Introduction
Colorectal cancer (CRC) represents one of the major
causes of morbidity and mortality throughout the world and is the
third most common form of human cancer worldwide (1,2).
Like other forms of cancer, CRC is characterized by angiogenesis,
which is a crucial event in promoting cancer growth, progression
and metastasis (3). Among the
various signaling molecules involved in the angiogenic process,
vascular endothelial growth factor (VEGF) and nitric oxide (NO) are
thought to be the key signaling molecules responsible for
neo-vascularization (4–6). Once hypersecreted, VEGF binds to its
type 2 receptor (VEGFR-2) and mediates the regulation of different
pathways in the target cells, mainly the phosphatidylinositol
3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of
rapamycin (mTOR) pathway (7,8) and
the phospho-p38 mitogen activated protein kinase (MAPK)-dependent
activation of nuclear factor κB (NF-κB) (9,10).
Activation of the NF-κB and Akt/mTOR pathways increases the levels
of the inducible nitric oxide synthase (iNOS) isoform, leading to
the release and accumulation of nitric oxide (NO) that acts as a
pro-angiogenic stimulus on the blood vessels and favors
neo-vascularization in solid tumors (5,11).
Thus, targeting the angiogenic and mitogenic pathways is a rational
and potentially effective strategy in the treatment of CRC
(12).
Rifaximin is a semi-synthetic antibiotic largely
used for the treatment of travelers' diarrhea and hepatic
encephalopathy (13,14). It is poorly absorbed on oral
administration (15) and as such
has an optimum safety profile. Apart from its antibiotic potential,
rifaximin has also been studied for its anti-inflammatory effects;
several studies have highlighted the anti-inflammatory potential of
rifaximin, which is mainly attributed to the inhibition of the
NF-κB signaling and NO release via activation of intestinal human
pregnane X (PXR) receptors (16,17).
The aim of the present study was to explore the
anti-proliferative and anti-migration effects of rifaximin, and to
evaluate the possible control of the angiogenic mediator release by
rifaximin using a human intestinal epithelial cell line to model
the intestinal barrier. The effect of rifaximin on VEGF and NO
signaling and the mechanisms involved were also investigated.
Materials and methods
Caco-2 cells were purchased from the European
Collection of Cell Cultures (ECACC, Public Health England Porton
Down, Salisbury, UK). Cell medium, drugs and reagents for
cell culture were purchased from Sigma-Aldrich (St. Louis, MO,
USA), unless otherwise specified. Instruments, reagents and
materials for western blot analysis were obtained from Bio-Rad
Laboratories (Milan, Italy). Rifaximin and ketoconazole were
purchased from Tocris Cookson, Inc. (Ballwin, MO, USA). Mouse
anti-total Akt, rabbit monoclonal anti-phospho-Akt (Ser473), rabbit
polyclonal anti-phospho-mTOR (pSer2448), rabbit polyclonal
anti-total p70S6K, rabbit polyclonal anti-phospho-p70S6K
(Thr421/Ser424, Thr389) and rabbit monoclonal anti-VEGF receptor
were purchased from Cell Signaling Technology (Euroclone, Pero,
Milan, Italy). Rabbit polyclonal anti-total mTOR was purchased from
Abcam (Cambridge, UK); mouse monoclonal anti-hypoxia-inducible
factor 1-α (HIF1-α) was purchased from Sigma-Aldrich (Milan,
Italy); rabbit polyclonal anti-matrix metalloprotease-2 and 9
(MMP-2 and MMP-9) and mouse anti-β-actin were purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA). Polyclonal rabbit
anti-mouse immunoglobulin G was procured from Dako (Glostrup,
Denmark), 32P-γ-ATP from Amersham Biosciences (Milan,
Italy), poly(deoxyinosinic-deoxycytidylic) acid (poly(dI-dC)), from
Boehringer Mannheim (Milan, Italy) and horseradish peroxidase (HRP)
from Dako (Milan, Italy). Chemiluminescence detection reagents were
purchased from Amersham Biosciences and custom oligonucleotides
were synthesized by TIB Molbiol (Boehringer-Mannheim, Mannheim,
Germany).
Cell culture
Caco-2 cells were cultured in 6-well plates in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS), 1% penicillin-streptomycin, 2 mM L-glutamate
and 1% non-essential amino acids. A total of 1×106
cells/well were plated and incubated for 24 h. Upon reaching
confluence, the cells were washed three times with
phosphate-buffered saline (PBS), detached with trypsin/ethylene
diamine tetraacetic acid (EDTA), plated in 10-cm diameter petri
dishes and allowed to adhere for 24 h. Subsequently, DMEM was
replaced with fresh medium and cells were treated for 72 h with
increasing concentrations of rifaximin (0.1, 1.0 and 10.0 μM)
dissolved in ultrapure and pyrogen-free sterile vehicle in the
presence or absence of the PXR antagonist ketoconazole (10 mM) at
different time-points depending upon the experiments. Rifaximin and
ketoconazole concentrations were selected based on the data from
the available literature (16,18)
and the pilot experiments (data not shown) that helped in
identifying the lowest effective concentrations.
Western blot analysis
Protein expression in the Caco-2 cells was evaluated
by western blot analysis. After the different treatments, cells
(1×106) were harvested, washed twice with ice-cold PBS
and centrifuged at 180 × g for 10 min at 4°C. The cell pellet
obtained after centrifugation was re-suspended in 100 μl ice-cold
hypotonic lysis buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM
KCl, 0.5 mM phenylmethylsulphonylfluoride, 1.5 μg/ml soybean
trypsin inhibitor, 7 μg/ml pepstatin A, 5 μg/ml leupeptin, 0.1 mM
benzamidine and 0.5 mM DTT). To lyse the cells, the suspension was
rapidly passed through a syringe needle five to six times and then
centrifuged for 15 min at 13,000 × g to obtain the cytoplasmic
fraction. The cytoplasmic fraction proteins were mixed with
non-reducing gel loading buffer [50 mM Tris, 10% sodium dodecyl
sulphate (SDS), 10% glycerol, 2 mg bromophenol/ml] at a 1:1 ratio,
boiled for 3 min and centrifuged at 10,000 × g for 10 min. The
protein concentration was determined using the Bradford assay and
50 μg of each sample was electrophoresed on a 12% discontinuous
polyacrylamide mini-gel. Proteins were then transferred onto
nitrocellulose membranes that had been saturated by incubation with
10% non-fat dry milk in 1X PBS overnight at 4°C with the following
antibodies: total Akt (1:1,000), phospho-Akt (1:2,000), total mTOR
(1:1,000), phospho-mTOR (1:1,000), total p70S6K (1:1,000),
phospho-p70S6K (1:1,000), anti-HIF-1α (1:500), anti-iNOS (1:1,000),
anti-VEGFR-2 (1:1,000), anti-MMP-2 (1:1,000), anti-MMP-9 (1:1,000),
anti-phospho-p38 (1:1,000), and anti-β-actin protein expression was
performed on total protein fractions of homogenates. Membranes were
then incubated with the specific secondary antibodies conjugated to
HRP. Immune complexes were identified by enhanced chemiluminescence
detection reagents and blots were analyzed by scanning densitometry
(GS-700 imaging densitometer; Bio-Rad Laboratories). Results were
expressed as optical density (OD) (arbitrary units; mm2)
and normalized against the expression of the housekeeping protein
β-actin.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was
performed to detect NF-κB activation in Caco-2 cells. Briefly, 10
mg of the cell extracts were incubated in a binding buffer (8 mM
HEPES, pH 7.0, 10% glycerol, 20 mM KCl, 4 mM MgCl2, 1 mM
sodium pyrophosphate) containing 1.0 mg of poly(dI-dC) and
32P-γ end-labeled probe with the following sequence: i)
5′ AAC TCC GGG AAT TTC CCT GGC CC 3′ and ii) 5′ GGG CCA GGG AAA TTC
CCG GAG TT 3′. Nuclear extracts were incubated for 15 min with
radiolabelled oligonucleotides (2.5–5.0×104 cpm) in 20
ml reaction buffer containing 2 mg poly(dI-dC), 10 mM Tris-HCl (pH
7.5), 100 mM NaCl, 1 mM EDTA, 1 mM DL-dithiothreitol, 1 mg/ml
bovine serum albumin (BSA) and 10% (v/v) glycerol. Nuclear
protein-oligonucleotide complexes were resolved by electrophoresis
on a 6% non-denaturing polyacrylamide gel in a Tris-borate-EDTA
buffer at 150 V for 2 h at 4°C. The gel was dried and
autoradiographed with an intensifying screen at −80°C for 20 h. The
relative bands were quantified by densitometric scanning using
VersaDoc (Bio-Rad Laboratories) and a computer program (Quantity
One Software; Bio-Rad Laboratories).
Nitric oxide quantification
NO was measured as nitrite
(NO2−) accumulation in the homogenates
derived from Caco-2 cells by a spectrophotometric assay based on
the Griess reaction. Griess reagent (1% sulphanilamide in
H2O plus 0.1% naphthyl ethylenediamine in
H3PO4) was added to an equal volume of
supernatant and the absorbance was measured at 550 nm. Nitrite
concentration (nM) was determined using a standard curve of
NaNO2.
Cell proliferation assay
Cell proliferation was evaluated by performing a
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT)
assay (19). Caco-2 cells
(5×104) were plated in 96-well plates and allowed to
adhere for 3 h. DMEM was then replaced with fresh medium and the
cells were untreated or treated with increasing concentrations of
rifaximin (0.1, 1 and 10 μM) in the presence or absence of
ketoconazole (10 mM). After 48 h, 25 μl MTT (5 mg/ml MTT in DMEM)
was added to the cells and the mixture was incubated for further 3
h at 37°C. Cells were then lysed and the dark blue crystals were
solubilized using a 125 μl solution containing 50% N,N-dimethyl
formamide and 20% (w/v) SDS (pH 4.5). The OD of each well was
determined using a Perkin-Elmer, Inc. (Waltham, MA, USA) microplate
spectrophotometer equipped with a 620-nm filter. Cell proliferation
in response to treatment was calculated using the following
equation: Cell proliferation at 48 h (%) = (OD treated/OD
untreated) × 100.
Wound healing assay
The wound healing assay was performed as previously
described by Renault-Mihara and colleagues, with some modifications
(20). Briefly, Caco-2 cells were
plated on a 6-well plate and allowed to adhere to the surface of
the wells. The cells were then scratched using a 200-μl sterile
pipette tip, washed with PBS and incubated with rifaximin (0.1–10
μM) for 48 h. After incubation, the cells were again washed twice
with PBS and fixed with 4% paraformaldehyde for 30 min. The cells
were washed three times with PBS and photographed in a bright field
using a Nikon Eclipse 80 (Nikon Instruments Europe, Kingston, UK)
microscope equipped with a high-resolution digital camera (Nikon
Digital Sight DS-U1; Nikon Corp.). The percentage of migration was
calculated by counting the number of cells that migrated into
scratched areas compared with cells that stayed in the peripheral
areas.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) for mouse
VEGF (Abcam, Cambridge, UK) was carried out on Caco-2 cell
supernatant at 24-h post treatment, according to the manufacturer's
protocol. Absorbance was measured on a microtiter plate reader.
VEGF levels were determined using the standard curve method.
Proliferating cell nuclear antigen
immunofluorescence
Caco-2 cells were plated onto glass slide chambers
coated with poly-D-lysine (3×104 cells/well) and
incubated for 24 h in the presence of rifaximin with or without
ketoconazole. After the treatment, cells were washed with
PBS-Triton 0.1% (T-PBS), fixed in 4% paraformaldehyde and then
incubated in 10% BSA/0.1% T-PBS solution for 90 min and for 1 h
with a 10% BSA/0.1% T-PBS solution of anti-proliferating cell
nuclear antigen (PCNA) antibody 1:100 (Abcam) for immunostaining.
Finally, the cells were incubated for 1 h in the dark with
fluorescein isothiocyanate conjugated anti-rabbit antibody 1:100
(Abcam). Nuclei were stained using Hoechst stain (1:5,000)
(Sigma-Aldrich) and images were captured using a camera (Nikon
digital sight DS-U1) connected to a microscope (Nikon eclipse 80i;
Nikon Instruments Europe). The analysis of RGB intensity was
performed using NIH software and quantification of PCNA+
proliferating cells was expressed as % of PCNA+
expressing cells per selected area (1 mm2).
Statistical analysis
Results were expressed as mean ± SEM of n=4
experiments in triplicate. Statistical analysis was performed using
parametric one way analysis of variance (ANOVA) and multiple
comparisons were performed using Bonferroni's post hoc test.
P-values <0.05 were considered to indicate a statistically
significant result.
Results
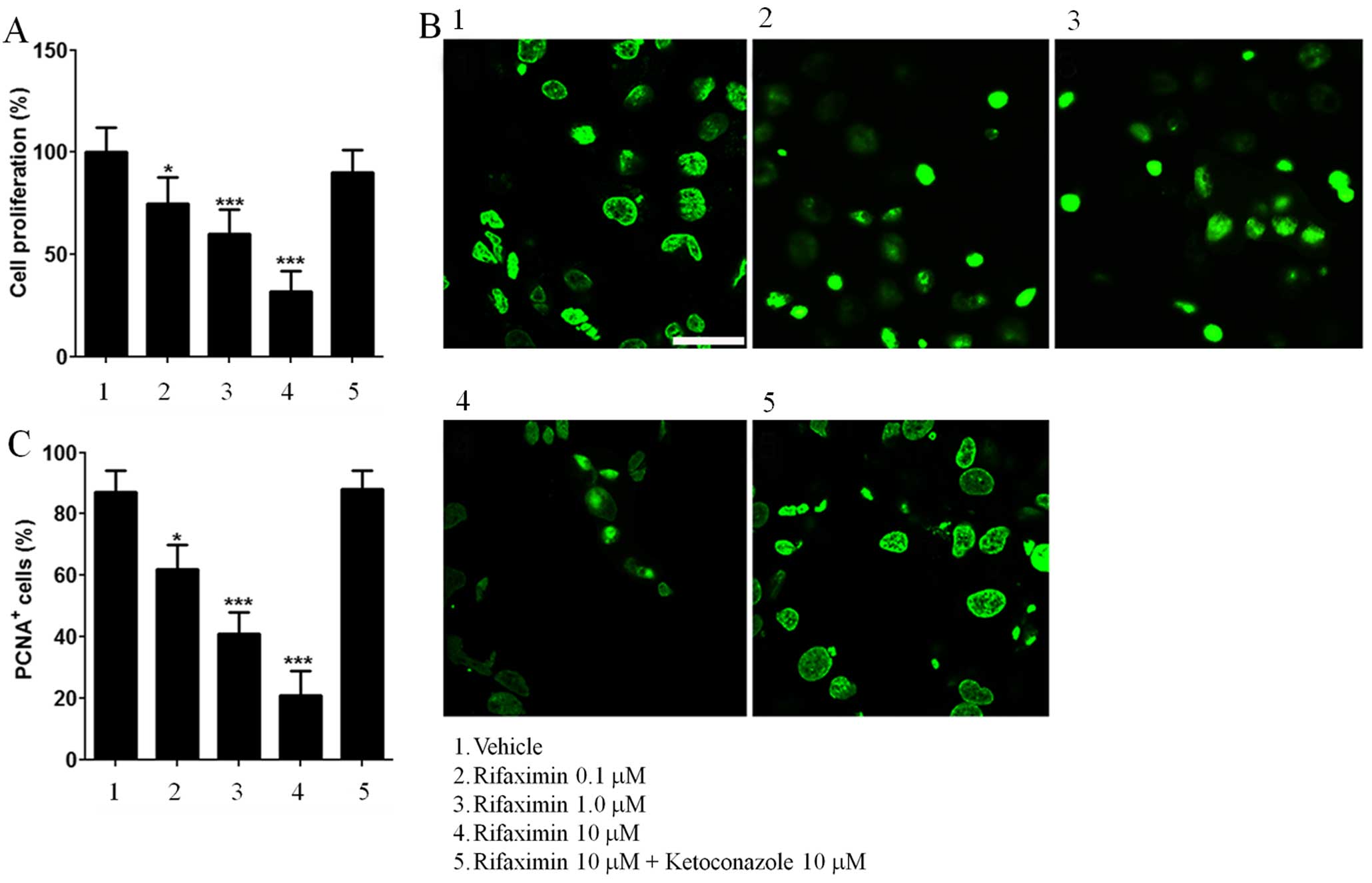
Cell proliferation in Caco-2 cells
The effect of rifaximin treatment on cell
proliferation and PCNA expression is shown in Fig. 1. Rifaximin 0.1, 1 and 10 μM caused
a significant and concentration-dependent reduction in Caco-2 cell
proliferation (−25, −40 and −68% vs. untreated cells; Fig. 1A), and this effect was inhibited by
ketoconazole. Similarly, the expression of PCNA was reduced in a
concentration-dependent manner by rifaximin 0.1, 1 and 10 μM (−29,
−53 and −76% vs. untreated cells; Fig.
1B and C) and completely abolished by ketoconazole.
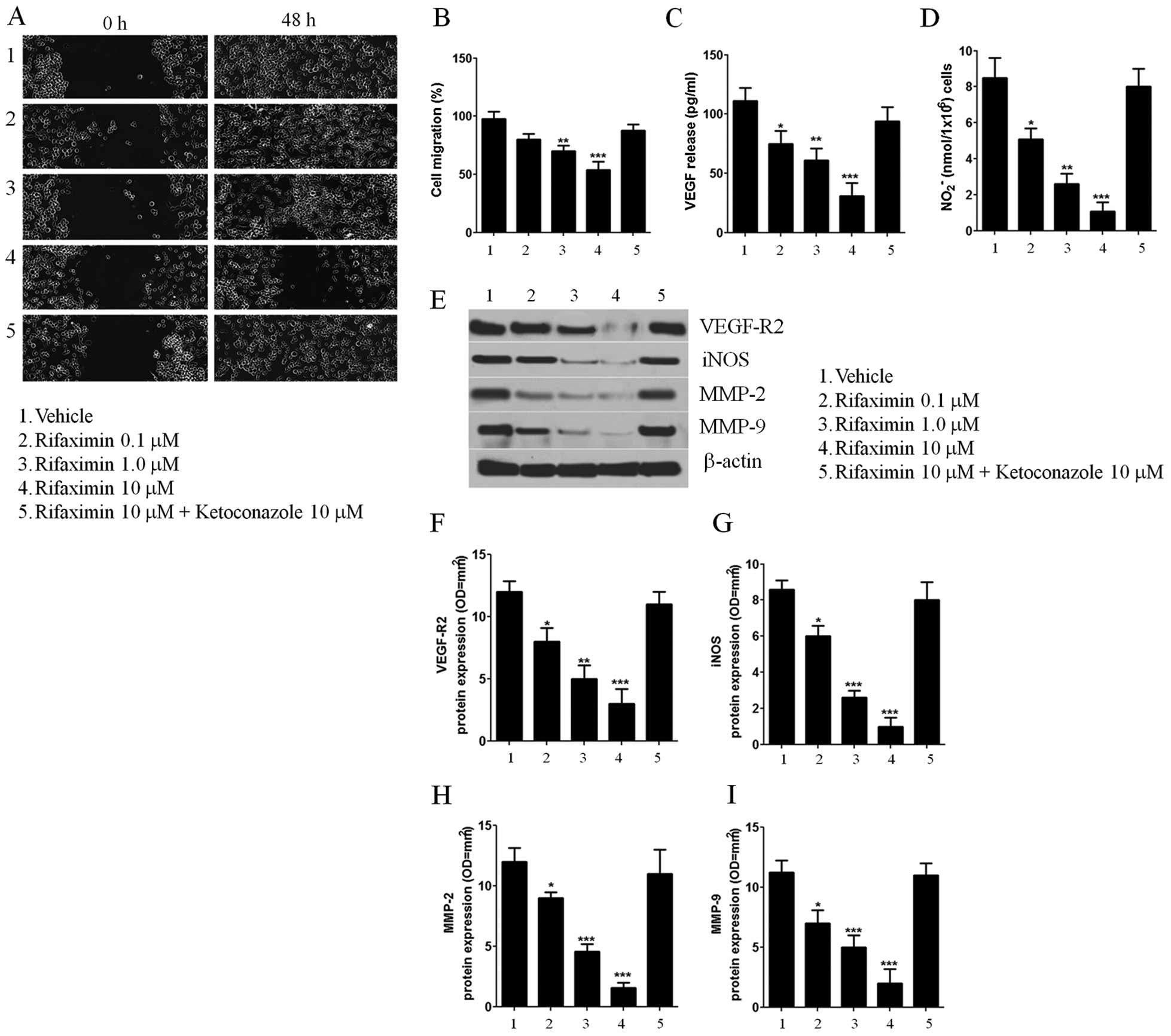
Cell migration, VEGF secretion, and
VEGFR-2, MMP-2 and MMP-9 expression
The wound healing assay was used to evaluate the
effect of rifaximin on Caco-2 cell migration. As indicated in panel
1 of Fig. 2A, untreated Caco-2
cells were able to invade and fully recolonize the scratched area
within 48 h of treatment. In contrast, the migration of cells
treated with rifaximin 0.1, 1 and 10 μM was significantly reduced
in a concentration-dependent manner (−18, −30 and −46%, vs.
untreated cells) (Fig. 2B). Also,
the distances between the borders of the wound were significantly
different compared to those measured in the untreated cells
(Fig. 2A, panels 2, 3 and 4). The
anti-migratory effect of rifaximin was completely counteracted by
ketoconazole (Fig. 2A, panel
5).
As shown in Fig. 2,
incubation with rifaximin 0.1, 1 and 10 μM resulted in a
significant downregulation of pro-angiogenic mediators released at
48 h, causing a significant and concentration-dependent decrease of
both VEGF secretion and NO release (−32, −45 −72 and −40, −69 and
−87%, respectively, vs. untreated cells) (Fig. 2C and D). Similarly, rifaximin
incubation significantly reduced the expression of VEGFR-2 and iNOS
protein (−33 −58, −65 and −40, −69, −78%, respectively, vs.
untreated cells) (Fig. 2E–G).
Moreover, the treatment caused a significant and
concentration-dependent inhibition of PXR-mediated MMP-2 and MMP-9
protein expression (−25, −62, −87 and −38, −56 and −78%,
respectively, vs. untreated cells) (Fig 2E, H and I).
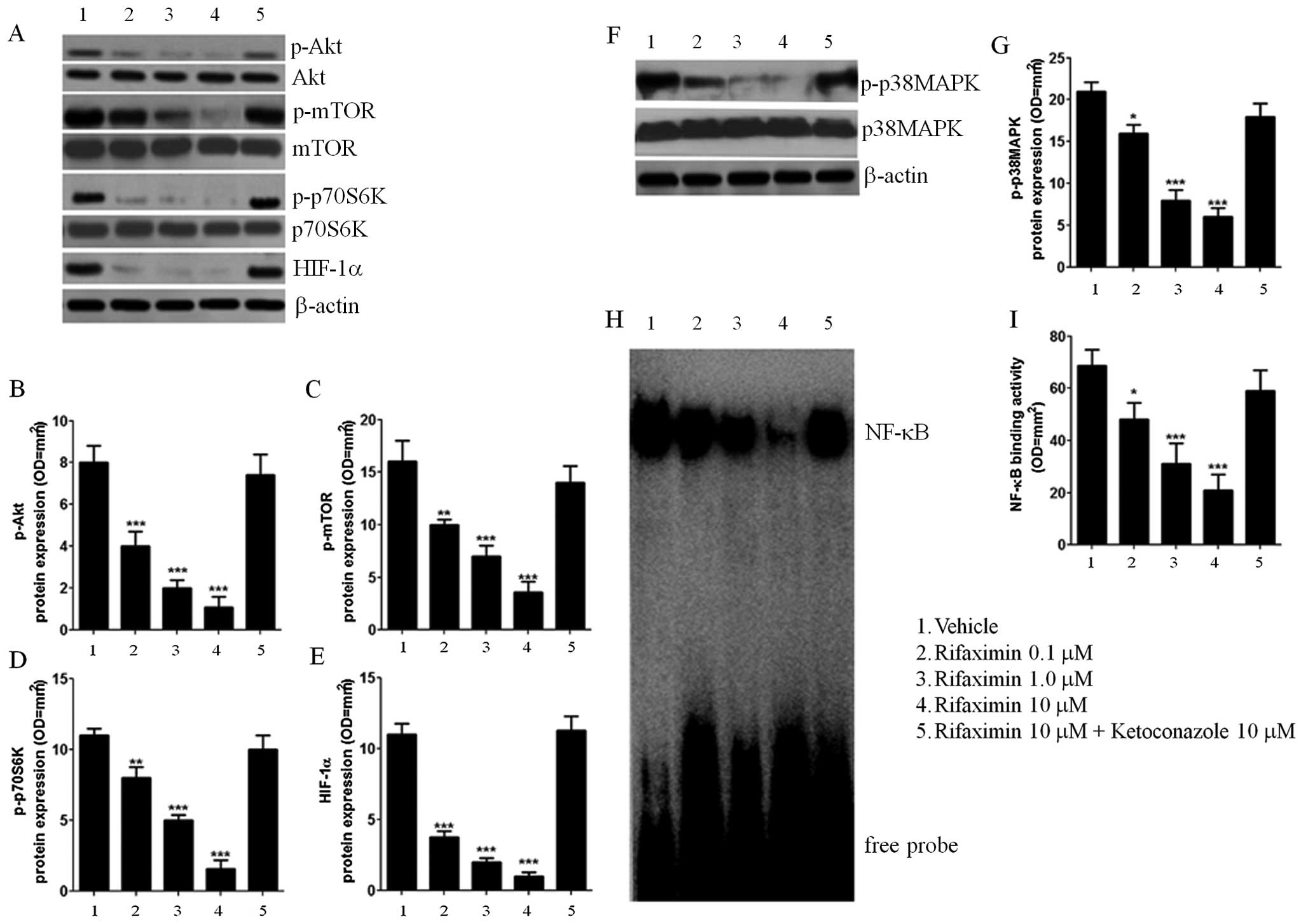
Akt/mTOR/p70S6K/HIF-1aandp38MAPK/NF-κBexpression
In order to further characterize the mechanisms
underlying the effect of rifaximin, the involvement of the
Akt/mTOR/p70S6K pathway was evaluated. Treatment with rifaximin
0.1, 1 and 10 μM resulted in a PXR-mediated and
concentration-dependent decrease in Akt phosphorylation (−50 −75
and −86% vs. untreated cells) and a significant reduction in the
phosphorylation rates of mTOR (−38, −56 and −78% vs. untreated
cells). Inhibition of p70S6K (−27, −55 and −85% vs. untreated
cells; Fig. 3A–D) was also seen at
24 h after rifaximin 0.1, 1 and 10 μM treatment. Rifaximin also
caused a significant and concentration-dependent decrease in HIF-1α
(−65, −82 and −92% vs. untreated cells); this effect was
counteracted by ketoconazole. Rifaximin 0.1, 1 and 10 μM
significantly blocked p38MAPK-phosphorylation (−24, −62 and −71%
vs. untreated cells; Fig 3F and G)
and inhibited NF-κB nuclear activation (−29, −55 and −61% vs.
untreated cells; Fig. 3H and I) as
seen by EMSA analysis; inhibition was significantly reversed by
ketoconazole treatment.
Discussion
In the present study, rifaximin caused a
concentration-dependent reduction of Caco-2 cell proliferation and
expression of PCNA vs. untreated cells. The migration of cells and
the expression of VEGF, VEGFR-2, NOS and iNOS were also reduced in
a concentration-dependent manner after treatment. Rifaximin
significantly blocked p38MAPK-phosphorylation, inhibited NF-κB
nuclear activation and p70S6K in Caco-2 cells. Moreover, MMP-2 and
MMP-9 levels, Akt phosphorylation, mTOR phosphorylation and HIF-1α
expression were also reduced in a concentration-dependent
manner.
PCNA is a marker of cell division and its
overexpression is associated with malignancy, infiltration of the
vasculature and tumor metastasis (21). It is considered to be a biomarker
of colorectal cancer (22). In
this study, rifaximin caused a progressive reduction of Caco-2 cell
proliferation and downregulated PCNA expression, thus, confirming
its antiproliferative effect. This effect was counteracted by the
selective PXR antagonist ketoconazole, suggesting a PXR-dependent
control of carcinoma cell growth rate.
The results of the present study also showed that
rifaximin effectively prevented the release of pro-angiogenic
mediators in Caco-2 cells and reduced the levels of VEGF, NO, VEGFR
and iNOS. Rifaximin-induced p38MAPK/NF-κB inhibition and reduction
in HIF-1α levels were significantly reversed by ketoconazole,
further confirming the involvement of PXR. HIF-1α is a
transcriptional modulator of pro-angiogenic factor release and its
expression is increased upon activation of the Akt/mTOR pathway
(23). Based on the results of the
present study, it can be stated that rifaximin acted at the PXR
site and further inhibited the release of VEGF and NO by negatively
interfering with both p38MAPK/NF-κB and p-Akt/p-mTOR-dependent
signaling pathways. Specifically, rifaximin markedly reduced the
phosphorylation of Akt, mTOR and p70S6K proteins in Caco-2 cells,
leading to inhibition of HIF-1α and blocking of the p38MAPK/ERK
phosphorylation signaling pathway, reducing the activation of
NF-κB. Also, rifaximin resulted in a significant and PXR-dependent
decrease in the expression of MMP-2 and MMP-9 in Caco-2 cells. MMPs
are involved in the growth and metastasis of cancer, specifically
by promoting angiogenesis, degrading the matrix barriers and
causing cell migration and proliferation (24). Thus, inhibition of MMP-2 and MMP-9
by rifaximin might be responsible for the observed reduction in the
migration of Caco-2 cells in vitro. Additionally, inhibition
of both NO and VEGF secretion by rifaximin may be responsible for
the consistent reduction of both iNOS and VEGFR expression in the
experimental setting. Cheng et al (16) studied the anti-inflammatory effect
of rifaximin in a colitis model and showed that rifaximin acted on
human PXR and caused NF-κB inhibition. Another study demonstrated
that rifaximin induced specific activation of PXR in PXR-humanized
mice and mediated the inflammation, cancer cell proliferation and
pro-apoptotic events in colon cancer (25). The results from the present study
are in line with these reports and further show that rifaximin is a
potent inhibitor of the release of angiogenic mediators.
One of the greatest advantages of this antibiotic is
its poor oral absorption and an optimum safety profile (23,26);
the unabsorbed drug stays in the gut, specifically in the colon,
long enough to locally exert its effects without any adverse
events.
Anti-angiogenic approaches in the treatment of colon
cancer have been extensively studied in the past decade, following
US Food and Drug Administration approval of the first anti-VEGF
monoclonal antibody, bevacizumab (27,28).
Despite their indisputable advantages as anticancer therapy,
anti-angiogenic compounds are limited by the complexity of the
converging pro-angiogenic pathways responsible for the
neo-vascularization process that makes angiogenesis in cancer
difficult to control, especially when the tumor starts developing
and metastasizing. This limits the use of anti-VEGF drugs, unless
they are used in combination with traditional chemotherapy
(29–31). Drugs capable of interfering with
the different steps of the pro-angiogenic and proliferative
processes in cancer cells are of special interest in an attempt to
identify an effective treatment. The results of the present study
indicate that rifaximin efficiently mediates the synergic
inhibition of multiple converging pathways involved in the growth
of cancer cells, pro-angiogenic mediator release and tissue
remodeling/invasion that could markedly increase the efficacy of
the anti-cancer therapy in CRC, when used in combination with VEGF
inhibitors. Based on the promising results seen with rifaximin
in vitro, further in vivo studies investigating a
possible multi-therapeutic approach with rifaximin plus traditional
VEGF inhibitors in CRC are warranted.
Acknowledgements
The authors would like to thank Nishad Parkar of
Springer Healthcare Communications for providing English and
scientific editing of the manuscript before submission. This
medical writing assistance was funded by Alfa Wasserman. C.C. is
post-doctoral fellow of the Fonds voor Wetenschappelijk Onderzoek
(FWO, Belgium).
References
|
1
|
Boyle P and Langman JS: ABC of colorectal
cancer: Epidemiology. BMJ. 321:805–808. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar :
|
|
3
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scaldaferri F, Vetrano S, Sans M, Arena V,
Straface G, Stigliano E, Repici A, Sturm A, Malesci A, Panes J, et
al: VEGF-A links angiogenesis and inflammation in inflammatory
bowel disease pathogenesis. Gastroenterology. 136:585–595.e585.
2009. View Article : Google Scholar
|
|
5
|
Ambs S, Merriam WG, Bennett WP,
Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG,
Billiar TR and Harris CC: Frequent nitric oxide synthase-2
expression in human colon adenomas: Implication for tumor
angiogenesis and colon cancer progression. Cancer Res. 58:334–341.
1998.PubMed/NCBI
|
|
6
|
Lala PK and Chakraborty C: Role of nitric
oxide in carcinogenesis and tumour progression. Lancet Oncol.
2:149–156. 2001. View Article : Google Scholar
|
|
7
|
Karar J and Maity A: PI3K/AKT/mTOR pathway
in angiogenesis. Front Mol Neurosci. 4:512011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pratheeshkumar P, Sreekala C, Zhang Z,
Budhraja A, Ding S, Son YO, Wang X, Hitron A, Hyun-Jung K, Wang L,
et al: Cancer prevention with promising natural products:
Mechanisms of action and molecular targets. Anticancer Agents Med
Chem. 12:1159–1184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gee E, Milkiewicz M and Haas TL: p38 MAPK
activity is stimulated by vascular endothelial growth factor
receptor 2 activation and is essential for shear stress-induced
angiogenesis. J Cell Physiol. 222:120–126. 2010. View Article : Google Scholar
|
|
10
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cianchi F, Cortesini C, Fantappiè O,
Messerini L, Schiavone N, Vannacci A, Nistri S, Sardi I, Baroni G,
Marzocca C, et al: Inducible nitric oxide synthase expression in
human colorectal cancer: Correlation with tumor angiogenesis. Am J
Pathol. 162:793–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reinacher-Schick A, Pohl M and Schmiegel
W: Drug insight: Antiangiogenic therapies for gastrointestinal
cancers - focus on monoclonal antibodies. Nat Clin Pract
Gastroenterol Hepatol. 5:250–267. 2008. View Article : Google Scholar
|
|
13
|
Mullen KD, Sanyal AJ, Bass NM, Poordad FF,
Sheikh MY, Frederick RT, Bortey E and Forbes WP: Rifaximin is safe
and well tolerated for long-term maintenance of remission from
overt hepatic encephalopathy. Clin Gastroenterol Hepatol.
12:1390–1397.e1392. 2014. View Article : Google Scholar
|
|
14
|
de la Cabada Bauche J and Dupont HL: New
developments in traveler's diarrhea. Gastroenterol Hepatol (NY).
7:88–95. 2011.
|
|
15
|
Calanni F, Renzulli C, Barbanti M and
Viscomi GC: Rifaximin: Beyond the traditional antibiotic activity.
J Antibiot (Tokyo). 67:667–670. 2014. View Article : Google Scholar
|
|
16
|
Cheng J, Shah YM, Ma X, Pang X, Tanaka T,
Kodama T, Krausz KW and Gonzalez FJ: Therapeutic role of rifaximin
in inflammatory bowel disease: Clinical implication of human
pregnane X receptor activation. J Pharmacol Exp Ther. 335:32–41.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mencarelli A, Renga B, Palladino G,
Claudio D, Ricci P, Distrutti E, Barbanti M, Baldelli F and
Fiorucci S: Inhibition of NF-κB by a PXR-dependent pathway mediates
counter-regulatory activities of rifaximin on innate immunity in
intestinal epithelial cells. Eur J Pharmacol. 668:317–324. 2011.
View Article : Google Scholar
|
|
18
|
Kota BP, Tran VH, Allen J, Bebawy M and
Roufogalis BD: Characterization of PXR mediated P-glycoprotein
regulation in intestinal LS174T cells. Pharmacol Res. 62:426–431.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar
|
|
20
|
Renault-Mihara F, Beuvon F, Iturrioz X,
Canton B, De Bouard S, Léonard N, Mouhamad S, Sharif A, Ramos JW,
Junier MP, et al: Phosphoprotein enriched in astrocytes-15 kDa
expression inhibits astrocyte migration by a protein kinase C
delta-dependent mechanism. Mol Biol Cell. 17:5141–5152. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guzi ska-Ustymowicz K, Pryczynicz A,
Kemona A and Czyzewska J: Correlation between proliferation
markers: PCNA, Ki-67, MCM-2 and antiapoptotic protein Bcl-2 in
colorectal cancer. Anticancer Res. 29:3049–3052. 2009.
|
|
22
|
Yang HB, Hsu PI, Chan SH, Lee JC, Shin JS
and Chow NH: Growth kinetics of colorectal adenoma-carcinoma
sequence: An immunohistochemical study of proliferating cell
nuclear antigen expression. Hum Pathol. 27:1071–1076. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cacciottolo TM, Kingdon A and Alexander
GJ: Rifaximin is largely safe and well tolerated but caution is
necessary when taken with statins. Clin Gastroenterol Hepatol.
12:17652014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cathcart J, Pulkoski-Gross A and Cao J:
Targeting matrix metalloproteinases in cancer: Bringing new life to
old ideas. Genes Dis. 2:26–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng J, Fang ZZ, Nagaoka K, Okamoto M, Qu
A, Tanaka N, Kimura S and Gonzalez FJ: Activation of intestinal
human pregnane X receptor protects against azoxymethane/dextran
sulfate sodium-induced colon cancer. J Pharmacol Exp Ther.
351:559–567. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirota SA: Understanding the molecular
mechanisms of rifaximin in the treatment of gastrointestinal
disorders: A focus on the modulation of host tissue function. Mini
Rev Med Chem. 16:206–217. 2015. View Article : Google Scholar
|
|
27
|
Li J and Saif MW: Current use and
potential role of bevacizumab in the treatment of gastrointestinal
cancers. Biologics. 3:429–441. 2009.PubMed/NCBI
|
|
28
|
Giantonio BJ, Catalano PJ, Meropol NJ,
O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA and Benson AB III;
Eastern Cooperative Oncology Group Study E3200. Bevacizumab in
combination with oxaliplatin, fluorouracil, and leucovorin
(FOLFOX4) for previously treated metastatic colorectal cancer:
Results from the Eastern Cooperative Oncology Group Study E3200. J
Clin Oncol. 25:1539–1544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hurwitz HI, Fehrenbacher L, Hainsworth JD,
Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF and
Kabbinavar F: Bevacizumab in combination with fluorouracil and
leucovorin: An active regimen for first-line metastatic colorectal
cancer. J Clin Oncol. 23:3502–3508. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar
|
|
31
|
Huber PE, Bischof M, Jenne J, Heiland S,
Peschke P, Saffrich R, Gröne HJ, Debus J, Lipson KE and Abdollahi
A: Trimodal cancer treatment: Beneficial effects of combined
antiangiogenesis, radiation, and chemotherapy. Cancer Res.
65:3643–3655. 2005. View Article : Google Scholar : PubMed/NCBI
|