Introduction
Breast cancer is one of the leading causes of
mortality among women in world due to various factors such as
aggressive invasion, early metastasis, resistance to existing
chemotherapeutic drugs and high mortality (1). Doxorubicin (Dox) is an anthracycline
of wide spectrum and an essential component of many treatment
regimens for tumors and it is broadly considered the most active
agent for breast cancer therapy (2). Dox shows a variety of molecular
mechanisms at cellular level to explain its role as intercalation
between two nitric bases of double DNA helix, generation of free
radicals leading to rupture of DNA strands, inhibition of the
respiratory chain enzymes in mitochondria, membrane lipid
oxidation, interference with DNA unwinding and helicase activity,
and induction of apoptosis in response to topoisomerase II
inhibition (3).
Apoptosis is a process of cell death in
multicellular organism and its regulation is important for normal
growth, homeostasis, development and cancer treatment. Alterations
of normal induction to apoptosis can cause growth of abnormal
cells, uncontrolled cell division and accumulation of mutations
(4). Therefore, regulation of
apoptosis plays an important role in the treatment of cancer
(5).
The group of B-cell lymphoma-2 protein (Bcl-2) is a
key regulator in the molecular mechanisms of apoptosis (6). This family includes protein function
as Bcl-2-associated protein X (Bax) promoter that induces and
accelerates cell death when associated with the formation of
Bax/Bax homodimer. Whereas, Bcl-2 and B-cell lymphoma extra-large
(Bcl-xL) antiapoptotic protein expression are repressed by Bax
through the formation of Bcl-2/Bax or Bcl-xL/Bax heterodimers
(7–9). Complex networks of interactions
between Bcl-2 family members both cytosolic and mitochondrial
determine the fate of the cell for death or survival (10).
Caspase family is a group of cysteine proteases that
activate apoptosis execution in two main pathways. The first
pathway is extrinsic death receptor-dependent and the second
pathway depends on mitochondria. Induction of both apoptotic
pathway are associated with the activation of caspases (11). Caspase-8 is the main death receptor
signaling caspase (12). Effector
caspase-3 is a frequently activated death protease that catalyzes
the specific cleavage of many key cellular proteins. Caspase-3
activation pathways have been identified to be both dependent and
non-dependent of mitochondrial cytochrome c release and
caspase-9 function (13).
Caspase-3 is required for some typical hallmarks of apoptosis and
it is indispensable for apoptotic chromatin condensation and DNA
fragmentation. Thus, caspase-3 is essential for processes
associated with cell rupture and the formation of apoptotic bodies
(14). Caspase-8 is an initiator
caspase and activates apoptosis when death receptors are stimulated
but it is also required by other apoptotic stimuli (15).
It has been proposed that production of ROS
(reactive oxygen species) leads to an imbalance between ROS
generation and degradation by cellular antioxidant mechanisms, this
deregulation is called oxidative stress (16,17).
High levels of ROS participate in genomic instability and lead to
aggressiveness and progression of carcinogenesis. This process is
accompanied by the activation of various gene and transcription
factors in cancer cells (18).
Superoxide radical is generated in many cellular processes mainly
by the electron transport chain into mitochondria (19).
Dox causes oxidative damage in tumor cells and it is
based on redox cycling accompanied by the release of iron from
cells. The drug-iron complex catalyzes O2 and
H2O2 conversion into more potent radicals.
Oxidative damage mechanism has been considered an important
antitumoral mechanism in cancer cells (3).
Superoxide dismutase 2 (SOD2) is a protein of the
mitochondrial matrix that catalyzes the formation of superoxide
radical into hydrogen peroxide (20). Overexpression of SOD2 results in a
marked suppression of cell growth (21). SOD2 and hydrogen peroxide levels
are higher in MDA-MB-231 than in MCF-7 cell lines (22).
Nuclear factor kappa-B (NF-κB) is a transcription
factor that regulates the expression of hundreds of genes that are
involved in regulating cell growth, differentiation, development
and apoptosis pathway in the cytoplasm (23,24).
Dox can activate the ubiquitin proteasome system that regulates the
NF-κB transcription factor and overactivation of NF-κB leads to an
increase of many cell functions that have been shown in several
tumor types (25).
The aim of the present study was to evaluate the
influence of Dox on apoptosis and oxidative stress in three breast
cancer cell lines the MCF-10F, which is non-tumorigenic not
expressing estrogen receptor (ER), progesterone receptor (PR) or
human epidermal growth factor receptor 2 (HER2); The MCF-7 cell
line, a tumorigenic triple-positive expressing ER, PR and HER2; and
MDA-MB-231, a tumorigenic triple-negative breast cancer cell
line.
Materials and methods
Cell lines and culture conditions
Three human breast cell lines were used for all the
experiments, the control cell line MCF-10F, a luminal-like
(estrogen receptor positive) MCF-7 and a triple-negative breast
cancer cell line MDA-MB-231. MCF-10F cell line (ATCC, Manassas, VA,
USA), a spontaneously immortalized breast epithelial cell line,
retains all the characteristics of normal epithelium in
vitro including anchorage-dependence, non-invasiveness and
non-tumorigenicity in nude mice. MCF-10F cell line was grown in
DMEM/F-12 (1:1) medium supplemented with antibiotics [100 U/ml
penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B (all
from Life Technologies, Grand Island, NY, USA)] and 10 μg/ml and 5%
equine serum (Biofluids, Rockville, MD, USA), 0.5 μg/ml
hydrocortisone (Sigma, St. Louis, MO, USA) and 0.02 μg/ml epidermal
growth factor (Collaborative Research, Bedford, MA, USA) were
added. The cell line MCF-7 was cultivated in the Dulbecco's
modified Eagle's medium (Sigma-Aldrich ChemieGmbH, Munich, Germany)
supplemented with 10% fetal calf serum (FCS; Sigma-Aldrich) at 37°C
and saturated air with 5% CO2. Human triple-negative
breast cancer cells MDA-MB-231 were purchased from the American
Type Culture Collection and were maintained in DMEM medium
supplemented with 10% FBS, 100 IU/ml penicillin, and 100 mg/ml
streptomycin and incubated at 37°C with CO2.
Drug treatment
Dox and dimethyl sulfoxide (DMSO) were purchased
from Sigma-Aldrich. Each Dox concentration was added when cells
reached 70% confluence and cultured for 24 and 48 h. Concentrations
of 1, 2, 4 and 8 μM were prepared with DMSO 0.05%.
Cell viability
Cell viability of MCF-10F, MCF-7 and MDA-MB-231 cell
lines was determined by using 3-(4,5-
dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) which
evaluated the percentage of viable cells. Cells were seeded into
24-well plate with concentration of 5×104 cells/well and
the following concentrations of Dox were tested 0, 1, 2, 4 and 8
μM. Four wells remained untreated as control. MTT assay was carried
out for 24 and 48 h after treatments. Mixing solution was prepared
using 2 mg of MTT (Sigma-Aldrich) powder in 1 ml PBS. The culture
medium was changed with 150 μl fresh media plus 50 μl MTT reagent
(2 mg/ml in PBS); the cell-free wells were considered as blank
controls. Cells were incubated at 37°C with CO2 at 5%
and a humidified atmosphere for 4 h. Then, the MTT solution was
removed and 200 μl of DMSO were added to each well. The plate was
maintained for 30 min at 37°C and then the OD (optical density) of
the wells was determined at 550 nm through a spectrophotometric
microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Western blot analysis
Cells were lysated with 0.5 ml lysis buffer (pH 7.2)
[Tris-Base (50 mM), EDTA (1 mM), NaCl (100 mM), PMSF (1 mM), and
centrifuged at 13,400 rpm for 10 min]. Cellular proteins from the
supernatant were dissolved in SDS-PAGE sample solution [60 mM Tris,
pH 6.5, 10% (w/v) glycerol, 5% (w/v) β-mercaptoethanol, 20% (w/v)
SDS, and 0.025% (w/v) bromophenol blue] and denatured by boiling
(100°C for 5 min). The total amount of protein used was 50 μg per
lane for Bax (sc-493), Bcl-2 (sc-7382), caspase-8 (sc-56070),
caspase-3 (sc-7148), SOD2 (sc-18503) and NF-κB (sc-7386) with
standard protein markers from Bio-Rad Laboratories (Hercules, CA,
USA). After fractionation by SDS-PAGE on gels (5×8 cm), proteins
were electroblotted onto polyvinylidene difluoride membrane using a
blotting apparatus (Bio-Rad Laboratories). Pre-stained SDS-PAGE
(standards) blots were blocked for 1 h in 5% defatted dry
milk-TBS-0.5% Tween and then incubated overnight at room
temperature with corresponding primary antibodies. The following
primary antibodies were used Bax (B-9) 1:4,000; Bcl-2 (N-19)
1:5,000; caspase-8 (8CSP03) 1:200; caspase-3 (H-277) 1:200; NF-κB
(4D1) 1:200; SOD2 (A-2) 1:200; β-actin (C-4) 1:5,000. Secondary
antibodies used were goat anti-mouse (Sc-2005) 1:5,000 and goat
anti-rabbit (Sc-2030) 1:5,000. All primary and secondary antibodies
were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Film was analyzed with Adobe Photoshop for Windows 7 software to
obtain the relative grade of luminescence to calculate the fold
expression.
Differential display reverse
transcriptase-PCR (DDRT-PCR) assessment
Total RNA was isolated using TRIzol reagent
(Invitrogen Corp., Long Island, NY, USA). The purified RNA sample
was first measured by a spectrophotometer (the ratio of absorbance
reading at 260/280 nm >1.8) and then electrophoresed on 1% (w/v)
agarose gel to check its quality and purity. RNA (2 μg) was used
for reverse transcriptase-polymerase chain reaction (DDRT-PCR). The
first-strand cDNA was synthesized with primer oligo-(dT) to
hybridize to 3′-poly-(A) tails. To confirm their similar expression
in all samples human β-actin was used as a control amplifier set.
Table I shows the primers of genes
selected for DDRT-PCR analyses, including the symbol and type of
primers of Bax, Bcl-xL, caspase-8, caspase-9, caspase-3, SOD2,
NF-κB and β-actin. All primers were obtained from Integrated DNA
Technologies (Coralville, IA, USA). To confirm the differential
gene expression 2 μl of cDNA and a varied number of PCR cycles (20,
25 and 30) were used to generate gene-specific probes. A linear
increase was observed in product generation in all cases
(log-phase). Then, 2 μl of cDNA and 30 cycles for Bax, Bcl-xL and
NF-κB; and 25 cycles for β-actin for PCR were used. Table II shows the protocol of DDRT-PCR
analyses, PCR step, temperature and time. In each PCR initial cycle
of 94°C for 10 min was necessary to activate Taq polymerase. Final
cycle of 72°C for 5 min was utilized to complete the amplicon
produced. The PCR product was run on a 2% (w/v) agarose gel with
ethidium bromide 5 mg/ml. Differentially expressed gene-specific
DNA bands were then photographed and analyzed with Adobe Photoshop
software to obtain the relative grade of luminescence to calculate
the fold-change of expression.
 | Table IPrimers of genes selected for
differential display reverse transcriptase-PCR (DDRT-PCR)
analysis. |
Table I
Primers of genes selected for
differential display reverse transcriptase-PCR (DDRT-PCR)
analysis.
| Gene name | PCR primer
sequencesa |
|---|
| β-actin | F: TGC CGA CAG GAT
GCA GAA G
R: GCC GAT CCA CAC GGA GTA CT |
| Bax | F: GCG AGT GTC TCA
AGC GCA TC
R: CCA GTT GAA GTT GCC GTC AGA A |
| Bcl-xL | F: CTG AAT CGG AGA
TGG AGA CC
R: TGG GAT GTC AGG TCA CTG AA |
|
Caspase-8 | F: CAT CCA GTC ACT
TTG CCA GA
R: GCA TCT GTT TCC CCA TGT TT |
|
Caspase-9 | F: CCA GAG ATT GCG
AAA CCA GAG G
R: GAG CAC CGA CAT CAC CAA ATT C |
|
Caspase-3 | F: CAG AAC TGG ACT
GTG GCA TTG
R: GCT TGT CGG CAT ACT GTT TCA |
| SOD2 | F: CCC TGG AAC CTC
ACA TCA AC
R: CCT TGC AGT GGA TCC TGA TT |
| NF-κB
(p65) | F: ATC TGC CGA GTG
AAC CGA AAC T
R: CCA GCC TGG TCC CGT GAA A |
 | Table IIProtocol of differential display
reverse transcriptase-PCR (DDRT-PCR). |
Table II
Protocol of differential display
reverse transcriptase-PCR (DDRT-PCR).
| Gene symbol | Cycles (no.) | PCR step |
Temperature/time |
|---|
| β-actin | 25 | Annealing | 58°C/30 sec |
| Bax | 30 | Annealing | 58°C/30 sec |
| Bcl-xL | 25 | Annealing | 55°C 30 sec |
|
Caspase-8 | 25 | Annealing | 56°C/30 sec |
|
Caspase-9 | 30 | Annealing | 58°C/30 sec |
|
Caspase-3 | 30 | Annealing | 58°C/30 sec |
| SOD2 | 25 | Annealing | 57°C/30 sec |
| NF-κB | 30 | Annealing | 58°C/30 sec |
Measurement of H2O2
concentration
To determine the hydrogen peroxide level the Amplex
Red Hydrogen Peroxide/Peroxidase assay kit purchased from Molecular
Probes (Eugene, OR, USA) was used. The protocol was according to
the manufacturer's procedure. HRP stock solution (10 U/ml) was
diluted (0–2 mU/ml) for the standard curve. A volume of 50 μl was
used for each reaction of individual wells of a microplate. The
microplate with reactions was incubated at room temperature for 30
min and protected from light. The absorbance was measured in a
microplate reader at 560 nm. The background was corrected for each
point subtracting the value derived from the no-HRP control.
Statistical analysis
Results of the cell growth assay were presented as
mean ± standard error (SE) in three independent experiments and the
comparison between treated and control groups was carried out by
ANOVA and Dunnett's test. P<0.05, P<0.01 and P<0.001 were
considered significant. Analysis of gene and protein expression was
performed using the Student's t-test to compare control with
treatment. IC50 dose at 50% was calculated by a
non-linear regression curve using GraphPad Prism 5.0 for Windows
(GraphPad Software, Inc., San Diego, CA, USA).
Results
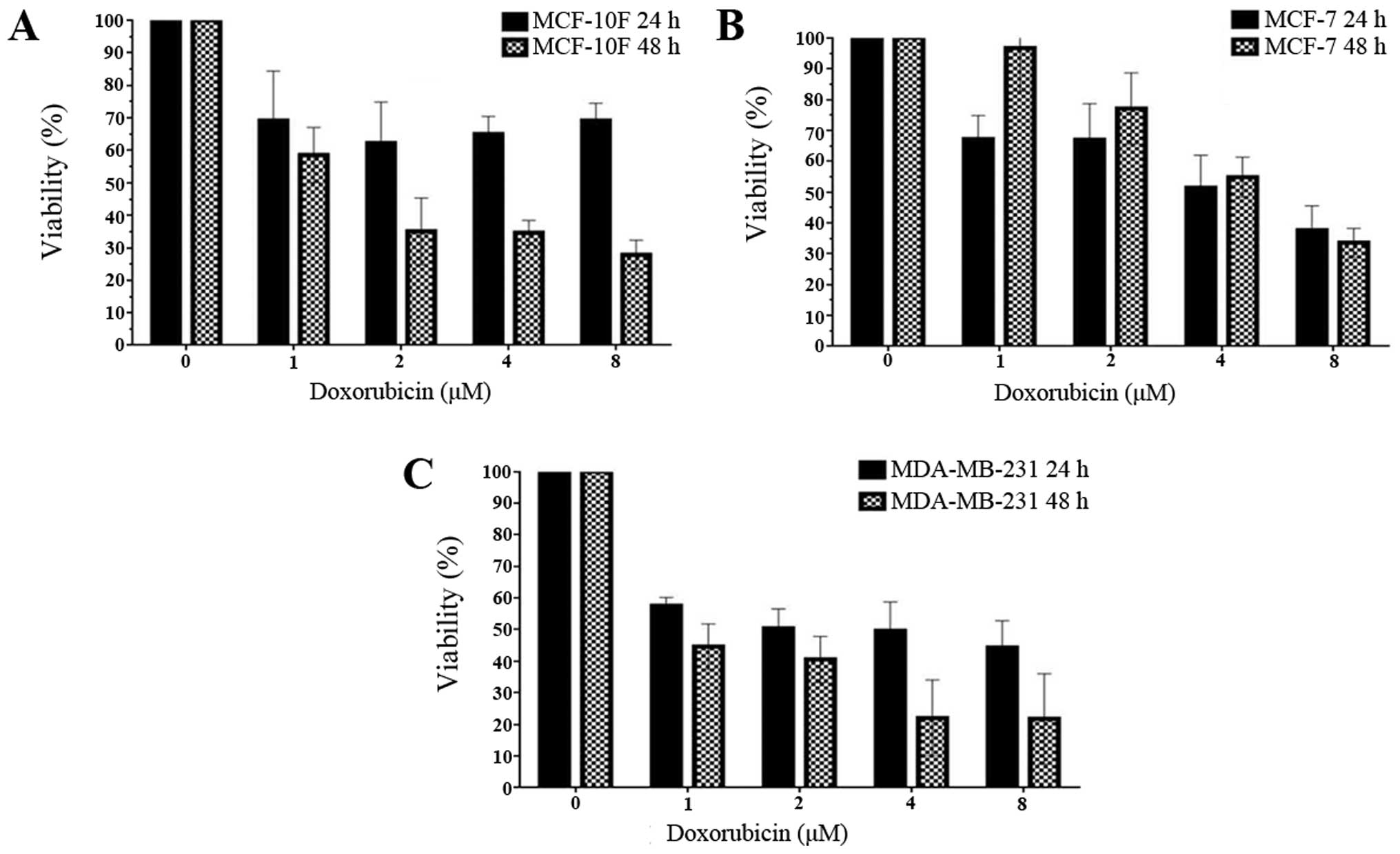
Cell viability and IC50
values
To study viability induced by Dox the breast cancer
cell lines MCF-10F, MCF-7 and MDA-MB-231 were used. They were
treated with increasing doses of Dox for 24 and 48 h and the
metabolic activity was quantified by MTT assay. Fig. 1 shows that the IC50
(drug concentration required to inhibit cell growth by 50%) was
determined by different concentrations (1, 2, 4 and 8 μM) in each
breast cancer cell line for 24 and 48 h. These values indicated
that IC50 was 1 μM for both MCF-10F and MDA-MB-231 cell
lines and for MCF-7 cell line it was 4 μM. This assay showed that
increased concentration of Dox decreased the viability of all three
cell lines in a time- and dose-dependent manner for 48 h. On the
other hand, MCF-7 cell line was dose-dependent after 24-h
treatment.
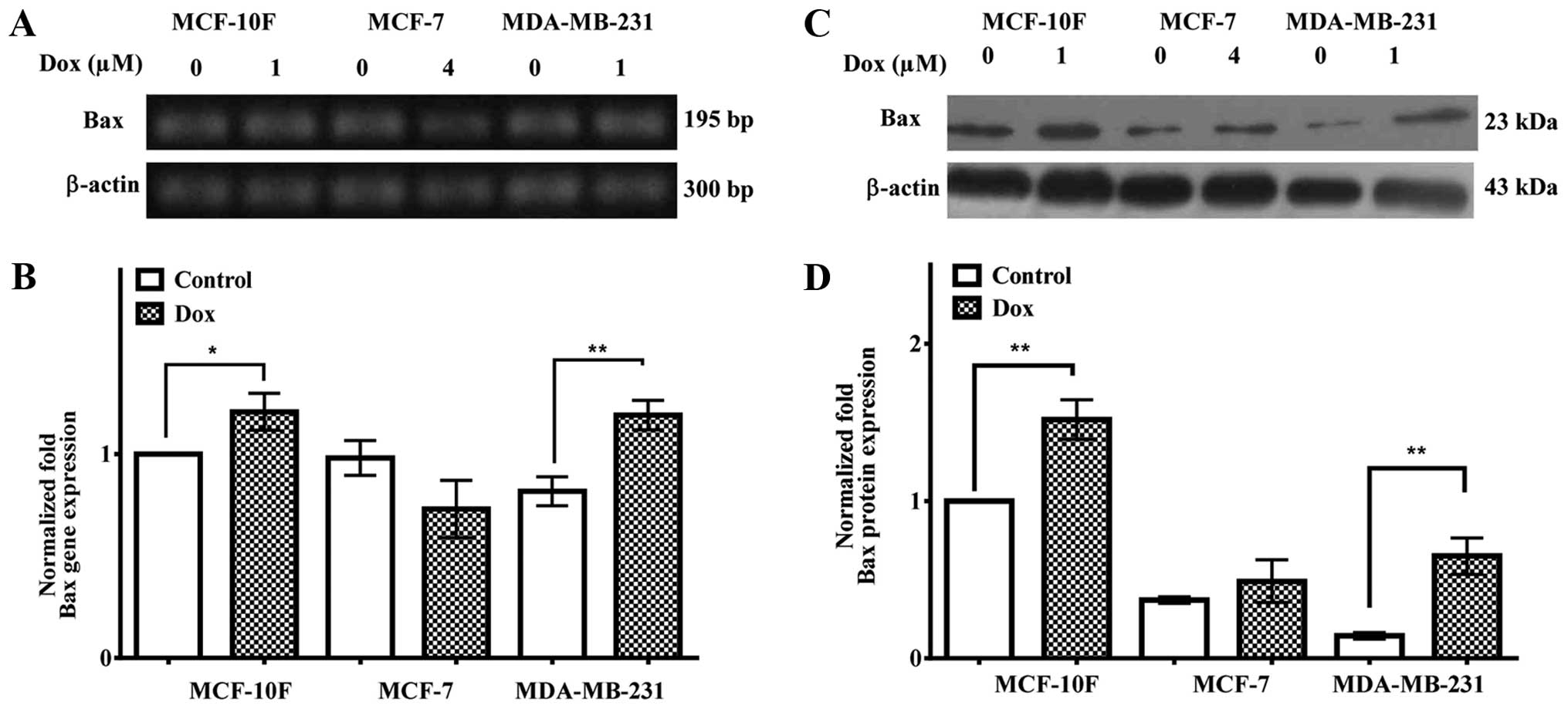
Bax gene and protein expression
Bax gene and protein expression in MCF-10F, MCF-7
and MDA-MB-231 cell lines were determined with 1, 4 and 1 μM of
Dox, respectively, for 48 h. Results in Fig. 2A and quantified in B showed that
Bax gene expression was upregulated in MCF-10F (P<0.05) and
MDA-MB-231 (P<0.05) cell lines but it was downregulated in the
MCF-7 cell line. Fig. 2C and
quantified in D indicated that Bax protein expression was
upregulated in MCF-10F and MDA-MB-231 cell lines (P<0.01) but
MCF-7 cells did not show any significant increase.
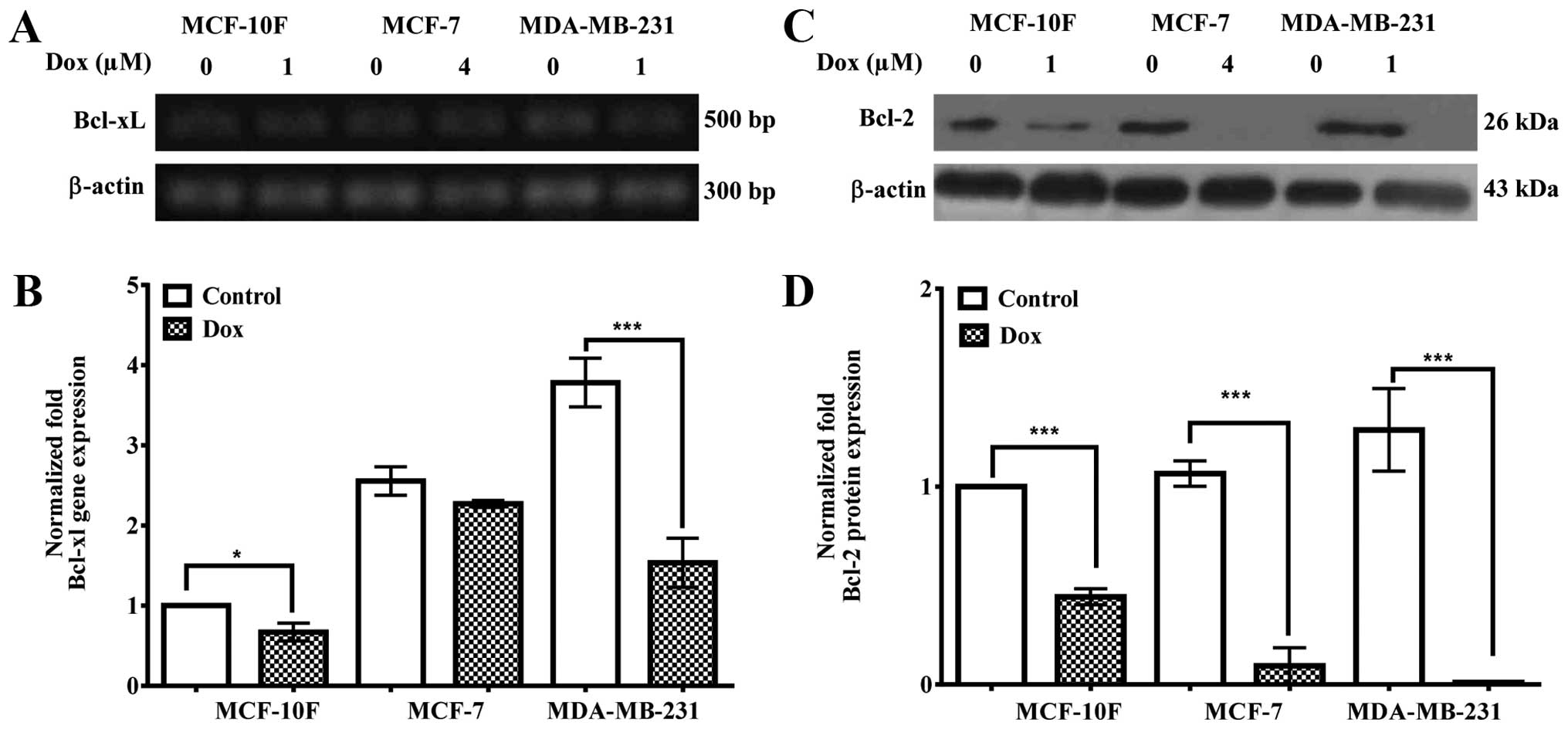
Bcl-xL gene and Bcl-2 protein
expression
Fig. 3A and
quantified in B indicate that Bcl-xL gene expression was
significantly (P<0.05) downregulated in MCF-10F and MDA-MB-231
(P<0.001) cells, but MCF-7 cells did not show significant
decrease. Fig. 3C and quantified
in D show that Bcl-2 protein expression was significantly
(P<0.001) decreased in all breast cancer cell lines (MCF-10F,
MCF-7 and MDA-MB-231).
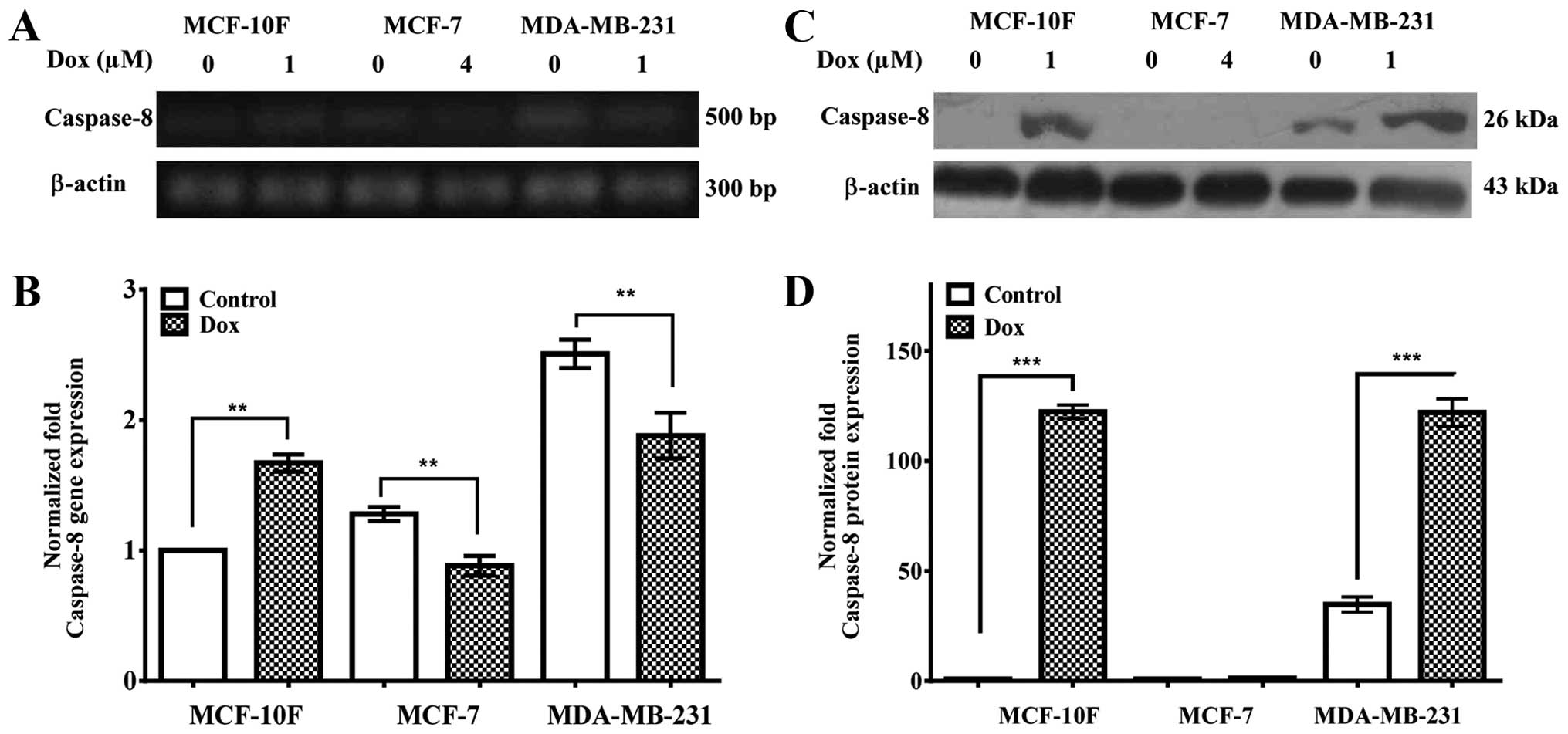
Caspase-8, caspase-9 and caspase-3 gene
and protein expression
Caspase-8, caspase-9 and caspase-3 gene and protein
expression were studied in MCF-10F, MCF-7 and MDA-MB-231 cell
lines. Fig. 4A and quantified in B
show that caspase-8 gene expression was upregulated in MCF-10F
(P<0.01), but it was downregulated in MCF-7 (P<0.01) and
MDA-MB-231 (P<0.01) cells. Fig.
4C and quantified in D indicated that caspase-8 protein
expression was significantly (P<0.001) upregulated in MCF-10F
and MDA-MB-231 cells when treated with Dox; though it was not
detected in MCF-7 cells.
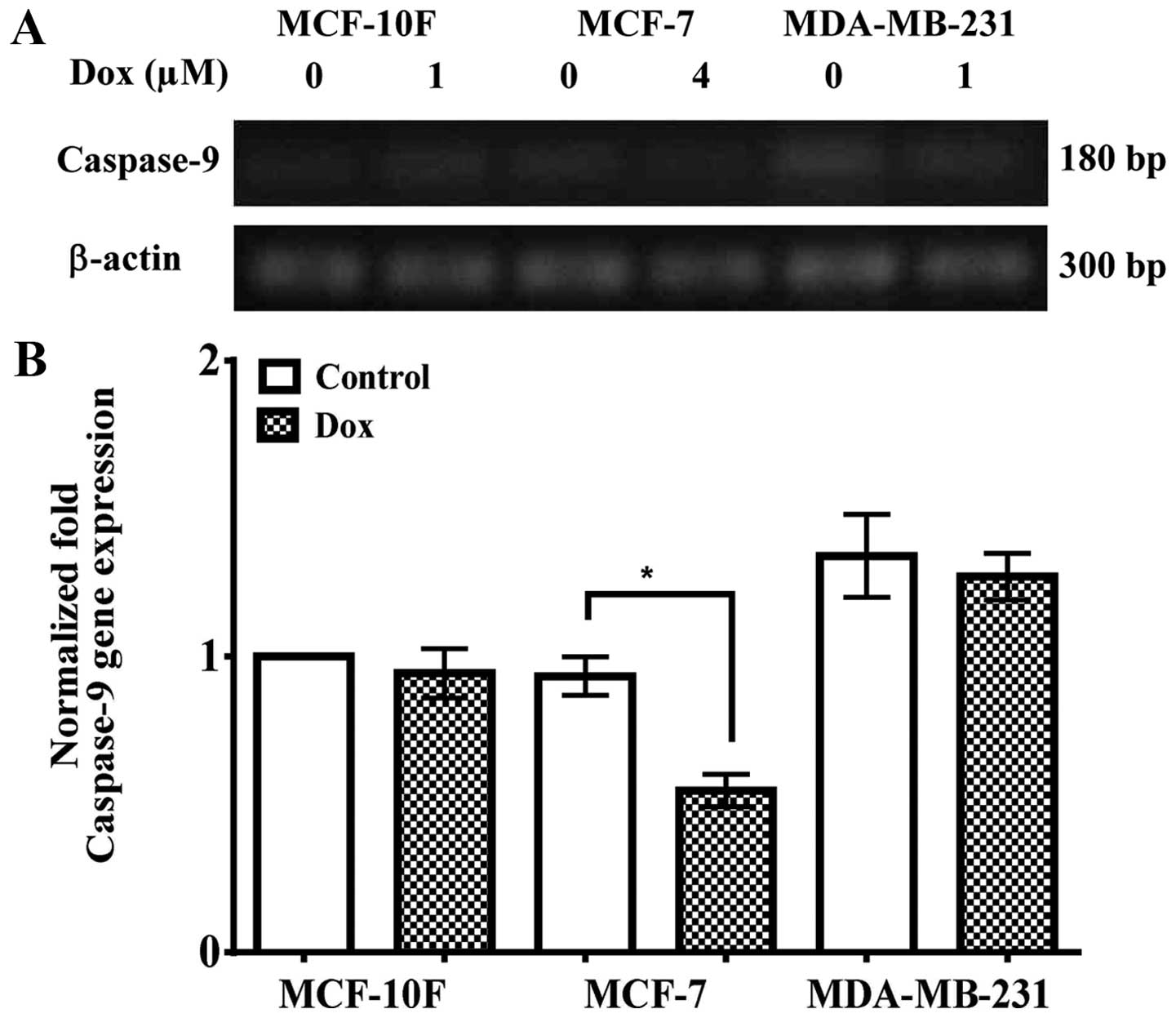
Fig. 5A and
quantified in B indicated that caspase-9 gene expression was higher
in MDA-MB-231 cell line than in MCF-10F and MCF-7 cell lines in
comparison with control cell lines. It also showed a decrease
(P<0.05) in caspase-9 gene expression in MCF-7 cells in
comparison to its counterparts. However, there was no difference in
MCF-10F and MDA-MB-231 cell lines.
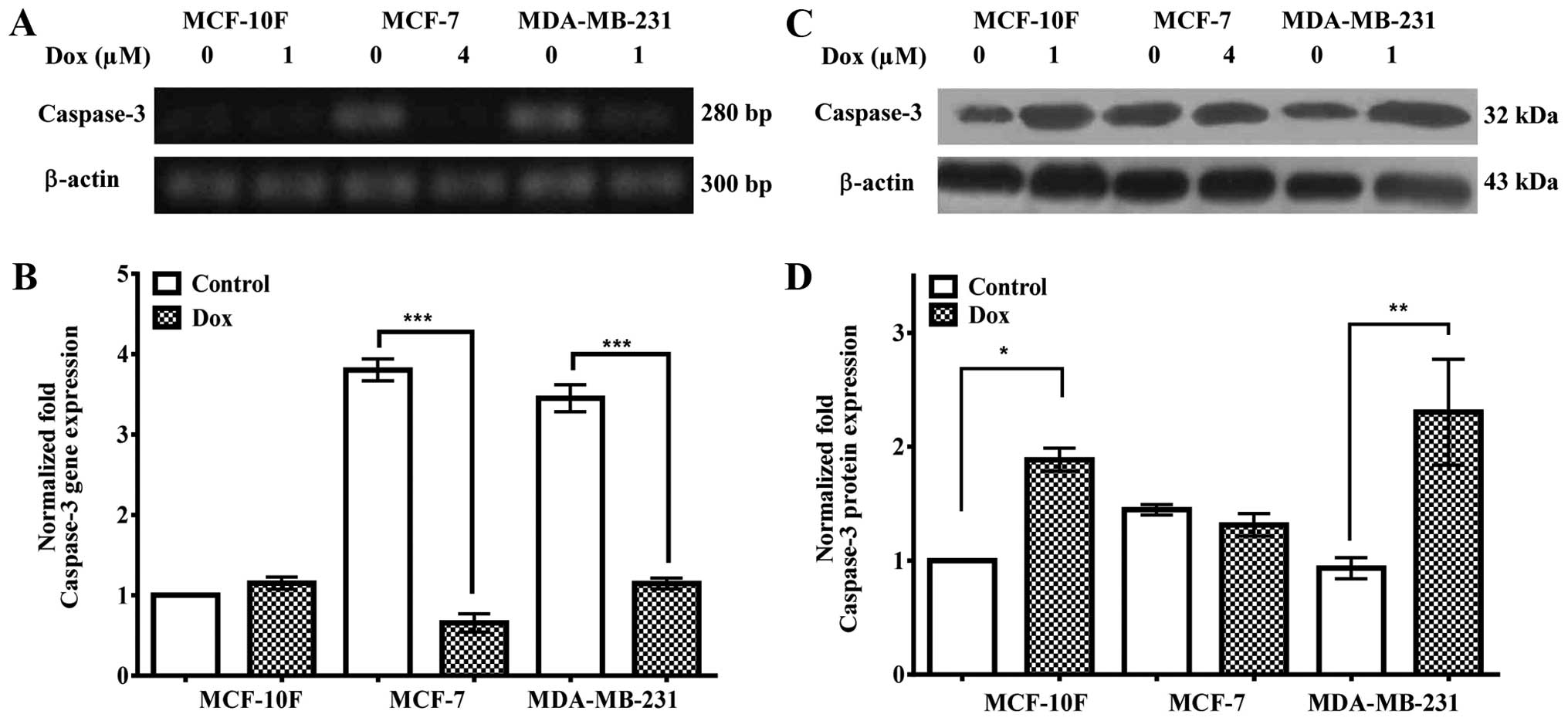
Fig. 6A and
quantified in B, indicate that caspase-3 gene expression was
downregulated in MCF-7 (P<0.001) and MDA-MB-231 (P<0.001)
cells. However, there was no difference in MCF-10F cell line. MCF-7
and MDA-MB-231 cells expressed higher level of caspase-3 gene
expression than MCF-10F control cells. Results in Fig. 6C and quantified in D, show an
increase in caspase-3 protein expression in MCF-10F (P<0.05) and
MDA-MB-231 (P<0.01) cells in comparison to its counterparts.
However, there was no significant difference in MCF-7 cells with
its counterpart.
SOD2 gene and protein expression
SOD2 gene and protein expression were analyzed in
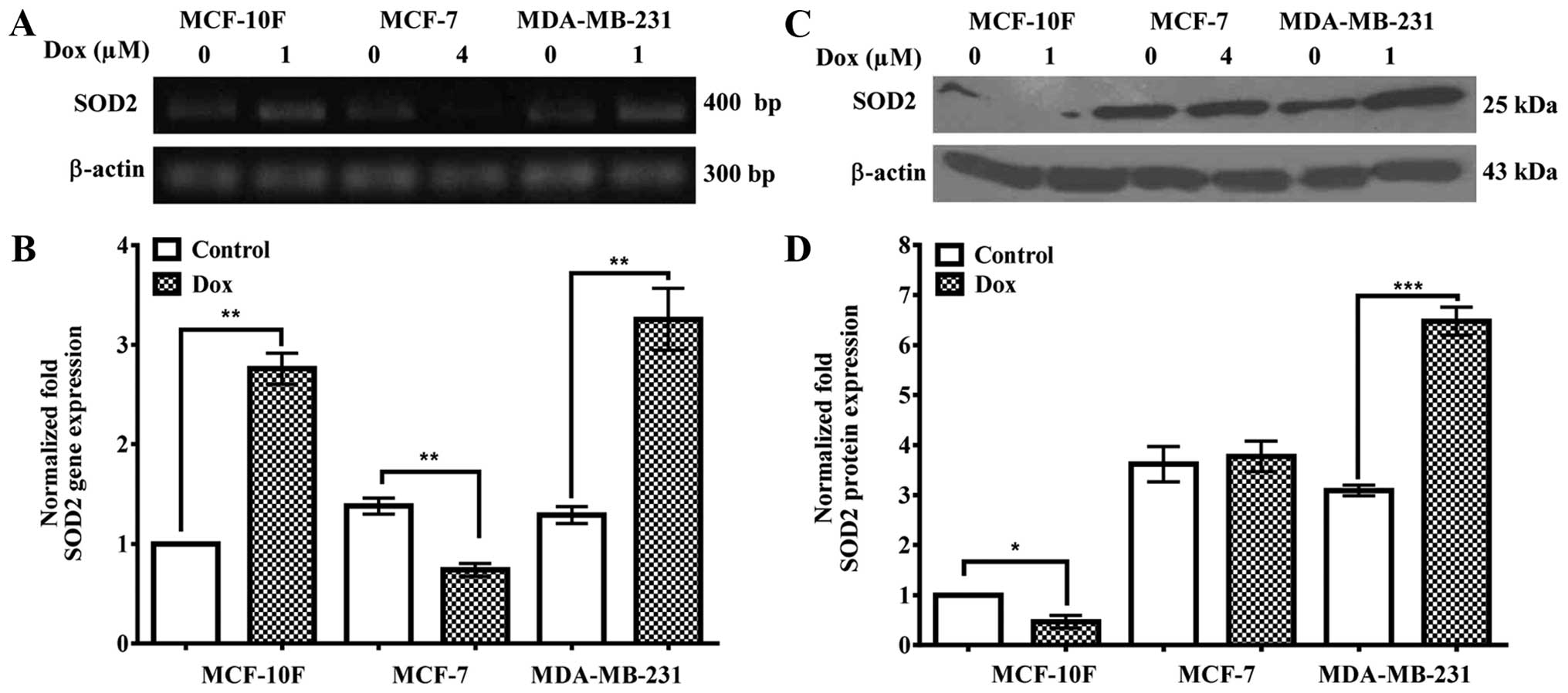
Dox-treated cell lines. Results in Fig. 7A and quantified in B, show an
increase (P<0.01) in SOD2 gene expression in MCF-10F and
MDA-MB-231 cells, whereas MCF-7 cells are downregulated
(P<0.01). Fig. 7C and
quantified in D showed a decrease in SOD2 protein expression
significantly (P<0.05) decreased in MCF-10F and it was
upregulated in MDA-MB-231 cells (P<0.001). MCF-7 cells did not
express any significant difference.
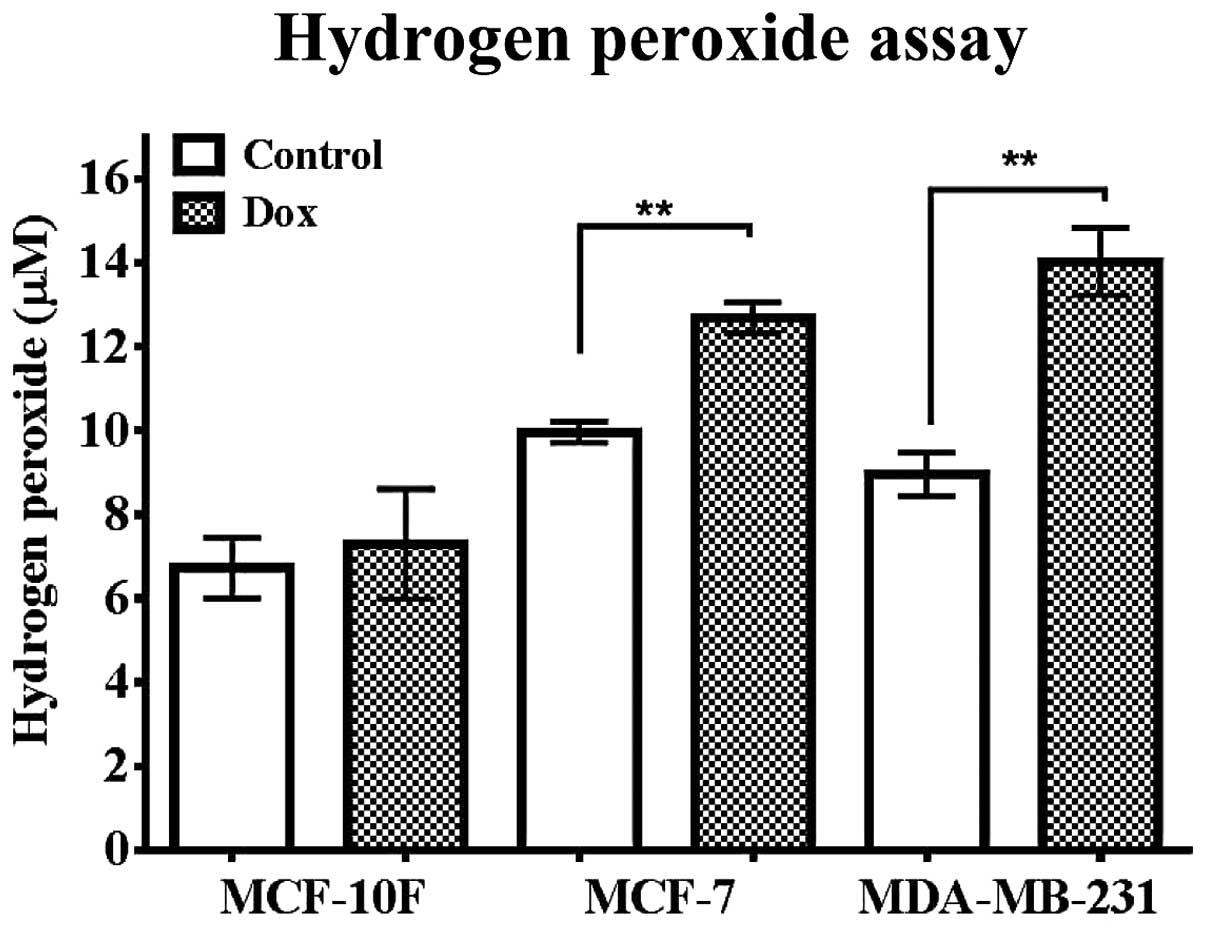
H2O2 level in
breast cancer cell lines exposed to Dox
The influence of Dox in H2O2
production was studied in MCF-10F, MCF-7 and MDA-MB-231 cell lines.
Fig. 8 shows that untreated MCF-7
cell line expressed higher level of H2O2 than
MCF-10F (P<0.05) control cells. MCF-10F cells did not express
significant differences in the production of
H2O2 when treated with Dox. MCF-7 (P<0.01)
and MDA-MB-231 (P<0.01) cells showed higher concentrations of
H2O2 production in comparison with its
counterparts.
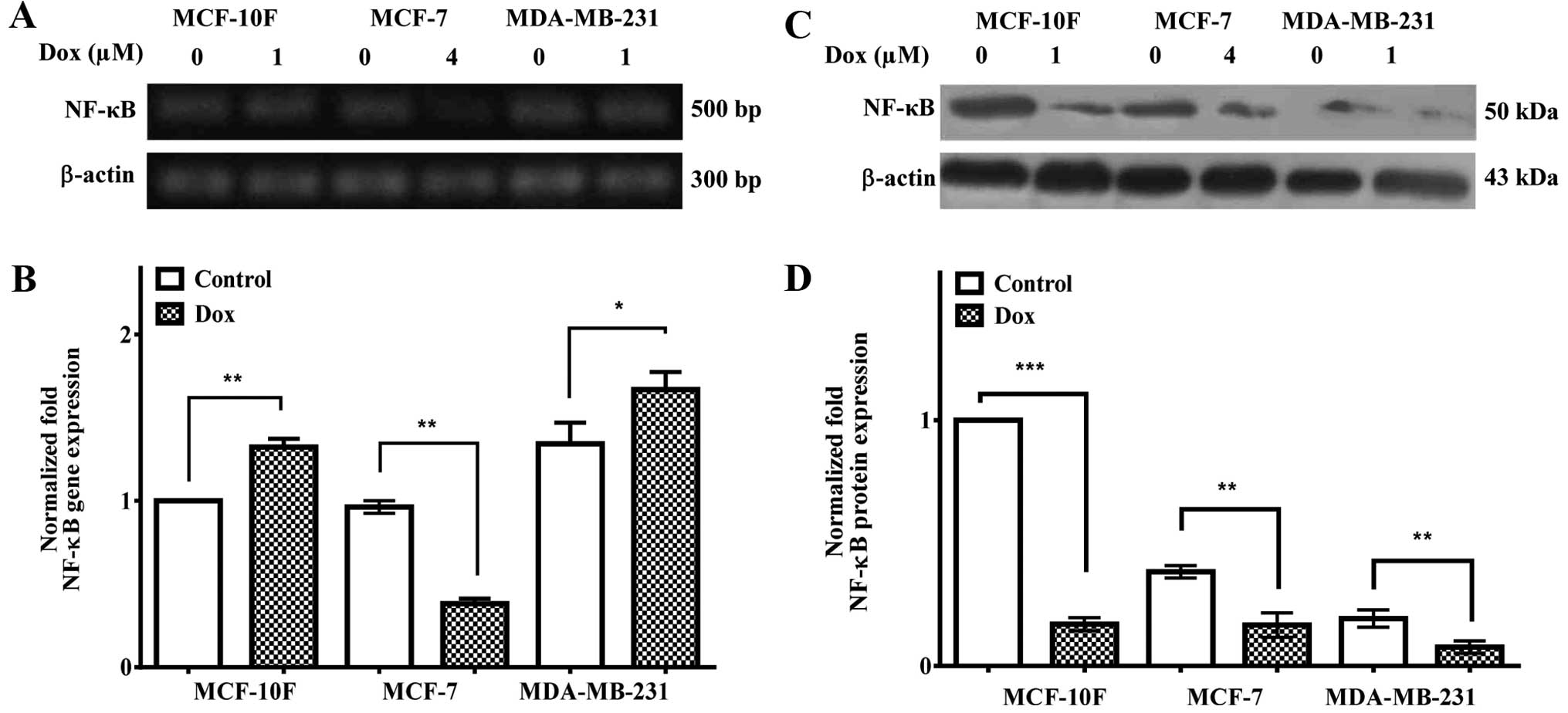
NF-κB gene and protein expression
Fig. 9A and
quantified in B, show increased NF-κB gene expression in MCF-10F
(P<0.01) and MDA-MB-231 (P<0.05) but it was downregulated in
MCF-7 (P<0.01). Results in Fig.
9C and quantified in D, showed decreased NF-κB protein
expression in MCF-10F (P<0.001), MCF-7 (P<0.01) and
MDA-MB-231 (P<0.01) cells. Non-treated MCF-7 and MDA-MB-231 cell
lines showed a lower protein expression in comparison to the
MCF-10F control cell line.
Discussion
Cytotoxic agents may induce apoptosis by initiating
death signaling pathways in susceptible target cells. Apoptosis is
induced by simultaneous or consequent activation of death receptor
systems, disturbance in mitochondrial function, proteolytic
processing of caspases, DNA damage and ROS damage (26). For evaluation of cytotoxicity
induced by Dox the MTT assay was applied at 24 and 48 h. Results
demonstrated that MCF-10F, MCF-7 and MDA-MB-231 cell viability was
clearly decreased in a dose-dependent manner. Thus, IC50
values were 1, 4 and 1 μM, respectively (Fig. 1) after 48-h treatment. The
IC50 for MCF-7 has been established to fluctuated
between 0.1 and 1.19 μM (27–29)
and it was found that MCF-7 cell line was more resistant than the
MDA-MB-231 cell line (30–32).
The effect of Dox on gene and protein expression of
Bax, Bcl-xL, Bcl-2, caspase-8, caspase-9 and caspase-3, all related
to apoptosis as well as SOD2 to oxidative stress parallel to
production of hydrogen peroxide were studied in MCF-10F, MCF-7 and
MDA-MB-231 cell lines. Results indicated that Bax gene and protein
expression was upregulated by Dox in MDA-MB-231, but not in MCF-7
cell line (33). Antitumoral drugs
apply their effects controlling the expression levels of numerous
members of the Bcl-2 family. Bcl-2 gene expression was
downregulated in MCF-10F and MDA-MB-231, but not in MCF-7 cell
line. Bcl-2 protein expression was decreased in the breast cancer
cell lines MCF-10F, MCF-7 and MDA-MB-231. Members of the Bcl-2
family are main regulators of cell death or cell survival. The
Bcl-2 family protein plays a significant role in apoptosis, either
as apoptotic activators such as Bax or as apoptotic inhibitors such
as Bcl-2 and Bcl-xL. Antiapoptotic Bcl-xL related gene expression
should lead to an inhibition of apoptosis in MCF-7 cell line.
Bcl-xL and Bcl-2 proteins are involved in apoptosis delay due to
the interaction with cytochrome c release (11,34).
Apoptosis induced by Dox was evaluated by caspase-8,
caspase-9 and caspase-3 gene and protein expression in three breast
cancer cell lines. Caspase-8 gene expression was downregulated in
MCF-7 and MDA-MB-231 and its protein expression was upregulated in
MCF-10F and MDA-MB-231 cell lines. However, it was not detected in
MCF-7 cell line. It has been indicated that caspase-8 accelerated
apoptosis, presumably by recruitment of other caspases such as -9
and 3- in B-lymphoid cells and breast cancer cells in a death,
receptor-independent manner (35–37).
It is also recruited by other apoptotic signals such as
detachment-induced cell death but it is not essential for apoptosis
induction (12).
The present study showed that caspase-9 gene
expression was higher in MDA-MB-231 than in MCF-10F and MCF-7
cells. Caspase-9 gene expression decreased in MCF-7 cells in
comparison to its counterpart when treated with Dox. Bcl-xL
interacts with apoptosome to inhibit apoptosis by the caspase-9
formation (38). It has been
demonstrated that MCF-7 and MDA-MB-231 cell lines initiated
apoptosis by caspase-9 and its expression was increased with
Dox-treatment (39,40). In the present study Dox did not
exert changes in gene expression in MDA-MB-231 cells.
Dox decreased caspase-3 gene expression in MCF-7 and
MDA-MB-231 cell lines. However, the protein expression increased in
MCF-10F and MDA-MB-231, but not in MCF-7 cell line. It was reported
that MCF-7 cells did not express detectable levels of caspase-3
(13,39), but contrary results also exist
(41–43). McGee et al (44) suggested that caspase-3-independant
apoptosis could be initiated by other effector caspases and then
they may take over the role of caspase-3 in mediating apoptosis in
MCF-7 cells.
Dox increased SOD2 gene expression in MDA-MB-231
cell line; however, it was decreased in MCF-7 cell line. SOD2
protein expression was downregulated in MCF-10F cells and was
upregulated in MDA-MB-231 cells. MCF-7 did not express significant
difference. SOD2 enzyme has been demonstrated to play an important
role in ROS damage by inhibiting cell proliferation (45). Cerutti et al (46) showed that SOD2 expression protected
breast cancer cell lines from an aggressive phenotype, therefore,
SOD2 overexpression is capable of inhibiting cell proliferation
in vitro. Cancer cells contain elevated ROS levels,
specifically H2O2 as a result of oncogenic
transformation and a product of SOD2 activity excess (22,47),
and the present study is in agreement with these authors.
Dox increased H2O2 production
in MCF-10F, MCF-7 and MDA-MB-231 cell lines in comparison to its
counterparts. MCF-10F cell line did not express significant
differences in the production of H2O2 when
treated with Dox. Chua et al (48) found similar results. It was
reported that high concentration of H2O2
induced an overexpression of specific oxidative related gene change
such as NF-κB. H2O2 production was related to
the phosphorylation of I kappa B-α which was degraded and then
activated NF-κB (49–51).
Dox increased the NF-κB gene expression in MCF-10F
and MDA-MB-231 but it decreased in MCF-7 cells. NF-κB protein
expression level decreased in all cell lines when treated with Dox.
It was confirmed that NF-κB inhibition sensitized apoptosis when
treated with Dox in various cancer cells e.g. breast cancer and
pancreatic carcinoma (52). NF-κB
expression plays an anti-apoptotic role in various cancer cells
such as breast cancer (53).
Dox decreased anti-apoptotic Bcl-2 protein
expression and affected oxidative stress by increasing hydrogen
peroxide production with simultaneously decrease NF-κB gene and
protein expression in MCF-7, a tumorigenic triple-positive cell
line. Results also indicated that Dox induced apoptosis by
upregulating Bax, caspase-8 and caspase-3 and downregulation of
Bcl-2 protein expression. On the contrary, ROS damage decreased by
increasing SOD2 gene and protein expression and hydrogen peroxide
production with parallel NF-κB protein expression decrease in
MDA-MB-231, tumorigenic triple-negative breast cancer cells. It can
be concluded that Dox activated apoptosis by inducing proteolytic
processing of Bcl-2 family, caspases and simultaneously decreased
oxidative stress by influencing ROS damage in MCF-7 and MDA-MB-231
cells.
Acknowledgements
The technical support of Guiliana Rojas, Georgina
Vargas Marchant and Leodán A. Crispin and helpful suggestions given
by Richard Ponce-Cusi are greatly appreciated. The present study
was supported by the Grant support FONDECYT#1120006 (G.M.C) and
MINEDUC-UTA (G.M.C).
References
|
1
|
Wu X, Liu X, Sengupta J, Bu Y, Yi F, Wang
C, Shi Y, Zhu Y, Jiao Q and Song F: Silencing of Bmi-1 gene by RNA
interference enhances sensitivity to doxorubicin in breast cancer
cells. Indian J Exp Biol. 49:105–112. 2011.PubMed/NCBI
|
|
2
|
Sinha BK, Mimnaugh EG, Rajagopalan S and
Myers CE: Adriamycin activation and oxygen free radical formation
in human breast tumor cells: Protective role of glutathione
peroxidase in adriamycin resistance. Cancer Res. 49:3844–3848.
1989.PubMed/NCBI
|
|
3
|
Minotti G, Menna P, Salvatorelli E, Cairo
G and Gianni L: Anthracyclines: Molecular advances and
pharmacologic developments in antitumor activity and
cardiotoxicity. Pharmacol Rev. 56:185–229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacobson MD, Weil M and Raff MC:
Programmed cell death in animal development. Cell. 88:347–354.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strobel T, Swanson L, Korsmeyer S and
Cannistra SA: BAX enhances paclitaxel-induced apoptosis through a
p53-independent pathway. Proc Natl Acad Sci USA. 93:14094–14099.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelly GL and Strasser A: The essential
role of evasion from cell death in cancer. Adv Cancer Res.
111:39–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chorna IV, Datsyuk LO and Stoika RS:
Expression of Bax, Bad and Bcl-2 proteins under x-radiation effect
towards human breast carcinoma MCF-7 cells and their
doxorubicin-resistant derivatives. Exp Oncol. 27:196–201.
2005.PubMed/NCBI
|
|
10
|
Lindsay J, Esposti MD and Gilmore AP:
Bcl-2 proteins and mitochondria - specificity in membrane targeting
for death. Biochim Biophys Acta. 1813:532–539. 2011. View Article : Google Scholar
|
|
11
|
Sharifi S, Barar J, Hejazi MS and Samadi
N: Doxorubicin changes Bax/Bcl-xL ratio, caspase-8 and 9 in breast
cancer cells. Adv Pharm Bull. 5:351–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wieder T, Essmann F, Prokop A, Schmelz K,
Schulze-Osthoff K, Beyaert R, Dörken B and Daniel PT: Activation of
caspase-8 in drug-induced apoptosis of B-lymphoid cells is
independent of CD95/Fas receptor-ligand interaction and occurs
downstream of caspase-3. Blood. 97:1378–1387. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang S, Zhou Q and Yang X: Caspase-3
status is a determinant of the differential responses to genistein
between MDA-MB-231 and MCF-7 breast cancer cells. Biochim Biophys
Acta. 1773:903–911. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kruidering M and Evan GI: Caspase-8 in
apoptosis: The beginning of ‘the end’? IUBMB Life. 50:85–90. 2000.
View Article : Google Scholar
|
|
16
|
Goffart S, von Kleist-Retzow JC and
Wiesner RJ: Regulation of mitochondrial proliferation in the heart:
Power-plant failure contributes to cardiac failure in hypertrophy.
Cardiovasc Res. 64:198–207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ridnour LA, Oberley TD and Oberley LW:
Tumor suppressive effects of MnSOD overexpression may involve
imbalance in peroxide generation versus peroxide removal. Antioxid
Redox Signal. 6:501–512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vera-Ramirez L, Sanchez-Rovira P,
Ramirez-Tortosa MC, Ramirez-Tortosa CL, Granados-Principal S,
Lorente JA and Quiles JL: Free radicals in breast carcinogenesis,
breast cancer progression and cancer stem cells. Biological bases
to develop oxidative-based therapies. Crit Rev Oncol Hematol.
80:347–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sosa V, Moliné T, Somoza R, Paciucci R,
Kondoh H and Leonart ME: Oxidative stress and cancer: An overview.
Ageing Res Rev. 12:376–390. 2013. View Article : Google Scholar
|
|
20
|
Miao L and St Clair DK: Regulation of
superoxide dismutase genes: Implications in disease. Free Radic
Biol Med. 47:344–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamarajugadda S, Cai Q, Chen H, Nayak S,
Zhu J, He M, Jin Y, Zhang Y, Ai L, Martin SS, et al: Manganese
superoxide dismutase promotes anoikis resistance and tumor
metastasis. Cell Death Dis. 4:e5042013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kattan Z, Minig V, Leroy P, Dauça M and
Becuwe P: Role of manganese superoxide dismutase on growth and
invasive properties of human estrogen-independent breast cancer
cells. Breast Cancer Res Treat. 108:203–215. 2008. View Article : Google Scholar
|
|
23
|
Kiningham KK, Cardozo ZA, Cook C, Cole MP,
Stewart JC, Tassone M, Coleman MC and Spitz DR: All-trans-retinoic
acid induces manganese superoxide dismutase in human neuroblastoma
through NF-kappaB. Free Radic Biol Med. 44:1610–1616. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen PM, Wu TC, Wang YC, Cheng YW, Sheu
GT, Chen CY and Lee H: Activation of NF-κB by SOD2 promotes the
aggressiveness of lung adenocarcinoma by modulating NKX2–1-mediated
IKKβ expression. Carcinogenesis. 34:2655–2663. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mantovani A: Molecular pathways linking
inflammation and cancer. Curr Mol Med. 10:369–373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fisher DE: Apoptosis in cancer therapy:
Crossing the threshold. Cell. 78:539–542. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lukyanova NY, Rusetskya NV, Tregubova NA
and Chekhun VF: Molecular profile and cell cycle in MCF-7 cells
resistant to cisplatin and doxorubicin. Exp Oncol. 31:87–91.
2009.PubMed/NCBI
|
|
28
|
Taherian A and Mazoochi T: Different
expression of extracellular signal-regulated kinases (ERK) 1/2 and
phospho-Erk proteins in MBA-MB-231 and MCF-7 cells after
chemotherapy with doxorubicin or docetaxel. Iran J Basic Med Sci.
15:669–677. 2012.PubMed/NCBI
|
|
29
|
Fornari FA, Randolph JK, Yalowich JC,
Ritke MK and Gewirtz DA: Interference by doxorubicin with DNA
unwinding in MCF-7 breast tumor cells. Mol Pharmacol. 45:649–656.
1994.PubMed/NCBI
|
|
30
|
Schneiderman RS, Shmueli E, Kirson ED and
Palti Y: TTFields alone and in combination with chemotherapeutic
agents effectively reduce the viability of MDR cell sub-lines that
over-express ABC transporters. BMC Cancer. 10:2292010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gupta P and Srivastava SK: Antitumor
activity of phenethyl isothiocyanate in HER2-positive breast cancer
models. BMC Med. 10:802012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mahéo K, Vibet S, Steghens JP, Dartigeas
C, Lehman M, Bougnoux P and Goré J: Differential sensitization of
cancer cells to doxorubicin by DHA: A role for lipoperoxidation.
Free Radic Biol Med. 39:742–751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tudor G, Aguilera A, Halverson DO, Laing
ND and Sausville EA: Susceptibility to drug-induced apoptosis
correlates with differential modulation of Bad, Bcl-2 and Bcl-xL
protein levels. Cell Death Differ. 7:574–586. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Varfolomeev EE, Schuchmann M, Luria V,
Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM,
Kemper OC, Kollet O, et al: Targeted disruption of the mouse
caspase 8 gene ablates cell death induction by the TNF receptors,
Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 9:267–276.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wesselborg S, Engels IH, Rossmann E, Los M
and Schulze-Osthoff K: Anticancer drugs induce caspase-8/FLICE
activation and apoptosis in the absence of CD95 receptor/ligand
interaction. Blood. 93:3053–3063. 1999.PubMed/NCBI
|
|
37
|
Liu WH and Chang LS: Fas/FasL-dependent
and -independent activation of caspase-8 in doxorubicin-treated
human breast cancer MCF-7 cells: ADAM10 down-regulation activates
Fas/FasL signaling pathway. Int J Biochem Cell Biol Dec.
43:1708–1719. 2011. View Article : Google Scholar
|
|
38
|
Hu Y, Benedict MA, Wu D, Inohara N and
Núñez G: Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent
caspase-9 activation. Proc Natl Acad Sci USA. 95:4386–4391. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liang Y, Yan C and Schor NF: Apoptosis in
the absence of Caspase 3. Oncogene. 20:6570–6578. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheah YH, Nordin FJ, Tee TT, Azimahtol HL,
Abdullah NR and Ismail Z: Antiproliferative property and apoptotic
effect of xanthorrhizol on MDA-MB-231 breast cancer cells.
Anticancer Res. 28(6A): 3677–3689. 2008.
|
|
41
|
Cui Q, Yu JH, Wu JN, Tashiro S, Onodera S,
Minami M and Ikejima T: P53-mediated cell cycle arrest and
apoptosis through a caspase-3-independent, but caspase-9-dependent
pathway in oridonin-treated MCF-7 human breast cancer cells. Acta
Pharmacol Sin. 28:1057–1066. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang HL, Chen CS, Chang WH, Lu FJ, Lai YC,
Chen CC, Hseu TH, Kuo CT and Hseu YC: Growth inhibition and
induction of apoptosis in MCF-7 breast cancer cells by Antrodia
camphorata. Cancer Lett. 231:215–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen JS, Konopleva M, Andreeff M, Multani
AS, Pathak S and Mehta K: Drug-resistant breast carcinoma (MCF-7)
cells are paradoxically sensitive to apoptosis. J Cell Physiol.
200:223–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McGee MM, Hyland E, Campiani G, Ramunno A,
Nacci V and Zisterer DM: Caspase-3 is not essential for DNA
fragmentation in MCF-7 cells during apoptosis induced by the
pyrrolo-1,5-benzoxazepine, PBOX-6. FEBS Lett. 515:66–70. 2002.
View Article : Google Scholar
|
|
45
|
Zhang Y, Zhang HM, Shi Y, Lustgarten M, Li
Y, Qi W, Zhang BX and Van Remmen H: Loss of manganese superoxide
dismutase leads to abnormal growth and signal transduction in mouse
embryonic fibroblasts. Free Radic Biol Med. 49:1255–1262. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cerutti P, Ghosh R, Oya Y and Amstad P:
The role of the cellular antioxidant defense in oxidant
carcinogenesis. Environ Health Perspect. 102(Suppl 10): 123–129.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Behrend L, Henderson G and Zwacka RM:
Reactive oxygen species in oncogenic transformation. Biochem Soc
Trans. 31:1441–1444. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chua PJ, Yip GW and Bay BH: Cell cycle
arrest induced by hydrogen peroxide is associated with modulation
of oxidative stress related genes in breast cancer cells. Exp Biol
Med (Maywood). 234:1086–1094. 2009. View Article : Google Scholar
|
|
49
|
Takada Y, Mukhopadhyay A, Kundu GC,
Mahabeleshwar GH, Singh S and Aggarwal BB: Hydrogen peroxide
activates NF-kappa B through tyrosine phosphorylation of I kappa B
alpha and serine phosphorylation of p65: evidence for the
involvement of I kappa B alpha kinase and Syk protein-tyrosine
kinase. J Biol Chem. 278:24233–24241. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kretz-Remy C, Mehlen P, Mirault ME and
Arrigo AP: Inhibition of I kappa B-alpha phosphorylation and
degradation and subsequent NF-kappa B activation by glutathione
peroxidase overexpression. J Cell Biol. 133:1083–1093. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Schreck R, Rieber P and Baeuerle PA:
Reactive oxygen intermediates as apparently widely used messengers
in the activation of the NF-kappa B transcription factor and HIV-1.
EMBO J. 10:2247–2258. 1991.PubMed/NCBI
|
|
52
|
Arlt A, Vorndamm J, Breitenbroich M,
Fölsch UR, Kalthoff H, Schmidt WE and Schäfer H: Inhibition of
NF-kappa B sensitizes human pancreatic carcinoma cells to apoptosis
induced by etoposide (VP16) or doxorubicin. Oncogene. 20:859–868.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang S, Kotamraju S, Konorev E, Kalivendi
S, Joseph J and Kalyanaraman B: Activation of nuclear factor-kappaB
during doxorubicin-induced apoptosis in endothelial cells and
myocytes is pro-apoptotic: The role of hydrogen peroxide. Biochem
J. 367:729–740. 2002. View Article : Google Scholar : PubMed/NCBI
|