Introduction
The incidence of thyroid cancer has increased
continuously worldwide for the past 3 decades (1,2);
particularly, the incidence increases dramatically in Chinese women
(3). Thyroid cancer is ranked the
fourth most common cancer among women in urban Beijing in 2012,
whereas it was not even in the top 10 cancers in 1999 (3). Papillary thyroid carcinoma (PTC)
exclusively accounts for the increase in the incidence of total
thyroid cancer, whereas the incidences of follicular thyroid
carcinoma (FTC), medullary thyroid carcinoma (MTC), and anaplastic
thyroid carcinoma (ATC) have not changed substantially (2). The mechanism underlying thyroid
cancer progression remains unclear. CXCL12/CXCR4/CXCR7 axis has
been found to play a critical role in cancer metastasis and
progression in many types of cancer (4), thus, may also contribute to thyroid
cancer progression.
CXCL12, which is also named as stromal cell-derived
factor-1 (SDF-1), is a CXC-chemokine and was originally identified
from bone marrow stromal cells (5). In addition to the well-recognized
receptor CXCR4 (6,7), CXCR7 has recently been identified as
another receptor for CXCL12 (8,9). The
molecular mechanism underlying the biological effect of CXCL12 is
associated with CXCR4-mediated activation of G protein-coupled
signaling molecules, including ERK1/2, MAPK, JNK and AKT (10,11).
The function of CXCR7 remains unclear; some reports suggested that
it may be a co-receptor for CXCR4 to enhance CXCL12-mediated
G-protein signaling (12,13); others indicated that CXCR7 may
behave as a decoy receptor to sequester CXCL12, resulting in a
CXCL12 gradient so to facilitate the stimulation of
CXCR4-associated downstream signaling (14,15).
The role of CXCL12/ CXCR4/CXCR7 axis in cancer metastasis and
progression has been increasingly recognized in many types of
cancer (4). Both in vitro
and in vivo studies have shown that the role of CXCL12/CXCR4
axis in cancer progression is mainly to facilitate metastasis and
mobilization of cancer cells (4).
The protein expression of CXCL12/CXCR4/CXCR7 has
been confirmed in multiple human cancers. In prostate cancer, CXCR7
expression increases as cancer aggressiveness increases, and CXCL12
level at the preferential metastatic sites, such as the bone,
liver, and kidney, were higher than that at non-metastatic tissue
(16,17). In breast cancer, CXCR4 is highly
expressed in cancer tissue but absent in normal breast tissue
(18). Strong CXCR7 expression has
been found in tumor-associated blood vessels in breast and lung
cancer tissue but not on normal vasculature (19). In thyroid cancer, the expressions
of CXCL12, CXCR4 and CXCR7 have also been detected in cancer tissue
(20–23). However, the contribution of
CXCL12/CXCR4/CXCR7 axis to thyroid cancer progression and the
possible CXCR4-mediated signaling pathways in thyroid cancer cells
remain unclear. The present study was performed to fill this
knowledge gap. The aim of this study was to investigate the
expression of CXCL12, CXCR4 and CXCR7 in malignant and benign
thyroid lesions and examine the role of CXCR4 and CXCR7 in human
thyroid cancer cell proliferation, migration and invasion.
Materials and methods
Tissue specimen
The present study was approved by the Institutional
Review Board of Shanghai Cancer Center of Fudan University. Signed
informed consent was obtained from each patient. Surgical tissue
specimens of PTC (40 cases), FTC (10 cases), MTC (10 cases), ATC
(10 cases), follicular adenoma (FA, 10 cases), Hashimoto's
thyroiditis (HT, 10 cases), and nodular goiter (NG, 10 cases) were
collected. The malignancy of thyroid cancer tissue was confirmed by
histopathological examination. The benign thyroid tissue specimens
were also confirmed to be free of malignancy. Paraffin-embedded
tissue specimens were used to prepare a tissue microarray (TMA) for
immunohistochemical staining. Each TMA contained 120 (12×10) cores
and 2 cores of each sample were printed on a TMA. The core size was
1 mm (diameter) × 4 μm (thickness).
Immunohistochemistry
TMAs were incubated with the following primary
antibodies: monoclonal mouse anti-human CXCR4 (1:400; Abcam,
Cambridge, MA, USA), anti-human CXCR7 (1:50; R&D Systems, Inc.,
Minneapolis, MN, USA), or anti-human CXCL12 (1:50; R&D
Systems). Briefly, 4-μm-thick sections of TMAs were transferred to
adhesive slides and maintained in a drying oven at 60°C for 2 h.
The standard heat epitope was deparaffinized using EZ Prep (Ventana
Medical Systems, Inc., Tucson, AZ, USA) at 75°C for 4 min. During
the heat pretreatment step, cell-conditioning solutions (Ventana
Medical Systems) containing Tris/Borate/EDTA were used at 100°C for
92 min. The antibodies were pre-diluted, and the tissue sections
were incubated with the diluted antibodies at 37°C for 16 min (for
CXCL12) and 30 min (for CXCR7). Subsequently, the tissue sections
were incubated with an appropriate reagent from the ultraView
Universal DAB kit (Ventana Medical Systems) at 37°C for 8 min and
counterstained with Harris's haematoxylin for 2 min. The stained
TMAs were observed under a microscope. The staining intensity
(CXCL12 in tumor cells and CXCR7 in endothelial cells) was scored
based on the following criteria: 0 represents no staining or faint
staining intensity in 10% cells; 1+ represents faint staining in
>10% of cells; 2+ represents moderate staining; 3+ represents
strong staining. The tissue specimen was considered positive for
CXCL12 or CXCR7 when the staining intensity score was 1+, 2+, or 3+
and negative when the score was 0.
Cell culture
Human thyroid cancer K1 cells were purchased from
Guangzhou Jenniobio Biotechnology Co., Ltd. (Guangzhou, China). The
cells were cultured in Dulbecco's modified Eagle's media (DMEM;
HyClone Laboratories, Inc., Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Invitrogen, Carlsbad, CA, USA), 100
units/ml penicillin and 100 μg/ml streptomycin (Thermo Fisher
Scientific, Waltham, MA, USA) at 37°C in 5% CO2.
Recombinant lentivirus construction and
transduction
Recombinant lentivirus expressing CXCR4 or CXCR7 was
constructed using the ViruaPower™ Lentiviral Expression systems
(Invitrogen). K1 cells were seeded in 24-well plates at the density
of 1×105 cells/well and incubated overnight. The culture
media were replaced with 2 ml fresh media containing 6 μg/ml
polybrene (Sigma, St. Louis, MO, USA) and the recombinant
lentivirus, and then the cells were incubated with polybrene and
the recombinant lentivirus at 37°C for 4 h. The media were replaced
with 2 ml fresh media and the cells were incubated overnight.
Lentivirus transduction efficiency was determined by lentivirus
expressing green fluorescent protein (GFP). Apparent GFP expression
appeared 48 h after transduction and GFP level reached the peak 72
h after transduction. Thus, K1 cells were cultured for 72 h after
transduction with recombinant lentivirus and then used for RT-PCR,
proliferation and Transwell invasion assay.
Transwell invasion assay
Cell invasion was determined by using 24-well
Transwell invasion chamber (8 μm pore size; Corning, Tewksbury, MA,
USA). A total of 200 μl cell suspensions in serum-free media
(5×104/well) were added in the upper chambers. The lower
chambers were filled with 800 μl DMEM media containing either 10%
FBS or 100 ng/ml CXCL12. After incubation for 24 h, the cells on
the upper surface of the membrane in the upper chamber were removed
by cotton swaps and the cells on the other side of the membrane
were fixed with methanol for 30 min, air dried and stained with
0.1% crystal violet solution for 10 min. The penetrated cells were
then counted under a microscope. Three to 5 fields were randomly
selected on each membrane and the average number of cells was used.
The experiment was repeated at least 3 times.
Cell proliferation
Cell proliferation was determined using the Cell
Counting kit-8 (CCK-8 kit; Dojindo Laboratories, Kumamoto, Japan).
Cells were plated in 96-well plates at a density of
8×103 cells/well in 100 μl serum-free media containing
100 ng/ml recombinant CXCL12 (PeproTech, Rocky Hill, NJ, USA) or
media containing 10% FBS. The cells were incubated for 24 or 48 h.
After incubation, 10 μl of CCK-8 solution was added to each well
and incubated at 37°C for 1–4 h. The media were then removed and
the dark blue formazan crystals were dissolved in 150 μl of DMSO.
The absorbance at 450 nm was measured in a microplate reader
(Corning Costar, Corning, NY, USA, USA). The experiment was
repeated independently at least 3 times.
Western blot analysis
Cells (2×105 cells/well) were cultured in
6-well plates and incubated at 37°C until confluency. After being
washed twice with ice-cold PBS, the cells were lysed in RIPA buffer
(Beyotime Institute of Biotechnology, Haimen, China) containing
protease inhibitor cocktail (1:1,000 dilution; Sigma) and
phosphatase inhibitor cocktail (1:1,000 dilution; Sigma). The cell
lysate was centrifuged at 12,000 × g for 10 min at 4°C. Protein
concentration in the supernatants was determined using a BCA kit
(Beyotime Institute of Biotechnology). A total of 30 μg protein
extract were separated by SDS-PAGE and transferred to PVDF
membranes. The membrane was blocked for 1 h at room temperature in
5% non-fat dry milk in TBST (10 mmol/l Tris, pH 8.0, 165 mmol/l
NaCl, 0.05% Tween-20). The membrane was then incubated with the
following primary antibodies: rabbit anti-human CXCR4, CXCR7,
p-Akt1 (B-1), p-ERK (B-5), β-actin, GAPDH and ERK1/2 (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) at room temperature for 1 h or
at 4°C overnight. After extensive washing, the membrane was
incubated with the secondary antibody, goat anti-rabbit IgG
conjugated with horseradish peroxidase (Cell Signaling Technology,
Danvers, MA, USA), at a dilution of 1:5,000 at room temperature for
1.5 h. Target proteins were visualized using an enhanced
chemiluminescence kit (Beyotime Institute of Biotechnology). For
quantification, the intensity of protein signal was evaluated using
Quantity One system (Bio-Rad Laboratories, Hercules, CA, USA). The
experiment was repeated at least 3 times.
Quantitative reverse-transcription
polymerase chain reaction
Total RNA was extracted from K1 cells using the
RNAiso Plus kit (Takara Bio, Shiga, Japan) according to the
manufacturer's instruction. A total of 500 ng total RNA was reverse
transcribed to cDNA using the reverse transcriptase M-MLV RT
(Takara) and random primers in a 20 μl reaction system. The RT
product (1 μl) was used as the template for quantitative PCR
(qPCR). The SYBR Premix Ex Taq™ II system (Takara Bio) was used for
qPCR reaction. The primer sequences are: MMP-2 forward,
5′-CGACCACAGCCAACTACGAT-3′ and reverse, 5′-GTCAGGAGAGGCCCCATAGA-3′;
MMP-9 forward, 5′-TCTATGGTCCTCGCCCTGAA-3′ and reverse,
5′-CATCGTCCACCGGACTCAAA-3′. The qPCR condition was 95°C for 2 min
and then 40 cycles of 95°C for 10 sec, 60°C for 30 sec, and 70°C
for 45 sec. Human β-actin was used as the internal control. The
qPCR was performed on the Applied Biosystems 7500 detection system.
Cycle threshold (Ct) values for all samples were determined. The
ΔΔCt method was used to calculate the expression levels of target
genes in experimental groups relative to control group.
Gel zymography
Culture media were mixed with non-reducing loading
buffer (4% SDS, 0.25 mmol/l Tris-HCl, 40% glycerol, 0.1%
bromophenol blue) at 1:3 ratio, and 15 μl of the mixture was loaded
in 10% SDS-PAGE gel containing 1 mg/ml gelatin. After
electrophoresis, the gel was washed twice, 45 min each time, in a
washing buffer (2.5% Triton X-100, 50 mmol/l Tris-HCl, 5 mmol/l
CaCl2, 1 μmol/l ZnCl2, pH 7.6), and then
washed again twice, 20 min each time, in a washing buffer (50
mmol/l Tris-HCl, 5 mmol/l CaCl2, 1 μmol/l
ZnCl2, pH 7.6). The gel was incubated in the reaction
buffer (50 mmol/l Tris-HCl, 5 mmol/l CaCl2, 1 μmol/l
ZnCl2, 0.02% Brij-35, pH 7.6) at 37°C for 42 h, stained
with Coomassie blue (0.05% Coomassie blue, 30% methanol, 10%
acetate) for 3 h, and de-stained with buffer A (30% methanol and
10% acetate), buffer B (20% methanol and 10% acetate) and buffer C
(10% methanol and 5% acetate) for 0.5, 1 h and 2 h,
respectively.
Statistical analysis
Continuous variables are presented as mean ±
standard deviation. The statistical analysis was performed using
the software SPSS 19.0. The Chi-square test and the Fisher's exact
test were used for categorical variables. P-value was 2-sided and
P<0.05 was considered statistically significant.
Results
The expression of CXCR4, CXCL12 and CXCR7
in thyroid tissue specimens
The expression of CXCR4, CXCL12 and CXCR7 varied in
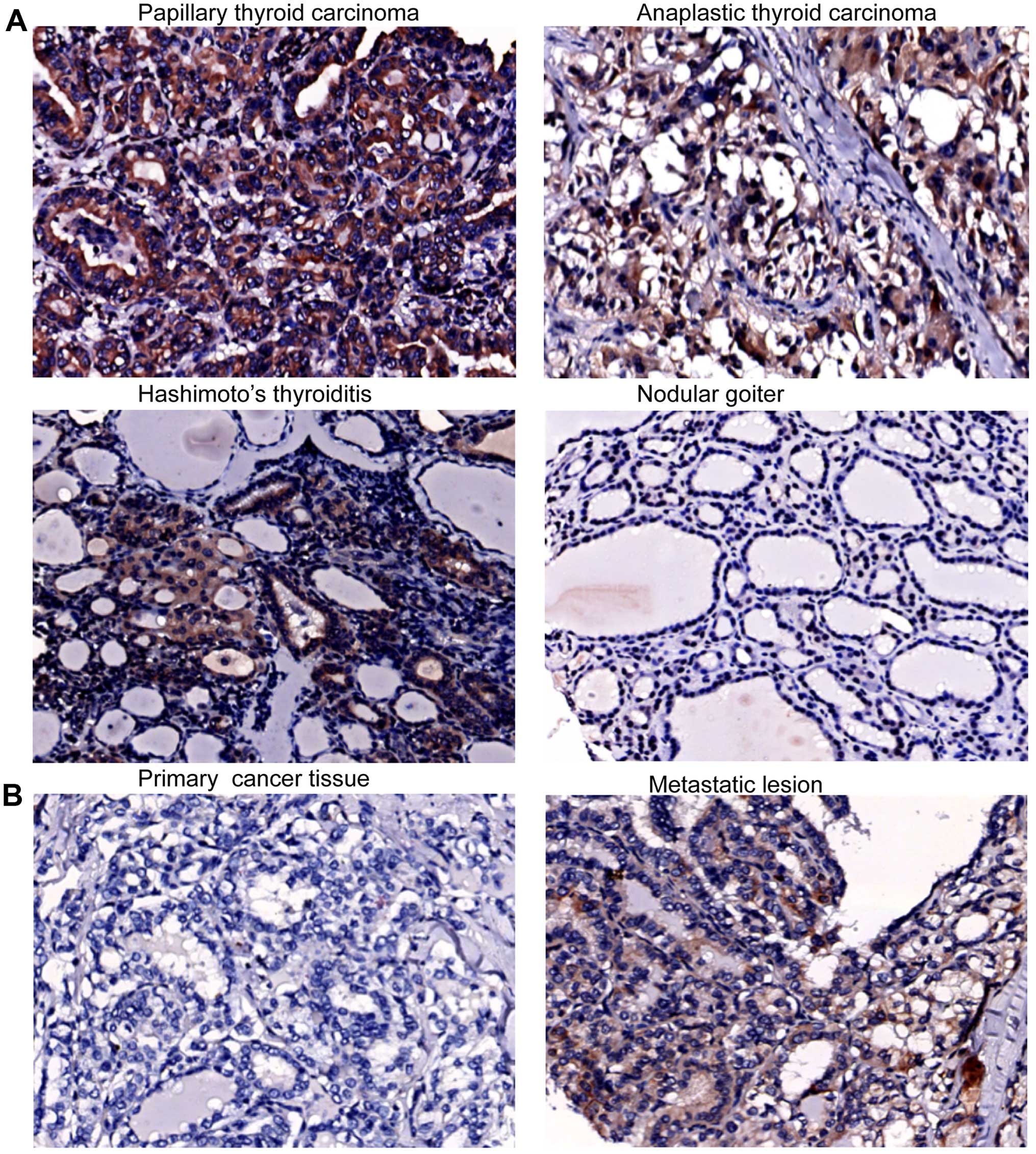
malignant and benign thyroid tissue specimens. CXCR4 was expressed
in 62.5% of PTC specimens and in 30–40% of other types of thyroid
malignancy, and benign lesions also expressed CXCR4 (Table I). The CXCR4 staining signals were
diffuse and appeared in the cytoplasm of cancer cells in PTC and
ATC specimens, and in HT and NG specimens, CXCR4 was predominantly
in follicular cells (Fig. 1A). The
proportion of specimens with positive CXCR4 in PTC with lymph node
metastasis (55%) was not significantly different from that (85%) in
PTC without lymph node metastasis (Table I). Notably, 3 cases of CXCR4
positive PTC with lymph node metastasis showed stronger CXCR4
staining in the metastatic lesions than in the primary thyroid
cancer tissue (Fig. 1B). CXCL12
expression was found in 75% of PTC specimens and 10% of FA
specimens, but was absent in FTC, MTC, ATC, HT and NG specimens
(Table I). Additionally, the
proportion of cases with positive CXCL12 (80%) in PTC with lymph
node metastasis was similar to that (70%) in PTC without lymph node
metastasis (Table I). CXCL12
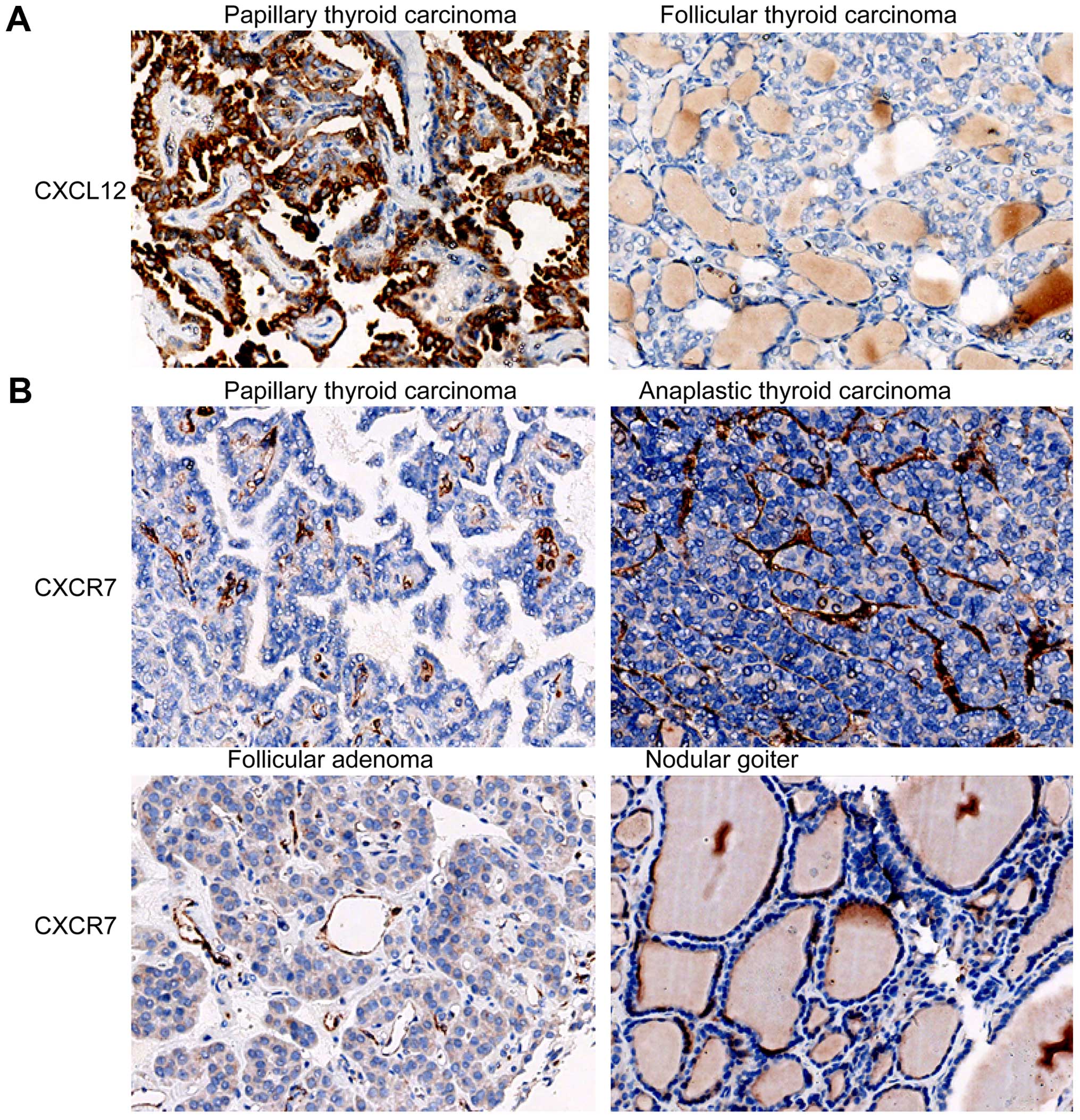
staining signals were in the cytoplasm of cancer cells (Fig. 2A). These results suggest that
papillary thyroid cancer cells may secret CXCL12, but other types
of thyroid cancer cells may not. CXCR7 was commonly expressed in
malignant thyroid tissue specimens. The proportions of cases with
positive CXCR7 in PTC (97.5%), FTC (100%), MTC (100%) and ATC
(100%) specimens were significantly higher than those in the benign
thyroid specimens, HT (20%) and NG (10%) (All P<0.05; Table I). The proportion of positive CXCR7
in FA was 80%. Notably, CXCR7 staining signals were mainly located
in the blood vessels in thyroid tissue (Fig. 2B).
 | Table IThe expression of CXCL12, CXCR7 and
CXCR4 in malignant and benign thyroid tissue specimens. |
Table I
The expression of CXCL12, CXCR7 and
CXCR4 in malignant and benign thyroid tissue specimens.
| Total number | CXCL12+ n
(%) | CXCR7+ n
(%) | CXCR4+ n
(%) |
|---|
| PTC | 40 | 30 (75) | 39 (97.5) | 25 (62.5) |
| LN− | 20 | 14 (70) | 19 (95) | 17 (85) |
| LN+ | 20 | 16 (80) | 20 (100) | 11 (55) |
| FTC | 10 | 0 (0) | 10 (100) | 3 (30) |
| MTC | 10 | 0 (0) | 10 (100) | 4 (40) |
| ATC | 10 | 0 (0) | 10 (100) | 4 (40) |
| FA | 10 | 1 (10)a | 8 (80) | 2 (20)a |
| HT | 10 | 0 (0)a | 2 (20)a | 4 (40) |
| NG | 10 | 0 (0)a | 1 (10)a | 0 (0)a |
Further examination of the staining intensity of the
positive specimens revealed that the intensity of CXCR7 staining
signals in the benign thyroid tissue specimens (FA, HT and NG) was
weak, and different types of thyroid malignancies presented various
CXCR7 staining intensity (Fig. 2
and Table II). The majority of
ATC specimens (90%) showed moderate or strong CXCR7 expression, and
for other types of thyroid malignancy, including PTC, FTC and MTC,
there was 50–70% of cases with moderate or strong CXCR7 staining
(Table II). CXCR4 expression
level was low in benign lesions including FA, HT and NG. There was
only one case of moderate or strong CXCR4 staining in the benign
lesions (Table II). All the
positive FTC specimens showed weak CXCR4 staining. In PTC, MTC and
ATC specimens, the proportions of weak staining were similar to
those of moderate or strong staining (Table II).
 | Table IIThe intensity of CXCL12, CXCR7 and
CXCR4 staining in thyroid tissue specimens. |
Table II
The intensity of CXCL12, CXCR7 and
CXCR4 staining in thyroid tissue specimens.
| | CXCL12 | CXCR7 | CXCR4 |
|---|
| |
|
|
|
|---|
| Total number | Weak n (%) | Moderate or strong
n (%) | Weak n (%) | Moderate or strong
n (%) | Weak n (%) | Moderate or strong
n (%) |
|---|
| PTC | 40 | 15 (37.5) | 15 (37.5) | 17 (42.5) | 22 (55) | 11 (27.5) | 14 (35) |
| LN− | 20 | 7 (35) | 7 (35) | 9 (45) | 10 (50) | 6 (30) | 11 (55) |
| LN+ | 20 | 8 (40) | 8 (40) | 8 (40) | 12 (60) | 6 (30) | 5 (25) |
| FTC | 10 | 0 (0) | 0 (0) | 5 (50) | 5 (50) | 3 (30) | 0 (0) |
| MTC | 10 | 0 (0) | 0 (0) | 3 (30) | 7 (70) | 2 (20) | 2 (20) |
| ATC | 10 | 0 (0) | 0 (0) | 1 (10) | 9 (90) | 2 (20) | 2 (20) |
| FA | 10 | 1 (10) | 0 (0) | 5 (50) | 3 (30) | 2 (20) | 0 (0) |
| HT | 10 | 0 (0) | 0 (0) | 1 (10) | 1 (10) | 3 (30) | 1 (10) |
| NG | 10 | 0 (0) | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) |
Overexpression of CXCR4 or CXCR7 in
thyroid cancer cells stimulates K1 cell invasion but does not
affect K1 cell proliferation
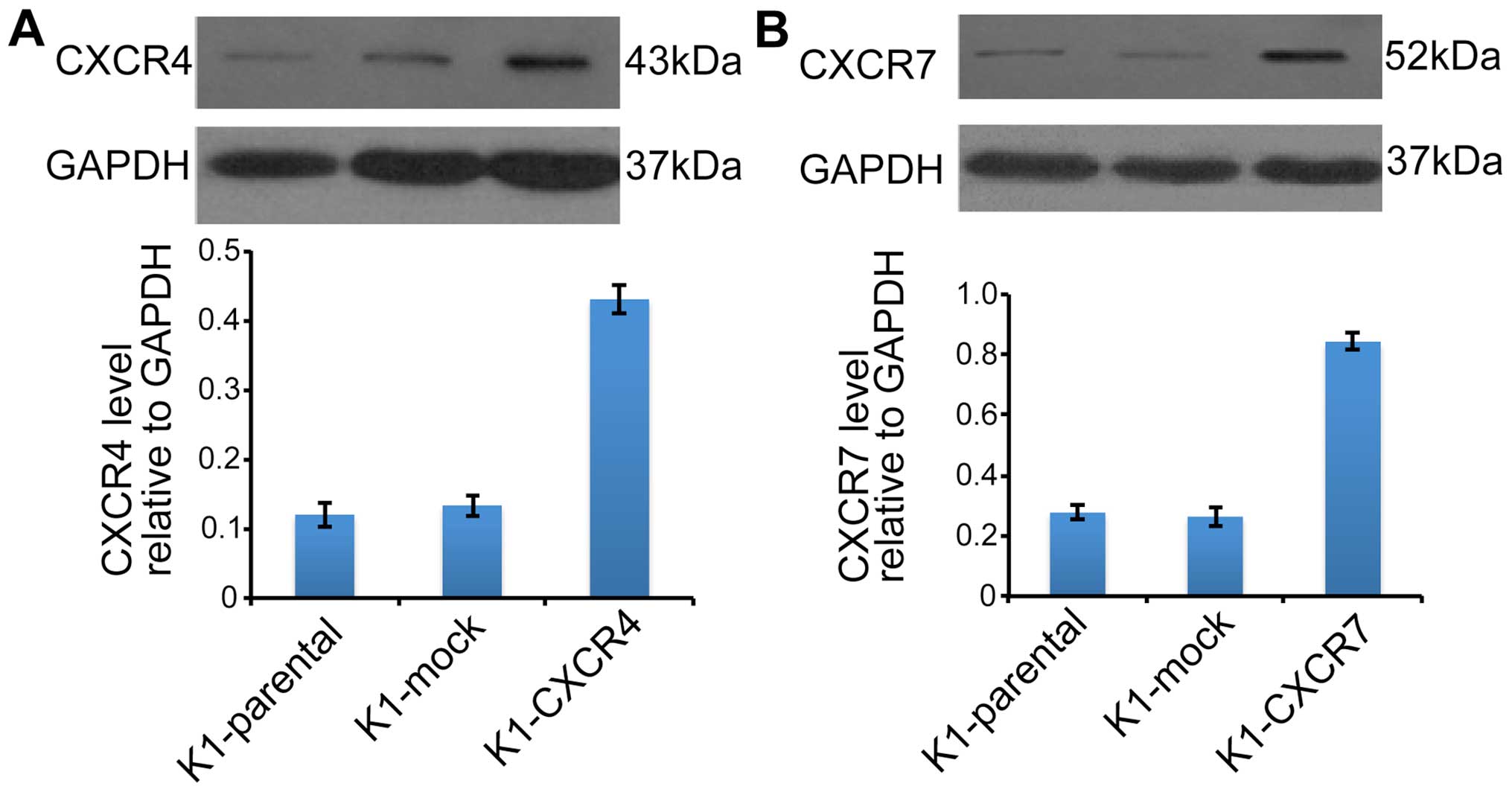
The protein levels of CXCR4 (Fig. 3A) and CXCR7 (Fig. 3B) were markedly increased in K1
cells transduced with CXCR4 or CXCR7 recombinant lentivirus. CXCL12
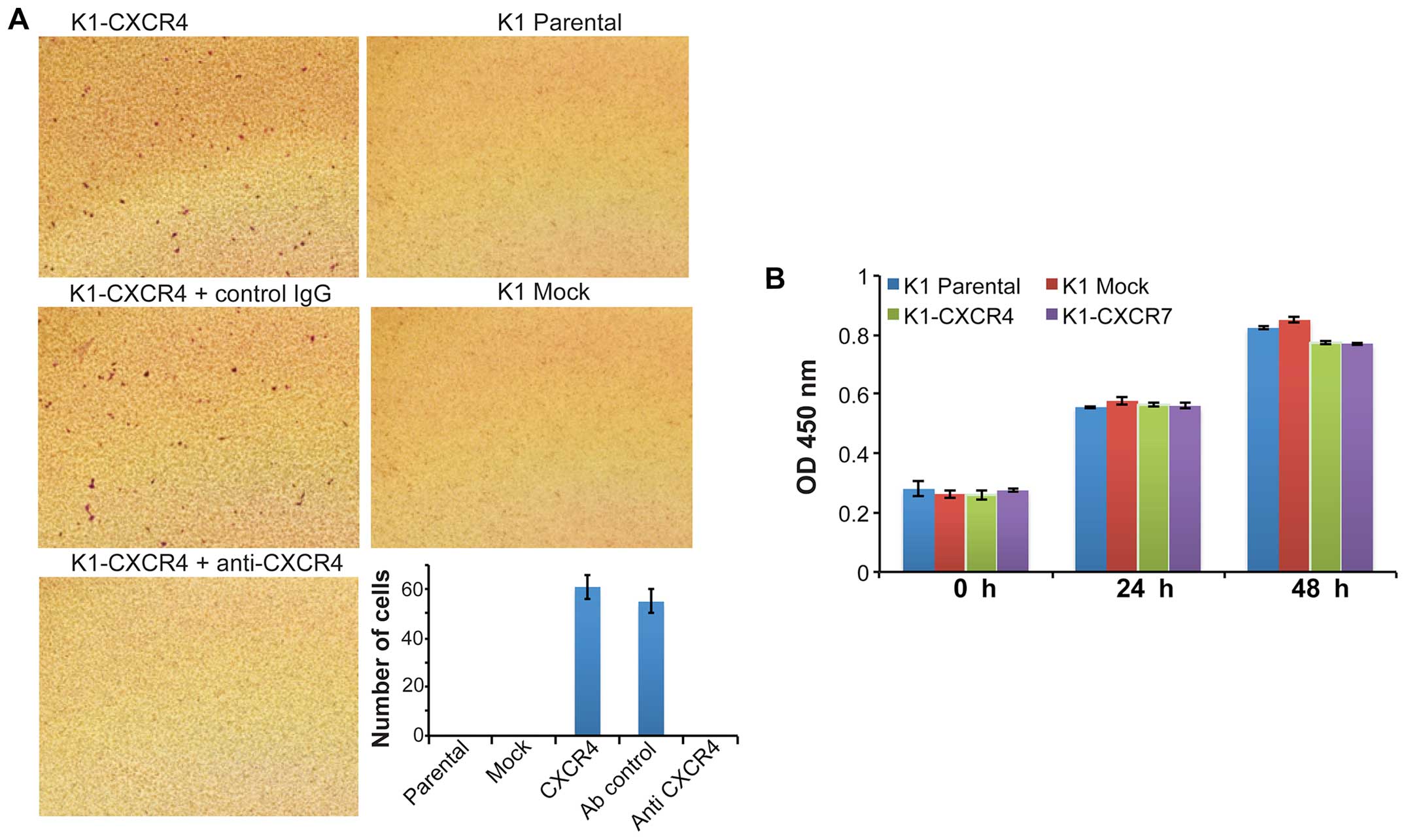
(100 ng/ml) markedly stimulated the invasion of K1 cells
overexpressing CXCR4 (K1-CXCR4); the CXCL12-induced K1-CXCR4
invasion was completely blocked by functional neutralizing antibody
against CXCR4 (Fig. 4A). However,
CXCL12 had no effects on the invasion of K1 cells overexpressing
CXCR7 (K1-CXCR7) (data not shown). In addition, 10% FBS did not
induce the invasion of K1-CXCR4 and K1-CXCR7 cells. These results
suggest that CXCR4 and CXCR7 may interact with CXCL12 differently
in K1 cells. CXCL12 did not stimulate the proliferation of K1-CXCR4
and K1-CXCR7 cells (data not shown). The proliferation rate of K1
cells in media containing 10% FBS was not affected by
overexpression of CXCR4 or CXCR7 (Fig.
4B).
Overexpression of CXCR4 or CXCR7
upregulates AKT and ERK phosphorylation and MMP-2 expression and
activity
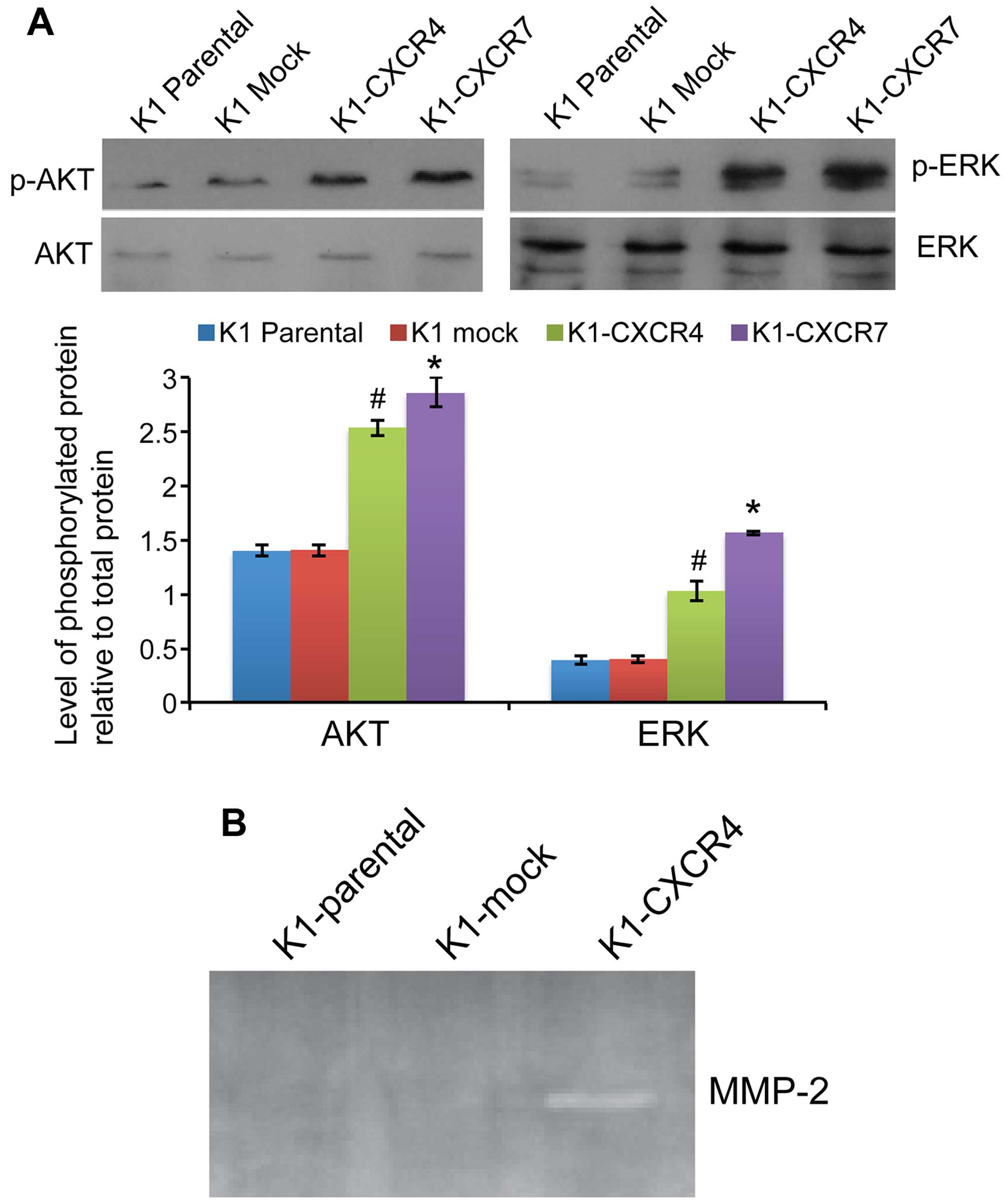
The phosphorylation levels of both AKT and ERK were
significantly increased in K1 cells overexpressing CXCR4 or CXCR7
compared with mock transfected cells (all P<0.05; Fig. 5A). RT-PCR revealed that
overexpression of CXCR4 markedly increased the mRNA expression of
matrix metalloproteinase (MMP)-2 in K1 cells by 908.2±10.9-fold
compared with K1-mock cells, whereas MMP-9 expression in K1 cells
was not affected by CXCR4 or CXCR7 overexpression. Consistently,
zymography assay showed that MMP-2 activity was markedly increased
in K1-CXCR4 cells compared with parental or mock-transfected K1
cells (Fig. 5B).
Discussion
In the present study, we found that CXCL12 was
exclusively expressed in PTC specimens but was not expressed in
benign thyroid specimens and other types of thyroid malignancy,
including FTC, MTC and ATC. These findings indicate that CXCL12 may
be an effective biomarker for PTC. Consistently, Chung et al
(20) recently demonstrated that
CXCL12 was exclusively overexpressed in human PTC regardless of
histological subtypes compared with non-cancerous thyroid lesions,
and the sensitivity and specificity of using CXCL12 as a diagnostic
marker for PTC was 90.8 and 96.8%, respectively (20). Jung et al (21) also reported that 90% of follicular
PTC specimens were positive for CXCL12, whereas only 10.5% of
follicular thyroid neoplasm specimens showed positive CXCL12. The
results of the present study showed that neither non-cancerous
thyroid lesions nor malignancy of FTC, MTC and ATC expressed
CXCL12, further supporting the exclusive expression of CXCL12 in
PTC and the diagnostic value of CXCL12 for PTC.
We found positive CXCL12 and CXCR7 staining in 75
and 97.5% of PTC specimens, respectively. Similarly, Liu et
al (22) showed that positive
immunohistochemical staining of CXCL12 and CXCR7 in 69.6 and 65.8%
of PTC specimens, respectively, and the expression of the ligand
and the receptor was positively correlated with lymph node
metastasis of PTC. Wagner et al (23) semi-quantitatively measured the
immunohistochemical staining intensity of CXCR7 in human PTC
specimens and found that PTCs with lymph node metastasis showed
high intensity of staining for CXCR7. In contrast, we did not find
any correlation of CXCR7 expression or the intensity of CXCR7
expression to PTC lymph node metastasis. The discrepancy may be
associated with the facts that antibodies against CXCR7 used in the
studies were from different manufacturers and/or minor difference
in immunohistochemical staining procedures could result in subtle
variation of staining. Thus, the association between CXCR7
expression and PTC lymph node metastasis needs to be further
investigated.
To the best of our knowledge, this study is the
first demonstrating that CXCR7 was also widely expressed in thyroid
malignancy of FTC, MTC and ATC in addition to PTC, and CXCR7 was
only weakly expressed in benign thyroid lesions. Notably, the
intensity of CXCR7 expression was particularly high in ATC. These
results indicate that CXCR7 function may vary in different types of
thyroid malignancy. We found that the CXCR7 staining signals were
mainly at endothelium of malignant thyroid tissue specimens.
Immunohistochemistry of human breast and lung cancer tissue also
revealed extensive CXCR7 expression on tumor-associated blood
vessels and cancer cells (19).
Thus, CXCR7 may contribute to thyroid cancer development by
regulating angiogenesis.
The present study found that CXCR4 was either absent
(NG) or expressed weakly in benign lesions (FA and HT) but showed
strong expression in PTC, MTC and ATC specimens. De Falco et
al (25) showed that CXCR4 was
overexpressed in ATC specimens compared with normal thyroid tissue
by real-time PCR and immunohistochemistry. Wagner et al
(23) found that high CXCR4
expression was significantly associated with large tumor size of
PTC specimens. Interestingly, we found that some PTC specimens
showed stronger CXCR4 staining in lymph node metastasis than in the
primary thyroid cancer tissue. Similarly, Lu et al (24) also showed that higher CXCR4
expression in the lymph node metastatic lesions than in the primary
tumor tissues of esophageal squamous cell cancer. These findings
indicate that CXCR4 positive thyroid cancer cells may have strong
migratory potential. Our results from the in vitro
experiments appear to support this hypothesis.
The in vitro experiments in this study showed
that CXCL12 induced K1-CXCR4 cell invasion, whereas did not affect
the invasion of K1 cells overexpressing CXCR7. These results
indicate that CXCR4 and CXCR7 may have distinct function in thyroid
cancer cells. CXCL12/CXCR4 axis-mediated migration has been
observed in human anaplastic thyroid cancer cells, and
CXCL12-induced anaplastic thyroid cancer cell migration is
associated with AKT and ERK activation (25,26).
Similarly, the present study also showed that AKT and ERK
phosphorylation was significantly increased in K1 cells
overexpressing CXCR4. Thus, CXCL12/CXCR4 axis appears to promote
thyroid cancer cell migration by activating AKT and ERK signaling
pathways. However, the function of CXCR7 in cancer development
remains unclear. CXCR7 alone may not directly trigger the
downstream signaling, instead, it may function as a co-receptor for
CXCR4 or as a decoy receptor to stimulate or enhance
CXCR4-associated downstream signaling (12–15).
The results of this study appear to support the co-receptor or
decoy receptor hypothesis regarding CXCR7 function, because CXCL12
failed to stimulate the invasion of K1 cells overexpressing CXCR7,
but significantly induced K1-CXCR4 cell invasion. Levoye et
al (12) investigated the
cooperation of CXCR4 and CXCR7 in T cells in the presence of CXCL12
found that CXCR7 alone did not trigger the CXCL12-induced
downstream signaling events, whereas co-expression of CXCR4 and
CXCR7 resulted in CXCR4/CXCR7 heterodimer formation, which
regulated CXCL12-promoted chemotaxis.
In addition, this study found that overexpression of
CXCR4 markedly induced MMP-2 expression and its activity in K1
cells. The association of CXCL12/CXCR4 axis and MMP-2 activation
has been indicated in CXCL12-induced lung alveolar epithelial cell
migration. Ghosh et al (27) found that blockade of CXCR4 by the
specific antagonist AMD-3100 inhibited epithelial cell migration
and decreased MMP-2 activity. Ying et al (28) also suggested that CXCL12/CXCR4 axis
might promote pancreatic cancer invasion by activating p38
mitogen-activated protein kinase and upregulating MMP-2 activity.
Thus, during thyroid cancer progression, CXCL12 may bind to CXCR4
to activate AKT and ERK signaling pathways, thus, upregulating
MMP-2 and ultimately promoting cancer cell migration and invasion.
CXCR7 may facilitate or enhance the binding of CXCL12 to CXCR4.
In conclusion, immunohistochemistry revealed that
CXCL12 was exclusively expressed in human PTC tissue and CXCR7 was
widely expressed in the endothelial cells of PTC, FTC, MTC, ATC and
FA tissue specimens, but only occasionally found in HT and NG
tissue specimens. CXCR4 was commonly expressed in thyroid cancer,
but only weakly expressed in benign thyroid lesions.
CXCL12/CXCR4/CXCR7 axis may contribute to thyroid cancer
development by regulating cancer cell migration/invasion via AKT
and ERK signaling and MMP-2 activation.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no.
81102044).
References
|
1
|
Kilfoy BA, Zheng T, Holford TR, Han X,
Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, et al:
International patterns and trends in thyroid cancer incidence,
1973–2002. Cancer Causes Control. 20:525–531. 2009. View Article : Google Scholar :
|
|
2
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013.PubMed/NCBI
|
|
3
|
Yang L, Yuan Y, Sun T, Li H and Wang N:
Population-based cancer incidence analysis in Beijing, 2008–2012.
Chin J Cancer Res. 27:13–21. 2015.PubMed/NCBI
|
|
4
|
Sun X, Cheng G, Hao M, Zheng J, Zhou X,
Zhang J, Taichman RS, Pienta KJ and Wang J: CXCL12/CXCR4/CXCR7
chemokine axis and cancer progression. Cancer Metastasis Rev.
29:709–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bleul CC, Fuhlbrigge RC, Casasnovas JM,
Aiuti A and Springer TA: A highly efficacious lymphocyte
chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med.
184:1101–1109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caruz A, Samsom M, Alonso JM, Alcami J,
Baleux F, Virelizier JL, Parmentier M and Arenzana-Seisdedos F:
Genomic organization and promoter characterization of human CXCR4
gene. FEBS Lett. 426:271–278. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta SK and Pillarisetti K: Cutting edge:
CXCR4-Lo: molecular cloning and functional expression of a novel
human CXCR4 splice variant. J Immunol. 163:2368–2372.
1999.PubMed/NCBI
|
|
8
|
Burns JM, Summers BC, Wang Y, Melikian A,
Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ,
et al: A novel chemokine receptor for SDF-1 and I-TAC involved in
cell survival, cell adhesion, and tumor development. J Exp Med.
203:2201–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Balabanian K, Lagane B, Infantino S, Chow
KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M and
Bachelerie F: The chemokine SDF-1/CXCL12 binds to and signals
through the orphan receptor RDC1 in T lymphocytes. J Biol Chem.
280:35760–35766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu DY, Tang CH, Yeh WL, Wong KL, Lin CP,
Chen YH, Lai CH, Chen YF, Leung YM and Fu WM: SDF-1alpha
up-regulates interleukin-6 through CXCR4, PI3K/Akt, ERK, and
NF-kappaB-dependent pathway in microglia. Eur J Pharmacol.
613:146–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roland J, Murphy BJ, Ahr B, Robert-Hebmann
V, Delauzun V, Nye KE, Devaux C and Biard-Piechaczyk M: Role of the
intra-cellular domains of CXCR4 in SDF-1-mediated signaling. Blood.
101:399–406. 2003. View Article : Google Scholar
|
|
12
|
Levoye A, Balabanian K, Baleux F,
Bachelerie F and Lagane B: CXCR7 heterodimerizes with CXCR4 and
regulates CXCL12-mediated G protein signaling. Blood.
113:6085–6093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sierro F, Biben C, Martínez-Muñoz L,
Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M,
et al: Disrupted cardiac development but normal hematopoiesis in
mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc
Natl Acad Sci USA. 104:14759–14764. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boldajipour B, Mahabaleshwar H, Kardash E,
Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q and Raz E:
Control of chemokine-guided cell migration by ligand sequestration.
Cell. 132:463–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dambly-Chaudière C, Cubedo N and Ghysen A:
Control of cell migration in the development of the posterior
lateral line: Antagonistic interactions between the chemokine
receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol. 7:232007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Shiozawa Y, Wang J, Wang Y, Jung
Y, Pienta KJ, Mehra R, Loberg R and Taichman RS: The role of CXCR7/
RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J
Biol Chem. 283:4283–4294. 2008. View Article : Google Scholar
|
|
17
|
Sun YX, Schneider A, Jung Y, Wang J, Dai
J, Wang J, Cook K, Osman NI, Koh-Paige AJ, Shim H, et al: Skeletal
localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis
blocks prostate cancer metastasis and growth in osseous sites in
vivo. J Bone Miner Res. 20:318–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miao Z, Luker KE, Summers BC, Berahovich
R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A,
Luker GD, et al: CXCR7 (RDC1) promotes breast and lung tumor growth
in vivo and is expressed on tumor-associated vasculature. Proc Natl
Acad Sci USA. 104:15735–15740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung SY, Park ES, Park SY, Song JY and
Ryu HS: CXC motif ligand 12 as a novel diagnostic marker for
papillary thyroid carcinoma. Head Neck. 36:1005–1012. 2014.
View Article : Google Scholar
|
|
21
|
Jung YY, Park IA, Kim MA, Min HS, Won JK
and Ryu HS: Application of chemokine CXC motif ligand 12 as a novel
diagnostic marker in preoperative fine-needle aspiration biopsy for
papillary thyroid carcinoma. Acta Cytol. 57:447–454. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Sun DX, Teng XY, Xu WX, Meng XP and
Wang BS: Expression of stromal cell-derived factor 1 and CXCR7 in
papillary thyroid carcinoma. Endocr Pathol. 23:247–253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wagner PL, Moo TA, Arora N, Liu YF,
Zarnegar R, Scognamiglio T and Fahey TJ III: The chemokine
receptors CXCR4 and CCR7 are associated with tumor size and
pathologic indicators of tumor aggressiveness in papillary thyroid
carcinoma. Ann Surg Oncol. 15:2833–2841. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu CL, Guo J, Gu J, Ge D, Hou YY, Lin ZW
and Ding JY: CXCR4 heterogeneous expression in esophageal squamous
cell cancer and stronger metastatic potential with CXCR4-positive
cancer cells. Dis Esophagus. 27:294–302. 2014. View Article : Google Scholar
|
|
25
|
De Falco V, Guarino V, Avilla E,
Castellone MD, Salerno P, Salvatore G, Faviana P, Basolo F, Santoro
M and Melillo RM: Biological role and potential therapeutic
targeting of the chemokine receptor CXCR4 in undifferentiated
thyroid cancer. Cancer Res. 67:11821–11829. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang JH, Hwang JH, Chung HK, Kim DW,
Hwang ES, Suh JM, Kim H, You KH, Kwon OY, Ro HK, et al: CXC
chemokine receptor 4 expression and function in human anaplastic
thyroid cancer cells. J Clin Endocrinol Metab. 88:408–416. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghosh MC, Makena PS, Gorantla V, Sinclair
SE and Waters CM: CXCR4 regulates migration of lung alveolar
epithelial cells through activation of Rac1 and matrix
metalloproteinase-2. Am J Physiol Lung Cell Mol Physiol.
302:L846–L856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ying X, Jing L, Ma S, Li Q, Luo X, Pan Z,
Feng Y and Feng P: GSK3β mediates pancreatic cancer cell invasion
in vitro via the CXCR4/MMP-2 pathway. Cancer Cell Int. 15:702015.
View Article : Google Scholar
|