Introduction
Y-box binding protein 1 (YB-1) was originally found
to initiate gene transcription (1). Its transcription is triggered by
binding between YB-1 and inverted CCAAT box, which is normally
located upstream of the TATA box in the promoter region. YB-1 has
been shown to be involved not only in the transcription of various
genes but also in cell viability and DNA repair (2). Furthermore, many studies demonstrate
that YB-1 is overexpressed in human cancer cell lines; therefore,
YB-1 may be a good target for cancer therapy (3). Shibahara et al (4) reported that most non-small lung
cancers overexpress YB-1. Therefore, silencing YB-1 may also
suppress the growth of non-small lung cancers.
Silencing the expression of a particular gene in
cancer cells is critical in cancer therapy, especially when the
gene in question is related to the growth and malignant alteration
of cancer cells. Therapeutic oligonucleotides including antisense
DNA and siRNA specifically silence protein expression by
interacting with target mRNA; thus, they are presumably more
efficient and less toxic than conventional low-molecular drugs
(5,6). As the target mRNAs are located in the
cytoplasm, it is essential for antisense DNA or siRNA to be
transported to the cytoplasm in order to have an effect.
Oligonucleotides themselves do not have the ability to be ingested
by cells; furthermore, they are quite fragile in biological fluids
because of enzymatic degradation and non-specific adsorption by
serum proteins. Therefore, they require a drug delivery system
(DDS) to protect them from such unfavorable interactions and
transport them to the cytoplasm. The most commonly used synthetic
DDS particles for oligonucleotide delivery are cationic lipids and
polymers, which form electrostatic complexes with negatively
charged oligonucleotides (7).
Although some of these cationic compounds are used for cellular
transfection in the laboratory, many problems must be overcome
before they can be used in humans, including low uptake efficiency
owing to a lack of targeting ability.
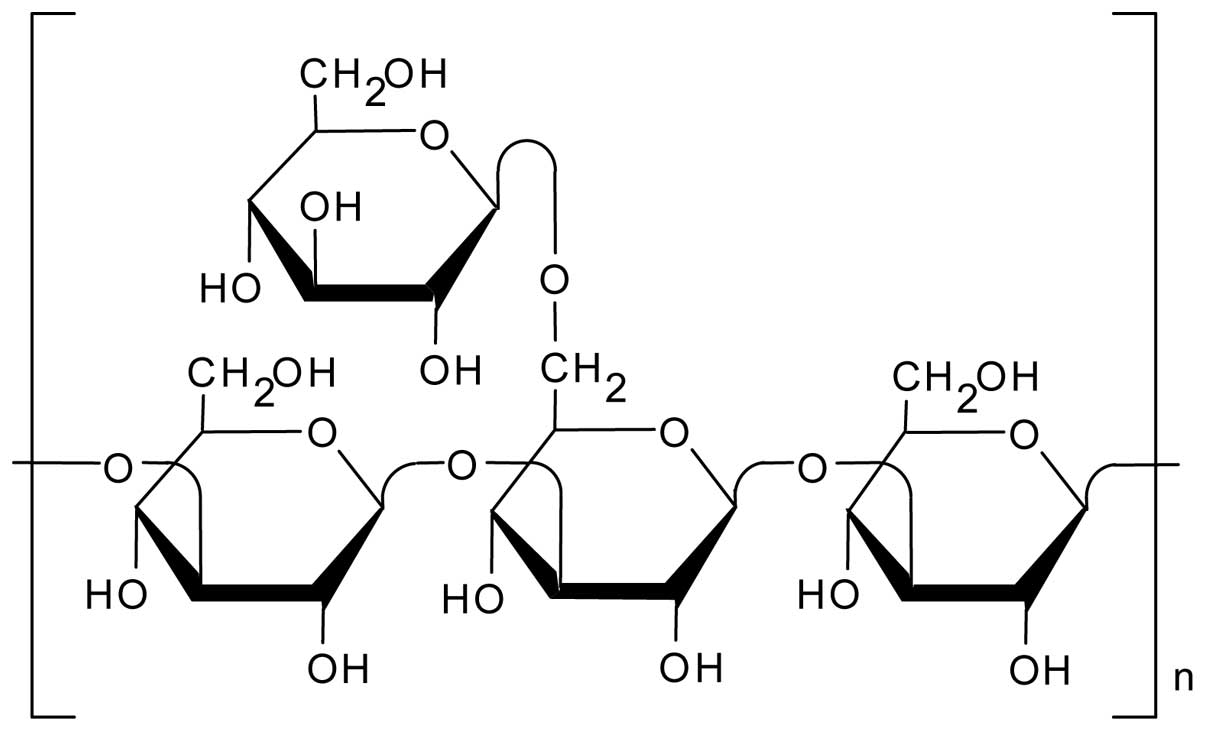
The natural polysaccharide schizophyllan (SPG)
(Fig. 1) has a main chain
comprising β-(1→3)-D-glucan and one β-(1→6)-D-glycosyl side chain
that links to the main chain at every three glucose residues
(8). We found that SPG forms a
complex with single-stranded homo-polynucleotides and examined the
fundamental properties of this complex (9–11).
We recently started to apply this complex to therapeutic
oligonucleotide delivery, including CpG DNA (12) and siRNA (13). For siRNA delivery, we demonstrated
that the SPG/siRNA complex can be recognized by a polysaccharide
receptor called Dectin-1 and subsequently enter the endocytic
pathway (13,14). This indicates SPG-mediated siRNA
delivery is one of the few known systems that possess cell-specific
targeting. Heyl et al (15)
demonstrated that Dectin-1 is widely expressed on human lung
tissues. Therefore, there is a strong possibility that some of the
lung cancers also express Dectin-1, meaning they could be treated
by delivering antisense oligonucleotides (AS-ODNs) to silence
YB-1.
Materials and methods
Preparation of antisense oligonucleotide
sequence for YB-1
YB-1 mRNA consisting of 1,561 bases was obtained
from the NCBI database (NCBI Reference Sequence: NM_004559.3)
(16). To find an effective
antisense sequence, we synthesized 153 different AS-ODNs in which
the length of the base was 25 and each sequence was designed to
match a part of the YB-1 mRNA sequence shifted from the 5′ end 10
by 10 bases. The AS-ODNs were designated AS0019-AS153 (Fig. 2).
SPG and complexation
SPG (average molecular weight, 4.5×105)
was kindly provided by Mitsui Sugar Co., Ltd. (Tokyo, Japan); this
is the same SPG used in other DDS studies (13). We prepared AS-ODNs/SPG complexes
from SPG and phosphorothioate AS-ODNs with the (dA)40
tail for YB-1. All phosphorothioate oligonucleotides including
AS-ODNs and Alexa546-labeled dA40 nucleotide were
synthesized by Gene Design Co., Ltd (Osaka, Japan) and purified by
high-performance liquid chromatography. SPG was dissolved in 0.25 N
NaOH (aq) for 2–5 days to dissociate the triple helix into a single
chain. The SPG solution, AS-ODNs in water, and phosphate-buffered
solution (330 mM NaH2PO4, pH 4.7) were mixed,
then the mixture (60 μM AS-ODNs, pH 7.4) was stored overnight at
4°C.
Lung cancer cell lines
The following 12 lung cancer cell lines were used:
B203L, PC9, A110L, A549, H1299, QG56, SQ1, B1203L, PC10, 904L, PC1
and A529L. Their characteristics and other information are
described elsewhere (17,18). All cell lines were cultured in
RPMI-1640 medium, GlutaMAX™ (Life Technologies, Tokyo, Japan) and
maintained in a 5% CO2 atmosphere at 37°C.
Cell viability assessment by the
water-soluble tetrazolium salt-8 (WST-8) assay
The cell viability assay has been previously
described (19). Briefly, PC9
cells (1×103) was seeded into 96-well plates. After 24
h, AS-ODNs were transiently transfected into the cells with 0.4l of
RNAiMax (Life Technologies) per well. Final concentration of
AS-ODNs is 50 nM in 120 μl of medium. For AS-ODNs/SPG complex, they
were directly added into the medium. After 96 h, the surviving
cells were stained with TetraColor One (Seikagaku Corp., Tokyo,
Japan) for 2–3 h at 37°C according to the manufacturer's
instructions. The absorbance was then measured at 450 nm.
Uptake of FITC-labeled SPG and
Alexa546-labeled dA40 nucleotide/SPG complex
Cells were seeded at 2.5×104 cells/well
in 24-well plates and incubated at 37°C under 5% CO2.
The cells were cultured in RPMI-1640 containing 10% FBS and 100
U/ml penicillin and 0.1 mg/ml streptomycin. After 24 h, 0.1 μM
FITC-labeled SPG (FITC-SPG) (13)
or 0.5 μM SPG complexed with Alexa546-labeled dA40
nucleotide (A546-dA40/SPG) was added to cells in the
presence of serum. After 8 h, cells treated with FITC-SPG were
washed twice with PBS and was observed with EVOS® FL
imaging system (Life Technologies). For competition assay, 10 μM
(20 times excess) SPG complexed with unlabeled dA40
nucleotide (dA40/SPG complex) was used to treat the
cells at the same time as the administration of 0.5 μM
A546-dA40/SPG. After 6 h, cells were washed twice with
PBS and was observed with EVOS® FL imaging system.
Western blot analysis
PC9 cells were suspended in lysis buffer with 10 mM
Tris-HCl (pH 7.9), 150 mM NaCl, 0.5% NP40, 1 mM PMSF and sonicated
for 10 sec. Whole cell lysate (2.5, 5 and 10 μg) were separated on
a 10% SDS-PAGE gel and transferred to a PVDF microporous membrane
(Millipore, Bedford, MA, USA). The membrane was immunoblotted with
anti-YB-1 antibody (20) at a
1:10,000 dilution, or with an anti-β-actin antibody (A5441;
Sigma-Aldrich) at a 1:10,000 dilution, for 1 h, and then incubated
with HRP-conjugated anti-rabbit IgG or anti-mouse IgG for 40 min.
Detection was performed using enhanced chemiluminescence (GE
Healthcare, Tokyo, Japan). Protein expression levels were
quantitated using Multi Gauge version 3.0 (Fujifilm, Tokyo,
Japan).
Results and Discussion
Selecting the antisense sequence
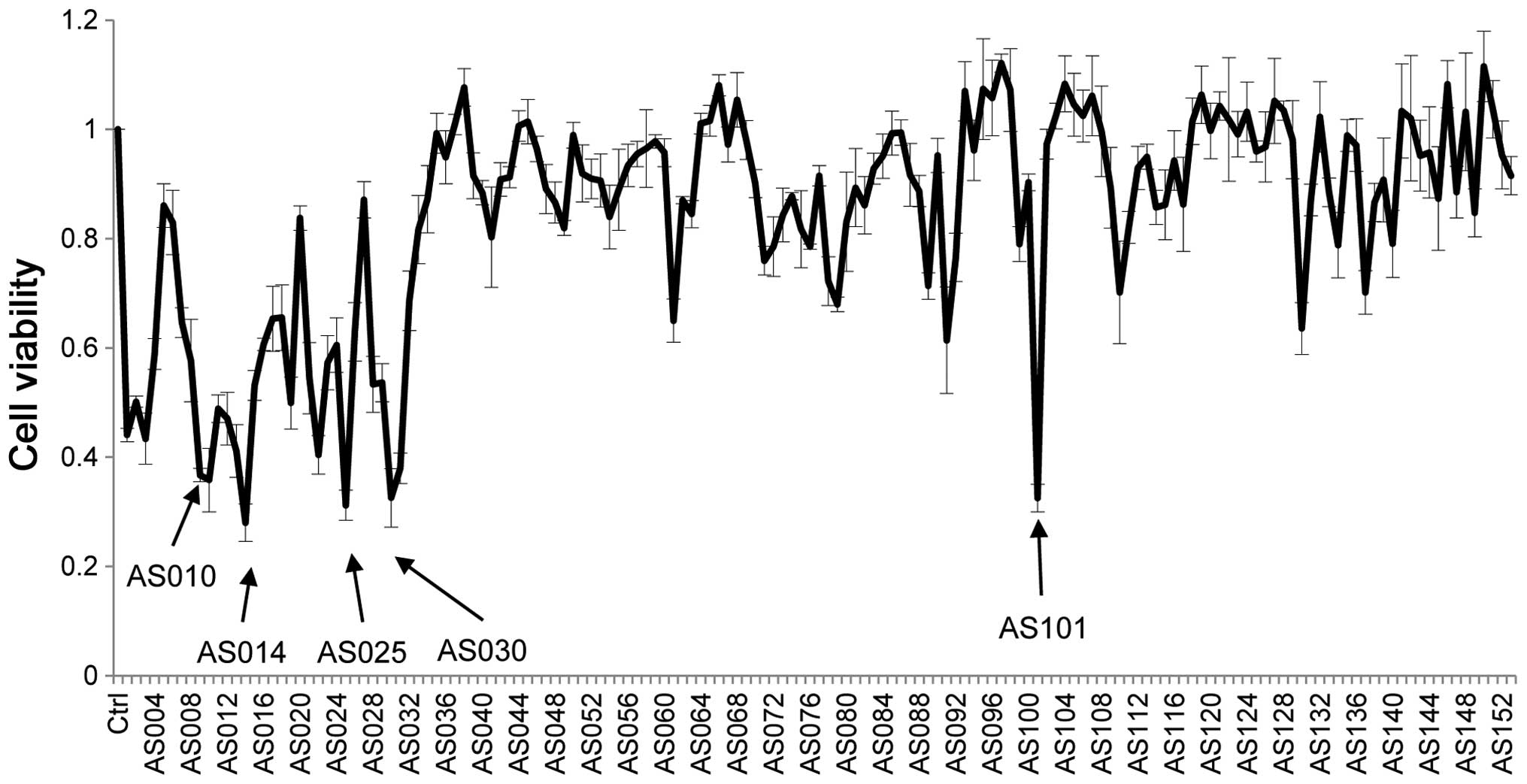
The cell viability of cancer cells treated with all
AS-ODNs transfected into PC9 cells is shown in Fig. 3; this is an in vitro screen
for cell viability. We used the RNAiMax reagent, a Lipofectamine
transfection reagent, which is a cationic liposome formulation that
forms positively charged polyion complexes with negatively charged
DNA or siRNA. Such complexes allow the bound DNA or siRNA to
eventually cross into the cytoplasm. As YB-1 is overexpressed in
PC9 cells (4) the observed
decreased cell viability is most likely due to YB-1 silencing by
AS-ODNs; moreover, the differences in cell viability reflect the
efficacy of individual AS-ODNs. The five most effective sequences
are indicated by arrows. Four AS-ODNs-AS010, AS014, AS025, and
AS030, which decreased the cell viability below one third are close
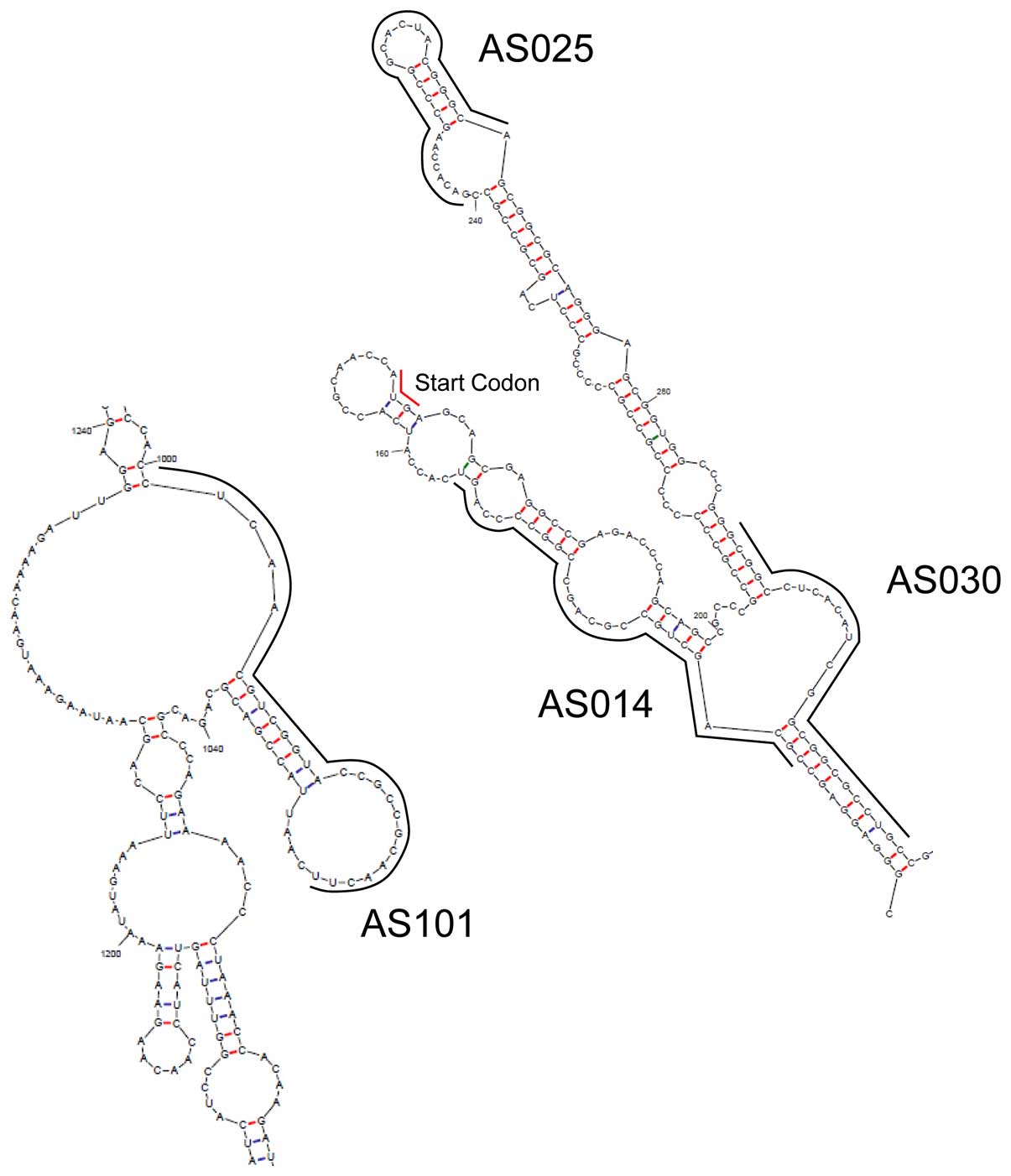
to the start codon, while AS101 is not (Fig. 4).
The recruitment of RNase H1 to the antisense
DNA/mRNA duplex is widely believed to be the key step for protein
silencing. However, there are few such sites on mRNAs, because the
binding site of mRNA must be a single chain and exposed to the
outside to make it easy for antisense DNA to bind the site. The
bulge/loop structure of YB-1 mRNA was estimated using UNAfold
(21); the result is shown in
Fig. 4, and the binding sites of
the four AS-ODNs are indicated by solid lines. All AS-ODNs binding
sites contain loop structures. Therefore, these loops are
hypothesized to be responsible for the YB-1 silencing; thus, after
hybridization, the double DNA/mRNA strand is presumably recruited
and cleaved by RNase H1.
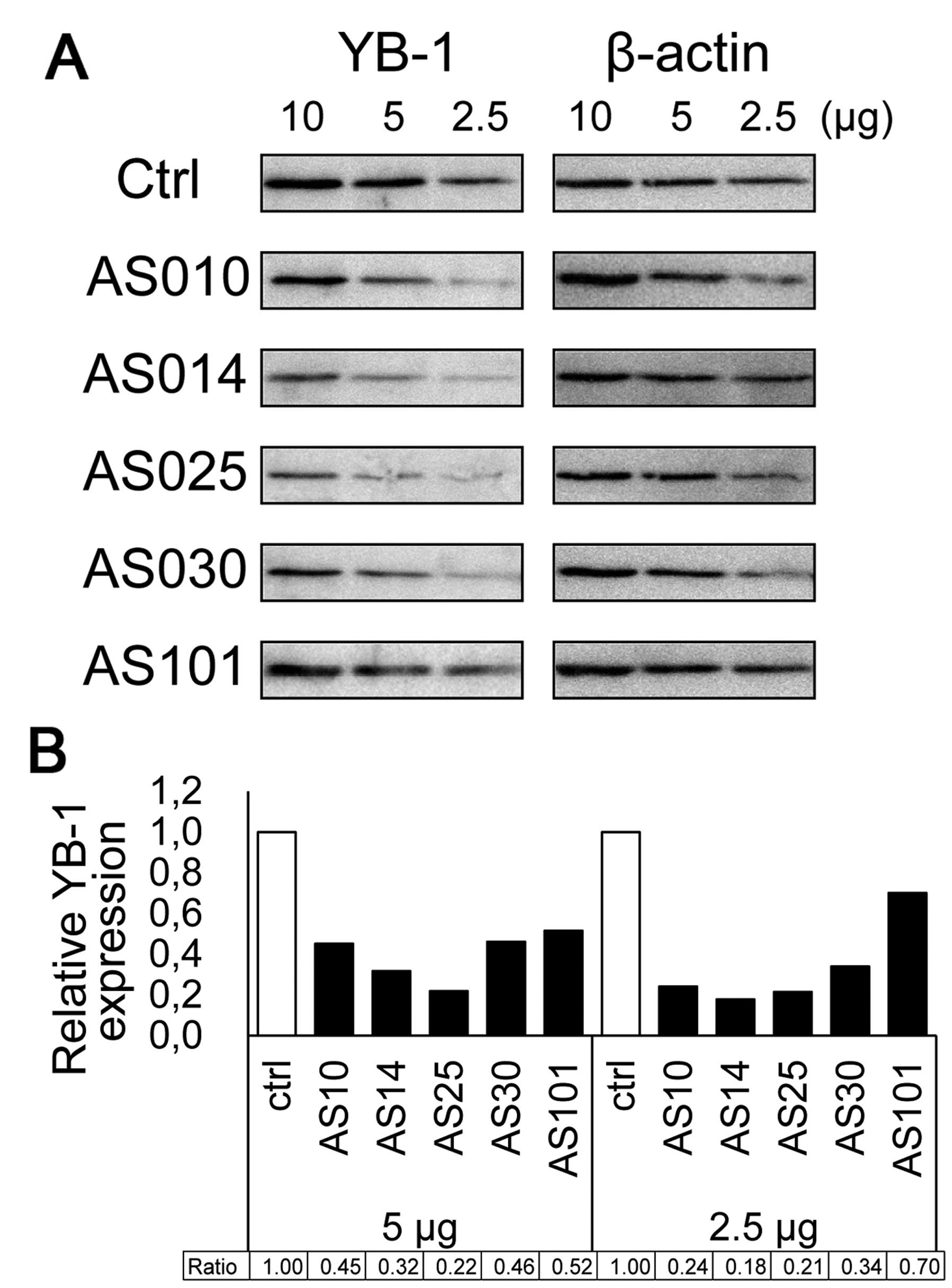
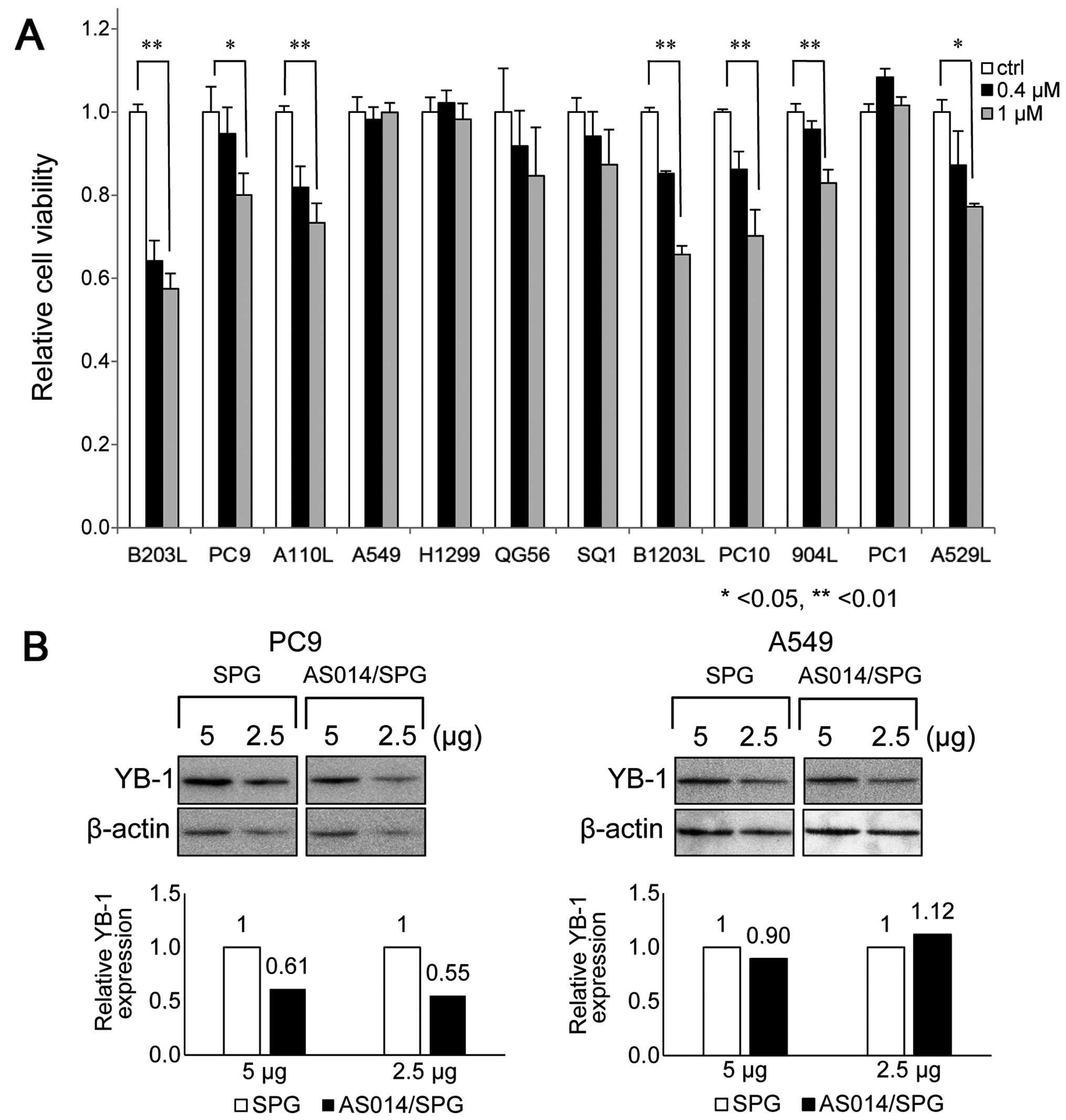
The results of western blot analysis comparing the
expression of five AS-ODNs silencing YB-1 are shown in Fig. 5A. Four AS-ODNs (AS10, AS14, AS25
and AS30) attenuated YB-1 expression to <50% of the control
(Fig. 5B). These results confirm
that the decreased cell viability is due to YB-1 silencing after
transfection. Western blot analysis indicated that AS014 and AS025
reduced the YB-1 expression very efficiently. As AS014 is closest
to the start codon region and exhibited considerably good efficacy
in both assays, we used AS014 hereafter.
Targeted delivery of AS-ODN with SPG
complexation
SPG forms a stoichiometric complex with particular
homo nucleotides such as poly(C) or poly(dA) via combination of
hydrogen bonding and hydrophobic interactions (9–11).
On the basis of our previous studies, we attached dA40
to the AS-ODN to enable complex formation with SPG and thus
efficient gene silencing (13,14).
When the attachment position is the 3′ end of the AS-ODNs, the
phosphorothioate linkage forms more stable complexes than the
phosphodiester ones (13).
Therefore, we attached phosphorothioate dA40 to the 3′
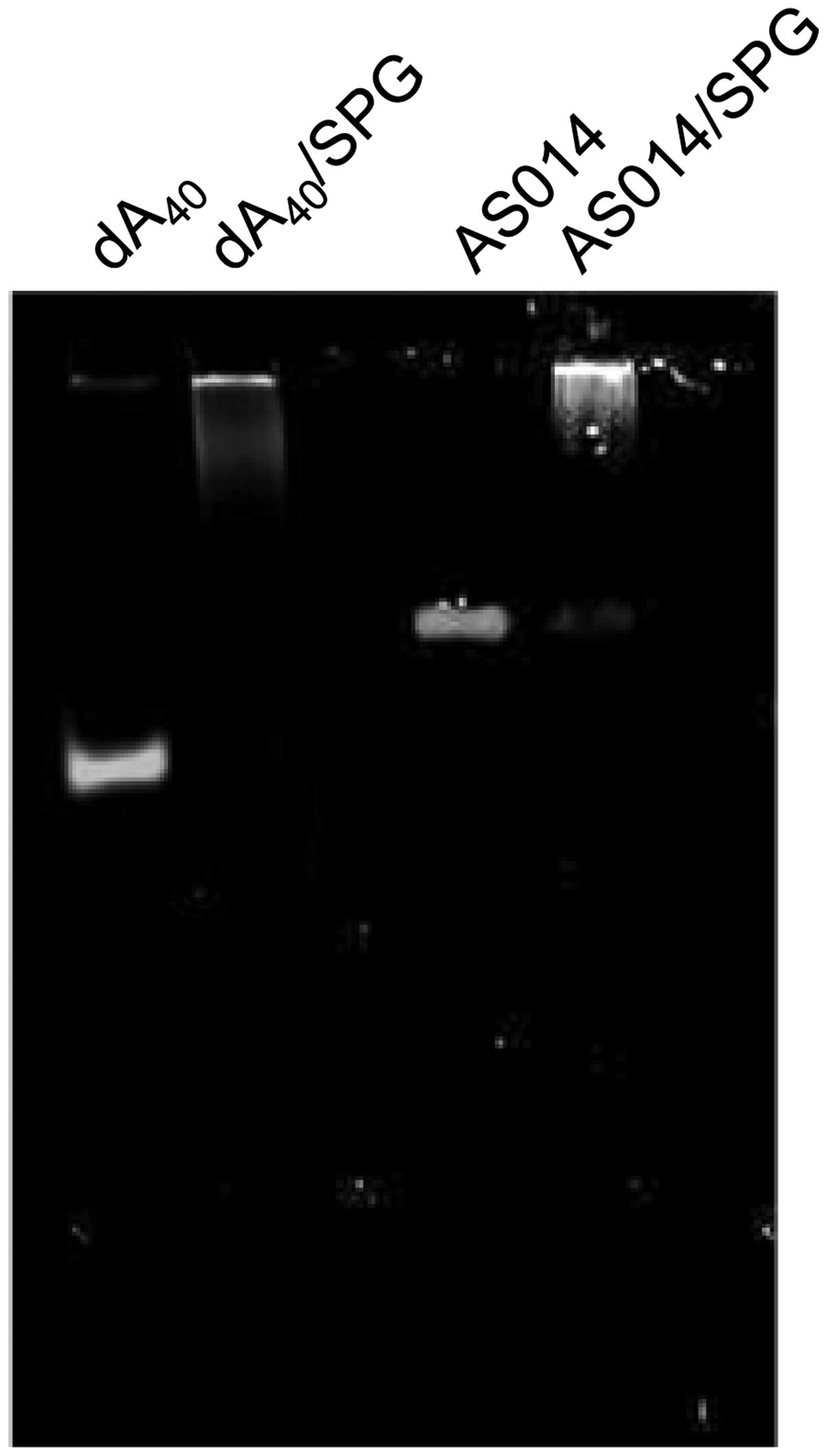
end of AS014 to form a complex with SPG. The exact stoichiometric
composition is (mG):(dA)=2:1, where mG is the main chain glucose
(9,10). However, in practice, we normally
prepare the complexes at an SPG rich composition. In the present
assay, we prepared the AS014-dA40/SPG complex at
(mG):(dA)=4:1. No free AS014-dA40 was observed in gel
electrophoresis (Fig. 6).
We previously demonstrated that the complex is taken
up by Dectin-1 expressing immunocytes. We recently cloned and
purified the extracellular domain of murine Dectin-1 and performed
binding affinity analysis between this protein and the SPG/DNA
complex by using quartz crystal microbalance (22). Phosphorothioate dA40
markedly enhanced the Dectin-1 binding affinity compared to that
with phosphodiester and also behaved differently in the Dectin-1
mutants in which the Trp221 and His223 residues in the 3′-terminal
exon were replaced with alanine. There appeared to be multiple
binding sites: the same site as SPG and an additional site(s) in
which phosphate anion specific electrostatic interactions were
mainly involved. This enhanced affinity of the phosphorothioate
DNA/SPG complex is another reason prompting its use in this
experiment.
The cell viability rates of various lung cancer
cells after the AS014/SPG complex was applied at 0.4 or 1.0 μM are
shown in Fig. 7. There are over
200 known lung cancer cell lines, from small to non-small cell
types, exceeding the number of other common epithelial cancers.
Among them, we selected 12 commonly available cell lines. AS014/SPG
complex application resulted in variably decreased cell viability
rates. As all of these cells overexpress YB-1, (4) which was also confirmed in the present
study (data not shown), the decreased cell viability is mainly
attributable to the number of AS014 molecules ingested and bound to
YB-1. However, it is unknown whether these cells express Dectin-1;
this issue is currently being investigated. The greatest decrease
in cell viability was observed in B203L, PC9, B1203L and PC10 cells
(Fig. 7A). AS014/SPG complex
decreased YB-1 expression ~40% in PC9 cells, but not A549 cells
(Fig. 7B). This results are
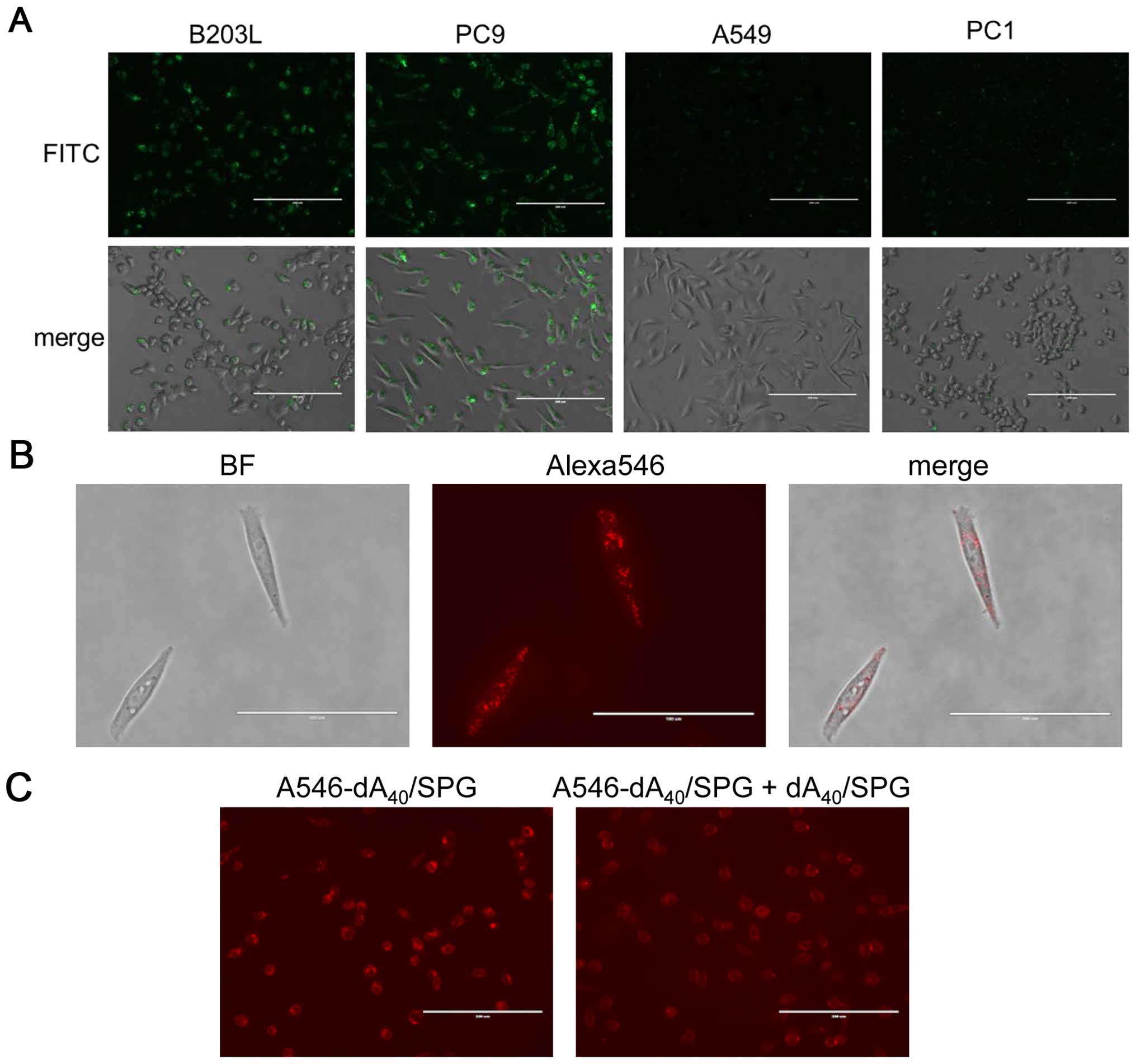
consistent with cell viability assessment (Fig. 7A). We selected B203L and PC9 as
well as A549 and PC1 cells, which showed no decrease in cell
viability, and performed fluorescent microscopy to observe
FITC-labeled SPG. SPG was actively ingested by B203L and PC9 but
not A549 or PC1 cells (Fig. 8A),
which is concordant with their cell viability. We also found
A546-dA40/SPG was ingested by PC9 cells (Fig. 8B). This ingestion was partially
abolished by addition of the dA40/SPG complex (Fig. 8C). As mentioned above, the Dectin-1
expression assay for these cells is ongoing. Nevertheless, it is
safe to conclude that AS014 was ingested by B203L and PC9 cells,
which was facilitated by the recognition of Dectin-1 for the
AS014/SPG complex. Furthermore, the results indicate AS014 silenced
the YB-1 expression, resulting in decreased cell viability.
In conclusion, five optimal antisense DNA sequences
for silencing YB-1 expression in lung cancers were selected from
among 153 candidates. We chose the one closest to the start codon,
AS014, and attached dA40 to the 3′ end to form a complex
with SPG. The resultant complexes were applied to 12 human-oriented
lung cancer cell lines to examine cell viability. Several cell
lines exhibited markedly decreased cell viability presumably due to
YB-1 silencing, which was corroborated by western blot analysis.
The cells exhibited such decrease also ingested SPG, suggesting the
AS014/SPG complex entered the cells via the Dectin-1 mediated
pathway.
References
|
1
|
Lasham A, Print CG, Woolley AG, Dunn SE
and Braithwaite AW: YB-1: Oncoprotein, prognostic marker and
therapeutic target? Biochem J. 449:11–23. 2013. View Article : Google Scholar
|
|
2
|
Ohga T, Koike K, Ono M, Makino Y, Itagaki
Y, Tanimoto M, Kuwano M and Kohno K: Role of the human Y
box-binding protein YB-1 in cellular sensitivity to the
DNA-damaging agents cisplatin, mitomycin C, and ultraviolet light.
Cancer Res. 56:4224–4228. 1996.PubMed/NCBI
|
|
3
|
Kuwano M, Uchiumi T, Hayakawa H, Ono M,
Wada M, Izumi H and Kohno K: The basic and clinical implications of
ABC transporters, Y-box-binding protein-1 (YB-1) and
angiogenesis-related factors in human malignancies. Cancer Sci.
94:9–14. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shibahara K, Sugio K, Osaki T, Uchiumi T,
Maehara Y, Kohno K, Yasumoto K, Sugimachi K and Kuwano M: Nuclear
expression of the Y-box binding protein, YB-1, as a novel marker of
disease progression in non-small cell lung cancer. Clin Cancer Res.
7:3151–3155. 2001.PubMed/NCBI
|
|
5
|
Antisense Drug Technology. Principles,
Strategies, and Applications. 2nd edition. Crooke ST: CRC Press;
Boca Raton, FL: 2007
|
|
6
|
Gene Silencing by RNA Interference:
Technology and Application. 1st edition. Sohail M: CRC Press; Boca
Raton, FL: 2004, View Article : Google Scholar
|
|
7
|
Mintzer MA and Simanek EE: Nonviral
vectors for gene delivery. Chem Rev. 109:259–302. 2009. View Article : Google Scholar
|
|
8
|
Harada T, Misaki A and Saito H: Curdlan: A
bacterial gelforming beta-1,3-glucan. Arch Biochem Biophys.
124:292–298. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakurai K and Shinkai S: Molecular
recognition of adenine, cytosine, and uracil in a single-stranded
RNA by a natural polysaccharide: Schizophyllan. J Am Chem Soc.
122:4520–4521. 2000. View Article : Google Scholar
|
|
10
|
Sakurai K, Mizu M and Shinkai S:
Polysaccharide-polynucleotide complexes. 2 Complementary
polynucleotide mimic behavior of the natural polysaccharide
schizophyllan in the macromolecular complex with single-stranded
RNA and DNA. Biomacromolecules. 2:641–650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sanada Y, Matsuzaki T, Mochizuki S,
Okobira T, Uezu K and Sakurai K: β-1,3-D-glucan
schizophyllan/poly(dA) triple-helical complex in dilute solution. J
Phys Chem B. 116:87–94. 2012. View Article : Google Scholar
|
|
12
|
Kobiyama K, Aoshi T, Narita H, Kuroda E,
Hayashi M, Tetsutani K, Koyama S, Mochizuki S, Sakurai K, Katakai
Y, et al: Nonagonistic Dectin-1 ligand transforms CpG into a
multitask nanoparticulate TLR9 agonist. Proc Natl Acad Sci USA.
111:3086–3091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mochizuki S, Morishita H and Sakurai K:
Macrophage specific delivery of TNF-α siRNA complexed with
β-1,3-glucan inhibits LPS-induced cytokine production in a murine
acute hepatitis model. Bioorg Med Chem. 21:2535–2542. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q, Ichimaru N, Higuchi S, Cai S, Hou
J, Fujino M, Nonomura N, Kobayashi M, Ando H, Uno A, et al:
Permanent acceptance of mouse cardiac allografts with CD40 siRNA to
induce regulatory myeloid cells by use of a novel polysaccharide
siRNA delivery system. Gene Ther. 22:217–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heyl KA, Klassert TE, Heinrich A, Müller
MM, Klaile E, Dienemann H, Grünewald C, Bals R, Singer BB and
Slevogt H: Dectin-1 is expressed in human lung and mediates the
proin-flammatory immune response to nontypeable Haemophilus
influenzae. MBio. 5:e01492–e14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mylona E, Melissaris S, Giannopoulou I,
Theohari I, Papadimitriou C, Keramopoulos A and Nakopoulou L:
Y-box-binding protein 1 (YB1) in breast carcinomas: Relation to
aggressive tumor phenotype and identification of patients at high
risk for relapse. Eur J Surg Oncol. 40:289–296. 2014. View Article : Google Scholar
|
|
17
|
Tanabe M, Izumi H, Ise T, Higuchi S,
Yamori T, Yasumoto K and Kohno K: Activating transcription factor 4
increases the cisplatin resistance of human cancer cell lines.
Cancer Res. 63:8592–8595. 2003.PubMed/NCBI
|
|
18
|
Izumi H, Takahashi M, Uramoto H, Nakayama
Y, Oyama T, Wang KY, Sasaguri Y, Nishizawa S and Kohno K:
Monocarboxylate transporters 1 and 4 are involved in the invasion
activity of human lung cancer cells. Cancer Sci. 102:1007–1013.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kashiwagi E, Izumi H, Yasuniwa Y, Baba R,
Doi Y, Kidani A, Arao T, Nishio K, Naito S and Kohno K: Enhanced
expression of nuclear factor I/B in oxaliplatin-resistant human
cancer cell lines. Cancer Sci. 102:382–386. 2011. View Article : Google Scholar
|
|
20
|
Takahashi M, Shimajiri S, Izumi H, Hirano
G, Kashiwagi E, Yasuniwa Y, Wu Y, Han B, Akiyama M, Nishizawa S, et
al: Y-box binding protein-1 is a novel molecular target for tumor
vessels. Cancer Sci. 101:1367–1373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zuker M: Mfold web server for nucleic acid
folding and hybridization prediction. Nucleic Acids Res.
31:3406–3415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mochizuki S, Morishita H, Adachi Y,
Yamaguchi Y and Sakurai K: Binding assay between murine Dectin-1
and β-glucan/DNA complex with quartz-crystal microbalance.
Carbohydr Res. 391:1–8. 2014. View Article : Google Scholar : PubMed/NCBI
|