Introduction
Multiple myeloma (MM) is a B cell malignancy
characterized by clonal proliferation of plasma cells in the bone
marrow (1). MM is the most common
hematological malignancy, second only to non-Hodgkin's lymphoma and
accounts for 10% of all hematological malignancies and 1% of all
cancers (2). MM treatment
comprises vincristine/adriamycin/dexamethasone or
melphalan/prednisolone chemotherapy and novel agents such as
thalidomide, lenalidomide, pomalidomide, and bortezomib (3,4).
However, MM remains an incurable disease with a 5-year survival
rate of ~35% (5). In addition,
most of the drugs used for MM treatment have side effects that
limit their utility. Thus, there remains an unmet need for novel
therapies for MM treatment.
The nuclear factor κB (NF-κB) pathway plays a
crucial role in the survival, growth, and drug resistance of
different types of cancers, including MM (6,7).
Constitutive NF-κB activity is present in human MM cell lines and
cells of the MM patients (8). The
NF-κB family includes RelA (p65), RelB, c-Rel, p50 (NF-κB1), and
p52 (NF-κB2) proteins (9). NF-κB
is typically a heterodimer composed of p50 and p65 subunits and is
constitutively present in the cytosol and the nucleus. In the
cytosol, NF-κB is inactivated by its association with inhibitor of
NF-κB (IκB) (10). Upon
stimulation, IκB is phosphorylated by IκB kinases marking it for
proteasomal degradation and thereby allowing nuclear translocation
of NF-κB (5,11). Then, NF-κB binds to specific DNA
sequences and promotes the transcription of its target genes
(12). The NF-κB pathway regulates
the gene expression of cell cycle regulators (c-Myc, cyclin D,
cyclin E, p21, and p27) and anti-apoptotic molecules [B cell
leukemia 2 (Bcl-2), B cell leukemia-xL (Bcl-xL), x-linked inhibitor
of apoptosis (XIAP), and c-IAP] (13,14).
Recent studies have reported that NF-κB inhibitors induced
apoptosis in hematopoietic tumor cells through downregulation of
anti-apoptotic proteins (15).
Therefore, the inhibition of NF-κB signaling is a potential target
for the treatment of MM.
Recently, plant and plant-derived drugs have been
recognized as one of the most attractive approaches for cancer
therapy (16). In addition, many
drugs derived from plants have been shown to be useful and
effective in sensitizing tumors to conventional agents, prolonging
survival time, and preventing the side effects of chemotherapy
(17,18). Mangiferin,
1,3,6,7-tetrahydroxyxanthone-C2-β-D-glucoside, is a compound
extracted from plants belonging to the Anacardiaceae and
Gentianaceae families, including Mangifera indica L.
(19). Mangiferin has been
reported to have various bioactivities, such as anti-oxidant,
antitumor, antidiabetic, anti-inflammatory, and immunomodulatory
activities (19). Previous studies
have revealed that mangiferin has anticancer effects in acute
myeloid leukemia (AML) cell lines (20). In addition, we showed that
mangiferin induced apoptosis by inhibiting the nuclear
translocation of NF-κB. However, the treatment of MM involves a
combination of two or three drugs, including adriamycin,
vincristine, and melphalan. In this study, we examined the effect
of the combination of mangiferin and conventional anticancer drugs
in MM cell lines.
Materials and methods
Materials
Mangiferin
(C19H18O11) and melphalan were
purchased from Sigma (St. Paul, MN, USA), and dissolved in dimethyl
sulfoxide. These reagents were dissolved in phosphate-buffered
saline (PBS) and filtered through 0.45-μm syringe filters (Iwaki
Glass, Tokyo, Japan) before use in the experiments described
below.
Adriamycin and vincristine were purchased from
Sigma. These reagents were dissolved in PBS and used for the
various assays described below.
Cell culture
IM9 cells and RPMI8226 were obtained from Health
Science Research Resources Bank (Osaka, Japan). IM9 cells were
cultured in RPMI-1640 medium (Sigma) containing 10% fetal bovine
serum (Gibco, Carlsbad, CA, USA), 100 μg/ml penicillin (Gibco), 100
U/ml streptomycin (Gibco), and 25 mM
4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (Wako, Osaka,
Japan). All cell lines were maintained at 37°C in an atmosphere
containing 5% CO2.
Trypan blue exclusion assay
The cells were plated in 96-well plates at
2×104 cells/ml and treated with mangiferin, anticancer
drugs, a combination of both, or without mangiferin (control).
After incubation, the cells were stained with trypan blue and the
number of stained cells was counted at days one, three, and
five.
Western blotting
Preparation of nuclear extracts for
NF-κB
The cells treated with mangiferin, anticancer drugs,
a combination of both, or without mangiferin (control) were washed
with cold PBS and lysed using a lysis buffer containing 100 mM
Tris-HCl (pH 7.4), 1 mM EDTA, 0.5% NP-40, 1 μM pepstatin, 1 μM
leupeptin, 2 mM sodium orthovanadate, 1 μM calpain inhibitor,
phosphatase inhibitor cocktail I/II, and 1 mM phenylmethylsulfonyl
fluoride (PMSF). The lysates were centrifuged at 14,000 rpm for 5
min, and the supernatant, which contained the cytoplasmic extracts,
was stored at −80°C. The nuclear pellet was resuspended in cold
nuclear extraction buffer for 30 min. The extract was centrifuged
at 14,000 rpm for 5 min, and the supernatant containing the nuclear
extract was obtained. The proteins were measured using the BCA
protein assay kit (Pierce, Rockford, IL, USA). Total cellular
proteins (30 μg of protein) from the cytoplasmic or the nuclear
extract were separated using 10% sodium dodecyl sulfate (SDS)
polyacrylamide gels. The proteins were transferred to polyvinyl
difluoride (PVDF) membranes (Amersham, Arlington Heights, IL, USA).
The membranes were blocked with 5% skim milk and incubated
overnight at 4°C with rabbit anti-human NF-κB p65 (Cell Signaling
Technology, Beverly, MA, USA) and rabbit anti-human lamin A/C
antibodies (Santa Cruz Biotechnologies, Santa Cruz, CA, USA). After
binding to an appropriate horseradish peroxidase-conjugated
secondary antibody, the proteins were visualized using Luminata
Forte Western HRP Substrate (Millipore, MA, USA) according to the
manufacturer's instructions.
Preparation of whole cell lysates
The cells treated with mangiferin, anticancer drugs,
a combination of both, or without mangiferin (control) were washed
with cold PBS and lysed with a lysis buffer containing 100 mM
Tris-HCl (pH 7.4), 1 mM EDTA, 0.5% NP-40, 1 μM pepstatin, 1 μM
leupeptin, 2 mM sodium orthovanadate, 1 μM calpain inhibitor,
phosphatase inhibitor cocktail I/II, and 1 mM PMSF. The proteins
were measured using the BCA protein assay kit (Pierce). The
extracts (30 μg of protein) were separated using 10% SDS
poly-acrylamide gels. The proteins were then transferred to PVDF
membranes (Amersham). The membranes were blocked with 5% skim milk
and incubated overnight at 4°C with each of the following
antibodies: rabbit anti-human phospho-p44/42 mitogen-activated
protein kinase (MAPK, ERK1/2), rabbit anti-human p44/42 MAPK
(ERK1/2), mouse anti-human phospho-IκB, rabbit anti-human IκB,
rabbit anti-human phospho-stress-activated protein kinase/c-jun
N-terminal kinase (JNK1/2), rabbit anti-human JNK1/2, rabbit
anti-human cyclin D, rabbit anti-human cyclin E, rabbit anti-human
XIAP, rabbit anti-human survivin (Cell Signaling Technology), mouse
anti-human β-actin (Sigma), rabbit anti-human p53, rabbit
anti-human p21, rabbit anti-human p27, rabbit anti-human PUMA,
rabbit anti-human NOXA, rabbit anti-human Bcl-xL, rabbit anti-human
Bax, rabbit anti-human Bim, and rabbit anti-human Bcl-2 (Santa Cruz
Biotechnologies). After binding of an appropriate horseradish
peroxidase-conjugated secondary antibody, the proteins were
visualized using Luminata Forte Western HRP Substrate (Millipore)
according to the manufacturer's instructions.
Flow cytometry
The cells treated with mangiferin, anticancer drugs,
a combination of both, or without mangiferin (control) were washed
with cold PBS, and fixed in 70% ethanol. The cells were resuspended
in PBS and 50 μg/ml propidium iodide was added. Then, the samples
were measured on a BD-LSR flow cytometer (BD Biosciences, CA, USA).
Cell cycles were analyzed on Cell Quest software (BD
Biosciences).
Analysis of apoptosis by flow
cytometry
Measurement of cells undergoing apoptosis was
performed with the Muse™ Annexin V and Dead Cell Assay kit (Merck
Millipore, Darmstadt, Germany), according to the manufacturer's
instructions. The cells were treated with mangiferin, anticancer
drugs, a combination of both, or without mangiferin (control) for
48 h. Then, Muse Annexin V and dead cell reagent was added. After
incubation for 20 min at room temperature, apoptotic cells were
applied to a Muse Cell Analyzer (Merck Millipore).
Measurement of the proteolytic activity
of caspase-3
The activity of caspase-3 was determined using the
caspase-3/CPP32 fluorometric assay kit (BioVision Mountain View,
CA, USA) according to the manufacturer's instructions. The cells
were treated with mangiferin, anticancer drugs, a combination of
both, or without mangiferin (control) for 36 h. Then, the cells
were washed in PBS and lysed using the lysis buffer provided in the
kit. The cell lysates were centrifuged at 14,000 rpm for 5 min, and
the reaction buffer containing 1 mM
Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin was added to the
supernatants and incubated at 37°C for 2 h. Subsequently, the
absorbance was measured using a fluorescence spectrophotometer
(Hitachi, Tokyo, Japan) at an emission wavelength of 505 nm and an
excitation wavelength of 400 nm.
Statistical analysis
All results are expressed as means ± standard
deviation of several independent experiments. Multiple comparisons
of the data were performed using analysis of variance with
Dunnett's test. P-values <5% were considered significant.
Results
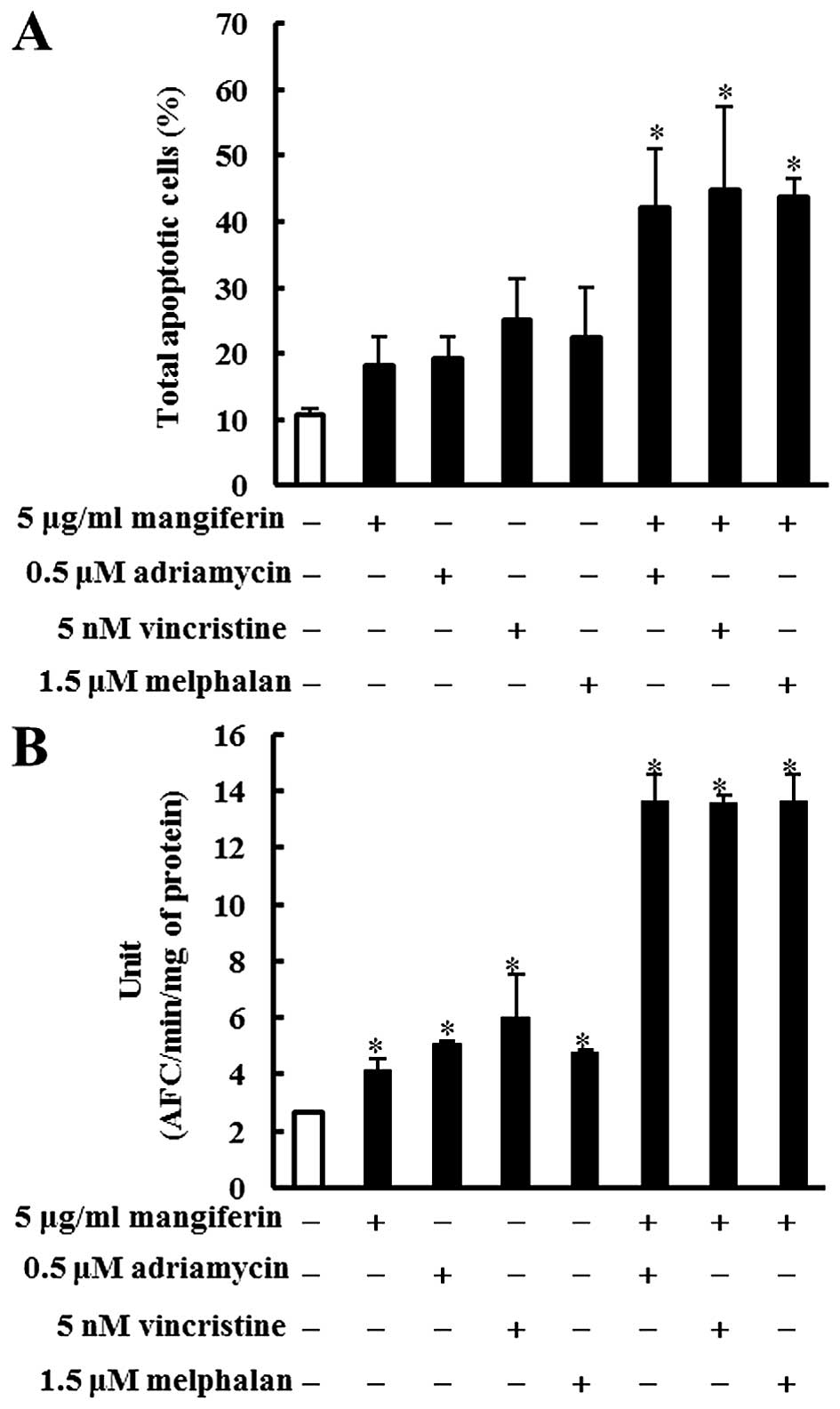
Mangiferin, adriamycin, vincristine, and
melphalan decrease the viability of the MM cell line
The effects of mangiferin and the three anticancer
drugs (adriamycin, vincristine, and melphalan) on the viability of
the MM cell lines (IM9 and RPMI8226) as determined by the trypan
blue exclusion assay are shown in Fig.
1. IM9 cells were treated in the absence (control) or presence
of mangiferin (5–50 μg/ml), adriamycin (0.1–10 μM), vincristine (5
nM-1 μM), or melphalan (1–5 μM). After three days, the viability of
IM9 cells treated with 5, 10, 25, and 50 μM mangiferin was 80.5,
76.3, 63.3 and 45.3%, respectively, whereas that after five days
was 67.2, 24.5, 19.3 and 16.3%, respectively. After three days, the
viability of IM9 cells treated with 5, 10, 25, and 50 μM adriamycin
was 85.5, 78.0, 45.4 and 28.9%, respectively, whereas that after
five days was 83.1, 74.3, 30.2 and 9.7%, respectively. Vincristine
and melphalan showed results similar to those observed with
adriamycin. In addition, RPMI8226 cells also showed results similar
to those observed with IM9 cells (Fig.
1B). These results indicated that mangiferin, adriamycin,
vincristine, and melphalan decreased the viability of MM cell lines
in a concentration-dependent manner.
 | Figure 1Mangiferin, adriamycin, vincristine,
and melphalan decrease the viability of IM9 cells and RPMI8226
cells. (A) IM9 cells were treated with mangiferin (5–50 μg/ml),
adriamycin (0.1–10 μM), vincristine (5 nm-1 μM), and melphalan (1–5
μM). (B) RPMI8226 cells were treated with mangiferin (25–200
μg/ml), adriamycin (0.1–10 μM), vincristine (5 nm-1 μM), and
melphalan (1–5 μM). Then, trypan blue exclusion assay was performed
in IM9 cells and RPMI8266 cells after one, three, and five days.
The results are expressed as the mean ± standard deviation (SD) of
three experiments performed in triplicate. *p<0.05
compared with control. |
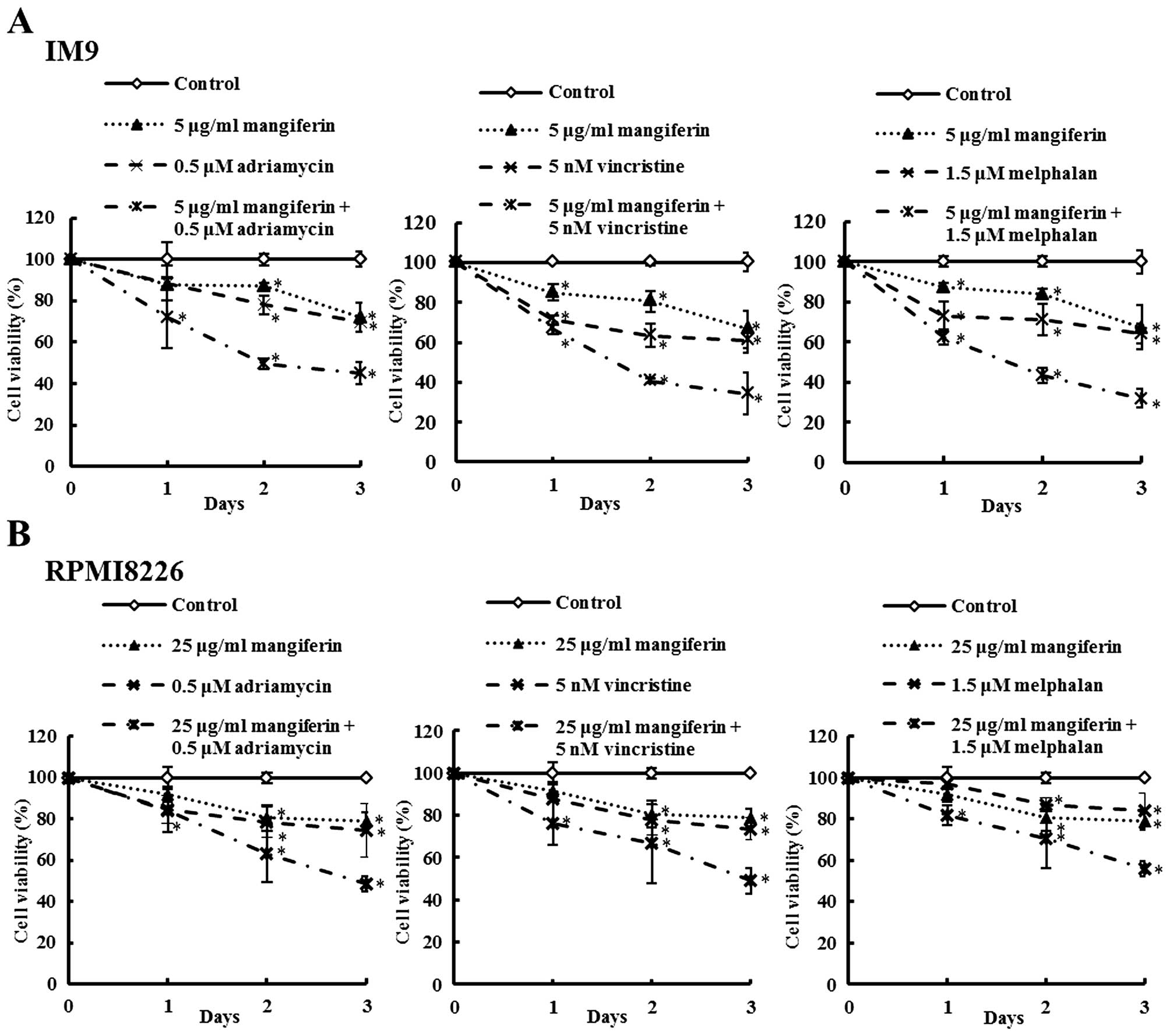
Mangiferin enhances the sensitivity of
human multiple myeloma cells to anticancer drugs
The effects of mangiferin, the three anticancer
drugs, and the combination of each anti-cancer drug and mangiferin
on the viability of the MM cell lines (IM9 and RPMI8226) were
determined by trypan blue exclusion assay and are shown in Fig. 2. IM9 cells were treated with either
mangiferin (5 μg/ml), adriamycin (0.5 μM), vincristine (5 nM),
melphalan (1.5 μM), or combination of mangiferin with an anticancer
drug. After three days, the viability of IM9 cells treated with 5
μg/ml mangiferin, 0.5 μM adriamycin, or combination of both was
72.0, 69.8 and 45.0%, respectively. The combination of mangiferin
and vincristine or melphalan showed results similar to those
observed with adriamycin. In addition, RPMI8226 cells also showed
results similar to those observed with IM9 cells (Fig. 2B). These results show that the
combination of mangiferin and an anticancer drug significantly
reduced the viability of the MM cell line in comparison to the use
of each of these drugs separately.
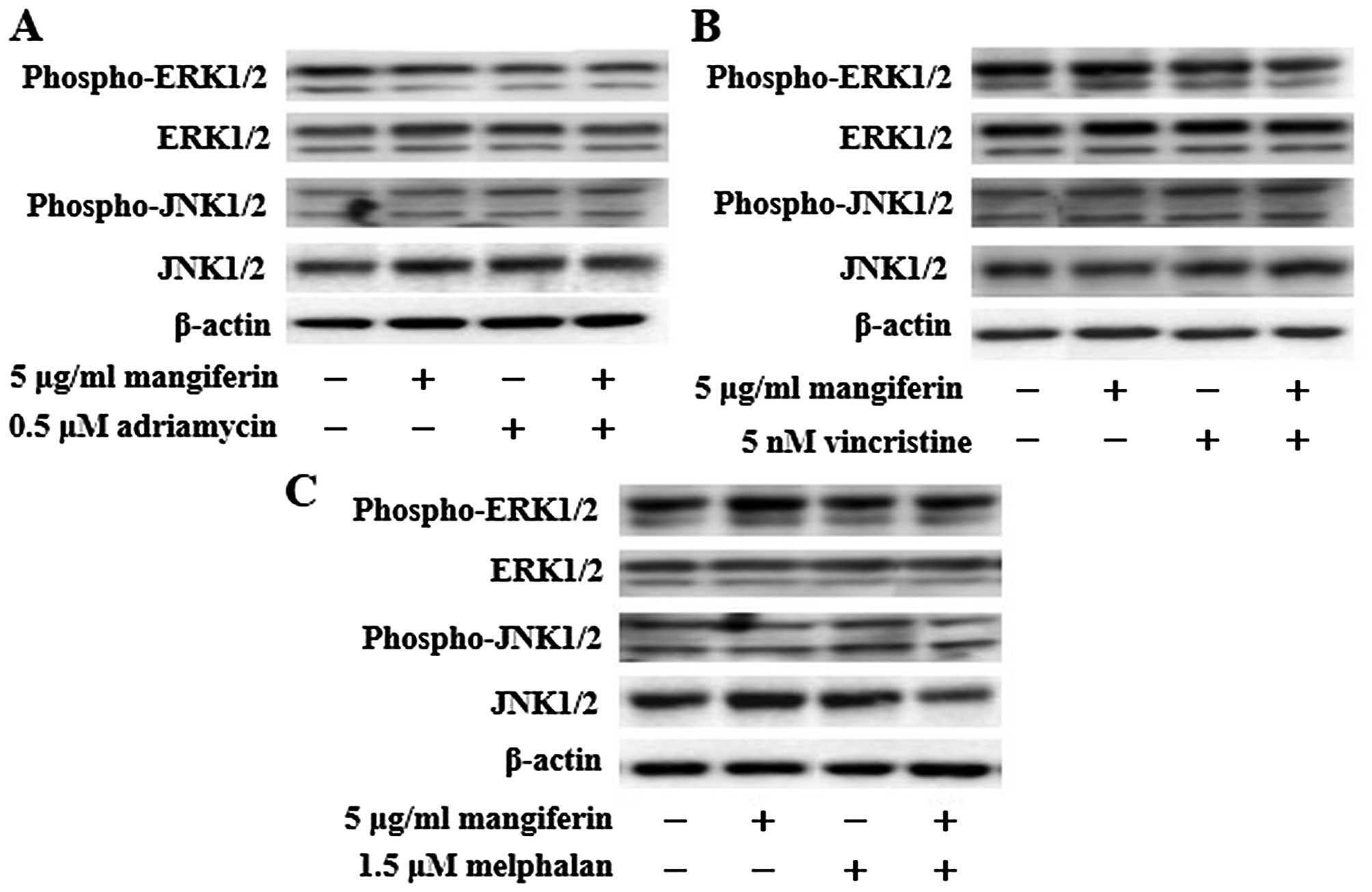
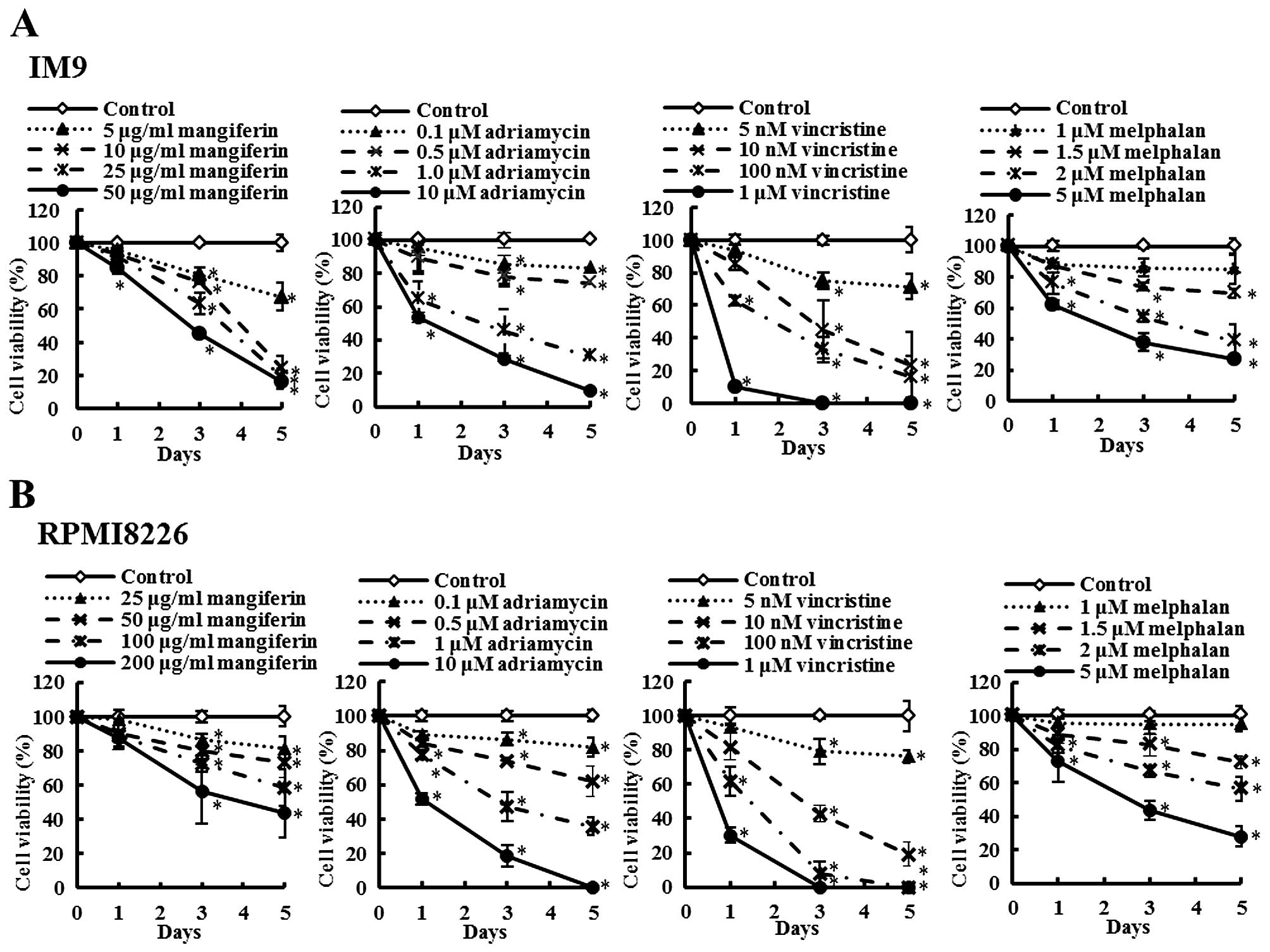
The combination of mangiferin and an
anticancer drug suppresses the nuclear translocation of NF-κB
In a previous study, we showed that mangiferin
inhibits the nuclear translocation of NF-κB in AML cell lines
(20). However, mangiferin did not
affect the levels of ERK1/2, Akt, and p38MAPK phosphorylation. To
clarify the molecular mechanisms underlying the effects of
combination of mangiferin and other anticancer drugs, we
investigated the nuclear translocation of NF-κB and expression of
phosphorylated IκB and IκB proteins by using western blotting. Our
results showed that the combination of mangiferin and each of the
other anticancer drugs significantly suppressed the nuclear
translocation of NF-κB (Fig. 3).
Further, we observed no changes in the levels of ERK1/2 and JNK1/2
phosphorylation (Fig. 4). These
results indicated that the decrease in the combination of
mangiferin and an anti-cancer drug induced cell viability was
attributed to inhibition of the NF-κB pathway.
 | Figure 3The combination of mangiferin and an
anticancer drug suppresses the nuclear translocation of NF-κB. (A)
IM9 cells were treated with mangiferin (5 μg/ml) or adriamycin (0.5
μM), vincristine (5 nM), and melphalan (1.5 μM), or a mixture of
mangiferin with an anticancer drug for three days. (B) RPMI8226
cells were treated with mangiferin (25 μg/ml) or adriamycin (0.5
μM), vincristine (5 nM), and melphalan (1.5 μM), or a mixture of
mangiferin with an anticancer drug for three days. The expression
of NF-κB, and, phospho-IκB was detected using western blotting. The
expression of IκB, Lamin A/C, and β-actin were used as internal
controls. |
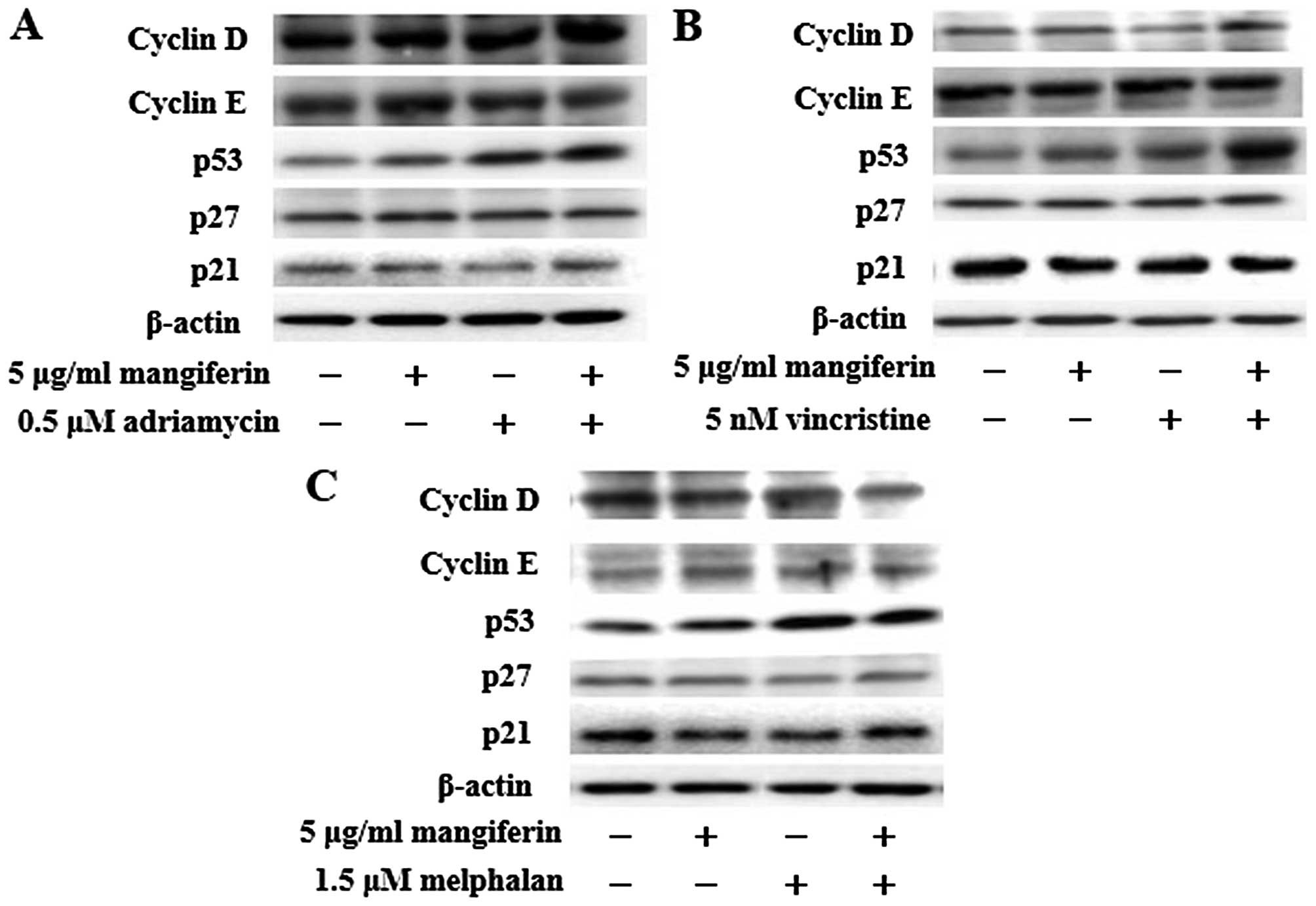
The combination of mangiferin and an
anticancer drug increases the expression of p53 and Noxa and
decreases the expression of XIAP, survivin, and Bcl-xL
proteins
NF-κB is a nuclear factor known to activate the
expression of genes involved in cell proliferation and cell
survival (anti-apoptotic proteins and pro-apoptotic proteins).
Therefore, we examined the expression of proteins involved in cell
proliferation and cell survival by using western blotting. Our
results showed that the combination of mangiferin and an anticancer
drug upregulated the expression of p53 and Noxa and downregulated
that of XIAP, survivin, and Bcl-xL proteins in comparison with
mangiferin alone (Figs. 5 and
6). However, we observed no
changes in the expression of cyclin D, cyclin E, p27, p21, Bcl-2,
Bax, Bim, and PUMA proteins.
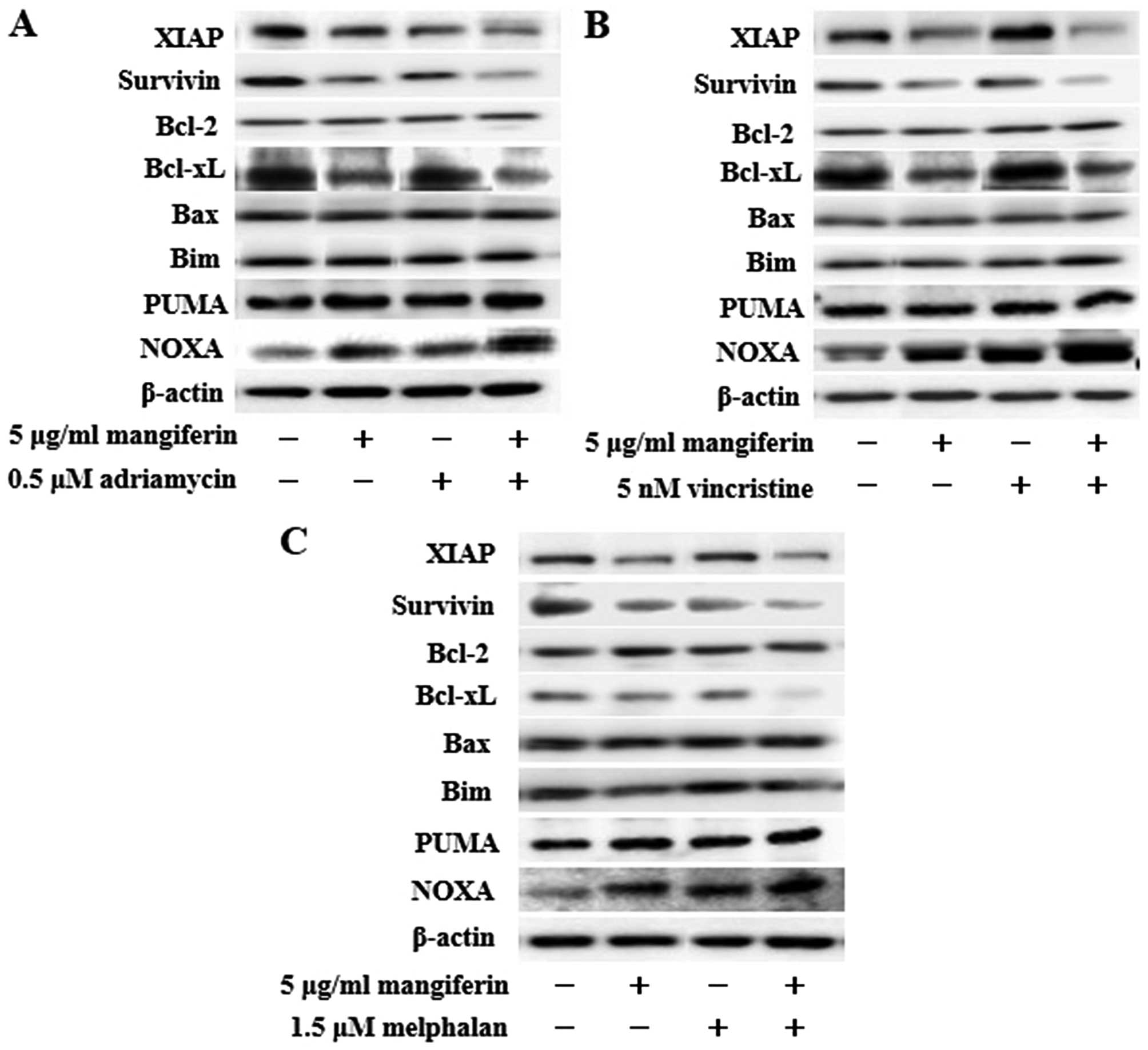 | Figure 6The combination of mangiferin and an
anticancer drug increase the expression of Noxa, and decrease the
expression of XIAP, survivin, and Bcl-xL proteins. IM9 cells were
treated with mangiferin (5 μg/ml) or (A) adriamycin (0.5 μM), (B)
vincristine (5 nM), and (C) melphalan (1.5 μM), or a mixture of
mangiferin with an anticancer drug for three days. The expression
of XIAP, survivin, Bcl-2, Bcl-xL, Bax, Bim, PUMA, and Noxa were
detected using western blotting. The expression of β-actin was used
as internal control. |
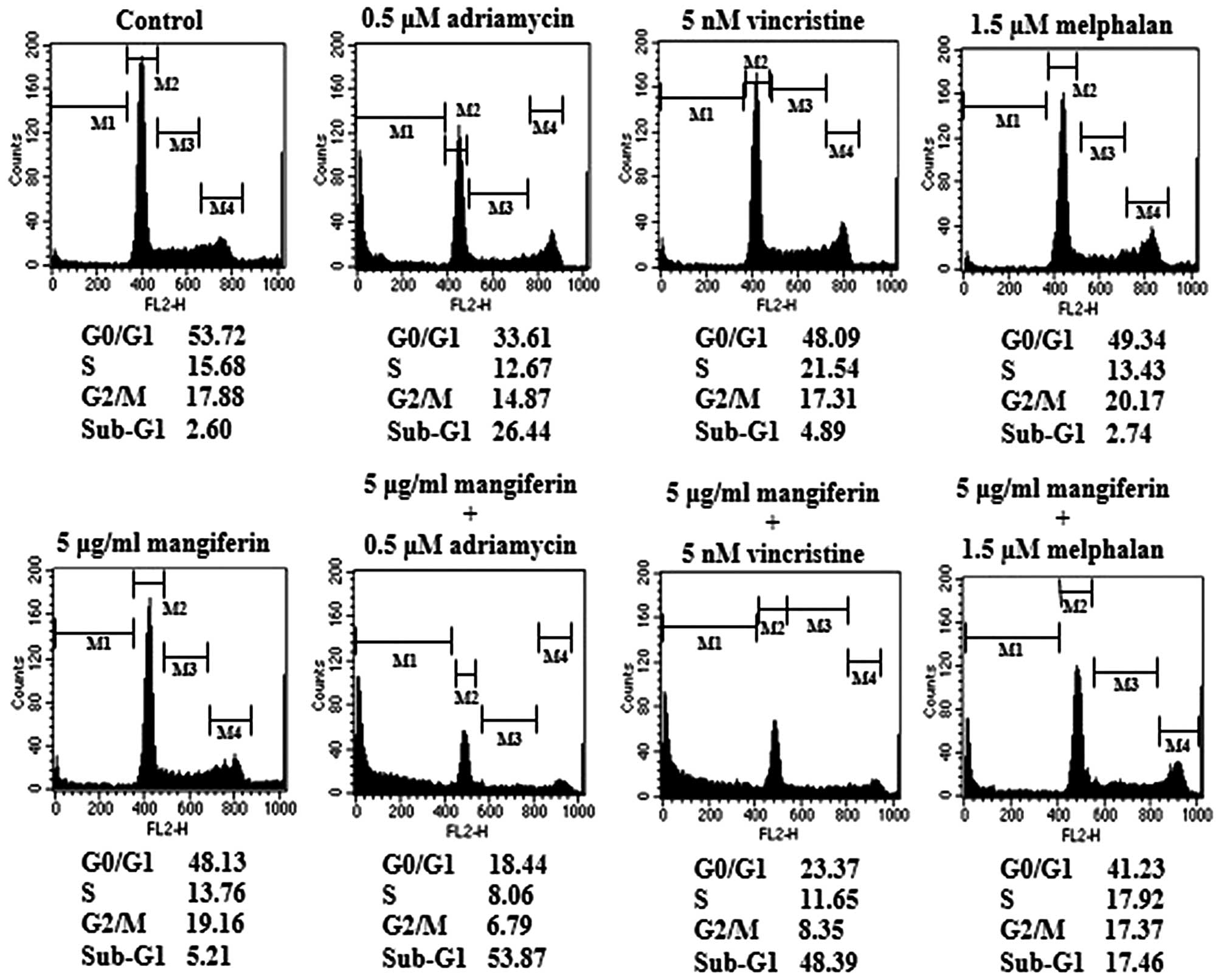
The combination of mangiferin and an
anticancer drug causes the accumulation of cells in the sub-G1
phase of the cell cycle
p53 plays important roles in various phases of the
cell cycle. Thus, we examined cell cycle regulation in IM9 cells
treated with a combination of mangiferin and each of the other
anticancer drugs by flow cytometry. Our results showed that the
combined treatment increased the accumulation of cell population in
the sub-G1 phase (Fig. 7). These
results are indicative of apoptosis.
The combination of mangiferin and an
anticancer drug induces apoptosis by activating caspase-3
We measured apoptotic cells using the Muse™ Annexin
V and Dead Cell Assay kit. IM9 cells were treated with a
combination of mangiferin and each of the other anticancer drugs
for two days. Our results showed that mangiferin increased the
number of apoptotic cells in a concentration-dependent manner
(Fig. 8A). Apoptosis is induced by
an interaction between various initiator and effector caspases.
Caspase-3 is a crucial effector of the apoptosis pathway. We
investigated caspase-3 activation in IM9 cells treated with a
combination of mangiferin and each of the other anticancer drugs by
using the caspase-3/CPP32 fluorometric assay kit. The combination
of mangiferin and an anticancer drug activated caspase-3 (Fig. 8B). These results showed that the
combined treatment induced apoptosis by activating caspase-3.
Discussion
Despite the development of increasingly effective
therapies, MM remains an incurable disease with an average survival
of 3–5 years following diagnosis. In addition, most of the
compounds used for MM treatment have side effects that limit their
utility. Presumably, the side effects of these compounds could be
decreased by reducing their dose and using them in combination with
another drug (21). The nuclear
factor κB (NF-κB) pathway plays a crucial role in the pathogenesis
of MM (22,23). Thus, inhibition of the NF-κB
pathway is a potential target for the treatment of MM. We have
previously shown that mangiferin induced apoptosis in AML cell
lines via inhibition of the NF-κB pathway (20). Additionally, it was reported that
mangiferin in combination with oxaliplatin counteract the
development of resistance to oxaliplatin in colon cancer cells by
reducing active NF-κB (24).
However, the effect of the combination of mangiferin and
conventional anticancer drugs in MM cell lines remain to be
clarified. In particular, the molecular mechanism has not been
elucidated thus far. In this study, we examined the effect of the
combination of mangiferin and conventional anticancer drugs in MM
cell lines.
We showed that mangiferin, adriamycin, vincristine,
and melphalan decrease the viability of MM cell lines. The
combination of mangiferin and each of the above-mentioned
anticancer drugs significantly reduced the viability of the MM cell
line in comparison with each of these drugs used alone.
Furthermore, our results showed that the combination treatment
significantly suppressed the nuclear translocation of NF-κB.
However, we observed no changes in the levels of ERK1/2 and JNK1/2
phosphorylation. In agreement with previous reports, constitutive
activation of NF-κB promoted multiple myeloma cell growth and
survival, and the NF-κB inhibitor dimethyl fumarate induced
apoptosis in MM cell lines (15,25).
In addition, other studies showed that celastrol induces
chemosensitization through downregulation of NF-κB in MM cell lines
(17). These results indicate that
the combination of mangiferin and other anticancer drugs exert
their effects on MM through downregulation of NF-κB pathway.
NF-κB initiates the transcriptional activation of
prosurvival genes and proliferation-promoting genes (26). We observed that the combination of
mangiferin and each of the other anticancer drugs significantly
increased the expression of p53 and Noxa and decreased the
expression of XIAP, survivin, and Bcl-xL, proteins. In addition,
the combination treatment caused the induction of apoptosis,
activation of caspase-3 and the accumulation of the cells in the
sub-G1 phase of the cell cycle. The tumor suppressor p53 induces
apoptosis by transactivation of its downstream apoptotic regulators
such as Noxa (27). Survivin, a
member of the inhibitor of apoptosis protein family, protects cells
from caspase-dependent apoptotic pathways. Survivin overexpression
has been reported in various hematopoietic and solid cancers
(28–30). XIAP is the most potent endogenous
direct inhibitor of caspases and is thus considered a key
physiological regulator of cell death. MM cells express high levels
of XIAP regulated by the NF-κB pathway (31). The Bcl-2 family member Bcl-xL is an
anti-apoptotic protein; Bcl-xL overexpression has been reported in
MM cell lines (32,33). These results suggest that the
combination of mangiferin and an anticancer drug induces apoptosis
by increasing the expression of p53 and Noxa and decreasing that of
XIAP, survivin, and Bcl-xL proteins via inhibition of the NF-κB
pathway.
In conclusion, our results showed that the
combination of mangiferin and an anticancer drug decreased the
viability of MM cell lines in comparison with each of these drugs
used separately. The decrease in the combination of mangiferin and
an anticancer drug induced cell viability was attributed to the
induction of apoptosis, activation of caspase-3, and the
accumulation of the cells in the sub-G1 phase of the cell cycle via
inhibition of nuclear translocation of NF-κB. Importantly, 40% of
multiple myeloma patients show constitutive activation of the NF-κB
pathway. Our findings showed that the combination of mangiferin and
an anticancer drug selectively inhibited the NF-κB pathway without
inhibiting other signaling factors. In addition, we found that
mangiferin enhanced the effect of conventional anticancer drugs
(adriamycin, vincristine, and melphalan) commonly used in multiple
myeloma treatment. Our results provided evidence of the potential
of the combination of mangiferin and an anticancer drug as a new
regime for the treatment of MM.
Acknowledgements
This study was supported in part by a Grant-in-Aid
for Scientific Research (C) from the Japan Society for the
Promotion of Science (JSPS) and by Ministry of Education, Culture,
Sports, Science, and Technology (MEXT)-Supported Program for the
Strategic Research Foundation at Private Universities,
2014–2018.
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
Bax
|
B cell leukemia-2 associated X
|
|
Bcl-2
|
B cell leukemia-2
|
|
Bcl-xL
|
B cell leukemia-xL
|
|
ERK1/2
|
extracellular signal-regulated kinase
1/2
|
|
IAP
|
inhibitors of apoptosis
|
|
IκB
|
inhibitor of κB
|
|
JNK1/2
|
c-Jun N-terminal protein kinase
1/2
|
|
MM
|
multiple myeloma
|
|
NF-κB
|
nuclear factor κB
|
|
PUMA
|
p53 upregulated modulator of
apoptosis
|
|
XIAP
|
X-linked inhibitor of apoptosis
protein
|
References
|
1
|
Raab MS, Podar K, Breitkreutz I,
Richardson PG and Anderson KC: Multiple myeloma. Lancet.
374:324–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bladé J, Cibeira MT and Rosiñol L: Novel
drugs for the treatment of multiple myeloma. Haematologica.
95:702–704. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajkumar SV: Treatment of multiple
myeloma. Nat Rev Clin Oncol. 8:479–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Zhang M, Wang M, He P, Liu X, Chen
L, Xi J, Wang M, Li J, Liu H, et al: Arsenic trioxide combined with
VCMP or VAD chemotherapy in patients with refractory or relapsed
multiple myeloma in a single institution of China. Indian J Hematol
Blood Transfus. 30:259–264. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Paramore A and Frantz S: Bortezomib. Nat
Rev Drug Discov. 2:611–612. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sethi G, Sung B and Aggarwal BB: Nuclear
factor-kappaB activation: From bench to bedside. Exp Biol Med
(Maywood). 233:21–31. 2008. View Article : Google Scholar
|
|
7
|
Tsubaki M, Takeda T, Ogawa N, Sakamoto K,
Shimaoka H, Fujita A, Itoh T, Imano M, Ishizaka T, Satou T, et al:
Over-expression of survivin via activation of ERK1/2, Akt, and
NF-κB plays a central role in vincristine resistance in multiple
myeloma cells. Leuk Res. 39:445–452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cormier F, Monjanel H, Fabre C, Billot K,
Sapharikas E, Chereau F, Bordereaux D, Molina TJ, Avet-Loiseau H
and Baud V: Frequent engagement of RelB activation is critical for
cell survival in multiple myeloma. PLoS One. 8:e591272013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karin M and Greten FR: NF-kappaB: Linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma C, Zuo W, Wang X, Wei L, Guo Q and Song
X: Lapatinib inhibits the activation of NF-κB through reducing
phosphorylation of IκB-α in breast cancer cells. Oncol Rep.
29:812–818. 2013.
|
|
11
|
Hideshima T, Ikeda H, Chauhan D, Okawa Y,
Raje N, Podar K, Mitsiades C, Munshi NC, Richardson PG, Carrasco
RD, et al: Bortezomib induces canonical nuclear factor-kappaB
activation in multiple myeloma cells. Blood. 114:1046–1052. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Panwalkar A, Verstovsek S and Giles F:
Nuclear factor-kappaB modulation as a therapeutic approach in
hematologic malignancies. Cancer. 100:1578–1589. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joyce D, Albanese C, Steer J, Fu M,
Bouzahzah B and Pestell RG: NF-kappaB and cell-cycle regulation:
The cyclin connection. Cytokine Growth Factor Rev. 12:73–90. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsubaki M, Ogawa N, Takeda T, Sakamoto K,
Shimaoka H, Fujita A, Itoh T, Imano M, Satou T and Nishida S:
Dimethyl fumarate induces apoptosis of hematopoietic tumor cells
via inhibition of NF-κB nuclear translocation and down-regulation
of Bcl-xL and XIAP. Biomed Pharmacother. 68:999–1005. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olaku O and White JD: Herbal therapy use
by cancer patients: A literature review on case reports. Eur J
Cancer. 47:508–514. 2011. View Article : Google Scholar :
|
|
17
|
Jeong SJ, Koh W, Kim B and Kim SH: Are
there new therapeutic options for treating lung cancer based on
herbal medicines and their metabolites? J Ethnopharmacol.
138:652–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang LH, Li Y, Yang SN, Wang FY, Hou Y,
Cui W, Chen K, Cao Q, Wang S, Zhang TY, et al: Gambogic acid
synergistically potentiates cisplatin-induced apoptosis in
non-small-cell lung cancer through suppressing NF-κB and MAPK/HO-1
signalling. Br J Cancer. 110:341–352. 2014. View Article : Google Scholar :
|
|
19
|
Luo F, Lv Q, Zhao Y, Hu G, Huang G, Zhang
J, Sun C, Li X and Chen K: Quantification and purification of
mangiferin from Chinese Mango (Mangifera indica L.) cultivars and
its protective effect on human umbilical vein endothelial cells
under H(2)O(2)-induced stress. Int J Mol Sci. 13:11260–11274. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shoji K, Tsubaki M, Yamazoe Y, Satou T,
Itoh T, Kidera Y, Tanimori Y, Yanae M, Matsuda H, Taga A, et al:
Mangiferin induces apoptosis by suppressing Bcl-xL and XIAP
expressions and nuclear entry of NF-κB in HL-60 cells. Arch Pharm
Res. 34:469–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Slawinska-Brych A, Zdzisinska B,
Mizerska-Dudka M and Kandefer-Szerszen M: Induction of apoptosis in
multiple myeloma cells by a statin-thalidomide combination can be
enhanced by p38 MAPK inhibition. Leuk Res. 37:586–594. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kannaiyan R, Hay HS, Rajendran P, Li F,
Shanmugam MK, Vali S, Abbasi T, Kapoor S, Sharma A, Kumar AP, et
al: Celastrol inhibits proliferation and induces chemosensitization
through down-regulation of NF-κB and STAT3 regulated gene products
in multiple myeloma cells. Br J Pharmacol. 164:1506–1521. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsubaki M, Komai M, Itoh T, Imano M,
Sakamoto K, Shimaoka H, Ogawa N, Mashimo K, Fujiwara D, Takeda T,
et al: Inhibition of the tumour necrosis factor-alpha autocrine
loop enhances the sensitivity of multiple myeloma cells to
anticancer drugs. Eur J Cancer. 49:3708–3717. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
du Plessis-Stoman D, du Preez J and van de
Venter M: Combination treatment with oxaliplatin and mangiferin
causes increased apoptosis and downregulation of NFκB in cancer
cell lines. Afr J Tradit Complement Altern Med. 8:177–184.
2011.
|
|
25
|
Trocoli A and Djavaheri-Mergny M: The
complex interplay between autophagy and NF-κB signaling pathways in
cancer cells. Am J Cancer Res. 1:629–649. 2011.
|
|
26
|
DiDonato JA, Mercurio F and Karin M: NF-κB
and the link between inflammation and cancer. Immunol Rev.
246:379–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu HJ, Liu L, Fan L, Zhang LN, Fang C,
Zou ZJ, Li JY and Xu W: The BH3-only protein Puma plays an
essential role in p53-mediated apoptosis of chronic lymphocytic
leukemia cells. Leuk Lymphoma. 54:2712–2719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsubaki M, Satou T, Itoh T, Imano M, Komai
M, Nishinobo M, Yamashita M, Yanae M, Yamazoe Y and Nishida S:
Over-expression of MDR1 and survivin, and decreased Bim expression
mediate multidrug-resistance in multiple myeloma cells. Leuk Res.
36:1315–1322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsubaki M, Komai M, Itoh T, Imano M,
Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K, Fujiwara D,
et al: By inhibiting Src, verapamil and dasatinib overcome
multidrug resistance via increased expression of Bim and decreased
expressions of MDR1 and survivin in human multidrug-resistant
myeloma cells. Leuk Res. 38:121–130. 2014. View Article : Google Scholar
|
|
30
|
Markovic O, Marisavljevic D,
Cemerikic-Martinovic V, Martinovic T, Filipovic B, Stanisavljevic
D, Zivković R, Hajder J, Stanisavljevic N and Mihaljevic B:
Survivin expression in patients with newly diagnosed nodal diffuse
large B cell lymphoma (DLBCL). Med Oncol. 29:3515–3521. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Desplanques G, Giuliani N, Delsignore R,
Rizzoli V, Bataille R and Barillé-Nion S: Impact of XIAP protein
levels on the survival of myeloma cells. Haematologica. 94:87–93.
2009. View Article : Google Scholar :
|
|
32
|
Lin J, Wu Y, Yang D and Zhao Y: Induction
of apoptosis and antitumor effects of a small molecule inhibitor of
Bcl-2 and Bcl-xl, gossypol acetate, in multiple myeloma in vitro
and in vivo. Oncol Rep. 30:731–738. 2013.PubMed/NCBI
|
|
33
|
Xu L, Yang D, Wang S, Tang W, Liu M, Davis
M, Chen J, Rae JM, Lawrence T and Lippman ME: (-)-Gossypol enhances
response to radiation therapy and results in tumor regression of
human prostate cancer. Mol Cancer Ther. 4:197–205. 2005.PubMed/NCBI
|