Introduction
Cancer is a leading cause of death in both more and
less economically developed countries; the burden is expected to
grow worldwide due to the growth and aging of the population and
improvements in early detection and treatment (1). Cancer is a major public health
problem in many parts of the world. It is currently the second
leading cause of death in the United States (1,2).
Breast cancer, is the most frequently occurring cancer in women and
some cases show slow growth with excellent prognosis, while
aggressive tumors have poor prognosis (1–3).
Breast tumors have also been identified into different subtypes
based on the expression of estrogen receptors (ER), progesterone
receptors (PR) and Her2 oncogene. The breast tumors that do not
express ER, PR or Her2 are called triple-negative breast cancers
and ~15% of the breast cancers fall into this category (3,4).
Apoptosis is a process of programmed cell death that
occurs in response to environmental stimuli and normal growth and
homeostasis, during development, embryogenesis, modification of
normal tissues, and appropriate strategy for prevention and
treatment of cancer (5,6). Bcl-2 protein family plays an
important role in the survival or death of a cell and in recent
years these proteins have been the target of many antitumor drugs
(7). Bax is a protein
pro-apoptotic that operates as an enhancer of apoptosis in contrast
to Bcl-2 with antiapoptotic properties. The interactions between
both in the cytosol and on mitochondria determine the fate of the
cell for death or survival (8).
Gene and protein expression of Bax in breast cancer cells increase
sensitivity to apoptotic stimuli and decreases tumor enlargement
while, Bcl-xL is another anti-apoptotic protein that inhibits
apoptosis and stimulates the progress of breast cancer (6,7,9). In
cancer cells, it has been demonstrated that the Bax/Bcl-2 ratio is
diminished favoring the survival of cells, but treatment with
anticancer drugs increased Bax/Bcl-2 ratio producing cell apoptosis
by the intrinsic pathway (6,10–12).
Caspase activity has been related to the different
pathways of apoptosis, caspase-8 and caspase-10 features of the
extrinsic pathway, whereas caspase-9 of the intrinsic pathway.
Caspase-8 and caspase-9 (among others) are initiators of apoptosis
and their activation triggers the caspase-3 effector, therefore,
their increase stimulate apoptosis (5,13,14).
On the contrary, nuclear factor kappa-light-chain-enhancer of
activated B cells (NF-κB) is a pro-inflammatory and pro-survival
transcription factor and it is known to be highly involved in the
initiation and progression of breast cancer. Dysregulation of NF-κB
activity alters the expression of cell death-regulating genes,
leading to the upregulation of antiapoptotic and pro-survival
genes, such as members of the Bcl-2 family. Protein inhibitors of
κB (IκB) suppress activation of NF-κB (15–17).
The inhibitors include IκBα, IκBβ, IκBɛ and IκBζ.
Noscapine is a phthalideisoquinoline alkaloid
derived from opium that has been used as an antitussive in humans
and in animal experiments. Some studies have shown anxiolytic
effects in mice. This is also a drug with low toxicity and good
tolerance, animal studies showed a wide margin of safety in both
acute and chronic treatment. Previous studies have shown that
noscapine is also an agent with antitumor properties that produces
alterations in cell cycle progression and consequently induces
apoptosis in cell lines of different cancers (18–20).
These features make it an attractive drug for the study in the
management of breast cancer. The aim of the present study was to
determine the effect of noscapine on gene and protein expression
involved in apoptosis in breast cancer cell lines compared to a
breast normal cell line.
Materials and methods
Cell lines and drug
In these studies three cell lines were used: i) a
control epithelial cell line breast MCF-10F spontaneously
immortalized; ii) a luminal-like adenocarcinoma triple positive
cell line MCF-7; and iii) breast cancer triple-negative cell line
MDA-MB-231. MCF-10F was grown in Dulbecco's modified Eagle's medium
(DMEM)/F-12 (1:1) supplemented with antibiotics 100 U/ml
penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B (all
from Life Technologies, Grand Island, NY, USA), 5% horse serum
(Biofluids, Rockville, MD, USA), 10 μg/ml insulin (Sigma-Aldrich,
St. Louis, MO, USA), 0.5 mg/ml hydrocortisone (Sigma) and 0.02
μg/ml epidermal growth factor (Collaborative Research, Inc.,
Bedford, MA, USA) at the temperature of 37°C and 5% concentration
of CO2. MCF-7 cell line was cultivated in the Dulbecco's
modified medium (Sigma-Aldrich, GmbH, Munich, Germany) supplemented
with antibiotics 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5
μg/ml amphotericin B and 10% fetal calf serum at the temperature of
37°C and 5% concentration of CO2. MDA-MB-231 cell line
was purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and was maintained DMEM supplemented with
antibiotics 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml
amphotericin B and 10% fetal bovine serum (FBS) at the temperature
of 37°C and 5% concentration of CO2. Noscapine (97%
purity) was obtained from Sigma-Aldrich. The noscapine stock
solution was prepared at 100 mM in dimethyl sulfoxide (DMSO)
(Sigma) and kept at −20°C until use.
MTT assay
The metabolic activity of living cells, as indicator
of cell viability, was determined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. The MCF-10F, MCF-7 and MDA-MB-231 cell lines were seeded in
24-well microplates (25×103 cells/well) and incubated in
culture medium for 24 and 48 h at temperature 37°C and 5%
concentration of CO2. After incubation the cells were
treated with 20, 40, 60, 80 and 100 μM of noscapine. Subsequently
to the treatment of cells, reduction of MTT was determined
following the manufacturer's instructions. The treatment groups
were compared with the control group and the results were expressed
as percentage of viable cells.
Western blotting
The cells were lysed with 1 ml of lysis buffer (Tris
Base 50 mM at pH 7.2, 1 mM EDTA, 100 mM NaCl, 1 mM PMSF, 1 mM
ortovanadate, 0.1% Triton X-100) and centrifuged at 10,000 rpm for
10 min. The supernatant with cellular proteins were dissolved in
SDS-PAGE solution [Tris 60 mM to pH 6.5, 10% (w/v) glycerol, 5%
(w/v) β-mercaptoethanol, 20% (w/v) SDS, and 0.025% (w/v)
bromo-phenol blue] and denatured by boiling (5 min) and the mixture
(30 sec) was stirred. The total amount of protein in each lane was
30 μg determined by the use of bicinchoninic acid method (Bio-Rad
Laboratories, Hercules, CA, USA) and BSA as standard, plus protein
standard markers (Bio-Rad Laboratories). After fractionation by
SDS-PAGE gels (7×14 cm), proteins were electro-transferred onto
PVDF membrane (Amersham Biosciences, Buckinghamshire, UK) using a
transfer apparatus (Bio-Rad Laboratories). The transferred
membranes were blocked for 2 h in 10% defatted milk-TBS-0.1% and
then incubated for 2 h at room temperature with the corresponding
primary antibody (1:200) Bcl-2 (C-2) sc-7382, Bax (N-20) sc-493,
caspase-8 (8CSP03) sc-56070, NF-κB p52 (C-5) sc-7386 and β-actin
(C4) sc-47778 followed by incubation with goat anti-mouse IgG-HRP
sc-2302 or goat anti-rabbit IgG-HRP sc-2004 secondary antibody
(1:5,000) in 5% milk dry defatted with TBS-0.1%-Tween. All steps
were performed at room temperature. The cell transfers were tested
with mouse anti-actin antibody as control. Immunoreactive bands
were displayed using the ECL™ detection method Western Blotting
detection reagent (Amersham Biosciences, Dübendorf, Switzerland)
and exposing the membrane to X-ray film. The experiments were
performed three times.
RNA extraction and cDNA synthesis
Total RNA was isolated using TRIzol reagent
(Invitrogen Corp., Carlsbad, CA, USA) according to the
manufacturer's recommendations. Total RNA (2 μg measured by
spectrophotometry to 260 nm wavelength) was reverse-transcribed to
cDNA using High Capacity cDNA reverse transcription kit (Applied
Biosystems, Carlsbad, CA, USA) and 10 units of RNase inhibitor
(Applied Biosystems).
Differential display-reverse
transcriptase-PCR(DD-RT-PCR)
Two microliters (μl) were used from cDNA obtained in
23 μl containing PCR Nucleotide Mix (Promega, Madison, WI, USA) and
5 μM of each primer for the target genes Bax, Bcl-xL,
caspase-8, caspase-9, NF-κB and IκBα.
β-actin was used as reference gene. The reaction was
performed in a Mastercycler personal (Eppendorf) with the following
conditions: 94°C for 10 min, followed by 25 cycles for melting
temperature of each primer (Table
I) for 30 sec, and 72°C for 30 sec, to finish 72°C for 5 min
(Table II). The PCR products were
run on a 2% (w/v) agarose gel with ethidium bromide (5 mg/ml) and
photographed and then analyzed.
 | Table IPrimers of genes selected for
differential display conventional PCR analysis. |
Table I
Primers of genes selected for
differential display conventional PCR analysis.
| Gene name | Primer
sequencesa | Product length
(bp)b |
|---|
| Bax | Forward:
GCGAGTGTCTCAAGCGCATC
Reverse: CCAGTTGAAGTTGCCGTCAGAA | 143 |
| Bcl-xL | Forward:
CTGAATCGGAGATGGAGACC
Reverse: TGGGATGTCAGGTCACTGAA | 211 |
|
Caspase-8 | Forward:
CATCCAGTCACTTTGCCAGA
Reverse: GCATCTGTTTCCCCATGTTT | 128 |
|
Caspase-9 | Forward:
CCAGAGATTGCGAAACCAGAGG
Reverse: GAGCACCGACATCACCAAATCC | 88 |
| NF-κB (Rel
A) | Forward:
ATCTGCCGAGTGAACCGAAACT
Reverse: CCAGCCTGGTCCCGTGAAA | 114 |
| IκBα | Forward:
CTCCGAGACTTTCGAGGAAATAC
Reverse: GCCATTGTAGTTGGTAGCCTTCA | 135 |
| β-actin | Forward:
ACTACCTCATGAAGATCCTC
Reverse: TAGAAGCATTTGCGGTGGACGATGG | 569 |
 | Table IIProtocol for PCR analysis. |
Table II
Protocol for PCR analysis.
| Gene name | Cycles no. | PCR step |
Temperature/time |
|---|
| Bax | 25 | Annealing | 58°C/30 sec |
| Bcl-xL | 25 | Annealing | 55°C/30 sec |
|
Caspase-8 | 25 | Annealing | 55°C/30 sec |
|
Caspase-9 | 25 | Annealing | 58°C/30 sec |
| NF-κB (Rel
A) | 25 | Annealing | 58°C/30 sec |
| IκBα | 25 | Annealing | 55°C/30 sec |
| β-actin | 25 | Annealing | 58°C/30 sec |
DNA fragmentation
The cell lines were lysed with 500 μl of lysis
buffer (Tris-HCl 10 mM at pH 8.0, 10 mM EDTA, 0.5% Triton X-100)
for 30 min on ice, followed by 1 h at 37°C in 1 μl of ribonuclease
A (10 mg/ml) (Sigma-Aldrich) and 2 h at 60°C with 25 μl of
proteinase K (20 mg/ml). Then, 500 μl of basic phenol-chloroform
(Winkler LTDA, Santiago, Chile) was added and centrifuged at 4°C
(13,200 rpm for 15 min). The supernatant was recovered and added
with isopropanol overnight at −20°C. It was centrifuged to 13,200
rpm for 15 min at 4°C and the pellet was suspended in 30 μl of
molecular water. The product was run on a 1.5% (w/v) agarose gel
with 5 mg/ml ethidium bromide and photographed.
Statistical analysis
The results were expressed as the average ± standard
error of the mean (SEM) and analyzed using one way ANOVA followed
by Dunnet's test. The P-value <0.05 was considered significant.
Inhibitor concentration at 50% (IC50) was calculated by
a non-linear regression curve using GraphPad Prism 6.0 for Windows
(GraphPad Software, Inc., San Diego, CA, USA).
Results
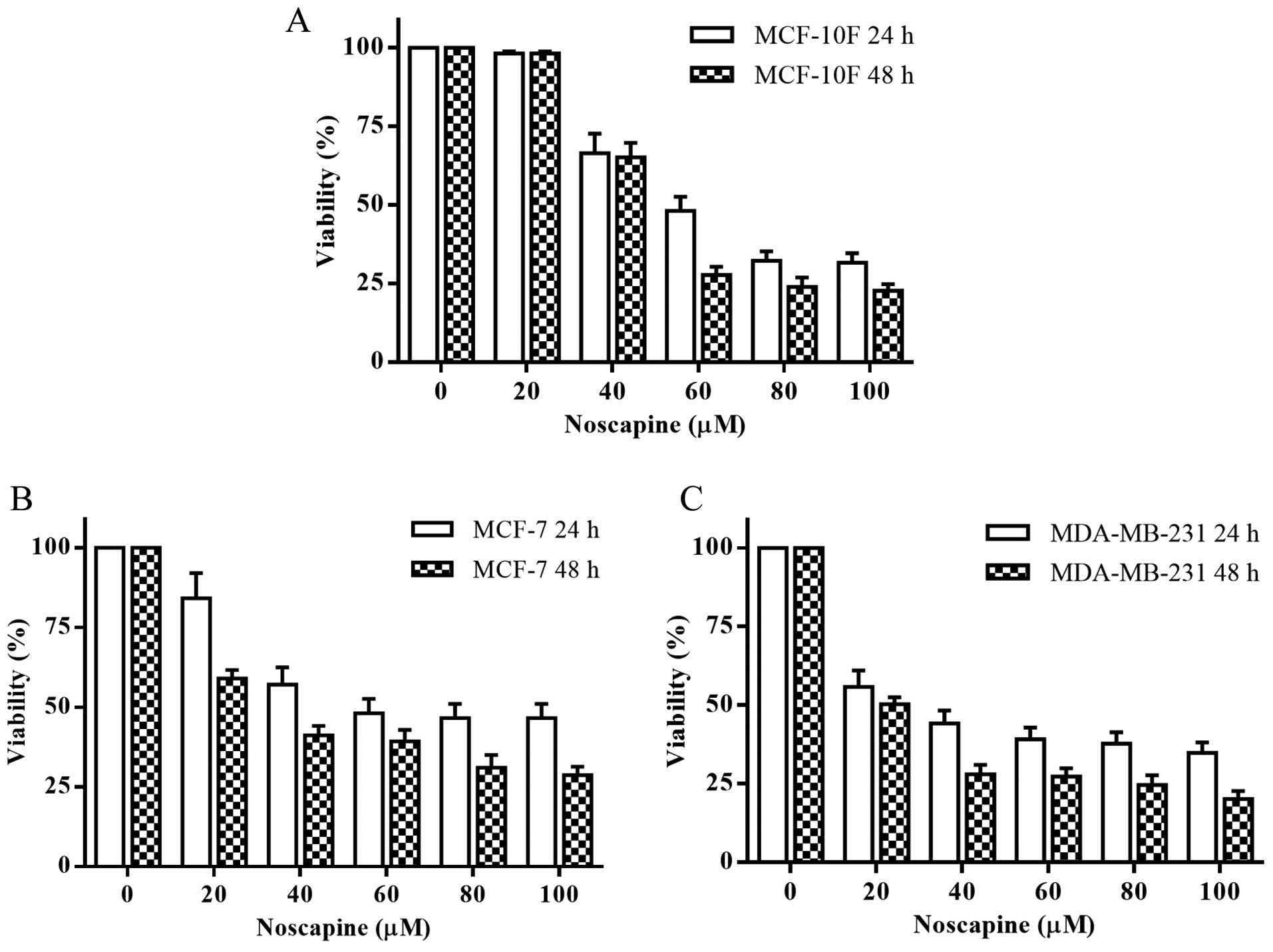
The MTT assay was used to determine the effect of
noscapine on cell viability in vitro. Noscapine effectively had a
dose-dependent cytotoxic effect after 24 and 48 h in MCF-10F, MCF-7
and MDA-MB-231 cell lines (Fig.
1). Noscapine had an IC50 of 58, 54 and 29 μM for
MCF-10F, MCF-7 and MDA-MB-231 cell lines at 24 h, respectively. The
IC50 was of 53, 30 and 20 μM for MCF-10F, MCF-7 and
MDA-MB-231 cell lines at 48 h, respectively. All the experiments
were performed with their respective IC50 at 48 h for
each cell line.
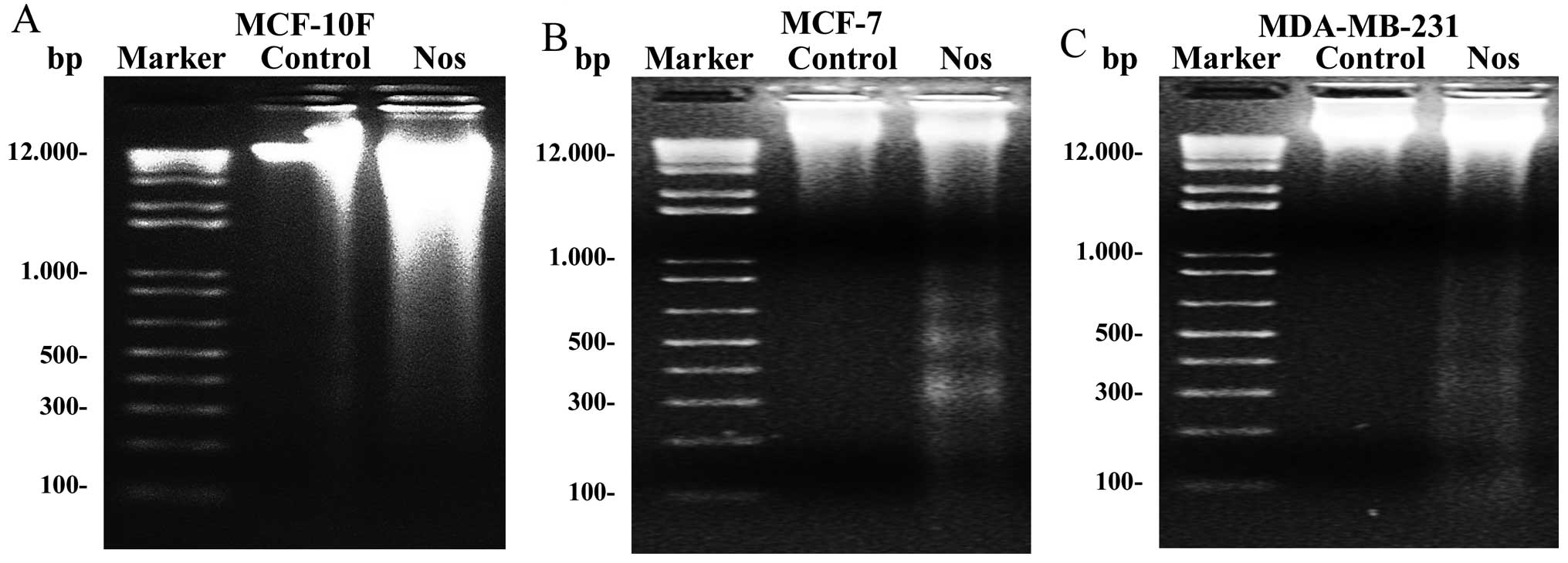
To determine whether apoptosis was the major
mechanism of cell death induced by noscapine, internucleosomal DNA
fragmentation was investigated in the three cell lines. Results
showed that control cell lines did not present fragmentation.
MCF-10F treated with noscapine did not show clearly ladder DNA
fragmentation (Fig. 2A). However,
results clearly showed that MCF-7, and MDA-MB-231 breast cancer
cell lines (Fig. 2B and C) treated
with noscapine had ladder DNA fragmentation.
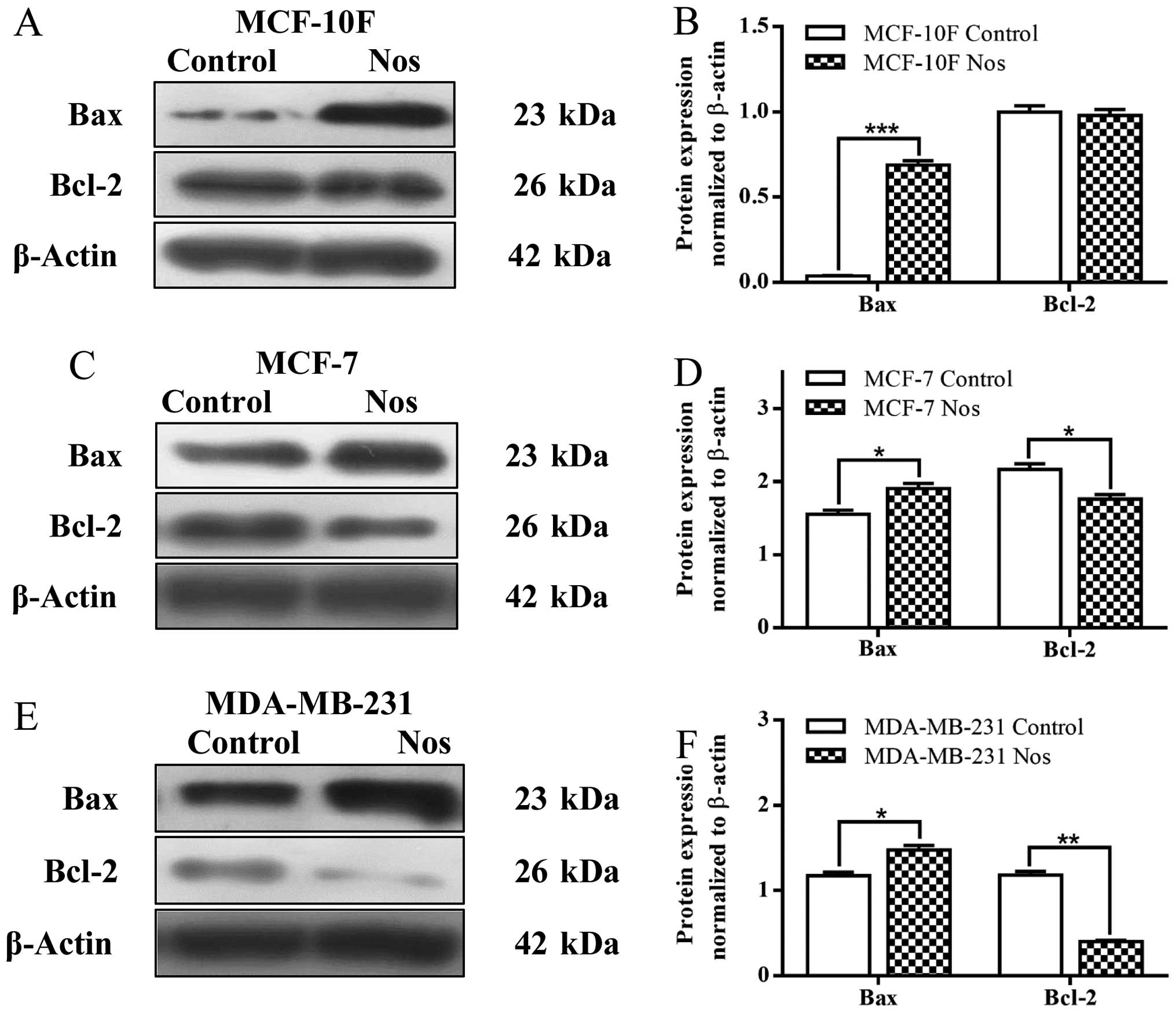
Results showed that noscapine significantly
(P<0.001) increased Bax protein expression but there was no
significance of Bcl-2 (Fig. 3A and
B) in MCF-10F cell line when compared to their counterparts.
Noscapine significantly (P<0.05) increased Bax protein
expression and significantly (P<0.05) decreased of Bcl-2 in
MCF-7 breast cancer cell line (Fig. 3C
and D). Noscapine also significantly (P<0.05) increased Bax
protein expression and it significantly (P<0.001) decreased
Bcl-2 protein expression in MDA-MB-231 breast cancer cell line
(Fig. 3E and F) in comparison to
their counterparts.
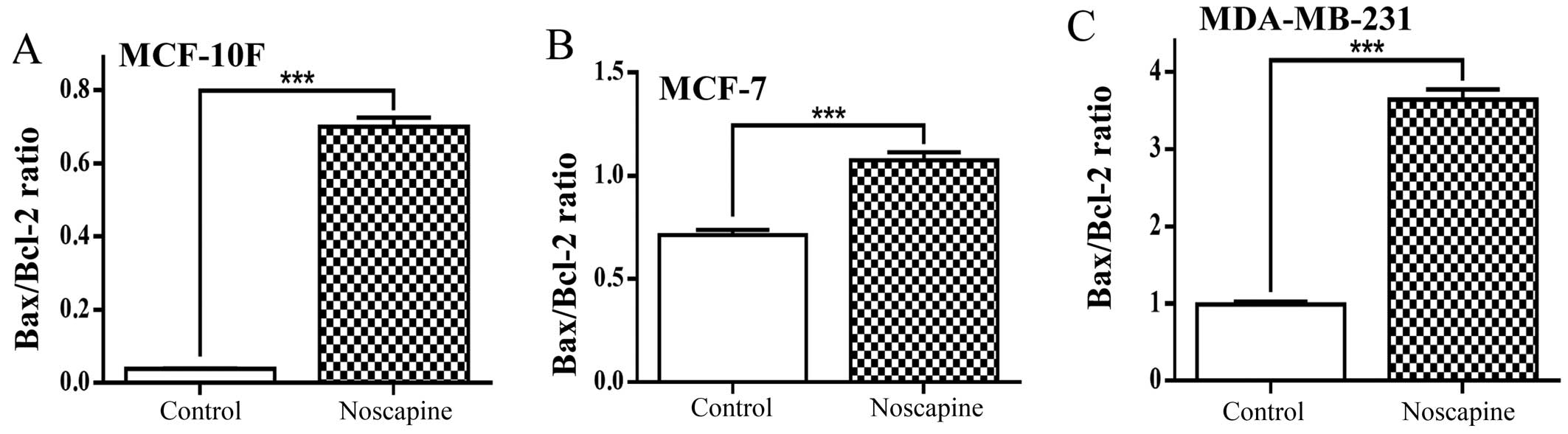
Fig. 4A shows
Bax/Bcl-2 ratio evaluated from protein expression in MCF-10F cells
treated with noscapine. The drug significantly (P<0.001)
increased the ratio in this cell line from 0.03 to 0.70 of the
proapoptotic protein Bax in relation to an antiapoptotic protein
Bcl-2. The MCF-7 cell line treated with noscapine also
significantly (P<0.001) increased the Bax/Bcl-2 ratio from 0.71
to 1.08 as seen in Fig. 4B.
Results showed that noscapine significantly (P<0.001) increased
Bax/Bcl-2 ratio from 0.99 to 3.64 in MDA-MB-231 cells (Fig. 4C).
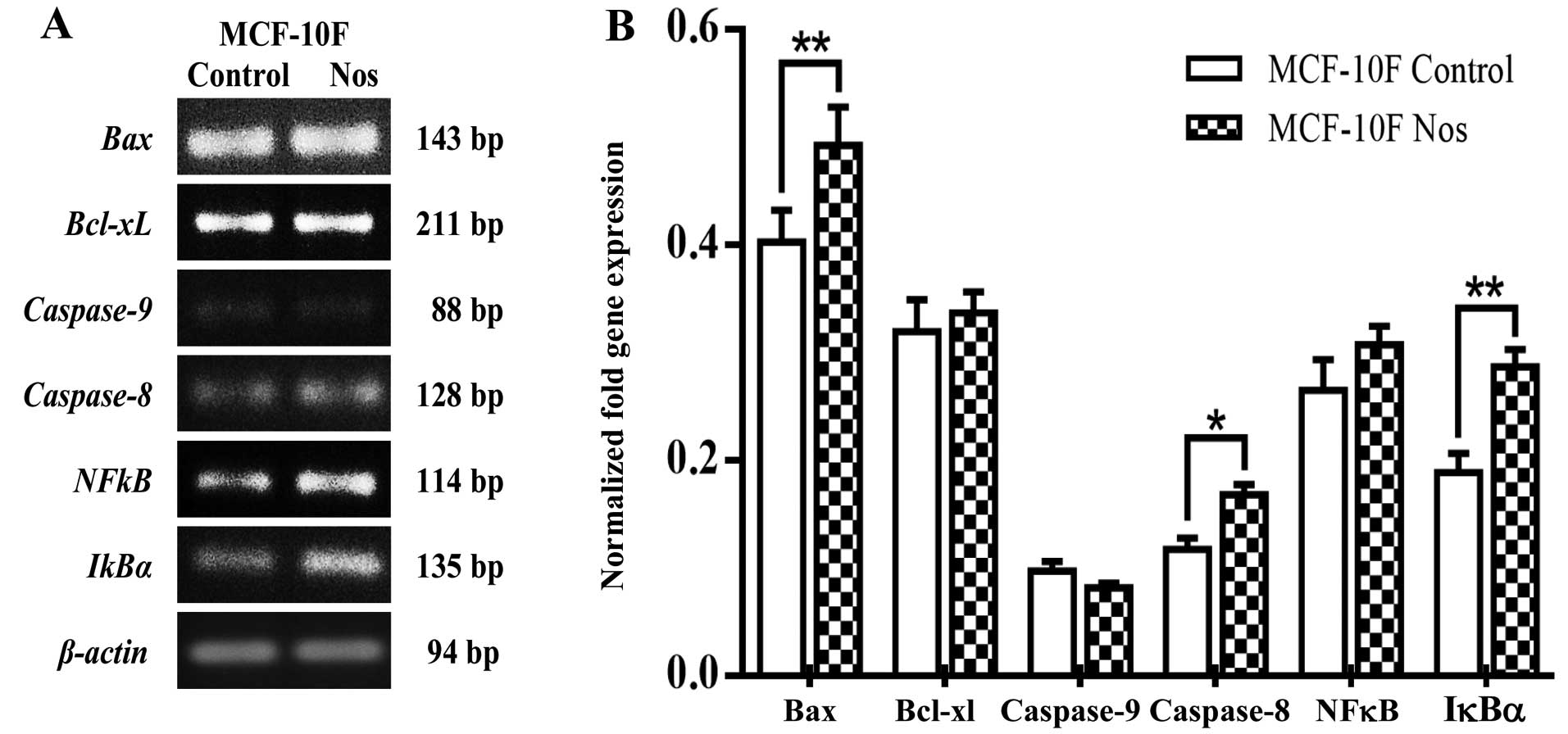
Studies on gene expression showed that
noscapine-treated MCF-10F cells significantly (P<0.05) increased
Bax, caspase-8 and IκBα (Fig. 5). However, there was no change in
Bcl-xL, caspase-9 and NF-κB by the effect of
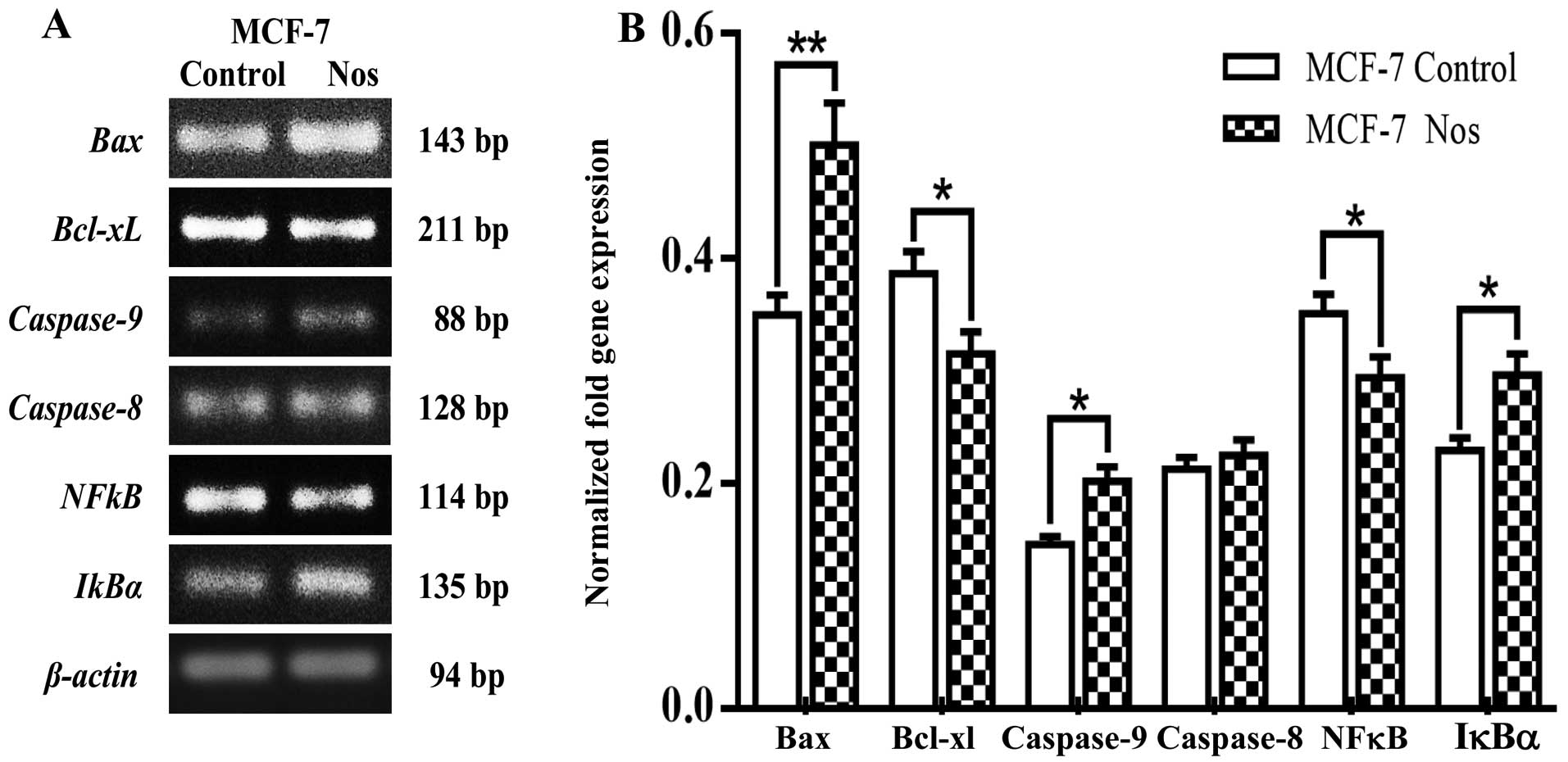
this drug. Fig. 6 shows that
noscapine significantly (P<0.05) increased Bax,
caspase-9 and IκBα, gene expression while
significantly (P<0.05) decreased the levels of Bcl-xL and
NF-κB in MCF-7. Caspase-8 did not show any significance by
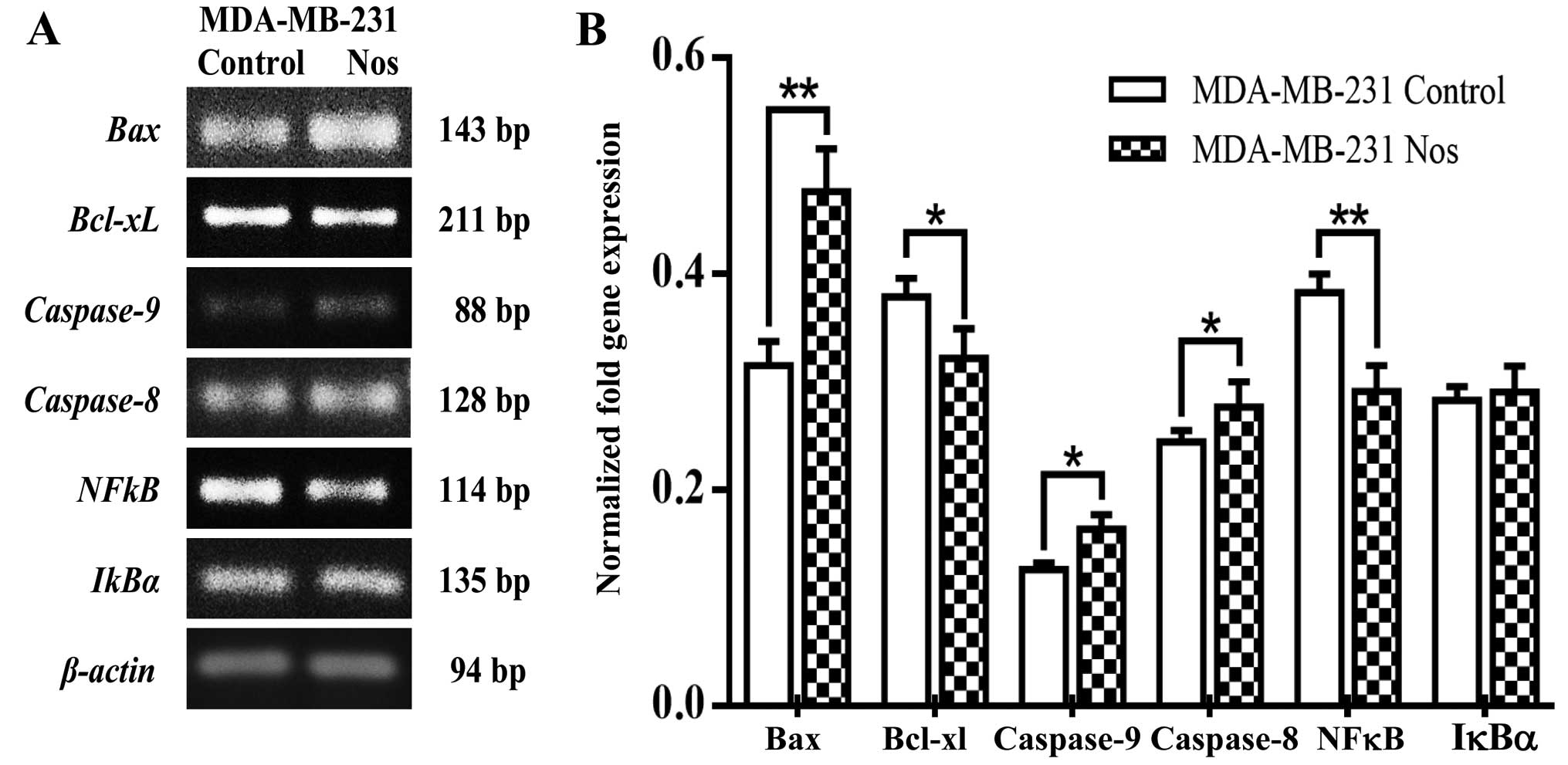
the effect of noscapine. Furthermore, noscapine treated- MDA-MB-231
cells significantly (P<0.05) increased Bax,
caspase-8, caspase-9 levels of gene expression, but
IκBα was not significantly different from its counterparts.
Noscapine significantly (P<0.05) decreased Bcl-xL and
NF-κB gene expression in MDA-MB-231 cells as observed in
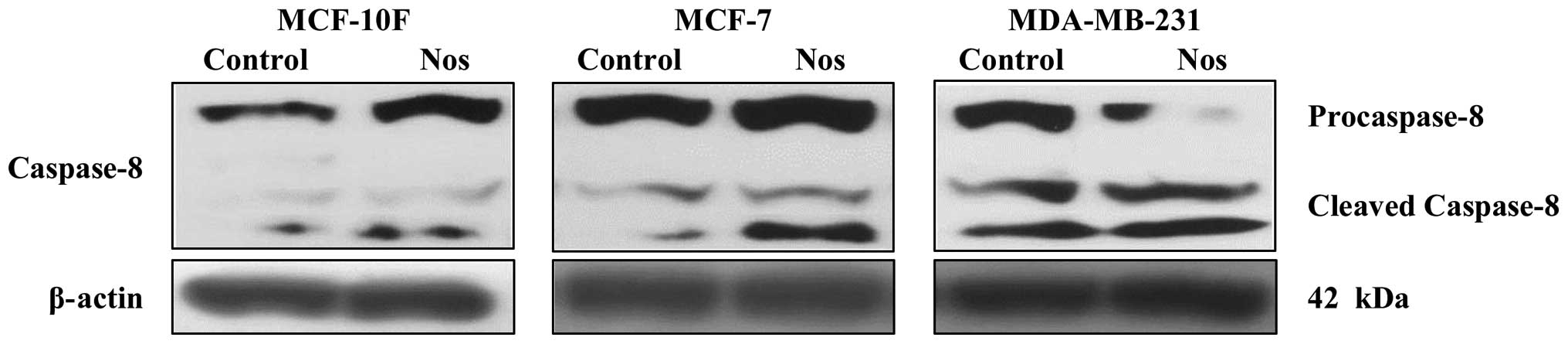
Fig. 7A and quantified in Fig. 7B. As shown in Fig. 8 noscapine-treated cell lines
exhibited increased cleavage of caspase-8 into cleaved caspase-8 in
MCF-7 and MDA-MB-231 compared to their respective controls. MCF-10F
cell line did not show cleavage.
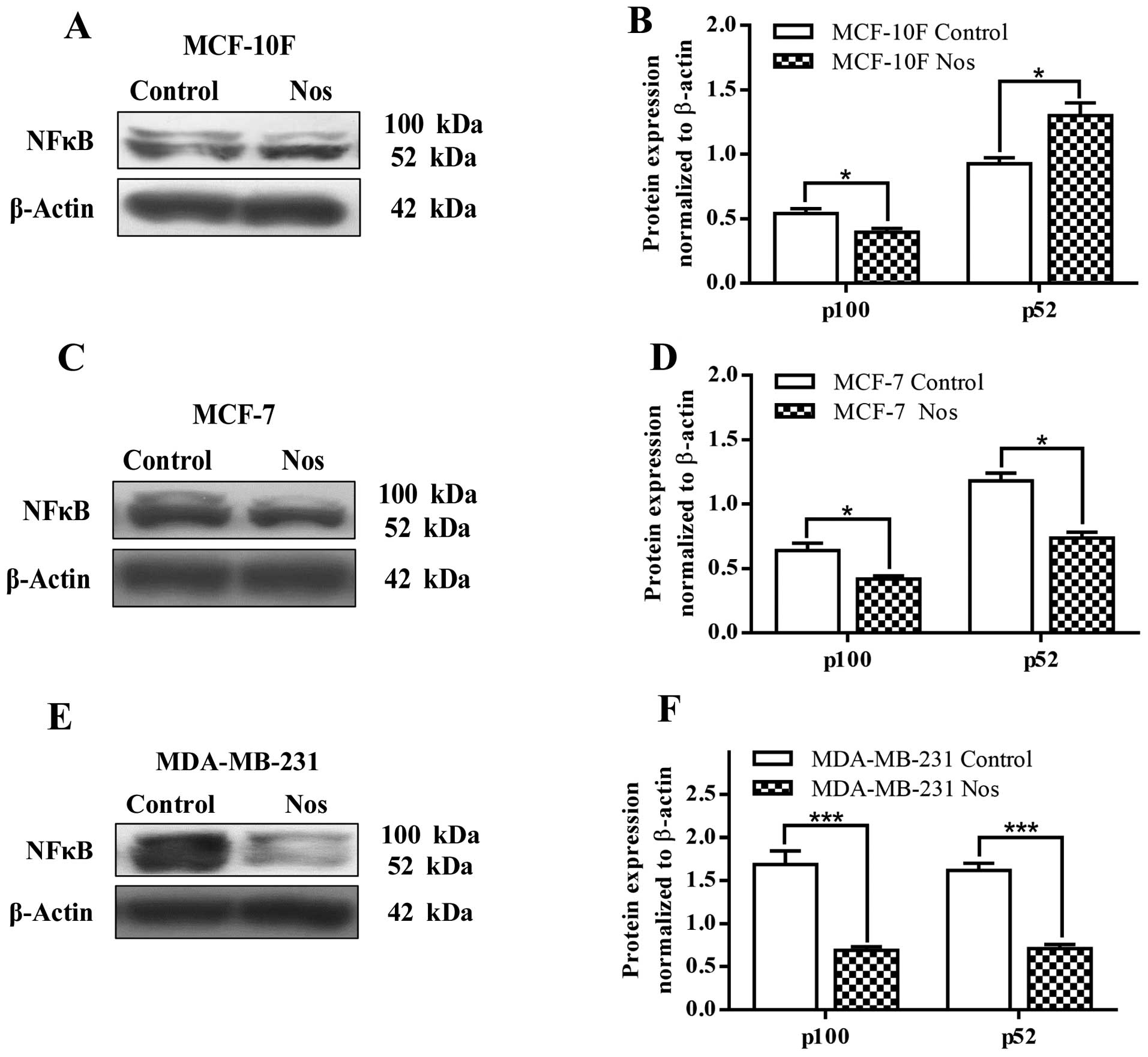
Fig. 9 shows NF-κB
(p52) protein expression that significantly (P<0.05) increased
in MCF-10F cells by noscapine and decreased NF-κB (p100) protein
expression when compared to its counterparts. After treatment with
noscapine, MCF-7 and MDA-MB-231 cell lines significantly (P<0.05
and P<0.001, respectively) decreased the levels of both p100 and
p52 (Fig. 9C–F).
Discussion
Our results showed that noscapine had a
dose-dependent cytotoxic effect after 24 and 48 h in the three cell
lines. The IC50 demonstrated that noscapine had specific
cytotoxic effect in MCF-7 and MDA-MB-231 breast cancer cell lines
requiring higher doses in MCF-10F normal cell line. It was also
demonstrated that triple-negative cells MDA-MB-231 was more
sensitive than triple-positive MCF-7 cells in treatment with
noscapine which agrees with the results of other studies (21,22).
Noscapine provides promise as an effective anticancer agent with
lower toxicity on normal cells.
Internucleosomal DNA fragmentation was investigated
in the three cell lines to determine whether apoptosis was the
major mechanism of cell death induced by noscapine.
Internucleosomal DNA fragmentation was observed in the three cell
lines studied. These results showed that cytotoxic effect of
noscapine was mediated by apoptosis, being clearly observed on
breast cancer cells. Previous research have shown that noscapine
induced apoptosis in vitro in various types of cancer such
as ovarian, neuroblastoma, colon, prostate and breast cancer among
others (18,20,23,24).
Noscapine increased Bax protein expression and there
was no significance of Bcl-2 in MCF-10F cell line when compared to
their counterparts. This drug increased Bax protein expression and
decreased Bcl-2 in MCF-7 and MDA-MB-231 breast cancer cell lines in
comparison to their counterparts. Similar results were observed by
other researchers and were related with Bax/Bcl-2 ratio (23,25).
Bax/Bcl-2 ratio was evaluated from protein expression in MCF-10F
cells treated with noscapine and increased ratio from 0.03 to 0.70
of Bax was found in relation to Bcl-2. The MCF-7 and MDA-MB-231
cells increased the ratio from 0.71 to 1.08 and from 0.99 to 3.64,
respectively. The decrease in ratio Bax/Bcl-2 is characteristic of
cancer cells where proapoptotic proteins are diminished compared to
antiapoptotic proteins suggesting that mitochondrial apoptosis
pathway is suppressed as indicated in other studies in different
types of cancer cells by several authors (20,23,25).
Noscapine-treated cells increased the expression of pro-apoptotic
genes and decreased anti-apoptotic gene expression. This finding
was consistent with the expression of proteins, suggesting that
apoptosis induced by noscapine was due to the increase of gene
expression of Bax and the decrease of Bcl-xL gene
expression.
Caspase-9 gene expression increased in MCF-7
and MDA-MB-231 cancer cell lines after treatment with noscapine
when compared to its counterpart. Caspase-8 increased gene
expression in MCF-10F and MDA-MB-231 but not in MCF-7. Based on
these data, it can be assumed that noscapine-induced apoptosis is
probably due to the involvement of caspase-8 and caspase-9,
activating the extrinsic and intrinsic apoptosis pathways as
reported previously (26–28). Noscapine exhibited an increased
cleavage of caspase-8 into cleaved caspase-8 in MCF-7 and
MDA-MB-231 compared to their respective controls. MCF-10F cells did
not show any cleavage. Studies have shown that NF-κB plays an
important role on gene expression that regulates cell death,
leading to the upregulation of antiapoptotic and pro-survival
genes, such as members of the Bcl-2 family and inhibitor apoptosis
proteins. Anti-apoptotic genes and proteins increased in breast
cancer cell lines mediated by NF-κB as well as decreased
proapoptotic gene and protein expression (29–31).
Our results showed that antiapoptotic gene and
protein expression diminished by the effect of noscapine, and
proapoptotic gene and protein expression increased. Both effects
probably are due not only to decreased NF-κB gene and protein
expression, but also to increase of IκBα induced by noscapine,
which suppresses activation of NF-κB. Thus, our results showed that
NF-κB gene and protein expression increased in MCF-7 and
MDA-MB-231 compared to MCF-10F cell line, but noscapine treatment
induced a decrease in MCF-7 and MDA-MB-231 breast cancer cell lines
compared to its counterparts. The translocation of NF-κB to the
nucleus is preceded by degradation of IκBα which is an inhibitor of
NF-κB (15,16,29,32).
The present results showed that noscapine increased IκBα
gene expression in MCF-10F and MCF-7 in comparison to its
counterparts. Furthermore, previous studies have shown high levels
of NF-κB polypeptides (both p100 and p52) in mammary carcinoma cell
lines and primary tumors compared to normal breast cells (17,33,34).
These results with noscapine in breast cancer cell lines are the
first to be published; however, studies have been reported in other
cancers (22,32).
We conclude that noscapine selectively induced
apoptosis in breast cancer cell lines, with little effect in normal
breast cell line and with stronger effect on triple negative breast
cancer cell line, possible through IκBα which inactivates NF-κB,
triggering the expression of genes and proteins and favoring
intrinsic and extrinsic apoptosis pathways.
Acknowledgements
The technical support of Guiliana Rojas, Georgina
Vargas Marchant and Leodán A. Crispin and helpful suggestions given
by Richard Ponce-Cusi are greatly appreciated. The present study
was supported by the Grant support FONDECYT #1120006 (G.M.C) and
MINEDUC-UTA (G.M.C).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tao Z, Shi A, Lu C, Song T, Zhang Z and
Zhao J: Breast cancer: Epidemiology and etiology. Cell Biochem
Biophys. 72:333–338. 2015. View Article : Google Scholar
|
|
4
|
Cadoo KA, Fornier MN and Morris PG:
Biological subtypes of breast cancer: Current concepts and
implications for recurrence patterns. Q J Nucl Med Mol Imaging.
57:312–321. 2013.PubMed/NCBI
|
|
5
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sharifi S, Barar J, Hejazi MS and Samadi
N: Doxorubicin changes Bax/Bcl-xL ratio, caspase-8 and 9 in breast
cancer cells. Adv Pharm Bull. 5:351–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ravi M, Tentu S, Baskar G, Rohan Prasad S,
Raghavan S, Jayaprakash P, Jeyakanthan J, Rayala SK and Venkatraman
G: Molecular mechanism of anti-cancer activity of phycocyanin in
triple-negative breast cancer cells. BMC Cancer. 15:7682015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li MX and Dewson G: Mitochondria and
apoptosis: Emerging concepts. F1000Prime Rep. 7:422015.PubMed/NCBI
|
|
9
|
Bargou RC, Daniel PT, Mapara MY, Bommert
K, Wagener C, Kallinich B, Royer HD and Dörken B: Expression of the
bcl-2 gene family in normal and malignant breast tissue: Low
bax-alpha expression in tumor cells correlates with resistance
towards apoptosis. Int J Cancer. 60:854–859. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vela L and Marzo I: Bcl-2 family of
proteins as drug targets for cancer chemotherapy: The long way of
BH3 mimetics from bench to bedside. Curr Opin Pharmacol. 23:74–81.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Besbes S, Mirshahi M, Pocard M and Billard
C: New dimension in therapeutic targeting of BCL-2 family proteins.
Oncotarget. 6:12862–12871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai L and Wang S: Targeting apoptosis
pathways for new cancer therapeutics. Annu Rev Med. 65:139–155.
2014. View Article : Google Scholar
|
|
13
|
Hata AN, Engelman JA and Faber AC: The
BCL2 family: Key mediators of the apoptotic response to targeted
anticancer therapeutics. Cancer Discov. 5:475–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao Y and Karin M: NF-kappaB in mammary
gland development and breast cancer. J Mammary Gland Biol
Neoplasia. 8:215–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khan S, Lopez-Dee Z, Kumar R and Ling J:
Activation of NF-κB is a novel mechanism of pro-survival activity
of glucocorticoids in breast cancer cells. Cancer Lett. 337:90–95.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Nag SA and Zhang R: Targeting the
NF-κB signaling pathways for breast cancer prevention and therapy.
Curr Med Chem. 22:264–289. 2015. View Article : Google Scholar
|
|
18
|
Ye K, Ke Y, Keshava N, Shanks J, Kapp JA,
Tekmal RR, Petros J and Joshi HC: Opium alkaloid noscapine is an
antitumor agent that arrests metaphase and induces apoptosis in
dividing cells. Proc Natl Acad Sci USA. 95:1601–1606. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahmoudian M and Rahimi-Moghaddam P: The
anti-cancer activity of noscapine: A review. Recent Patents
Anticancer Drug Discov. 4:92–97. 2009. View Article : Google Scholar
|
|
20
|
Li S, He J, Li S, Cao G, Tang S, Tong Q
and Joshi HC: Noscapine induced apoptosis via downregulation of
survivin in human neuroblastoma cells having wild type or null p53.
PLoS One. 7:e400762012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aneja R, Vangapandu SN, Lopus M,
Viswesarappa VG, Dhiman N, Verma A, Chandra R, Panda D and Joshi
HC: Synthesis of micro-tubule-interfering halogenated noscapine
analogs that perturb mitosis in cancer cells followed by cell
death. Biochem Pharmacol. 72:415–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chougule MB, Patel AR, Jackson T and Singh
M: Antitumor activity of Noscapine in combination with Doxorubicin
in triple negative breast cancer. PLoS One. 6:e177332011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang ZR, Liu M, Peng XL, Lei XF, Zhang JX
and Dong WG: Noscapine induces mitochondria-mediated apoptosis in
human colon cancer cells in vivo and in vitro. Biochem Biophys Res
Commun. 421:627–633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Afzali M, Ghaeli P, Khanavi M, Parsa M,
Montazeri H, Ghahremani MH and Ostad SN: Non-addictive opium
alkaloids selectively induce apoptosis in cancer cells compared to
normal cells. Daru. 23:162015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu M, Luo XJ, Liao F, Lei XF and Dong WG:
Noscapine induces mitochondria-mediated apoptosis in gastric cancer
cells in vitro and in vivo. Cancer Chemother Pharmacol. 67:605–612.
2011. View Article : Google Scholar
|
|
26
|
Chougule M, Patel AR, Sachdeva P, Jackson
T and Singh M: Anticancer activity of Noscapine, an opioid alkaloid
in combination with cisplatin in human non-small cell lung cancer.
Lung Cancer. 71:271–282. 2011. View Article : Google Scholar :
|
|
27
|
Heidari N, Goliaei B, Moghaddam PR,
Rahbar-Roshandel N and Mahmoudian M: Apoptotic pathway induced by
noscapine in human myelogenous leukemic cells. Anticancer Drugs.
18:1139–1147. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gonçalves A, Braguer D, Carles G, André N,
Prevôt C and Briand C: Caspase-8 activation independent of
CD95/CD95-L interaction during paclitaxel-induced apoptosis in
human colon cancer cells (HT29-D4). Biochem Pharmacol.
60:1579–1584. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barham W, Chen L, Tikhomirov O, Onishko H,
Gleaves L, Stricker TP, Blackwell TS and Yull FE: Aberrant
activation of NF-κB signaling in mammary epithelium leads to
abnormal growth and ductal carcinoma in situ. BMC Cancer.
15:6472015. View Article : Google Scholar
|
|
30
|
Li F, Zhang J, Arfuso F, Chinnathambi A,
Zayed ME, Alharbi SA, Kumar AP, Ahn KS and Sethi G: NF-κB in cancer
therapy. Arch Toxicol. 89:711–731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sethi G, Ahn KS and Aggarwal BB: Targeting
nuclear factor-kappa B activation pathway by thymoquinone: Role in
suppression of antiapoptotic gene products and enhancement of
apoptosis. Mol Cancer Res. 6:1059–1070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sung B, Ahn KS and Aggarwal BB: Noscapine,
a benzyliso-quinoline alkaloid, sensitizes leukemic cells to
chemotherapeutic agents and cytokines by modulating the NF-kappaB
signaling pathway. Cancer Res. 70:3259–3268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cogswell PC, Guttridge DC, Funkhouser WK
and Baldwin AS Jr: Selective activation of NF-kappa B subunits in
human breast cancer: Potential roles for NF-kappa B2/p52 and for
Bcl-3. Oncogene. 19:1123–1131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wertz IE: TNFR1-activated NF-κB signal
transduction: Regulation by the ubiquitin/proteasome system. Curr
Opin Chem Biol. 23:71–77. 2014. View Article : Google Scholar : PubMed/NCBI
|