Introduction
Colorectal cancer (CRC) is the third most lethal
malignancy in the world, and the major cause of death of CRC
patients is metastasis (1).
Furthermore, it is known that the potential for a tumor cell to
metastasize depends on its interactions with the homeostatic
factors that promote tumor-cell growth, survival, angiogenesis,
invasion and metastasis (2).
During tumor metastasis, two key events are invasion and metastatic
colonization. Both of these events occur because of interactions
between the cancer cells and the stromal microenvironment. Tumor
cell invasion of the surrounding extracellular matrix (ECM), which
involves migration and intravasation, is the early stage of
metastasis (3). It is known that
ECM degradation is an important stage of tumor metastasis that is
regulated by matrix metalloproteinases (MMPs) (4). Among the MMP family members, MMP-2
and MMP-9 contribute to the degradation of the ECM and play
important roles in cancer cell migration and invasion (5). In addition, vascular endothelial
(VE)-cadherin is an important molecule that modulates cell-cell and
cell-ECM contact permeability and extravasation (6). Moreover, when cancer cells leave the
original tumor organ and enter the blood or lymphatic circulation
via intravasation, the cells will migrate to and invade a
metastatic target organ, which is referred to as extravasation.
During this process, adhesion molecules (e.g., E-selectin and
ICAM-1) play a key role in regulating the adhesion of tumor cells
to endothelial cells (2,7). Inhibiting cell migration, invasion
and adhesion may be an effective strategy for improving the
prognosis of CRC.
The transcription factor NF-κB plays an important
role in cell metastasis and invasion signaling pathways (8). Several genes that are known to be
involved in tumor metastasis, such as the genes encoding MMP-2,
MMP-9, E-selectin, ICAM-1, and VE-cadherin, are regulated by NF-κB.
All of these factors are downstream targets of the NF-κB signaling
pathway. When NF-κB is abnormally overexpressed, these
metastasis-relevant genes may be activated, leading to the
initiation of cancer cell metastasis.
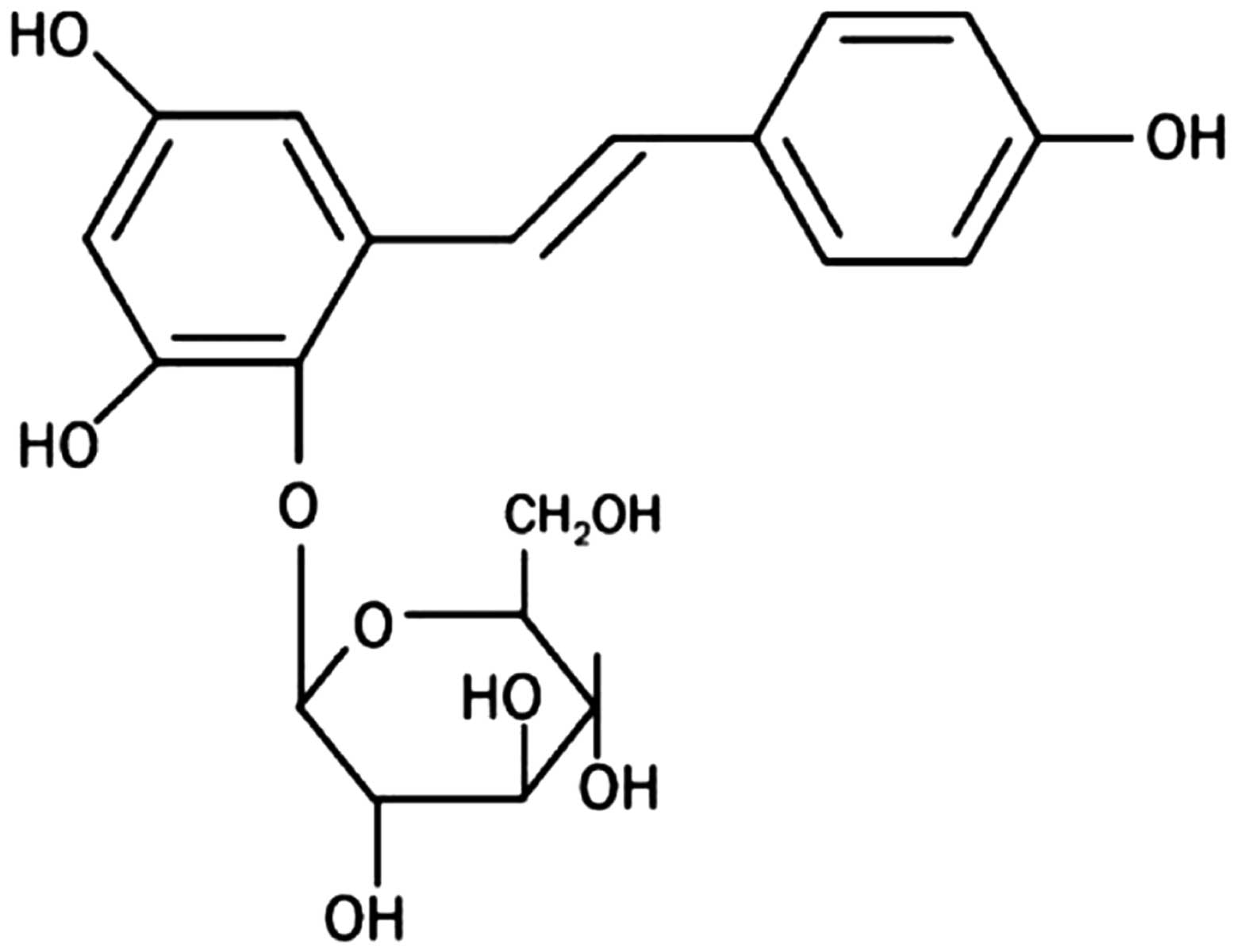
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucopyranoside (THSG) is one
of the most abundant components of Polygonum multiflorum
Thunb (He-Shou-Wu), a traditional Chinese medicine and medicinal
food component (9). Many
physiological studies demonstrated that THSG has various protective
effects against acute and chronic colitis, neurodegenerative
disorders and atherosclerosis (10–12).
However, cellular studies to investigate the effects of THSG on
anticancer and antimetastasis are still limited. THSG belongs to
stilbenoids with a hydroxystilbene structure (Fig. 1) (9). The resveratrol, piceatannol and
pterstilbene types of stilbenoids can inhibit metastasis through
suppressing migration and invasion in vivo and in
vitro (10–12). These stilbenoids all are
hydroxylated derivatives of stilbene and have a C6-C2-C6 structure.
Additionally, THSG has a specific glycoside structure (9). A previous study showed that THSG has
antitumor and antimetastatic activity via inhibiting DNA synthesis
in Lewis lung carcinoma tumors and human umbilical vein endothelial
cells (13). Since THSG is
biologically active, more cellular studies to clarify the potential
effects of THSG on anticancer and anti-metastasis are worth further
investigation.
The aims of this study were to determine whether
THSG exerts anti-metastatic effects and whether this effect on
human CRC cells involves the regulation of cell migration, invasion
or adhesion. Wounding healing assays, cell invasion assays, and
measurements of the activity and protein expression of MMPs such as
MMP-2 and MMP-9 were analyzed in previous studies to examine the
migration and invasion ability (14–17);
these techniques were also employed in this study. In addition,
cell adhesion capacity, the levels of adhesion molecules such as
ICAM-1 and E-selectin, and transepithelial electrical resistance
(TEER) were measured to evaluate cell adhesion and invasion
abilities in this study. In addition, whether THSG regulates the
expression of metastasis-associated molecules including MMP-2,
MMP-9, E-selectin and ICAM by altering NF-κB activation was
analyzed in this study. These experiments helped to elucidate how
THSG regulates the migration and invasion of human CRC cells and
the molecular signal transduction pathways involved in this
process.
Materials and methods
Chemicals and reagents
THSG (HPLC purity 95%) was purchased from Zhongxin
Pharmaceuticals (Tianjin, China). Antisera against E-selectin,
ICAM-1 phosphorylated (p-) VE-cadherin, p-IκB and NF-κB were
purchased from Abcam (Cambridge, MA, USA). Antisera against MMP-2
and MMP-9 were obtained from GeneTex, Inc. (San Antonio, TX, USA).
2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF) was
purchased from Life Technologies GmbH (Darmstadt, Germany).
Cell cultures and treatment
In this study, the HT-29 human colorectal carcinoma
cell line was used as a model of CRC metastasis (18), and EA.hy926 human endothelial cells
were used as an experimental model of invasion and adhesion
(19). HT-29 cells and EA.hy926
cells were purchased from the Bioresource Collection and Research
Center (Hsinchu, Taiwan). The HT-29 and EA.hy926 cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum and 1% penicillin/streptomycin at 37°C in a humidified
atmosphere containing 5% CO2.
In our pre-tests, various THSG concentrations
ranging from 10 μM to 50 mM were applied for 24 or 48 h for cell
viability analysis. The pre-test results showed that 1, 5 or 10 mM
THSG treatment significantly decreased the levels of both MMP-2 and
E-cadherin in HT-29 cells without reducing cell viability (data not
shown). Thus, 1, 5 and 10 mM THSG were predominantly used in our
further experiments. After plating and incubating the cells for 24
h, the cells were treated with THSG diluted in dimethyl sulfoxide
(DMSO) for various biochemical analyses. Cells treated with DMSO
alone were used as a control group.
Cell viability analysis
Cell viability was evaluated using the
3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
reduction assay as described by Debizot and Lang (20). HT-29 cells were incubated in 3-cm
plates (1×106 cells) for 24 h and treated with 1, 5, 10
or 50 mM THSG for 24, 48 or 72 h. Then, MTT (5 mg/ml) dye was
added, and the optical density (OD) was measured at 570 nm.
Wound healing assay
To investigate the effects of THSG on the migration
ability of human colorectal carcinoma cells, a wound healing assay
was conducted. The protocol for the wound-healing assay was a
modified version of a protocol described by Ang et al
(21). HT-29 cells were cultured
on 3-cm plates (1×106 cells) for 24 h, and a
micropipette tip was used to make a uniform scratch in the center
of the well. The cells were then washed with PBS. Various
concentrations of THSG (1, 5 or 10 mM) were added to the respective
wells for the indicated duration (24, 48 or 72 h), and the
morphology of HT-29 cells was observed under an inverted
fluorescence microscope (Olympus IX51 Microscope, Olympus Optical
Co. Ltd., Tokyo, Japan). The width of the wound area was measured
using ImageJ software to determine the cell migration distance. The
wound closure rate for a given treatment period was calculated as
follows: % wound area closure = (wound area of control group-wound
area of treatment group)/wound area of control group × 100. The
migration ability of HT-29 cells is suppressed when the wound
closure rate is lower.
Invasion assay
The invasion assay is a method for assessing
intravasation and extravasation abilities. A Matrigel solution (50
μl) was added to the wells of a 24-well Transwell plate that was
incubated at 37°C for 30 min. Next, HT-29 cells were resuspended in
serum-free RPMI-1640 medium (5×104 cells/3-cm plate) in
the absence or presence of THSG (1, 5 or 10 mM) in the upper
chamber. RPMI-1640 medium (500 μl) containing 10% FBS was added to
the lower chamber. After incubation for 24 h, the invading cells
that had migrated to the lower surface of the filter membrane were
stained with 0.2% crystal violet for 15 min. The number of invading
cells on the lower surface of the filter membrane was counted under
an inverted fluorescence microscope (Olympus IX51 Microscope,
Olympus Optical Co. Ltd.) using NIH ImageJ software (22).
TEER measurement
TEER is a quantitative measurement of the barrier
integrity of a monolayer (23).
For the TEER measurement, EA.hy926 cells (5×104
cells/well) were cultured for 24 h in RPMI-1640 medium containing
1, 5 or 10 mM THSG. The TEER values were obtained by subtracting
the TEER of the cell culture dish groove from the TEER in the
presence of a cell layer. These measurements were recorded using a
Millicell-ERS voltohmmeter (Millipore Continental Water Systems,
Bedford, MA, USA).
Adhesion assay
The adhesion assay protocol was a modified version
of a protocol described by Braut-Boucher et al (24). EA.hy926 cells (1×106
cells) were cultured on 3-cm plates and exposed to 1, 5 or 10 mM
THSG for 6 h. The EA.hy926 cells were then washed in PBS and
co-cultured with HT-29 cells labeled with 10 μM BCECF for 1 h.
After washing in PBS, the morphology of BCECF-stained HT-29 cells
was evaluated under an inverted fluorescence microscope (Olympus
IX51 Microscope, Olympus Optical Co. Ltd.). Furthermore, the
BCECF-stained cells were collected and measured fluorometrically
using an ELISA reader at an OD of 580 nm.
Analysis of the expression of proteins
regulating cell migration, adhesion, and invasion and NF-κB
activation
Approximately 5×105 HT-29 cells/3-cm
plate, which were used for the MMP-2, MMP-9, p-VE-cadherin, p-IκB,
IκB, and cytoplasmic and nuclear NF-κB expression analyses or
5×105 EA.hy926 cells/3-cm plate, which were used for the
E-selectin, ICAM-1, p-IκB, and cytoplasmic and nuclear NF-κB
expression analyses, were incubated in a 12-well plate in the
presence of 0, 1, 5 or 10 mM THSG for 24 h.
The cells were washed twice with cold PBS and then
harvested in 200 μl of lysis buffer containing 10 mM Tris-HCl, 5 mM
EDTA, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), and 20 μg/ml
aprotinin at pH 7.4. The cellular protein levels were determined
using the method described by Lowry et al (25). For each sample, 10–20 mg of
cellular proteins were applied to 10% sodium dodecyl sulfate (SDS)
polyacrylamide gels (26). After
electrophoresis, the proteins that had separated on the gels were
transferred to polyvinylidene difluoride membranes (27). The membranes were then incubated
with anti-MMP-2, anti-MMP-9, anti-E-selectin, anti-ICAM-1,
anti-p-VE-cadherin, anti-p-IκB, anti-IκB or anti-NF-κB antibodies
at 37°C for 1 h, followed by incubation with a
peroxidase-conjugated secondary antibody. The bands were visualized
using hydrogen peroxide/diaminobenzidine tetrahydrochloride or an
enhanced chemiluminescence detection kit (Amersham Life Science,
Buckinghamshire, UK); then, the band densities were quantified
using an AlphaImager 2000 imaging system (Alpha Innotech, San
Leandro, CA, USA). The protein level of the control group was
regarded as 100%.
NF-κB DNA-binding activity assay
Nuclear extracts were obtained from cell pellets
using an NE-PER extraction kit (Thermo Scientific, Rockford, IL,
USA) according to the manufacturer's instructions. The NF-κB
DNA-binding activity of the nuclear fraction was determined using
an NF-κB (p65) transcription factor activity assay kit (Cayman
Chemical Co., Ann Arbor, MI, USA) according to the manufacturer's
instuctions.
Statistical analysis
The data were analyzed using the statistical
analysis software SPSS for Windows, version 20.0 (SPSS Inc.,
Chicago, IL, USA). One-way analysis of variance (ANOVA) and
Duncan's multiple range tests were used to evaluate the
significance of the differences between two mean values. A p-value
<0.05 was considered to be statistically significant.
Results
THSG suppresses the migration of human
colorectal carcinoma cells
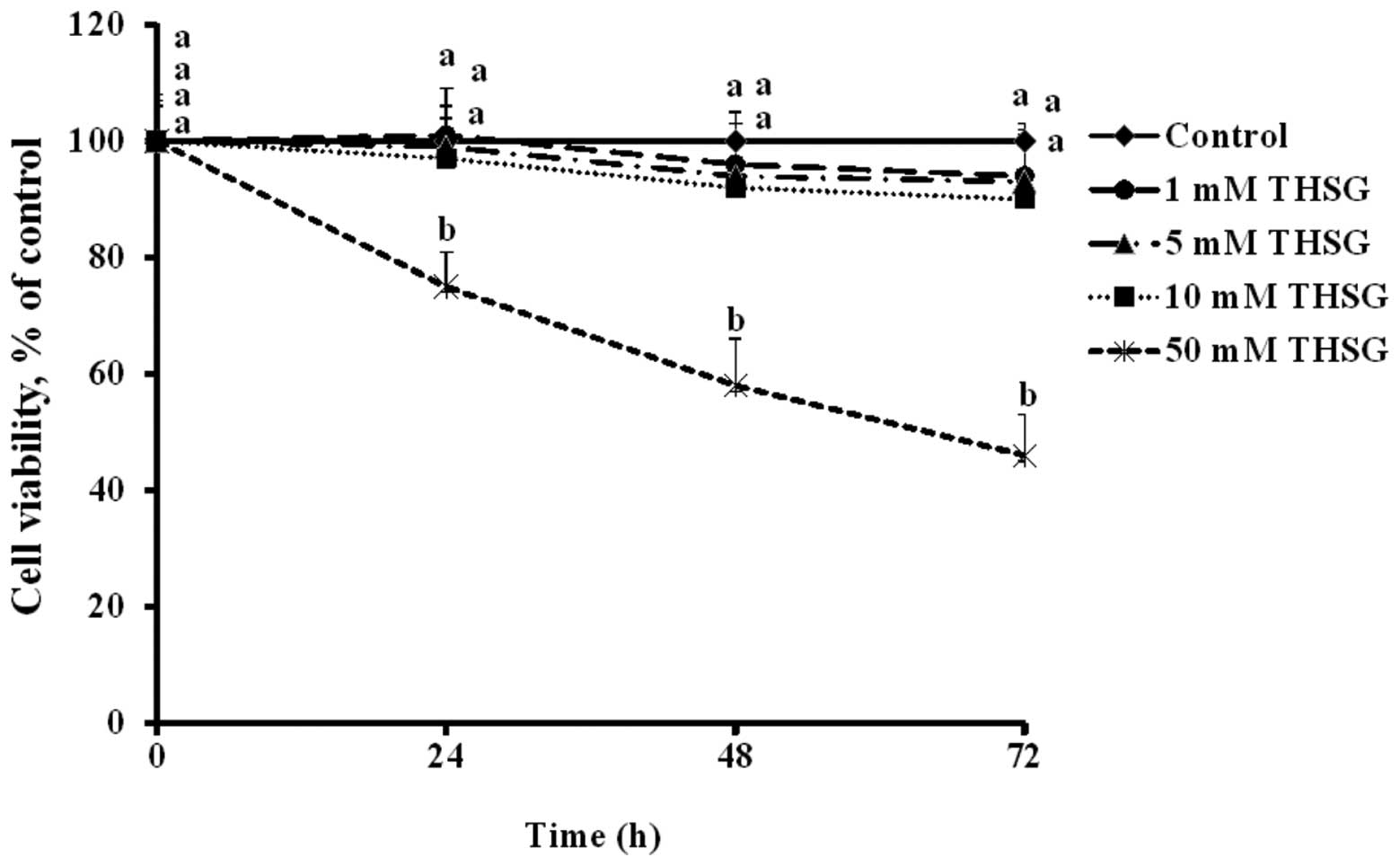
The cell viability of HT-29 cells treated with 1, 5
or 10 mM THSG did not significantly differ from the control cells
during the 72-h incubation period. However, the viability of the
cells treated with 50 mM THSG for 24, 48 or 72 h was significantly
lower than that of the controls (p<0.05) (Fig. 2).
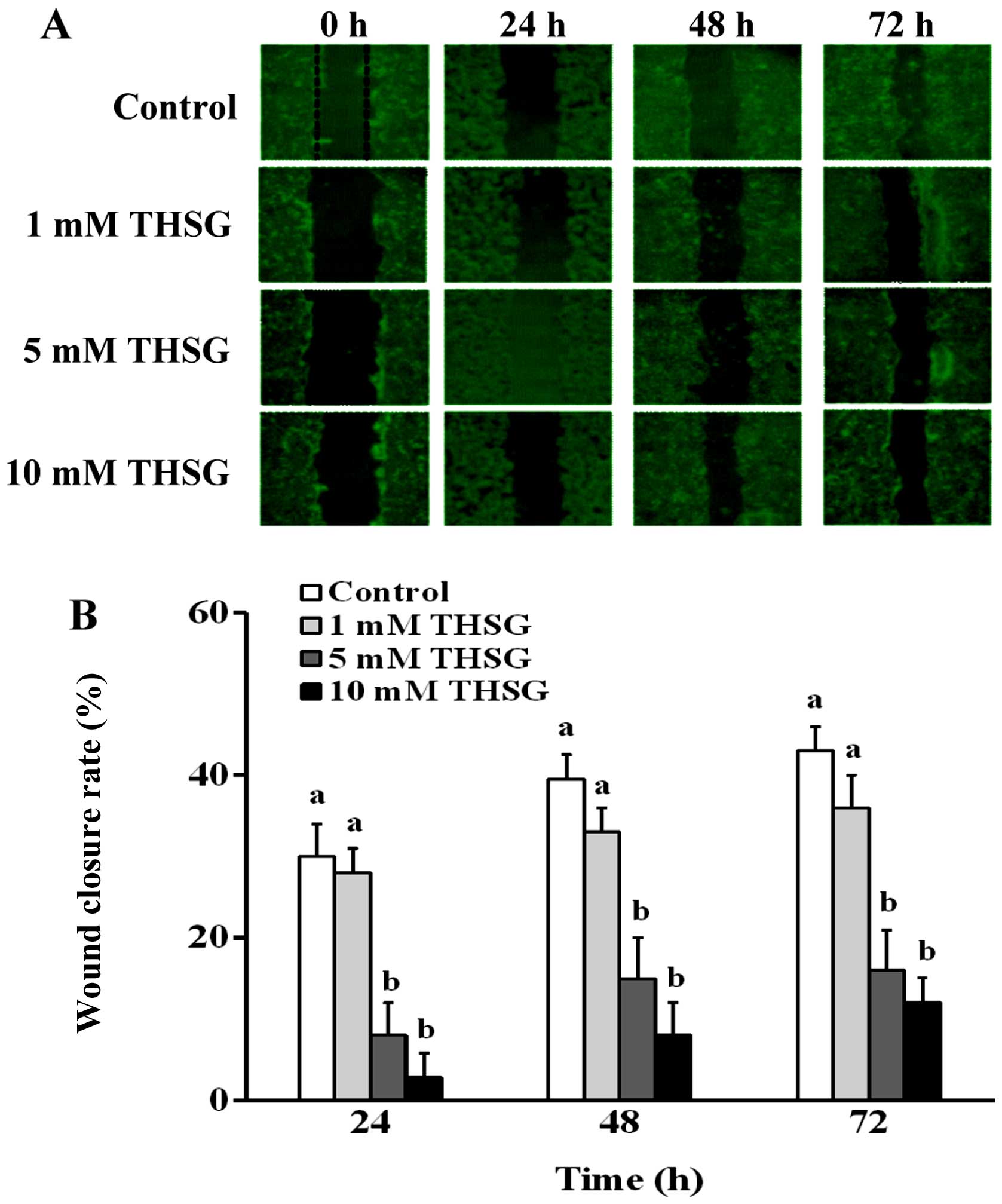
To determine whether the migration of HT-29 cells is
suppressed by THSG, a monolayer wound healing assay was conducted.
As shown in Fig. 3A, after the
HT-29 cells were incubated in various concentrations of THSG for
24, 48 or 72 h, the wound healing areas varied. When HT-29 cells
were treated with 5 or 10 mM THSG for 24, 48 or 72 h, their
migration was inhibited by 22–27 and 27–31%, respectively (Fig. 3B). These results indicated that
THSG significantly decreased HT-29 cell migration.
THSG reduces the invasiveness of human
colorectal carcinoma cells and their passage across human
endothelial cells
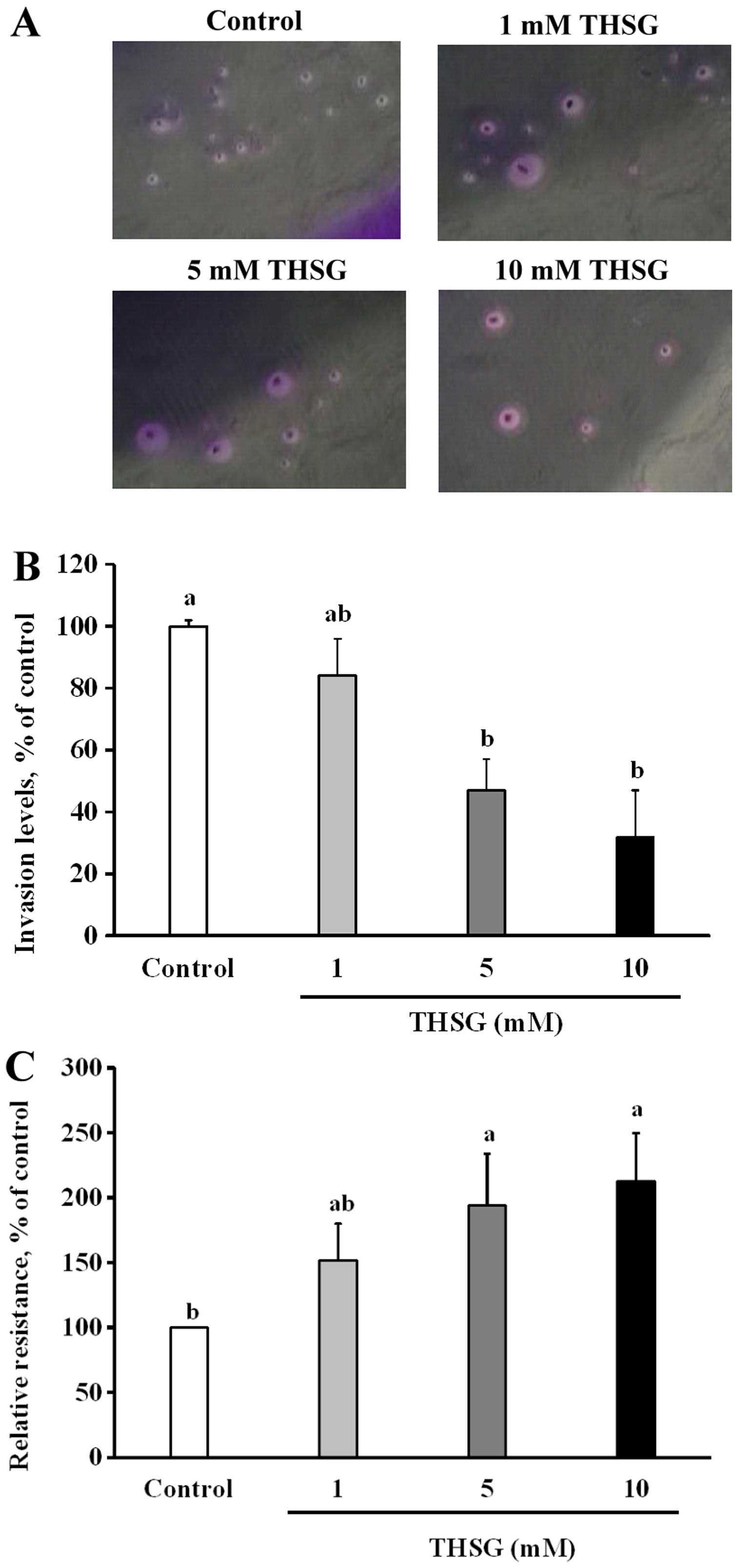
The results of a versatile Transwell Matrigel
invasion assay indicated that when HT-29 cells were incubated in
various concentrations of THSG for 24 h, the percentages of
invading cells were lower than those of the control cells (Fig. 4A and B). The relative abundances of
invading HT-29 cells were 84±12, 47±17 and 32±15% of the control
levels after treatment for 24 h with 1, 5 and 10 mM THSG,
respectively. Cell invasion was significantly suppressed in all of
the THSG-treated groups compared with the control group (100%)
(p<0.05). This result shows that THSG may reduce inter-colon
cell migration and invasion by HT-29 cells.
The effect of THSG on the TEER of EA.hy926 cells was
also analyzed. As shown in Fig.
4C, treatment of EA.hy926 cells with 5 or 10 mM THSG
significantly increased the TEER values to 194±40 and 213±37% of
the control levels (100%), respectively (p<0.05). This result
shows that THSG may increase the inter-epithelial cell TEER, thus
decreasing EA.hy926 cell-cell permeability, intravasation, and
extravasation.
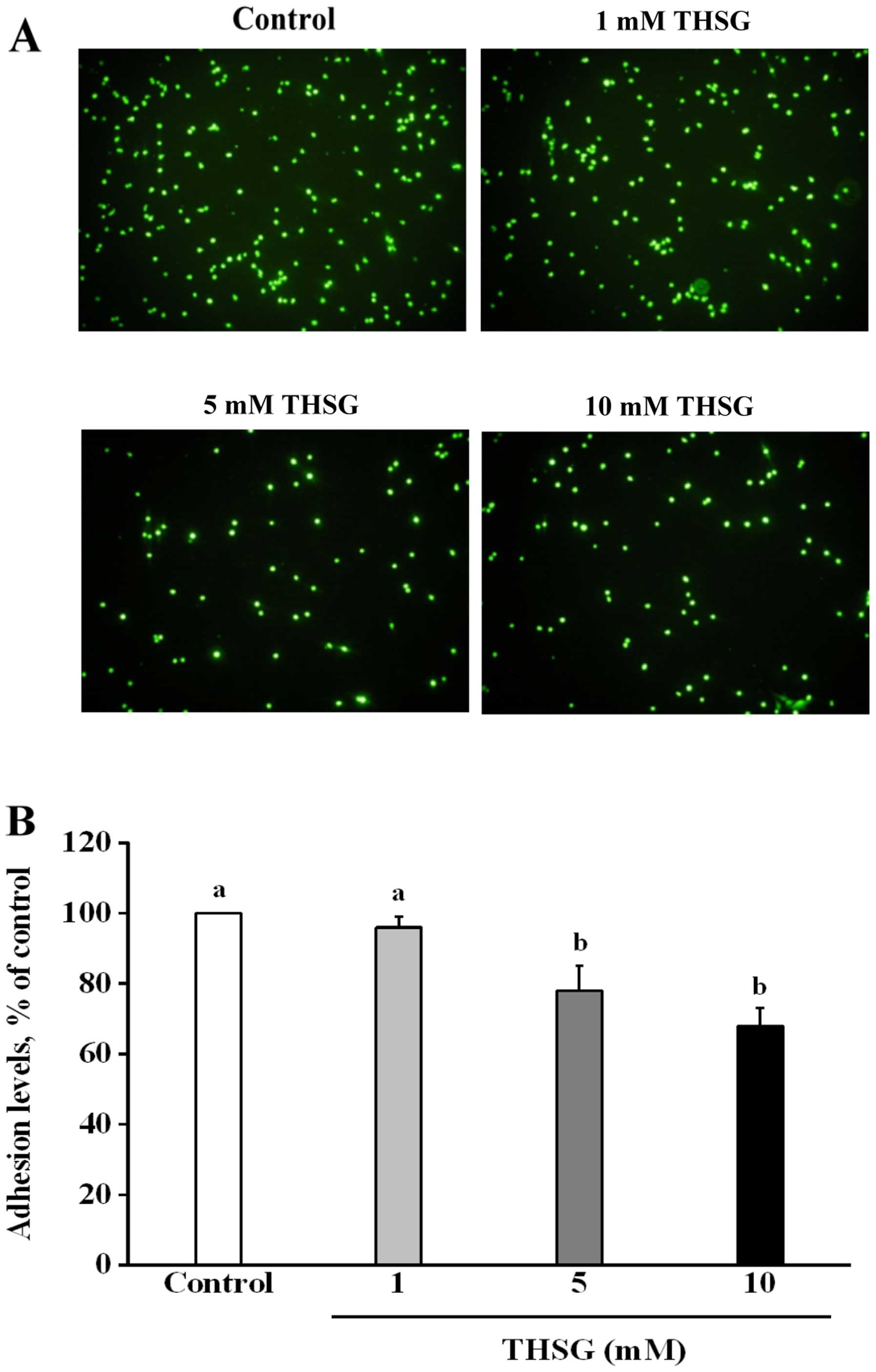
THSG regulates the adhesion of human
colorectal carcinoma cells to human endothelial cells
The fluorescence microscopy data shown in Fig. 5A indicate that treatment of
EA.hy926 cells with various concentrations of THSG reduced the
number of adherent cells in a dose-dependent manner after
co-culture with HT-29 cells. As shown in Fig. 5B, the percentages of EA.hy926 cells
that adhered to HT-29 cells were 96±3, 88±7 and 68±5% for the 1, 5,
and 10 mM treatment groups, respectively, relative to the control
group (100%). Percentages of adherent cells in the 5 and 10 mM THSG
groups were significantly lower than that in the control group
(100%) after the 24-h incubation period (p<0.05). These results
demonstrate that THSG can decrease the ability of HT-29 cells to
adhere to EA.hy926 cells, thereby reducing cancer cell adhesion to
a metastatic target organ.
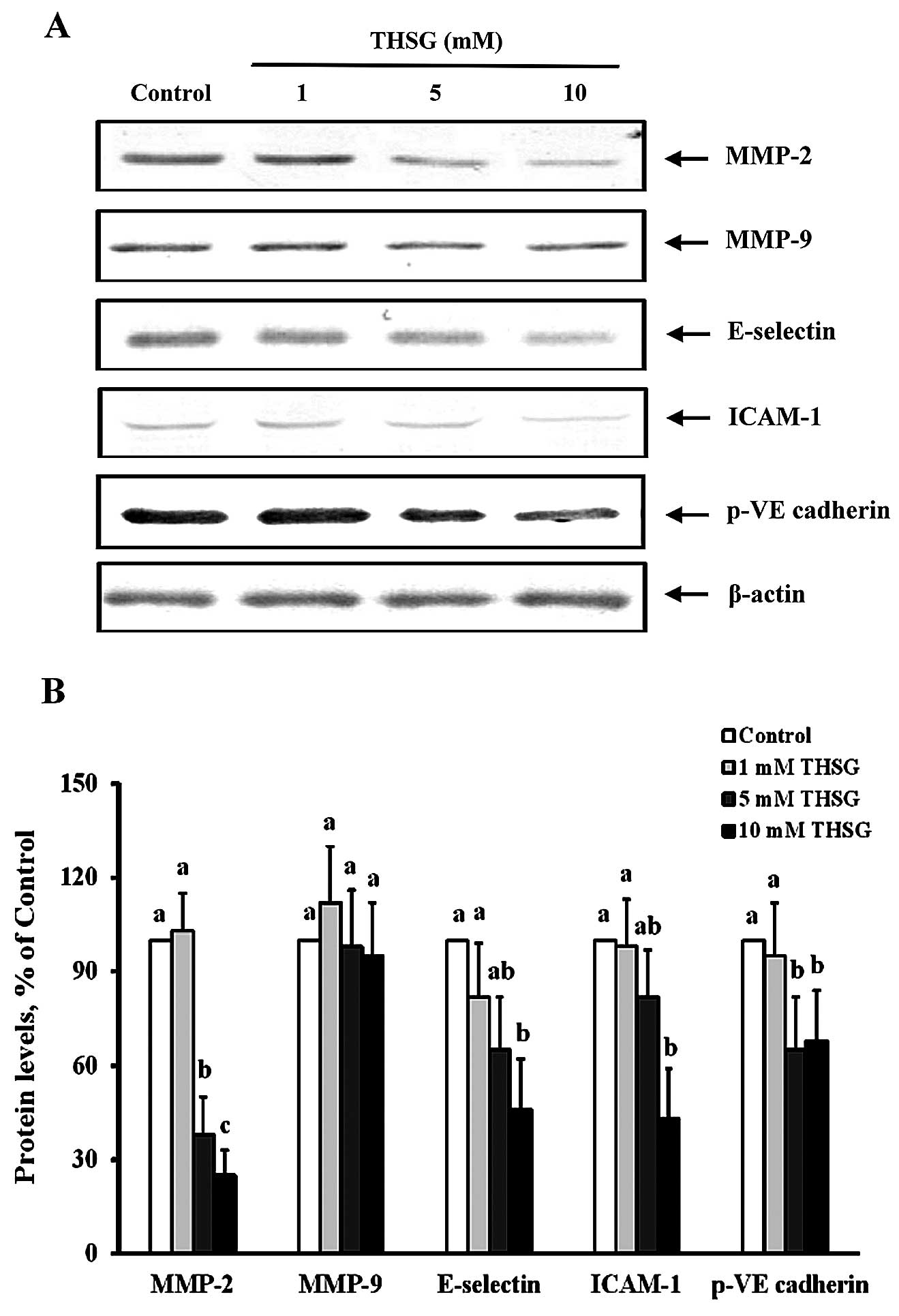
THSG suppresses the levels of
metastasis-associated regulatory proteins
To elucidate the effects of THSG on the
metastasis-related processes of migration, adhesion and invasion by
HT-29 cells, we analyzed the expression of the proteins involved in
these events. As shown in Fig. 6,
HT-29 cells treated with 5 or 10 mM THSG for 24 h displayed MMP-2
protein levels that were significantly reduced to 38±6 and 25±8% of
the control levels (100%), respectively (p<0.05). However, after
treatment of HT-29 cells with various concentrations of THSG for 24
h, the MMP-9 protein level was not significantly altered compared
with the control treatment. Fig. 6
also shows the levels of p-VE-cadherin, an important protein in
cell-cell adherens junctions, following treatment with 5 or 10 mM
THSG. The levels of p-VE-cadherin in the 5 and 10 mM THSG treatment
groups were significantly reduced to 35±14 and 42±16% of the
control values (100%), respectively. These results indicate that
THSG can regulate cell migration and intravasation by suppressing
MMP-2 expression and VE-cadherin phosphorylation.
With respect to cell adhesion, the protein levels of
E-selectin and ICAM-1, both of which act as adhesion molecules in
EA.hy926 cells, were significantly reduced to 62±14 and 43±13% of
the control levels (100%), respectively, in the 10 mM THSG
treatment group (p<0.05) (Fig.
6). These results indicate that the expression of these
adhesion molecules was suppressed by THSG and that this compound
may perform an important anti-metastatic function.
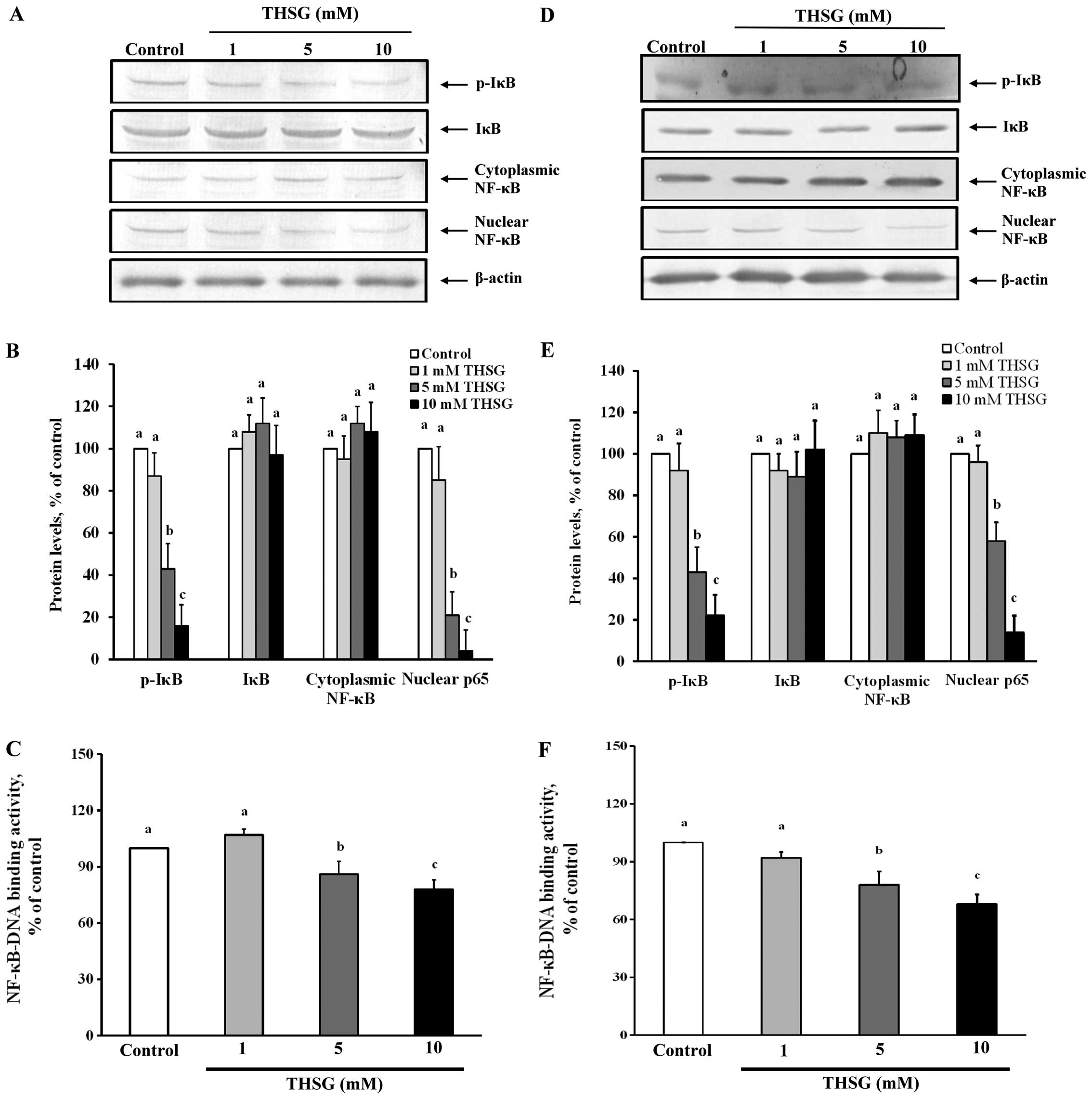
THSG reduces NF-κB activation in HT-29
cells and EA.hy926 cells
In this study, to examine whether the regulation of
MMP-2, MMP-9, E-selectin and ICAM-1 expression by THSG is dependent
on the inhibition of NF-κB activation, both immunoblotting assays
for an NF-κB regulatory molecule and NF-κB-DNA binding activity
assays were performed. Fig. 7
shows the effects of THSG on NF-κB activation in HT-29 cells and
EA.hy926 cells. According to the immunoblotting results, the levels
of p-IκB and nuclear NF-κB were significantly reduced after
treatment of HT-29 cells with 5 or 10 mM THSG for 24 h compared
with the control treatment (100%) (p<0.05) (Fig. 7A and B). This result indicates that
5 and 10 mM THSG can reduce the translocation of NF-κB from the
cytoplasm to the nucleus in HT-29 cells. Fig. 7C shows that the NF-κB-DNA binding
activity of the HT-29 cells treated with 5 or 10 μM THSG was
significantly reduced to approximately 14–22% of the control levels
(100%) (p<0.05).
Similar results were observed in the EA.hy926 cells.
Treatment of EA.hy926 cells with 5 or 10 mM THSG for 24 h
significantly inhibited the phosphorylation of IκB and reduced the
nuclear NF-κB level compared with the control treatment (100%)
(p<0.05) (Fig. 7D and E). The
NF-κB-DNA binding activity in the EA.hy926 cells was also inhibited
by the 5 and 10 mM THSG treatments (22–32%) compared with the
control group (100%) (p<0.05) (Fig.
7F). These results indicate that THSG can suppress the
activation of NF-κB and regulate the protein expression of its
downstream targets in HT-29 cells and EA.hy926 cells.
Discussion
In this study, THSG was shown to significantly
suppress HT-29 cell migration, invasion and adhesion (Figs. 3Figure 4–5) and to significantly regulate the
expression of metastasis-associated proteins by suppressing the
activation of NF-κB (Figs. 6 and
7). These findings are novel in
terms of the anti-metastatic effects of THSG. Specifically, THSG is
a hydroxylated derivative of stilbene that is an important
stilbenoid compound. In terms of the anti-metastatic activities of
stilbenoids, both resveratrol and its metabolite piceatannol were
shown to significantly suppress cell migration and invasion by
reducing wound healing and invasion abilities and MMP-2 and MMP-9
expression via the inhibition of PI3K3/Akt/NF-κB and AP-1 signaling
in brain, prostate, breast, and pancreatic cancer cells (14–16,28).
Additionally, pterostilbene can decrease cell migration and
invasion by suppressing PI3K/NF-κB-mediated MMP-2 and MMP-9
expression in oral squamous cell carcinoma and hepatocellular
carcinoma cells (16). In this
study, our results showed that THSG treatment significantly
decreased wound healing and invasion abilities and MMP-2 expression
in HT-29 cells. Moreover, THSG significantly suppressed the ability
of cancer cells to adhere to endothelial cells and reduced the
levels of adhesion molecules (including ICAM-1 and E-selectin) and
cell-cell permeability (based on TEER). This study provides data
demonstrating that THSG inhibits cell migration, invasion, and
adhesion, and these findings indicate that THSG can inhibit
metastasis by reducing HT-29 cell adhesion to endothelial cells and
cell-cell permeability.
Notably, resveratrol, piceatannol and pterostilbene
possess a C6-C2-C6 structure. The optimal concentration of these
three stilbenes for inhibiting cell migration and invasion is ~5–80
μM. However, THSG possesses an additional glycoside moiety
(Fig. 1). Glycoside residues
usually play an important role in the pharmacokinetic parameters of
natural products. However, glycosylation increases the
hydrophilicity of agly-cone and promote its excretion via urine
(13). Previous studies showed
that 300 μM-24.6 mM THSG can prevent cardiotoxicity and upregulate
melanin synthesis in various types of mouse cardiomyocytes and
B16F1 melanoma cells (17,29). In this study, THSG exhibited an
effective dose of 5–10 mM for anti-metastatic activity.
Some stilbenoids regulate MMP-2 or MMP-9 expression
and others inhibit cell invasive ability; however, the molecular
regulatory mechanism underlying these effects remains unclear. In
this study, THSG not only suppressed cell migration and invasion
but also inhibited molecular signaling by proteins involved in
metastasis and NF-κB pathway activation. Nearly 50% of all CRC
patients develop colorectal liver metastasis, which is the main
cause of death among patients with CRC (1). Therefore, in addition to research on
targeted anti-metastasis therapy, the search for a herb or
phytochemical to treat metastatic colon cancer is a new direction
of research. THSG could potentially be used to prevent CRC
metastasis.
When tumor cells undergo invasion and migration,
they alter their surrounding ECM and basement membrane (BM)
proteins to create a route for cell migration, and this process is
regulated by MMPs (4). It is well
known that MMP inhibitors block endothelial cell activities that
are essential for cell proliferation and invasion (30). A previous study showed that
fucoidan exerts a concentration-dependent inhibitory effect on the
invasion and migration of A549 human lung cancer cells by
decreasing the activity of MMP-2 (31). Gefitinib was found to inhibit MMP-9
and MMP-2 secretion and mRNA expression, suppressing the metastasis
of colon cancer HT-29 cells (32).
Gallic acid suppressed PC-3 human prostate cancer cell migration
and invasion by inhibiting the MMP-2 and MMP-9 signaling pathways
(33). Our results showed that
HT-29 cells treated with 5 or 10 mM THSG exhibited significantly
reduced MMP-2 protein levels (Fig.
6). The suppressive effect of THSG on MMP-2 expression may be
an important contributor to its anti-migratory effect.
Regarding tumor metastasis, tumor cells degrade the
ECM via the regulation of MMP expression as well as other
mechanisms, thereby allowing the cells to break away from the
primary tumor. In the metastatic process, primary tumor cells
undergo epithelial-mesenchymal transition (EMT), which involves
morphogenetic changes that enhance cell motility and plasticity,
allowing the cells to migrate to the circulation to become
circulating tumor cells (34).
Then, these circulating tumor cells roll along the endothelial cell
surface, on which E-selectin is expressed (35). This event allows tumor cells to
transmigrate from the endothelium to a metastatic site (36). In addition, ICAM-1 plays a critical
role in regulating metastasis. Inhibiting ICAM-1 has been shown to
block the invasion of lung cancer cells in vitro (37). A previous study showed that
cannabidiol elicited a concentration-dependent increase in ICAM-1
expression, inhibiting the adhesion and extravasation of A549,
H358, and H460 lung cancer cells (38). When cancer cells leave the original
tumor organ, they intravasate into the circulatory system to
migrate to the metastatic target organ. Adhesion molecules (e.g.,
E-selectin and ICAM-1) play a key role in regulating tumor
adhesion, which is involved in this process (2). The E-selectin-mediated adhesion of
tumor cells to the vascular endothelium was shown to be crucial in
the progression of extravasation and metastasis in previous studies
(39). In this study, we found
that 10 mM THSG significantly inhibited the expression of both
E-selectin and ICAM-1 (Fig. 6).
Therefore, THSG can reduce HT-29 cell adhesion by decreasing the
E-selectin and ICAM-1 levels.
Additionally, our results demonstrated that THSG
dose-dependently reduced endothelial permeability, as indicated by
the TEER values (Fig. 5), and
suppressed the phosphorylation of VE-cadherin (Fig. 6) in HT-29 cells. These results
provide compelling evidence that THSG can suppress the
extravasation of HT-29 cells. It is known that cadherins are
transmembrane proteins with various important functions during
cell-cell adhesion in normal and cancerous cells (40). Among the cadherin family members,
VE-cadherin plays an important role in endothelial cell adherens
junctions and the regulation of vascular permeability, invasion and
angiogenesis during tumor progression and metastasis. Endothelial
permeability- and junction-related proteins play important roles in
extravasation (41). VE-cadherin
is a strictly endothelial-specific adhesion molecule located at the
junctions between endothelial cells. These junctions are associated
with cell-cell adhesion structures and enable intercellular
communication. The phosphorylation of VE-cadherin inactivates this
protein and increases cell monolayer permeability, promoting tumor
invasiveness (42). Moreover,
endothelial cell-cell permeability can be monitored via the TEER,
which potentially serves as an indicator of cancer cell
extravasation (43). The
expression of MMPs and junction proteins is important for cell
membrane permeability and tumor cell extravasation (44). A previous study demonstrated that
Capsosiphon fulvescens, a green alga, significantly
suppresses cell invasion by decreasing MMP-2 and MMP-9 expression
and increasing TEER in human gastric cancer AGS cells (45). THSG also inhibits MMP-2 expression
and increases TEER in HT-29 cells, suppressing extravasation and
metastasis. Our results show that THSG, which is the active
principle component of Polygonum multiflorum, exerts
anti-migratory effects by suppressing the expression of MMP-2. This
compound also performs anti-invasion functions by inhibiting the
expression of VE-cadherin and reducing epithelial permeability by
elevating TEER. Moreover, THSG displays anti-adhesion properties
that are mediated by the inhibition of E-selectin and ICAM-1
protein expression.
In this study, THSG was significantly affected by
the expression of specific cell migration-, invasion- and
adhesion-associated genes, e.g., MMP-2, E-selectin and ICAM, via
the regulation of NF-κB activation, resulting in decreased protein
levels of MMP-2, E-selectin and ICAM in HT-29 and EA.hy926 cells
(Fig. 6). NF-κB is sequestered in
the cytoplasm by its interaction with the inhibitory protein IκB.
Exposure to a variety of external stimuli causes the
phosphorylation of IκBα, which leads to the disassociation of NF-κB
from IκBα (46). Activated NF-κB
then translocates to the nucleus, where it binds to the cis-acting
κB enhancer element of target genes such as MMP-2, MMP-9,
E-selectin and ICAM and activates their transcription (47–49).
Previous studies have shown that inhibiting NF-κB activity in human
prostate cancer cells decreases their metastatic abilities by
suppressing cell invasion via the downregulation of MMPs (50). As shown in Fig. 7, NF-κB pathway activation is
downregulated in response to THSG treatment. These results indicate
that NF-κB is an important cellular target of THSG for
metastasis-associated gene regulation.
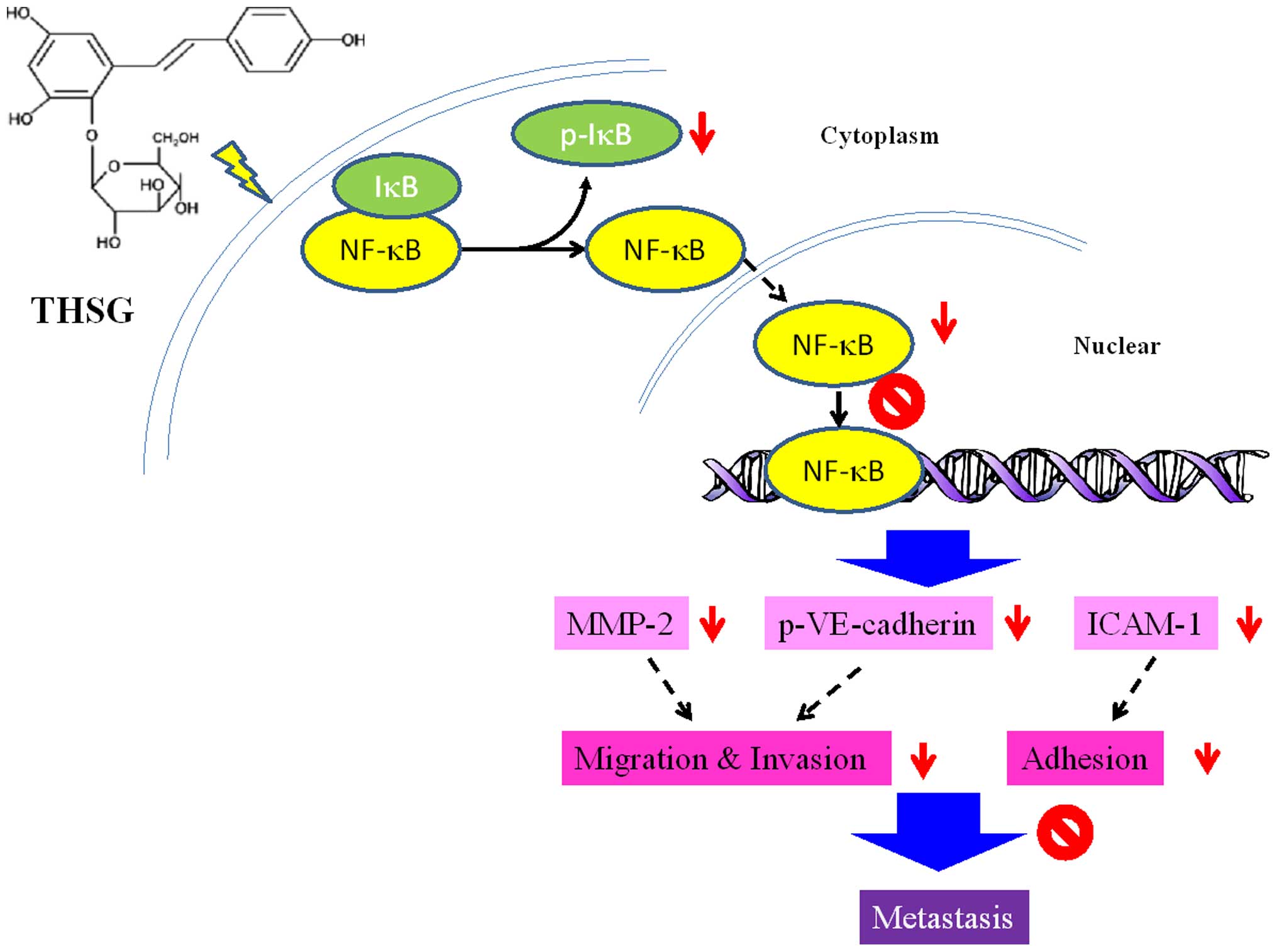
In conclusion, as shown in Fig. 8, our results support a model in
which THSG suppresses CRC cell metastasis. THSG significantly
altered the expression of specific migration-related genes, e.g.,
MMP-2 and ICAM-1, via the downregulation NF-κB activation,
resulting in inhibited migration, invasion and adhesion by HT-29
and EA.hy926 cells. Thus, THSG may reduce the metastatic ability of
colon cancer cells. In HT-29 cells, the mechanisms of action of
THSG may involve the inhibition of cell migration, adhesion,
intravasation and extravasation, as well as the regulation of
protein expression. THSG might serve as a potential anti-metastatic
agent.
Acknowledgements
This study was supported by E-Da Hospital
(EDAHT102009) in Kaohsiung, Taiwan.
Abbreviations:
|
THSG
|
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside
|
|
MMP
|
matrix metalloproteinase
|
|
TEER
|
transepithelial electrical
resistance
|
|
ICAM-1
|
intercellular adhesion molecule-1
|
|
ECM
|
extracellular matrix
|
|
VE
|
vascular endothelial
|
|
BCECF
|
2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein
|
|
PMSF
|
phenylmethylsulfonyl fluoride
|
|
SDS
|
sodium dodecyl sulfate
|
|
BM
|
basement membrane
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics for hispanics/latinos. CA Cancer J Clin. 62:283–298.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fidler IJ: Critical determinants of
metastasis. Semin Cancer Biol. 12:89–96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duffy MJ, Maguire TM, Hill A, McDermott E
and O'Higgins N: Metalloproteinases: Role in breast carcinogenesis,
invasion and metastasis. Breast Cancer Res. 2:252–257. 2000.
View Article : Google Scholar
|
|
5
|
Zhang L, Shi J, Feng J, Klocker H, Lee C
and Zhang J: Type IV collagenase (matrix metalloproteinase-2 and
-9) in prostate cancer. Prostate Cancer Prostatic Dis. 7:327–332.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nelson CM, Pirone DM, Tan JL and Chen CS:
Vascular endothelial-cadherin regulates cytoskeletal tension, cell
spreading, and focal adhesions by stimulating RhoA. Mol Biol Cell.
15:2943–2953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi H, Boelte KC and Lin PC:
Endothelial cell adhesion molecules and cancer progression. Curr
Med Chem. 14:377–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shishodia S and Aggarwal BB: Nuclear
factor-kappaB activation mediates cellular transformation,
proliferation, invasion angiogenesis and metastasis of cancer.
Cancer Treat Res. 119:139–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv G, Lou Z, Chen S, Gu H and Shan L:
Pharmacokinetics and tissue distribution of
2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside from traditional
Chinese medicine Polygonum multiflorum following oral
administration to rats. J Ethnopharmacol. 137:449–456. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Zhao L, Han T, Chen S and Wang J:
Protective effects of
2,3,5,4′-tetrahydroxystilbene-2-O-beta-d-glucoside, an active
component of Polygonum multiflorum Thunb, on experimental colitis
in mice. Eur J Pharmacol. 578:339–348. 2008. View Article : Google Scholar
|
|
11
|
Sun FL, Zhang L, Zhang RY and Li L:
Tetrahydroxystilbene glucoside protects human neuroblastoma SH-SY5Y
cells against MPP+-induced cytotoxicity. Eur J
Pharmacol. 660:283–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu QL, Xiao JH, Ma R, Ban Y and Wang JL:
Effect of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside on
lipo-protein oxidation and proliferation of coronary arterial
smooth cells. J Asian Nat Prod Res. 9:689–697. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kimura Y and Okuda H: Effects of naturally
occurring stilbene glucosides from medicinal plants and wine, on
tumour growth and lung metastasis in Lewis lung carcinoma-bearing
mice. J Pharm Pharmacol. 52:1287–1295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jayasooriya RG, Lee YG, Kang CH, Lee KT,
Choi YH, Park SY, Hwang JK and Kim GY: Piceatannol inhibits
MMP-9-dependent invasion of tumor necrosis factor-α-stimulated
DU145 cells by suppressing the Akt-mediated nuclear factor-κB
pathway. Oncol Lett. 5:341–347. 2013.
|
|
15
|
Song NR, Hwang MK, Heo YS, Lee KW and Lee
HJ: Piceatannol suppresses the metastatic potential of MCF10A human
breast epithelial cells harboring mutated H-ras by inhibiting MMP-2
expression. Int J Mol Med. 32:775–784. 2013.PubMed/NCBI
|
|
16
|
Lin CW, Chou YE, Chiou HL, Chen MK, Yang
WE, Hsieh MJ and Yang SF: Pterostilbene suppresses oral cancer cell
invasion by inhibiting MMP-2 expression. Expert Opin Ther Targets.
18:1109–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang SH, Wang WQ and Wang JL: Protective
effect of tetrahydroxystilbene glucoside on cardiotoxicity induced
by doxorubicin in vitro and in vivo. Acta Pharmacol Sin.
30:1479–1487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang WL and Frucht H: Activation of the
PPAR pathway induces apoptosis and COX-2 inhibition in HT-29 human
colon cancer cells. Carcinogenesis. 22:1379–1383. 2001. View Article : Google Scholar
|
|
19
|
Zhang XM, Lv YG, Chen GB, Zou Y, Lin CW,
Yang L, Guo P and Lin MP: Effect of mild hypothermia on breast
cancer cells adhesion and migration. Biosci Trends. 6:313–324.
2012.
|
|
20
|
Denizot F and Lang R: Rapid colorimetric
assay for cell growth and survival. Modifications to the
tetrazolium dye procedure giving improved sensitivity and
reliability. J Immunol Methods. 89:271–277. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ang SF, Zhao ZS, Lim L and Manser E: DAAM1
is a formin required for centrosome re-orientation during cell
migration. PLoS One. 5:130642010. View Article : Google Scholar
|
|
22
|
Vincent C, Siddiqui TA and Schlichter LC:
Podosomes in migrating microglia: Components and matrix
degradation. J Neuroinflammation. 9:1902012. View Article : Google Scholar :
|
|
23
|
Gopal PK, Prasad J, Smart J and Gill HS:
In vitro adherence properties of Lactobacillus rhamnosus DR20 and
Bifidobacterium lactis DR10 strains and their antagonistic activity
against an enterotoxigenic Escherichia coli. Int J Food Microbiol.
67:207–216. 2001. View Article : Google Scholar
|
|
24
|
Braut-Boucher F, Pichon J, Rat P, Adolphe
M, Aubery M and Font J: A non-isotopic, highly sensitive,
fluorimetric, cell-cell adhesion microplate assay using calcein
AM-labeled lymphocytes. J Immunol Methods. 178:41–51. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
26
|
Towbin H, Staehelin T and Gordon J:
Electrophoretic transfer of proteins from polyacrylamide gels to
nitrocellulose sheets: Procedure and some applications. Proc Natl
Acad Sci USA. 76:4350–4354. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li W, Ma J, Ma Q, Li B, Han L, Liu J, Xu
Q, Duan W, Yu S, Wang F, et al: Resveratrol inhibits the
epithelial-mesenchymal transition of pancreatic cancer cells via
suppression of the PI-3K/Akt/NF-κB pathway. Curr Med Chem.
20:4185–4194. 2013. View Article : Google Scholar
|
|
29
|
Jiang Z, Xu J, Long M, Tu Z, Yang G and He
G: 2, 3, 5, 4′-tetrahydroxystilbene-2-O-beta-D-glucoside (THSG)
induces melanogenesis in B16 cells by MAP kinase activation and
tyrosinase upregulation. Life Sci. 85:345–350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Murphy AN, Unsworth EJ and
Stetler-Stevenson WG: Tissue inhibitor of metalloproteinases-2
inhibits bFGF-induced human microvascular endothelial cell
proliferation. J Cell Physiol. 157:351–358. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee H, Kim JS and Kim E: Fucoidan from
seaweed Fucus vesiculosus inhibits migration and invasion of human
lung cancer cell via PI3K-Akt-mTOR pathways. PLoS One.
7:e506242012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Toda D, Ota T, Tsukuda K, Watanabe K,
Fujiyama T, Murakami M, Naito M and Shimizu N: Gefitinib decreases
the synthesis of matrix metalloproteinase and the adhesion to
extracellular matrix proteins of colon cancer cells. Anticancer
Res. 26A:129–134. 2006.
|
|
33
|
Liu KC, Huang AC, Wu PP, Lin HY, Chueh FS,
Yang JS, Lu CC, Chiang JH, Meng M and Chung JG: Gallic acid
suppresses the migration and invasion of PC-3 human prostate cancer
cells via inhibition of matrix metalloproteinase-2 and -9 signaling
pathways. Oncol Rep. 26:177–184. 2011.PubMed/NCBI
|
|
34
|
Bonnomet A, Brysse A, Tachsidis A, Waltham
M, Thompson EW, Polette M and Gilles C: Epithelial-to-mesenchymal
transitions and circulating tumor cells. J Mammary Gland Biol
Neoplasia. 15:261–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dimitroff CJ, Lechpammer M, Long-Woodward
D and Kutok JL: Rolling of human bone-metastatic prostate tumor
cells on human bone marrow endothelium under shear flow is mediated
by E-selectin. Cancer Res. 64:5261–5269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barthel SR, Hays DL, Yazawa EM, Opperman
M, Walley KC, Nimrichter L, Burdick MM, Gillard BM, Moser MT,
Pantel K, et al: Definition of molecular determinants of prostate
cancer cell bone extravasation. Cancer Res. 73:942–952. 2013.
View Article : Google Scholar :
|
|
37
|
Huang WC, Chan ST, Yang TL, Tzeng CC and
Chen CC: Inhibition of ICAM-1 gene expression, monocyte adhesion
and cancer cell invasion by targeting IKK complex: Molecular and
functional study of novel alpha-methylene-gamma-butyrolactone
derivatives. Carcinogenesis. 25:1925–1934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ramer R, Bublitz K, Freimuth N, Merkord J,
Rohde H, Haustein M, Borchert P, Schmuhl E, Linnebacher M and Hinz
B: Cannabidiol inhibits lung cancer cell invasion and metastasis
via intercellular adhesion molecule-1. FASEB J. 26:1535–1548. 2012.
View Article : Google Scholar
|
|
39
|
Köhler S, Ullrich S, Richter U and
Schumacher U: E-/P-selectins and colon carcinoma metastasis: First
in vivo evidence for their crucial role in a clinically relevant
model of spontaneous metastasis formation in the lung. Br J Cancer.
102:602–609. 2010. View Article : Google Scholar :
|
|
40
|
Hazan RB, Qiao R, Keren R, Badano I and
Suyama K: Cadherin switch in tumor progression. Ann NY Acad Sci.
1014:155–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Canel M, Serrels A, Frame MC and Brunton
VG: E-cadherin-integrin crosstalk in cancer invasion and
metastasis. J Cell Sci. 126:393–401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Birchmeier W and Behrens J: Cadherin
expression in carcinomas: Role in the formation of cell junctions
and the prevention of invasiveness. Biochim Biophys Acta.
1198:11–26. 1994.PubMed/NCBI
|
|
43
|
Ludwig T, Ossig R, Graessel S, Wilhelmi M,
Oberleithner H and Schneider SW: The electrical resistance
breakdown assay determines the role of proteinases in tumor cell
invasion. Am J Physiol Renal Physiol. 283:F319–F327. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Park HS, Kim GY, Choi IW, Kim ND, Hwang
HJ, Choi YW and Choi YH: Inhibition of matrix metalloproteinase
activities and tightening of tight junctions by diallyl disulfide
in AGS human gastric carcinoma cells. J Food Sci. 76:T105–T111.
2011. View Article : Google Scholar
|
|
45
|
Kim YM, Kim IH and Nam TJ: Inhibition of
AGS human gastric cancer cell invasion and proliferation by
Capsosiphon fulvescens glycoprotein. Mol Med Rep. 8:11–16.
2013.PubMed/NCBI
|
|
46
|
Bours V, Bonizzi G, Bentires-Alj M, Bureau
F, Piette J, Lekeux P and Merville M: NF-kappaB activation in
response to toxical and therapeutical agents: Role in inflammation
and cancer treatment. Toxicology. 153:27–38. 2000. View Article : Google Scholar
|
|
47
|
Cota-Gomez A, Flores NC, Cruz C, Casullo
A, Aw TY, Ichikawa H, Schaack J, Scheinman R and Flores SC: The
human immunodeficiency virus-1 Tat protein activates human
umbilical vein endothelial cell E-selectin expression via an
NF-kappa B-dependent mechanism. J Biol Chem. 277:14390–14399.
2002.PubMed/NCBI
|
|
48
|
Orr AW, Sanders JM, Bevard M, Coleman E,
Sarembock IJ and Schwartz MA: The subendothelial extracellular
matrix modulates NF-kappaB activation by flow: A potential role in
atherosclerosis. J Cell Biol. 169:191–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xue H, Sun K, Xie W, Hu G, Kong H, Wang Q
and Wang H: Etanercept attenuates short-term
cigarette-smoke-exposure-induced pulmonary arterial remodelling in
rats by suppressing the activation of TNF-α/NF-κB signal and the
activities of MMP-2 and MMP-9. Pulm Pharmacol Ther. 25:208–215.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Palayoor ST, Youmell MY, Calderwood SK,
Coleman CN and Price BD: Constitutive activation of IkappaB kinase
alpha and NF-kappaB in prostate cancer cells is inhibited by
ibuprofen. Oncogene. 18:7389–7394. 1999. View Article : Google Scholar : PubMed/NCBI
|