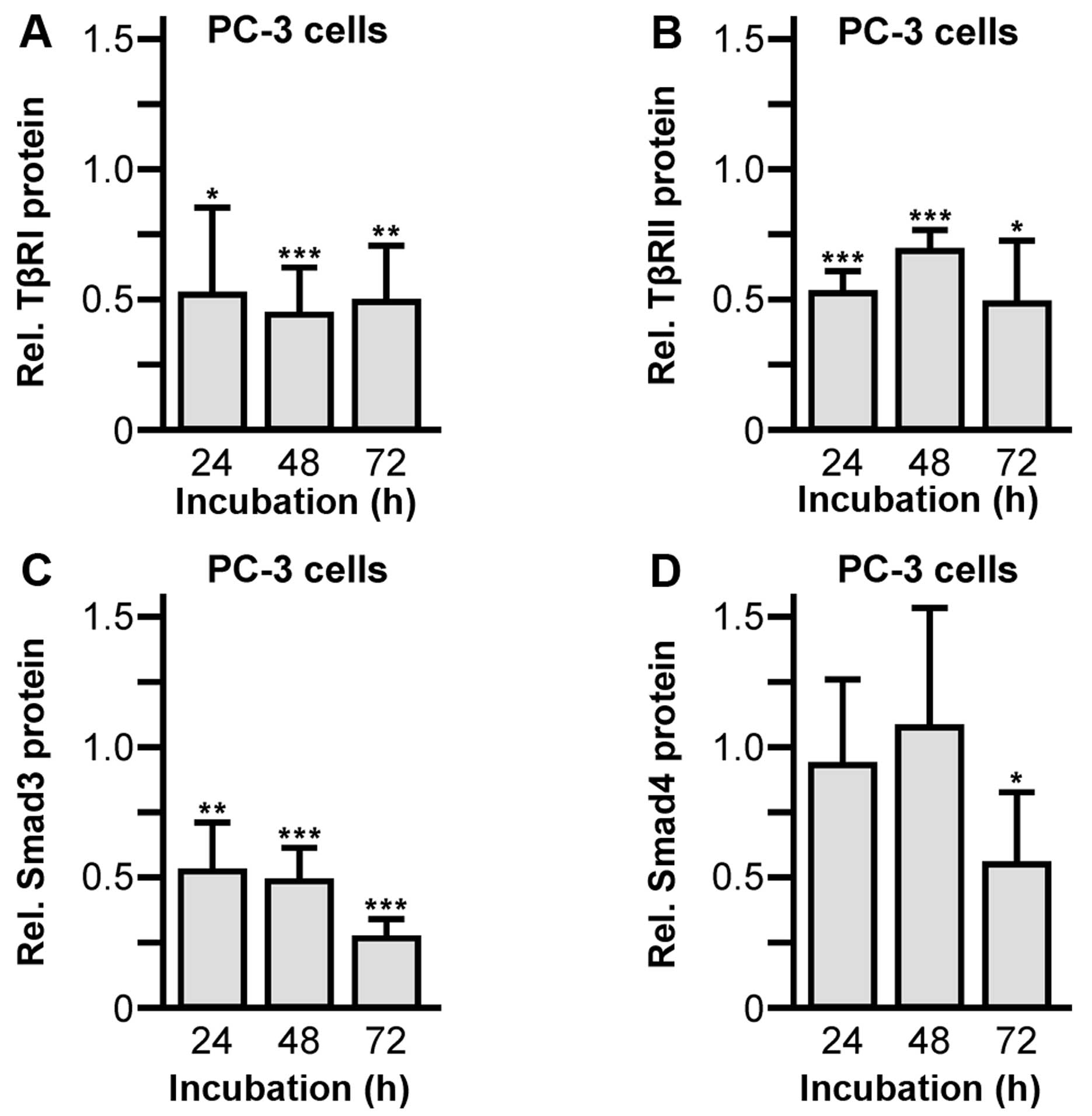
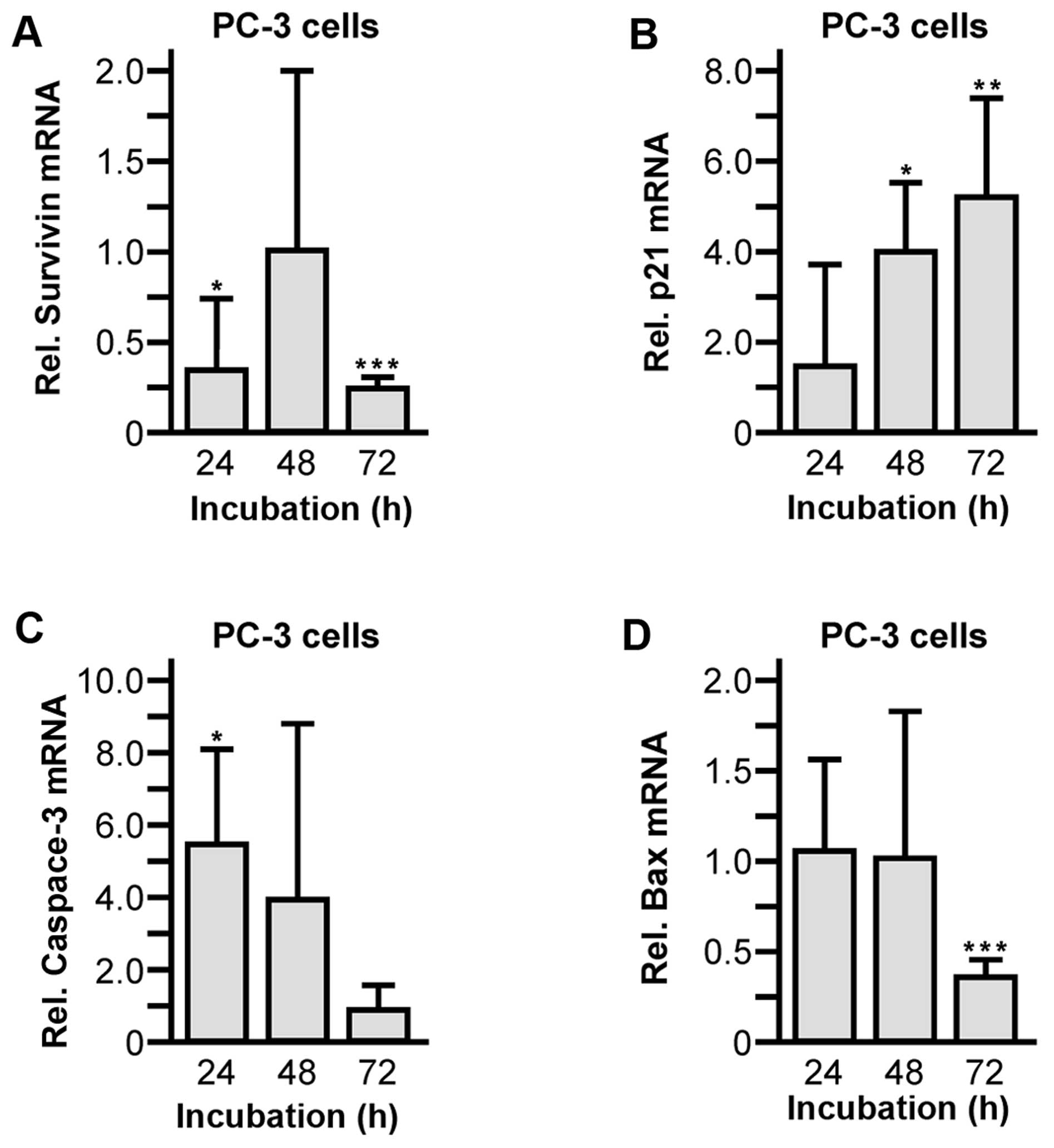
|
1
|
Labrie F, Luu-The V, Lin SX, Simard J,
Labrie C, El-Alfy M, Pelletier G and Bélanger A: Intracrinology:
Role of the family of 17 beta-hydroxysteroid dehydrogenases in
human physiology and disease. J Mol Endocrinol. 25:1–16. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Piao YS, Wiesenfeld P, Sprando R and
Arnold JT: TGFβ1 alters androgenic metabolites and hydroxysteroid
dehydrogenase enzyme expression in human prostate reactive stromal
primary cells: Is steroid metabolism altered by prostate reactive
stromal microenvironment? J Steroid Biochem Mol Biol. 138:206–213.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: COU-AA-301 Investigators: Abiraterone and increased survival
in metastatic prostate cancer. N Engl J Med. 364:1995–2005. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miller WL: Steroidogenic enzymes.
Disorders of the Human Adrenal Cortex. Flück CE and Miller WL:
Karger; Basel: pp. 1–18. 2008, View Article : Google Scholar
|
|
5
|
Yin L and Hu Q: CYP17 inhibitors -
abiraterone, C17,20-lyase inhibitors and multi-targeting agents.
Nat Rev Urol. 11:32–42. 2014. View Article : Google Scholar
|
|
6
|
Pia A, Vignani F, Attard G, Tucci M,
Bironzo P, Scagliotti G, Arlt W, Terzolo M and Berruti A:
Strategies for managing ACTH dependent mineralocorticoid excess
induced by abiraterone. Cancer Treat Rev. 39:966–973. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Richards J, Lim AC, Hay CW, Taylor AE,
Wingate A, Nowakowska K, Pezaro C, Carreira S, Goodall J, Arlt W,
et al: Interactions of abiraterone, eplerenone, and prednisolone
with wild-type and mutant androgen receptor: A rationale for
increasing abiraterone exposure or combining with MDV3100. Cancer
Res. 72:2176–2182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Harshman LC and Taplin ME: Abiraterone
acetate: Targeting persistent androgen dependence in
castration-resistant prostate cancer. Adv Ther. 30:727–747. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stanbrough M, Bubley GJ, Ross K, Golub TR,
Rubin MA, Penning TM, Febbo PG and Balk SP: Increased expression of
genes converting adrenal androgens to testosterone in
androgen-independent prostate cancer. Cancer Res. 66:2815–2825.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai C, Chen S, Ng P, Bubley GJ, Nelson PS,
Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, et al:
Intratumoral de novo steroid synthesis activates androgen receptor
in castration-resistant prostate cancer and is upregulated by
treatment with CYP17A1 inhibitors. Cancer Res. 71:6503–6513. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mostaghel EA, Marck BT, Plymate SR,
Vessella RL, Balk S, Matsumoto AM, Nelson PS and Montgomery RB:
Resistance to CYP17A1 inhibition with abiraterone in
castration-resistant prostate cancer: Induction of steroidogenesis
and androgen receptor splice variants. Clin Cancer Res.
17:5913–5925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Small EJ, Baron AD, Fippin L and Apodaca
D: Ketoconazole retains activity in advanced prostate cancer
patients with progression despite flutamide withdrawal. J Urol.
157:1204–1207. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brossard D, Zhang Y, Haider SM, Sgobba M,
Khalid M, Legay R, Duterque-Coquillaud M, Galera P, Rault S,
Dallemagne P, et al: N-substituted piperazinopyridylsteroid
derivatives as abiraterone analogues inhibit growth and induce
pro-apoptosis in human hormone-independent prostate cancer cell
lines. Chem Biol Drug Des. 82:620–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soifer HS, Souleimanian N, Wu S,
Voskresenskiy AM, Collak FK, Cinar B and Stein CA: Direct
regulation of androgen receptor activity by potent CYP17 inhibitors
in prostate cancer cells. J Biol Chem. 287:3777–3787. 2012.
View Article : Google Scholar :
|
|
16
|
Bruno RD, Gover TD, Burger AM, Brodie AM
and Njar VC: 17alpha-Hydroxylase/17,20 lyase inhibitor VN/124-1
inhibits growth of androgen-independent prostate cancer cells via
induction of the endoplasmic reticulum stress response. Mol Cancer
Ther. 7:2828–2836. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong KK, Engelman JA and Cantley LC:
Targeting the PI3K signaling pathway in cancer. Curr Opin Genet
Dev. 20:87–90. 2010. View Article : Google Scholar :
|
|
18
|
Traish AM and Morgentaler A: Epidermal
growth factor receptor expression escapes androgen regulation in
prostate cancer: A potential molecular switch for tumour growth. Br
J Cancer. 101:1949–1956. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura Y, McNamara K and Sasano H:
Estrogen receptor expression and its relevant signaling pathway in
prostate cancer: A target of therapy. Curr Mol Pharmacol.
5:392–400. 2012. View Article : Google Scholar
|
|
20
|
Steiner MS and Barrack ER: Transforming
growth factor-beta 1 overproduction in prostate cancer: Effects on
growth in vivo and in vitro. Mol Endocrinol. 6:15–25.
1992.PubMed/NCBI
|
|
21
|
Danielpour D: Functions and regulation of
transforming growth factor-beta (TGF-beta) in the prostate. Eur J
Cancer. 41:846–857. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu B and Kyprianou N: Transforming growth
factor beta and prostate cancer. Cancer Treat Res. 126:157–173.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stope MB, Rönnau C, Schubert T, Staar D,
Bradl J, Ziegler P, Streitbörger A, Kroeger N, Zimmermann U,
Walther R, et al: Transforming growth factor β in prostate cancer:
Cellular effects and basic molecular mechanisms. Urologe A.
52:378–383. 2013.(In German). View Article : Google Scholar
|
|
24
|
Shariat SF, Kattan MW, Traxel E, Andrews
B, Zhu K, Wheeler TM and Slawin KM: Association of pre- and
postoperative plasma levels of transforming growth factor beta(1)
and interleukin 6 and its soluble receptor with prostate cancer
progression. Clin Cancer Res. 10:1992–1999. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carroll AG, Voeller HJ, Sugars L and
Gelmann EP: p53 oncogene mutations in three human prostate cancer
cell lines. Prostate. 23:123–134. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Bokhoven A, Varella-Garcia M, Korch C,
Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS
and Bokhoven van A: Molecular characterization of human prostate
carcinoma cell lines. Prostate. 57:205–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coxon JP, Oades GM, Kirby RS and Colston
KW: Zoledronic acid induces apoptosis and inhibits adhesion to
mineralized matrix in prostate cancer cells via inhibition of
protein prenylation. BJU Int. 94:164–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang F, Yang Z, Yu D, Wang J, Li R and
Ding G: Sepia ink oligopeptide induces apoptosis in prostate cancer
cell lines via caspase-3 activation and elevation of Bax/Bcl-2
ratio. Mar Drugs. 10:2153–2165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raja Singh P, Arunkumar R, Sivakamasundari
V, Sharmila G, Elumalai P, Suganthapriya E, Brindha Mercy A,
Senthilkumar K and Arunakaran J: Anti-proliferative and apoptosis
inducing effect of nimbolide by altering molecules involved in
apoptosis and IGF signalling via PI3K/Akt in prostate cancer (PC-3)
cell line. Cell Biochem Funct. 32:217–228. 2014. View Article : Google Scholar
|
|
30
|
Muenchen HJ, Poncza PJ and Pienta KJ:
Different docetaxel-induced apoptotic pathways are present in
prostate cancer cell lines LNCaP and PC-3. Urology. 57:366–370.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fares M, Abou-Seri SM, Abdel-Aziz HA,
Abbas SE, Youssef MM and Eladwy RA: Synthesis and antitumor
activity of pyrido [2,3-d]pyrimidine and pyrido[2,3-d]
[1,2,4]triazolo[4,3-a] pyrimidine derivatives that induce apoptosis
through G1 cell-cycle arrest. Eur J Med Chem. 83:155–166. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hsu JL, Liu SP, Lee CC, Hsu LC, Ho YF,
Huang HS and Guh JH: A unique amidoanthraquinone derivative
displays antiproliferative activity against human
hormone-refractory metastatic prostate cancers through activation
of LKB1-AMPK-mTOR signaling pathway. Naunyn Schmiedebergs Arch
Pharmacol. 387:979–990. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weiss M, Gümbel D, Hanschmann EM,
Mandelkow R, Gelbrich N, Zimmermann U, Walther R, Ekkernkamp A,
Sckell A, Kramer A, et al: Cold atmospheric plasma treatment
induces anti-proliferative effects in prostate cancer cells by
redox and apoptotic signaling pathways. PLoS One. 10:e01303502015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Erler JT, Cawthorne CJ, Williams KJ,
Koritzinsky M, Wouters BG, Wilson C, Miller C, Demonacos C,
Stratford IJ and Dive C: Hypoxia-mediated down-regulation of Bid
and Bax in tumors occurs via hypoxia-inducible factor 1-dependent
and -independent mechanisms and contributes to drug resistance. Mol
Cell Biol. 24:2875–2889. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang H, Sun L, Xiao X, Xie R, Liu C, Wang
Y, Wei Y, Zhang H and Liu L: Krüppel-like factor 8 contributes to
hypoxia-induced multidrug resistance in gastric cancer cells.
Cancer Sci. 105:1109–1115. 2014. View Article : Google Scholar : PubMed/NCBI
|