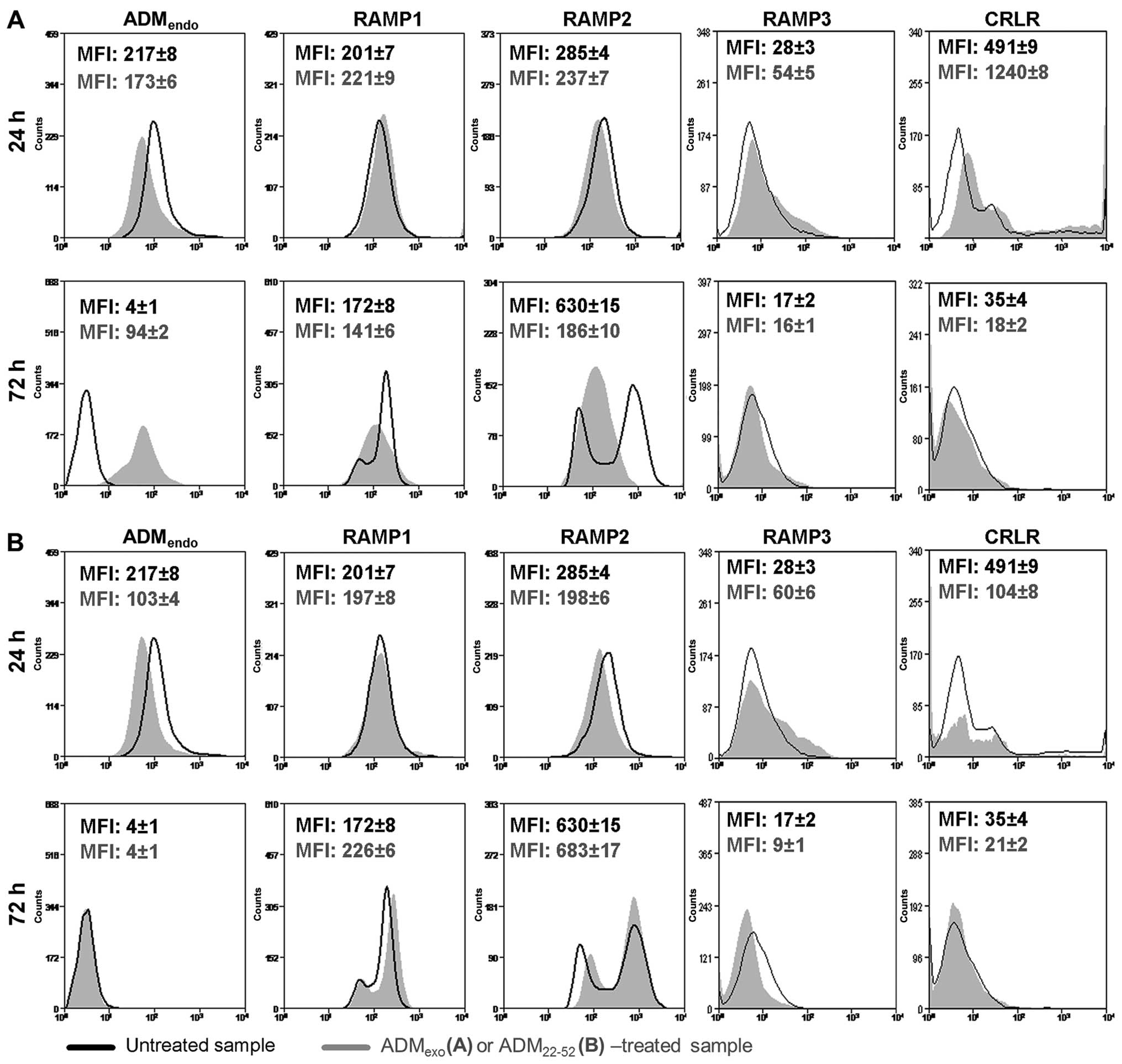
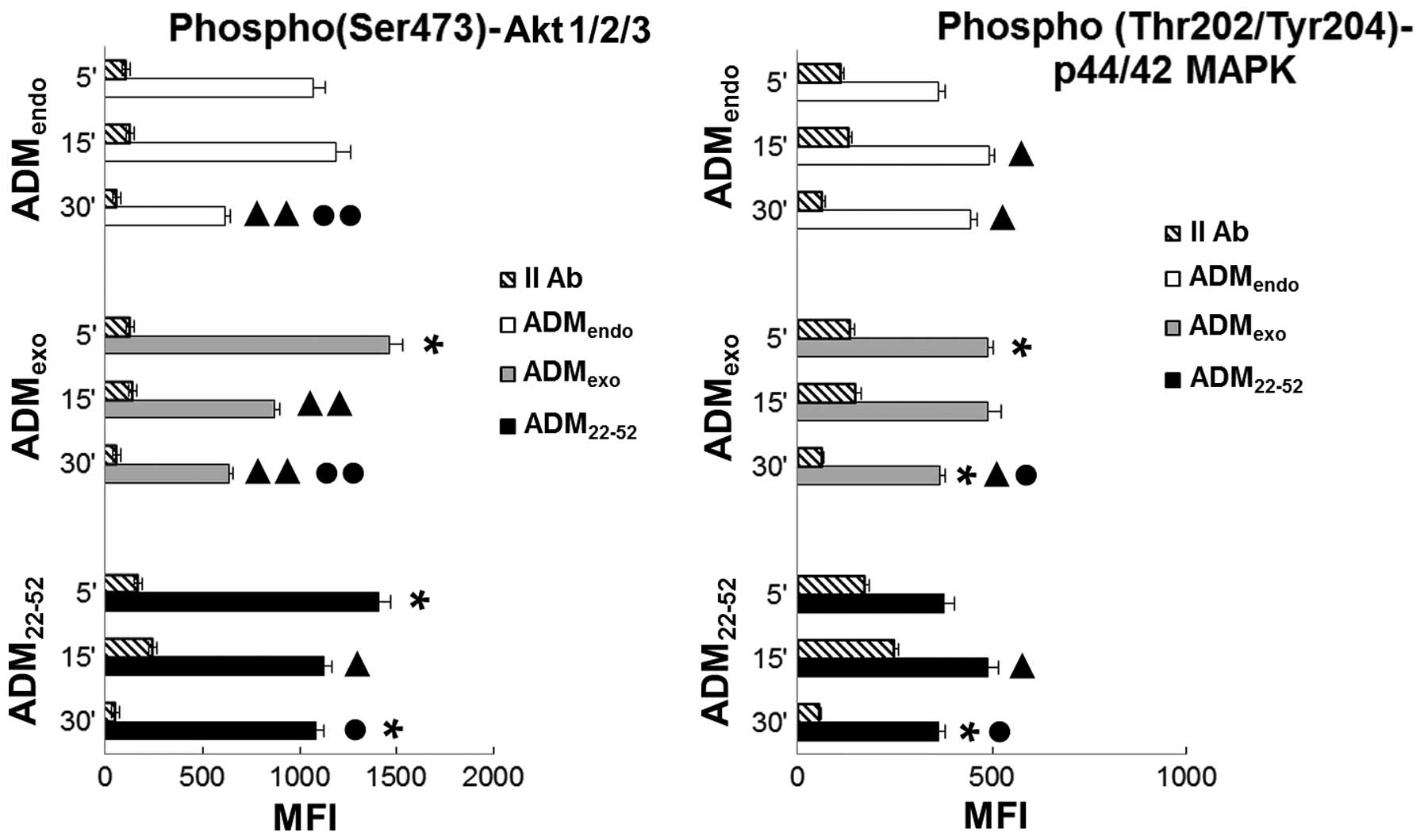
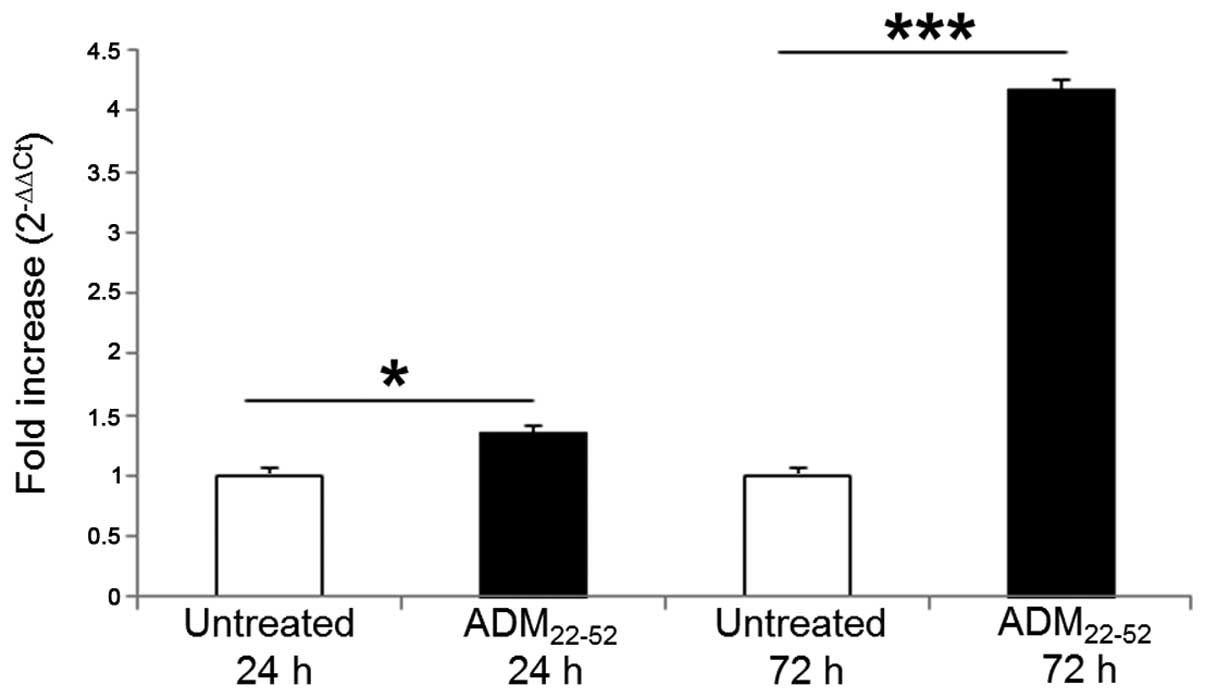
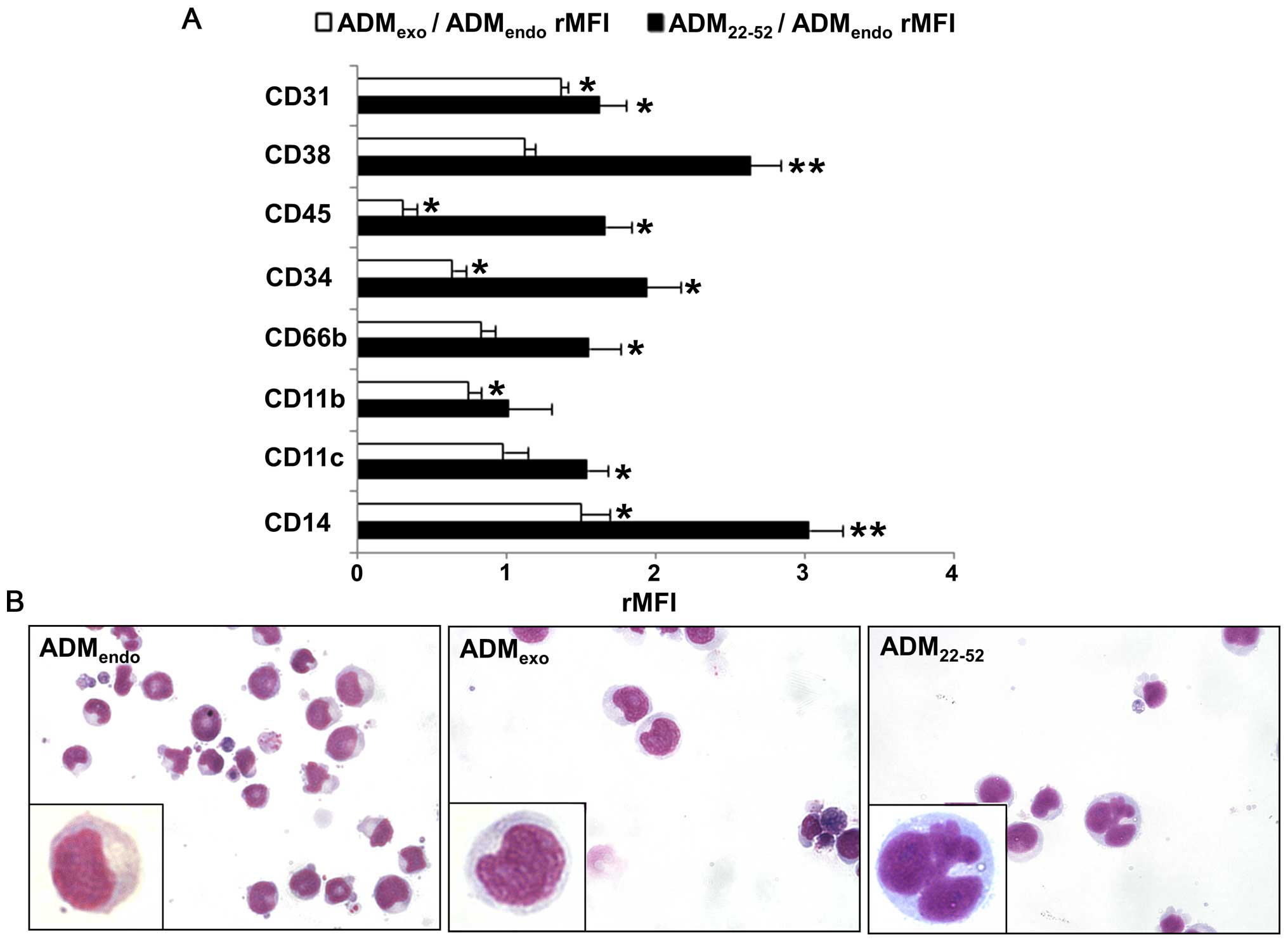
|
1
|
Korf K, Wodrich H, Haschke A, Ocampo C,
Harder L, Gieseke F, Pollmann A, Dierck K, Prall S, Staege H, et
al: The PML domain of PML-RARα blocks senescence to promote
leukemia. Proc Natl Acad Sci USA. 111:12133–12138. 2014. View Article : Google Scholar
|
|
2
|
Nitto T and Sawaki K: Molecular mechanisms
of the anti-leukemia activities of retinoid and arsenic. J
Pharmacol Sci. 126:179–185. 2014. View Article : Google Scholar
|
|
3
|
Kitamura K, Kangawa K, Kawamoto M, Ichiki
Y, Nakamura S, Matsuo H and Eto T: Adrenomedullin: A novel
hypotensive peptide isolated from human pheochromocytoma. 1993.
Biochem Biophys Res Commun. 425:548–555. 2012. View Article : Google Scholar : PubMed/NCBI
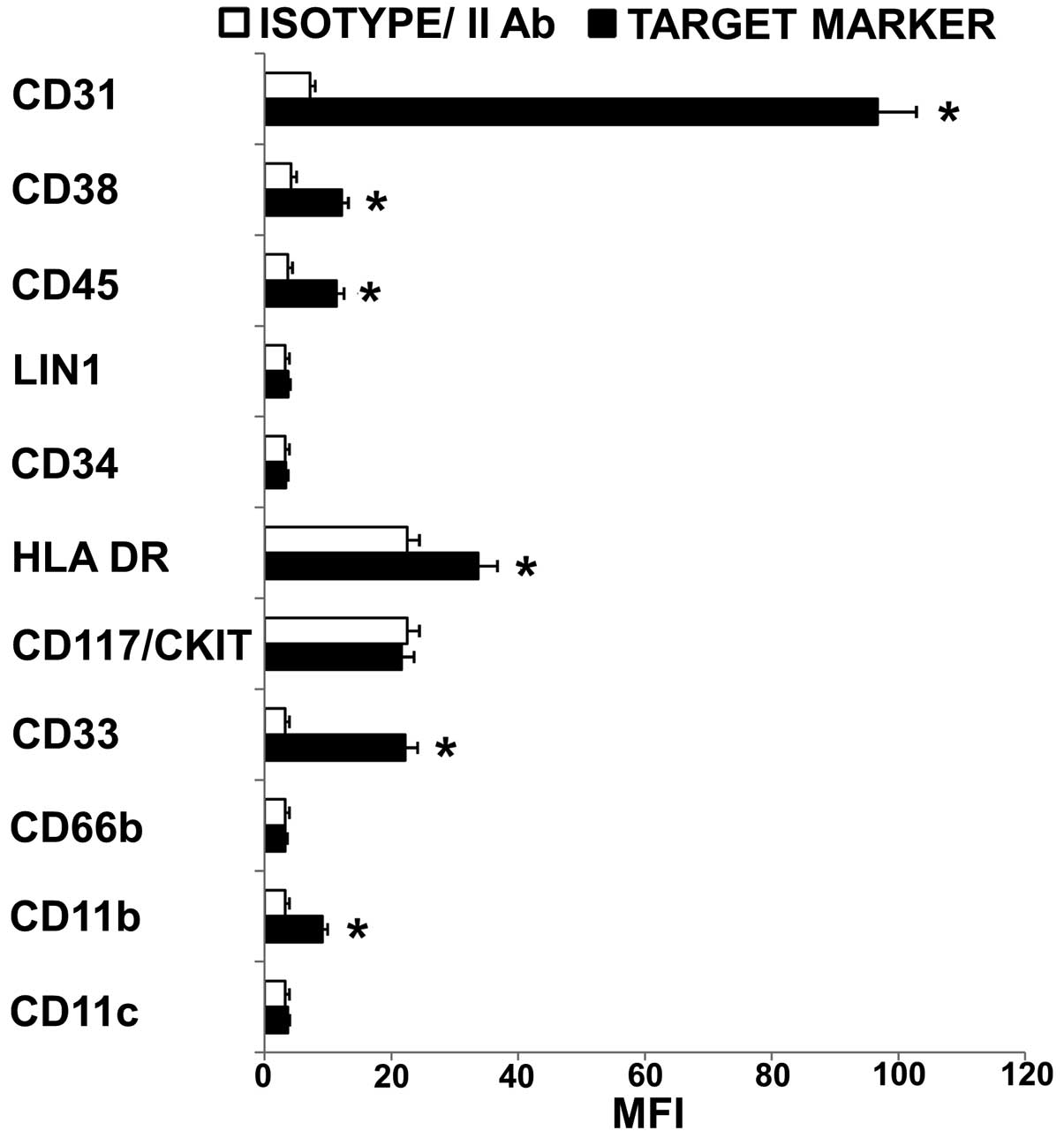
|
|
4
|
Kitamura K, Kangawa K and Eto T:
Adrenomedullin and PAMP: Discovery, structures, and cardiovascular
functions. Microsc Res Tech. 57:3–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Andreis PG, Mazzocchi G, Rebuffat P and
Nussdorfer GG: Effects of adrenomedullin and proadrenomedullin
N-terminal 20 peptide on rat zona glomerulosa cells. Life Sci.
60:1693–1697. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
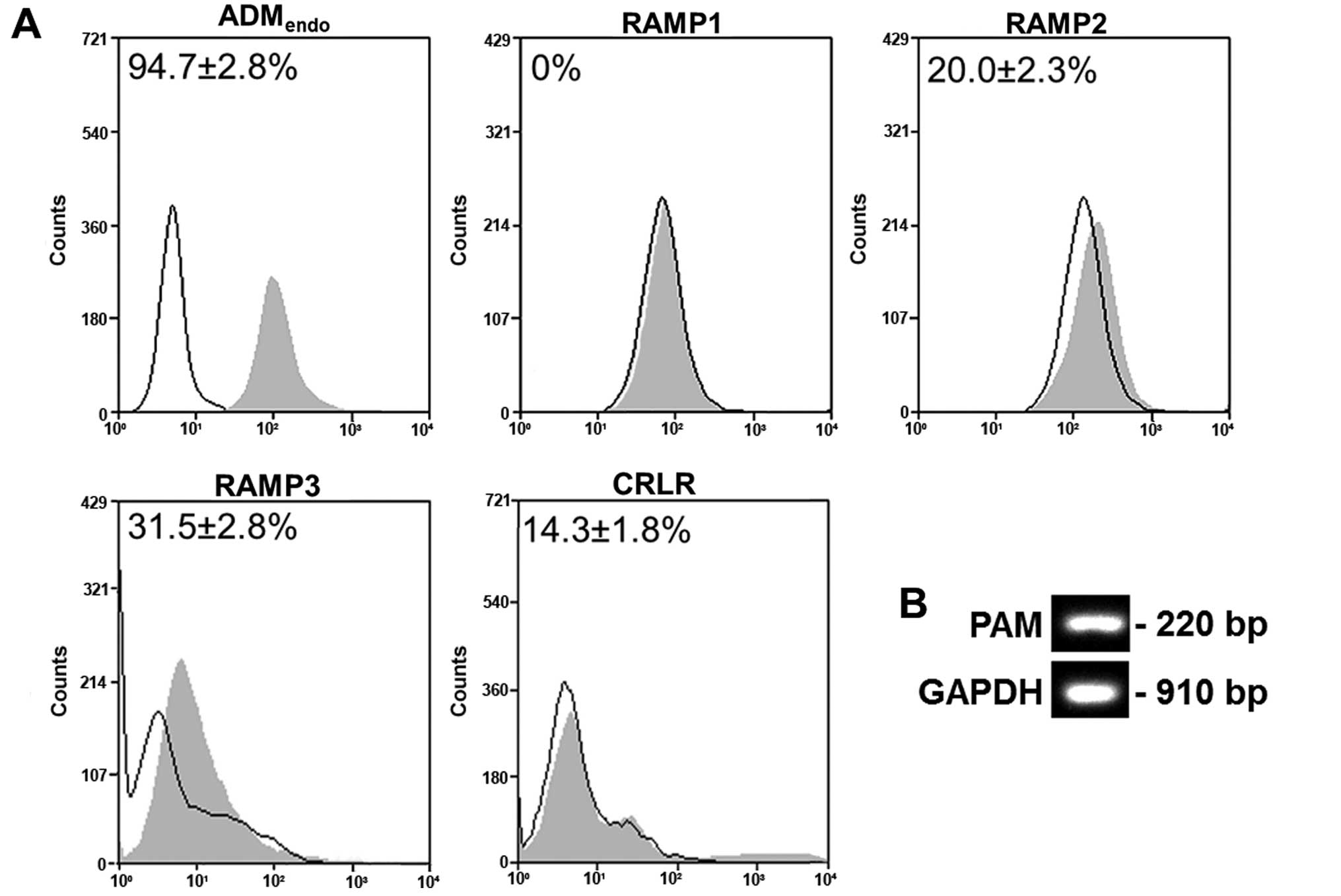
Dickerson IM: Role of CGRP-receptor
component protein (RCP) in CLR/RAMP function. Curr Protein Pept
Sci. 14:407–415. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shindo T, Sakurai T, Kamiyoshi A,
Ichikawa-Shindo Y, Shimoyama N, Iinuma N, Arai T and Miyagawa S:
Regulation of adrenomedullin and its family peptide by RAMP system:
lessons from genetically engineered mice. Curr Protein Pept Sci.
14:347–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tixier E, Leconte C, Touzani O, Roussel S,
Petit E and Bernaudin M: Adrenomedullin protects neurons against
oxygen glucose deprivation stress in an autocrine and paracrine
manner. J Neurochem. 106:1388–1403. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
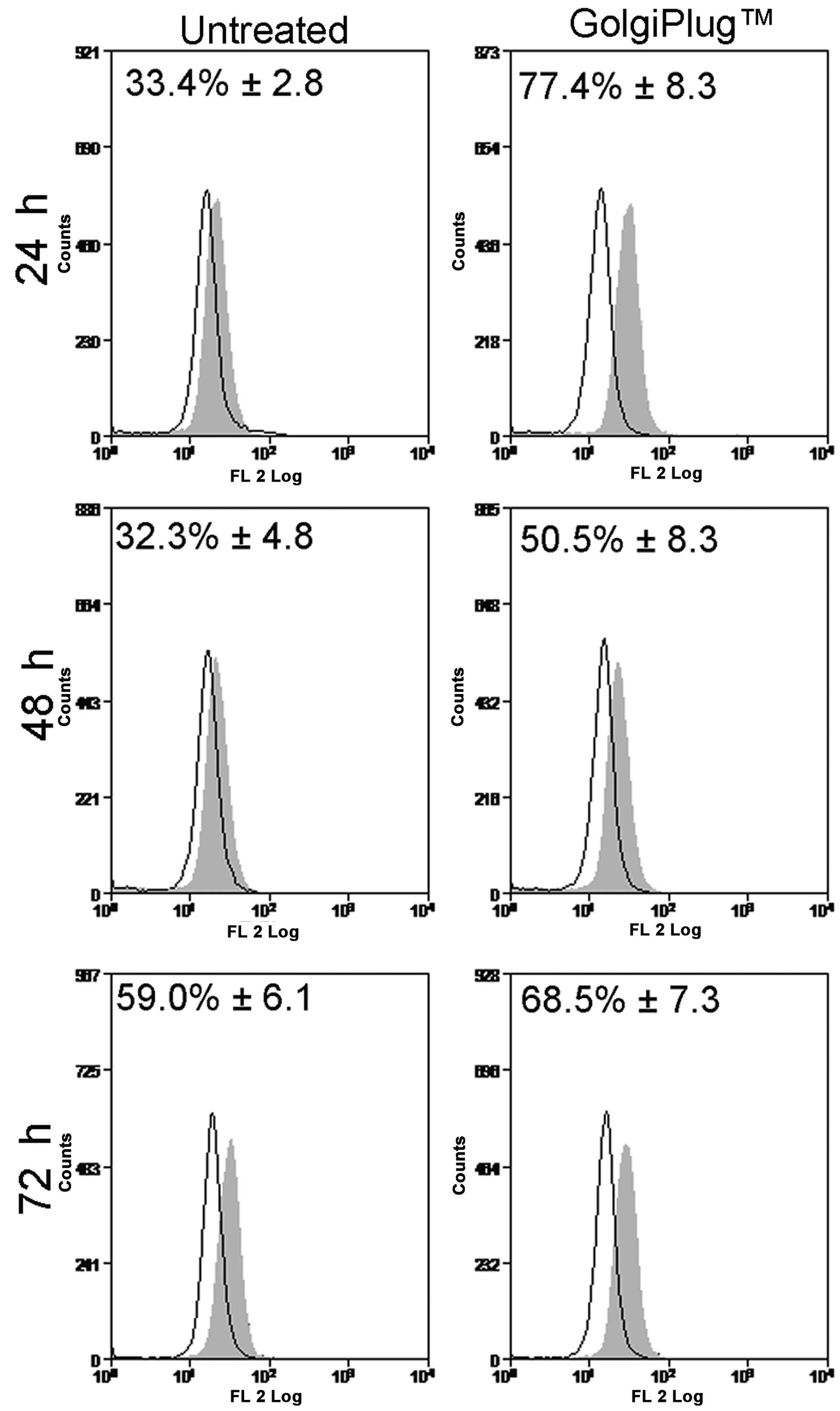
|
Shichiri M and Hirata Y: Regulation of
cell growth and apoptosis by adrenomedullin. Hypertens Res.
26(Suppl): S9–S14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Larráyoz IM, Martínez-Herrero S,
García-Sanmartín J, Ochoa-Callejero L and Martínez A:
Adrenomedullin and tumour microenvironment. J Transl Med.
12:3392014. View Article : Google Scholar : PubMed/NCBI
|
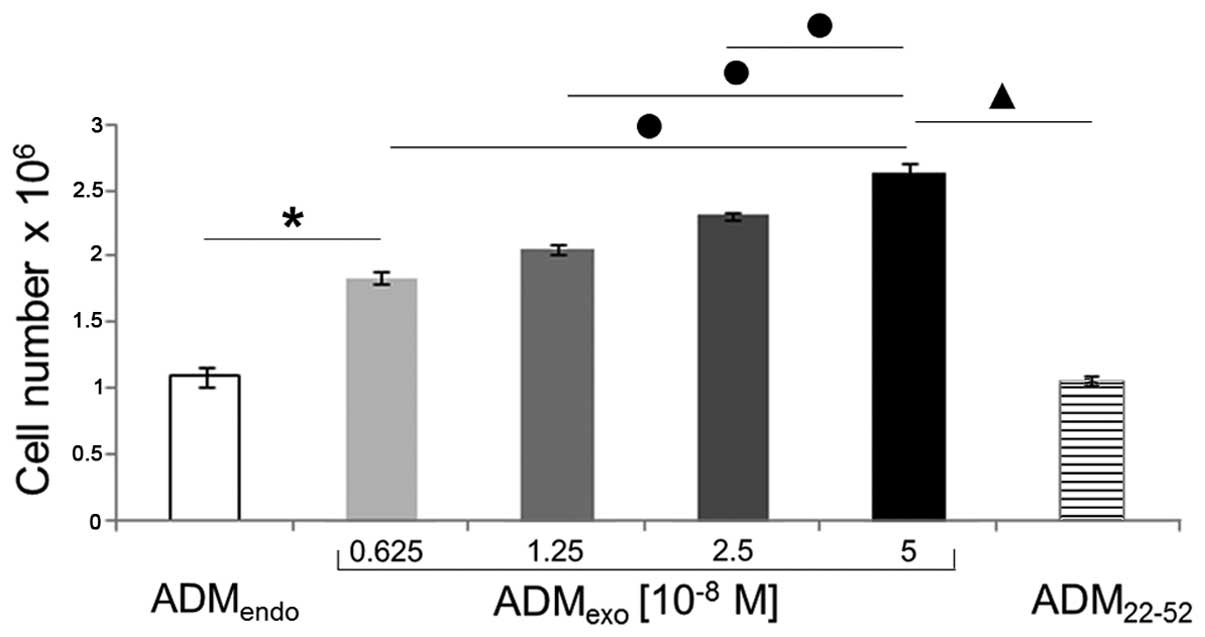
|
11
|
Berenguer-Daizé C, Boudouresque F, Bastide
C, Tounsi A, Benyahia Z, Acunzo J, Dussault N, Delfino C, Baeza N,
Daniel L, et al: Adrenomedullin blockade suppresses growth of human
hormone-independent prostate tumor xenograft in mice. Clin Cancer
Res. 19:6138–6150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nikitenko LL, Fox SB, Kehoe S, Rees MC and
Bicknell R: Adrenomedullin and tumour angiogenesis. Br J Cancer.
94:1–7. 2006. View Article : Google Scholar
|
|
13
|
Rullé S, Ah Kioon MD, Asensio C, Mussard
J, Ea HK, Boissier MC, Lioté F and Falgarone G: Adrenomedullin, a
neuropeptide with immunoregulatory properties induces semi-mature
tolerogenic dendritic cells. Immunology. 136:252–264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hojo Y, Ikeda U, Ohya K, Ichida M, Kario
K, Takahashi M, Ikeda M, Minota S, Isumi Y, Minamino N, et al:
Interaction between monocytes and vascular endothelial cells
induces adrenomedullin production. Atherosclerosis. 155:381–387.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kubo A, Minamino N, Isumi Y, Kangawa K,
Dohi K and Matsuo H: Adrenomedullin production is correlated with
differentiation in human leukemia cell lines and peripheral blood
monocytes. FEBS Lett. 426:233–237. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakayama M, Takahashi K, Murakami O,
Murakami H, Sasano H, Shirato K and Shibahara S: Adrenomedullin in
monocytes and macrophages: Possible involvement of
macrophage-derived adrenomedullin in atherogenesis. Clin Sci
(Lond). 97:247–251. 1999. View Article : Google Scholar
|
|
17
|
Del Pup L, Belloni AS, Carraro G, De
Angeli S, Parnigotto PP and Nussdorfer GG: Adrenomedullin is
expressed in cord blood hematopoietic cells and stimulates their
clonal growth. Int J Mol Med. 11:157–160. 2003.PubMed/NCBI
|
|
18
|
De Angeli S, Del Pup L, Febas E, Conconi
MT, Tommasini M, Di Liddo R, Albertin G, Parnigotto PP and
Nussdorfer GG: Adrenomedullin and endothelin-1 stimulate in vitro
expansion of cord blood hematopoietic stem cells. Int J Mol Med.
14:1083–1086. 2004.PubMed/NCBI
|
|
19
|
Baxter SS, Carlson LA, Mayer AM, Hall ML
and Fay MJ: Granulocytic differentiation of HL-60 promyelocytic
leukemia cells is associated with increased expression of Cul5. In
Vitro Cell Dev Biol Anim. 45:264–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ziolkowska A, Budzynska K, Trejter M,
Tortorella C, Belloni AS and Malendowicz LK: Effects of
adrenomedullin and its fragment 22–52 on basal and ACTH-stimulated
secretion of cultured rat adrenocortical cells. Int J Mol Med.
11:613–615. 2003.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Paietta E, Goloubeva O, Neuberg D, Bennett
JM, Gallagher R, Racevskis J, Dewald G, Wiernik PH and Tallman MS;
Eastern Cooperative Oncology Group. A surrogate marker profile for
PML/RAR alpha expressing acute promyelocytic leukemia and the
association of immunophenotypic markers with morphologic and
molecular subtypes. Cytometry B Clin Cytom. 59:1–9. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guglielmi C, Martelli MP, Diverio D, Fenu
S, Vegna ML, Cantù-Rajnoldi A, Biondi A, Cocito MG, Del Vecchio L,
Tabilio A, et al: Immunophenotype of adult and childhood acute
promyelocytic leukaemia: Correlation with morphology, type of PML
gene breakpoint and clinical outcome. A cooperative Italian study
on 196 cases. Br J Haematol. 102:1035–1041. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taetle R, Ostergaard H, Smedsrud M and
Trowbridge I: Regulation of CD45 expression in human leukemia
cells. Leukemia. 5:309–314. 1991.PubMed/NCBI
|
|
25
|
Carrigan SO, Weppler AL, Issekutz AC and
Stadnyk AW: Neutrophil differentiated HL-60 cells model Mac-1
(CD11b/CD18)-independent neutrophil transepithelial migration.
Immunology. 115:108–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim KH, Seoh JY and Cho SJ: Phenotypic and
functional analysis of HL-60 cells used in opsonophagocytic-killing
assay for Streptococcus pneumoniae. J Korean Med Sci. 30:145–150.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Veselská R, Zitterbart K, Auer J and
Neradil J: Differentiation of HL-60 myeloid leukemia cells induced
by all-trans retinoic acid is enhanced in combination with caffeic
acid. Int J Mol Med. 14:305–310. 2004.PubMed/NCBI
|
|
28
|
Newman PJ, Berndt MC, Gorski J, White GC
II, Lyman S, Paddock C and Muller WA: PECAM-1 (CD31) cloning and
relation to adhesion molecules of the immunoglobulin gene
superfamily. Science. 247:1219–1222. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brouwer RE, Hoefnagel J, Borger van Der
Burg B, Jedema I, Zwinderman KH, Starrenburg IC, Kluin-Nelemans HC,
Barge RM, Willemze R and Falkenburg JH: Expression of
co-stimulatory and adhesion molecules and chemokine or apoptosis
receptors on acute myeloid leukaemia: High CD40 and CD11a
expression correlates with poor prognosis. Br J Haematol.
115:298–308. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Howard M, Grimaldi JC, Bazan JF, Lund FE,
Santos-Argumedo L, Parkhouse RM, Walseth TF and Lee HC: Formation
and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen
CD38. Science. 262:1056–1059. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muller WA, Weigl SA, Deng X and Phillips
DM: PECAM-1 is required for transendothelial migration of
leukocytes. J Exp Med. 178:449–460. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deaglio S, Morra M, Mallone R, Ausiello
CM, Prager E, Garbarino G, Dianzani U, Stockinger H and Malavasi F:
Human CD38 (ADP-ribosyl cyclase) is a counter-receptor of CD31, an
Ig superfamily member. J Immunol. 160:395–402. 1998.PubMed/NCBI
|
|
33
|
Dianzani U, Funaro A, DiFranco D,
Garbarino G, Bragardo M, Redoglia V, Buonfiglio D, De Monte LB,
Pileri A and Malavasi F: Interaction between endothelium and
CD4+CD45RA+ lymphocytes. Role of the human
CD38 molecule. J Immunol. 153:952–959. 1994.PubMed/NCBI
|
|
34
|
Rocchi P, Boudouresque F, Zamora AJ,
Muracciole X, Lechevallier E, Martin PM and Ouafik L: Expression of
adrenomedullin and peptide amidation activity in human prostate
cancer and in human prostate cancer cell lines. Cancer Res.
61:1196–1206. 2001.PubMed/NCBI
|
|
35
|
Zudaire E, Martínez A, Garayoa M, Pío R,
Kaur G, Woolhiser MR, Metcalfe DD, Hook WA, Siraganian RP, Guise
TA, et al: Adrenomedullin is a cross-talk molecule that regulates
tumor and mast cell function during human carcinogenesis. Am J
Pathol. 168:280–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miyashita K, Itoh H, Sawada N, Fukunaga Y,
Sone M, Yamahara K, Yurugi T and Nakao K: Adrenomedullin promotes
proliferation and migration of cultured endothelial cells.
Hypertens Res. 26(Suppl): S93–S98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Belloni AS, Trejter M, Malendowicz LK and
Nussdorfer GG: Adrenomedullin stimulates proliferation and inhibits
apoptosis of immature rat thymocytes cultured in vitro. Peptides.
24:295–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Iwasaki H, Eguchi S, Shichiri M, Marumo F
and Hirata Y: Adrenomedullin as a novel growth-promoting factor for
cultured vascular smooth muscle cells: Role of tyrosine
kinase-mediated mitogen-activated protein kinase activation.
Endocrinology. 139:3432–3441. 1998.PubMed/NCBI
|
|
39
|
Andreis PG, Markowska A, Champion HC,
Mazzocchi G, Malendowicz LK and Nussdorfer GG: Adrenomedullin
enhances cell proliferation and deoxyribonucleic acid synthesis in
rat adrenal zona glomerulosa: Receptor subtype involved and
signaling mechanism. Endocrinology. 141:2098–2104. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ikeda U, Kanbe T, Kawahara Y, Yokoyama M
and Shimada K: Adrenomedullin augments inducible nitric oxide
synthase expression in cytokine-stimulated cardiac myocytes.
Circulation. 94:2560–2565. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jacob A, Wu R and Wang P: Regulation of
RAMP expression in diseases. Adv Exp Med Biol. 744:87–103. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Poyner DR, Sexton PM, Marshall I, Smith
DM, Quirion R, Born W, Muff R, Fischer JA and Foord SM:
International Union of Pharmacology. XXXII. The mammalian
calcitonin gene-related peptides, adrenomedullin, amylin, and
calcitonin receptors. Pharmacol Rev. 54:233–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gibbons C, Dackor R, Dunworth W, Fritz-Six
K and Caron KM: Receptor activity-modifying proteins: RAMPing up
adrenomedullin signaling. Mol Endocrinol. 21:783–796. 2007.
View Article : Google Scholar
|
|
44
|
McLatchie LM, Fraser NJ, Main MJ, Wise A,
Brown J, Thompson N, Solari R, Lee MG and Foord SM: RAMPs regulate
the transport and ligand specificity of the
calcitonin-receptor-like receptor. Nature. 393:333–339. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Steelman LS, Pohnert SC, Shelton JG,
Franklin RA, Bertrand FE and McCubrey JA: JAK/STAT, Raf/MEK/ERK,
PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis.
Leukemia. 18:189–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
47
|
Hsu J, Shi Y, Krajewski S, Renner S,
Fisher M, Reed JC, Franke TF and Lichtenstein A: The AKT kinase is
activated in multiple myeloma tumor cells. Blood. 98:2853–2855.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakatani K, Thompson DA, Barthel A, Sakaue
H, Liu W, Weigel RJ and Roth RA: Up-regulation of Akt3 in estrogen
receptor-deficient breast cancers and androgen-independent prostate
cancer lines. J Biol Chem. 274:21528–21532. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hayashi H, Tsuchiya Y, Nakayama K, Satoh T
and Nishida E: Down-regulation of the PI3-kinase/Akt pathway by ERK
MAP kinase in growth factor signaling. Genes Cells. 13:941–947.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kohroki J, Nishiyama T, Nakamura T and
Masuho Y: ASB proteins interact with Cullin5 and Rbx2 to form E3
ubiquitin ligase complexes. FEBS Lett. 579:6796–6802. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kile BT, Schulman BA, Alexander WS, Nicola
NA, Martin HM and Hilton DJ: The SOCS box: A tale of destruction
and degradation. Trends Biochem Sci. 27:235–241. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fay MJ, Longo KA, Ka rathanasis GA, Shope
DM, Mandernach CJ, Leong JR, Hicks A, Pherson K and Husain A:
Analysis of CUL-5 expression in breast epithelial cells, breast
cancer cell lines, normal tissues and tumor tissues. Mol Cancer.
2:402003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kalla C, Scheuermann MO, Kube I, Schlotter
M, Mertens D, Döhner H, Stilgenbauer S and Lichter P: Analysis of
11q22-q23 deletion target genes in B-cell chronic lymphocytic
leukaemia: Evidence for a pathogenic role of NPAT, CUL5, and
PPP2R1B. Eur J Cancer. 43:1328–1335. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Abdullah M and Seman Z: Role of signaling
pathways in acute myeloid leukemia. Myeloid Leukemia-Basic
Mechanisms of Leukemogenesis. Koschmieder S and Krug U: InTech;
Rijeka: pp. 429–448. 2011
|
|
56
|
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR,
Pfeffer LM and Donner DB: NF-kappaB activation by tumour necrosis
factor requires the Akt serine-threonine kinase. Nature. 401:82–85.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fukumoto S, Hsieh CM, Maemura K, Layne MD,
Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, et al:
Akt participation in the Wnt signaling pathway through Dishevelled.
J Biol Chem. 276:17479–17483. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Sykes SM, Lane SW, Bullinger L,
Kalaitzidis D, Yusuf R, Saez B, Ferraro F, Mercier F, Singh H,
Brumme KM, et al: AKT/FOXO signaling enforces reversible
differentiation blockade in myeloid leukemias. Cell. 146:697–708.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Marcinkowska E, Garay E, Gocek E, Chrobak
A, Wang X and Studzinski GP: Regulation of C/EBPbeta isoforms by
MAPK pathways in HL60 cells induced to differentiate by
1,25-dihy-droxyvitamin D3. Exp Cell Res. 312:2054–2065. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Trayner ID, Bustorff T, Etches AE, Mufti
GJ, Foss Y and Farzaneh F: Changes in antigen expression on
differentiating HL60 cells treated with dimethylsulphoxide,
all-trans retinoic acid, alpha1,25-dihydroxyvitamin D3
or 12-O-tetradecanoyl phorbol-13-acetate. Leuk Res. 22:537–547.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bellón T, López-Rodríguez C, Rubio MA,
Jochems G, Bernabeu C and Corbi AL: Regulated expression of p150,95
(CD11c/CD18; αX/β2) and VLA-4 (CD49d/CD29; α4/β1) integrins during
myeloid cell differentiation. Eur J Immunol. 24:41–47. 1994.
View Article : Google Scholar
|
|
62
|
Park DJ, Chumakov AM, Vuong PT, Chih DY,
Gombart AF, Miller WH Jr and Koeffler HP: CCAAT/enhancer binding
protein epsilon is a potential retinoid target gene in acute
promyelocytic leukemia treatment. J Clin Invest. 103:1399–1408.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
van Lochem EG, van der Velden VH, Wind HK,
te Marvelde JG, Westerdaal NA and van Dongen JJ: Immunophenotypic
differentiation patterns of normal hematopoiesis in human bone
marrow: Reference patterns for age-related changes and
disease-induced shifts. Cytometry B Clin Cytom. 60:1–13. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lewandowski D, Linassier C, Iochmann S,
Degenne M, Domenech J, Colombat P, Binet C and Hérault O:
Phosphatidylinositol 3-kinases are involved in the all-trans
retinoic acid-induced upregulation of CD38 antigen on human
haematopoietic cells. Br J Haematol. 118:535–544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hansen PB, Kjaersgaard E, Johnsen HE, Gram
J, Pedersen M, Nikolajsen K and Hansen NE: Different membrane
expression of CD11b and CD14 on blood neutrophils following in vivo
administration of myeloid growth factors. Br J Haematol. 85:50–56.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Gallay N, Anani L, Lopez A, Colombat P,
Binet C, Domenech J, Weksler BB, Malavasi F and Herault O: The role
of platelet/endothelial cell adhesion molecule 1 (CD31) and CD38
antigens in marrow microenvironmental retention of acute
myelogenous leukemia cells. Cancer Res. 67:8624–8632. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lemischka IR: Microenvironmental
regulation of hematopoietic stem cells. Stem Cells. 15(Suppl 1):
63–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chiarini F, Lonetti A, Evangelisti C,
Buontempo F, Orsini E, Evangelisti C, Cappellini A, Neri LM,
McCubrey JA and Martelli AM: Advances in understanding the acute
lymphoblastic leukemia bone marrow microenvironment: From biology
to therapeutic targeting. Biochim Biophys Acta. ppi:
S0167-4889(15)00293-1. 2015 View Article : Google Scholar
|
|
69
|
Bakondi B, Shimada IS, Perry A, Munoz JR,
Ylostalo J, Howard AB, Gregory CA and Spees JL: CD133 identifies a
human bone marrow stem/progenitor cell sub-population with a
repertoire of secreted factors that protect against stroke. Mol
Ther. 17:1938–1947. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chute JP, Muramoto GG, Dressman HK, Wolfe
G, Chao NJ and Lin S: Molecular profile and partial functional
analysis of novel endothelial cell-derived growth factors that
regulate hematopoiesis. Stem Cells. 24:1315–1327. 2006. View Article : Google Scholar
|
|
71
|
Williams CA and Lavik EB: Engineering the
CNS stem cell microenvironment. Regen Med. 4:865–877. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kocemba KA, van Andel H, de Haan-Kramer A,
Mahtouk K, Versteeg R, Kersten MJ, Spaargaren M and Pals ST: The
hypoxia target adrenomedullin is aberrantly expressed in multiple
myeloma and promotes angiogenesis. Leukemia. 27:1729–1737. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Takahashi K, Morimoto R, Hirose T, Satoh F
and Totsune K: Adrenomedullin 2/intermedin in the
hypothalamopituitary-adrenal axis. J Mol Neurosci. 43:182–192.
2011. View Article : Google Scholar
|
|
74
|
Tsuruda T, Kato J, Kitamura K, Kuwasako K,
Imamura T, Koiwaya Y, Tsuji T, Kangawa K and Eto T: Adrenomedullin:
A possible autocrine or paracrine inhibitor of hypertrophy of
cardiomyocytes. Hypertension. 31:505–510. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shichiri M, Fukai N, Ozawa N, Iwasaki H
and Hirata Y: Adrenomedullin is an autocrine/paracrine growth
factor for rat vascular smooth muscle cells. Regul Pept.
112:167–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lah JJ and Frishman WH: Adrenomedullin: A
vasoactive and natriuretic peptide with therapeutic potential.
Heart Dis. 2:259–265. 2000.
|
|
77
|
Mazzocchi G, Albertin G and Nussdorfer GG:
Adrenomedullin (ADM), acting through ADM(22-52)-sensitive
receptors, is involved in the endotoxin-induced hypotension in
rats. Life Sci. 66:1445–1450. 2000. View Article : Google Scholar
|
|
78
|
Albertin G, Carraro G, Parnigotto PP,
Conconi MT, Ziolkowska A, Malendowicz LK and Nussdorfer GG: Human
skin keratinocytes and fibroblasts express adrenomedullin and its
receptors, and adrenomedullin enhances their growth in vitro by
stimulating proliferation and inhibiting apoptosis. Int J Mol Med.
11:635–639. 2003.PubMed/NCBI
|
|
79
|
Rebuffat P, Macchi C, Malendowicz LK and
Nussdorfer GG: Up-regulation of adrenomedullin gene expression in
the regenerating rat adrenal cortex. Int J Mol Med. 20:551–555.
2007.PubMed/NCBI
|