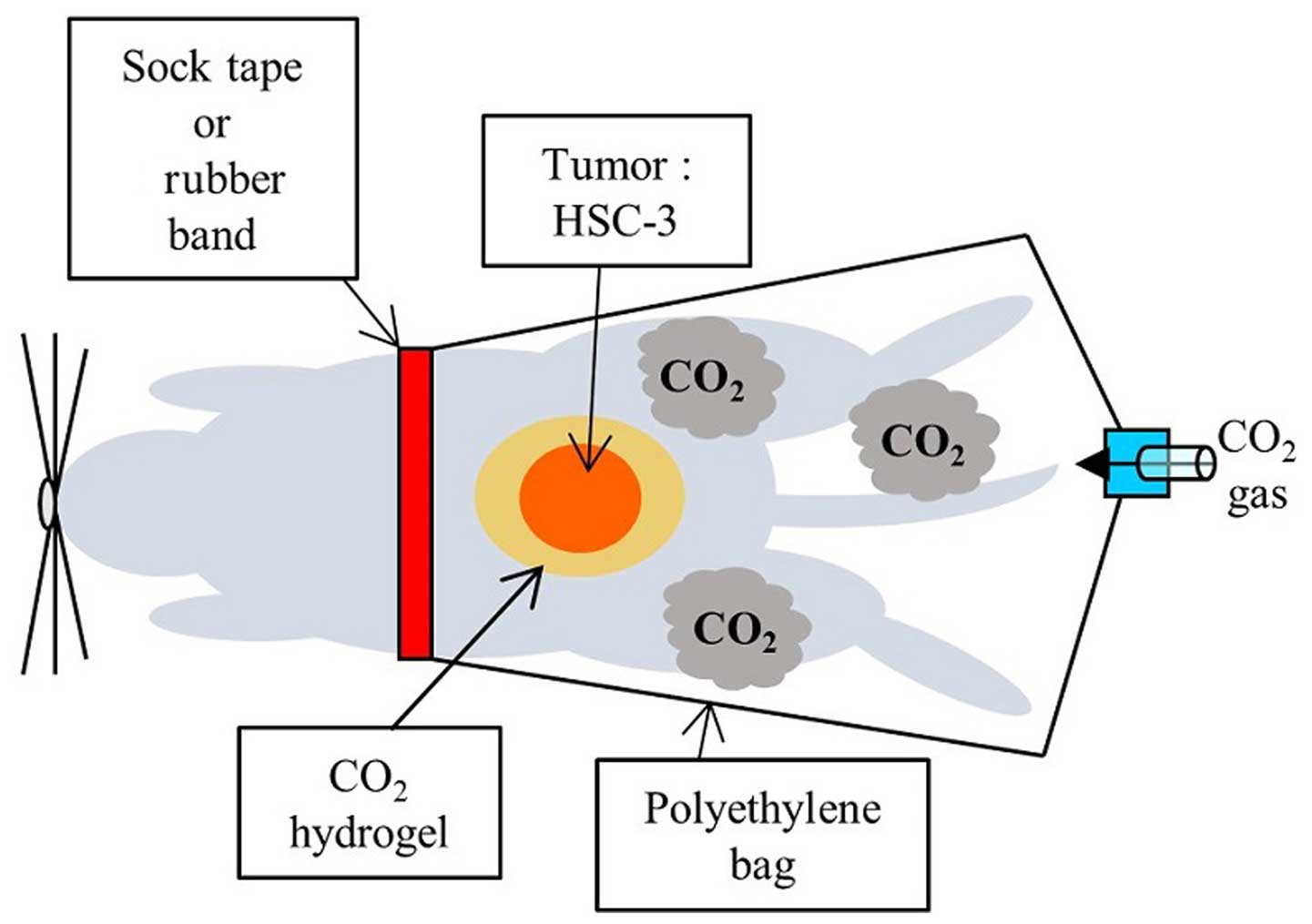
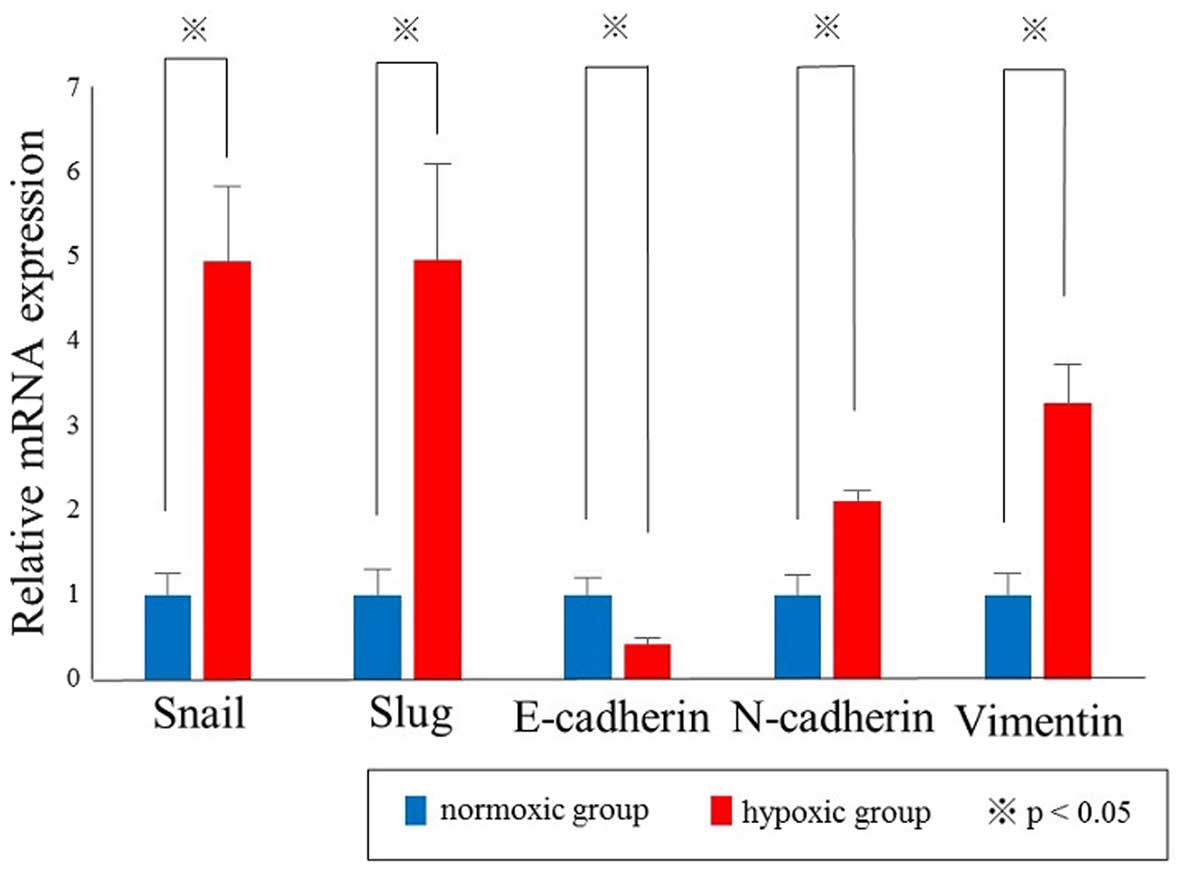
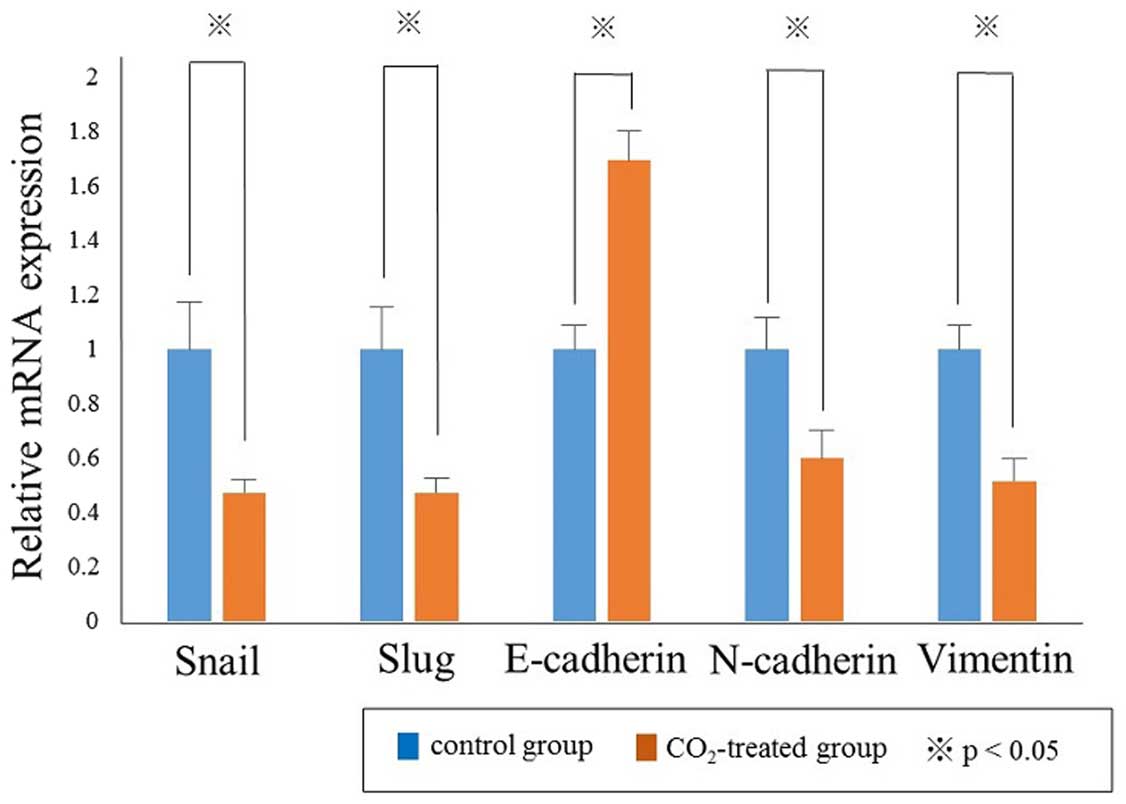
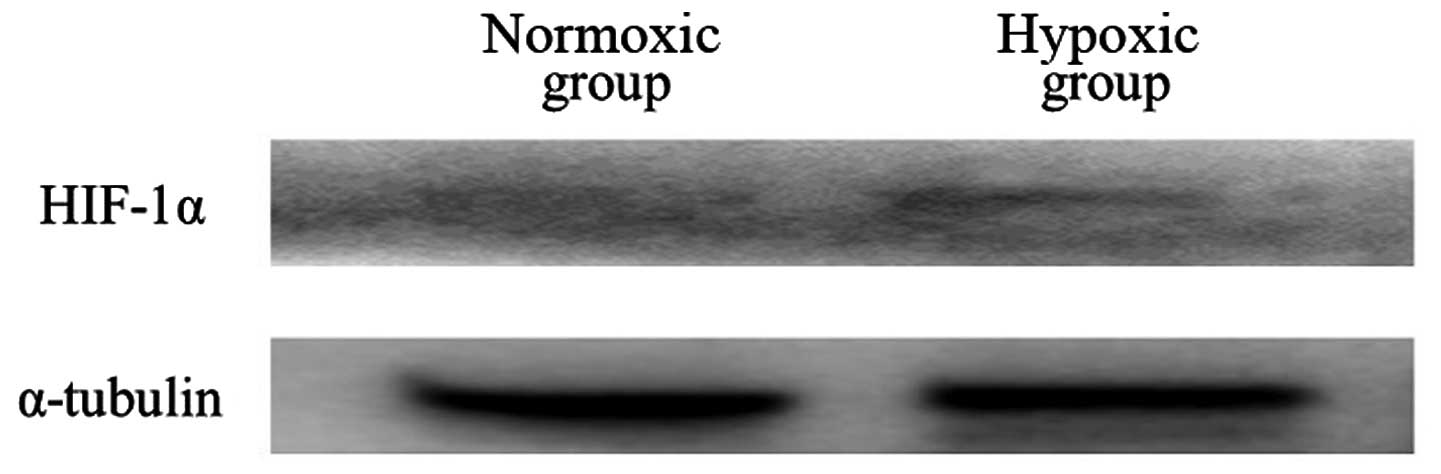
|
1
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316. 2009.
View Article : Google Scholar
|
|
2
|
Kume K, Haraguchi M, Hijioka H, Ishida T,
Miyawaki A, Nakamura N and Ozawa M: The transcription factor Snail
enhanced the degradation of E-cadherin and desmoglein 2 in oral
squamous cell carcinoma cells. Biochem Biophys Res Commun.
430:889–894. 2013. View Article : Google Scholar
|
|
3
|
Sasahira T, Kirita T, Yamamoto K, Ueda N,
Kurihara M, Matsushima S, Bhawal UK, Bosserhoff AK and Kuniyasu H:
Transport and Golgi organisation protein 1 is a novel tumour
progressive factor in oral squamous cell carcinoma. Eur J Cancer.
50:2142–2151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Savagner P: Leaving the neighborhood:
Molecular mechanisms involved during epithelial-mesenchymal
transition. BioEssays. 23:912–923. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
7
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
8
|
Ikenouchi J, Matsuda M, Furuse M and
Tsukita S: Regulation of tight junctions during the
epithelium-mesenchyme transition: Direct repression of the gene
expression of claudins/occludin by Snail. J Cell Sci.
116:1959–1967. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
da Silva SD, Morand GB, Alobaid FA, Hier
MP, Mlynarek AM, Alaoui-Jamali MA and Kowalski LP:
Epithelial-mesenchymal transition (EMT) markers have prognostic
impact in multiple primary oral squamous cell carcinoma. Clin Exp
Metastasis. 32:55–63. 2015. View Article : Google Scholar
|
|
10
|
Fan CC, Wang TY, Cheng YA, Jiang SS, Cheng
CW, Lee AY and Kao TY: Expression of E-cadherin, Twist, and p53 and
their prognostic value in patients with oral squamous cell
carcinoma. J Cancer Res Clin Oncol. 139:1735–1744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Faleiro-Rodrigues C, Macedo-Pinto I,
Pereira D and Lopes CS: Prognostic value of E-cadherin
immunoexpression in patients with primary ovarian carcinomas. Ann
Oncol. 15:1535–1542. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang
DC and Yang JM: Expression of E-cadherin and beta-catenin in
gastric carcinoma and its correlation with the clinicopatho-logical
features and patient survival. World J Gastroenterol. 8:987–993.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Richmond PJ, Karayiannakis AJ, Nagafuchi
A, Kaisary AV and Pignatelli M: Aberrant E-cadherin and
alpha-catenin expression in prostate cancer: Correlation with
patient survival. Cancer Res. 57:3189–3193. 1997.PubMed/NCBI
|
|
14
|
Bondi J, Bukholm G, Nesland JM, Bakka A
and Bukholm IR: An increase in the number of adhesion proteins with
altered expression is associated with an increased risk of cancer
death for colon carcinoma patients. Int J Colorectal Dis.
21:231–237. 2006. View Article : Google Scholar
|
|
15
|
Tseng RC, Lee SH, Hsu HS, Chen BH, Tsai
WC, Tzao C and Wang YC: SLIT2 attenuation during lung cancer
progression deregulates beta-catenin and E-cadherin and associates
with poor prognosis. Cancer Res. 70:543–551. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li YY, Zhou CX and Gao Y: Snail regulates
the motility of oral cancer cells via RhoA/Cdc42/p-ERM pathway.
Biochem Biophys Res Commun. 452:490–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wushou A, Pan HY, Liu W, Tian Z, Wang LZ,
Shali S and Zhang ZY: Correlation of increased twist with lymph
node metastasis in patients with oral squamous cell carcinoma. J
Oral Maxillofac Surg. 70:1473–1479. 2012. View Article : Google Scholar
|
|
18
|
Zhao D, Tang XF, Yang K, Liu JY and Ma XR:
Over-expression of integrin-linked kinase correlates with aberrant
expression of Snail, E-cadherin and N-cadherin in oral squamous
cell carcinoma: Implications in tumor progression and metastasis.
Clin Exp Metastasis. 29:957–969. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou J, Tao D, Xu Q, Gao Z and Tang D:
Expression of E-cadherin and vimentin in oral squamous cell
carcinoma. Int J Clin Exp Pathol. 8:3150–3154. 2015.PubMed/NCBI
|
|
20
|
Hockel M, Schlenger K, Aral B, Mitze M,
Schaffer U and Vaupel P: Association between tumor hypoxia and
malignant progression in advanced cancer of the uterine cervix.
Cancer Res. 56:4509–4515. 1996.PubMed/NCBI
|
|
21
|
Jing SW, Wang YD, Chen LQ, Sang MX, Zheng
MM, Sun GG, Liu Q, Cheng YJ and Yang CR: Hypoxia suppresses
E-cadherin and enhances matrix metalloproteinase-2 expression
favoring esophageal carcinoma migration and invasion via hypoxia
inducible factor-1 alpha activation. Dis Esophagus. 26:75–83. 2013.
View Article : Google Scholar
|
|
22
|
Krishnamachary B, Zagzag D, Nagasawa H,
Rainey K, Okuyama H, Baek JH and Semenza GL: Hypoxia-inducible
factor-1-dependent repression of E-cadherin in von Hippel-Lindau
tumor suppressor-null renal cell carcinoma mediated by TCF3,
ZFHX1A, and ZFHX1B. Cancer Res. 66:2725–2731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krishnamachary B, Berg-Dixon S, Kelly B,
Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi
P, et al: Regulation of colon carcinoma cell invasion by
hypoxia-inducible factor 1. Cancer Res. 63:1138–1143.
2003.PubMed/NCBI
|
|
24
|
Lv L, Yuan J, Huang T, Zhang C, Zhu Z,
Wang L, Jiang G and Zeng F: Stabilization of Snail by HIF-1α and
TNF-α is required for hypoxia-induced invasion in prostate cancer
PC3 cells. Mol Biol Rep. 41:4573–4582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang F, Chang M, Shi Y, Jiang L, Zhao J,
Hai L, Sharen G and Du H: Down-regulation of hypoxia-inducible
factor-1 suppresses malignant biological behavior of
triple-negative breast cancer cells. Int J Clin Exp Med.
7:3933–3940. 2014.
|
|
26
|
Wang N, Dong CR, Jiang R, Tang C, Yang L,
Jiang QF, Chen GG and Liu ZM: Overexpression of HIF-1α,
metallothionein and SLUG is associated with high TNM stage and
lymph node metastasis in papillary thyroid carcinoma. Int J Clin
Exp Pathol. 7:322–330. 2013.
|
|
27
|
Takeda D, Hasegawa T, Ueha T, Imai Y,
Sakakibara A, Minoda M, Kawamoto T, Minamikawa T, Shibuya Y, Akisue
T, et al: Transcutaneous carbon dioxide induces mitochondrial
apoptosis and suppresses metastasis of oral squamous cell carcinoma
in vivo. PLoS One. 9:e1005302014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Matsui T, Ota T, Ueda Y, Tanino M and
Odashima S: Isolation of a highly metastatic cell line to lymph
node in human oral squamous cell carcinoma by orthotopic
implantation in nude mice. Oral Oncol. 34:253–256. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okada Y, Akisue T, Hara H, Kishimoto K,
Kawamoto T, Imabori M, Kishimoto S, Fukase N, Onishi Y and Kurosaka
M: The effect of bevacizumab on tumour growth of malignant fibrous
histiocytoma in an animal model. Anticancer Res. 30:3391–3395.
2010.PubMed/NCBI
|
|
30
|
Jordan RC and Daley T: Oral squamous cell
carcinoma: new in sights. J Can Dent Assoc. 63:517–518. 521–525.
1997.
|
|
31
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smith A, Teknos TN and Pan Q: Epithelial
to mesenchymal transition in head and neck squamous cell carcinoma.
Oral Oncol. 49:287–292. 2013. View Article : Google Scholar :
|
|
33
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wheelock MJ, Shintani Y, Maeda M, Fukumoto
Y and Johnson KR: Cadherin switching. J Cell Sci. 121:727–735.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Peinado H, Portillo F and Cano A:
Transcriptional regulation of cadherins during development and
carcinogenesis. Int J Dev Biol. 48:365–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mattijssen V, Peters HM, Schalkwijk L,
Manni JJ, van ‘t Hof-Grootenboer B, de Mulder PH and Ruiter DJ:
E-cadherin expression in head and neck squamous-cell carcinoma is
associated with clinical outcome. Int J Cancer. 55:580–585. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thomas PA, Kirschmann DA, Cerhan JR,
Folberg R, Seftor EA, Sellers TA and Hendrix MJ: Association
between keratin and vimentin expression, malignant phenotype, and
survival in postmenopausal breast cancer patients. Clin Cancer Res.
5:2698–2703. 1999.PubMed/NCBI
|
|
41
|
Yokoyama K, Kamata N, Hayashi E, Hoteiya
T, Ueda N, Fujimoto R and Nagayama M: Reverse correlation of
E-cadherin and snail expression in oral squamous cell carcinoma
cells in vitro. Oral Oncol. 37:65–71. 2001. View Article : Google Scholar
|
|
42
|
Tang CH and Tsai CC: CCL2 increases MMP-9
expression and cell motility in human chondrosarcoma cells via the
Ras/Raf/ MEK/ERK/NF-κB signaling pathway. Biochem Pharmacol.
83:335–344. 2012. View Article : Google Scholar
|
|
43
|
Barrallo-Gimeno A and Nieto MA:
Evolutionary history of the Snail/Scratch superfamily. Trends
Genet. 25:248–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Parent AE, Newkirk KM and Kusewitt DF:
Slug (Snai2) expression during skin and hair follicle development.
J Invest Dermatol. 130:1737–1739. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Murray SA, Oram KF and Gridley T: Multiple
functions of Snail family genes during palate development in mice.
Development. 134:1789–1797. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li G, Satyamoorthy K and Herlyn M:
N-cadherin-mediated intercellular interactions promote survival and
migration of melanoma cells. Cancer Res. 61:3819–3825.
2001.PubMed/NCBI
|
|
48
|
Vuoriluoto K, Haugen H, Kiviluoto S,
Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB and Ivaska J:
Vimentin regulates EMT induction by Slug and oncogenic H-Ras and
migration by governing Axl expression in breast cancer. Oncogene.
30:1436–1448. 2011. View Article : Google Scholar
|
|
49
|
Hashimoto T, Soeno Y, Maeda G, Taya Y,
Aoba T, Nasu M, Kawashiri S and Imai K: Progression of oral
squamous cell carcinoma accompanied with reduced E-cadherin
expression but not cadherin switch. PLoS One. 7:e478992012.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mandal M, Myers JN, Lippman SM, Johnson
FM, Williams MD, Rayala S, Ohshiro K, Rosenthal DI, Weber RS,
Gallick GE, et al: Epithelial to mesenchymal transition in head and
neck squamous carcinoma: Association of Src activation with
E-cadherin down-regulation, vimentin expression, and aggressive
tumor features. Cancer. 112:2088–2100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang S, Zhou X, Wang B, Zhang K, Liu S,
Yue K, Zhang L and Wang X: Loss of VHL expression contributes to
epithelial-mesenchymal transition in oral squamous cell carcinoma.
Oral Oncol. 50:809–817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Teppo S, Sundquist E, Vered M, Holappa H,
Parkkisenniemi J, Rinaldi T, Lehenkari P, Grenman R, Dayan D,
Risteli J, et al: The hypoxic tumor microenvironment regulates
invasion of aggressive oral carcinoma cells. Exp Cell Res.
319:376–389. 2013. View Article : Google Scholar
|
|
53
|
Wu XY, Fu ZX and Wang XH: Effect of
hypoxia-inducible factor 1-α on Survivin in colorectal cancer. Mol
Med Rep. 3:409–415. 2010.
|
|
54
|
Zhang Q, Zhang ZF, Rao JY, Sato JD, Brown
J, Messadi DV and Le AD: Treatment with siRNA and antisense
oligonucleotides targeted to HIF-1alpha induced apoptosis in human
tongue squamous cell carcinomas. Int J Cancer. 111:849–857. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sakai Y, Miwa M, Oe K, Ueha T, Koh A,
Niikura T, Iwakura T, Lee SY, Tanaka M and Kurosaka M: A novel
system for transcutaneous application of carbon dioxide causing an
‘artificial Bohr effect’ in the human body. PLoS One. 6:e241372011.
View Article : Google Scholar
|