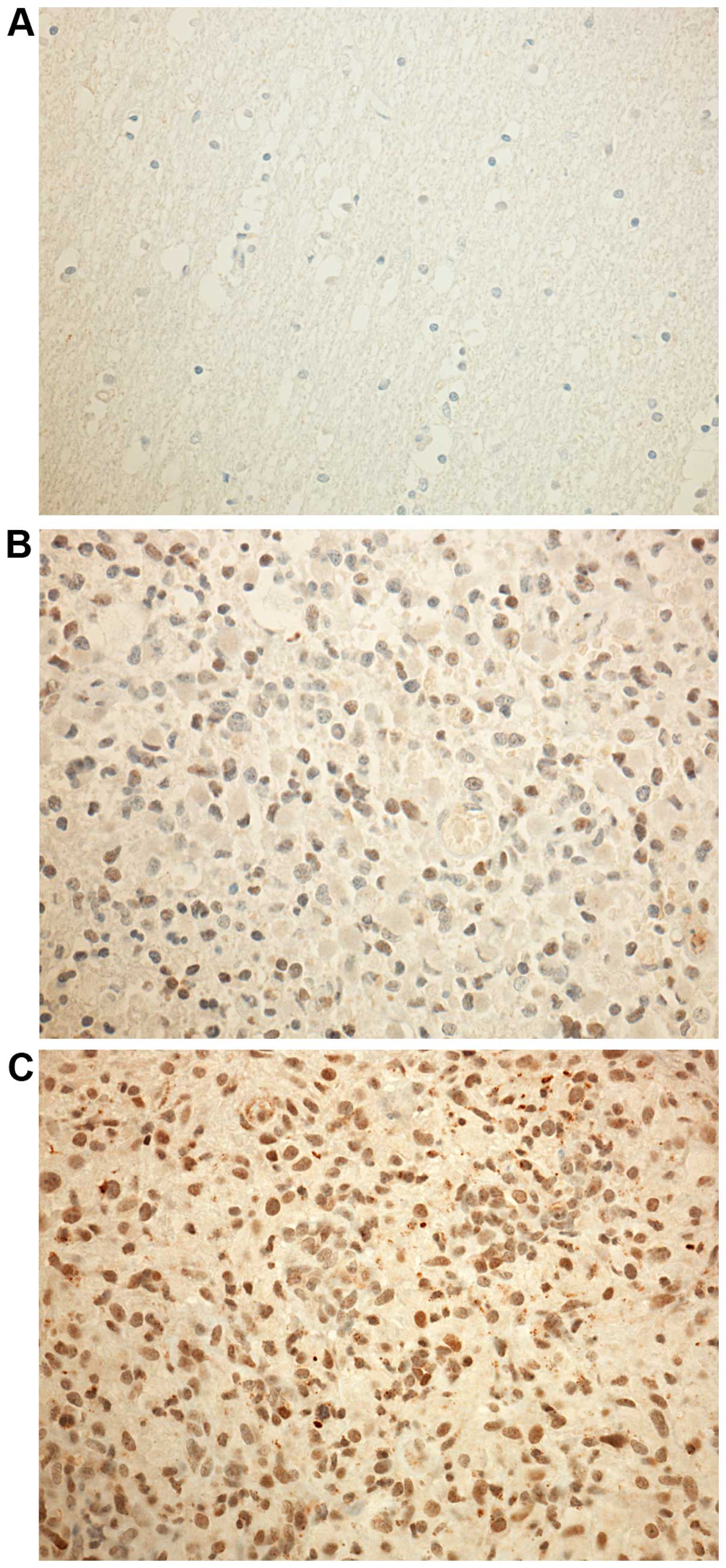
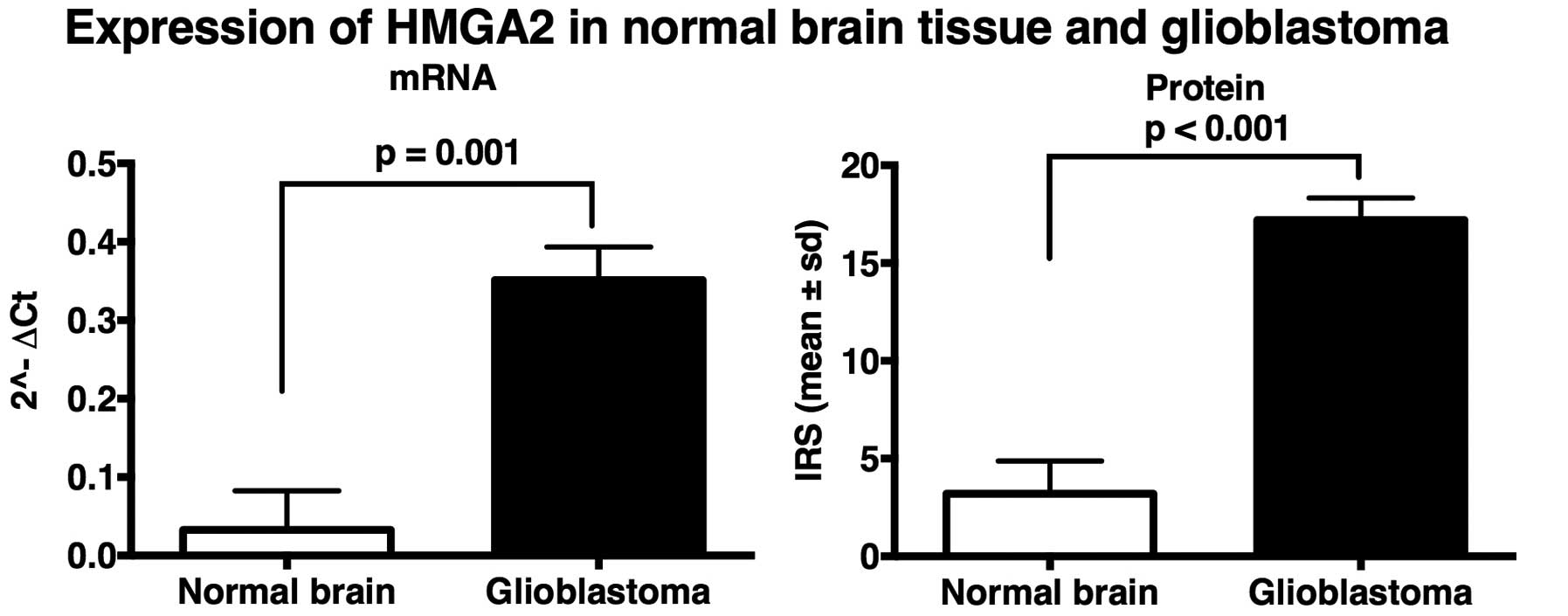
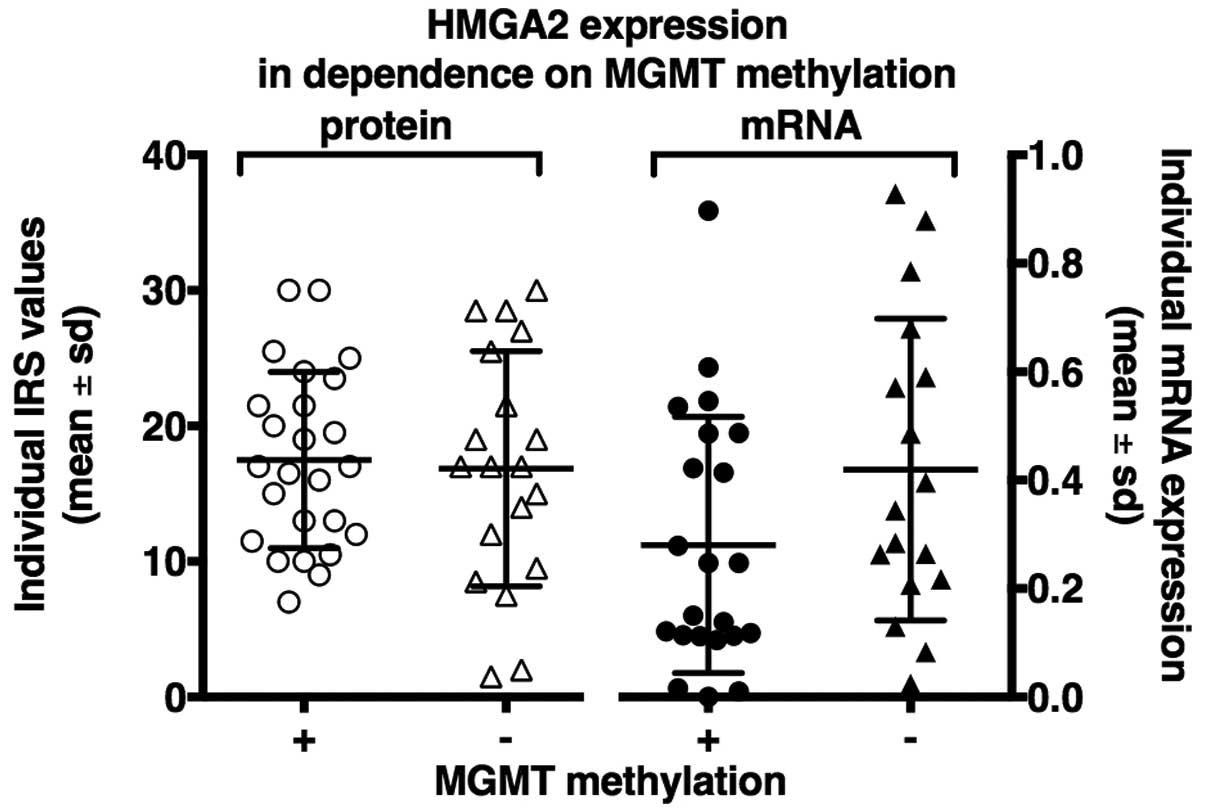
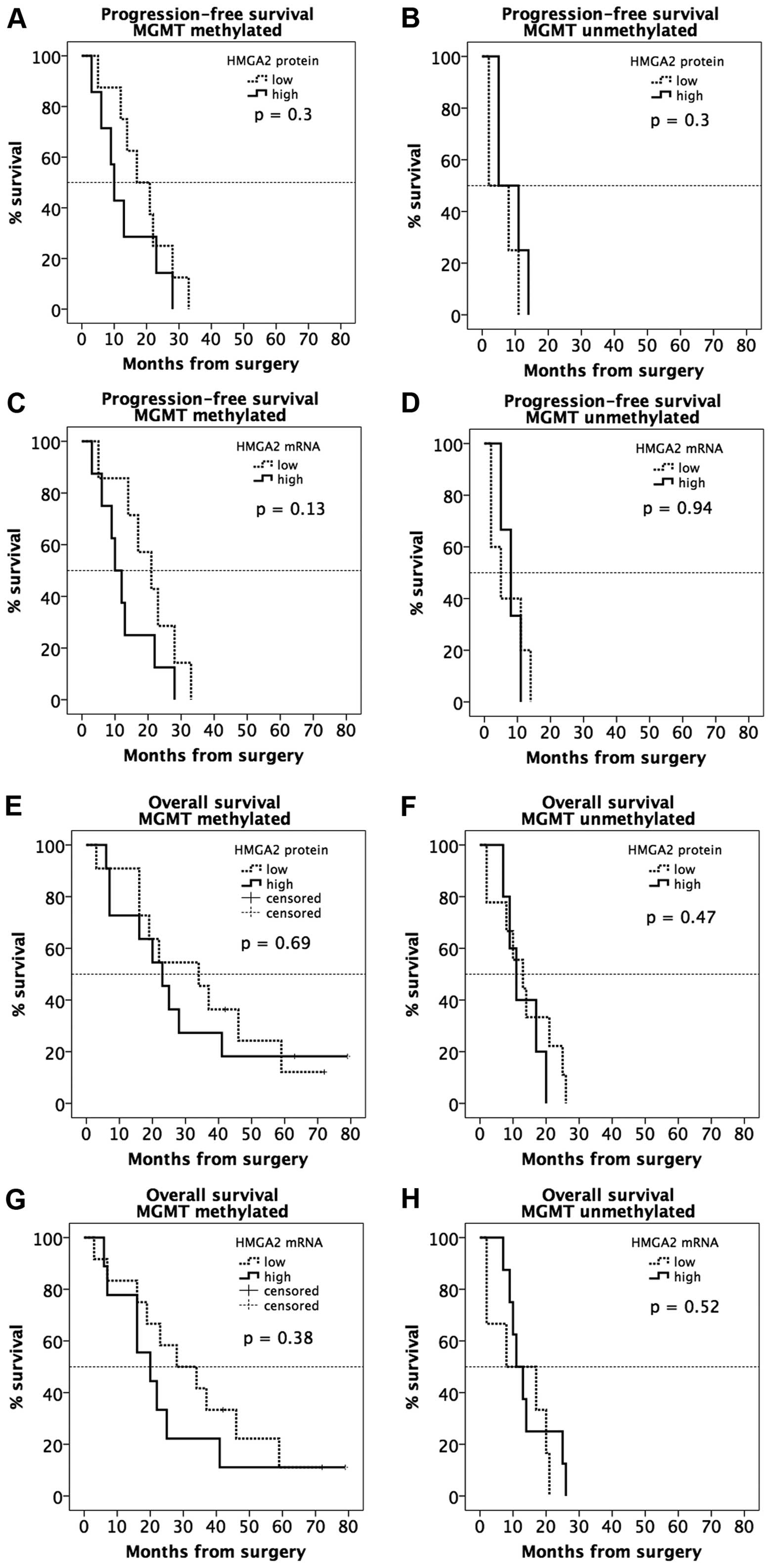
|
1
|
Grosschedl R, Giese K and Pagel J: HMG
domain proteins: Architectural elements in the assembly of
nucleoprotein structures. Trends Genet. 10:94–100. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monzen K, Ito Y, Naito AT, Kasai H, Hiroi
Y, Hayashi D, Shiojima I, Yamazaki T, Miyazono K, Asashima M, et
al: A crucial role of a high mobility group protein HMGA2 in
cardiogenesis. Nat Cell Biol. 10:567–574. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang X, Liu X, Li AYJ, Chen L, Lai L, Lin
HH, Hu S, Yao L, Peng J, Loera S, et al: Overexpression of HMGA2
promotes metastasis and impacts survival of colorectal cancers.
Clin Cancer Res. 17:2570–2580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fusco A and Fedele M: Roles of HMGA
proteins in cancer. Nat Rev Cancer. 7:899–910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akai T, Ueda Y, Sasagawa Y, Hamada T, Date
T, Katsuda S, Iizuka H, Okada Y and Chada K: High mobility group
I-C protein in astrocytoma and glioblastoma. Pathol Res Pract.
200:619–624. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shell S, Park SM, Radjabi AR, Schickel R,
Kistner EO, Jewell DA, Feig C, Lengyel E and Peter ME: Let-7
expression defines two differentiation stages of cancer. Proc Natl
Acad Sci USA. 104:11400–11405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo L, Chen C, Shi M, Wang F, Chen X, Diao
D, Hu M, Yu M, Qian L and Guo N: Stat3-coordinated
Lin-28-let-7-HMGA2 and miR-200-ZEB1 circuits initiate and maintain
oncostatin M-driven epithelial-mesenchymal transition. Oncogene.
32:5272–5282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee YS and Dutta A: The tumor suppressor
microRNA let-7 represses the HMGA2 oncogene. Genes Dev.
21:1025–1030. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Q, Liu T, Zheng S, Gao X, Lu M,
Sheyhidin I and Lu X: HMGA2 is down-regulated by microRNA let-7 and
associated with epithelial-mesenchymal transition in oesophageal
squamous cell carcinomas of Kazakhs. Histopathology. 65:408–417.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fedele M, Battista S, Kenyon L,
Baldassarre G, Fidanza V, Klein-Szanto AJ, Parlow AF, Visone R,
Pierantoni GM, Outwater E, et al: Overexpression of the HMGA2 gene
in transgenic mice leads to the onset of pituitary adenomas.
Oncogene. 21:3190–3198. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian ZR, Asa SL, Siomi H, Siomi MC,
Yoshimoto K, Yamada S, Wang EL, Rahman MM, Inoue H, Itakura M, et
al: Overexpression of HMGA2 relates to reduction of the let-7 and
its relationship to clinicopathological features in pituitary
adenomas. Mod Pathol. 22:431–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizzi C, Cataldi P, Iop A, Isola M, Sgarra
R, Manfioletti G and Giancotti V: The expression of the
high-mobility group A2 protein in colorectal cancer and surrounding
fibroblasts is linked to tumor invasiveness. Hum Pathol.
44:122–132. 2013. View Article : Google Scholar
|
|
13
|
Malek A, Bakhidze E, Noske A, Sers C,
Aigner A, Schäfer R and Tchernitsa O: HMGA2 gene is a promising
target for ovarian cancer silencing therapy. Int J Cancer.
123:348–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motoyama K, Inoue H, Nakamura Y, Uetake H,
Sugihara K and Mori M: Clinical significance of high mobility group
A2 in human gastric cancer and its relationship to let-7 microRNA
family. Clin Cancer Res. 14:2334–2340. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Cello F, Hillion J, Hristov A, Wood LJ,
Mukherjee M, Schuldenfrei A, Kowalski J, Bhattacharya R, Ashfaq R
and Resar LM: HMGA2 participates in transformation in human lung
cancer. Mol Cancer Res. 6:743–750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu B, Pang B, Hou X, Fan H, Liang N,
Zheng S, Feng B, Liu W, Guo H, Xu S, et al: Expression of
high-mobility group AT-hook protein 2 and its prognostic
significance in malignant gliomas. Hum Pathol. 45:1752–1758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ashar HR, Fejzo MS, Tkachenko A, Zhou X,
Fletcher JA, Weremowicz S, Morton CC and Chada K: Disruption of the
architectural factor HMGI-C: DNA-binding AT hook motifs fused in
lipomas to distinct transcriptional regulatory domains. Cell.
82:57–65. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tallini G, Vanni R, Manfioletti G,
Kazmierczak B, Faa G, Pauwels P, Bullerdiek J, Giancotti V, Van Den
Berghe H and Dal Cin P: HMGI-C and HMGI(Y) immunoreactivity
correlates with cytogenetic abnormalities in lipomas, pulmonary
chondroid hamartomas, endometrial polyps, and uterine leiomyomas
and is compatible with rearrangement of the HMGI-C and HMGI(Y)
genes. Lab Invest. 80:359–369. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giannini G, Di Marcotullio L, Ristori E,
Zani M, Crescenzi M, Scarpa S, Piaggio G, Vacca A, Peverali FA,
Diana F, et al: HMGI(Y) and HMGI-C genes are expressed in
neuroblastoma cell lines and tumors and affect retinoic acid
responsiveness. Cancer Res. 59:2484–2492. 1999.PubMed/NCBI
|
|
20
|
Chiou GY, Chien CS, Wang ML, Chen MT, Yang
YP, Yu YL, Chien Y, Chang YC, Shen CC, Chio CC, et al: Epigenetic
regulation of the miR142-3p/interleukin-6 circuit in glioblastoma.
Mol Cell. 52:693–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morishita A, Zaidi MR, Mitoro A,
Sankarasharma D, Szabolcs M, Okada Y, D'Armiento J and Chada K:
HMGA2 is a driver of tumor metastasis. Cancer Res. 73:4289–4299.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: European Organisation for Research and Treatment
of Cancer Brain Tumour and Radiation Oncology Groups; National
Cancer Institute of Canada Clinical Trials Group: Effects of
radiotherapy with concomitant and adjuvant temozolomide versus
radiotherapy alone on survival in glioblastoma in a randomised
phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet
Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weller M, Pfister SM, Wick W, Hegi ME,
Reifenberger G and Stupp R: Molecular neuro-oncology in clinical
practice: A new horizon. Lancet Oncol. 14:e370–e379. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Neubeck C, Seidlitz A, Kitzler HH,
Beuthien-Baumann B and Krause M: Glioblastoma multiforme: Emerging
treatments and stratification markers beyond new drugs. Br J
Radiol. 88:201503542015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okonogi N, Shirai K, Oike T, Murata K,
Noda SE, Suzuki Y and Nakano T: Topics in chemotherapy,
molecular-targeted therapy, and immunotherapy for newly-diagnosed
glioblastoma multiforme. Anticancer Res. 35:1229–1235.
2015.PubMed/NCBI
|
|
26
|
Weller M, Felsberg J, Hartmann C, Berger
H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn
JC, et al: Molecular predictors of progression-free and overall
survival in patients with newly diagnosed glioblastoma: A
prospective translational study of the German Glioma Network. J
Clin Oncol. 27:5743–5750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SM, Woo JS, Jeong CH, Ryu CH, Lim JY
and Jeun SS: Effective combination therapy for malignant glioma
with TRAIL-secreting mesenchymal stem cells and lipoxygenase
inhibitor MK886. Cancer Res. 72:4807–4817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Macdonald DR, Kiebert G, Prados M, Yung A
and Olson J: Benefit of temozolomide compared to procarbazine in
treatment of glioblastoma multiforme at first relapse: Effect on
neurological functioning, performance status, and health related
quality of life. Cancer Invest. 23:138–144. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Venur VA, Peereboom DM and Ahluwalia MS:
Current medical treatment of glioblastoma. Cancer Treat Res.
163:103–115. 2015. View Article : Google Scholar
|
|
30
|
Mabray MC, Barajas RF Jr and Cha S: Modern
brain tumor imaging. Brain Tumor Res Treat. 3:8–23. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hoelzinger DB, Mariani L, Weis J, Woyke T,
Berens TJ, McDonough WS, Sloan A, Coons SW and Berens ME: Gene
expression profile of glioblastoma multiforme invasive phenotype
points to new therapeutic targets. Neoplasia. 7:7–16. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perego C, Vanoni C, Massari S, Raimondi A,
Pola S, Cattaneo MG, Francolini M, Vicentini LM and Pietrini G:
Invasive behaviour of glioblastoma cell lines is associated with
altered organisation of the cadherin-catenin adhesion system. J
Cell Sci. 115:3331–3340. 2002.PubMed/NCBI
|
|
33
|
Sturm D, Witt H, Hovestadt V, Khuong-Quang
DA, Jones DT, Konermann C, Pfaff E, Tönjes M, Sill M, Bender S, et
al: Hotspot mutations in H3F3A and IDH1 define distinct epigenetic
and biological subgroups of glioblastoma. Cancer Cell. 22:425–437.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Badie B and Schartner J: Role of microglia
in glioma biology. Microsc Res Tech. 54:106–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giese A, Bjerkvig R, Berens ME and
Westphal M: Cost of migration: Invasion of malignant gliomas and
implications for treatment. J Clin Oncol. 21:1624–1636. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beije N, Kraan J, Taal W, van der Holt B,
Oosterkamp HM, Walenkamp AM, Beerepoot L, Hanse M, van Linde ME,
Otten A, et al: Prognostic value and kinetics of circulating
endothelial cells in patients with recurrent glioblastoma
randomised to bevacizumab plus lomustine, bevacizumab single agent
or lomustine single agent. A report from the Dutch Neuro-Oncology
Group BELOB trial. Br J Cancer. 113:226–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deviers A, Ken S, Filleron T, Rowland B,
Laruelo A, Catalaa I, Lubrano V, Celsis P, Berry I, Mogicato G, et
al: Evaluation of the lactate-to-N-acetyl-aspartate ratio defined
with magnetic resonance spectroscopic imaging before radiation
therapy as a new predictive marker of the site of relapse in
patients with glioblastoma multiforme. Int J Radiat Oncol Biol
Phys. 90:385–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hutterer M, Nowosielski M, Haybaeck J,
Embacher S, Stockhammer F, Gotwald T, Holzner B, Capper D, Preusser
M, Marosi C, et al: A single-arm phase II Austrian/German
multi-center trial on continuous daily sunitinib in primary
glioblastoma at first recurrence (SURGE 01-07). Neuro Oncol.
16:92–102. 2014. View Article : Google Scholar
|
|
39
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection, and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar
|
|
40
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nestler U, Lutz K, Pichlmeier U, Stummer
W, Franz K, Reulen HJ and Bink A: 5-ALA Glioma Study Group:
Anatomic features of glioblastoma and their potential impact on
survival. Acta Neurochir (Wien). 157:179–186. 2015. View Article : Google Scholar
|
|
42
|
Matsukado Y, MacCarty CS and Kernohan JW:
The growth of glioblastoma multiforme (astrocytomas, grades 3 and
4) in neurosurgical practice. J Neurosurg. 18:636–644. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Becker KP and Yu J: Status quo -
standard-of-care medical and radiation therapy for glioblastoma.
Cancer J. 18:12–19. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Y, Shete S, Etzel CJ, Scheurer M,
Alexiou G, Armstrong G, Tsavachidis S, Liang FW, Gilbert M, Aldape
K, et al: Polymorphisms of LIG4, BTBD2, HMGA2, and RTEL1 genes
involved in the double-strand break repair pathway predict
glioblastoma survival. J Clin Oncol. 28:2467–2474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Halle B, Marcusson EG, Aaberg-Jessen C,
Jensen SS, Meyer M, Schulz MK, Andersen C and Kristensen BW:
Convection-enhanced delivery of an anti-miR is well-tolerated,
preserves anti-miR stability and causes efficient target
de-repression: A proof of concept. J Neurooncol. 126:47–45. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stupp R, van den Bent MJ and Hegi ME:
Optimal role of temozolomide in the treatment of malignant gliomas.
Curr Neurol Neurosci Rep. 5:198–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gilbert MR, Wang M, Aldape KD, Stupp R,
Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT,
et al: Dose-dense temozolomide for newly diagnosed glioblastoma: A
randomized phase III clinical trial. J Clin Oncol. 31:4085–4091.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee J, Ha S, Jung CK and Lee HH:
High-mobility-group A2 overexpression provokes a poor prognosis of
gastric cancer through the epithelial-mesenchymal transition. Int J
Oncol. 46:2431–2438. 2015.PubMed/NCBI
|