1. Introduction
Head and neck malignant carcinoma is the world's
fifth most common cancer with incidence exceeding half a million
annually (1,2). Oral squamous cell carcinoma (OSCC)
represents 95% of head and neck malignant carcinoma (3). As a low-grade and well-differentiated
verrucous variant of OSCC, oral verrucous carcinoma (OVC) accounts
for 2–12% of all oral carcinomas with a 5-year survival rate of
only approximately 50%, and is receiving increasing attention
(4).
OVC is a malignant tumor characterized by slow
exophytic growth, usually presenting cauliflower-like and pebbly
mamillated warty lesions (5). It
shows a typical ‘pushing border’ (light and electron) microscopic
feature with a local invasive pattern and rare regional and distant
metastases (6). The history of OVC
can be traced back to as early as 1948 when it was first described
by Lauren V. Ackermann (also referred to as ‘Ackermann's tumor’ or
‘verrucous carcinoma of Ackermann’) (7). Its pathology was not studied
independently until mid-1980s (8).
Although ensuing research on diagnosis and treatment of OVC was
largely triggered at the beginning of this century (9), the research progress is still far
from satisfactory. For instance, the differentiation of OVC from
OSCC is important regarding their different molecular mechanisms
and prognoses. However, it is currently difficult to differentiate
them by simply observing clinical and pathological features because
OVC has similar biological behavior to OSCC, including tendency to
local invasion, insidious lymph node metastasis and occurrence of
malignant lesions (10). These
similarities usually cause clinical misdiagnosis and mistreatment
(11). Undoubtedly, it is critical
to seek reliable molecular markers of OVC to resolve such
challenges.
Over the past several decades, there have been
numerous studies concerning precise diagnosis and effective
treatment of OVC (12–14). The authors' research group has been
investigating this type of cancer since 1992 (14). As a timely and detailed review
about OVC is still lacking at present, this paper aims to deliver
an overview of OVC with emphasis on recent research developments.
It covers almost all subfields of OVC, including its etiology,
clinical manifestations and pathology, molecular mechanism,
diagnosis and differential diagnosis, and treatments, followed by a
detailed discussion on main challenges confronted in the field and
promising measures for resolving them. This review is expected to
offer a useful guide for research development and clinical practice
of OVC.
2. Etiology
OVC has complex etiology which depends on a variety
of factors (15,16). There exist strong associations
between OVC and alcohol consumption, smoking, areca nut chewing and
oral microbiota (17–21). These factors may act individually
or synergistically in oral carcinogenesis. OVC also has a
relationship with undesirable prosthesis, earlier injuries and
scars, and chronic inflammation. Moreover, it may occur as a result
of deterioration of premalignant lesions, including oral verrucous
leukoplakia, oral lichen planus, oral submucous fibrosis (OSF),
odontogenic keratocyst (22).
Alcohol and smoking related carcinogens are two main
well-established risk factors for oral cancers including OVC
(18). Excessive alcohol
consumption can increase incidence of OVC because alcohol may act
as a solvent that promotes movement of carcinogens via oral
cellular membranes, as the consumption has the capability to change
intracellular metabolism of the epithelial cells, causing
impairment of cellular function (e.g., reduced mitochondrial
function and enhanced DNA alkylation) in the initial phase of oral
carcinogenesis (23,24). Similar to alcohol consumption,
smoking is another potential factor that may induce OVC (19). In fact, there exist over 300
carcinogens, i.e., aromatic hydrocarbon benz-pyrene and the tobacco
specific nitrosamines (TSNs), in tobacco smoke or its water-soluble
components that will leach into saliva. These carcinogens interfere
with DNA replication by generating DNA adducts, primarily 06 methyl
Guanine, damaging replicating cells of the immune response
(25,26).
Areca nut extracts contain various carcinogens, such
as N-nitroso amines. These carcinogens cause DNA single-strand
breaks and mutations, facilitating tumor formation and growth.
Furthermore, arecoline in areca nut extracts has genetic toxicity
and teratogenicity on a variety of cells, playing an important role
in oral carcinogenesis (20).
Oral microbiota may present a non-ignorable role in
oral carcinogenesis through their impacts on local metabolism of
alcohol and smoking-related carcinogens. It was found that five
bacterial phyla, including Firmicutes, Proteobacteria,
Bacteroidetes, Actinobacteria, and Fusobacteria, are associated
with oral cancer (21). They
activate alcohol and smoking related carcinogens locally. Oral
bacteria can convert ethanol to acetaldehyde, an in vitro
and in vivo genotoxin, exposing the oral and
gastrointestinal tract directly to carcinogenic acetaldehyde after
alcohol use (27). The bacteria
may function in enhanced activation of carcinogenic nitrosamines
from tobacco smoking because in vitro common oral microbes
activate nitrosodiethylamine (NDEA, a tobacco smoke nitrosamine) to
its carcinogenic (IARC, Group 2A) adduct-forming hydroxylated
product (28).
Among other potential causes of OVC, of interest is
the controversial and inconclusive pathogenic role of human
papillomavirus (HPV) (29–31). Some researchers considered that HPV
was a possible pathogeny of OVC (32–35).
Noble-Topham et al reported the detection of HPV DNA in 12
(48%) of 25 OVC patients. Specifically, HPV 6b/11 DNA, HPV 16 DNA,
HPV 18 DNA, and HPV 16 DNA plus HPV 18 DNA were detected in one
(4%), one (4%), nine (36%), and one (4%) cases, respectively. The
detection of HPV 18 DNA in 40% of OVCs reveals an association
between HPV and OVC although the potential etiologic and prognostic
significance of HPV in OVC deserves further exploration (34). On the contrary, other scholars
argued that the role of HPV might be occasional as there was no
verified correlation between OVC and HPV in their work (36–38).
For instance, de Spíndula-Filho et al examined the role of
HPV in cellular proliferation in OVC based on quantitative analyses
of 39 OSCCs, 8 OVCs and 9 normal mucosa samples. No correlation
between HPV and OVC was established in this study because all
samples tested were negative for HPV (36). Evidently, there is conflicting
research regarding the role of HPV. Further studies on
determination of appropriate sample size and use of highly
sensitive molecular biology techniques (e.g., polymerase chain
reaction) are expected to produce new information in order to gain
further understanding on the topic.
3. Clinical manifestations and
pathology
Clinical manifestations
OVC often occurs in buccal mucosa, tongue, lip,
gingiva, alveolar ridge and mouth floor (39), exhibiting a predilection for
elderly males, especially those over the age of sixty (40,41).
Its predominant clinical manifestations are exophytic mass and
papillary appearance. Due to its slow growth which contributes to
long medical history (up to several years) and to the local
aggression that leads to rare regional or distant metastasis, OVC
has a relatively good prognosis (42). According to clinical manifestations
and prognosis, Tang et al first divided OVC into three
types: exogenic type, cystoid type, and infiltrative type (14,43).
The exogenic type of OVC is characterized by exophytic growth,
cauliflower-like warty lesion and slow tumor growth. However, the
other two types of OVC grow rapidly, forming bean dreg-like white
dry keratosis, accompanying poor prognosis compared to the exogenic
type of OVC.
Pathology
Pathological features in optical
microscopy
OVC epithelial cells are well differentiated with
weak cell atypia. In optical microscopy, the squamous epithelium of
OVC shows highly proliferative, papillary appearance and excess
aceratosis. The highly proliferative epithelial pegs show swelling
and blunt ends in the shape of liquid droplets. All epithelial pegs
are infiltrated to the connective tissue in the same depth, forming
pushing borders (44). Many
lymphocytes and plasma cells are also infiltrated into the
connective tissue in which cancer cells may degenerate or become
necrosis or be swallowed by phagocytic cells, resulting in
carcinoma cell destruction. Between squamous epithelium and
connective tissue, the majority of components of epithelial
basement membrane (BM) of OVC remains integrated.
Pathological features in electron
microscopy
The pathological features of OVC can be reflected by
its stereology in the electron microscopy. The stereology of OVC
observed under a transmission electron microscope usually shows
thick and intact basement membrane of the cancer with obviously
thicker substrate than the normal cells in local areas. With
increased inflammatory cells (e.g., lymphocytes and plasma cells),
the basement membrane is disrupted in some cases. The
ultrastructural pathological features of the exogenic type of OVC
in the electron microscopy are well differentiated epithelial cells
with keratocyst, large and regular nucleus with obvious nucleolus,
no pseudopodia on the membrane and no cytoplasmic vacuolation.
However, for the cystoid type and infiltrative type of OVC, they
have poorly-differentiated epithelial cell with obvious
heteromorphism, large, irregular and lobulated nucleus, clear
pseudopodia on the membrane and obvious cytoplasmic vacuolation
(43).
4. Molecular mechanisms
The development of OVC is a multistep process
involving the accumulation of multiple genetic alterations
modulated by genetic predisposition and environmental influences
such as tobacco use, alcohol consumption, microbial infections, and
chronic inflammation. All of these factors can result in a wide
range of genetic alterations and epigenetic modifications that can
be detected in a range of molecular studies. Exploration of
molecular mechanism is important for reducing the morbidity and
mortality and for improving long-term survival rate of OVC. It
mainly focuses on seeking definitive and effective molecular
biomarkers which are widely used to identify the evolution of
dysplasia lesions to cancer. Up to now, a large number of studies
have been carried out to reveal the molecular mechanism of OVC from
perspectives of genetics and epigenetics (45,46).
Genetics
The molecular mechanism of OVC is closely associated
with its genetics. Genetic alterations are involved in
polymorphism, point mutation, deletion, and other alterations.
Previous investigation mainly focused on gene profiling (47,48).
As a special type of OSCC, OVC has its own specific
clinical manifestations and pathological features. Further
understanding of the molecular mechanisms of OVC requires gene
expression differentiation between OVC and OSCC. In fact, many
genes express differentially between OVC and OSCC, and some of them
are closely related to cancer progression of OVC. To identify key
genes that regulate and control the biological behavior of OVC,
Wang et al differentiated gene expression profiles between
OVC and OSCC (49). The cancer
tissues and the matched normal oral mucosa tissues from 5 OVC
patients and 6 OSCC patients were analyzed using the Affymetrix
HG-U133 Plus 2.0. The function and biological pathways of gene were
profiled with the Ingenuity Systems IPA software. It was found that
167 genes expressed differentially between OVC and OSCC. Among
them, 108 genes were upregulation and 59 genes were downregulation.
Compared with their matched normal mucosa tissues, 39 common genes
were expressed differentially (22 upregulation, 17 downregulation)
between OVC and OSCC. Some of these 39 genes were related to the
networking functions including cellular movement, genetic disorder,
inflammatory response and immune cell trafficking. Between OVC and
OSCC, 8 of the 39 genes, namely ADAMTS12 (a disintegrin and
metalloproteinase with thrombospondin motifs), COL4A1 (α1 type IV
collagen), COL4A2 (α2 type IV collagen), INHBA (inhibin, βA), MMP1
(matrix metalloproteinase 1), SERPINE1 (serpin peptidase inhibitor,
clade E, member 1), TGFBI (transforming growth factor, β-induced),
and HLF (human lactoferrin), were expressed differentially and
considered effective biomarkers in differentiating OVC and
OSCC.
Epigenetics
The cellular and physiological trait variations of
OVC may not involve changes in DNA sequence. Carcinogenesis is a
multistep process modulated by a number of epigenetics
modifications (50). Prior
research devoted much effort to identification of molecular
mechanism of OVC from epigenetics perspective according to number
and percentages of molecules in each functional category, including
tumor growth (cell cycle acceleration and proliferation), tumor
suppression (antitumor defense and apoptosis), angiogenesis and
tumor invasion and metastasis. The corresponding biomarkers for
diagnosis of OVC are summarized in Table I.
 | Table IPotential markers for OVC (in
previous studies). |
Table I
Potential markers for OVC (in
previous studies).
| Classification | Marker | Function | Expression in OVC
compared to normal tissues (NT) or OSCC | Effects | Refs. |
|---|
| Tumor growth | Cyclin B1 | Regulating cell
cycle (G2-M phase) |
OSCC>OVC>NT | Differentiation of
OVC from OSCC and prognosis of OVC | 36 |
| Cyclin-D1 | Regulating cell
cycle (G1-S phase) |
Poorly-differentiated
OSCC>moderately-differentiated
OSCC>OVC>well-differentiated OSCC | Histological
grading of OSCC and differentiation of OVC from OSCC | 52 |
| PCNA | Regulating cell
cycle (late G1-S phase) | Well-differentiated
OSCC>OVC>OVH>NT | Prognosis of
OVC | 53 |
| Ki67 | Regulating cell
cycle (G1-S-G2 phase) | OSCC>OVC | Prognosis of OVC
within which OSCC arises | 54 |
| αB-crystallin | Anti-apoptosis |
OSCC>OVC>NT | Carcinogenesis by
controlling activation of caspase-3 | 55 |
| SKp2 | Regulating cell
cycle (G1 phase) | OVC,
OSCC>NT | Prognosis of
OVC | 56 |
| Mutant p53 | Contributing to
oncogenesis instead of suppressing tumor |
OSCC>OVC>NT | Differentiation of
OVC from OSCC and histological grading of OSCC at invasive front
regions | 57 |
| p63 | Maintaining
epithelial cell regeneration and homeostasis | OSCC>OVC | Diagnosis of
OVC | 58 |
| Tumor
suppression | p16 | Preventing cells
from going through G1-S phase, inhibiting DNA synthesis and cell
proliferation | OVC>OSCC;
OVC>dysplastic epithelium | Pathogenesis of OVC
with overexpression of p16 caused by inactivation of pRb | 59 |
| p21 | Mediating growth
arrest (G1 and S phases) and inhibiting DNA synthesis | OVH>OVC | Pathogenesis of
OVC | 60 |
| p27 | Stopping/reducing
the cell division cycle (G1 phase) | Dysplastic
lesions>OVC>OSCC | Pathogenesis of
OVC | 59 |
| PTEN | Restraining cell
growth in the G1 phase, apoptosis and impeding cell invasion and
metastasis | NT>OVC,
OSCC | Diagnosis of
OVC | 61 |
| NQO1 and SOD | Antioxidation,
anti-aging, and detoxification | OVC>OSCC | Differentiation of
OVC from OSCC | 62 |
| iNOS | Overproduction of
iNOS suppressing tumor growth and inducing apoptosis | OVH=OSF>OVC | (Pre)malignant
carcinogenesis and prognosis of OVC | 63 |
| Angiogenesis | VEGF | Inducing blood
vessel growth and formation of vascular cavity | OSCC>OVC | Differentiation of
OVC from OSCC | 62 |
| Tumor invasion and
metastasis | MMPs | Degrading
extracellular matrix and basement membrane | Absence of MMP-7,
-9 and -12 in OVC rather than OSCC | Differentiation,
diagnosis and prognosis of OVC | 71 |
| | | MMP-2, MMP-9:
high-grade OSCC>low-grade OSCC>OVC>NT | Differentiation of
OVC and OSCC and histological grading of OSCC at invasive front
regions | 57 |
| | | MMP-10:
OSCC>OVC>NT | Differentiation of
OVC and OSCC at invasive front regions | 72 |
| Basement membrane
(BM) proteins | A supporting pad
for epithelial cells, connecting epithelial tissues and connective
tissues | Laminin:
OED>OVC>OSCC; collagen IV: OVC>OED; discontinuities of
laminin, collagen IV and fibronectin: OED>OVC | Tumor invasion
indicated by BM loss. Differentiation of OVC from OSCC and OED | 73 |
| Moesin | Cross-linkers
affecting cell-cell recognition and signaling and cell
movement | Well-differentiated
OSCC>OED>OVC> moderately-differentiated
OSCC>poorly-differentiated OSCC | Differentiation of
OVC from OED and OSCC | 74 |
| Laminin-332 γ2 | A component of BM
associated with cell migration and tumor invasion | Well-differentiated
OSCC>OVC | Differentiation of
OVC from well-differentiated OSCC | 75 |
Tumor growth (cell cycle acceleration and
proliferation) markers
Cell cycle refers to eukaryotic cells with continued
division from the end of mitotic cycle growing to the end of next
mitotic cycle. Cancer cells often have an abnormal mitotic cycle.
Cell proliferation, differentiation, senescence and apoptosis are
closely related to the cell cycle regulatory machinery (51). The markers associated with the
dysregulation of the cell cycle machinery usually indicate cancer
progression. As shown in Table I,
the most intensively investigated tumor growth markers for OVC
diagnosis are cyclins including cyclin-B1 (36) and cyclin-D1 (52), proliferating cell nuclear antigen
(PCNA) (53), Ki67 (54), αB-crystallin (55), S-phase kinase-interacting protein 2
(SKp2) (56), mutant p53 (57) and p63 (58). Most of these markers express in a
decreasing order from OSCC through OVC to normal mucosal tissue.
Note that the expression levels of some tumor growth markers,
(e.g., Cyclin-D1 and PCNA in Table
I) remain controversial in well-differentiated OSCC and a part
of OVC with strong tendency to local invasion.
Tumor suppressor markers (antitumor
defense and apoptosis)
During the cell cycle, cyclins control the
progression of cancer cells by activating cyclin-dependent kinase
(CDK). The progression of OVC may be restrained by a series of CDK
inhibitors, e.g., INK4 (Inhibitor of CDK4, including p15, p16, p18
and p19) and Kip (Kinase inhibition protein, such as p21, p27, and
p57) (59,60). Other tumor suppression markers
include proteins phosphatase and tensin homologue (PTEN) (61), quinone oxidoreductase 1 (NQO1),
superoxide dismutase (SOD) (62),
and inducible nitric oxide synthase (iNOS) (63). As presented in Table I, the majority of the markers have
a declining expression order from oral premalignant lesions, such
as dysplastic epithelium, OVH, OSF and oral epithelial dysplasia
(OED), through OVC to OSCC. It is worth noting that the typical
tumor suppressor protein, wild-type p53, is absent in Table I because its role as a marker for
OVC is still unconfirmed.
Angiogenesis markers
Angiogenesis is crucial in the occurrence,
development and prognosis of tumor. Angiogenesis markers may have
the potential for diagnosis and prevention of carcinomas (64). The markers of angiogenesis may be
used for the prognosis and treatment of OVC. As shown in Table I, the vascular endothelial growth
factor (VEGF) family is thought to be one of the strongest
angiogenesis simulators that induce blood vessel growth. It also
induces formation of vascular cavity and increases vascular
permeability. Hence, VEGF is regarded as a marker of metabolism and
transformation of OVC (62).
Tumor invasion and metastasis
markers
Tumor cells break through the extracellular matrix
and basement membrane, which is an important step during the
process of tumor invasion and metastasis (65). Many matrix metallo proteinases
(MMPs) play significant roles in this process, including MMP-1, -2,
-7, -9, -10, -12, -13, -14, -19, and -26 (66–68).
Moreover, basement membrane, composed of laminin, collagen IV and
fibronectin (69), is a
continuous, insoluble but flexible structure located between the
basal surface of epithelium and connective tissue. As a selective
barrier for molecules, basement membrane is closely related to cell
differentiation, cell migration, and tumorigenesis (70). Table
I shows different expression levels of tumor invasion and
metastatic potential markers (71–75)
for OVC.
5. Diagnosis and differential diagnosis
Diagnosis
The diagnosis of OVC includes two aspects: clinical
and pathological diagnosis. In the clinical aspect, OVC usually has
a characteristic exophytic mass, cauliflower-like warty lesion and
slow growth. On the pathological examination, the most important
and typical pathological features of OVC are infiltration of all
rete pegs to the connective tissue in the same depth which forms
pushing borders. These features can be used to diagnose some OVC
cases with acceptable accuracy. However, for accurate diagnosis,
multiple factors except for clinical and pathological features
should be considered to eliminate the influence of other lesions on
discrimination, such as OSCC within hybrid VC. First, as
pathological diagnosis is subjective, different explanations may
occur for the same phenomenon. Second, collection of remarkable
characteristic CT and MRI images of OVC may substantially improve
the diagnosis. Third, reliable genes and proteins may be sought as
diagnostic markers for OVC. Lastly, the medical history and
clinical manifestations can serve as good references for the
diagnosis.
Differential diagnosis
Although much effort has been spent on differential
diagnosis of OVC, gold diagnosis standards or specific diagnostic
markers are still lacking. The main reasons are as follows: First,
OVC is similar to many diseases in clinical and pathological
aspects. Different OVC cases may show different biological
behaviors. Second, for the same OVC, it may be diagnosed
differently when pathological examination is performed on different
sites. Third, hybrid verrucous carcinoma, composed of OVC and
differently-differentiated OSCC, may exist. This type of carcinoma
has more aggressive invasion nature with incidence rate up to 20%
(76). Obviously, it is crucial to
make a differential diagnosis between OVC and other similar
diseases for improving treatment and prognosis.
OVC and oral verrucous hyperplasia
(OVH)
OVC and OVH are two distinctive oral verrucous
lesions in the clinico-pathology in spite of their similar
morphologies in the clinical and histopathological aspects
(77,78). From the clinical aspect, both of
them have a thick, extensive, white plaque, or exophytic verrucous
appearance. The most common sites for the two lesions are buccal
mucosa, tongue and lip. However, to differentiate them effectively,
some histopathological features may be used because OVC has the
explicit ‘pushing broader’ feature with destructive extrapolation
edges at the junction of lower connective tissues, whereas OVH does
not show invasion of the hyperplastic epithelium into the lamina
propria compared with adjacent normal mucosal epithelium. Further
differentiation can also be achieved with the assistance of
biomarkers. For instance, CD34, α-smooth muscle actin and HuR
protein have the capability to diagnose OVC and OVH (79,80).
OVC and oral squamous papilloma
(OSP)
Oral squamous papilloma (OSP) shares similar
morphology to OVC. OSP and OVC are often clinically present as
exophytic, cauliflower and papillary forms. From the
histopathological point of view, it is possible to differentiate
OSP from OVC. For OVC, all rete pegs of the epithelium tend to
project into the underlying connective tissue, at more or less the
same level, forming ‘pushing border.’ OSP often presents as many
long, thin and finger-like projections which extend above the
mucosal surface. Each finger-like projection, which contains a
central connective tissue, is lined by stratified squamous
epithelium. The upper epithelial cells of OSP have pyknotic and
crenated nuclei, which are often surrounded by edematous or
optically clear zone, known as ‘koilocytic’ cell (81). The differentiation can also be
achieved by using some proteins as markers. These proteins include
the cytokeratins (CKs) family (e.g., CK 10, 13, 14 and 16), whose
expression relates to the biological behavior of both lesions
(82).
OVC and OSCC
As aforementioned, OVC has a strong tendency to
local invasion whereas metastasis is rarely seen (83). OVC has some pathological
similarities to OSCC, especially for well-differentiated OSCC and
OVC. Aiming at assessing and validating biomarkers for better
understanding of the genesis and molecular mechanisms of OVC and
OSCC, Pentenero et al (84)
found OVC and OSCC could be differently characterized using
chromosomal instability biomarkers. The difference in
aggressiveness and prognosis of OVC and OSCC was reflected by DNA
index characteristics. Some tumor genes and molecular markers
including Cyclin-D1, laminin-332 γ2, PCNA, moesin, MMP-2, MMP-9 can
also be used for comparative evaluation of OVC and OSCC, especially
for OVC and well-differentiated OSCC, guiding clinicians to make an
accurate diagnosis (52,53,57,74,75).
OVC and oral hybrid verrucous
carcinoma
Oral hybrid verrucous carcinoma (VC) is a neoplasm
composed of OVC and differently-differentiated OSCC (85). For example, well-differentiated
OSCC was identified within OVC and invaded the underlying
connective tissue and bone (76).
Unlike OVC, oral hybrid VC is staged and graded similar to OSCC.
However, the proportion of conventional OVC component may vary and
the prognosis of hybrid VC with high proportion of OVC may have
better prognosis than OSCC. Due to high similarity in staging and
grading, incision biopsy is extremely unreliable to diagnose and
differentiate oral hybrid VC from OVC (86). For diagnosis of this hybrid tumor,
it is necessary to examine an adequate biopsy sample extending to
the underlying bone for examination of the periosteum and the
mucosa-connective tissue interface.
6. Treatment
The general treatment principles of OVC are
consistent with OSCC, but the treatment of OVC has its own
characteristics. Since the first report of OVC there have been
debates regarding the treatment of choice for this tumor. The
treatment regimens mainly include surgery, chemotherapy,
radiotherapy or combinations, cryotherapy, and shave excision.
However, surgery for wide lesion area usually results in uncosmetic
appearance and dysfunction. Chemotherapy or radiotherapy may have
poor response and anaplastic transformation, and thus questionable
effectiveness. Regarding these issues, unconventional treatment
modalities have been put forward in recent years. They include
photodynamic therapy and CO2 laser therapy. The details
of these treatment regimens for OVC are summarized in Table II.
 | Table IITreatment regimens for OVC (in
previous studies). |
Table II
Treatment regimens for OVC (in
previous studies).
| Treatment
regimen | Number of
patients/gender | Time/age | Specific treatment
modalities and additional information | Results (recurrence
rate, RR; disease-free survival, DFS; overall survival rate,
OSR) | Refs. |
|---|
| Surgery | 101/M: 79, F:
22 | 1990 to 2000/53.9
(average) | Surgery for
patients with no history of head and neck treatment | RR: 68% (first-time
surgery), salvage rate for recurrent tumors: 66.7%, DFS: 77.6% (5
years) | 91 |
| Surgery | 38/M: 36, F: 2 | 1996 to 2002/51
(median) | Staging work-up and
preoperative evaluation (e.g., computed tomography of head and neck
area and blood chemistry) before surgery | RR: 0, OSR: 94.7%
(3 years) | 92 |
| Surgery | 40/M: 38, F: 2 | 1991 to 2002/53.8
(average) | / | Control rate: 94.9%
(first-time surgery); OSR: 89.9% (5 years) | 93 |
| Surgery | 86/M: 52, F:
34 | 1990 to 2012/64.1
(average) | Enlarged resection
of pure lesions performed in 1.0 cm to 1.5 cm outside the mass
edge | RR: 3.5%
(first-time surgery); 0 (second-time surgery) (5 years) | 94 |
| Surgery/surgery +
radiation/radiation | 2350 head and neck
VC (1314 OVC)/M: 1410, F: 940 | 1985 to 1996/69
(median) | Early stage:
surgery (85.8%); advanced stage: surgery (56.9%), surgery +
radiation (16.3%), radiation (12.5%) | SR: 73.7% (5
years); for localized oral cavity tumors, SR: surgery: 85.7%,
surgery + radiation: 68.4% radiation: 41.8% (5 years) | 12 |
| Radiotherapy | 53/M: 29, F:
24 | 1985 to 1987/<35
(1.9%); 36–59 (47.2%); >60 (50.9%) | Radiotherapy given
either as external beam radiotherapy or interstitial implantation,
or as a combination of the two | RR: 30.2%; DFS:
66%, OSR: 86% (5 years); No anaplastic transformation in recurrence
cases | 97 |
| Radiotherapy | 107/M: 75, F:
32 | 1977 to 1987/50–59
(37.3%); 60–69 (27.1%) | Different stage
tumors receiving different dosage, fractions, time, and
equipment | SR: 100% (stage I),
68% (stage I1), 35% (stage 111), 26% (stage IV) (5 years); RR:
48.6% | 98 |
| High-dose- rate
(HDR) brachytherapy | 1/M | /85 | A dose of 48 Gy in
12 fractions three times per week | Tumor disappeared
without lymphadenopathy after 5 months | 99 |
| Chemotherapy
(methotrexate) | 12/M: 3, F: 9 | 1972 to 2010/79
(median) | Different stage
tumors receiving different dosages by using various routes
(intra-arterial injection, intramuscular injection and intravenous
injection) | 7 patients: good
responses; 4 patients: partial responses; 1 patient: no response.
Additional treatments needed for patients with no response after
one or two cycles | 44 |
| Chemotherapy
(capecitabine) | 2/F | 1990/71;
2002/75 | Two times a day for
one cycle, namely 2 weeks on and 1 week off, at a dose of 1000
mg | Both lesions
achieving nearly complete resolution within 3 weeks (dramatic
response); time for a durable partial response: first patient: 1
year, second patient: 6 months | 103 |
| Intra-arterial
chemotherapy (methotrexate) | 15/M | /55 | 50 mg per day for a
mean period of 7.5 days, followed by 25 mg per week for 10
weeks | Tumor markedly
regressed and finally entirely disappeared after 2.5 months, RR: 0
(43 months) | 104 |
| Intra-arterial
chemotherapy (methotrexate) | 1/M | /68 | 25 mg per day for
11 days, folinic acid given intramuscularly 6 mg every 6 h during
the period | Tumor disappeared
after 1.5 months; an ulcer recurred after 5 years and restored by
surgical intervention with a nasolabial flap | 105 |
|
Radiochemotherapy | 5/M: 2, F: 3 | /74 (median) | Radiotherapy
(median, 56 Gy) + chemotherapy (vinblastine 2 mg (day 1);
methotrexate 50 mg (day 2); bleomycin 15 mg (days 2 and 3), and
repetition at 2–3 week intervals) | 5 patients cured
and 1 patient died (within a median 2.92 years) | 106 |
| Surgery/surgery +
radiochemotherapy | 15/M: 5, F: 10 | 1981 to 1997/76.9
(average) | One group (A):
surgery; the other group (B): surgery + radiochemotherapy | DSF: A: 78%, B: 33%
(5 years); A: 52%, B: 33% (10 years); anaplastic transformation
occasionally occurred during treatments of OVC | 107 |
| Surgery/surgery +
chemotherapy, radiotherapy, or both | 12/M: 5, F: 7 | 1980 to
2000/67.8±3.7 (average) | One group (A):
surgery; the other group (B): surgery + chemotherapy, radiotherapy,
or both | Local control rate:
A: 86.6%, B: 82.1%, SR: A: 91.3%, B: 92.3% (5 years) | 108 |
| Cryotherapy and
shave excision | 20 (26 lesions: 17
OVH, 9 OVC)/M: 12, F: 8 | /45–91 | Shave excision +
spraying liquid nitrogen (40–50 sec)+ thawing (30–60 sec)+ repeated
freeze-thaw cycle 3 times | Tumors disappeared
and lesions healed after 3–4 weeks; RR: 33.3% (23 month);
Recurrence cases cured with the same technique | 112 |
| Photodynamic
therapy (PDT) | 1/M | /56 | Multiple 3-min
fractionated irradiations (1000 sec) with a light emitting diode
red light at 635±5 nm +20% 5-aminolevulinic acid (1.5 or 2 h) | Extraoral tumor
disappeared after 6 cycles; intraoral tumor disappeared after 22
cycles; no recurrence within 6 months | 115 |
| CO2
laser therapy | 1/F | /76 | One session of
CO2 laser SmartXide DEKA (Firenze-Italy) (wave length:
10.600 nm, power: 8 W, repetition rate: 80 Hz, pulse width: 1000
msec) | No recurrence and
metastasis within the 2-year follow-up | 116 |
| CO2
laser therapy | 2/F | 2002/72;
2003/70 | A focused laser
beam (wave length: 10.6 μm, power: 6W) + a defocused beam | Tumor and lesion
disappeared after 11 months; no recurrence within 3 years | 117 |
Surgery
Surgery has been considered the preferred treatment
for OVC (87,88). The aim of surgery is to eradicate
the tumor without disabling function. For the exogenic type of OVC,
surgical excision is the first-line method due to its controlled
size, rare tumor recurrence, and good prognosis. However, for the
hybrid type of OVC, the surgical excision should be progressive.
The excision boundary needs careful estimation because the excision
sizes for the hybrid type of OVC are usually much broader.
Incomplete or excessive resection often accelerates tumor growth,
leading to anaplastic transformation, poor function and difficult
reconstruction. In this case, surgery (e.g., primary tumor
resection and neck dissection) combined with radiotherapy and
chemotherapy may be appropriate to minimize tumor recurrence and
undesired prognosis (88–90). Table
II shows the uses of surgery for OVC since mid-1980s, clearly
demonstrating its effectiveness after treatment (91–94).
Radiotherapy
OVC was initially thought to be somewhat
radioresistant in the oral cavity or the larynx (95). It was reported the local recurrence
rate could reach as high as 57% by following radiotherapy,
resulting from the high incidence rate of multiple primary tumors.
The anaplastic transformation may also occur in >10% of OVC
cases (96). In fact, the
treatment policy mainly depends on the extension of the primary
tumor and on the regional nodal involvement. The patients who
undergo surgery are usually in Stage I or II, whereas radiotherapy
(or combined with surgery) appears more suitable for patients in
Stage III or IV, the advanced tumor stages which are not an
indication for surgery (97). It
was shown that the 5-year actuarial survival of patients with OVC
treated by primary radiotherapy did not show any significant
difference when compared to that of patients treated by surgery
(98). In this regard, the role of
radiation in promoting anaplastic transformation, a risk which is
certainly over-emphasized, seems questionable and warrants further
verification. Table II shows
representative satisfactory results obtained by using radiotherapy
for OVC treatment (97–99). Overall, radiotherapy was deemed
less effective but an acceptable alternative treatment regimen for
OVC.
Chemotherapy
Up to now, few reports have focused on the
efficiency of chemotherapy schemes applied to OVC (100). Surgery and radiation are the
major treatments for the exogenic type of OVC. However, for some
OVC with strong tendency to local invasion, chemotherapy may be
another cost-effective alternative treatment for patients, which
usually improves the quality of life considerably. For instance,
intra-arterial chemotherapy, featured by convenient dosing,
excellent drug activity and acceptable toxicity profile, is
effective in some OVC patients. Chemotherapeutic drugs have the
capacity to evoke rapid and clinically significant sustained
response which can be well tolerated in the patients. Moreover, the
persistent and greater exposure of the tumor region to the drugs
may induce rapid tumor shrinkage and achieve alleviation in a short
time with reduced systemic toxicity (101,102). As shown in Table II, methotrexate and capecitabine
are the desirable drugs for OVC treatments (103–105).
Radiochemotherapy
There is a controversy over the outcomes of clinical
treatments by radiochemotherapy. For example, Strojan et al
reported that the simultaneous intensification of chemotherapy was
useful for reducing the radiotherapy dose, which is of benefit to
minimization of toxic side effects induced by the treatment
(106). Yoshimura et al
compared different treatment approaches for 15 patients having OVC
at the Shimane Medical University Hospital (107). The results showed that the
disease-free survival rates of surgery alone and surgery combined
with radiotherapy and chemotherapy were superior to radiotherapy,
chemotherapy or their combinations. Surgery was considered the
first choice of treatment for OVC, and radiotherapy combined with
chemotherapy was regarded as the second most preferable treatment
when the patient does not fit for surgery, refuses surgery, or has
inoperable tumor. Overall, radiochemotherapy has acceptable
therapeutic results (108).
Cryotherapy and shave excision
Cryotherapy is an effective and acceptable treatment
method for oral precancerous and cancerous lesions including oral
leukoplakia (OL), OVH, OVC and OSCC (109,110). It destroys lesional tissues
mainly by disrupting cell membrane and by damaging protein, enzyme
and vasculature, resulting in cells swelling, rupturing, or
dehydrating. Cryotherapy is capable of reducing blood, scar and
pain, and decreasing the occurrence of secondary infections
(111). However, cryotherapy does
not involve tissue excision and thus lacks precision. It is
difficult to judge the final volume of tissue necrosis.
Furthermore, OVC lesions are usually bulky and fungating. To obtain
complete lesion regression, combined use of cryotherapy and shave
excision is demanded (112). In
practice, cryotherapy is not the predominant treatment method for
OVC, but it is easy, safe, and conservative in OVC treatment.
Photodynamic therapy
Photodynamic therapy (PDT), also known as
photochemotherapy (PCT), or phototherapy, was first introduced into
oral cancer treatment in the mid-1980s (113). It is a minimally invasive and
negligibly toxic technique that has shown great potential in recent
years in the treatment of oral precancerous and cancerous lesions,
oral premalignant and malignant disorders, including OL, oral
erythroleukoplakia (OEL), OVH, OVC, OSCC, and bacterial and fungal
infections (114). In general,
PDT mediates tumor destruction by three mechanisms. Firstly, the
free radicals and singlet oxygen kill tumor cells directly.
Secondly, PDT can damage the tumor-associated vasculature, causing
thrombus formation and subsequent tumor infarction. Thirdly,
PDT-destroyed tumor tissues release tumor specific antigens that
activate an immune response against the residual tumor cells. Since
PDT has simple procedure with minimal pre-treatment, high efficacy,
little or no scar formation, high patient compliance, low
invasiveness, and slight side effects, it has played a significant
role in the management of OVC (Table
II) (115).
CO2 laser therapy
Since the early 1970s, carbon dioxide
(CO2) laser therapy has been introduced to treat
patients having oral lesions. The advantages of this treatment
include short surgical time, effective wound sterilization, fast
hemostasis and healing process, little pain, sealing of adjacent
lymphatic vessels, reduced spread of malignant cells and
anti-metastasis. These advantages have been partially confirmed by
reported studies in Table II
(116,117).
7. Discussion
OVC has received increasing attention in the past
decades, which has been demonstrated by much effort spent on its
etiology, clinical manifestations, pathology, diagnosis and
treatment. There were evident advancements in this field, covering
from etiological analysis to effective treatment. In particular,
exploration of molecular mechanism and diagnosis of OVC have been
largely promoted by employing multiple biomarkers. With advancement
of understanding of the mechanism and diagnosis, various treatment
regimens have been developed for OVC patients. The most notable
ones include surgery, radiotherapy, and chemotherapy, which have
already showed desired results in many cases. Other unconventional
modalities such as cryotherapy and shave excision, photodynamic
therapy, laser and immune therapies further enhanced the treatment
effectiveness although they were often recognized as a means of
auxiliary approach. Without doubt, these progresses will warrant
effective prevention and better treatment of OVC. However, it
should be noted that this field is still facing three challenges
from primary sub-fields of the research and clinical practice of
OVC, namely multifactorial etiology, complex molecular mechanism,
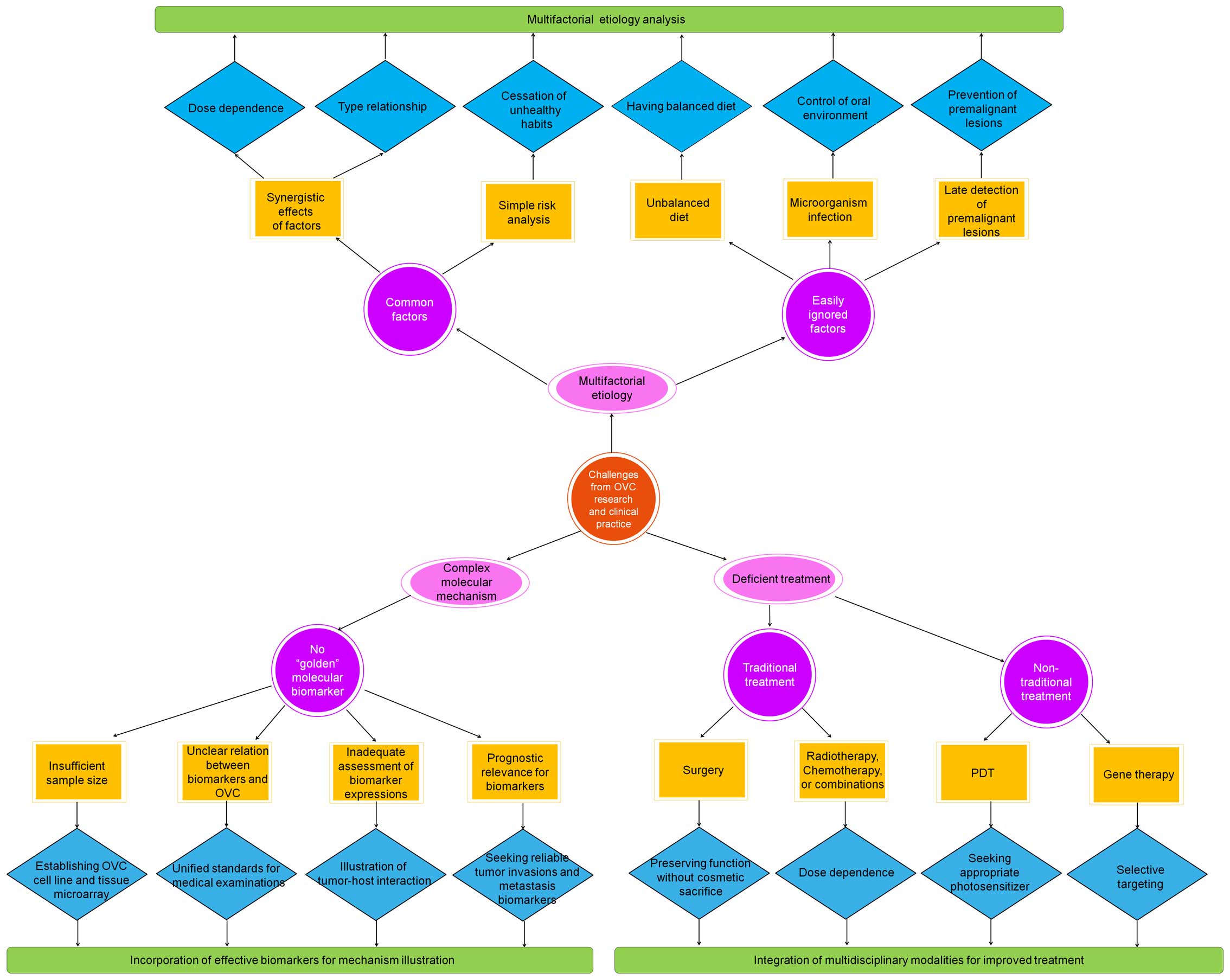
and deficient treatment (Fig. 1).
To resolve these challenges, more effort on the multifactorial
etiology analysis, incorporation of effective biomarkers for
mechanism illustration, and integration of multidisciplinary
modalities for improved treatment is desired.
Multifactorial etiology analysis
As aforementioned, the etiology of OVC is
multifactorial. The most important etiological factors are excess
consumption of alcohol, tobacco, and areca nut usage. However, it
was difficult to explain the increasing incidence of OVC with those
common risk factors alone. This is because, on the one hand, these
factors often act synergistically and therefore, their
dose-dependence and type relationship are hard to determine. On the
other hand, there is a lack of detailed risk analysis of these
habits. The cessation of these habits may prevent the development
of second primary tumors that arise independently, but it is
useless for multiple primary tumors that are caused by migration of
already transformed clone of cells (118).
Apart from the above risk factors, other factors
that predispose towards the development of OVC involve unbalanced
diet, i.e., an antioxidant-deficient diet. This finding can be
demonstrated by the advantages of consumption of fruit and
vegetables. Another easily ignored factor in association with OVC,
as discussed in the etiology section, is microorganism infection,
which requires control of oral environment. Microbes have the
potential of being used as a diagnostic indicator although the
relationship between microflora and oral malignancy, and how
microorganisms interact with the oral mucosa at a cellular level
deserve further investigation. Finally, late detection of
premalignant oral lesions has evolved into another important
etiological factor. Successful inhibition of development of
premalignant oral lesions toward OVC would considerably reduce the
risk of OVC. This can be achieved by combining commercial
diagnostic aids and adjunctive techniques besides conventional oral
examination for screening of patients for signs of oral cancer and
precancerous lesions. A large number of oral cancer screening and
case-finding aids or adjuncts (e.g., toluidine blue, brush
cytology, tissue reflectance and autofluorescence) have already
been developed and used to assist in the screening of healthy
patients for evidence of occult cancerous change or to assess the
biologic potential of clinically abnormal mucosal lesions (119,120). Altogether, it is necessary to
enforce multifactorial etiology analysis to reduce the morbidity
and even mortality of OVC.
Incorporation of effective biomarkers for
mechanism illustration
There is no doubt that the molecular mechanism of
OVC remains the focus of attention. To further the understanding of
the mechanism, reliable molecular markers associated with the
occurrence, progression and prognosis of OVC should be sought
regarding the complexity of oral carcinogenesis. For this goal,
various molecular markers have been proposed for use. However,
clear molecular markers as a golden diagnostic standard are still
absent. This fact is attributed to several reasons. Firstly, due to
insufficient sample size, some markers showed low predictive values
which fail to reach significance. It is thus necessary to establish
an OVC cell line and tissue microarray. Secondly, incomplete
knowledge for the relation between biomarkers and OVC may cause
‘superficial’ understanding of their roles, which are often
questionable (121). Taking VEGF
as an example, there is no identified close correlation between its
expression and microvessel density (MVD) (122). Prior work showed the oral
carcinomas did not react to experimental anti-angiogenetic therapy
and the mean MVD revealed no relationship with the survival rate
(123). Thirdly, the assessment
of role of expressions of biomarkers like proteins is inadequate.
The expressions appear to be more important than the markers
themselves. A good example is p53, whose molecules up- or
downstream on the apoptotic pathway were found to be more
important. It indicates that further exploration of the field has
to consider the tumor-host interaction. Fourthly, the prognostic
relevance, usually evaluated based on a long-term follow-up, has
not been provided for evaluation of markers of the tumor invasion
and metastasis (e.g., MMPs). Since the relevance may illustrate
another area of local interaction between oral cancer and its host
in utilizing proteolytic enzymes for peritumoral matrix degradation
and tumor spread, it actually indicates another direction for
seeking reliable biomarkers. Overall, more attention should be
directed at the role of molecular markers for deep understanding of
the molecular mechanism of OVC, which essentially requires good
incorporation of effective biomarkers in association with
histopathology, molecular profiling with well-established clinical
parameters, and prognostic analysis.
Integration of multidisciplinary
modalities for improved treatment
In principle, the choice of treatment for OVC
depends on many factors. Current clinical applications involve use
of a variety of treatment modes. The most extensively used regimens
are surgery, radiotherapy, chemotherapy and radiochemotherapy,
which, as discussed before, have already showed desired results.
Specifically, surgery represents the first choice of treatment for
OVC. It aims at preserving functions without cosmetic sacrifice and
its efficacy relies on multiple factors including primary site,
location, size, proximity to bone, and depth of infiltration. For
example, the use of marginal mandibulectomy and mandibulotomy for
tumors that approach or involve the mandible requires special
attention to the mechanism of bone involvement. The success of
surgery also depends on the role of the surgeon which represents an
unnegligible factor throughout the life history of an OVC patient
and on the techniques involved during surgery. Advanced
technologies, such as rapid prototyping combined with X-ray
tomography, are expected to remedy the disadvantages of surgery
(124–126). For radiotherapy, chemotherapy,
and radiochemotherapy, they are usually regarded as the next most
preferable treatment when surgery is inappropriate. Employment of
either or both of them will contribute to the increase of the
overall survival of patients with OVC once dose dependencies of
radiation and drugs associated with the drug delivery system are
established (127).
Despite considerable advances in the above
traditional modalities, the survival of patients with OVC still
needs improvement. Unconventional approaches provide alternative
ways for treatment of OVC. Among them, PDT is especially promising
because of its better prognosis than radiotherapy and
chemoradiotherapy (128). For
application of PDT, an ideal photosensitizer should be administered
easily and safely, targeted appropriately, illuminated and
activated at clinically useful wavelengths, pain-free, and obtained
easily to achieve apoptosis and tumor necrosis with vascular
cessation for clinical operation. The success of PDT also requires
accurate dosimetry and suitable illumination devices and
sufficiently defined treatment parameters. Thus, interactions
between clinical applications and technological innovations and
interdisciplinary research approaches should be pursued to overcome
the difficulties and challenges for PDT.
Gene therapy is another very promising method as it
introduces new genetic material into targeted cells without
poisoning non-targeted tissues for treatment (129). The general strategies utilized in
a gene therapy approach for cancer include gene addition therapy,
gene excision therapy, antisense RNA technique, immunotherapy,
‘suicide’ gene therapy. For OVC, gene therapy is currently under
investigation in clinical trials (130). Although it has a rather high
requirement for selective targeting of tumor cells associated with
multiple etiological factors, exploitation of the principle and
selective targeting of tumor cells are feasible as our
understanding of the molecular mechanisms of OVC progresses. Also,
regarding that OVC is an attractive tumor target due to its
frequent genetic mutations and accessibility for intratumoral
administration, the safety and efficacy of gene therapy for
prevention and treatment of OVC can be further enhanced by phase
clinical studies and trials.
Overall, as OVC is characterized by multifactorial
etiology and incomplete understanding of molecular mechanism, a
variety of treatment modalities exist and may complement one
another well. Integration of multidisciplinary modalities, such as
surgery, chemotherapy, radiotherapy and/or unconventional methods,
either sequentially or concurrently is highly recommended for OVC
treatment.
8. Conclusions
As a verrucous variant of OSCC, OVC has received
increasing attention recently. This paper offers a systematic
review on its etiology, clinical manifestations and pathology,
molecular mechanism, diagnosis and differential diagnosis and
treatment. It clearly shows that the enormous effort spent in the
past decades has contributed to significant advancements in this
field, ranging from etiological analysis to development of various
regimens for treatment. Nevertheless, this field also faces three
great challenges from primary sub-fields of the research and
clinical practice of OVC, namely multifactorial etiology, complex
molecular mechanism, and deficient treatment.
From the point of view of etiology, common risk
factors alone cannot adequately account for the increasing
incidence of OVC. Instead, other factors that predispose towards
the development of OVC, namely unbalanced diet, microorganism
infection, and late detection of premalignant oral lesions, warrant
further analysis. From the perspective of the molecular mechanism
of OVC, incorporation of effective biomarkers in association with
histopathology and molecular profiling with well-established
clinical parameters, and prognostic analysis of OVC deserves more
attention for deep understanding of the mechanism. Lastly, to
promote effectiveness and efficacy of OVC treatment, it is
necessary to integrate multidisciplinary modalities, such as
surgery, chemotherapy, radiotherapy and/or unconventional methods
(e.g., PDT), either sequentially or concurrently considering their
potential complements to each other. In brief, continuous effort on
the multifactorial etiology analysis and molecular mechanism
through pursuing effective biomarkers will offer key insights into
OVC pathogenesis which leads the treatment with integration of
multidisciplinary modalities.
Acknowledgements
This work was partially supported by the Natural
Science Foundation of Qinghai Province of China (Grant 2013-z-908),
the National Natural Science Foundation of China (Grant 81300841)
and the Grant from Science and Technology Department of Hunan
Province of China (Grant 2013SK5075). The authors would like to
extend their sincere appreciation to Dr Shan Gao at Suzhou Ribo
Life Science Co. Ltd and Dr Zhiwei Peng at School of Minerals
Processing and Bioengineering, Central South University for helpful
discussions.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujii M: Multidisciplinary approach for
the treatment of head and neck cancer. Int J Clin Oncol.
19:209–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rivera C and Venegas B: Histological and
molecular aspects of oral squamous cell carcinoma (Review). Oncol
Lett. 8:7–11. 2014.PubMed/NCBI
|
|
4
|
Chaisuparat R, Limpiwatana S, Kongpanitkul
S, Yodsanga S and Jham BC: The Akt/mTOR pathway is activated in
verrucous carcinoma of the oral cavity. J Oral Pathol Med. Jan
17–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamala K, Sankethguddad S and Sujith SG:
Verrucous carcinoma of oral cavity - a case report with review of
literature. IJHSR. 5:330–334. 2015.
|
|
6
|
Gokavarapu S, Parvataneni N, Charan CR,
Puthamakula S, Kulkarni G and Reddy BS: Multi centricity of oral
verrucous carcinoma: A case series of 22 cases. Indian J
Otolaryngol Head Neck Surg. 67:138–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ackerman LV: Verrucous carcinoma of the
oral cavity. Surgery. 23:670–678. 1948.PubMed/NCBI
|
|
8
|
Wang Y, Wu QG and Zhen LF: Clinical
pathology study of oral verrucous carcinoma. Chin J Stomatol.
20:65–68. 1985.
|
|
9
|
Tang ZG, Li JY, Su T, Yao ZG, Shen ZH and
Li HB: The clinic study on oral verrucous carcinoma. J Oral
Maxillofac Surg. 12:87–88. 2002.
|
|
10
|
Rahali L, Omor Y, Mouden K, Mahdi Y,
Elkacemi H, Elmajjaoui S, Latib R, Kebdani T, Boujida MN and
Benjaafar N: Oral verrucous carcinoma complicating a repetitive
injury by the dental prosthesis: A case report. Pan Afr Med J.
20:2972015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zargaran M, Eshghyar N, Baghaei F and
Moghimbeigi A: Assessment of cellular proliferation in oral
verrucous carcinoma and well-differentiated oral squamous cell
carcinoma using Ki67: A non-reliable factor for differential
diagnosis? Asian Pac J Cancer Prev. 13:5811–5815. 2012. View Article : Google Scholar
|
|
12
|
Koch BB, Trask DK, Hoffman HT, Karnell LH,
Robinson RA, Zhen W and Menck HR; Commission on Cancer, American
College of Surgeons; American Cancer Society. National survey of
head and neck verrucous carcinoma: Patterns of presentation, care,
and outcome. Cancer. 92:110–120. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vallonthaiel AG, Singh MK, Dinda AK,
Kakkar A, Thakar A and Das SN: Expression of cell cycle associated
proteins p53, pRb, p16, p27 and correlation with survival: A
comparative study on squamous cell carcinoma and verrucous
carcinoma. Appl Immunohistochem Mol Morphol. 24:193–200. 2016.
View Article : Google Scholar
|
|
14
|
Tang Z, Xie X, Li J, Liu X, Yao Z and Zhao
S: A clinic study on oral verrucous carcinoma phenotypes. Chin J
Dent Res. 8:57–61. 2005.
|
|
15
|
Pravda C, Srinivasan H, Koteeswaran D and
Manohar LA: Verrucous carcinoma in association with oral submucous
fibrosis. Indian J Dent Res. 22:6152011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Joshi P, Dutta S, Chaturvedi P and Nair S:
Head and neck cancers in developing countries. Rambam Maimonides
Med J. 5:e00092014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Agnihotri A and Agnihotri D: Verrucous
carcinoma: A study of 10 cases. Indian J Oral Sci. 3:79–83. 2012.
View Article : Google Scholar
|
|
18
|
Schutze M, Boeing H, Pischon T, Rehm J,
Kehoe T, Gmel G, Olsen A, Tjønneland AM, Dahm CC, Overvad K, et al:
Alcohol attributable burden of incidence of cancer in eight
European countries based on results from prospective cohort study.
BMJ. 342:d15842011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson N: Tobacco use and oral cancer: A
global perspective. J Dent Educ. 65:328–339. 2001.PubMed/NCBI
|
|
20
|
Zhang X and Reichart PA: A review of betel
quid chewing, oral cancer and precancer in Mainland China. Oral
Oncol. 43:424–430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahn J, Chen CY and Hayes RB: Oral
microbiome and oral and gastrointestinal cancer risk. Cancer Causes
Control. 23:399–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta S, Kumar K, Raviprakash SM and
Arunkumar KV: Ackerman's tumor of the oral cavity: A study of four
cases with its conglomerate appearance. J Dent Specialities.
3:92–95. 2015.
|
|
23
|
La Vecchia C, Tavani A, Franceschi S, Levi
F, Corrao G and Negri E: Epidemiology and prevention of oral
cancer. Oral Oncol. 33:302–312. 1997. View Article : Google Scholar
|
|
24
|
Andrade JO, Santos CA and Oliveira MC:
Associated factors with oral cancer: A study of case control in a
population of the Brazil's Northeast. Rev Bras Epidemiol.
18:894–905. 2015.(In Portuguese). View Article : Google Scholar
|
|
25
|
Aslesh OP, Paul S, Paul L and Javasree AK:
High prevalence of tobacco use and associated oral mucosal lesion
among interstate male migrant workers in urban Kerala, India. Iran
J Cancer Prev. 8:e38762015. View Article : Google Scholar
|
|
26
|
Al-Jaber A, Al-Nasser L and El-Metwally A:
Epidemiology of oral cancer in Arab countries. Saudi Med J.
37:249–255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Langevin F, Crossan GP, Rosado IV, Arends
MJ and Patel KJ: Fancd2 counteracts the toxic effects of naturally
produced aldehydes in mice. Nature. 475:53–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verna L, Whysner J and Williams GM:
N-nitrosodiethylamine mechanistic data and risk assessment:
Bioactivation, DNA-adduct formation, mutagenicity, and tumor
initiation. Pharmacol Ther. 71:57–81. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee SY, Cho NH, Choi EC, Baek SJ, Kim WS,
Shin DH and Kim SH: Relevance of human papilloma virus (HPV)
infection to carcinogenesis of oral tongue cancer. Int J Oral
Maxillofac Surg. 39:678–683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Campisi G and Giovannelli L: Controversies
surrounding human papilloma virus infection, head & neck vs
oral cancer, implications for prophylaxis and treatment. Head Neck
Oncol. 1:82009. View Article : Google Scholar :
|
|
31
|
Herrero R, Castellsagué X, Pawlita M,
Lissowska J, Kee F, Balaram P, Rajkumar T, Sridhar H, Rose B,
Pintos J, et al; IARC Multicenter Oral Cancer Study Group. Human
papillomavirus and oral cancer: The International Agency for
Research on Cancer multicenter study. J Natl Cancer Inst.
95:1772–1783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Samman M and Sethi N: Oral verrucous
pre-malignant lesions and HPV. Clin Otolaryngol. 40:292–293. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
del Pino M, Bleeker MC, Quint WG, Snijders
PJ, Meijer CJ and Steenbergen RD: Comprehensive analysis of human
papillomavirus prevalence and the potential role of low-risk types
in verrucous carcinoma. Mod Pathol. 25:1354–1363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Noble-Topham SE, Fliss DM, Hartwick RW,
McLachlin CM, Freeman JL, Noyek AM and Andrulis IL: Detection and
typing of human papillomavirus in verrucous carcinoma of the oral
cavity using the polymerase chain reaction. Arch Otolaryngol Head
Neck Surg. 119:1299–1304. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fujita S, Senba M, Kumatori A, Hayashi T,
Ikeda T and Toriyama K: Human papillomavirus infection in oral
verrucous carcinoma: Genotyping analysis and inverse correlation
with p53 expression. Pathobiology. 75:257–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
de Spíndula-Filho JV, da Cruz AD,
Oton-Leite AF, Batista AC, Leles CR, de Cássia Gonçalves Alencar R,
Saddi VA and Mendonça EF: Oral squamous cell carcinoma versus oral
verrucous carcinoma: An approach to cellular proliferation and
negative relation to human papillomavirus (HPV). Tumour Biol.
32:409–416. 2011. View Article : Google Scholar
|
|
37
|
Patel KR, Chernock RD, Zhang TR, Wang X,
El-Mofty SK and Lewis JS Jr: Verrucous carcinomas of the head and
neck, including those with associated squamous cell carcinoma, lack
transcriptionally active high-risk human papillomavirus. Hum
Pathol. 44:2385–2392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Samman M, Wood H, Conway C, Berri S,
Pentenero M, Gandolfo S, Cassenti A, Cassoni P, Al Ajlan A, Barrett
AW, et al: Next-generation sequencing analysis for detecting human
papillomavirus in oral verrucous carcinoma. Oral Surg Oral Med Oral
Pathol Oral Radiol. 118:117–125.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Waskowska J, Koszowski R,
Raczkowska-Siostrzonek A and Stemplewska K: Verrucous carcinoma of
the tongue-a rare case study. Cent Eur J Med. 7:145–148. 2012.
|
|
40
|
Rodrigues J, Vaz OP, Salelkar RS, Ramani
A, Falari S and Veeresh HM: A rare case of verrucous carcinoma on
the dorsum of the tongue. Int J Adv Case Rep. 2:530–531. 2015.
|
|
41
|
Garcia NG, Oliveira DT, Hanemann JA and
Pereira AA: Oral verrucous carcinoma mimicking a chronic
candidiasis: A case report. Case Rep Oncol Med.
2012:1902722012.PubMed/NCBI
|
|
42
|
Alkan A, Bulut E, Gunhan O and Ozden B:
Oral verrucous carcinoma: A study of 12 cases. Eur J Dent.
4:202–207. 2010.PubMed/NCBI
|
|
43
|
Liu O, Zhang H, Wang Y, Quan H, Zhang J,
Zhou J, Zuo J, Tang J, Fang X, Wang W, et al: Stereology study of
oral verrucous carcinoma. J BUON. 17:343–349. 2012.PubMed/NCBI
|
|
44
|
Karagozoglu KH, Buter J, Leemans CR,
Rietveld DH, van den Vijfeijken S and van der Waal I: Subset of
patients with verrucous carcinoma of the oral cavity benefit from
treatment with methotrexate. Br J Oral Maxillofac Surg. 50:513–518.
2012. View Article : Google Scholar
|
|
45
|
Terada T: Multiple verrucous carcinomas of
the oral cavity. J Maxillofac Oral Surg. 14(Suppl 1): 393–396.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Angadi VC and Angadi PV: GLUT-1
immunoexpression in oral epithelial dysplasia, oral squamous cell
carcinoma, and verrucous carcinoma. J Oral Sci. 57:115–122. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mallick S, Breta M, Gupta SD, Dinda AK,
Mohanty BK and Singh MK: Angiogenesis, proliferative activity and
DNA ploidy in oral verrucous carcinoma: A comparative study
including verrucous hyperplasia and squamous cell carcinoma. Pathol
Oncol Res. 21:1249–1257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Samman M, Wood HM, Conway C, Stead L, Daly
C, Chalkley R, Berri S, Senguven B, Ross L, Egan P, et al: A novel
genomic signature reclassifies an oral cancer subtype. Int J
Cancer. 137:2364–2373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang YH, Tian X, Liu OS, Fang XD, Quan HZ,
Xie S, Gao S and Tang ZG: Gene profiling analysis for patients with
oral verrucous carcinoma and oral squamous cell carcinoma. Int J
Clin Exp Med. 7:1845–1852. 2014.PubMed/NCBI
|
|
50
|
Burgio E and Migliore L: Towards a
systemic paradigm in carcinogenesis: Linking epigenetics and
genetics. Mol Biol Rep. 42:777–790. 2015. View Article : Google Scholar
|
|
51
|
Patil GB, Hallikeri KS, Balappanavar AY,
Hongal SG, Sanjaya PR and Sagari SG: Cyclin B1 overexpression in
conventional oral squamous cell carcinoma and verrucous carcinoma-
A correlation with clinicopathological features. Med Oral Patol
Oral Cir Bucal. 18:e585–e590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Angadi PV and Krishnapillai R: Cyclin D1
expression in oral squamous cell carcinoma and verrucous carcinoma:
Correlation with histological differentiation. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 103:e30–e35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sakurai K, Urade M, Takahashi Y, Kishimoto
H, Noguchi K, Yasoshima H and Kubota A: Increased expression of
c-erbB-3 protein and proliferating cell nuclear antigen during
development of verrucous carcinoma of the oral mucosa. Cancer.
89:2597–2605. 2000. View Article : Google Scholar
|
|
54
|
Terada T: Squamous cell carcinoma arising
within verrucous carcinoma of the oral cavity: A case report. Int J
Clin Exp Pathol. 5:363–366. 2012.PubMed/NCBI
|
|
55
|
Quan H, Tang Z, Zhao L, Wang Y, Liu O, Yao
Z and Zuo J: Expression of αB-crystallin and its potential
anti-apoptotic role in oral verrucous carcinoma. Oncol Lett.
3:330–334. 2012.PubMed/NCBI
|
|
56
|
Wang RQ and Tang ZG: Expression of Skp2
and p27 in oral verrucous carcinoma. J Oral Sci Res. 30:230–234.
2014.
|
|
57
|
Mohtasham N, Babakoohi S, Shiva A, Shadman
A, Kamyab-Hesari K, Shakeri MT and Sharifi-Sistani N:
Immunhistochemical study of p53, Ki-67, MMP-2 and MMP-9 expression
at invasive front of squamous cell and verrucous carcinoma in oral
cavity. Pathol Res Pract. 209:110–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Odar K, Kocjan BJ, Hošnjak L, Gale N,
Poljak M and Zidar N: Verrucous carcinoma of the head and neck -
not a human papillomavirus-related tumour? J Cell Mol Med.
18:635–645. 2014. View Article : Google Scholar
|
|
59
|
Saito T, Nakajima T and Mogi K:
Immunohistochemical analysis of cell cycle-associated proteins p16,
pRb, p53, p27 and Ki-67 in oral cancer and precancer with special
reference to verrucous carcinomas. J Oral Pathol Med. 28:226–232.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin HP, Wang YP and Chiang CP: Expression
of p53, MDM2, p21, heat shock protein 70, and HPV 16/18 E6 proteins
in oral verrucous carcinoma and oral verrucous hyperplasia. Head
Neck. 33:334–340. 2011.
|
|
61
|
Odar K, Boštjančič E, Gale N, Glavač D and
Zidar N: Differential expression of microRNAs miR-21, miR-31,
miR-203, miR-125a-5p and miR-125b and proteins PTEN and p63 in
verrucous carcinoma of the head and neck. Histopathology.
61:257–265. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ray JG, Mukherjee S, Pattanayak Mohanty S
and Chaudhuri K: Oral verrucous carcinoma - a misnomer?
Immunohistochemistry based comparative study of two cases. BMJ Case
Rep. 2011.bcr11201034792011.
|
|
63
|
Chen YK, Hsuen SS and Lin LM: Increased
expression of inducible nitric oxide synthase for human oral
submucous fibrosis, verrucous hyperplasia, and verrucous carcinoma.
Int J Oral Maxillofac Surg. 31:419–422. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chawla H, Urs AB and Augustine J:
Association of macrophages with angiogenesis in oral epithelial
dysplasia, oral verrucous carcinoma, and oral squamous cell
carcinoma: An immunohistochemical study. Appl Immunohistochem Mol
Morphol. Dec 9–2015.(Epub ahead of print). View Article : Google Scholar
|
|
65
|
Al-Azri AR, Gibson RJ, Bowen JM, Stringer
AM, Keefe DM and Logan RM: Involvement of matrix metalloproteinases
(MMP-3 and MMP-9) in the pathogenesis of irinotecan-induced oral
mucositis. J Oral Pathol Med. 44:459–467. 2015. View Article : Google Scholar
|
|
66
|
Kerkelä E, Ala-aho R, Klemi P, Grénman S,
Shapiro SD, Kähäri VM and Saarialho-Kere U: Metalloelastase
(MMP-12) expression by tumour cells in squamous cell carcinoma of
the vulva correlates with invasiveness, while that by macrophages
predicts better outcome. J Pathol. 198:258–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ala-aho R, Grénman R, Seth P and Kähäri
VM: Adenoviral delivery of p53 gene suppresses expression of
collagenase-3 (MMP-13) in squamous carcinoma cells. Oncogene.
21:1187–1195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lawal AO, Adisa AO, Kolude B and Adeyemi
BF: Immunohistochemical expression of MMP-2 and MMP-8 in oral
squamous cell carcinoma. J Clin Exp Dent. 7:e203–e207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kierszenbaum AL and Tres L: Histology and
Cell Biology, an Introduction to Pathology. 3rd edition. Elsevier;
Philadelphia, PA: 2012
|
|
70
|
van der Zee JA, van Eijck CHJ, Hop WCJ,
Biermann K, Dicheva BM, Seynhaeve ALB, Koning GA, Eggermont AM and
Ten Hagen TL: Tumour basement membrane laminin expression predicts
outcome following curative resection of pancreatic head cancer. Br
J Cancer. 107:1153–1158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Impola U, Uitto VJ, Hietanen J, Hakkinen
L, Zhang L, Larjava H, Isaka K and Saarialho-Kere U: Differential
expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and
metallo-elastase (MMP-12) in oral verrucous and squamous cell
cancer. J Pathol. 202:14–22. 2004. View Article : Google Scholar
|
|
72
|
Kadeh H, Saravani S, Heydari F, Keikha M
and Rigi V: Expression of matrix metalloproteinase-10 at invasive
front of squamous cell carcinoma and verrucous carcinoma in the
oral cavity. Asian Pac J Cancer Prev. 16:6609–6613. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Arduino PG, Carrozzo M, Pagano M,
Broccoletti R, Scully C and Gandolfo S: Immunohistochemical
expression of basement membrane proteins of verrucous carcinoma of
the oral mucosa. Clin Oral Investig. 14:297–302. 2010. View Article : Google Scholar
|
|
74
|
Kobayashi H, Sagara J, Masumoto J, Kurita
H, Kurashina K and Taniguchi S: Shifts in cellular localization of
moesin in normal oral epithelium, oral epithelial dysplasia,
verrucous carcinoma and oral squamous cell carcinoma. J Oral Pathol
Med. 32:344–349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zargaran M, Eshghyar N, Vaziri PB and
Mortazavi H: Immunohistochemical evaluation of type IV collagen and
laminin-332 γ2 chain expression in well-differentiated oral
squamous cell carcinoma and oral verrucous carcinoma: A new
recommended cut-off. J Oral Pathol Med. 40:167–173. 2011.
View Article : Google Scholar
|
|
76
|
Kolokythas A, Rogers TM and Miloro M:
Hybrid verrucous squamous carcinoma of the oral cavity: Treatment
considerations based on a critical review of the literature. J Oral
Maxillofac Surg. 68:2320–2324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ho PS, Chen PL, Warnakulasuriya S, Shieh
TY, Chen YK and Huang IY: Malignant transformation of oral
potentially malignant disorders in males: A retrospective cohort
study. BMC Cancer. 9:260–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hsue SS, Wang WC, Chen CH, Lin CC, Chen YK
and Lin LM: Malignant transformation in 1458 patients with
potentially malignant oral mucosal disorders: A follow-up study
based in a Taiwanese hospital. J Oral Pathol Med. 36:25–29. 2007.
View Article : Google Scholar
|
|
79
|
Paral KM, Taxy JB and Lingen MW: CD34 and
α smooth muscle actin distinguish verrucous hyperplasia from
verrucous carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol.
117:477–482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Habiba U, Kitamura T, Yanagawa-Matsuda A,
Hida K, Higashino F, Ohiro Y, Totsuka Y and Shindoh M: Cytoplasmic
expression of HuR may be a valuable diagnostic tool for determining
the potential for malignant transformation of oral verrucous
borderline lesions. Oncol Rep. 31:1547–1554. 2014.PubMed/NCBI
|
|
81
|
Alan H, Agacayak S, Kavak G and Ozcan A:
Verrucous carcinoma and squamous cell papilloma of the oral cavity:
Report of two cases and review of literature. Eur J Dent.
9:453–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Oliveira MC, Silveira EJ, Godoy GP, Amorim
RF, Costa AL and Queiroz LM: Immunohistochemical evaluation of
intermediate filament proteins in squamous papilloma and oral
verrucous carcinoma. Oral Dis. 11:288–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Klieb HBE and Raphael SJ: Comparative
study of the expression of p53, Ki67, E-cadherin and MMP-1 in
verrucous hyperplasia and verrucous carcinoma of the oral cavity.
Head Neck Pathol. 1:118–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pentenero M, Donadini A, Di Nallo E,
Maffei M, Marino R, Familiari U, Broccoletti R, Castagnola P,
Gandolfo S and Giaretti W: Distinctive chromosomal instability
patterns in oral verrucous and squamous cell carcinomas detected by
high-resolution DNA flow cytometry. Cancer. 117:5052–5057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gokavarapu S, Rao SLM, Tantravahi US,
Gundimeda SD, Rao TS and Murthy S: Oral hybrid verrucous carcinoma:
A clinical study. Indian J Surg Oncol. 5:257–262. 2014. View Article : Google Scholar
|
|
86
|
Gokavarapu S, Chandrasekhara Rao LM,
Patnaik SC, Parvataneni N, Raju KV, Chander R and Kumar KA:
Reliability of incision biopsy for diagnosis of oral verrucous
carcinoma: A multivariate clinicopathological study. J Maxillofac
Oral Surg. 14:599–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Verma DK, Bansal S, Gupta D and Bansal A:
Neck dissection in verrucous carcinoma: A surgical dilemma. IJSS
Case Rep Rev. 1:42–45. 2015.
|
|
88
|
Sadasivan A, Thankappan K, Rajapurkar M,
Shetty S, Sreehari S and Iyer S: Verrucous lesions of the oral
cavity treated with surgery: Analysis of clinico-pathologic
features and outcome. Contemp Clin Dent. 3:60–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sciubba JJ and Helman JI: Current
management strategies for verrucous hyperkeratosis and verrucous
carcinoma. Oral Maxillofac Surg Clin North Am. 25:77–82. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Candau-Alvarez A, Dean-Ferrer A,
Alamillos-Granados FJ, Heredero-Jung S, García-García B,
Ruiz-Masera JJ, Arévalo-Arévalo R, Zafra-Camacho F and
Valenzuela-Salas B: Verrucous carcinoma of the oral mucosa: An
epidemiological and follow-up study of patients treated with
surgery in 5 last years. Med Oral Patol Oral Cir Bucal.
19:e506–e511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Walvekar RR, Chaukar DA, Deshpande MS, Pai
PS, Chaturvedi P, Kakade A, Kane SV and D'Cruz AK: Verrucous
carcinoma of the oral cavity: A clinical and pathological study of
101 cases. Oral Oncol. 45:47–51. 2009. View Article : Google Scholar
|
|
92
|
Kang CJ, Chang JTC, Chen TM, Chen IH and
Liao CT: Surgical treatment of oral verrucous carcinoma. Chang Gung
Med J. 26:807–812. 2003.
|
|
93
|
Huang TT, Hsu LP, Hsu YH and Chen PR:
Surgical outcome in patients with oral verrucous carcinoma:
Long-term follow-up in an endemic betel quid chewing area. ORL J
Otorhinolaryngol Relat Spec. 71:323–328. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Wang Z, Li DX, Liu MD, Zhong M and Sun CF:
Eighty-six cases of oral verrucous carcinomaclinical and
pathological analysis. Chin J Pract Stomatol. 7:367–369. 2014.
|
|
95
|
Proffitt SD, Spooner TR and Kosek JC:
Origin of undifferentiated neoplasm from verrucous epidermal
carcinoma of oral cavity following irradiation. Cancer. 26:389–393.
1970. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ferlito A, Rinaldo A and Mannarà GM: Is
primary radiotherapy an appropriate option for the treatment of
verrucous carcinoma of the head and neck? J Laryngol Otol.
112:132–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Vidyasagar MS, Fernandes DJ, Kasturi DP,
Akhileshwaran R, Rao K, Rao S, Rao RV and Solomon JG: Radiotherapy
and verrucous carcinoma of the oral cavity. A study of 107 cases
Acta Oncol. 31:43–47. 1992. View Article : Google Scholar
|
|
98
|
Jyothirmayi R, Sankaranarayanan R,
Varghese C, Jacob R and Nair MK: Radiotherapy in the treatment of
verrucous carcinoma of the oral cavity. Oral Oncol. 33:124–128.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
van Gestel KM, Buurman DJ, Pijls R,
Kessler PA, van den Ende PL, Hoffmann AL and Troost EG: Locally
advanced verrucous carcinoma of the oral cavity: Treatment using
customized mold HDR brachytherapy instead of hemi-maxillectomy.
Strahlenther Onkol. 189:894–898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tanaka J, Yoshida K, Takahashi M and
Suzuki M: A case of verrucous carcinoma of the tongue, effectively
treated with preoperative chemotherapy (UFT, CDDP, PEP) and
irradiation. Gan To Kagaku Ryoho. 19:525–527. 1992.(In Japanese).
PubMed/NCBI
|
|
101
|
Galmarini FC, Galmarini CM, Sarchi MI,
Abulafia J and Galmarini D: Heterogeneous distribution of tumor
blood supply affects the response to chemotherapy in patients with
head and neck cancer. Microcirculation. 7:405–410. 2000. View Article : Google Scholar
|
|
102
|
World Health Organization. WHO Handbook
for reporting results of cancer treatment. World Health
Organization; Geneva: 1979
|
|
103
|
Salesiotis A, Soong R, Diasio RB, Frost A
and Cullen KJ: Capecitabine induces rapid, sustained response in
two patients with extensive oral verrucous carcinoma. Clin Cancer
Res. 9:580–585. 2003.PubMed/NCBI
|
|
104
|
Wu CF, Chen CM, Shen YS, Huang IY, Chen
CH, Chen CY, Shieh TY and Sheen MC: Effective eradication of oral
verrucous carcinoma with continuous intraarterial infusion
chemotherapy. Head Neck. 30:611–617. 2008. View Article : Google Scholar
|
|
105
|
Sheen MC, Sheu HM, Lai FJ, Lin SD, Wu CF,
Wang YW and Lan CC: A huge verrucous carcinoma of the lower lip
treated with intra-arterial infusion of methotrexate. Br J
Dermatol. 151:727–729. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Strojan P, Soba E, Budihna M and Auersperg
M: Radio-chemotherapy with Vinblastine, Methotrexate, and Bleomycin
in the treatment of verrucous carcinoma of the head and neck. J
Surg Oncol. 92:278–283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yoshimura Y, Mishima K, Obara S, Nariai Y,
Yoshimura H and Mikami T: Treatment modalities for oral verrucous
carcinomas and their outcomes: Contribution of radiotherapy and
chemotherapy. Int J Clin Oncol. 6:192–200. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ogawa A, Fukuta Y, Nakajima T, Kanno SM,
Obara A, Nakamura K, Mizuki H, Takeda Y and Satoh M: Treatment
results of oral verrucous carcinoma and its biological behavior.
Oral Oncol. 40:793–797. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Kawczyk-Krupka A, Waśkowska J,
Raczkowska-Siostrzonek A, Kościarz-Grzesiok A, Kwiatek S, Straszak
D, Latos W, Koszowski R and Sieroń A: Comparison of cryotherapy and
photodynamic therapy in treatment of oral leukoplakia. Photodiagn
Photodyn Ther. 9:148–155. 2012. View Article : Google Scholar
|
|
110
|
Yu CH, Lin HP, Cheng SJ, Sun A and Chen
HM: Cryotherapy for oral precancers and cancers. J Formos Med
Assoc. 113:272–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Lin HP, Chen HM, Cheng SJ, Yu CH and
Chiang CP: Cryogun cryotherapy for oral leukoplakia. Head Neck.
34:1306–1311. 2012. View Article : Google Scholar
|
|
112
|
Yeh CJ: Treatment of verrucous hyperplasia
and verrucous carcinoma by shave excision and simple cryosurgery.
Int J Oral Maxillofac Surg. 32:280–283. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kennedy JC, Pottier RH and Pross DC:
Photodynamic therapy with endogenous protoporphyrin IX: Basic
principles and present clinical experience. J Photochem Photobiol
B. 6:143–148. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lin HP, Chen HM, Yu CH, Yang H, Wang YP
and Chiang CP: Topical photodynamic therapy is very effective for
oral verrucous hyperplasia and oral erythroleukoplakia. J Oral
Pathol Med. 39:624–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Chen HM, Chen CT, Yang H, Lee MI, Kuo MYP,
Kuo YS, Wang YP, Tsai T and Chiang CP: Successful treatment of an
extensive verrucous carcinoma with topical 5-aminolevulinic
acid-mediated photodynamic therapy. J Oral Pathol Med. 34:253–256.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Galimberti D, Galimberti G, Pontón Montaño
A, Jácome LR and Galimberti R: Oral verrucous carcinoma treated
with carbon dioxide laser. J Eur Acad Dermatol Venereol.
24:976–977. 2010. View Article : Google Scholar
|
|
117
|
Azevedo LH, Galletta VC, de Paula Eduardo
C, de Sousa SO and Migliari DA: Treatment of oral verrucous
carcinoma with carbon dioxide laser. J Oral Maxillofac Surg.
65:2361–2366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
van Houten VM, Leemans CR, Kummer JA,
Dijkstra J, Kuik DJ, van den Brekel MW, Snow GB and Brakenhoff RH:
Molecular diagnosis of surgical margins and local recurrence in
head and neck cancer patients: A prospective study. Clin Cancer
Res. 10:3614–3620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Patton LL, Epstein JB and Kerr AR:
Adjunctive techniques for oral cancer examination and lesion
diagnosis: A systematic review of the literature. J Am Dent Assoc.
139:896–905. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lingen MW, Kalmar JR, Karrison T and
Speight PM: Critical evaluation of diagnostic aids for the
detection of oral cancer. Oral Oncol. 44:10–22. 2008. View Article : Google Scholar :
|
|
121
|
Carlile J, Harada K, Baillie R, Macluskey
M, Chisholm DM, Ogden GR, Schor SL and Schor AM: Vascular
endothelial growth factor (VEGF) expression in oral tissues:
Possible relevance to angiogenesis, tumour progression and field
cancerisation. J Oral Pathol Med. 30:449–457. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tae K, El-Naggar AK, Yoo E, Feng L, Lee
JJ, Hong WK, Hittelman WN and Shin DM: Expression of vascular
endothelial growth factor and microvessel density in head and neck
tumorigenesis. Clin Cancer Res. 6:2821–2828. 2000.PubMed/NCBI
|
|
123
|
Gleich LL, Zimmerman N, Wang YO and
Gluckman JL: Angiogenic inhibition for the treatment of head and
neck cancer. Anticancer Res. 18:2607–2609. 1998.PubMed/NCBI
|
|
124
|
Peng Q, Tang Z, Liu O and Peng Z: Rapid
prototyping-assisted maxillofacial reconstruction. Ann Med.
47:186–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Saunders RE and Derby B: Inkjet printing
biomaterials for tissue engineering: Bioprinting. Int Mater Rev.
59:430–448. 2014. View Article : Google Scholar
|
|
126
|
Maire E and Withers PJ: Quantitative X-ray
tomography. Int Mater Rev. 59:1–43. 2014. View Article : Google Scholar
|
|
127
|
Germershaus O, Lühmann T, Rybak J-C,
Ritzer J and Meinel L: Application of natural and semi-synthetic
polymers for the delivery of sensitive drugs. Int Mater Rev.
60:101–131. 2015. View Article : Google Scholar
|
|
128
|
Konopka K and Goslinski T: Photodynamic
therapy in dentistry. J Dent Res. 86:694–707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xi S and Grandis JR: Gene therapy for the
treatment of oral squamous cell carcinoma. J Dent Res. 82:11–16.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Rao RS, Patil S, Ghosh S and Kumari K:
Current aspects and future strategies in oral cancer research: A
review. J Med Radiol Pathol Surg. 1:8–13. 2015.
|