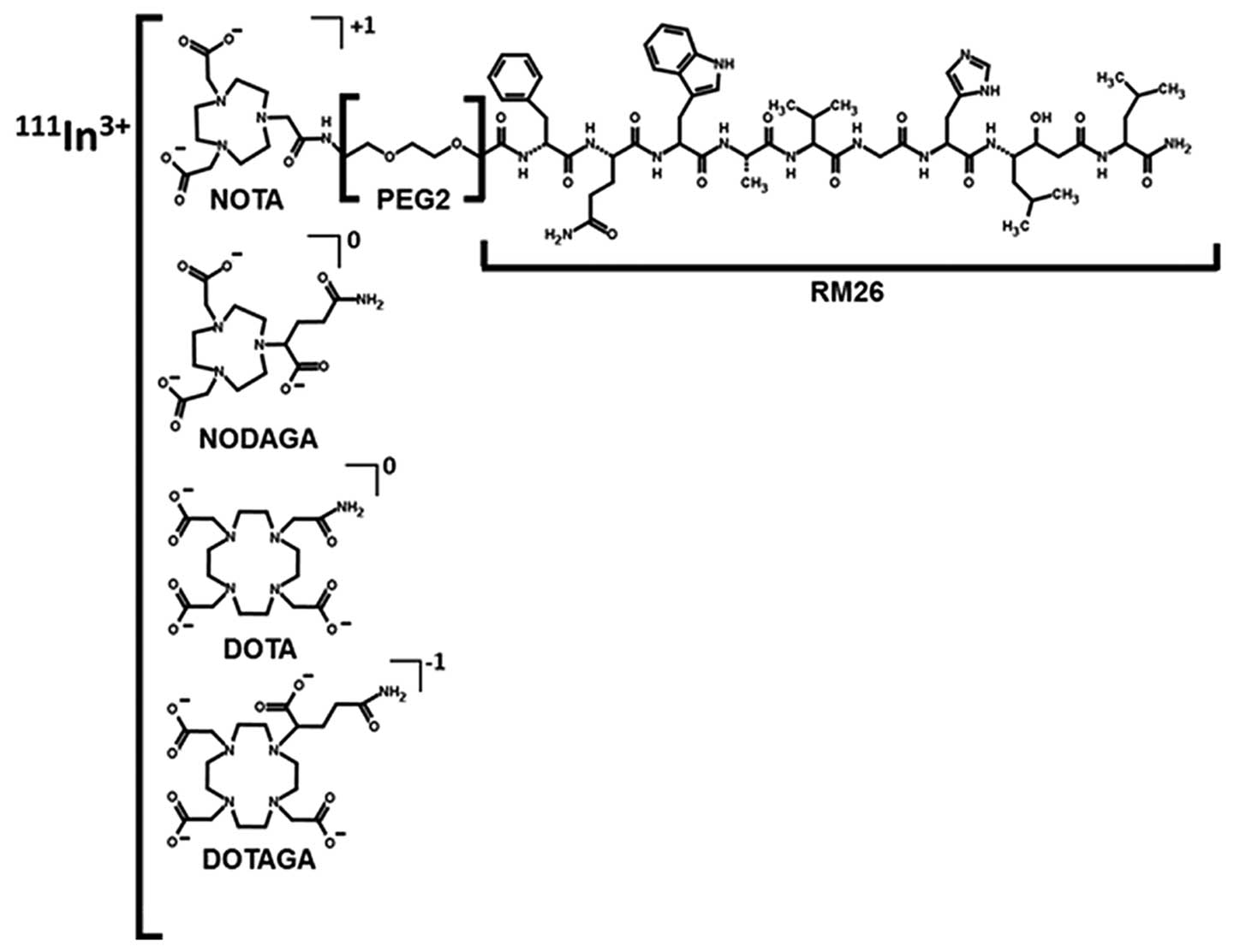
Introduction
Bombesin analogs, which show significant potential
in the diagnosis and therapy of prostate cancer, have been widely
investigated in recent years. The attractiveness of these peptides
is determined by their ability to bind selectively and avidly to
gastrin-releasing peptide receptors (GRPRs), which are ectopically
expressed in several tumors, including prostate and breast cancer
(1). Imaging of GRPR expression in
prostate cancer using high-affinity radiolabeled bombesin analogs
could therefore complement the current prostate-specific antigen
(PSA)-based screening methods, which have limited standalone
diagnostic specificity and can generate false positive and false
negative results (2). This type of
imaging could also provide an answer to the discrepancy between the
histological prevalence and clinical disease encountered in
biopsies (3). Moreover, it could
allow preoperative assessment of lymph node involvement and other
metastases, which are crucial preconditions for adequate staging
and treatment selection (4).
However, the detection of subcentimeter metastases is challenging
and requires imaging solutions that are characterized by high
sensitivity (5).
The potential benefit of radionuclide molecular
imaging using peptide-based targeting vectors is supported by the
success reported for somatostatin analogs, which also paved the way
for the design and development of several bombesin derivatives in
the last decade. While the initial consensus was that
internalization of the receptor-ligand complex is a decisive
precondition for optimal imaging and therapy (6), the superior results obtained using
somatostatin antagonists (7) led
to a paradigm shift in bombesin analog development, from agonists
to antagonists. The study by Cescato and co-workers showed that
bombesin-based antagonists had superior tumor targeting and
pharmacokinetic characteristics compared to agonists without
eliciting a physiological response (8).
Recently, we have investigated a high-affinity
antagonistic analog of bombesin [RM26, (D-Phe6, Sta13,
Leu14)-bombesin(6–14)] conjugated to a
1,4,7-triazacyclononane-N, N′,N″-triacetic acid (NOTA) chelator via
a diethylene glycol (PEG2) spacer
(NOTA-PEG2-RM26). This construct was labeled with
68Ga, 111In and Al18F and showed
favorable pharmacokinetic properties (9,10).
The high affinity to GRPR (KD in the picomolar range)
suggested that no further modifications of the peptide sequence are
required (9). Nonetheless, several
other parameters known to influence the targeting and
biodistribution properties of radiopeptides could be employed to
obtain higher sensitivity and specificity (11). Such parameters include overall and
local distribution of charge and lipophilic patches and can be
tuned by modifications to the spacers, chelating moieties and
radionuclides.
Therefore, in a further attempt to optimize the
biodistribution profile for possible clinical use, several
modifications in the length of spacers and different chelating
agents have been explored. The effects of the length of PEG spacers
as hydrophilicity modifiers (NOTA-PEGn-RM26, n=2,3,4,6)
were shown to be minor (12).
However, the use of different macrocyclic chelators for the
labeling of RM26 with 68Ga had a profound influence on
the biodistribution profile of bombesin analogs, appreciably
altering the blood clearance, tumor uptake and kidney retention of
radioactivity (13). In this
regard, the constructs containing the triaza chelators NOTA and
1,4,7-triazacyclononane,1-glutaric acid-4,7-acetic acid (NODAGA),
namely 68Ga-NOTA-PEG2-RM26 and
68Ga-NODAGA-PEG2-RM26 provided better imaging
properties (higher tumor-to-organ ratios) compared to
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and
1,4,7,10-tetraazacyclododecane,1-(glutaric acid)-4,7,10-triacetic
acid (DOTAGA) coupled analogs. The higher tumor uptake and faster
clearance from blood and healthy tissues indicated that NOTA is the
superior chelator for 68Ga labeling of RM26 (NOTA >
NODAGA > DOTA > DOTAGA) for imaging of prostate cancer using
positron emission tomography (PET) (13).
To date, despite its value as a high-end diagnostic
tool, PET imaging is limited by the large infrastructure required
for the production of β+-emitting radioisotopes and the
higher cost of imaging equipment, making PET an expensive
technology (14). Single-photon
emission computed tomography (SPECT) cameras, on the other hand,
are more widely available, and there is a broader array of
available and less-expensive SPECT radiotracers compared to PET
tracers. Moreover, SPECT radionuclides have a longer half-life,
which allows for imaging at later time points when better contrast
can be achieved. Additionally, SPECT radionuclides can be
transported to distant hospitals and imaging centers. One commonly
used SPECT radionuclide in clinical context is 111In
(2.8 days half-life).
Different radionuclides have also been known to
impact the behavior of radiopharmaceuticals (15,16).
It is increasingly evident that the influence of radionuclides is
intimately connected to the chelator moiety. Therefore, matching
the isotopes and chelators could substantially improve the
pharmacokinetic properties of radiotracers (17). A good match depends on a multitude
of factors, including the coordination number of the metal ion,
chelator denticity, radionuclide-chelator complex geometry,
oxidation state of the metal ion, and the rate of complex formation
and dissociation (18).
The aim of the current study was to evaluate the
influence of the radionuclide-chelator complex on the
biodistribution and targeting properties of
111In-labeled bombesin antagonist RM26 and to identify
an optimal construct for SPECT imaging of GRPR expression. For this
purpose, four different constructs containing NOTA, NODAGA, DOTA
and DOTAGA macrocyclic chelators [further denoted as
X-PEG2-RM26, X=NOTA, NODAGA, DOTA, DOTAGA (Fig. 1)] were radiolabeled with
111In and evaluated in vitro and in
vivo.
Materials and methods
Peptide synthesis
X-PEG2-RM26 (RM26= [D-Phe6, Sta13,
Leu14]Bombesin[6–14], X= NOTA, DOTA, NODAGA and DOTAGA) were
synthesized by manual solid-phase peptide synthesis (SPPS) using
standard Fmoc/t-Bu conditions, as previously described (9,13).
The identity was confirmed by HPLC/MS using a Kinetex 2.6 μm C18
(50×3.0 mm) column and a 2.5-min, 5–60% acetonitrile/water (0.05%
formic acid) gradient. Purity was determined by UV-HPLC (220 nm)
and was >96.5% for all conjugates (13).
Radiolabeling
All 111In labelings and quality controls
were performed based on protocols developed and presented
previously (9). Briefly, an
aqueous solution of 10 nmol (10 μl in Milli-Q water) of
X-PEG2-RM26 was buffered with 80 μl (0.2 M, pH 5.5) of
ammonium acetate (Merck). The buffers for 111In labeling
were purified from metal contamination using Chelex 100 resin
(Bio-Rad Laboratories). After the addition of 60 MBq (80–150 μl in
0.05 M hydrochloric acid) of 111In (Covidien), the
reaction mixture was incubated for 10 min at 90°C.
The yield, radiochemical purity and in vitro
stability studies of 111In-X-PEG2-RM26 were
analyzed using instant thin-layer chromatography (ITLC) strips
(150-771 Dark Green, Tec-Control Chromatography strips from Biodex
Medical Systems). Citric acid (0.2 M, pH 2.0) was used as the
running buffer. In this system, free indium and its complexes
migrate with the solvent front (Rf=1.0), while peptides
remain at the origin (Rf=0.0). The system was previously
cross-validated using radioHPLC and radioSDS-PAGE (sodium dodecyl
sulfate-polyacrylamide gel electrophoresis) (9,10).
To test the labeling stability,
111In-X-PEG2-RM26 was incubated for 1 h at
room temperature in the presence of 1000-fold molar excess of EDTA
disodium salt (Sigma). The samples were analyzed by ITLC.
Before in vivo studies, the reaction mixtures
of 111In-X-PEG2-RM26 were additionally
purified using solid phase extraction. Briefly, the reaction
mixtures were diluted with 3 ml of deionized water and passed
through a 1 ml Oasis HLB cartridge (Waters). The cartridge was then
washed with 5 ml of deionized water. The radiolabeled product was
eluted with 1 ml of 1:1 EtOH/water.
In vitro studies
GRPR-expressing PC-3 human prostate cancer cells
(ATCC) were cultured in RPMI media complemented with 10% fetal calf
serum, 2 mM L-glutamine and PEST (penicillin 100 IU/ml) (all from
Biochrom AG). The cells were detached using trypsin-EDTA solution
(0.05% trypsin, 0.02% EDTA in buffer; Biochrom AG). All experiments
were performed in triplicate and 0.7×106 cells/dish were
seeded two days before the experiment.
For the in vitro binding specificity study,
PC-3 cells were incubated with 1 nM
111In-X-PEG2-RM26 solutions for 1 h at 37°C.
One set of dishes in each experiment was pre-incubated with
100-fold excess of unlabeled peptide, added 10 min before the
addition of the radiolabeled compounds. After being washed once
with serum-free media, cells were treated with 0.5 ml trypsin
solution. Cell-associated radioactivity was measured in an
automated gamma-counter (3-inch NaI(Tl) detector, 1480 Wizard,
Wallac Oy) and presented as percentage from added
radioactivity.
Cellular processing was performed on PC-3 cells,
which were incubated with 2 nM of
111In-X-PEG2-RM26 at 37°C. At predetermined
time points (1, 2, 4, 8, and 24 h after the start of incubation),
the incubation medium was discarded, the cells were washed and the
membrane-bound and internalized radioactivity was collected using
the acid wash method previously described (9).
The half-inhibitory concentration (IC50)
was estimated for natIn-loaded metallopeptides by
complete displacement experiments using the universal BN
radioligand 125I-Tyr4-BBN (Perkin Elmer) and
increasing concentrations of the metal-lopeptides (0–1000 nM). Cell
monolayers were incubated with
natIn-X-PEG2-RM26 in the presence of 0.1 pmol
(~200,000 cpm) 125I-Tyr4-BBN for 5 h at 4°C.
After incubation, the cells were harvested, and cell-associated
radioactivity was determined as described above. The half-maximal
inhibitory concentration values were calculated by fitting the data
by nonlinear regression using GraphPad Prism software (GraphPad
Software Inc.).
The kinetic binding studies were performed using
LigandTracer Yellow Instruments (Ridgeview Instruments AB) at room
temperature, as previously described (19). Briefly, a Petri dish (Nunclon,
diameter 100 mm, containing 3 ml culture medium) with PC-3 cells
was attached to the rotating table of the instrument. After a
10-min baseline run, 111In-X-PEG2-RM26 was
added to the medium to obtain a ligand concentration of 0.3 nM, and
the uptake curve was recorded for 200 min. Thereafter, the ligand
concentration was increased to 10 nM, and the uptake curve was
recorded for another 150 min. Then,
111In-X-PEG2-RM26-containing medium was
aspirated, 3 ml of fresh medium were added, and the dissociation
curve was followed overnight. Interaction analysis and calculation
of the equilibrium dissociation constant (KD) were
performed with TracerDrawer software (Ridgeview Instruments
AB).
In vivo studies
All animal experiments were planned and performed
according to the national legislation on the protection of
laboratory animals, and the study plans were approved by the local
committee for animal research ethics. Groups of 4 mice per data
point were used. The biodistribution and targeting to GRPRs of
111In-X-PEG2-RM26 were evaluated in female
BALB/c nu/nu mice (weight: 21±1 g) bearing PC-3 xenografts
(107 cells/mouse, implanted 2 weeks before the
experiment). The average tumor size was 0.32±0.17 g at the time of
the experiment.
To study tumor targeting, mice bearing PC-3
xenografts were intravenously injected into the tail vein with 45
pmol of 111In-X-PEG2-RM26 (30 kBq, 100 μl).
The injected peptide dose was adjusted by dilution with non-labeled
X-PEG2-RM26. The mice were euthanized at 4 and 24 h
post-injection (p.i.) by intraperitoneal injection of a
Ketalar-Rompun solution (10 mg/ml Ketalar and 1 mg/ml Rompun; 20 μl
of solution per gram of body weight). Blood samples were collected
by heart puncture. The organs of interest were collected and
weighed, and their radioactivity content was measured in a
gamma-counter. The organ uptake values were expressed as a
percentage of injected dose per gram of tissue weight (% ID/g).
Small animal SPECT/CT imaging
Whole body scans of the subjects, injected with
111In-X-PEG2-RM26 (45 pmol, 300 kBq) were
performed using the Triumph™ Trimodality System (TriFoil Imaging,
Inc., Northridge, CA, USA) at 4 h and 24 h p.i. The subjects were
euthanized by CO2 asphyxiation immediately before being
placed in the camera. A computed tomography (CT) acquisition was
first carried out to position the body of the animal in the camera
at the following parameters: field of view (FOV), 80 mm;
magnification, 1.48; one projection and 512 frames for 2.13 min.
Subsequently, SPECT acquisition was performed in same position with
the following parameters: FOV, 8 cm; 75A10 collimators (5 pinhole);
acquisition energy window over 150–250 keV; 32 projections.
CT raw files were reconstructed by Filter Back
Projection (FBP). SPECT raw data were reconstructed by FLEX SPECT
software, which uses an ordered Subset Expectation Maximization
(OSEM) iterative reconstruction algorithm. SPECT and CT data were
fused and analyzed using PMOD v3.508 (PMOD Technologies Ltd.,
Zurich, Switzerland). Coronal SPECT-CT images of the scans were
presented as maximum intensity projections (MIP) in RGB color scale
to obtain a visual confirmation of the biodistribution results.
Statistics
Statistical analyses were performed by unpaired,
two-tailed t-test using GraphPad Prism (version 4.00 for windows
GraphPad Software, San Diego, CA, USA). P-values <0.05 were
considered significant.
Results
Labeling chemistry
All constructs were successfully labeled with
111In, and the average yield was above 97% (Table I). Therefore, no additional
purification was required for in vitro studies. Stability
was evaluated in the presence of both 1000-fold molar excess of
EDTA and in PBS for 1 h at room temperature, and the subsequent
ITLC analysis showed stable coupling of 111In for all
conjugates (Table I).
 | Table ILabeling and stability of
111In-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA and
DOTAGA)a. |
Table I
Labeling and stability of
111In-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA and
DOTAGA)a.
| NOTA | NODAGA | DOTA | DOTAGA |
|---|
| Labeling yield for
111In | 98.5±0.5 | 99.6±0.2 | 99.2±0.8 | 97.6±2.3 |
| Purity after
HLB | 100 | 100 | 100 | 100 |
| Release in the
presence of excess EDTA | 3.7±0.2 | 6.2±0.3 | 0.4±0.5 | 2.4±3.3 |
| Release in PBS | 1.5±0.6 | 1.7±0.1 | 0 | 0.9±0.6 |
In vitro characterization
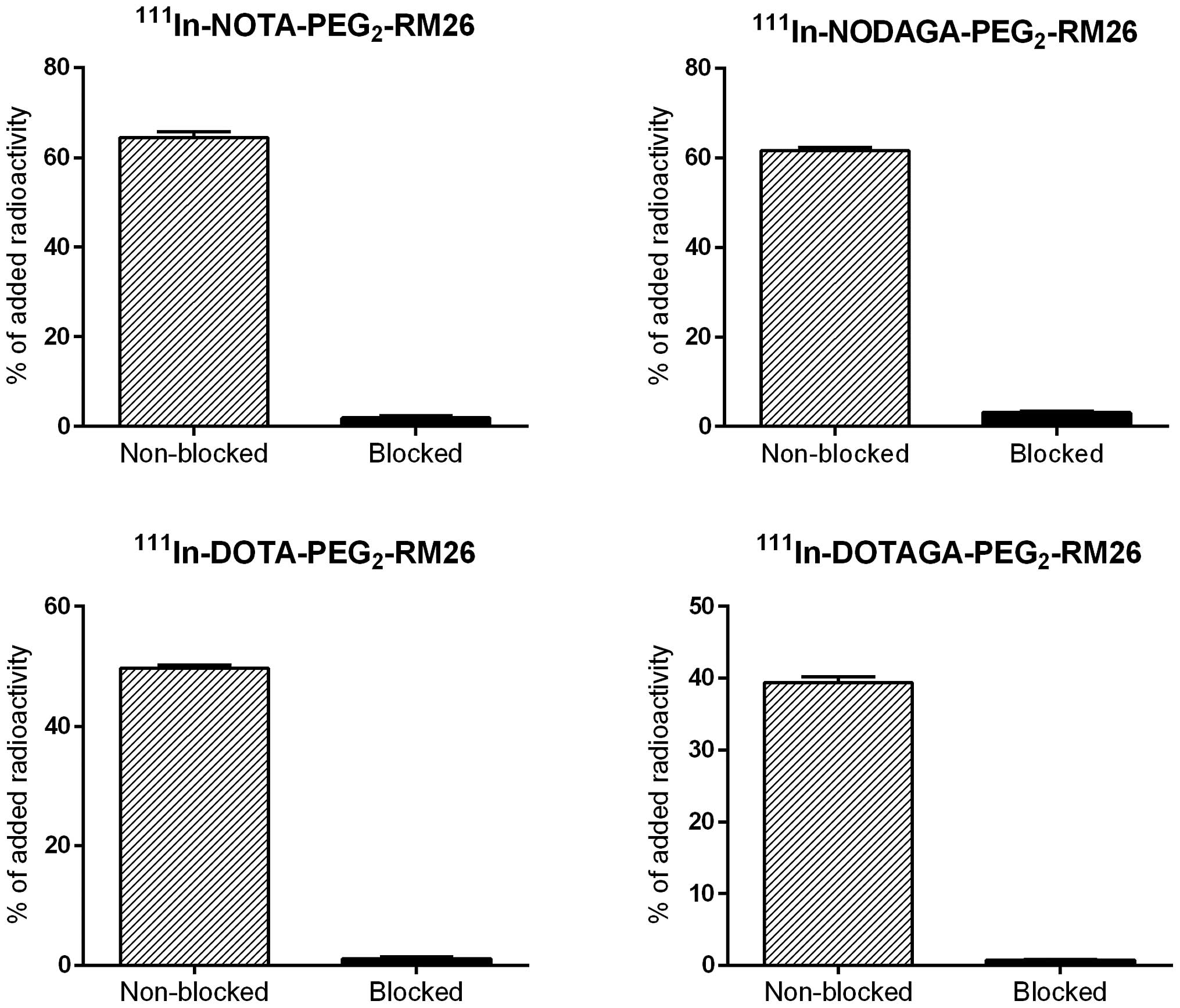
The results of binding specificity tests are
presented in Fig. 2.
Pre-saturation of receptors by adding a large molar excess of
non-labeled peptide caused a significant (p<1.3×10−7)
reduction in the radioactivity bound to GRPR-expressing PC-3 cells,
demonstrating that the uptake was receptor mediated.
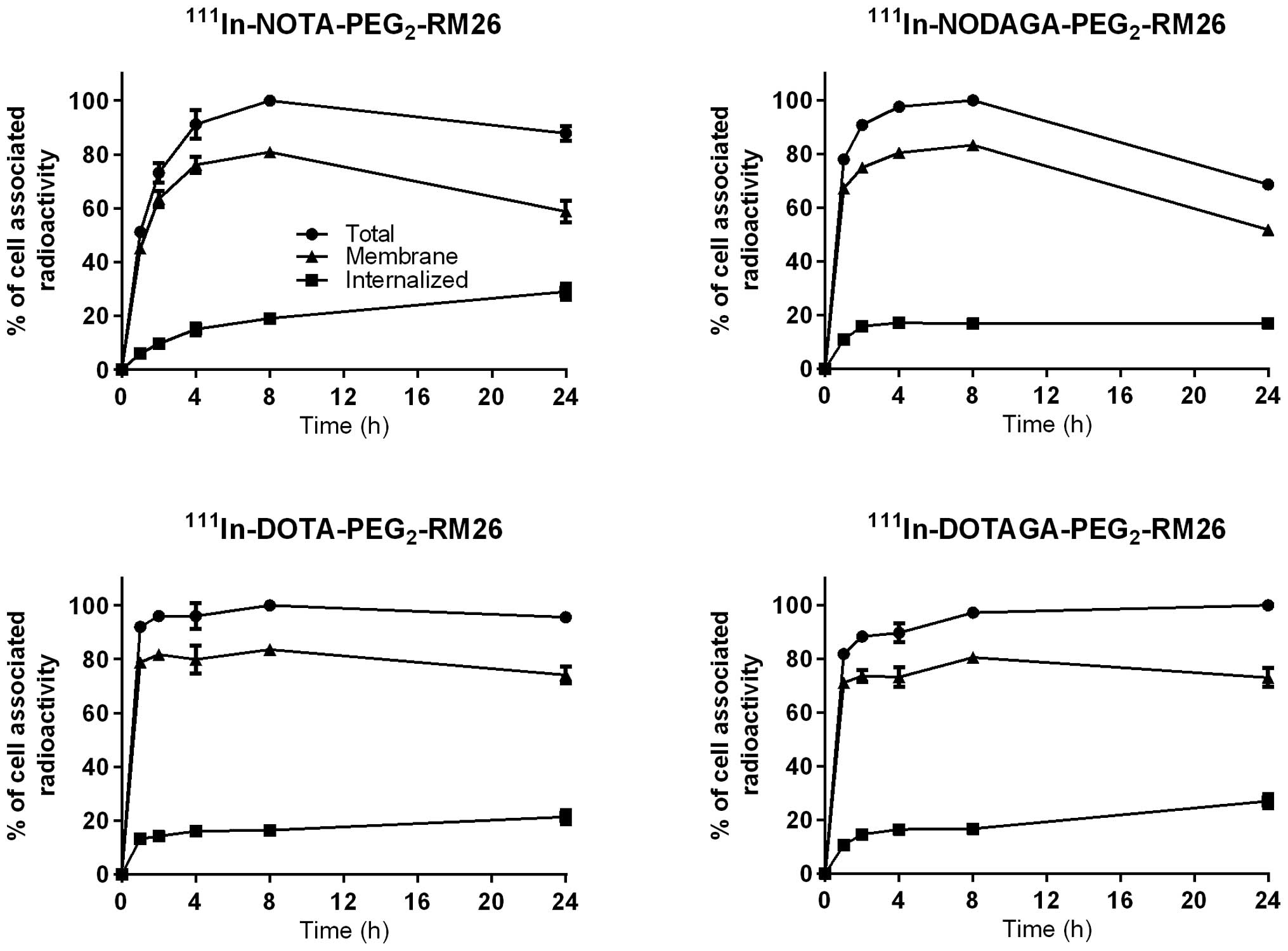
Data concerning cellular processing of
111In-X-PEG2-RM26 by PC-3 cells are presented
in Fig. 3. The rapid binding of
all four conjugates to PC-3 cells was accompanied by slow
internalization, reaching 33% of cell-associated radioactivity for
111In-NOTA-PEG2-RM26; 25% for
111In-NODAGA-PEG2-RM26; 22% for
111In-DOTA-PEG2-RM26; and 27% for
111In-DOTAGA-PEG2-RM26 after 24 h of
incubation at 37°C. The pattern of cell-associated radioactivity
over time for 111In-NODAGA-PEG2-RM26 differed
from the patterns observed for the other conjugates, despite the
fact they were tested simultaneously. Maximum cell- associated
radioactivity was reached after 8 h of incubation for all
conjugates. For 111In-NODAGA-PEG2-RM26, the
cell-associated radioactivity decreased by 30% from the maximum at
24 h, while for the other conjugates it plateaued.
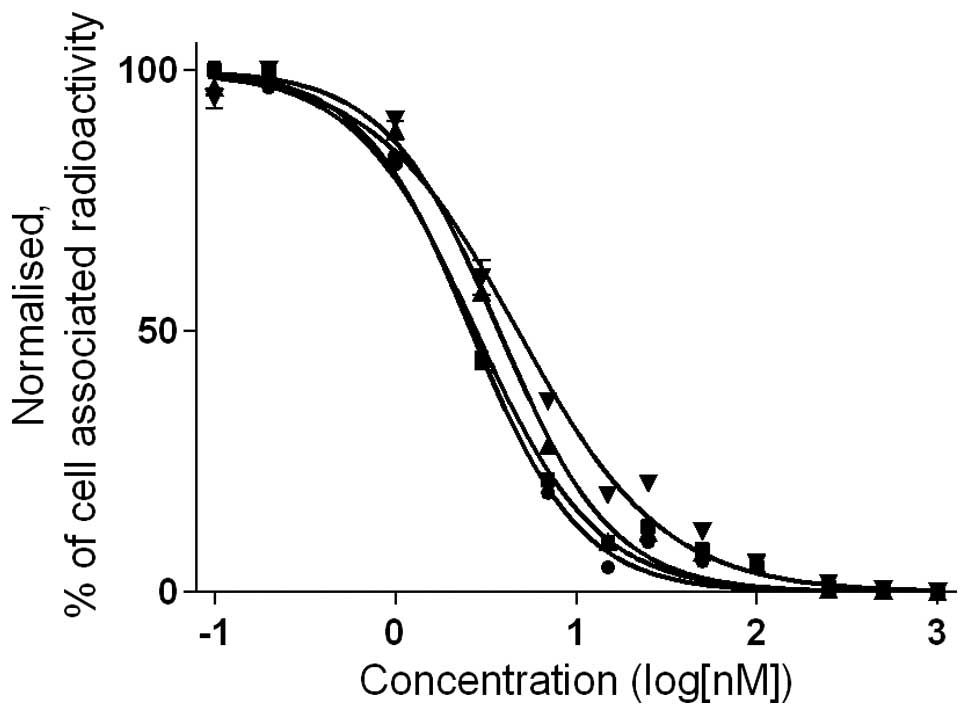
The half-maximal inhibitory concentration
(IC50) was determined for natIn-loaded
analogs using 125I-Tyr4-BBN as a displacement
radioligand (Table II and
Fig. 4). All IC50
values were in the low nanomolar range. However, chelator-dependent
differences in affinity could be seen. The IC50 values
of natIn-X-PEG2-RM26 were lower for
positively charged NOTA (2.6±0.1 nM), followed by neutral NODAGA
and DOTA complexes (3.7±0.2 and 2.8±0.2 nM, respectively). The
highest IC50 values (lowest affinity) were obtained for
the negatively charged natIn-DOTAGA-PEG2-RM26
(4.8±0.5 nM).
 | Table IIInhibition of
125I-Tyr4-BBN binding to PC-3 cells with
natIn-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA and DOTAGA). |
Table II
Inhibition of
125I-Tyr4-BBN binding to PC-3 cells with
natIn-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA and DOTAGA).
|
111In-X-PEG2-RM26 | Competitive binding
assay-IC50 (nM)a | Real-time binding
kinetics (LigandTracer)b |
|---|
|
|---|
| ka
(M−1sec−1) | kd
(sec−1) | KD
(nM) |
|---|
| NOTA | 2.6±0.1 | (1.7±0.7)
×105 | (3.1±0.6)
×10−5 | 0.21±0.09 |
| DOTA | 2.8±0.2 | (1.1±0.2)
×105 | (3.1±1.6)
×10−5 | 0.37±0.08 |
| NODAGA | 3.7±0.2 | (0.4±0.1)
×105 | (1.3±1.4)
×10−5 | 0.36±0.11 |
| DOTAGA | 4.8±0.5 | (1.4±0.1)
×105 | (6.1±1.4)
×10−5 | 0.44±0.05 |
The binding affinity of
111In-X-PEG2-RM26 to PC-3 cells was measured
using LigandTracer Yellow instruments (Table II). The calculated KD
values were in the subnanomolar range and had the same pattern as
the IC50 values, i.e., the affinity of
111In-NOTA-PEG2-RM26 was the highest and the
affinity of 111In-DOTAGA-PEG2-RM26 was the
lowest. It should be noted that all values were in a very narrow
range.
In vivo studies
Biodistribution of radiolabeled conjugates and
comparison of their in vivo tumor targeting were performed
in female BALB/c nu/nu mice bearing PC-3 xenografts 4 and 24 h
p.-i. (Table III). Data obtained
for 111In-NOTA-PEG2-RM26 at 24 h p.i. were in
good agreement with previously published results (9). All conjugates demonstrated rapid
whole body and blood clearance via kidney excretion. The rapid
blood clearance indicated good stability of radiolabeled compounds
toward transchelation to blood proteins. All conjugates
demonstrated rapid and nearly equal uptakes in tumors 4 h p.i.
However, the radioactivity retention in tumors at 24 h p.i.
differed. While the average radioactivity concentration decreased
by 25–30% for the NOTA-and NODAGA-containing variants, it dropped
by 40% for the DOTAGA conjugate and by 60% for the DOTA
conjugate.
 | Table IIIBiodistribution of
111In-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA,
DOTAGA) in PC-3-xenografted BALB/c nu/nu mice 4 and 24 h p.i. |
Table III
Biodistribution of
111In-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA,
DOTAGA) in PC-3-xenografted BALB/c nu/nu mice 4 and 24 h p.i.
| NOTA | NODAGA | DOTA | DOTAGA |
|---|
|
|
|
|
|
|---|
| Organ | 4 h p.i. | 24 h p.i. | 4 h p.i. | 24 h p.i. | 4 h p.i. | 24 h p.i. | 4 h p.i. | 24 h p.i. |
|---|
| Blood |
0.0535±0.003a,b | 0.008±.002b,c | 0.15±0.01c | 0.012±0.003b,c | 0.13±0.02c | 0.023±0.004 | 0.07±0.02 | 0.025±0.008 |
| Lung | 0.20±0.04b,c | 0.06±0.03 | 0.18±0.03b,c | 0.06±0.06 | 0.08±0.01 | 0.022±0.008 | 0.08±0.02 | 0.05±0.02 |
| Liver | 2.4±0.2a,b,c | 1. 5±0.2a,b,c | 1.2±0.2c | 0.41±0.08 | 1.0±0.3c | 0.30±0.08 | 0.57±0.10 | 0.4±0.1 |
| Spleen | 0.7±0.1b | 0.29±0.05c | 0.6±0.1b | 0.28±0.07c | 1.1±0.3 | 0.4±0.1 | 0.7±0.3 | 0.6±0.1 |
| Pancreas | 2.2±0.2a,b,c | 0.076±0.009a,b,c | 16±2b,c | 0.22±0.05b,c | 0.19±0.0c | 0.02±0.01 | 0.11±0.02 | 0.021±0.004 |
| Stomach | 1.1±0.3b,c | 0.09±0.03a,b,c | 2.0±0.7b,c | 0.19±0.08b,c | 0.15±0.0c | 0.03±0.01 | 0.07±0.01 | 0.02±0.01 |
| Intestines | 1.1±0.1a,b,c | 0.09±0.01b,c | 2.9±0.6b,c | 0.10±0.03b,c | 0.06±0.02 | 0.02±0.01 | 0.06±0.01 | 0.02±0.01 |
| Kidney | 3.6±0.3a,b,c | 1.3±0.3a,b | 6.5±0.8b,c | 2.6±0.4b,c | 2.4±0.2c | 0.63±0.02c | 2.9±0.2 | 1.7±0.3 |
| Tumor | 2.5±0.9 | 1.7±0.5a | 3.6±0.8c | 2.7±0.6b,c | 3.4±0.6c | 1.27±0.2 | 2.3±0.4 | 1.4±0.3 |
| Muscle | 0.06±0.01b,c | 0.020±0.005a,b,c | 0.05±0.02b,c | 0.006±0.003 | 0.016±0.003 | 0.002±0.004 | 0.012±0.005 | 0.008±0.006 |
| Bone | 0.11±0.03b,c | 0.07±0.02 | 0.08±0.02c | 0.04±0.02 | 0.05±0.03 | 0.03±0.02 | 0.03±0.01 | 0.03±0.02 |
| Carcassd | 4.4±0.2a,b,c | 0.9±0.3 | 12.6±0.8b,c | 0.65±0.06b | 1.81±0.4 | 0.4±0.1 | 1.5±0.5 | 0.5±0.3 |
The pattern of radioactivity distribution in normal
organs was different for the studied conjugates. The accumulated
radioactivity in the liver and GRPR-expressing organs was
significantly higher for 111In-NOTA-PEG2-RM26
and 111In-NODAGA-PEG2-RM26 compared to
111In-DOTA-PEG2-RM26 and
111In-DOTAGA-PEG2-RM26.
111In-NOTA-PEG2-RM26 showed the highest
initial uptake and the slowest clearance of radioactivity from the
liver. 111In-DOTA-PEG2-RM26 had a
significantly lower retention of radioactivity in kidneys at both 4
and 24 h p.i. In addition, DOTA- and DOTAGA-conjugates had a
significantly lower radioactivity concentration in muscle and bones
than the other two variants at both time points. There was a clear
chelator-dependent difference in the uptake of radioconjugates in
receptor-positive organs (pancreas, stomach and small intestines)
at 4 h p.i., when DOTA- and DOTAGA-containing variants showed a
significantly lower uptake. At 4 h p.i., the pancreatic uptake of
radioactivity was one order of magnitude higher for NOTA- and two
orders higher for NODAGA-coupled analogs compared to DOTA- and
DOTAGA-containing variants.
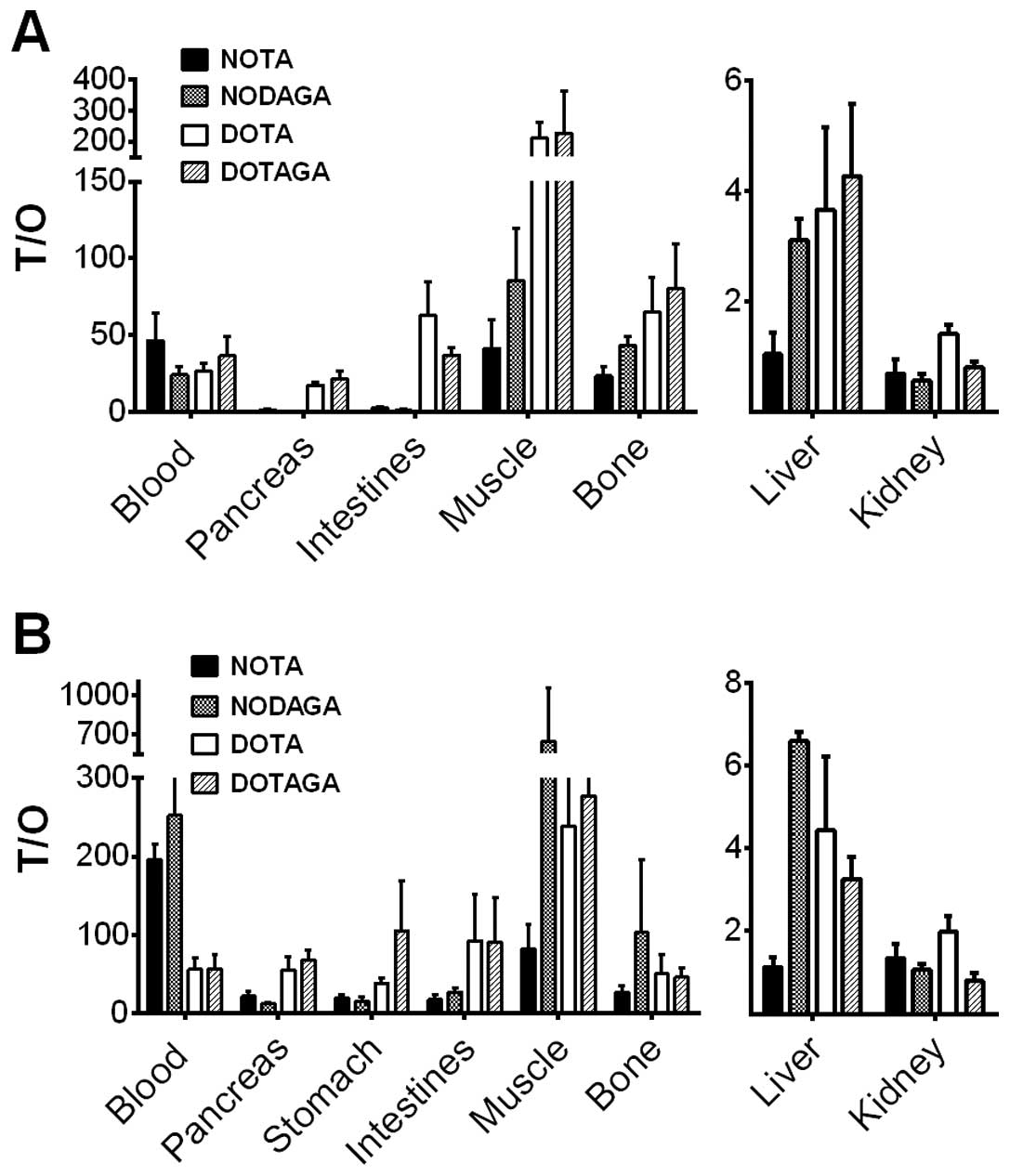
Tumor-to-organ ratios are presented in Fig. 5 and Table IV. Remarkably, DOTA- and
DOTAGA-coupled analogs provided significantly higher tumor-to-lung,
tumor-to-pancreas, tumor-to-stomach, tumor-to-small intestines,
tumor-to-muscle and tumor-to-bone ratios compared to NOTA- and
NODAGA-containing variants at 4 h p.i. The radioactivity
accumulation in tumors at 4 h p.i. was exceeded only by kidney
uptake in the case of 111In-DOTAGA-PEG2-RM26,
and it was higher than that in all normal organs, including
excretory organs, for 111In-DOTA-PEG2-RM26.
The 24 h p.i. tumor-to-organ ratios were >1 for all conjugates
except for the tumor-to-kidney ratios for NOTA, NODAGA and DOTAGA
conjugates and the tumor-to-liver ratio for NOTA conjugate. The
washout of radioactivity from normal organs, including
receptor-positive organs, was more rapid than from tumors. The
NODAGA conjugate demonstrated the highest tumor-to-blood
(significantly over DOTA and DOTAGA conjugates), tumor-to-muscle,
and tumor-to-bone ratios at 24 h p.i. among the tested
conjugates.
 | Table IVTumor-to-normal-tissue ratios of
111In-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA and
DOTAGA) in PC-3-xenografted BALB/c nu/nu mice 4 h and 24 h p.i. |
Table IV
Tumor-to-normal-tissue ratios of
111In-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA and
DOTAGA) in PC-3-xenografted BALB/c nu/nu mice 4 h and 24 h p.i.
| NOTA | NODAGA | DOTA | DOTAGA |
|---|
|
|
|
|
|
|---|
| Organ | 4 h p.i. | 24 h p.i. | 4 h p.i. | 24 h p.i. | 4 h p.i. | 24 h p.i. | 4 h p.i. | 24 h p.i. |
|---|
| Blood | 47±18 | 196±20b,c | 24±5 | 253±93b,c | 27±6 | 56±14 | 36±13 | 58±17 |
| Lung | 13±6a,b,c | 29±9b | 20±1b,c | 82±49 | 44±7c | 64±18c | 30±5 | 33±14 |
| Liver | 1±0.4a,b,c | 1±0.2a,b,c | 3.1±0.4 | 6.6±0.2c | 4±1 | 4±2 | 4±1 | 3.3±0.5 |
| Spleen | 4±1a | 6±1a,c | 6±1b | 10±1b,c | 3.1±0.6 | 4±2 | 4±2 | 2.5±0.7 |
| Pancreas | 1.1±0.5a,b,c | 22±6a,b,c | 0.23±0.04b,c | 12±2b,c | 18±2 | 55±17 | 21±5 | 69±13 |
| Stomach | 2.3±0.6b,c | 20±4b,c | 1.8±0.6b,c | 16±6b,c | 24±6c | 39±7 | 35±4 | 106±64 |
| Small
intestines | 2±1b,c | 18±7b,c | 1.3±0.4b,c | 27±5 | 62.63±22c | 93±58 | 37±5 | 92±56 |
| Kidney | 0.7±0.2b | 1.3±0.3c | 0.6±0.1b,c | 1.1±0.2b | 1.41±0.2c | 2.0±0.4c | 0.8±0.1 | 0.8±0.2 |
| Muscle | 42±19b,c | 83±30a | 86±34b | 646±408 | 212±52 | 284±126 | 228±136 | 277±252 |
| Bone | 23±6a,b,c | 27±8 | 43±6c | 104±92 | 65±22 | 51±25 | 80±29 | 46±12 |
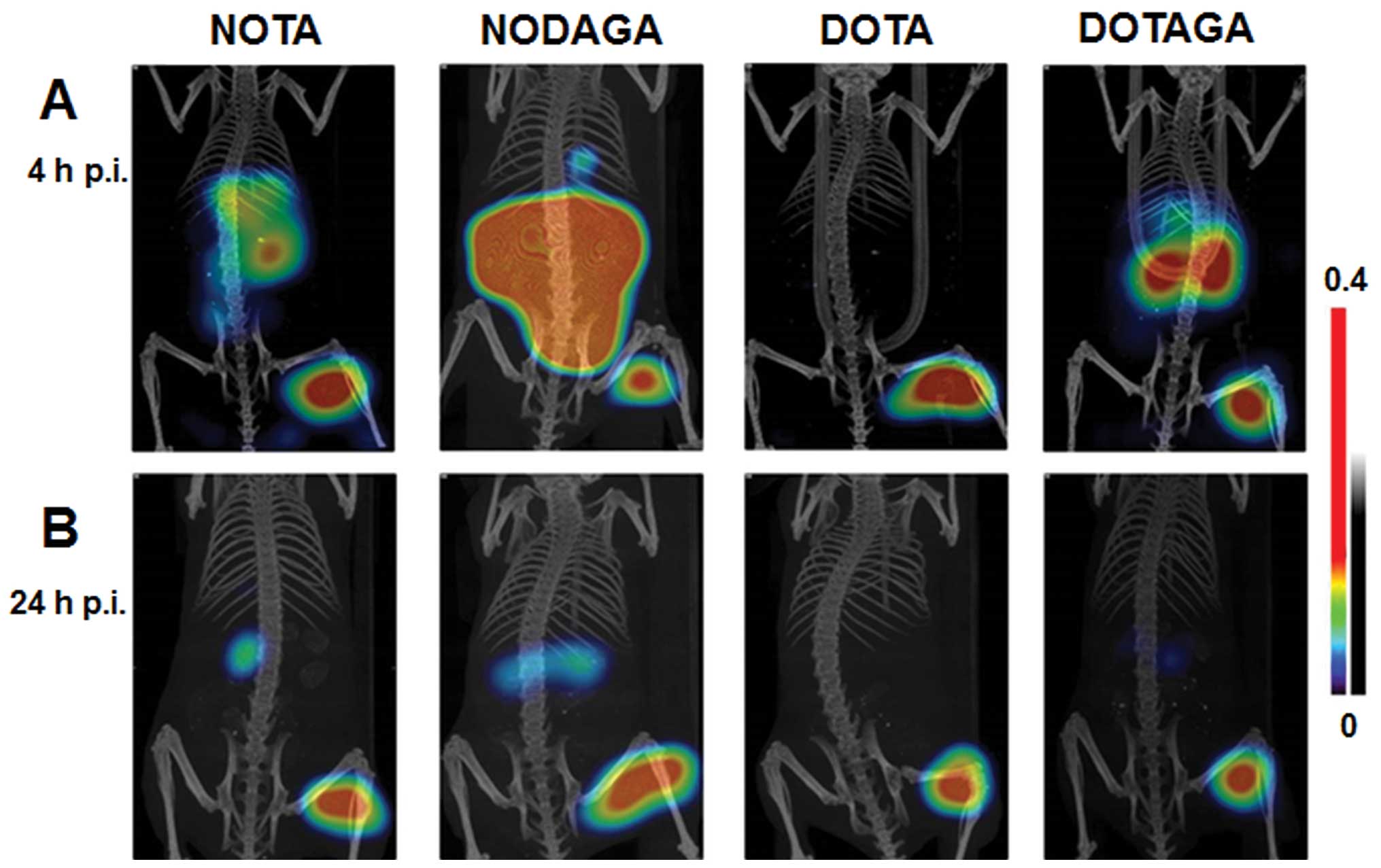
Imaging studies
Fig. 6 shows the
coronal gamma camera scans acquired 4 and 24 h after the i.v.
injection of 111In-X-PEG2-RM26 (X=NOTA,
NODAGA, DOTA, DOTAGA) into mice bearing PC-3 xenografts. The
maximum intensity projection (MIP) images confirmed the capacity of
all radio-conjugates to clearly visualize GRPR expression. The
higher uptake of NOTA and NODAGA at the earlier time point in
receptor-positive organs in the abdomen can also be seen in the
gamma camera images. The significantly higher tumor-to-kidney
ratios obtained at 4 h p.i. for
111In-DOTA-PEG2-RM26 resulted in superior
images, where only the tumor uptake was visualized. At 24 h p.i.,
signals from tumors in all studied conjugates dominated, while
traces of radioactivity accumulation were visible in the kidneys
for all conjugates except the DOTA-containing one.
Discussion
We have recently reported the influence of chelating
moieties on the pharmacokinetic properties of
68Ga-labeled bombesin antagonist RM26
(D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2) (13). The four constructs, which differed
only in the chelating moieties
(68Ga-X-PEG2-RM26, X=NOTA, NODAGA, DOTA,
DOTAGA), showed significantly different biodistribution profiles,
where 68Ga-NOTA-PEG2-RM26 was the most
promising agent for PET imaging in terms of tumor uptake, blood and
whole body clearance, level of renal reabsorption of radioactivity,
and tumor-to-nontumor ratios.
Despite the significant progress in the clinical
application of PET during last decade, SPECT is still used for over
75% of all nuclear imaging procedures. Taking into account new
developments in SPECT/CT, the number of established SPECT cameras
and the low price of SPECT relative to PET, the development of
SPECT tracers remains relevant. The above-mentioned conjugates
(X-PEG2-RM26, X=NOTA, NODAGA, DOTA, DOTAGA) could be
easily labeled with indium-111, a three-valent radiometal suitable
for SPECT. An additional advantage of 111In is its
longer half-life (2.8 days), which allows for imaging
investigations at later time points (up to 2–3 days after
administration). The investigations at later time points could
potentially increase sensitivity due to the clearance of non-bound
radioactivity from blood and healthy tissues. For prostate cancer,
the detection of low abdominal lymph node involvement, when the
enlargement could not be detected by CT or magnetic resonance
tomography (MRT), is an ultimate goal for nuclear imaging. It was
demonstrated that for secure SPECT detection of lesions with a
diameter of 8 mm, an effective uptake ratio of 84 is required
(20).
At the same time, it is known that changing the
radiometal could dramatically influence the affinity and
biodistribution profile, leading to altered imaging properties even
when radiometals have the same valency (21). Ga3+-DOTATOC had a 5-fold
higher affinity to somatostatin receptor type 2 (SSTR2) and a
2-fold higher tumor uptake compared to Y3+-DOTATOC
(22). These pronounced
differences were attributed to the different coordination
geometries of Ga3+ and Y3+ with DOTA, which
resulted in conformational differences in the D-Phe1
residue (23). Indium-111 has an
identical oxidation state (+III) as the previously studied
gallium-68 but is different in size, having a larger ionic radius
(0.92 Å) compared to that of gallium (0.65 Å). The coordination
numbers of these metals are also different. Gallium is
hexacoordinated to DOTA, the chelator adopting a pseudo-octahedral
cis geometry with one free carboxylate group deprotonated at
physiological pH, whereas indium is octacoordinated to DOTA,
exhibiting, in this case, a somewhat distorted square-antiprismatic
geometry (16,22). Both GaIII and
InIII are hexacoordinated to NOTA, forming trigonal
prisms (24). However, indium-NOTA
complexes have a noticeably distorted geometry (25). Some structural data for
GaIII and InIII complexes with NODAGA and
DOTAGA can be drawn from the aforementioned parent NOTA and DOTA
chelators. Taking the above in consideration, the imaging
properties of the indium-111 labeled anti-GRPR antagonistic analog
PEG2-RM26 coupled to different chelators should be
re-evaluated.
All four compounds (X-PEG2-RM26, X=NOTA,
NODAGA, DOTA, DOTAGA) (Fig. 1)
were successfully radiolabeled with 111In, had almost
identical quantitative yields and maintained in vitro
binding specificity to GRPR-expressing PC-3 cells (Fig. 2). The cellular processing study
confirmed the antagonistic properties of all conjugates, showing
low internalization that reached 30% of cell-associated
radioactivity after 24 h. The similar internalization patterns
indicated that chelators had no major effect on the cellular
processing of analogs, although some differences could be observed.
The NOTA-containing conjugate had the highest internalized
radioactivity fraction and the NODAGA-containing conjugate
demonstrated a decrease of cell-associated radioactivity between 8
and 24 h of incubation (Fig. 3).
It is of interest that the NODAGA-containing conjugate demonstrated
the best radioactivity retention in tumors, which lead to superior
tumor-to-blood and tumor-to-muscle ratios at 24 h p.i.
The binding properties of
natIn-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA,
DOTAGA) correlated with the net charge of radionuclide-chelator
complexes, as shown by the competitive binding assay. While all
IC50 values were in the low nanomolar range, the
presence of a positive charge at the N terminus in the case of
111In-NOTA-PEG2-RM26 had a positive effect on
binding affinity, while a negative charge
(111In-DOTAGA-PEG2-RM26) resulted in lower
affinity (Fig. 4 and Table II). These results are similar to
those obtained for 68Ga-labeled conjugates and
somatostatin analogs (13,26,27).
Ligand-receptor binding was also measured in real-time using
LigandTracer instruments. The obtained KD values were in
the subnanomolar range and had the same pattern as the
IC50 values, confirming the favorable influence of an
N-terminus positive charge on the binding affinity of the
peptides.
The biodistribution of
111In-X-PEG2-RM26 (X=NOTA, NODAGA, DOTA,
DOTAGA) in mice was characterized by a rapid clearance of
radioactivity from the blood and non-GRPR-expressing organs
(Table III). The excretion of
radioactivity was predominantly via kidney ultrafiltration.
Interestingly, the radioactivity uptake in the liver was
significantly higher for 111In-NOTA-PEG2-RM26
with a positively charged metal complex compared to the other
conjugates at both time points. Elevated liver uptake of lipophilic
peptide-based radiopharmaceuticals is a known phenomenon (21) and was taken in account when we
designed this imaging probe (9).
Our rationale was that polyethylene glycol-containing linkers
together with chelators should increase the local hydrophilicity of
the probe (leading to decreased hepatic uptake), and, at the same
time, not disturb the lipophilic part of the probe that is
responsible for receptor recognition. This study endorsed this
approach. It should also be noted that the conjugates containing
NODAGA and DOTA that form neutral complexes with indium-111 had a
two-fold lower liver uptake compared to the NOTA conjugate.
Moreover, the liver uptake of the DOTAGA-containing variant that
forms a negatively charged complex with indium-111 was 6-fold lower
than the one seen for the positively charged NOTA conjugate. It
should be mentioned that similar differences in affinities and
liver uptake were found for gallium-labeled variants (13). Taken together, we can assume that,
when increasing local hydrophilicity of the probe with the aim of
decreasing hepatobiliary uptake, preference should be given to
neutral moieties.
The uptake in receptor-positive organs (pancreas,
stomach and small intestine) was significantly higher for the NOTA-
and NODAGA-containing analogs. Notably, the uptake of radioactivity
in the pancreas (the most abundantly GRPR-expressing organ), was
ten-fold higher for 111In-NOTA-PEG2-RM26 and
100-fold higher for 111In-NODAGA-PEG2-RM26
compared to DOTA- and DOTAGA-coupled variants at 4 h p.i.
Nonetheless, tumor uptake of indium-111 labeled conjugates was
nearly equal, correlating with their similar affinity values. We
can speculate that the discrepancy between pancreatic and tumor
uptake is due to interspecies differences between mouse and human
GRPR. Maina et al reported a 13-fold difference between the
binding affinities of radiolabeled bombesin analog Z-070 to human
GRPR compared to mouse GRPR, whereas another bombesing analog
Demobesin1 showed no difference in binding affinity to human and
mouse GRPR (28). A similar uptake
pattern with differences in the pancreas that did not match the
tumor uptake in mice was also seen for other radiolabeled bombesin
analogs (29,30). However, the fourth extracellular
domain of GRPR responsible for the binding of bombesin analogs was
reported to be identical for humans and mice (31). In that case, the discrepancy
between pancreatic and tumor uptake could reflect differences in
off-target interactions due to different complex geometries. This
could also explain the differences between the present study and
the data published for gallium-68-labeled conjugates where a strong
correlation between affinity and pancreatic and tumor uptake was
found (13).
The higher initial uptake and slower clearance for
111In-NOTA-PEG2-RM26 in the liver is
consistent with previous results, which indicated that the presence
of a positive local charge at the N terminus of the peptide
increases the hepatic uptake of radioactivity (21). Remarkably, the kidney retention of
radioactivity was significantly lower for
111In-DOTA-PEG2-RM26 compared to the other
analogs. Overall, the tumor-to-organ ratios (except for the
tumor-to-blood ratio for the NOTA conjugate) were significantly
higher in most organs for the conjugates containing tetraza
chelators (DOTA and DOTAGA) at 4 h p.i.
111In-DOTA-PEG2-RM26 had the best
biodistribution properties at this time point (Table IV). The tumor uptake of
radioactivity exceeded the uptake in anatomically relevant organs
for prostate cancer (muscle, bone, intestine) and excretory organs
(liver and kidneys). This was confirmed by microSPECT/CT images
(Fig. 6). The higher uptake of
radioactivity in the pancreas, stomach and small intestine for
111In-NODAGA-PEG2-RM26 and higher liver
uptake for 111In-NOTA-PEG2-RM26 resulted in
an increased abdominal background. The significantly higher
tumor-to-kidney ratio resulted in the best imaging contrast for
111In-DOTA-PEG2-RM26 at 4 h p.i., when the
only visualized structure was the tumor.
At later time points, despite the significant
differences in tumor-to-nontumor ratios (see Fig. 5 and Table IV), all conjugates provided high
contrast images of GRPR-expressing tumors due to efficient
clearance of radioactivity from normal organs together with a
significantly slower release from the tumors (Fig. 6). Nonetheless, taking into account
the above-mentioned requirement of a high tumor-to-nontumor ratio
for clear detection of small lesions (20), we should note that at the later
time point of 24 h p.i., the NODAGA conjugate demonstrated the
superior biodistribution profile, with the highest contrast to
blood and anatomically relevant organs for prostate cancer, such as
muscle and bones.
In conclusion, the radionuclide-chelator complex
had a profound influence on the biodistribution and targeting
properties of 111In-labeled bombesin antagonist RM26 to
GRPR. The net charge of the radionuclide-chelator complex
influenced the binding affinity and liver uptake of the
radioconjugates. The geometry of the radionuclide-chelator complex
appeared to have an even more profound effect on the
biodistribution profile, accounting for the 4 h p.i. superiority of
tetraza chelators for 111In-labeled radioconjugates.
However, at 24 h p.i., 111In-NODAGA-PEG2-RM26
provided the best tumor-to-organ ratios for blood and organs that
are anatomically relevant for prostate cancer, and it represents a
suitable candidate for SPECT imaging of GRPR-expressing tumors.
Acknowledgements
This research was financially supported by grants
from the Swedish Cancer Society (Cancerfonden) and the Swedish
Research Council (Vetenskapsrådet).
References
|
1
|
Smith CJ, Volkert WA and Hoffman TJ:
Gastrin releasing peptide (GRP) receptor targeted
radiopharmaceuticals: A concise update. Nucl Med Biol. 30:861–868.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thompson IM, Pauler DK, Goodman PJ, Tangen
CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford
ED, et al: Prevalence of prostate cancer among men with a
prostate-specific antigen level < or =4.0 ng per milliliter. N
Engl J Med. 350:2239–2246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scardino PT, Weaver R and Hudson MA: Early
detection of prostate cancer. Hum Pathol. 23:211–222. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bubendorf L, Schöpfer A, Wagner U, Sauter
G, Moch H, Willi N, Gasser TC and Mihatsch MJ: Metastatic patterns
of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol.
31:578–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Abiraj K, Thorek DL, Waser B,
Smith-Jones PM, Honer M, Reubi JC and Maecke HR: Evolution of
bombesin conjugates for targeted PET imaging of tumors. PLoS One.
7:e440462012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bodei L, Paganelli G and Mariani G:
Receptor radionuclide therapy of tumors: A road from basic research
to clinical applications. J Nucl Med. 47:375–377. 2006.PubMed/NCBI
|
|
7
|
Ginj M, Zhang H, Waser B, Cescato R, Wild
D, Wang X, Erchegyi J, Rivier J, Mäcke HR and Reubi JC:
Radiolabeled somatostatin receptor antagonists are preferable to
agonists for in vivo peptide receptor targeting of tumors. Proc
Natl Acad Sci USA. 103:16436–16441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cescato R, Maina T, Nock B, Nikolopoulou
A, Charalambidis D, Piccand V and Reubi JC: Bombesin receptor
antagonists may be preferable to agonists for tumor targeting. J
Nucl Med. 49:318–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Varasteh Z, Velikyan I, Lindeberg G,
Sörensen J, Larhed M, Sandström M, Selvaraju RK, Malmberg J,
Tolmachev V and Orlova A: Synthesis and characterization of a
high-affinity NOTA-conjugated bombesin antagonist for GRPR-targeted
tumor imaging. Bioconjug Chem. 24:1144–1153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Varasteh Z, Aberg O, Velikyan I, Lindeberg
G, Sörensen J, Larhed M, Antoni G, Sandström M, Tolmachev V and
Orlova A: In vitro and in vivo evaluation of a (18)F-labeled high
affinity NOTA conjugated bombesin antagonist as a PET ligand for
GRPR-targeted tumor imaging. PLoS One. 8:e819322013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Varshney R, Hazari PP, Fernandez P, Schulz
J, Allard M and Mishra AK: 68Ga-labeled bombesin analogs for
receptor-mediated imaging. Theranostics, Gallium-68, and Other
Radionuclides: A Pathway to Personalized Diagnosis and Treatment.
Baum RP and Rösch F: Springer; Heidelberg, New York, Dordrecht,
London: pp. 221–256. 2013, View Article : Google Scholar
|
|
12
|
Varasteh Z, Rosenström U, Velikyan I,
Mitran B, Altai M, Honarvar H, Rosestedt M, Lindeberg G, Sörensen
J, Larhed M, et al: The effect of mini-PEG-based spacer length on
binding and pharmacokinetic properties of a 68Ga-labeled
NOTA-conjugated antagonistic analog of bombesin. Molecules.
19:10455–10472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Varasteh Z, Mitran B, Rosenström U,
Velikyan I, Rosestedt M, Lindeberg G, Sörensen J, Larhed M,
Tolmachev V and Orlova A: The effect of macrocyclic chelators on
the targeting properties of the 68Ga-labeled gastrin releasing
peptide receptor antagonist PEG2-RM26. Nucl Med Biol. 42:446–454.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Müller C and Schibli R: Single photon
emission computed tomography tracer. Molecular Imaging in Oncology.
Schober O and Riemann B: Springer; Heidelberg, New York, Dordrecht,
London: pp. 65–105. 2013, View Article : Google Scholar
|
|
15
|
Ginj M and Maecke HR: Radiometallo-labeled
peptides in tumor diagnosis and therapy. Metal Ions in Biological
Systems: Metal Complexes in Tumor Diagnosis and as Anticancer
Agents. Sigel A and Sigel H: 42. FontisMedia SA and Marcel Dekker,
Inc; pp. 109–142. 2004
|
|
16
|
Heppeler A, André JP, Buschmann I, Wang X,
Reubi JC, Hennig M, Kaden TA and Maecke HR: Metal-ion-dependent
biological properties of a chelator-derived somatostatin analogue
for tumour targeting. Chemistry. 14:3026–3034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartholomä MD, Louie AS, Valliant JF and
Zubieta J: Technetium and gallium derived radiopharmaceuticals:
Comparing and contrasting the chemistry of two important
radiometals for the molecular imaging era. Chem Rev. 110:2903–2920.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lozza C, Navarro-Teulon I, Pèlegrin A,
Pouget JP and Vivès E: Peptides in receptor-mediated radiotherapy:
From design to the clinical application in cancers. Front Oncol.
3:2472013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu B, Varasteh Z, Orlova A, Andersson K,
Larhammar D and Björkelund H: Detecting ligand interactions with G
protein-coupled receptors in real-time on living cells. Biochem
Biophys Res Commun. 441:820–824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eckelman WC, Kilbourn MR and Mathis CA:
Specific to nonspecific binding in radiopharmaceutical studies:
It's not so simple as it seems! Nucl Med Biol. 36:235–237. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tolmachev V and Orlova A: Influence of
labelling methods on biodistribution and imaging properties of
radiolabelled peptides for visualisation of molecular therapeutic
targets. Curr Med Chem. 17:2636–2655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heppeler A, Froidevaux S, Macke HR,
Jermann E, Behe M, Powell P and Hennig M: Radiometal-labelled
macrocyclic chelator-derivatised somatostatin analogue with superb
tumour targeting properties and potential for receptor rmediated
internal radiotherapy. Chemistry. 5:1974–1981. 1999. View Article : Google Scholar
|
|
23
|
Deshmukh MV, Voll G, Kühlewein A, Mäcke H,
Schmitt J, Kessler H and Gemmecker G: NMR studies reveal structural
differences between the gallium and yttrium complexes of
DOTA-D-Phe1-Tyr3-octreotide. J Med Chem. 48:1506–1514. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Broan CJ, Cox JPL, Craig AS, Kataky R,
Parker D, Harrison A, Randall A and Ferguson GJ: Structure and
solution stability of indium and gallium complexes of
1,4,7-triazacyclononanetriacetate and of yttrium complexes of
1,4,7,10-tetraazacyclododecanetetraacetate and related ligands:
Kinetically stable complexes for use in imaging and
radioimmunotherapy. X-Ray molecular structure of the indium and
gallium complexes of 1,4,7-triazacyclononane-1,4,7-triacetic acid.
J Chem Soc Perkin Trans. 2:87–99. 1991. View Article : Google Scholar
|
|
25
|
Matthews RC, Parker D, Ferguson G, Kaitner
B, Harisson A and Royle L: Synthesis and structure of stable indium
and gallium complexes of
(R)-1,4,7-tris(2′-methylcarboxymethyl)-triazacyclononane.
Polyhedron. 10:1951–1953. 1991. View Article : Google Scholar
|
|
26
|
Abiraj K, Mansi R, Tamma ML, Fani M,
Forrer F, Nicolas G, Cescato R, Reubi JC and Maecke HR: Bombesin
antagonist-based radioligands for translational nuclear imaging of
gastrin-releasing peptide receptor-positive tumors. J Nucl Med.
52:1970–1978. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gourni E, Mansi R, Jamous M, Waser B,
Smerling C, Burian A, Buchegger F, Reubi JC and Maecke HR:
N-terminal modifi-cations improve the receptor affinity and
pharmacokinetics of radiolabeled peptidic gastrin-releasing peptide
receptor antagonists: Examples of 68Ga- and 64Cu-labeled peptides
for PET imaging. J Nucl Med. 55:1719–1725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maina T, Nock BA, Zhang H, Nikolopoulou A,
Waser B, Reubi JC and Maecke HR: Species differences of bombesin
analog interactions with GRP-R define the choice of animal models
in the development of GRP-R-targeting drugs. J Nucl Med.
46:823–830. 2005.PubMed/NCBI
|
|
29
|
Fournier P, Dumulon-Perreault V,
Ait-Mohand S, Tremblay S, Bénard F, Lecomte R and Guérin B: Novel
radiolabeled peptides for breast and prostate tumor PET imaging:
(64)Cu/and (68) Ga/NOTA-PEG-[D-Tyr(6),
βAla(11),Thi(13),Nle(14)]BBN(6-14). Bioconjug Chem. 23:1687–1693.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lane SR, Nanda P, Rold TL, Sieckman GL,
Figueroa SD, Hoffman TJ, Jurisson SS and Smith CJ: Optimization,
biological evaluation and microPET imaging of copper-64-labeled
bombesin agonists, [64Cu-NO2A-(X)-BBN(7-14)NH2], in a prostate
tumor xenografted mouse model. Nucl Med Biol. 37:751–761. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tokita K, Katsuno T, Hocart SJ, Coy DH,
Llinares M, Martinez J and Jensen RT: Molecular basis for
selectivity of high affinity peptide antagonists for the
gastrin-releasing peptide receptor. J Biol Chem. 276:36652–36663.
2001. View Article : Google Scholar : PubMed/NCBI
|