Introduction
Stress responses and related psychosocial factors
(e.g., hopelessness and social support) can be of significant
prognostic value for cancer progression (1). Stress is defined as ‘the state of
threatened or perceived as threatened homeostasis, associated with
activation of the stress system, mainly comprised by the
hypothalamic-pituitary-adrenal axis and the arousal/sympathetic
nervous systems’ (2). Widely
accepted as being a complex construct, stress, is divided into
several stages: i) the stressors which are the event potentially
leading to the psycho-physiological features associated with
stress, ii) the mediators which can involve the appraisal of, as
well as coping with, the stressor, iii) the moderators where this
can include the social support being available as well as the
personality and resource of the individual, and finally, iv) the
stress response itself which includes activation of the
hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic
nervous system (3–6). The physiological response to stress
usually involves activation of the HPA axis above the basal level,
resulting in increased synthesis and secretion of glucocorticoids
(GC) (such as cortisol) as well as activation of the sympathetic
nervous system resulting in the secretion of catecholamines (such
as adrenalin) (7). Concerning
cancer prognosis, certain psychosocial factors such as little
social support and hopelessness were found in many prospective
studies and reviews to predict prognosis in cancer, independent of
confounders such as cancer stage and treatments (1,8,9).
Such responses are thought to partly affect cancer progression via
the effects of glucocorticoids on cancer cells. Several studies
have found that cortisol administration leads to greater cell
invasiveness in some cancer cell lines (10).
Cortisol exerts its actions by binding to and
activating glucocorticoid receptors (11,12).
The glucocorticoid receptor (GR) is a nuclear receptor that acts as
a phosphoprotein and a transcription factor modulating
transcriptional events. To date, the cloning of four splice
variants of the GR gene have been reported: GRα, GRβ, GRγ and GR-P
(13). Moreover, growth
arrest-specific transcript 5 (GAS5) encodes a single strand
non-coding RNA (ncRNA). GAS5 ncRNA can be a repressor for the GR by
acting as a decoy glucocorticoid response element (GRE), competing
with DNA GREs for binding to the GR. GAS5 ncRNA thus acts as a
negative regulator by preventing GRs from binding to their DNA GRE
and compromising the normal functions of the GR-cortisol complex
(14).
Abnormal regulation of the immune system by
stressors, whether suppression of anticancer cellular immunity or
enhancement of pro-inflammatory cytokines (15,16)
may result in significant adverse health consequences for tumour
proliferation and metastatic events (17). In addition, patients diagnosed with
cancer face psychosocial stressors that can lead to abnormal levels
of cortisol (18). Indeed,
nocturnal cortisol and cortisol variability show significant
dysregulation in ovarian cancer patients, and this dysregulation is
associated with greater functional disability, fatigue, and
vegetative depression (19). In
some other cancers, little cortisol variability was also found to
predict poor prognosis (20).
The neurohormone OT is involved in various aspects
of social cognition and prosocial behaviour such as trust (21,22).
Central OT exerts anxiolytic and anti-depressive effects by
activating its cognate receptor OTR which belongs to the GPCR
superfamily (23). Early studies
in a rodent model have shown that oxytocin decreases blood pressure
and lowers circulating cortisol levels (24). Intranasal OT benefits some aspects
of socio-emotional functioning (21). Furthermore, one study found that OT
also interacts synergistically with social support in relation to
cortisol secretion: during stress, people who received both OT and
social support had lower cortisol levels than those receiving
either treatment alone or no treatment (25). A number of studies proposed that OT
can act as a negative regulator of cell proliferation in human
breast carcinomas (26), human
central nervous system tumours (27), and human osteosarcoma cell lines
(28). Human endometrial
carcinomas also express OTR, and OT inhibits the proliferation of
endometrial cancer cells (29).
Intraperitoneal administration of OT resulted in the reduction of
intraperitoneal dissemination of ovarian carcinoma cells in a mouse
model (30). Thus, there is
regulatory cross-talk between OT and cortisol both at the
socio-behavioural and at the systemic levels, which may also have
consequences at the tumour level.
However, to this date, little is known about
potential cross-talk between cortisol and oxytocin in the context
of cancer in general and in ovarian cancer specifically. Because of
the often grave prognosis of ovarian cancer due to it being
diagnosed frequently at advanced stages (31), and since it is easy to administer
OT, it seemed important to examine the effects of OT and cortisol
on ovarian cancer cells first in vitro. Based on their
opposing effects, we hypothesised that the activity of cortisol in
ovarian cancer cells might be compromised if OTR signalling is
activated.
Materials and methods
Patients
Clinical samples were of ovarian origin (n=12) and
were taken from patients admitted to the First Department of
Obstetrics and Gynecology, Papageorgiou General Hospital, Medical
School, Aristotle University, Thessaloniki, Greece. Ethical
approval was obtained by the local authority and Brunel University.
The majority of ovarian cancers were deemed to be grade 3 stage III
(poorly differentiated and involving the whole peritoneal cavity,
not just confined to ovaries/tubes or pelvis) (10/12). Control
samples (n=10) were also used in this study, obtained from patients
undergoing total hysterectomy and bilateral salpingo-oopherectomy
for benign reasons. None of the two groups (ovarian cancer and
control) received hormone replacement therapy, and ovarian cancer
patients were all post-menopausal. Table I provides further information on
the stage, grade, age and CA125 status of ovarian cancer
patients.
 | Table IPatient details (age, stage, grade,
CA125) recruited for this study. |
Table I
Patient details (age, stage, grade,
CA125) recruited for this study.
| Histology | Grade | Stage | Age (years) | CA125 |
|---|
| Serous | 3 | IIIC | 64 | 474 |
| Serous | 3 | IIIC | 48 | 4,350 |
| Serous | 3 | IIIC | 61 | 858 |
| Serous | 2 | IIIC | 54 | 537 |
| Serous | 3 | IIIC | 69 | 534 |
| Serous | 3 | IV | 65 | UKN |
| Serous | 3 | IIIC | 75 | 242 |
| Serous | 3 | IIIC | 65 | 478 |
| Serous | 3 | IIIC | 56 | UKN |
| Serous | 3 | IIIC | 64 | 2,657 |
| Serous | 3 | IIIC | 64 | 339 |
| Serous | 2 | IIIC | 56 | 542 |
Cell culture
SKOV3, and MDAH-2774 ovarian cancer cell lines (from
American Type Culture Collection, USA), PEO1 (gift from Dr Helen
Coley, University of Surrey) were cultured in RPMI phenol red-free
complete media (Gibco) containing 10% fetal bovine serum (FBS), 5%
penicillin/streptomycin (P/S) solution (5,000 μg/ml) and 5% Gibco
100X non-essential amino acids (NEAA) at 37°C and 5%
CO2. For cell treatments, cells were seeded overnight
into 6-well plates with 2 ml complete media before media were
aspirated and wells washed with PBS solution. The cells were
incubated for 3 h in serum free media (phenol red-free media with
5% NEAA and 5% P/S, without FBS). The cells were then treated with
vehicle (NS), oxytocin (OT) and/or cortisol (C) to make a final
concentration of 100 nM. Cell concentration and viability values
were determined using a Countess™ automated cell counter based upon
the method of trypan blue exclusion (Invitrogen, Paisley,
Renfrewshire, UK), as previously described (32).
Wound healing assay
A solid line spanning the diameter of each well on a
6-well plate was drawn on the reverse side before cells were seeded
at equal density and treated as stated above. The ‘wound’ was
created using a 200-μl yellow pipette tip (Fisher) and scratching a
line through the cells which was perpendicular to the line drawn
along the well. Images of each wound at 0 hours (h), 6, 12 and 18 h
after treatment were inspected by the Olympus IX71 Microscope and
the images captured using the Photometrics Cool Snap™ CF camera.
Percentage migration of cells into the wound after 18 h was
calculated using the following formula: 1 − average width of wound
at 18 h/average width of wound at 0 h*100.
RNA isolation, cDNA synthesis and
quantitative RT-PCR
Total RNA was isolated using an RNA extraction kit
(Sigma-Aldrich, UK), according to the manufacturer's instructions.
RNA concentration was determined by spectrophotometric analysis
(NanoDrop; Thermo Scientific, UK) and agarose gel electrophoresis.
RNA (500 ng) was reverse-transcribed into cDNA using 5 IU/μl RNase
H reverse transcriptase (Invitrogen). Relative expression of the
genes of interest was assessed by quantitative PCR (Q-PCR) on an
ABI7400 instrument (Applied Biosystems) and xxpress®
(BJS Biotechnologies) using SYBR® Green-PCR reaction
mixture (Sigma-Aldrich) and the primers for GR, GAS5 as previously
described (33). For OTR the
primers used were: (sense) 5′-TTACAATCACTA GGATGGCTACAA-3′;
(anti-sense) 5′-CATTTACATTCCCAC CAACAATTTAA-3′. As a negative
control for all the reactions, distilled water was used in place of
the cDNA. RNAs were assayed from two to three independent
biological replicates. The RNA levels were expressed as a ‘relative
quantification’ using the housekeeping gene 18S RNA (RQ) value.
‘ΔCt method’ was employed for comparing relative expression results
between treatments in Q-PCR (34).
Protein extraction from SKOV3, PEO1 and
MDAH2774 cells
Ovarian cancer cells were cultured to 80%
confluence, and in the presence or absence of OT, cortisol,
cortisol and oxytocin (100 nM for 48 h). Cells were then lysed
using 200 μl 1X Laemmli buffer (Sigma-Aldrich) and denatured for 5
min at 100°C before they were cooled on ice.
Western immunoblotting
Samples were separated on an SDS-10% polyacrylamide
gel and the proteins were transferred to a nitrocellulose membrane.
The membrane was blocked in TBS containing 5% dried milk powder
(w/v) and 0.1% Tween-20, for 1 h at room temperature. After three
washes with TBS-0.1% Tween-20, the nitrocellulose membranes were
incubated with primary antibodies against caspase-3, Beclin-2 and
GAPDH (Cell Signaling Technology). All primary antisera were used
at a 1:1,000 dilution overnight at 4°C. The membranes were washed
thoroughly for 30 min with TBS-0.1% Tween, before incubation with
the secondary HRP-conjugated immunoglobulin (1:2,000) for 1 h at
room temperature and further washing for 30 min with TBS-0.1%
Tween-20. Antibody complexes were visualised as previously
described (33).
Ovarian tissue microarray
Unstained paraffin tissue micro-array slides
containing multiple ovarian carcinoma and normal tissue micro-array
(70 cases of ovarian carcinoma, 5 cases of tumour adjacent normal
ovary and 5 normal ovarian tissue from different biopsies; Biomax
USA) were used for this study.
The paraffin-embedded slides were deparaffinised and
rehydrated by a series of washes in reducing concentrations of
ethanol (100, 95, 70 and 50%) followed by rinsing in tap water for
10 min. Antigen retrieval was accomplished by incubating the slide
in sodium citrate (pH 6.0) for 20 min in a microwave. Slides were
washed in 0.4% of PBS-T for 5 min and then incubated for 15 min in
the PBS containing 0.3% H2O2 to stop the
interference of the endogenous peroxidase activity. Blocking was
carried out with 5% goat serum, followed by overnight incubation
with primary GRα antibody. The following day, after several washes
with PBS, slides were incubated with HRP conjugate-secondary
antibody for 60 min. Further washing in PBS-T was carried out for
20 min before performing staining. Slides were then subjected to
DAB staining, counterstained with haematoxylin and washed with 0.1%
sodium bicarbonate. The extent of staining was scored based on the
proportion of cells stained positive for GRα, as follows: 0, <5%
of cells; 1, 5–25% of cells; 2, 26–50% of cells; 3, 51–75%; and 4,
>75% of cells. Scoring was calculated from the mean of the two
independently conducted assessments.
GR luciferase reporter assay
SKOV-3 cells were incubated in 12-well plates with
McCoy's 5A medium supplemented with 1.5 mM L-glutamine and 2.2 g/l
sodium bicarbonate (Hyclone, Logan UT, USA (1 ml/well). The cells
were co-transfected with 1 μg of pGRE-Luc vector (a gift from Dr
John A. Cidlowski, NIEHS) and 0.5 μg of the pRL-TK vector (Renilla
luciferase, Promega, Madison, WI, USA) to correct for transfection
efficiency in transfection media containing OptiMed and
Lipofectamine 2000 (Invitrogen) as described previously (35). The transfection medium were
replaced after 6 h with fresh culture medium containing 100 nM
cortisol, 100 nM oxytocin, alone or in combination. The cells were
grown overnight until 90% confluent. Cell extracts were assayed
using a dual-luciferase reporter assay system (Promega) following
the manufacturer's instructions. Firefly and Renilla luciferase
activities were measured for 10 sec each, respectively, using a
CLARIOstar luminometer and the data were analyzed with Mars
software (BMG Labtechnologies Inc., Durham, NC, USA). The relative
luciferase activity level of each treatment (n=3) was expressed as
the ratio of firefly/Renilla luciferase activity values.
In silico analysis of gene expression
from microarray data
Oncomine (www.onocomine.org) is an online database consisting of
previously published patient microarray data available to the
public. We used this in silico dataset to compare the
expression of OTR in normal and ovarian cancer tissues.
Statistical analysis
Statistical analysis was performed by the Student's
t-test. A value of P<0.05 was regarded as statistically
significant. For the immunohistochemistry studies, a Student's
t-test where the assumptions of equal variances were not met, we
used Levine's test, which uses often non-integer degrees of
freedom. Q-PCR and western blot analysis data are reported as the
mean ± SEM.
Results
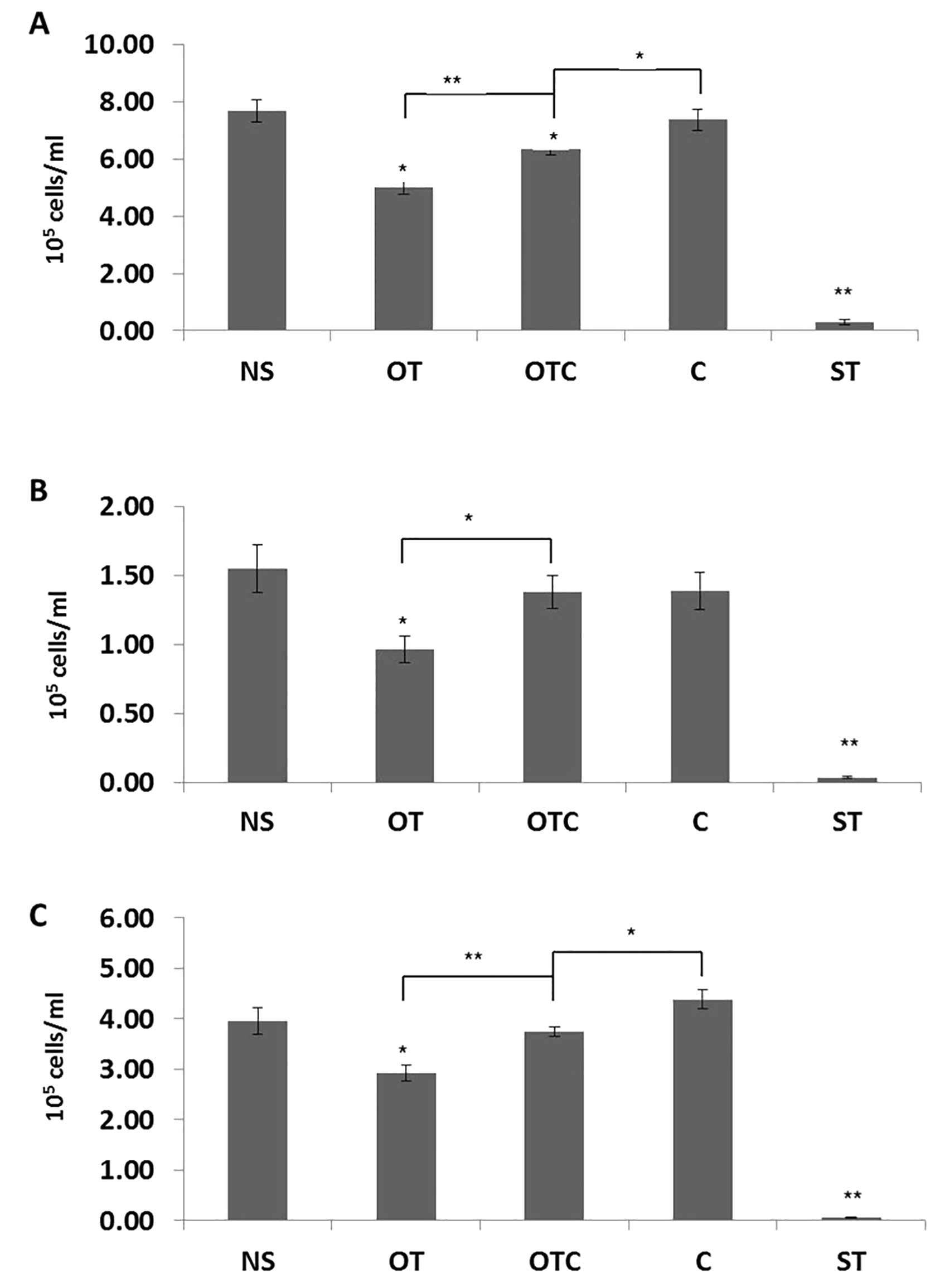
Cortisol inhibits the anti-proliferative
effects of OT in vitro
SKOV3, PEO1 and MDAH-2774 ovarian cancer cells were
treated for 48 h with oxytocin (OT), cortisol (C), and cortisol
plus oxytocin (C+OT) at 100 nM. This concentration was chosen in
accordance with previous studies that demonstrated that 100 nM
cortisol doses simulate stress conditions in vitro and this
resembles physiological levels of circulating steroid in
vivo (36). OT concentration
was also chosen at 100 nM as it is the concentration at which the
OTR was maximally activated in a number of in vitro studies
(30). Staurosporine (ST) at 100
nM was also used as an extra control agent for reduction of cell
proliferation (37). In SKOV3 and
MDAH-2774 cells, OT partially, but significantly, reversed the
proliferative effects of cortisol when compared to the effects of
cortisol alone (Fig. 1A and C). In
all three cell lines used, OT alone was able to significantly
reduce the proliferation of ovarian cancer cells (Fig. 1). The extent of the inhibition
varied, with OT having a more profound effect on PEO1 and SKOV3
cells.
Effects of cortisol and OT on cell
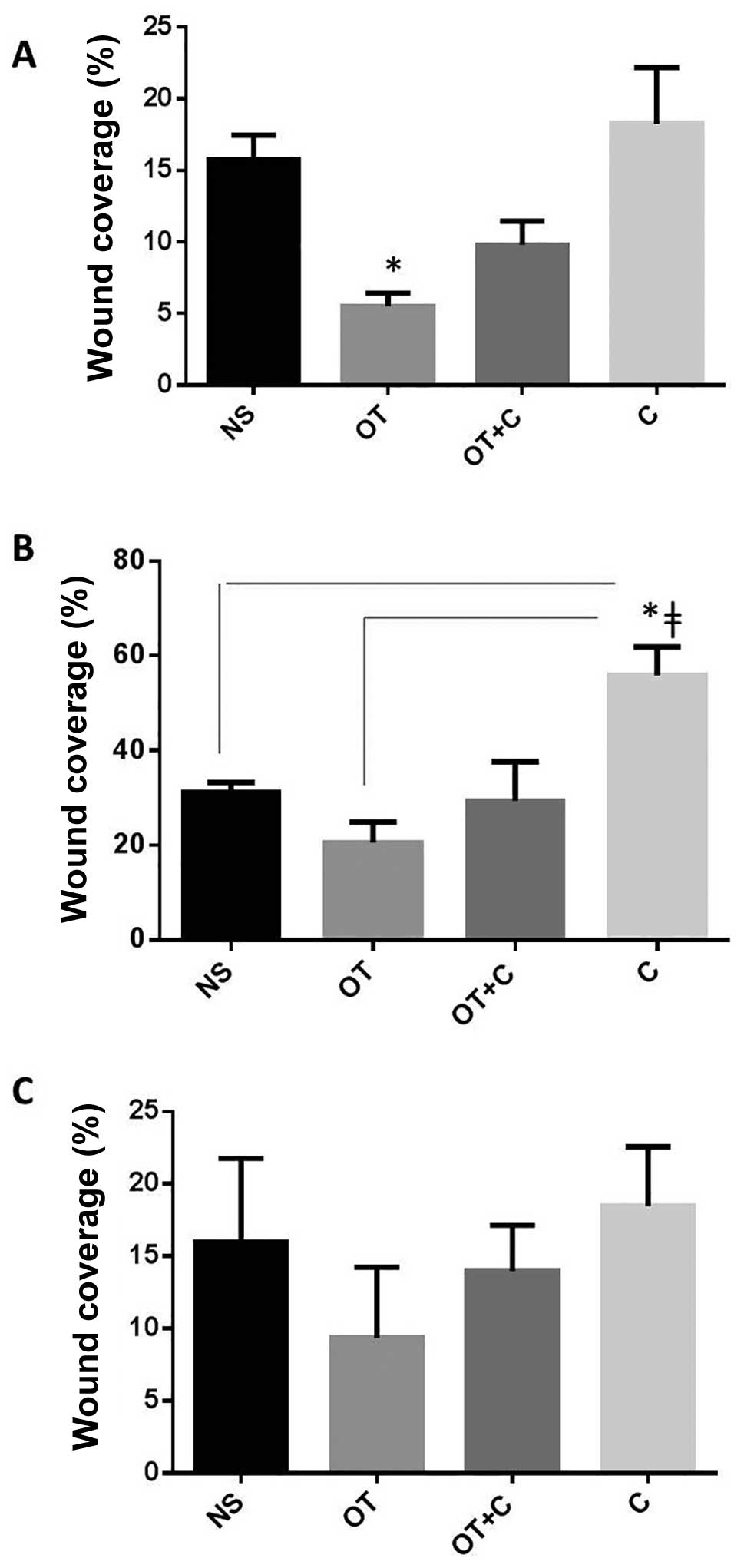
migration
We then assessed the effects of C and OT on cell
migration in scratch conditions. OT significantly reduced the
migratory ability of SKOV3 cells when compared to controls
(Fig. 2A), whereas in PEO1 cells,
C alone induced a significant cell migration compared to controls
and to OT treated cells (Fig. 2B).
In MDAH2774, although the differences did not reach statistical
significance, they followed a similar trend towards inhibition of
cell migration by OT and induction by C (Fig. 2C).
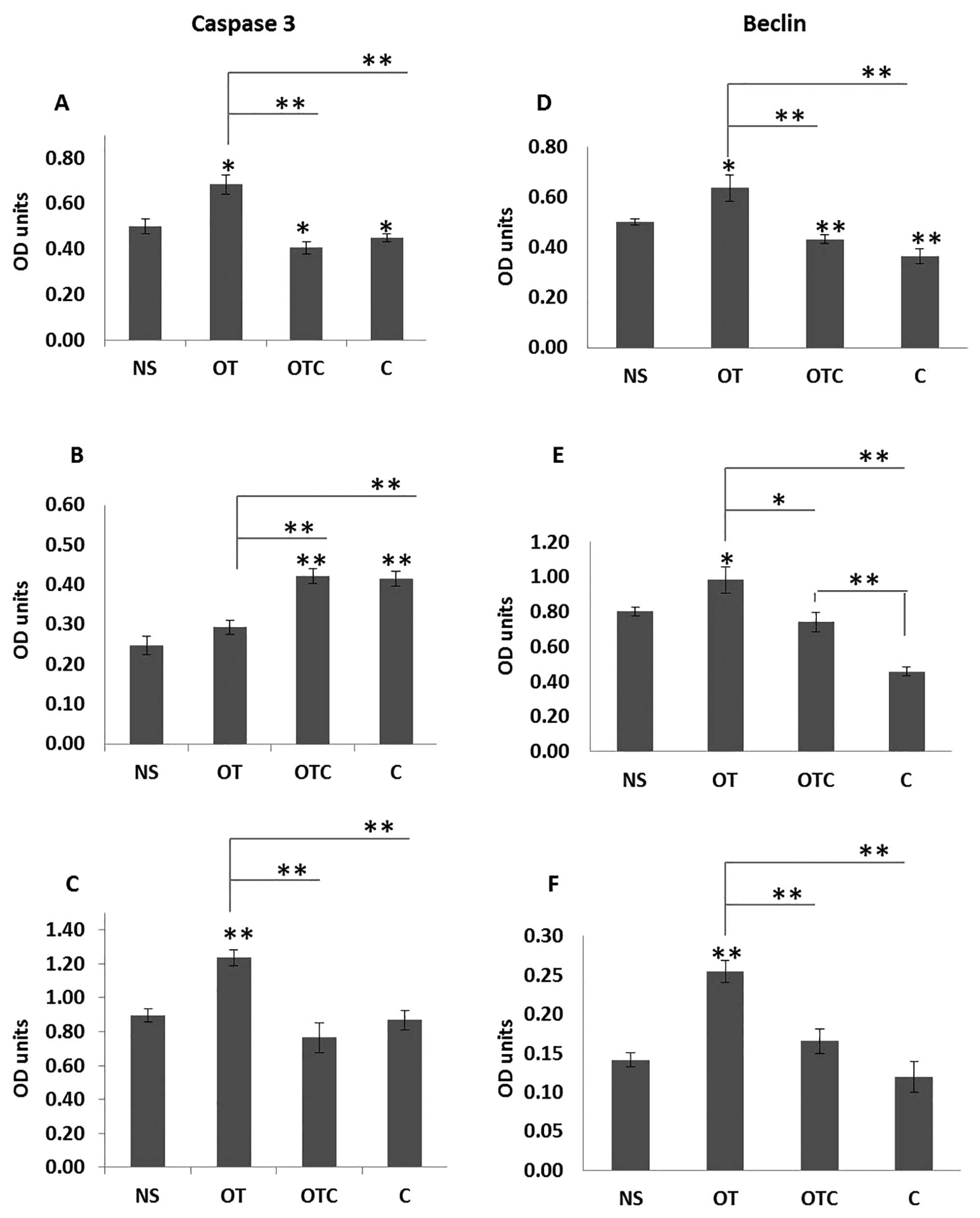
Effects of cortisol and OT on
apoptosis
To further understand the potential pro-apoptotic
mechanisms of OT in vitro, we measured the levels of
apoptosis-associated proteins in the presence or absence of
cortisol.
After exposure to OT±C and C alone for 48 h, cleaved
caspase-3 over total caspase-3 and Beclin-1 over GAPDH protein
levels were examined by western blotting. Quantitative analysis of
cleaved caspase-3 over total caspase-3 bands by scanning
densitometry revealed a significant increase in this ratio in OT
treated SKOV3 (56% P<0.01) and MDAH-2774 (76% P<0.01) cells
compared to cortisol alone (Fig. 3A
and C). Surprisingly, in PEO1 cells, cortisol treatment induced
more cleavage of caspase-3 when compared to OT (Fig. 3B). The effects of cortisol remained
unaltered in the presence of oxytocin in all three cell lines, thus
suggesting that there is no involvement of caspase-3 as a
cross-talk mechanism in these models between C and OT.
We next measured the expression of the
autophagy-related protein Beclin-1. Autophagy is a highly conserved
cellular process that is involved in several catabolic processes,
including degradation of long-lived proteins and organelles, and
cell death (38). Although
autophagy is initiated as a protective response to stress, the
constitutive activation of autophagy can lead to cell death by
excessive self-degradation of essential cellular components
(38,39). Beclin-1 protein levels were
significantly increased in all three OT-treated cell lines when
compared to cortisol alone or to cortisol and oxytocin (Fig. 3D–F). In all cell lines, OT appeared
to reverse the reduction of Beclin-1 that was due to cortisol,
since Beclin-1 levels were moderately elevated in SKOV3 and
MDAH-2774 cells in cortisol and OT treated samples when compared to
cortisol alone. Moreover, in PEO1, the induction of Beclin-1 was
significant in the same preparations (Fig. 3E), thus suggesting that the effects
of OT+C on cell proliferation described above may have been
mediated by a potential cross-talk with mechanisms regulating
autophagy and subsequently cell death.
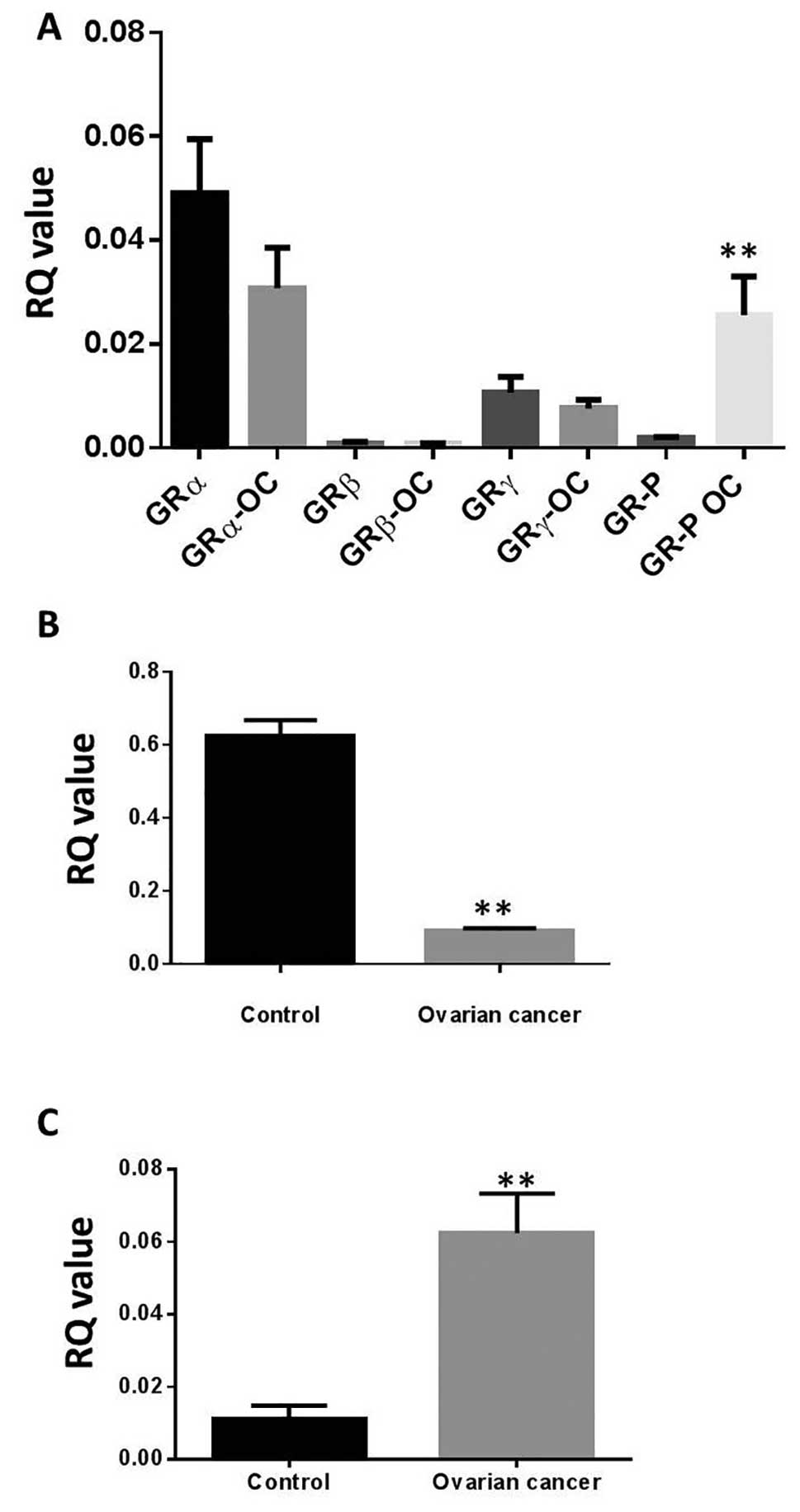
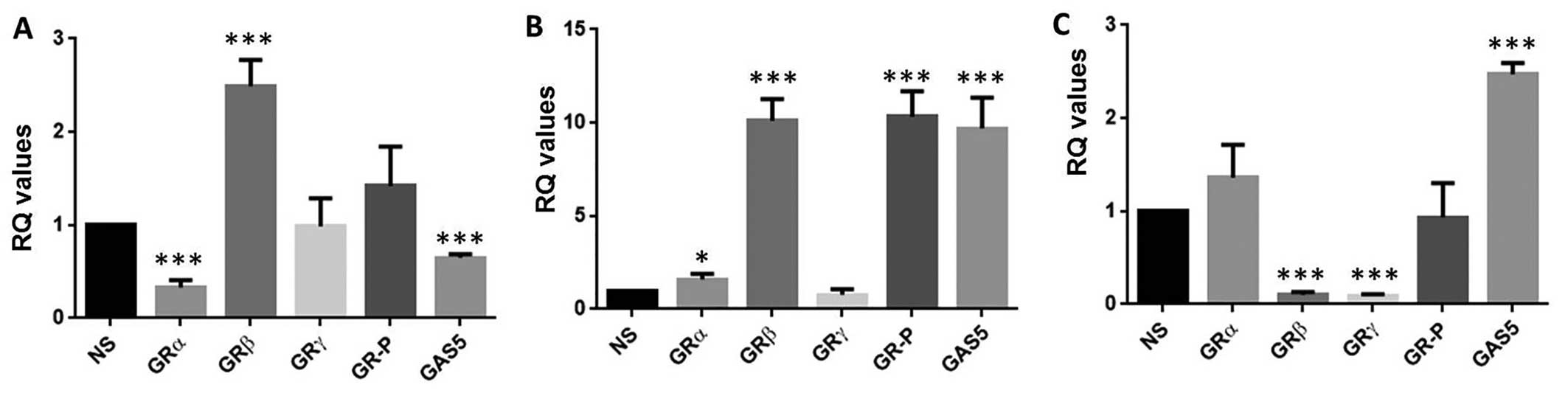
Expression of OTR, GRs and GAS5 in
ovarian cancer patients
Quantitative RT-PCR revealed that GAS5 and all GR
variants were expressed at the ovarian level (Fig. 4). No apparent differences in the
expression of GRα, GRβ, or GRγ between control (n=10) and ovarian
cancer (n=12) patients were found. However, GR-P was significantly
upregulated in ovarian cancer patients when compared to the control
group (Fig. 4A). Moreover, the
pseudo-GRE GAS5 was significantly downregulated in ovarian cancer
(Fig. 4B), whereas OTR was
significantly upregulated in the same cohort (Fig. 4C) when compared to controls. We
also analysed the expression of gene copy number of OTR, using
Oncomine datasets. OTR gene copy number showed an increased
expression in the Bonome dataset in ovarian carcinoma patients
(n=185) against normal ovarian surface epithelium (n=10; data not
shown).
We then expanded on these observations at protein
level using tissue microarray slides containing 70 samples of
ovarian cancer and 10 samples of normal tissue. Results are
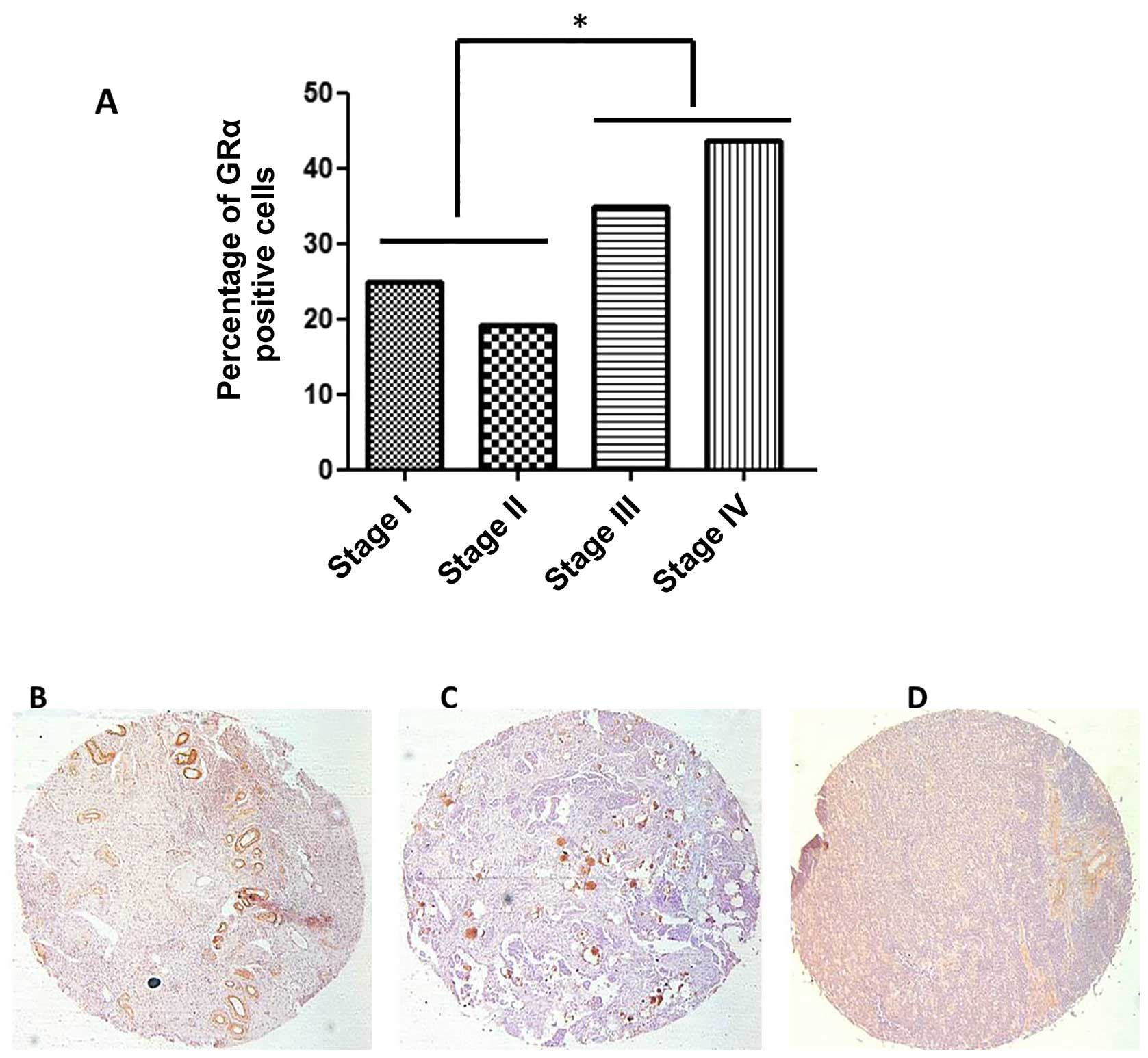
presented as a percentage of total cells positive for GRα
expression. The immunohistochemistry study shows 49% positive
staining for GRα in the normal ovaries (n=10) and 31% in malignant
tissues [n=70; χ2 (1) = 1.28; P>0.5]. Of the
malignant tissues, 31% of epithelial origin ovarian cancers were
stained positive for GRα and 26% of germ cell cancers were positive
for GRα.
In the sample 43.9% were at stage I, 21.1% stage II,
28.1 stage III and 7% stage IV. Because of the relatively small
sample and to increase statistical power, we grouped the patients
in early stages (I and II) versus late (III and IV; Fig. 5A). After log transforming the GRα
expression, the late stage patients had significantly higher
expression of GRα (1.2) than early stage patients [0.4;
t(54.3)=2.5; P=0.015]. There was no further correlation of GRα
expression with clinico-pathological features such as age and
histological type of tumour.
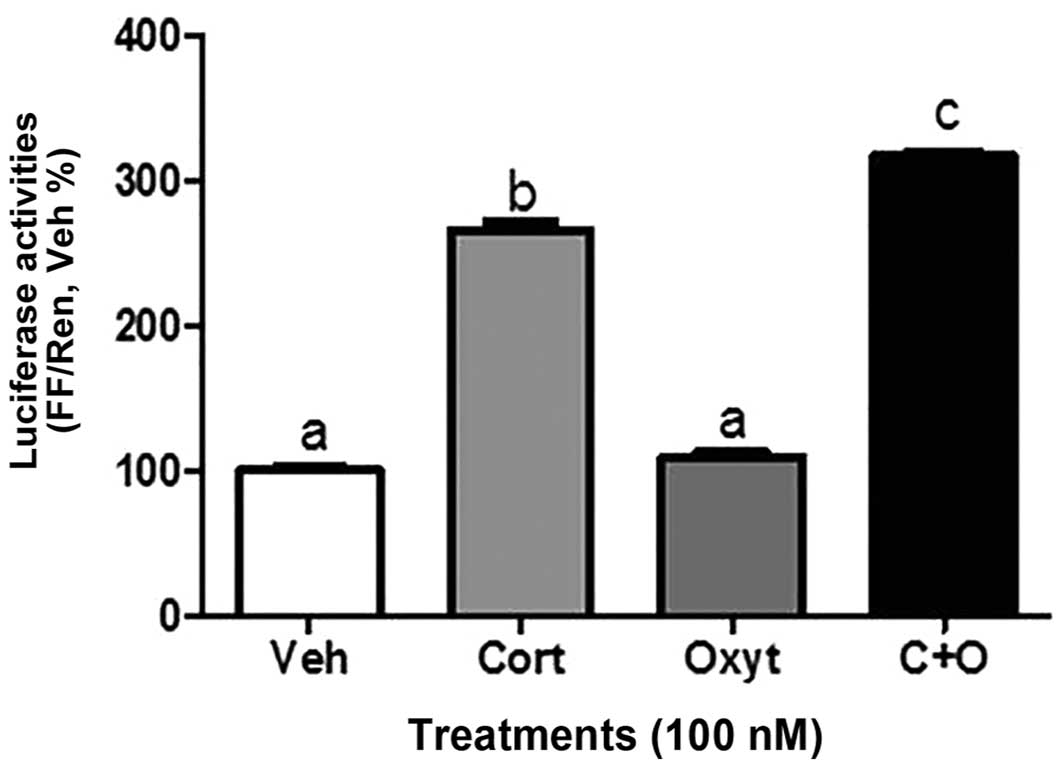
Transactivation of GR by OTR and effects
of OT and C on GR splicing in vitro
The effects of C and OT alone or in combination on
transactivation of human GR were assessed using a GRE-luciferase
reporter system in SKOV3 cells. Treatment of SKOV3 cells for 16 h
with 100 nM OT did not induce any changes in the luciferase
activity, whereas C alone exerted a significant increase.
Interestingly, when SKOV3 cells were treated with C+OT, the
increase in GRE activity was significantly higher compared to the
effects of C alone (Fig. 6). The
effect was not additive.
OT drives differential splicing of GR isoforms in a
cell-specific manner. In SKOV3 (Fig.
7A) and PEO1 (Fig. 7B) cells,
OT significantly induced GRβ expression, whereas only in PEO1, the
expression of GR-P was significantly augmented. With regards to
GAS5, it was induced in PEO1 (Fig.
7B) and MDAH-2774 cells (Fig.
7C).
Given the increase of OTR seen in the clinical
samples, and since this may result from the cancer-induced stress
responses as well, we tested the hypothesis that cortisol, a stress
hormone, might affect OTR expression directly. SKOV3, PEO1 and
MDAH-774 were treated for 48 h with cortisol, in an attempt to
resemble a sustained moderate stress environment in vitro.
When SKOV3, PEO1 and MDAH-774 cells were treated with cortisol (100
nM), the expression of OTR was significantly upregulated by 5-fold,
2-fold and in 81%, respectively, when compared to basal levels
(data not shown).
Discussion
In this study, we provide to the best of our
knowledge, for the first time, evidence for cross-talk between a
stress hormone (cortisol) and a ‘social’ hormone (OT) at the
molecular level in ovarian cancer cells. These findings have wider
implications especially for ovarian cancer patients who frequently
exhibit stress, depression, anxiety, and poorer overall quality of
life (QOL) (40,41). A major finding of this study is the
inhibitory role that oxytocin can exert over the cortisol effects
in tumour cells. We demonstrate that OT reversed the effects of
cortisol by inducing autophagy, as evident from the upregulation of
Beclin-1. Interestingly, there is a correlation of decreased
expression of Beclin-1 with poorer outcomes in patients with
ovarian carcinoma (42); and
between decreased expression of Beclin-1 with the development of
epithelial ovarian tumours (43).
This is of particular importance since synthetic glucocorticoids
can promote cell survival in epithelial tumours, including breast
and ovarian cancers. For example, cortisol can enhance the invasive
potential of SKOV3 ovarian cancer cells (10). Moreover, to prevent
chemotherapy-related side effects, dexamethasone is routinely
administered to patients with ovarian cancer. In a recent clinical
study, ovarian cancer patients receiving dexamethasone showed a
significant induction of pro-cancer cell survival genes, including
serum and glucocorticoid-regulated kinase 1 (SGK1) and map kinase
phosphatase 1 (MKP1)/dual specificity phosphatase 1 (DUSP1) at the
tumour site. It appears therefore that glucocorticoids (GCs) may
decrease chemotherapy effectiveness via induction of anti-apoptotic
gene expression (44). In another
study, dexamethasone not only induced therapy resistance of primary
ovarian carcinomas in vivo, it also led to a faster basal
growth of the xenografts (45).
Collectively, these data suggest that while the anti-emetic effects
by glucocorticoids may be of benefit to patients to tolerate the
side effects of the treatment, their counteracting of cytotoxic
treatments may minimize the treatment-induced growth retardation of
ovarian cancer.
Furthermore, in the cell lines SKOV3 and MDAH-2774
OT induced caspase-3, indicative of initiating apoptotic events. A
similar effect has been observed in rat neuronal cells (46). However, the effects of cortisol on
caspase-3 cleavage remained unaltered in the presence of oxytocin
in all three cell lines, implying that OT exerts a direct apoptotic
effect, involving a particular caspase, independent of cortisol.
Over the past decade, a substantial amount of data implicates OT in
proliferative and anti-proliferative effects in vitro, and
here we have shown that OT can exert a cytostatic/cytotoxic effect
in ovarian cancer cells. The OTR, is another ‘promiscuous’ GPCR in
terms of its capacity to induce multiple signalling pathways
involving PLC, cAMP, IP3, MAPK to name a few. Here we provide
evidence for a unique cross-talk between OTR and GR, since OT can
transactivate the GR gene when in the presence of cortisol. This
can have implications in GR splicing events. For example, OT can
compromise GR signalling by inducing multiple splicing isoforms
(GRβ and GRγ) that can act in a dominant negative manner, including
the induction of the pseudo-GRE GAS5.
It is possible therefore for OT to act in a cell- or
tissue-specific manner and exert a dual role as a result. In the
only in vivo study, intraperitoneal administration of OT
resulted in the reduction of intraperitoneal dissemination of
ovarian cancer cells followed by suppression of MMP2 and increases
in expression of E-cadherin (30).
Breastfeeding - a state where OT is markedly elevated for more than
one year, reduces the risk of developing ovarian cancer compared
with never breast-feeding (47),
and may also reduce endometrioid ovarian cancer risk to a greater
extent than other subtypes (48,49).
Our results are also in line with other studies showing
anti-proliferative effects of OT in several, but not all, cancer
cell types (26–28).
These data have wider implications in stress
management for cancer patients. The severe emotional distress
accompanying a diagnosis of cancer generally and ovarian cancer
specifically and its initial treatment has been extensively
documented (40). In ovarian
cancer, social support has been related to higher NK cytotoxicity
in PBMC and tumour-infiltrating lymphocytes (TIL), whereas distress
was related to lower NK cytotoxicity in TIL (50). Ovarian cancer patients suffering
from chronic stress, depression and low social support have
increased MMP-9 levels in tumour-associated macrophages (51), of importance for tumour invasion
and metastasis. Moreover, there is a substantial body of
longitudinal research relating initial social support to lower
morbidity and mortality from a variety of cancers (8). The results observed in the present
study provide important evidence for possible mechanisms linking
social support to better cancer prognosis since social support is
positively related to OT levels (52) and our results propose that OT may
minimise the anti-apoptotic effects or stress-related cortisol in
ovarian cancer.
The three ovarian cancer cell lines exhibit
differences in GR expression levels but similarities in the
response to OT (proliferation) and wound (mechanical scratch
assay). SKOV3 human ovarian clear cell adenocarcinoma cells were
derived from the ascites of a Caucasian 64-year-old female. PEO1 is
an adherent ovarian cancer cell line derived from a malignant
effusion from the peritoneal ascites of a patient with a poorly
differentiated serous adenocarcinoma. MDAH-2774 cells are of human
ovarian endometrioid adenocarcinoma origin, isolated from the
ascites of an untreated female patient. MDAH-2774 cells show a
trend towards triploidy, particularly trisomy of chromosomes 1, 2,
3, 6, 11, 12, 16 and X and monosomy of chromosomes 17 and 21. SKOV3
have mutations in TP53 and PIK3CA genes, whereas MDAH2774 have
mutations in TP53, PIK3CA, KRAS, BRCA1 (silent) and BRCA2 (silent)
(53). It should also be noted
that there is heterogeneity in cultured cells and the SKOV3 cell
line has been shown to contain cells with high and low invasive and
migratory potential (54). This
may provide an explanation of the variability seen in experiments
involving SKOV3, PEO1 and MDAH-2774 cell lines.
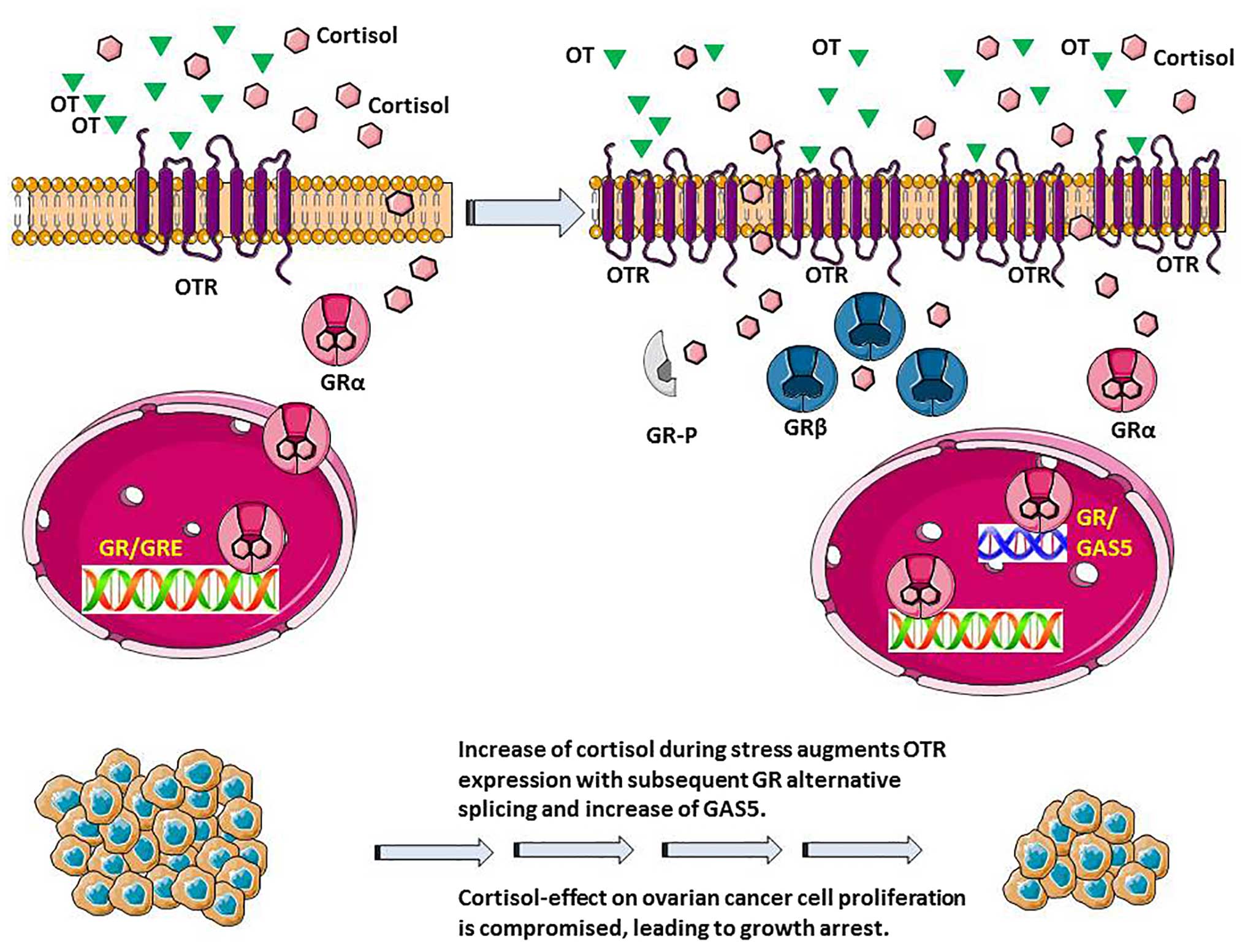
We have provided conclusive evidence that cortisol
can induce OTR expression at concentrations that mimic a stressful
milieu in vitro. This could be a compensatory defensive and
regulatory response. In our study, there was also an induction of
OTR in ovarian cancer patients compared to controls and, a slight
decrease in GRα. We propose that OT may exert a dual beneficial
effect: at the CNS level allowing patients to cope better with
stress by seeking social support and by possibly feeling less
lonely on one hand, and at the ovarian level by partially-reversing
the deleterious effects of glucocorticoids on tumour cell
viability, on the other; whilst retaining the useful anti-emetic
effects of glucocorticoids (Fig.
8). However, this study did not include direct evidence linking
these two levels in patients; including assessment of their
psychosocial profile. Future studies need to examine prospectively
the relationship between social support, systemic and in
situ levels of OT and cortisol, and prognosis in ovarian cancer
patients. This will enable to extend the observations found in this
study and then point at possible clinical implications.
References
|
1
|
Chida Y and Steptoe A: Positive
psychological well-being and mortality: A quantitative review of
prospective observational studies. Psychosom Med. 70:741–756. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pervanidou P and Chrousos GP: Metabolic
consequences of stress during childhood and adolescence.
Metabolism. 61:611–619. 2012. View Article : Google Scholar
|
|
3
|
Gidron Y and Ronson A: Psychosocial
factors, biological mediators, and cancer prognosis: A new look at
an old story. Curr Opin Oncol. 20:386–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thaker PH and Sood AK: Neuroendocrine
influences on cancer biology. Semin Cancer Biol. 18:164–170. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith SM and Vale WW: The role of the
hypothalamic-pituitary-adrenal axis in neuroendocrine responses to
stress. Dialogues Clin Neurosci. 8:383–395. 2006.
|
|
6
|
Kaptein A and Weinman J: Health
Psychology: Some Introductory Remarks. Malden: Blackwell
Publishing; 2004
|
|
7
|
Geronikolou SA, Chamakou A, Mantzou A,
Chrousos G and Kanaka-Gantenbein C: Frequent cellular phone use
modifies hypothalamic-pituitary-adrenal axis response to a cellular
phone call after mental stress in healthy children and adolescents:
A pilot study. Sci Total Environ. 536:182–188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nausheen B, Gidron Y, Peveler R and
Moss-Morris R: Social support and cancer progression: A systematic
review. J Psychosom Res. 67:403–415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watson M, Homewood J, Haviland J and Bliss
JM: Influence of psychological response on breast cancer survival:
10-year follow-up of a population-based cohort. Eur J Cancer.
41:1710–1714. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sood AK, Bhatty R, Kamat AA, Landen CN,
Han L, Thaker PH, Li Y, Gershenson DM, Lutgendorf S and Cole SW:
Stress hormone-mediated invasion of ovarian cancer cells. Clin
Cancer Res. 12:369–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rhen T and Cidlowski JA: Antiinflammatory
action of glucocorticoids - new mechanisms for old drugs. N Engl J
Med. 353:1711–1723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Bosscher K, Vanden Berghe W and
Haegeman G: The interplay between the glucocorticoid receptor and
nuclear factor-kappaB or activator protein-1: Molecular mechanisms
for gene repression. Endocr Rev. 24:488–522. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tissing WJE, Meijerink JPP, den Boer ML
and Pieters R: Molecular determinants of glucocorticoid sensitivity
and resistance in acute lymphoblastic leukemia. Leukemia. 17:17–25.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Non-coding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3:ra82010. View Article : Google Scholar
|
|
15
|
Inbar S, Neeman E, Avraham R, Benish M,
Rosenne E and Ben-Eliyahu S: Do stress responses promote leukemia
progression? An animal study suggesting a role for epinephrine and
prostaglandin-E2 through reduced NK activity. PLoS One.
6:e192462011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maes M, Song C, Lin A, De Jongh R, Van
Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, et
al: The effects of psychological stress on humans: Increased
production of pro-inflammatory cytokines and a Th1-like response in
stress-induced anxiety. Cytokine. 10:313–318. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Powell DJH, Liossi C, Moss-Morris R and
Schlotz W: Unstimulated cortisol secretory activity in everyday
life and its relationship with fatigue and chronic fatigue
syndrome: A systematic review and subset meta-analysis.
Psychoneuroendocrinology. 38:2405–2422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Volden PA and Conzen SD: The influence of
glucocorticoid signaling on tumor progression. Brain Behav Immun.
30(Suppl): S26–S31. 2013. View Article : Google Scholar
|
|
19
|
Weinrib AZ, Sephton SE, Degeest K, Penedo
F, Bender D, Zimmerman B, Kirschbaum C, Sood AK, Lubaroff DM and
Lutgendorf SK: Diurnal cortisol dysregulation, functional
disability, and depression in women with ovarian cancer. Cancer.
116:4410–4419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sephton SE, Lush E, Dedert EA, Floyd AR,
Rebholz WN, Dhabhar FS, Spiegel D and Salmon P: Diurnal cortisol
rhythm as a predictor of lung cancer survival. Brain Behav Immun.
30(Suppl): S163–S170. 2013. View Article : Google Scholar
|
|
21
|
Ebner NC, Maura GM, Macdonald K, Westberg
L and Fischer H: Oxytocin and socioemotional aging: Current
knowledge and future trends. Front Hum Neurosci. 7:4872013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kosfeld M, Heinrichs M, Zak PJ,
Fischbacher U and Fehr E: Oxytocin increases trust in humans.
Nature. 435:673–676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neumann ID and Landgraf R: Balance of
brain oxytocin and vasopressin: Implications for anxiety,
depression, and social behaviors. Trends Neurosci. 35:649–659.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uvnäs-Moberg K: Oxytocin may mediate the
benefits of positive social interaction and emotions.
Psychoneuroendocrinology. 23:819–835. 1998. View Article : Google Scholar
|
|
25
|
Heinrichs M, Baumgartner T, Kirschbaum C
and Ehlert U: Social support and oxytocin interact to suppress
cortisol and subjective responses to psychosocial stress. Biol
Psychiatry. 54:1389–1398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thibonnier M, Conarty DM, Preston JA,
Plesnicher CL, Dweik RA and Erzurum SC: Human vascular endothelial
cells express oxytocin receptors. Endocrinology. 140:1301–1309.
1999.PubMed/NCBI
|
|
27
|
Copland JA, Ives KL, Simmons DJ and Soloff
MS: Functional oxytocin receptors discovered in human osteoblasts.
Endocrinology. 140:4371–4374. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bussolati G, Cassoni P, Ghisolfi G, Negro
F and Sapino A: Immunolocalization and gene expression of oxytocin
receptors in carcinomas and non-neoplastic tissues of the breast.
Am J Pathol. 148:1895–1903. 1996.PubMed/NCBI
|
|
29
|
Cassoni P, Marrocco T, Deaglio S, Sapino A
and Bussolati G: Biological relevance of oxytocin and oxytocin
receptors in cancer cells and primary tumors. Ann Oncol. 12(Suppl
2): S37–S39. 2001. View Article : Google Scholar
|
|
30
|
Morita T, Shibata K, Kikkawa F, Kajiyama
H, Ino K and Mizutani S: Oxytocin inhibits the progression of human
ovarian carcinoma cells in vitro and in vivo. Int J Cancer.
109:525–532. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Longuespée R, Boyon C, Desmons A, Vinatier
D, Leblanc E, Farré I, Wisztorski M, Ly K, D'Anjou F, Day R, et al:
Ovarian cancer molecular pathology. Cancer Metastasis Rev.
31:713–732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bourton E, Foster H, Plowman P, Harvey A
and Parris C: Hypersensitivity of BRCA1 heterozygote lymphoblastoid
cells to gamma radiation and PARP inhibitors. J Genet Syndr Gene
Ther. 4:1462013.
|
|
33
|
Mparmpakas D, Zachariades E, Sotiriadis G,
Goumenou A, Harvey AJ, Gidron Y and Karteris E: Differential
expression of placental glucocorticoid receptors and growth
arrest-specific transcript 5 in term and preterm pregnancies:
Evidence for involvement of maternal stress. Obstet Gynecol Int.
2014:2392782014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Foster HA, Davies J, Pink RC, Turkcigdem
S, Goumenou A, Carter DR, Saunders NJ, Thomas P and Karteris E: The
human myometrium differentially expresses mTOR signalling
components before and during pregnancy: Evidence for regulation by
progesterone. J Steroid Biochem Mol Biol. 139:166–172. 2014.
View Article : Google Scholar
|
|
35
|
Kelder J, Azevedo R, Pang Y, de Vlieg J,
Dong J and Thomas P: Comparison between steroid binding to membrane
progesterone receptor alpha (mPRalpha) and to nuclear progesterone
receptor: Correlation with physicochemical properties assessed by
comparative molecular field analysis and identification of
mPRalpha-specific agonists. Steroids. 75:314–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mparmpakas D, Zachariades E, Goumenou A,
Gidron Y and Karteris E: Placental DEPTOR as a stress sensor during
pregnancy. Clin Sci (Lond). 122:349–359. 2012. View Article : Google Scholar
|
|
37
|
Harvey AJ, Pennington CJ, Porter S, Burmi
RS, Edwards DR, Court W, Eccles SA and Crompton MR: Brk protects
breast cancer cells from autophagic cell death induced by loss of
anchorage. Am J Pathol. 175:1226–1234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumar D, Shankar S and Srivastava RK:
Rottlerin-induced autophagy leads to the apoptosis in breast cancer
stem cells: Molecular mechanisms. Mol Cancer. 12:1712013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baehrecke EH: Autophagy: Dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Arden-Close E, Gidron Y and Moss-Morris R:
Psychological distress and its correlates in ovarian cancer: A
systematic review. Psychooncology. 17:1061–1072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schrepf A, Clevenger L, Christensen D,
DeGeest K, Bender D, Ahmed A, Goodheart MJ, Dahmoush L, Penedo F,
Lucci JA III, et al: Cortisol and inflammatory processes in ovarian
cancer patients following primary treatment: Relationships with
depression, fatigue, and disability. Brain Behav Immun. 30(Suppl):
S126–S134. 2013. View Article : Google Scholar :
|
|
42
|
Lin H-X, Qiu H-J, Zeng F, Rao H-L, Yang
G-F, Kung H-F, Zhu XF, Zeng YX, Cai MY and Xie D: Decreased
expression of Beclin 1 correlates closely with Bcl-xL expression
and poor prognosis of ovarian carcinoma. PLoS One. 8:e605162013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shen Y, Li DD, Wang LL, Deng R and Zhu XF:
Decreased expression of autophagy-related proteins in malignant
epithelial ovarian cancer. Autophagy. 4:1067–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Melhem A, Yamada SD, Fleming GF, Delgado
B, Brickley DR, Wu W, Kocherginsky M and Conzen SD: Administration
of glucocorticoids to ovarian cancer patients is associated with
expression of the anti-apoptotic genes SGK1 and MKP1/DUSP1 in
ovarian tissues. Clin Cancer Res. 15:3196–3204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang C, Kolb A, Mattern J, Gassler N,
Wenger T, Herzer K, Debatin KM, Büchler M, Friess H, Rittgen W, et
al: Dexamethasone desensitizes hepatocellular and colorectal
tumours toward cytotoxic therapy. Cancer Lett. 242:104–111. 2006.
View Article : Google Scholar
|
|
46
|
Erbaş O, Oltulu F and Taşkiran D:
Amelioration of rotenone-induced dopaminergic cell death in the
striatum by oxytocin treatment. Peptides. 38:312–317. 2012.
View Article : Google Scholar
|
|
47
|
Colditz GA and Rosner B: Cumulative risk
of breast cancer to age 70 years according to risk factor status:
Data from the Nurses' Health Study. Am J Epidemiol. 152:950–964.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Danforth KN, Tworoger SS, Hecht JL, Rosner
BA, Colditz GA and Hankinson SE: Breastfeeding and risk of ovarian
cancer in two prospective cohorts. Cancer Causes Control.
18:517–523. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Modugno F, Ness RB and Wheeler JE:
Reproductive risk factors for epithelial ovarian cancer according
to histologic type and invasiveness. Ann Epidemiol. 11:568–574.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lutgendorf SK, Sood AK, Anderson B, McGinn
S, Maiseri H, Dao M, Sorosky JI, De Geest K, Ritchie J and Lubaroff
DM: Social support, psychological distress, and natural killer cell
activity in ovarian cancer. J Clin Oncol. 23:7105–7113. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lutgendorf SK, Weinrib AZ, Penedo F,
Russell D, DeGeest K, Costanzo ES, Henderson PJ, Sephton SE,
Rohleder N, Lucci JA III, et al: Interleukin-6, cortisol, and
depressive symptoms in ovarian cancer patients. J Clin Oncol.
26:4820–4827. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Light KC, Grewen KM and Amico JA: More
frequent partner hugs and higher oxytocin levels are linked to
lower blood pressure and heart rate in premenopausal women. Biol
Psychol. 69:5–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Beaufort CM, Helmijr JCA, Piskorz AM,
Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van
IJcken WF, Heine AA, Smid M, et al: Ovarian cancer cell line panel
(OCCP): Clinical importance of in vitro morphological subtypes.
PLoS One. 9:e1039882014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bai H, Li H, Li W, Gui T, Yang J, Cao D
and Shen K: The PI3K/AKT/mTOR pathway is a potential predictor of
distinct invasive and migratory capacities in human ovarian cancer
cell lines. Oncotarget. 6:25520–25532. 2015. View Article : Google Scholar : PubMed/NCBI
|