J, Wang Y, Kang Q, Luo Q, et al: Wnt antagonist SFRP3 inhibits
the differentiation of mouse hepatic progenitor cells. J Cell
Biochem. 108:295–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang MH, Chen CL, Chau GY, Chiou SH, Su
CW, Chou TY, Peng WL and Wu JC: Comprehensive analysis of the
independent effect of twist and snail in promoting metastasis of
hepatocellular carcinoma. Hepatology. 50:1464–1474. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mima K, Hayashi H, Kuroki H, Nakagawa S,
Okabe H, Chikamoto A, Watanabe M, Beppu T and Baba H:
Epithelial-mesenchymal transition expression profiles as a
prognostic factor for disease-free survival in hepatocellular
carcinoma: Clinical significance of transforming growth factor-β
signaling. Oncol Lett. 5:149–154. 2013.
|
|
18
|
Yamada T, Yoshikawa M, Kanda S, Kato Y,
Nakajima Y, Ishizaka S and Tsunoda Y: In vitro differentiation of
embryonic stem cells into hepatocyte-like cells identified by
cellular uptake of indocyanine green. Stem Cells. 20:146–154. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamo N, Yasuchika K, Fujii H, Hoppo T,
Machimoto T, Ishii T, Fujita N, Tsuruo T, Yamashita JK, Kubo H, et
al: Two populations of Thy1-positive mesenchymal cells regulate in
vitro maturation of hepatic progenitor cells. Am J Physiol
Gastrointest Liver Physiol. 292:G526–G534. 2007. View Article : Google Scholar
|
|
20
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mongan NP and Gudas LJ: Diverse actions of
retinoid receptors in cancer prevention and treatment.
Differentiation. 75:853–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Connolly RM, Nguyen NK and Sukumar S:
Molecular pathways: Current role and future directions of the
retinoic acid pathway in cancer prevention and treatment. Clin
Cancer Res. 19:1651–1659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lo-Coco F, Ammatuna E, Montesinos P and
Sanz MA: Acute promyelocytic leukemia: Recent advances in diagnosis
and management. Semin Oncol. 35:401–409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arisi MF, Starker RA, Addya S, Huang Y and
Fernandez SV: All trans-retinoic acid (ATRA) induces
re-differentiation of early transformed breast epithelial cells.
Int J Oncol. 44:1831–1842. 2014.PubMed/NCBI
|
|
25
|
de Almeida Vasconcelos Fonseca EM, Chagas
CE, Mazzantini RP, Heidor R, Ong TP and Moreno FS: All-trans and
9-cis retinoic acids, retinol and beta-carotene chemopreventive
activities during the initial phases of hepatocarcinogenesis
involve distinct actions on glutathione S-transferase positive
preneoplastic lesions remodeling and DNA damage. Carcinogenesis.
26:1940–1946. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song K, Li W, Wang H, Wang H, Liu T, Ning
R and Wang L: Investigation of coculture of human adipose-derived
stem cells and mature adipocytes. Appl Biochem Biotechnol.
167:2381–2387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zanatta G, Steffens D, Braghirolli DI,
Fernandes RA, Netto CA and Pranke P: Viability of mesenchymal stem
cells during electrospinning. Braz J Med Biol Res. 45:125–130.
2012. View Article : Google Scholar
|
|
28
|
Fan TT, Cheng Y, Wang YF, Gui SY, Chen FH,
Zhou Q and Wang Y: A novel all-trans retinoid acid derivative
N-(3-trifluoromethyl-phenyl)-retinamide inhibits lung
adenocarcinoma A549 cell migration through down-regulating
expression of myosin light chain kinase. Asian Pac J Cancer Prev.
15:7687–7692. 2014. View Article : Google Scholar
|
|
29
|
Zhou RJ, Liao WG, Yang ZZ, Min J and Xiao
Y: Effects and mechanisms of ATRA on proliferation, cell cycle of
lung carcinoma cellline A549. Acta Academiae Medicinae Militaris
Tertiae. 14:1399–1401. 2007.
|
|
30
|
Nitto T and Sawaki K: Molecular mechanisms
of the antileukemia activities of retinoid and arsenic. J Pharmacol
Sci. 126:179–185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okuno M, Kojima S, Matsushima-Nishiwaki R,
Tsurumi H, Muto Y, Friedman SL and Moriwaki H: Retinoids in cancer
chemoprevention. Curr Cancer Drug Targets. 4:285–298. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tania M, Khan MA and Fu J: Epithelial to
mesenchymal transition inducing transcription factors and
metastatic cancer. Tumour Biol. 35:7335–7342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Jin ZY, Liu CH, Xie F, Lin XS and
Huang Q: MicroRNA-21 regulates biological behavior by inducing EMT
in human cholangiocarcinoma. Int J Clin Exp Pathol. 8:4684–4694.
2015.PubMed/NCBI
|
|
34
|
Ye Z, Zhou M, Tian B, Wu B and Li J:
Expression of lncRNA-CCAT1, E-cadherin and N-cadherin in colorectal
cancer and its clinical significance. Int J Clin Exp Med.
8:3707–3715. 2015.PubMed/NCBI
|
|
35
|
Zhou J, Tao D, Xu Q, Gao Z and Tang D:
Expression of E-cadherin and vimentin in oral squamous cell
carcinoma. Int J Clin Exp Pathol. 8:3150–3154. 2015.PubMed/NCBI
|
|
36
|
Ting HJ, Deep G, Jain AK, Cimic A,
Sirintrapun J, Romero LM, Cramer SD, Agarwal C and Agarwal R:
Silibinin prevents prostate cancer cell-mediated differentiation of
naive fibroblasts into cancer-associated fibroblast phenotype by
targeting TGF beta2. Mol Carcinog. 54:730–741. 2015. View Article : Google Scholar
|
|
37
|
Thaiparambil JT, Bender L, Ganesh T, Kline
E, Patel P, Liu Y, Tighiouart M, Vertino PM, Harvey RD, Garcia A,
et al: Withaferin A inhibits breast cancer invasion and metastasis
at sub-cytotoxic doses by inducing vimentin disassembly and serine
56 phosphorylation. Int J Cancer. 129:2744–2755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee J, Hahm ER, Marcus AI and Singh SV:
Withaferin A inhibits experimental epithelial-mesenchymal
transition in MCF-10A cells and suppresses vimentin protein level
in vivo in breast tumors. Mol Carcinog. 54:417–429. 2015.
View Article : Google Scholar
|
|
39
|
Tian Q, Xue Y, Zheng W, Sun R, Ji W, Wang
X and An R: Overexpression of hypoxia-inducible factor 1α induces
migration and invasion through Notch signaling. Int J Oncol.
47:728–738. 2015.PubMed/NCBI
|
|
40
|
Wang YP, Yu GR, Lee MJ, Lee SY, Chu IS,
Leem SH and Kim DG: Lipocalin-2 negatively modulates the
epithelial-to-mesenchymal transition in hepatocellular carcinoma
through the epidermal growth factor (TGF-beta1)/Lcn2/Twist1
pathway. Hepatology. 58:1349–1361. 2013. View Article : Google Scholar : PubMed/NCBI
|