Introduction
Bladder cancer is the most common malignant tumor of
the urinary system worldwide, accounting for 74,000 new cases and
16,000 deaths annually in 2015 (1). Despite intense efforts toward
improving the therapeutic success in treating bladder cancer, ~10%
non-muscle-invasive bladder cancer ultimately progressed to
muscle-invasive bladder cancer (2). Tendency to invade and transfer is
believed to be a major cause of treatment failure in bladder
cancer.
Epithelial-to-mesenchymal transition (EMT) is of
great necessity for the initiation of the metastatic cascade. The
process confers migratory, invasive and stem-like properties upon
cells (3), and epithelial
polarized cells ultimately turn into motile mesenchymal-appearing
cells (4). In epithelial cells
during EMT, cellular phenotype has a series of changes, including
the loss of cell-cell contacts, cell polarity and epithelial
markers, particularly E-cadherin. Moreover, cells acquire
mesenchymal-appearing properties. Additionally, cellular
cytoskeleton change during EMT confers mesenchymal phenotypes, such
as increased motility (5,6). The induction of EMT can cause cancer
cells to invade through the basement membrane and colonize into the
distant solid organs. Hence, the EMT process is essential for the
invasion-metastasis cascade of cancer.
The Hedgehog (Hh) signaling pathway has been
implicated to vertebrate development, cell differentiation and
carcinogenesis (7). It was
reported that the Hh signaling pathway is aberrantly expressed in
various cancers, including breast cancer (8), prostate cancer (9) and lung cancer (10). Moreover, accumulating evidence
shows that Gli-1, a crucial factor in the Hedgehog (Hh) signaling
pathway, could increase the expression of N-cadherin and Snail,
while decrease the expression of E-cadherin (11). Furthermore, Hedgehog signaling
pathway enhanced invasive and metastatic potential of
hepatocellular carcinoma (12).
These results suggest that there may be a close association in the
occurrence of EMT and metastasis with Hedgehog signaling
pathway.
Tetrandrine (TET), isolated from traditional Chinese
medicine Stephaniae, is widely used for a broad spectrum of
pharmacological events, including antihypertension, antisilicosis
and antiarrhythmia. Accumulating evidence has shown the strong
anticancer effect of tetrandrine. For many tumors such as prostate
cancer (13,14), colon cancer (15,16),
gastric cancer (17), esophageal
squamous carcinoma (18),
tetrandrine exhibited beneficial impact on reversion of multi-drug
resistance (18,19), apoptosis induction, cell cycle
arrest, inhibition of angiogenesis, chemosensitization, inhibition
of metastasis (20), involved in
ROS/AKT pathway (21), Wnt/β
signaling pathway (22), intrinsic
apoptotic signaling pathway, and mitogen-activated protein kinase
activation.
Li et al (23) showed that tetrandrine exerts
anticancer effects against bladder cancer via growth inhibition and
apoptosis induction. In spite of its anticancer potential, however,
the anti-metastasis effect and the underlying mechanism of
tetrandrine on bladder cancer has not yet been elucidated. In
particular, there have been no studies showing whether tetrandrine
inhibits the Hh signaling pathway to regulate the EMT in bladder
cancer. The aim of the present study was to focus on the potential
of tetrandrine as an inhibitor of the Hh pathway, which reverses
EMTs and inhibits migration and invasion, in bladder cancer.
Materials and methods
Reagents and cell culture
Tetrandrine
(C38H42N2O6) was
purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved
with 0.1 mol/l HCl at a concentration of 25 mg/ml. Stock solutions
were stored at −20°C and diluted with medium in certain proportions
immediately prior to use. Antibodies against E-cadherin,
N-cadherin, vimentin, Slug, Gli-1, β-actin and
peroxidase-conjugated secondary antibodies were obtained from Cell
Signaling Technology, Inc. (Beverly, MA, USA).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were obtained from Sigma Chemical Co. (St. Louis, MO, USA). The
enhanced chemiluminescence (ECL) detection system was obtained from
Amersham Life Science, Inc. (Arlington Heights, IL, USA).
Human bladder cancer cell lines 5637, T24, 253, BV,
J82 and UC3, and bladder epithelial SVHUC-1 cell were obtained from
the American Type Culture Collection (Manassas, VA, USA). These
cell lines were cultured in 1640/Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY,
USA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA, USA)
at 37°C in a humidified atmosphere with 5% CO2.
Cell proliferation assay
A modified MTT assay was used to detect cell
proliferation viability. Briefly, 5637 and T24 cells were seeded at
a density of 1×104 cells with 90% density in 96-well
plates and treated with increasing doses of tetrandrine for 24 h.
Then, 20 μl MTT dye solution (5.0 mg/ml) was added to each well and
incubated at 37°C for 4 h. After incubation, the culture medium was
discarded and the cells were lysed with dimethyl sulfoxide to
dissolve the formazan crystals. The optical density (OD) of each
well was detected at a wavelength of 490 nm using a 96-well
microplate reader (Bio-Rad, Hercules, CA, USA). The percentage of
cell growth inhibition resulting from tetrandrine was calculated
as: [(OD490control cells−OD490treated
cells)/OD490control cells] × 100. The experiments
were performed in triplicate.
Wound healing assay
Bladder cancer 5637 or T24 cells were seeded onto
6-well plates. When the cell density reached >90%, scratch
wounds were made across the monolayer using the tip of a 200-μl
pipette. Then the wounded cultures were incubated in a serum-free
medium with various concentrations of tetrandrine at different
times, and five random fields (×100) were chosen from each scratch
wound and visualized by microscopy to assess the ability of cell
migration. The experiments were performed in triplicate.
Transwell migration assay
Transwell migration assays were performed using
bladder cancer 5637 and T24 cells after treatment with tetrandrine.
Cells (5637: 6×104 or T24: 3×104) with 200 μl
serum-free medium were plated onto the top chamber, and 800 μl of
medium supplemented with 10% fetal calf serum were added to the
lower chamber. After incubation at 37°C for various times, cells
adhering to the top chambers were wiped out with a cotton swab. The
migratory cells on the lower surface of the filter were fixed with
4% paraformaldehyde and stained with 0.1% crystal violet (Beyotime,
Shanghai, China). Cells that had migrated to the lower surface were
counted in five randomly chosen visual fields under a microscope at
×100 magnification. All of the data were obtained from three
independent experiments.
Matrigel invasion assay
The impact of tetrandrine on the invasion of bladder
cancer cells was determined by Matrigel invasion assay using a
Millicell chamber (Millipore, Billerica, MA, USA). The membrane
(polycarbonic membrane, 6.5 mm diameter, 8-μm pore size) in the
upper chamber was coated with 50 μl Matrigel (Matrigel: serum-free
medium 1:5). After incubation at 37°C for 5 h, the cells (5637:
12×104 or T24: 6×104 in 200 μl medium of
serum starvation, respectively) were treated by the same procedures
as described above, similarly to the Transwell migration assay.
Quantitative real-time PCR assay
5637 and T24 cells were cultured in the experimental
conditions and their total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) following the manufacturer's
protocol. Then complementary DNA (cDNA) was synthesized using a
PrimerScript RT reagent kit (Takara, Dalian, China). Additionally,
the relative levels of target gene messenger RNA (mRNA) transcript
were detected by quantitative real-time PCR assay (qRT-PCR) using
the SYBR Green Master Mix. The sequences of primers for the PCR
amplification were forward, 5′-AGCGTGAGCCTG AATCTGTG-3′ and
reverse, 5′-CAGCATGTACTGGGCTTTGAA-3′ for Gli-1 (188 bp); forward,
5′-CGAGAGCTACACGT T CACGG-3′ and reverse,
5′-GGGTGTCGAGGGAAAAATAGG-3′ for E-cadherin (119 bp); forward,
5′-TCAGGCGTCTG TAGAGGCTT-3′ and reverse,
5′-ATGCACATCCTTCGATAAGACTG-3′ for N-cadherin (94 bp); forward,
5′-GACGCCATCA ACACCGAGTT-3′ and reverse,
5′-CTTTGTCGTTGGTTAGCTGGT-3′ for vimentin (238 bp); forward,
5′-CGAACTGGACA CACATACAGTG-3′ and reverse,
5′-CTGAGGATCTCTGGTTGTGGT-3′forSlug(87bp);forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′ for β-actin (250 bp). All assays were
performed in triplicate and were calculated on the basis of the
ΔΔCt method. The n-fold change in mRNAs expression was determined
according to the method of 2-ΔΔCt.
Western blotting
Briefly, the bladder cancer cells were harvested 24
h after the tetrandrine treatment, and lysed on ice for 10 min with
a lysis buffer [10 mmol/l Tris-HCl (pH 7.4), 150 mmol/l NaCl, 0.1%
sodium dodecyl sulfate (SDS), 1 mmol/l ethylenediaminetetraacetic
acid, 1 mmol/l ethylene glycol tetraacetic acid, 0.3 mmol/l
phenylmethylsulfonyl fluoride, 0.2 mmol/l sodium orthovanadate, 1%
NP-40, 10 mg/ml leupeptin and 10 mg/ml aprotinin]. After
centrifugation, the clarified protein lysates (~30–60 μg) were
separated by SDS-polyacrylamide gel (10%) and transferred to
polyvinylidene membranes (Millipore, Bedford, MA, USA).
Immunoblotting was subsequently performed with the primary antibody
against E-cadhrein, N-cadherin, vimentin, as well as Slug, and
β-actin overnight at 4°C. The membranes were then washed and
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibody at room temperature (25°C). Ultimately, the protein bands
were visualized with ECL substrate and exposed to X-ray film.
Immunofluorescence microscopy
The immunofluorescence staining of F-actin was
performed following the manufacturer's protocol. In brief, cells on
slides were washed and fixed with 4% paraformaldehyde for 15 min.
After being washed, cells were permeabilized with 0.1% Triton X-100
for 5 min. Then cells were stained with 20 μl F-actin for 30 min
and DAPI dye for 5 min. Nikon (Tokyo, Japan) A1 confocal microscope
was used for the observation of micrographs.
Plasmid transfection
Gli-1 cDNA was cloned into pcDNA3.1 vector. When
bladder cancer 5637 or T24 cells achieved 70–80% confluency for
plasmid transfection, the cells were transfected with X-treme Gene
HP DNA transfection reagent (Roche, Germany) for 48 h according to
the manufacturer's instructions, and harvested for the turnover
experiments.
Statistical analysis
GraphPad Prism (vesion 5.0) software was used for
all statistical analyses, and Student's t-test (two-sided) was used
for comparisons involving only two groups. A value of P<0.05 was
considered statistically significant.
Results
The antiproliferative effect of
tetrandrine in bladder cancer 5637 and T24 cells
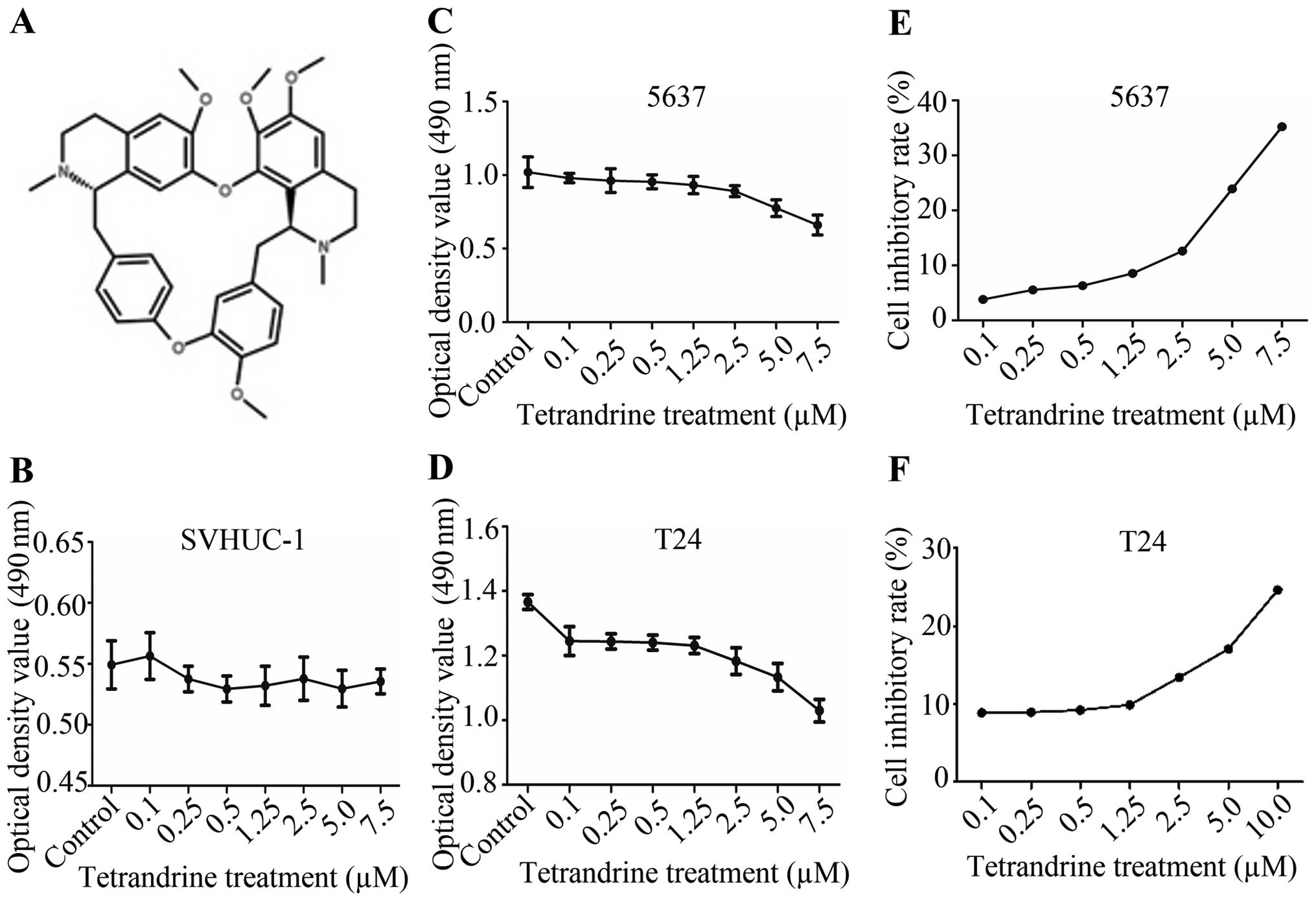
Fig. 1A shows the
chemical structure of tetrandrine with the molecular structural
formula C38H42N2O6 and
a molecular weight of 622.74988 g/mol. The MTT assays showed that
tetrandrine did not have a significant inhibitory effect on normal
bladder epithelial SVHUC-1 cells (Fig.
1B), which indicated that tetrandrine had low cytotoxity on
normal cells. While the growth of bladder cancer 5637 and T24 cells
was significantly inhibited by tetrandrine at a concentration of
≥0.5 μM, and at cell density >90% (Fig. 1C–F). Based on the above,
tetrandrine at 0.5 μM (a <10% inhibitory rate) was chosen as the
appropriate dose in the subsequent studies, to exclude the
suppressing interference from bladder cancer proliferation by
tetrandrine.
Tetrandrine suppresses the migration and
invasion of bladder cancer 5637 and T24 cells
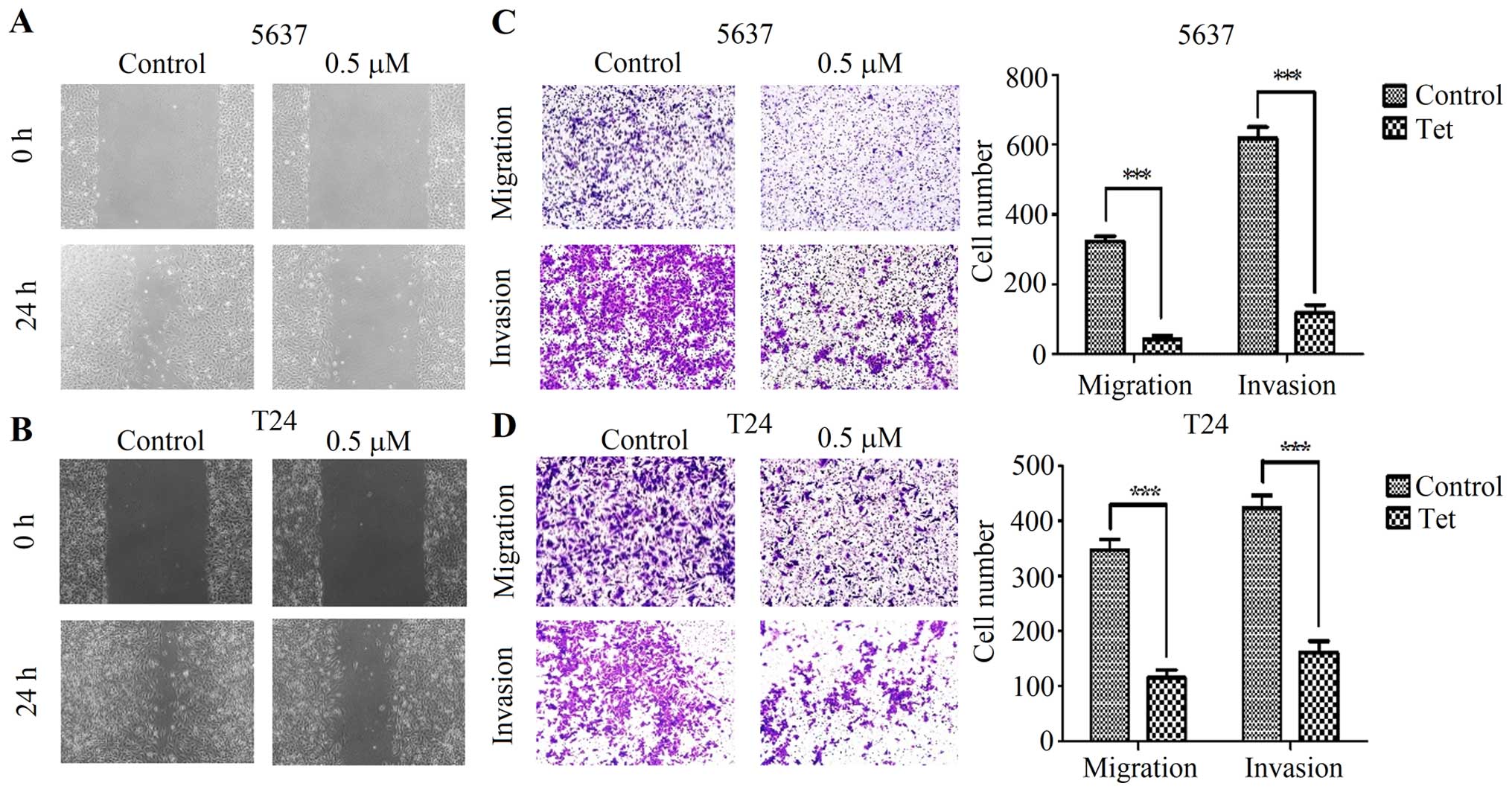
In order to determine the function of tetrandrine on
bladder cancer cell migration, wound healing assay and Transwell
migration assay were assessed under a microscope at 0 and 24 h. The
results showed that the scratches of the cells treated with
tetrandrine were much wider than that in control group (Fig. 2A and B). Furthermore, tetrandrine
in the 5637 cells decreased cell migratory ability after 24 h
(Fig. 2C). Similarly, treatment of
the T24 cells with tetrandrine resulted in a prominent decrease in
migration compared to the control group (Fig. 2D). These results indicated that
tetrandrine was able to repress migration in the human bladder
cancer 5637 and T24 cells. Next, using a Matrigel invasion assay,
we focused on whether tetrandrine could affect the invasiveness of
bladder cancer cells. The results showed that tetrandrine
significantly decreased invasive capability of 5637 and T24 cells
(Fig. 2C and D).
These results indicated that tetrandrine might play
a crucial role in inhibiting the migration and invasion potential
of human bladder cancer cells, as presented by the observation in
the wound-healing and Transwell assays.
Tetrandrine reverses
epithelial-mesenchymal transition (EMT) in human bladder cancer
cells
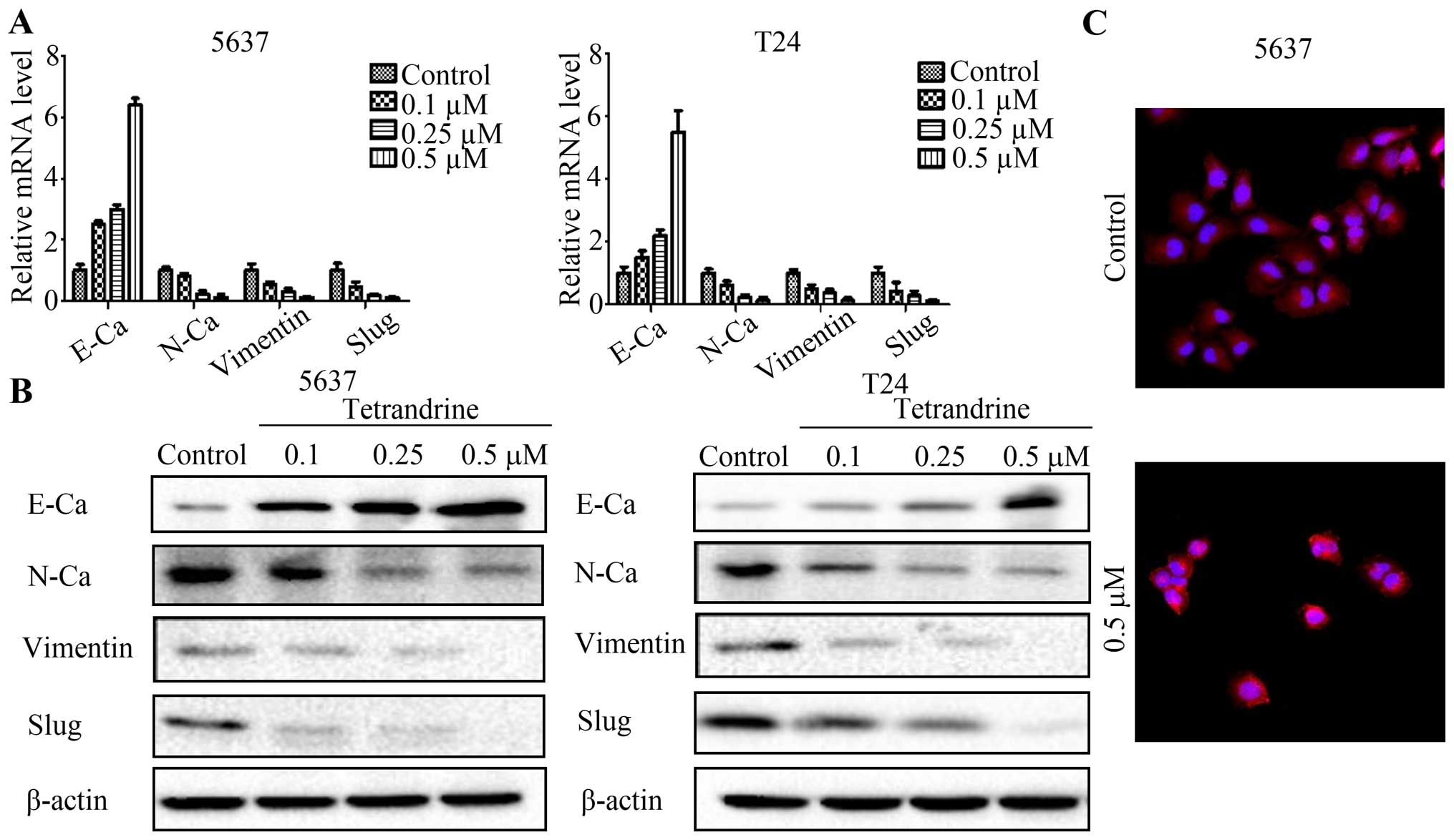
EMT is essential in tumorigenesis and cancer
progression (24). We, therefore,
examined the effect of tetrandrine on the mRNA and protein
expression of EMT markers in bladder cancer cells. The results
showed that the expression of N-cadherin, vimentin and Slug
reduced, while the expression of E-cadherin increased in a
concentration-dependent manner (Fig.
3A and B), suggesting that tetrandrine could block EMT. Then we
detected the stress fibers (F-actin) in bladder cancer 5637 cells
treated with tetrandrine, or not, with confocal immunofluorescence
microscopy. Interestingly, we found that in tetrandrine-treated
cells, the bundled F-actin (stress fibers) drastically decreased
(Fig. 3C), which indicated the
mobility of 5637 cells was inhibited by tetrandrine.
Tetrandrine downregulates the expression
of Gli-1 in bladder cancer 5637 and T24 cells
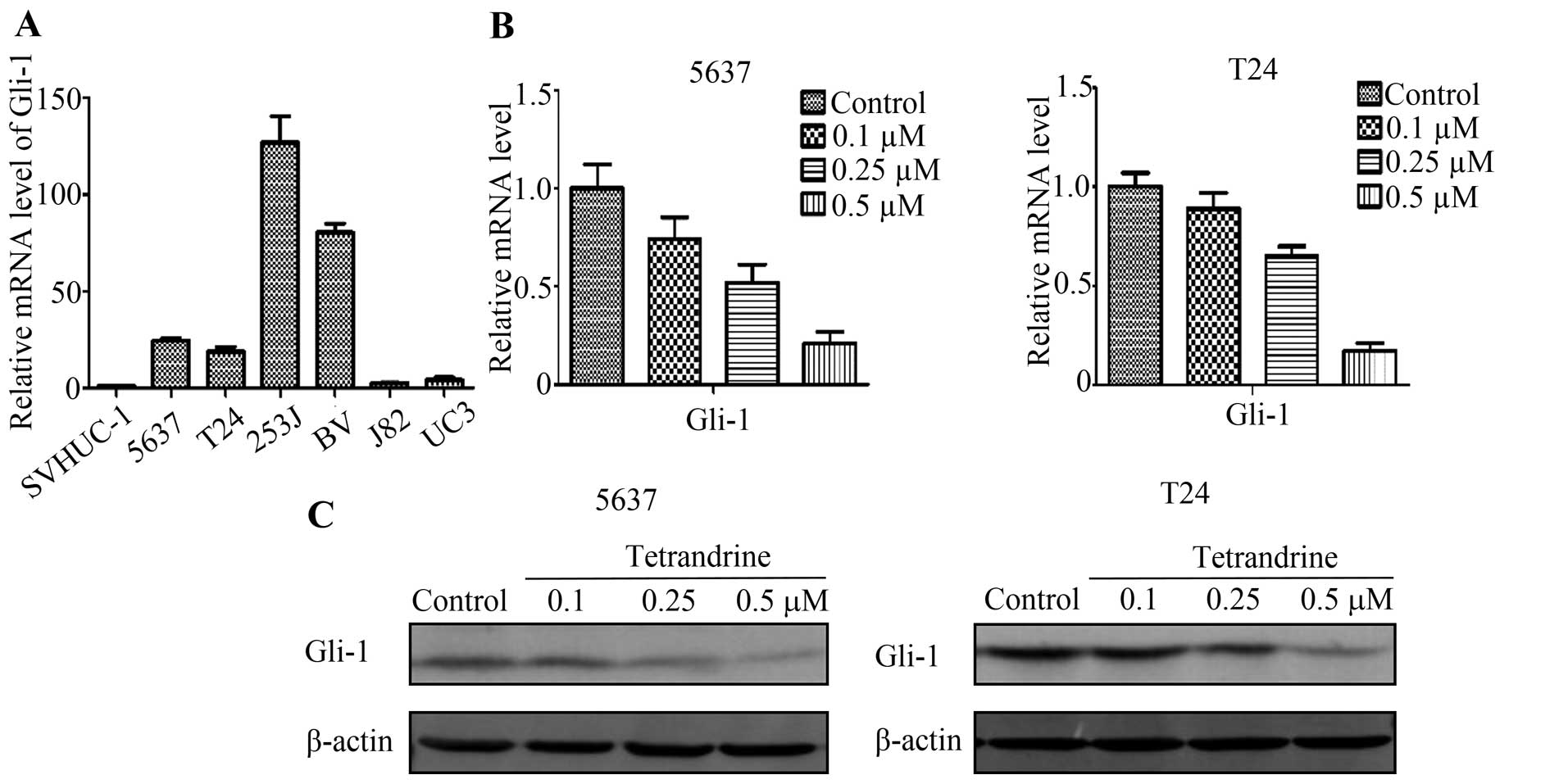
Hedgehog (Hh) signaling pathway has been reported to
play important roles in tumorigenesis and progression of several
human cancers (25–27). Gli-1 is a key member of Hh
signaling pathway signaling (28).
Therefore, we detected Gli-1 level in normal bladder epithelial
cells and 6 bladder cancer cell lines (5637, T24, 253, BV, J82,
UC3). Total RNA was extracted from these cell lines, and through
quantitative real-time PCR analysis, we found that expression of
Gli-1 in normal bladder epithelial cells is significantly lower
than that in bladder cancer cells (Fig. 4A), which indicated that the
relative level of Gli-1 is upregulated in bladder cancer cells.
To confirm the ability of tetrandrine to inhibit the
Hh signaling pathway, 5637 and T24 cells treated with tetrandrine
for 24 h were used to examine changes in the Gli-1 level. As shown
in Fig. 4B and C, tetrandrine
downregulated Gli-1 expression in a dose-dependent manner in mRNA
and protein levels. These observations indicated that Hh signaling
pathway may play an important role in the pathogenesis of bladder
cancer.
Tetrandrine inhibits migration and
invasion of bladder cancer cells at least partly through
downregulating Gli-1
To determine whether tetrandrine negatively regulate
Gli-1 to modulate tumor migration and invasion of bladder cancer,
we overexpressed Gli-1 by transiently transfecting Gli-1 plasmid
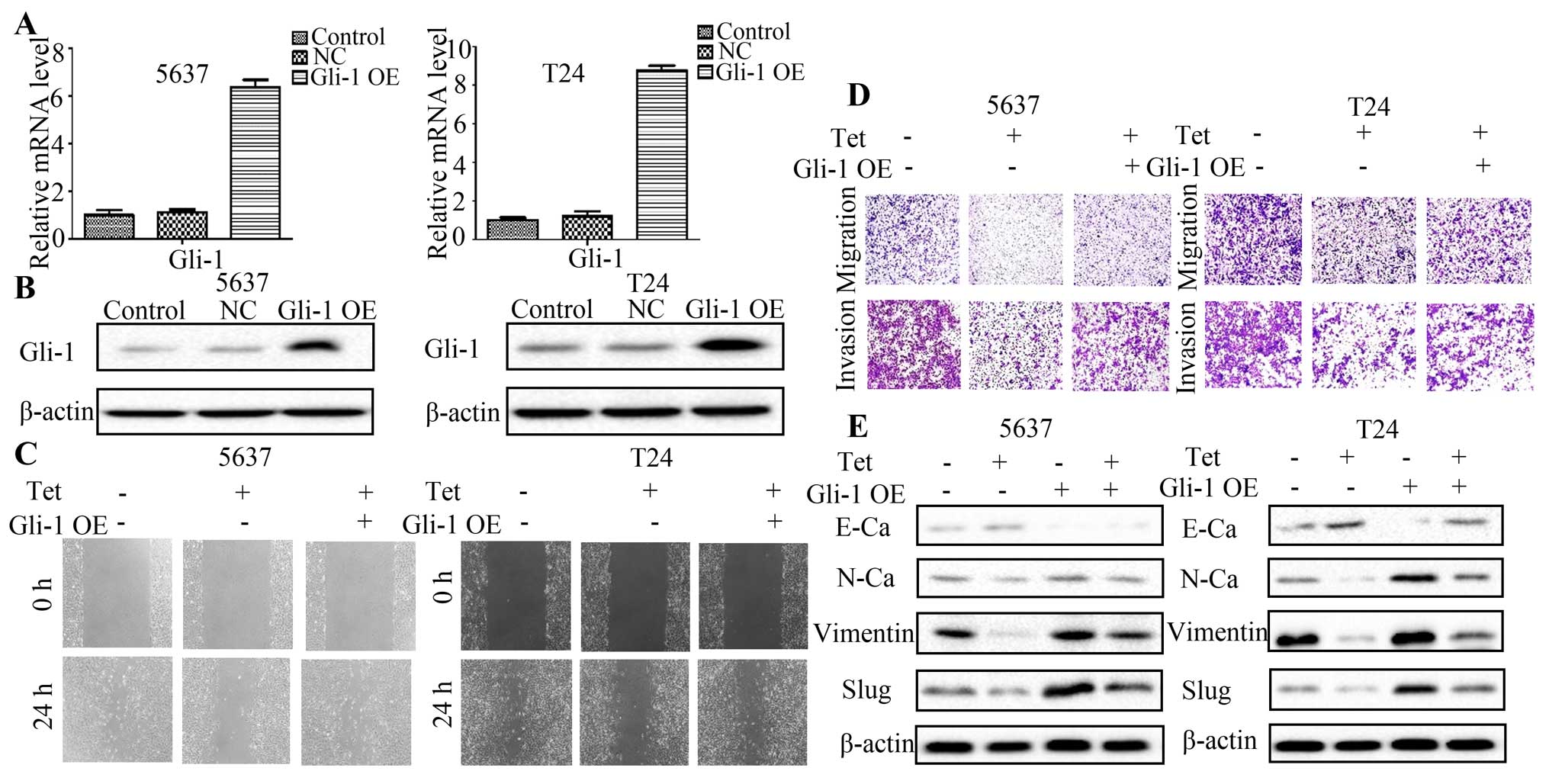
into 5637 and T24 cells (Fig. 5A and
B). Tetrandrine decreased the width of scratches and reduced
the cell number in the lower transwell chamber, while combined
Gli-1 overexpression potentiated the migration and invasion ability
of 5637 and T24 cells (Fig. 5C and
D). These results indicated that Gli-1 participated in the
antitumor effect of tetrandrine on migration and invasion in
bladder cancer. Through western blot analysis of EMT markers, we
further found that the elevated level of E-cadherin and the reduced
expression of N-cadherin, vimentin and Slug by tetrandrine could be
partially reversed when combined with the Gli-1 overexpression in
bladder cancer cells (Fig. 5E).
Taken together, these results suggest a deeper concept that
tetrandrine might inhibit bladder cancer migration and invasion,
and reverse EMT at least partly through negatively regulating
Gli-1.
Discussion
Numerous studies in the literature have shown that
tetrandrine exhibits strong antitumor effect against various
cancer. However, there have been only a limited number of studies
on the anti-metastasis activity of tetrandrine on cancer cells. It
has been reported that tetrandrine significantly inhibited tumor
metastasis in a mouse model of stage IV breast cancer, involving
the endothelial cell-specific molecule-1 (ESM-1), integrin β5
protein and intercellular cell adhesion molecule-1 (ICAM-1). In our
earlier study (data not shown), we found that
tetrandrine could inhibit metastatic phenotype of prostate cancer
cells by regulating AKT/mTOR/MMP-9 signaling pathway. In this
study, our results demonstrated that tetrandrine significantly
inhibited bladder cancer migration and invasion, suggesting that
tetrandrine might be a potential therapeutic method for bladder
cancer.
Epithelial-mesenchymal transition (EMT) is a process
in which epithelial cells lose their characteristics and gain
mesenchymal features. EMT could result in invasion of surrounding
stroma, intravasation, dissemination and colonization of distant
sites. Therefore, EMT may confer metastatic characteristics on
carcinomas to some extent. Studies have shown that EMT has a
positive impact on HCC invasion and metastasis (29). A hallmark of EMT is the
downregulation of E-cadherin (30). E-cadherin is a cell adhesion
molecule involved in the cell-cell adhesion. The decreased level of
E-cadherin is considered to indicate elevated metastatic activity
of cancer. While the increased expression of N-cadherin, vimentin
and Slug are correlated with poor prognosis and tumor
aggressiveness (31,32). In our study, we observed an induced
expression of E-cadherin while a reduced expression of N-cadherin,
vimentin, Slug and a decreased mobility in bladder cancer cells
treated with tetrandrine, suggesting a potential role of
tetrandrine in the blockade of EMT involved in early
metastasis.
Hedgehog (Hh) pathway has been verified to play a
momentous role in embryonic development and the tumor progression.
Previous studies have shown that the Gli-1 is a vital effector of
the Hedgehog pathway (33). High
expression of Gli-1 has a positive correlation with the
invasiveness of breast cancer (34), and with the poor prognosis of
disease (35). Additionally, we
found that the mRNA level of Gli-1 in 6 different bladder cancer
cells is significantly higher than that in bladder epithelial
SVHUC-1 cells. Studies indicated that aberrant activation of
Hedgehog (Hh) could facilitate cancer EMT, invasion, and metastasis
(36). In gastric cancer, Hh
signaling pathway potentiates metastasis by positively regulating
Akt, EMT and MMP-9 pathway (37).
Additionally, it was reported that hypoxia can upregulate Gli-1 to
induce EMT and to enhance tumor invasiveness in pancreatic cancer
(38). Accumulating evidence
indicates that Hedgehog signaling pathway especially Gli-1 is
correlated with EMT, and tumor invasion and metastasis. However,
the roles of Gli-1 in the tetrandrine-induced EMT and invasion of
bladder cancer cells have not been clarified. Our findings show
that tetrandrine combined with Gli-1 overexpression could attenuate
the anti-metastatic capability of tetrandrine on bladder cancer.
Furthermore, the expression of E-cadherin was down while the
N-cadherin, vimentin and Slug levels recovered to some extent.
These findings deepen the concept that Hedgehog signaling is a
fundamental driver of tumor metastasis in bladder cancer, thus
Hedgehog pathway blockage by tetrandrine is a promising novel
treatment strategy for unresectable forms of bladder cancer.
Taken together, our above results demonstrated that
tetrandrine inhibits bladder cancer cell migration and invasion,
and reverses EMT in vitro by negatively regulating Gli-1. It
is suggested that Gli-1 could be potential therapeutic target of
tetrandrine against bladder cancer.
Acknowledgements
This study was partially supported by the
Institutional Scientific Development Foundation of the First
Affiliated Hospital of Xi'an Jiaotong University.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW and
Kurth K: Predicting recurrence and progression in individual
patients with stage Ta T1 bladder cancer using EORTC risk tables: a
combined analysis of 2596 patients from seven EORTC trials. Eur
Urol. 49:465–466; discussion 475–477. 2006. View Article : Google Scholar
|
|
3
|
Nisticò P, Bissell MJ and Radisky DC:
Epithelial-mesenchymal transition: General principles and
pathological relevance with special emphasis on the role of matrix
metalloproteinases. Cold Spring Harb Perspect Biol. 4:42012.
View Article : Google Scholar
|
|
4
|
Chang CJ, Chao CH, Xia W, Yang JY, Xiong
Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, et al: p53 regulates
epithelial-mesenchymal transition and stem cell properties through
modulating miRNAs. Nat Cell Biol. 13:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie D, Gore C, Liu J, Pong RC, Mason R,
Hao G, Long M, Kabbani W, Yu L, Zhang H, et al: Role of DAB2IP in
modulating epithelial-to-mesenchymal transition and prostate cancer
metastasis. Proc Natl Acad Sci USA. 107:2485–2490. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gjerdrum C, Tiron C, Høiby T, Stefansson
I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT,
et al: Axl is an essential epithelial-to-mesenchymal
transition-induced regulator of breast cancer metastasis and
patient survival. Proc Natl Acad Sci USA. 107:1124–1129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Li YY, Wu YY and Nie YQ:
Expression and clinical significance of hedgehog signaling pathway
related components in colorectal cancer. Asian Pac J Cancer Prev.
13:2319–2324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flemban A and Qualtrough D: The potential
role of hedgehog signaling in the luminal/basal phenotype of breast
epithelia and in breast cancer invasion and metastasis. Cancers
(Basel). 7:1863–1884. 2015. View Article : Google Scholar
|
|
9
|
Lin H, Jackson GA, Lu Y, Drenkhahn SK,
Brownstein KJ, Starkey NJ, Lamberson WR, Fritsche KL, Mossine VV,
Besch-Williford CL, et al: Inhibition of Gli/hedgehog signaling in
prostate cancer cells by “cancer bush” Sutherlandia frutescens
extract. Cell Biol Int. 40:131–142. 2016. View Article : Google Scholar
|
|
10
|
Huang L, Walter V, Hayes DN and Onaitis M:
Hedgehog-GLI signaling inhibition suppresses tumor growth in
squamous lung cancer. Clin Cancer Res. 20:1566–1575. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Deng W, Nail CD, Bailey SK, Kraus
MH, Ruppert JM and Lobo-Ruppert SM: Snail induction is an early
response to Gli1 that determines the efficiency of epithelial
transformation. Oncogene. 25:609–621. 2006.
|
|
12
|
Chen JS, Huang XH, Wang Q, Huang JQ, Zhang
LJ, Chen XL, Lei J and Cheng ZX: Sonic hedgehog signaling pathway
induces cell migration and invasion through focal adhesion
kinase/AKT signaling-mediated activation of matrix
metalloproteinase (MMP)-2 and MMP-9 in liver cancer.
Carcinogenesis. 34:10–19. 2013. View Article : Google Scholar
|
|
13
|
Chaudhary P and Vishwanatha JK: c-Jun
NH2-terminal kinase-induced proteasomal degradation of c-FLIPL/S
and Bcl2 sensitize prostate cancer cells to Fas- and
mitochondria-mediated apoptosis by tetrandrine. Biochem Pharmacol.
91:457–473. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu W, Kou B, Ma ZK, Tang XS, Lv C, Ye M,
Chen JQ, Li L, Wang XY and He DL: Tetrandrine suppresses
proliferation, induces apoptosis, and inhibits migration and
invasion in human prostate cancer cells. Asian J Androl.
17:850–853. 2015.PubMed/NCBI
|
|
15
|
Wu K, Zhou M, Wu QX, Yuan SX, Wang DX, Jin
JL, Huang J, Yang JQ, Sun WJ, Wan LH, et al: The role of IGFBP-5 in
mediating the anti-proliferation effect of tetrandrine in human
colon cancer cells. Int J Oncol. 46:1205–1213. 2015.
|
|
16
|
Wu JM, Chen Y, Chen JC, Lin TY and Tseng
SH: Tetrandrine induces apoptosis and growth suppression of colon
cancer cells in mice. Cancer Lett. 287:187–195. 2010. View Article : Google Scholar
|
|
17
|
Qin R, Shen H, Cao Y, Fang Y, Li H, Chen Q
and Xu W: Tetrandrine induces mitochondria-mediated apoptosis in
human gastric cancer BGC-823 cells. PLoS One. 8:e764862013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang TH, Wan JY, Gong X, Li HZ and Cheng
Y: Tetrandrine enhances cytotoxicity of cisplatin in human
drug-resistant esophageal squamous carcinoma cells by inhibition of
multi-drug resistance-associated protein 1. Oncol Rep.
28:1681–1686. 2012.PubMed/NCBI
|
|
19
|
Sun YF and Wink M: Tetrandrine and
fangchinoline, bisbenzylisoquinoline alkaloids from Stephania
tetrandra can reverse multidrug resistance by inhibiting
P-glycoprotein activity in multidrug resistant human cancer cells.
Phytomedicine. 21:1110–1119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao JL, Ji X, He TC, Zhang Q, He K, Zhao
Y, Chen SH and Lv GY: Tetrandrine suppresses cancer angiogenesis
and metastasis in 4T1 tumor bearing mice. Evid Based Complement
Alternat Med. 2013:2650612013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiao W, Jiang Y, Men Q, Yuan L, Huang Z,
Liu T, Li W and Liu X: Tetrandrine induces G1/S cell cycle arrest
through the ROS/Akt pathway in EOMA cells and inhibits angiogenesis
in vivo. Int J Oncol. 46:360–368. 2015.
|
|
22
|
He BC, Gao JL, Zhang BQ, Luo Q, Shi Q, Kim
SH, Huang E, Gao Y, Yang K, Wagner ER, et al: Tetrandrine inhibits
Wnt/β-catenin signaling and suppresses tumor growth of human
colorectal cancer. Mol Pharmacol. 79:211–219. 2011. View Article : Google Scholar :
|
|
23
|
Li X, Su B, Liu R, Wu D and He D:
Tetrandrine induces apoptosis and triggers caspase cascade in human
bladder cancer cells. J Surg Res. 166:e45–e51. 2011. View Article : Google Scholar
|
|
24
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar
|
|
25
|
Amakye D, Jagani Z and Dorsch M:
Unraveling the therapeutic potential of the Hedgehog pathway in
cancer. Nat Med. 19:1410–1422. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Briscoe J and Thérond PP: The mechanisms
of Hedgehog signalling and its roles in development and disease.
Nat Rev Mol Cell Biol. 14:416–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harris LG, Pannell LK, Singh S, Samant RS
and Shevde LA: Increased vascularity and spontaneous metastasis of
breast cancer by hedgehog signaling mediated upregulation of cyr61.
Oncogene. 31:3370–3380. 2012. View Article : Google Scholar :
|
|
28
|
Zhang J, Zhang GX, Chen FF, He BS, Ye F
and Pan XL: Effect of Helicobacter pylori lipopolysaccharide on
expression of Gli and Ptch-1 proteins in sonic hedgehog signaling
pathway of gastric mucosa GES-1 cells. Zhejiang Da Xue Xue Bao Yi
Xue Ban. 42:543–549. 2013.In Chinese. PubMed/NCBI
|
|
29
|
Reichl P, Haider C, Grubinger M and
Mikulits W: TGF-β in epithelial to mesenchymal transition and
metastasis of liver carcinoma. Curr Pharm Des. 18:4135–4147. 2012.
View Article : Google Scholar
|
|
30
|
Mallini P, Lennard T, Kirby J and Meeson
A: Epithelial-to-mesenchymal transition: What is the impact on
breast cancer stem cells and drug resistance. Cancer Treat Rev.
40:341–348. 2014. View Article : Google Scholar
|
|
31
|
Satelli A and Li S: Vimentin in cancer and
its potential as a molecular target for cancer therapy. Cell Mol
Life Sci. 68:3033–3046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Voulgari A and Pintzas A:
Epithelial-mesenchymal transition in cancer metastasis: Mechanisms,
markers and strategies to overcome drug resistance in the clinic.
Biochim Biophys Acta. 1796:75–90. 2009.PubMed/NCBI
|
|
33
|
Li X, Ma Q, Duan W, Liu H, Xu H and Wu E:
Paracrine sonic hedgehog signaling derived from tumor epithelial
cells: A key regulator in the pancreatic tumor microenvironment.
Crit Rev Eukaryot Gene Expr. 22:97–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jeng KS, Sheen IS, Jeng WJ, Yu MC, Hsiau
HI and Chang FY: High expression of Sonic Hedgehog signaling
pathway genes indicates a risk of recurrence of breast carcinoma.
Onco Targets Ther. 7:79–86. 2013. View Article : Google Scholar
|
|
35
|
ten Haaf A, Bektas N, von Serenyi S, Losen
I, Arweiler EC, Hartmann A, Knüchel R and Dahl E: Expression of the
glioma-associated oncogene homolog (GLI) 1 in human breast cancer
is associated with unfavourable overall survival. BMC Cancer.
9:2982009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abidi A: Hedgehog signaling pathway: a
novel target for cancer therapy: vismodegib, a promising
therapeutic option in treatment of basal cell carcinomas. Indian J
Pharmacol. 46:3–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK,
Kim HK, Kim JS and Oh SC: Sonic hedgehog pathway promotes
metastasis and lymphangiogenesis via activation of Akt, EMT, and
MMP-9 pathway in gastric cancer. Cancer Res. 71:7061–7070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan
W, Sun Q, Xu J, Wu Z, et al: Hedgehog signaling regulates hypoxia
induced epithelial to mesenchymal transition and invasion in
pancreatic cancer cells via a ligand-independent manner. Mol
Cancer. 12:662013. View Article : Google Scholar : PubMed/NCBI
|