Introduction
Epithelial ovarian cancer remains the most
devastating gynecologic cancer. Although 40–60% of patients achieve
complete clinical responses to first-line chemotherapy treatment,
~50% of these patients relapse within 5 years; only 10–15% of
patients who present with advanced disease achieve long-term
remission (1,2). The standard of care for first-line
chemotherapy treatment is paclitaxel in combination with a
platinum-based compound, or platinum-based therapy alone (3). Chemotherapy induces significant side
effects and has limited efficacy, such as drug resistance and rapid
recurrence, respectively, which subsequently reduce patient
survival rates (4,5). Hence, more effort is needed to
understand the mechanisms of drug resistance to anticancer drugs.
Resistance to chemotherapy for ovarian cancer potentially involves
several mechanisms (6).
P-glycoprotein, which is encoded by the gene, multiple drug
resistance 1 (MDR1), functions as a transmembrane efflux
pump for the elimination and disposition of xenobiotics, including
the anticancer drugs paclitaxel and cisplatin, which implies a
major role in multidrug resistance (7). MDR1 over-expression in certain
tumor cells has been associated with protection against anticancer
agents (8). Therefore, altering
MDR1 expression could improve the patient’s clinical outcome
(9–11).
Pregnane X receptor (PXR), a member of the nuclear
receptor superfamily, has been shown to mediate the genomic effects
of several steroid hormones, including progesterone, pregnenolone,
and estrogen, and of xenobiotics (12–18).
PXR regulation involves a specific DNA sequence, the PXR-responsive
element, which is found in various genes, including the upstream
region of the cytochrome (P450) 3A (CYP3A) gene family
(12,14,17),
which codes for monooxygenases responsible for the oxidative
metabolism of certain endogenous substrates and xenobiotics
(19,20), and MDR1 (21,22).
Because the PXR pathway is activated by a large number of
prescription drugs designed to treat infection, cancer, convulsion,
and hypertension (23), PXR is
thought to play roles in drug metabolism/efflux and drug-drug
interaction. For example, PXR activation is reportedly involved in
regulating cell cycle proliferation and apoptosis inhibition
(24–27). PXR was previously shown as a
possible prognostic factor that might feasibly identify patients at
risk of recurrence or death from epithelial ovarian cancer
(28), however, PXR function in
ovarian cancer remains unknown, especially in combination with
anticancer drugs. Paclitaxel and cisplatin, which are widely used
as first-line chemotherapy drugs in ovarian cancer (3–5),
could act as PXR ligands (29).
In addition, we previously showed that
downregulating the constitutive androstane receptor (CAR) through
RNA interference significantly promoted cell growth inhibition and
enhancement of apoptosis in the presence of anticancer agents,
paclitaxel and cisplatin (30).
CAR as well as PXR is activated by a variety of endogenous and
exogenous ligands including drugs, insecticides, pesticides, and
nutritional compounds (31) and
functions as a xenobiotic receptor that regulates detoxification
and clearance of xenobiotics (32). Therefore, both PXR and CAR might
play some roles in drug resistance.
We therefore examined the effects of combining a
ligand and antagonist for PXR with anticancer drugs on ovarian
cancer cells, and the potential contribution of PXR downregulation
by RNA interference toward increasing drug sensitivity and
overcoming drug resistance in this study. We also examined the
relationship of PXR with another xenobiotics receptor CAR in the
drug resistance function for ovarian cancer.
Materials and methods
Materials
Phthalic acid bis (2-ethylhexel ester) (phthalate),
5-pregneno-3β-ol-20-one (pregnenolone), ketoconazole, paclitaxel,
cisplatin, and dimethyl sulfoxide (DMSO) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Each drug was dissolved in DMSO
and stored at −20°C before use. The final concentration of DMSO was
0.1%; 0.1% DMSO was used for the negative control.
Cell culture
Ovarian cancer cell lines, SKOV-3, OVCAR-3, CaOV-3,
and BG-1 cells, and human hepatoma cell line, HepG2 cells were
obtained from the Health Science Research Resources Bank (Osaka,
Japan). These ovarian cancer cell lines were derived from patients
with ovarian adenocarcinoma. SKOV-3 cells and CAOV-3 were from
patients previously treated with chemotherapy, BG-1 cells from an
untreated patient, and OVCAR-3 cell line from a patient who did not
respond to chemotherapy (33).
SKOV-3 and OVCAR-3 cells have been shown to be relatively resistant
to platinum and taxanes, BG-1 cells to be resistant to platinum,
and CAOV-3 to be sensitive to both platinum and taxanes (33,34).
Cells were cultured in Dulbecco’s modified Eagle’s medium
(Invitrogen, Carlsbad, CA, USA) with 10% charcoal-stripped fetal
bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel)
and 1% penicillin/streptomycin (Invitrogen). The cells were
cultured under a humidified 5% CO2 atmosphere at
37°C.
Quantitative real-time
reverse-transcription polymerase chain reaction (PCR)
Total RNA was isolated using the RNeasy Mini kit
(Qiagen, Hilden, Germany) according to the manufacturer’s protocol,
then stored at −80°C. The concentration and purity of isolated RNA
was determined using a spectrophotometer (Thermo Fisher Scientific
Inc., Waltham, MA, USA). Reverse transcription was performed using
the High Capacity cDNA Reverse Transcription kit with RNA inhibitor
(Applied Biosystems, Foster City, CA, USA). Complementary DNA was
obtained through the GeneAmp PCR System 9700 (Applied Biosystems).
cDNA isolated from each of the different drug groups was analyzed
by real-time PCR using TaqMan universal master mix containing
different primers (Applied Biosystems). Primers for MDR1, CYP3A4,
and PXR (Applied Biosystems) were normalized to the 18S
housekeeping gene. After 50 cycles, mRNA expression was calculated
by the comparative CT-value method. The negative control was 0.1%
DMSO.
Transient transfection studies
The (CYP3A4)3-tk-chloramphenicol acetyl
transferase (CAT) was generated by inserting three copies of
double-strand oligonucleotides containing the CYP3A4
(5′-GGGTCAGCAAGTTCA-3′). The (MDR1)3-tk-CAT was
generated by inserting three copies of a double-strand
oligonucleotide containing the MDR1 (5′-AGGTCAAGTTAGTTCA-3′) as
described before (29). CaOV-3
cells were transfected with 1μg of a reporter gene construct
[(CYP3A4)3-tk-CAT or (MDR1)3-tk-CAT]. In all
experiments, liposome-mediated transfections used Lipofectamine
(Life Technologies, Gaithersburg, MD, USA) according to the
manufacturer’s instructions. Transfected cells were treated with
DMSO alone or with anticancer drugs paclitaxel (5 μM), cisplatin
(10 μM), PXR ligands pregnenolone (10 μM) or phthalate (10 μM),
with or without RNA interference for 36 h. Cell extracts were
prepared and assayed for CAT activity. Amount of CAT was determined
using a CAT ELISA Kit (Roche Diagnostics Co., Tokyo, Japan)
according to the manufacturer’s instructions.
RNA interference
The siRNA cocktail targeting human PXR (cat. no.
sc-44057), human CAR (cat. no. sc-39918), and negative control
cocktail (cat. no. sc-37007), which consists of a scrambled
sequence that does not lead to the specific degradation of any
cellular message, were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). Cells were transfected with PXR siRNA, CAR
siRNA or control siRNA using the siRNA Reagent system (Santa Cruz
Biotechnology) according to the manufacturer’s protocol.
MTT assay
Cells were plated 5×103 in a 96-well
plate in at least triplicate samples for each experimental
condition. Twenty-four hours later, cells were treated with
anticancer drugs paclitaxel (5 μM) or cisplatin (10 μM) with or
without RNA interference or in the presence or absence of
pregnenolone or ketoconazole for 24, 48 and 72 h. We used a
commercial MTT cell proliferation kit (Bioassay Systems, Hayward,
CA, USA) and followed its instructions. After each incubation time,
15 μl 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) was added into each well. After a 4-h incubation at 37°C, 150
μl solubilization solution was added to each well and the plate was
gently agitated for 1 h at room temperature. Absorbance was
measured at 595 nm using an Imark microplate reader (Bio-Rad,
Philadelphia, PA, USA).
Apoptosis assay
Apoptosis was examined using a terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL) assay that employed the ApopTag Plus Peroxidase In Situ
Apoptosis Detection kit (EMD Millipore, Billerica, MA, USA)
following the manufacturer’s protocol. Briefly, cells were grown on
sterilized glass coverslips in a 6-well culture plate overnight,
and then exposed to the different experimental drug groups. Cells
were then stained and mounted with Vectashield mounting medium in
the presence of the nuclear stain 4′,6-diamidino-2-phenylindole
(DAPI). Afterwards, TUNEL- and DAPI-positive cells were counted
using fluorescence microscopy. The ratio of TUNEL-positive to
DAPI-positive cells was calculated.
Statistical analysis
Statistical analysis was performed as one-way
analysis of variance with post-hoc testing analysis and the
Student’s t-test using SPSS Statistics 19.0 software (IBM, Armonk,
NY, USA). All data are expressed as mean ± SD. P<0.05 was
considered statistically significant.
Results
Effect of paclitaxel and cisplatin on
MDR1 and CYP3A4 mRNA expression and PXR-mediated transcription in
ovarian cancer cell lines
Expression of PXR has been reported in SKOV-3 and
OVCAR-8 ovarian cancer cells (27). We examined PXR expression in
OVCAR-3, CaOV-3, SKOV-3 and BG-1 cells using RT-PCR. PXR was
strongly expressed in SKOV-3 and CaOV-3 cells, moderately expressed
in OVCAR-3 cells, and not expressed in BG-1 cells (Fig. 1A). To investigate the effect of PXR
ligands (including anticancer drugs) on expression of CYP3A4 and
MDR1 in vitro, we examined mRNA levels of CYP3A4 and MDR1 in
SKOV-3, CaOV-3, and BG-1 cells that had been exposed to
pregnenolone, phthalate, paclitaxel, and cisplatin, which could
activate PXR-mediated transcription through CYP3A4 and MDR1
promoters (28). In SKOV-3 and
CaOV-3 cells, CYP3A4 mRNA levels were significantly increased in
the presence of PXR ligands (Fig.
1B). Pregnenolone and phthalate had significantly positive
effects on CYP3A4 expression compared with paclitaxel or cisplatin.
In contrast, the MDR1 level was significantly and strongly
increased in the presence of paclitaxel or cisplatin compared with
pregnenolone and phthalate. MDR1 and CYP3A4 levels did not change
in response to any PXR ligands in BG-1 cells (which did not express
PXR). PXR ligands also enhanced PXR-mediated transcription through
both MDR and CYP3A4 promoters in CaOV-3 cells (Fig. 1C). Paclitaxel and cisplatin had
significant effects on PXR-mediated transcription through the MDR
promoter compared with pregnenolone and phthalate. In contrast,
pregnenolone and phthalate strongly enhanced PXR-mediated
transcription through CYP3A4 promoter compared with paclitaxel or
cisplatin.
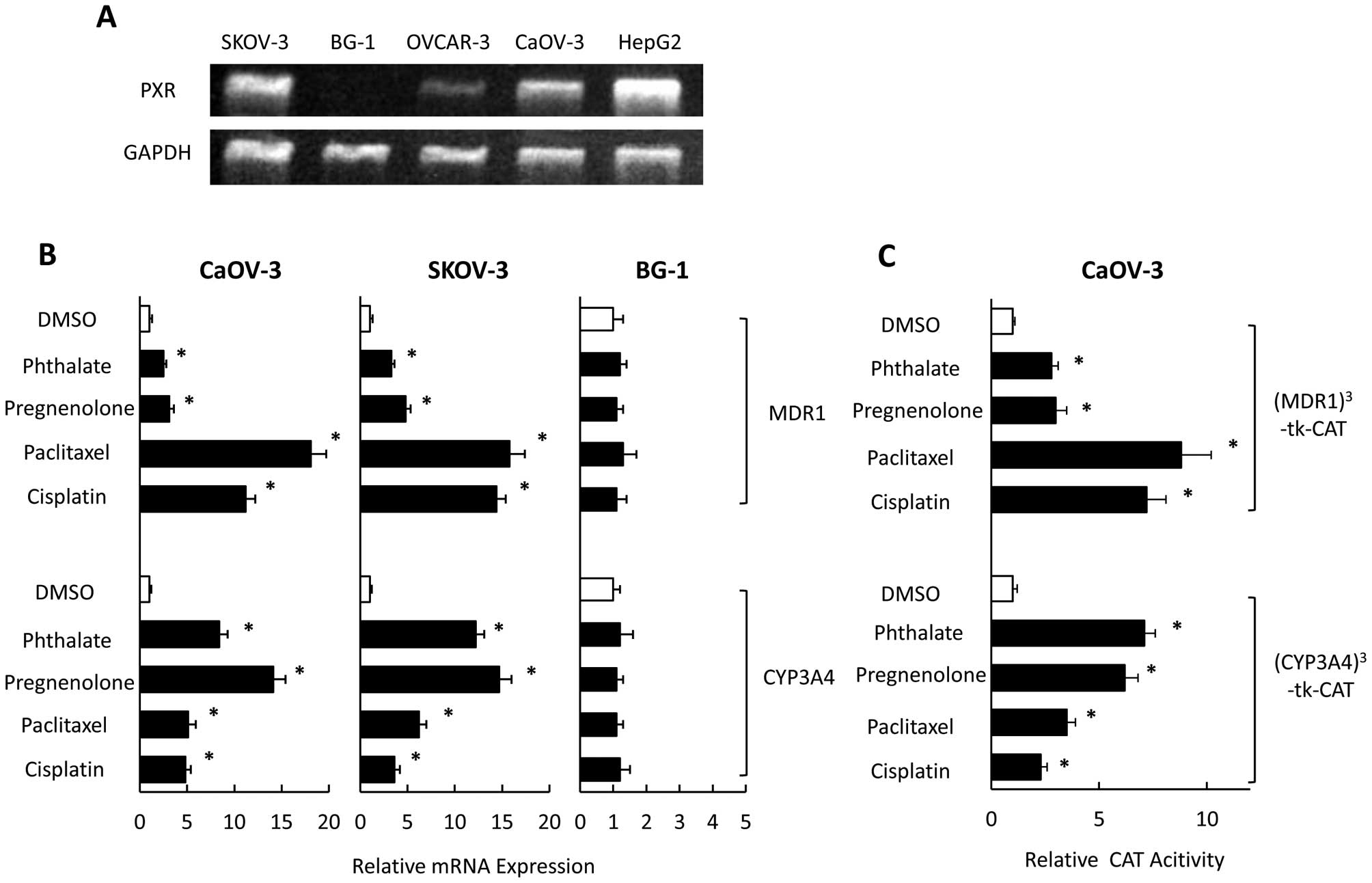 | Figure 1Effect of anticancer drugs on the
mRNA expression of MDR1 and CYP3A4 and PXR-mediated transcription
in ovarian cancer cell lines. The expression of PXR and
GAPDH mRNAs were analyzed using RT-PCR in ovarian cancer
cell lines, SKOV-3, BG-1, OVCAR-3, and CaOV-3 (A). CaOV-3, SKOV-3,
and BG-1 cells were treated with pregnenolone, phthalate,
paclitaxel, cisplatin, or DMSO for 24 h and analyzed for the mRNA
expression using quantitative real-time PCR (B). CaOV-3 cells were
cotransfected with reporter gene constructs,
(MDR1)3-tk-CAT or (CYP3A4)3-tk-CAT and
treated with pregnenolone, phthalate, paclitaxel, cisplatin, or
DMSO for 36 h. CAT levels were determined by an ELISA kit (C).
Results show mean ± SD of 5 independent experiments
(*P<0.01 vs. DMSO group). |
Effect of PXR siRNA on MDR1 and CYP3A4
mRNA expression and PXR-mediated transcription in CaOV-3 cells
We examined the effect of downregulating PXR by
using siRNA on expression of MDR1 and CYP3A4, and PXR-mediated
transcription in the presence of paclitaxel or cisplatin in CaOV-3
cells. First, we used RT-PCR to confirm that PXR mRNA was not
detected in the PXR-siRNA transfected CaOV-3 cells (Fig. 2A). The effects of PXR ligands,
including paclitaxel and cisplatin, on MDR1 expression were
strongly inhibited in PXR siRNA-transfected cells, and we saw no
positive effects of PXR ligands on CYP3A4 expression in PXR
siRNA-transfected cells (Fig. 2B),
nor any non-specific effects of siRNA. PXR expression did not
significantly differ between cells treated with control siRNA and
those not treated with any siRNA (data not shown). We also examined
the effects of PXR siRNAs on PXR-mediated transcription. In control
siRNA-transfected cells, PXR ligands significantly activated native
PXR-mediated transcription; whereas the PXR siRNA-transfected cells
showed no PXR-mediated transactivation in the presence of PXR
ligands, including paclitaxel and cisplatin (Fig. 2C).
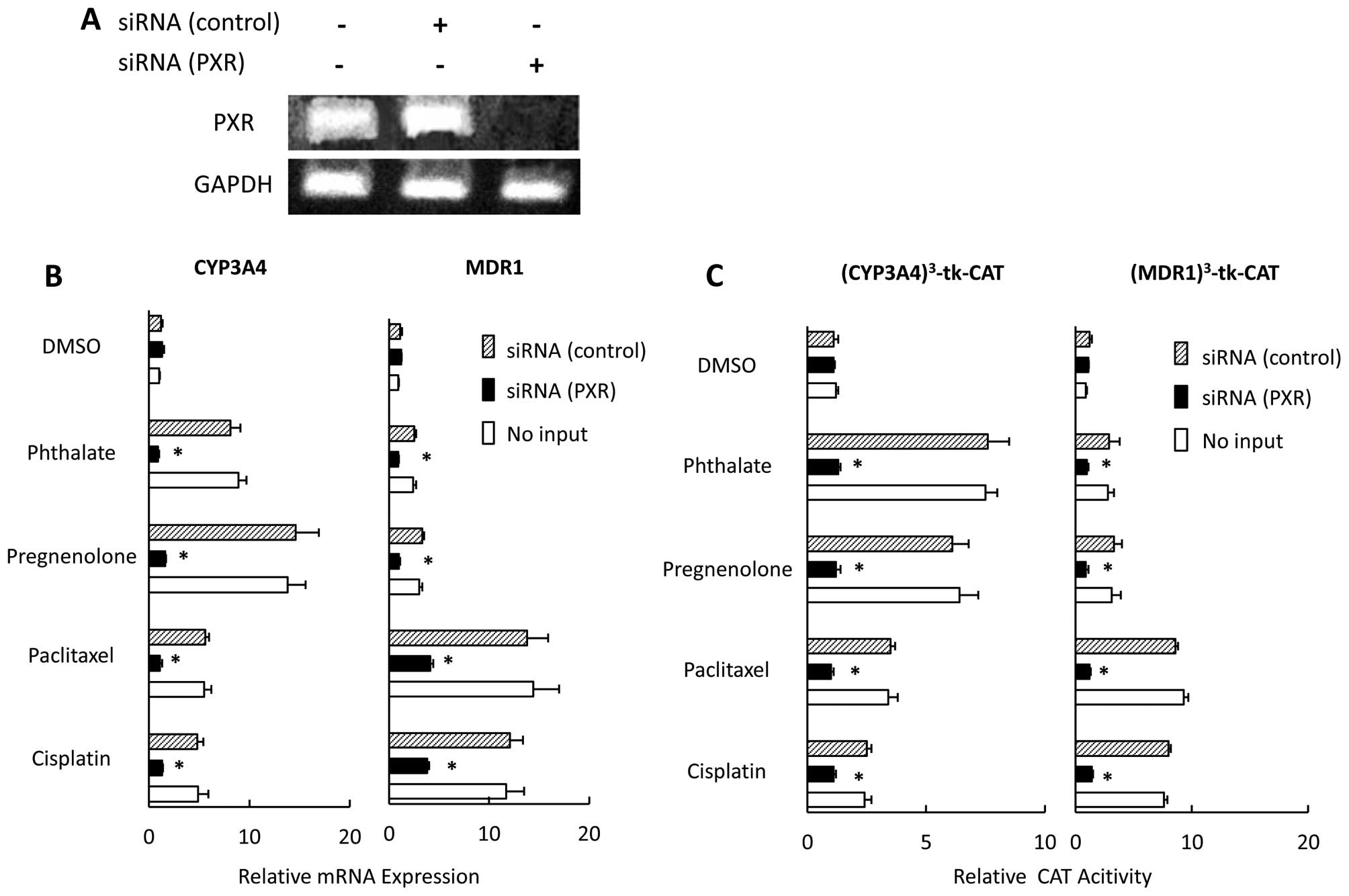 | Figure 2Effect of PXR siRNA on the
expression of MDR1 and CYP3A4 and PXR-mediated transcription in
CaOV-3 cells. CaOV-3 cells were transfected with PXR- or control
siRNA (A). CaOV-3 cells were transfected with PXR- or control siRNA
and treated with pregnenolone, phthalate, paclitaxel, cisplatin, or
DMSO for 36 h. CYP3A4 and MDR1 mRNA expression were
analyzed by quantitative real-time PCR. Each experiment was
performed in triplicate, 4 times (internal control, 18S; negative
control, DMSO). Changes in gene expression were calculated as
ratios of target gene to internal control (B). CaOV-3 cells were
cotransfected with PXR- or control siRNAs, or no siRNA and a
reporter gene construct, (MDR1)3-tk-CAT or
(CYP3A4)3-tk-CAT; and then treated with pregnenolone,
phthalate, paclitaxel, cisplatin or DMSO for 36 h. CAT levels were
determined by ELISA (C). Results, mean ± SD of 5 independent
experiments (*P<0.01 vs. control siRNA group). |
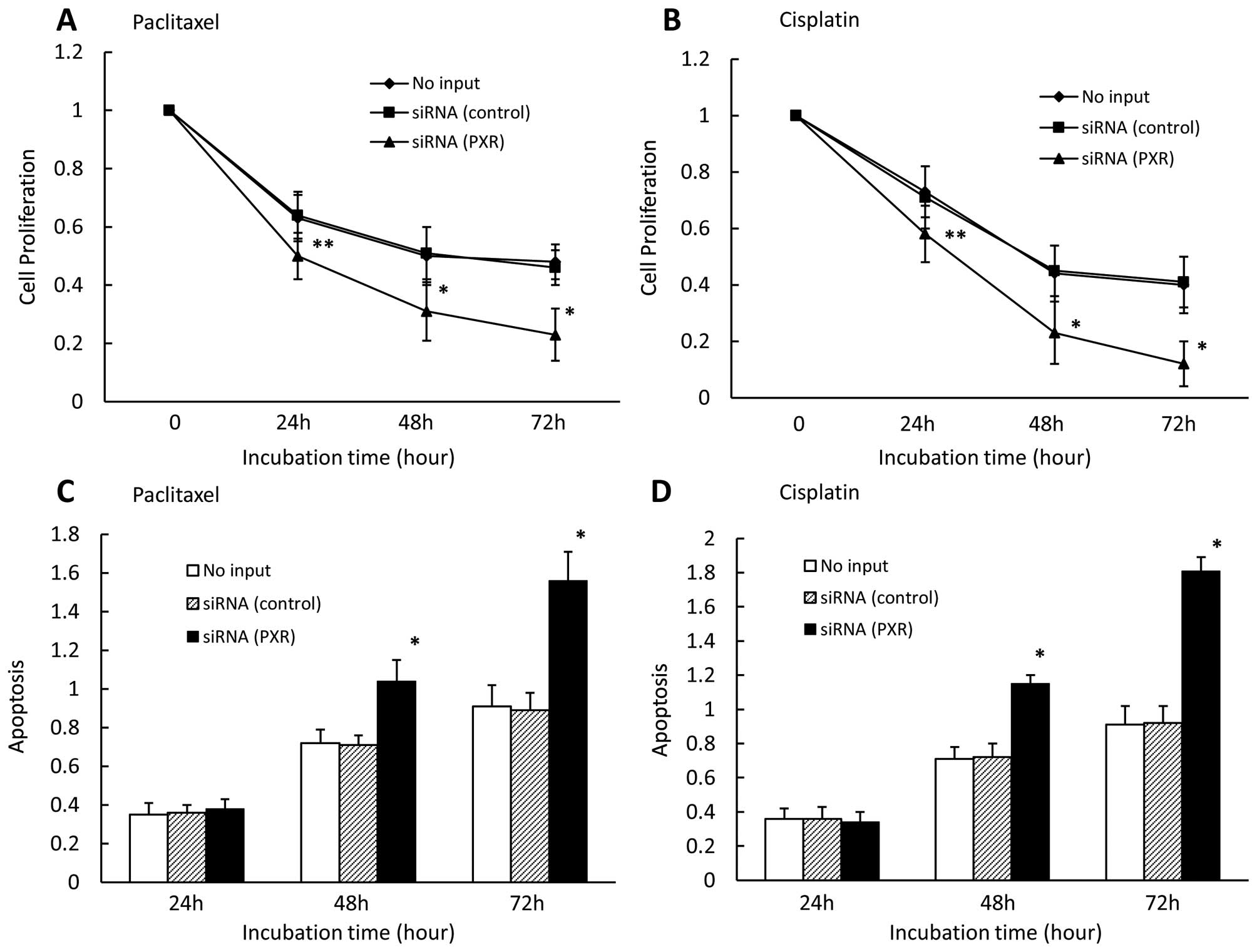
Effect of PXR siRNA on cell proliferation
and apoptosis in CaOV-3 cells
We also examined the effect of downregulated PXR on
cell proliferation and apoptosis in the presence of paclitaxel or
cisplatin in CaOV-3 cells. We found that down-regulated PXR
significantly enhanced cell growth inhibition in the presence of
paclitaxel (Fig. 3A) and cisplatin
(Fig. 3B) for 24, 48, and 72 h.
Cell growth did not significantly differ between control
siRNA-transfected cells and untransfected cells. We also observed
that downregulated PXR significantly enhanced apoptosis in PXR
siRNA-transfected cells compared with cells transfected with
control siRNA or without siRNA in the presence of paclitaxel
(Fig. 3C) and cisplatin (Fig. 3D) for 48 and 72 h. Apoptosis rates
did not significantly differ between control siRNA-transfected
cells and untransfected cells.
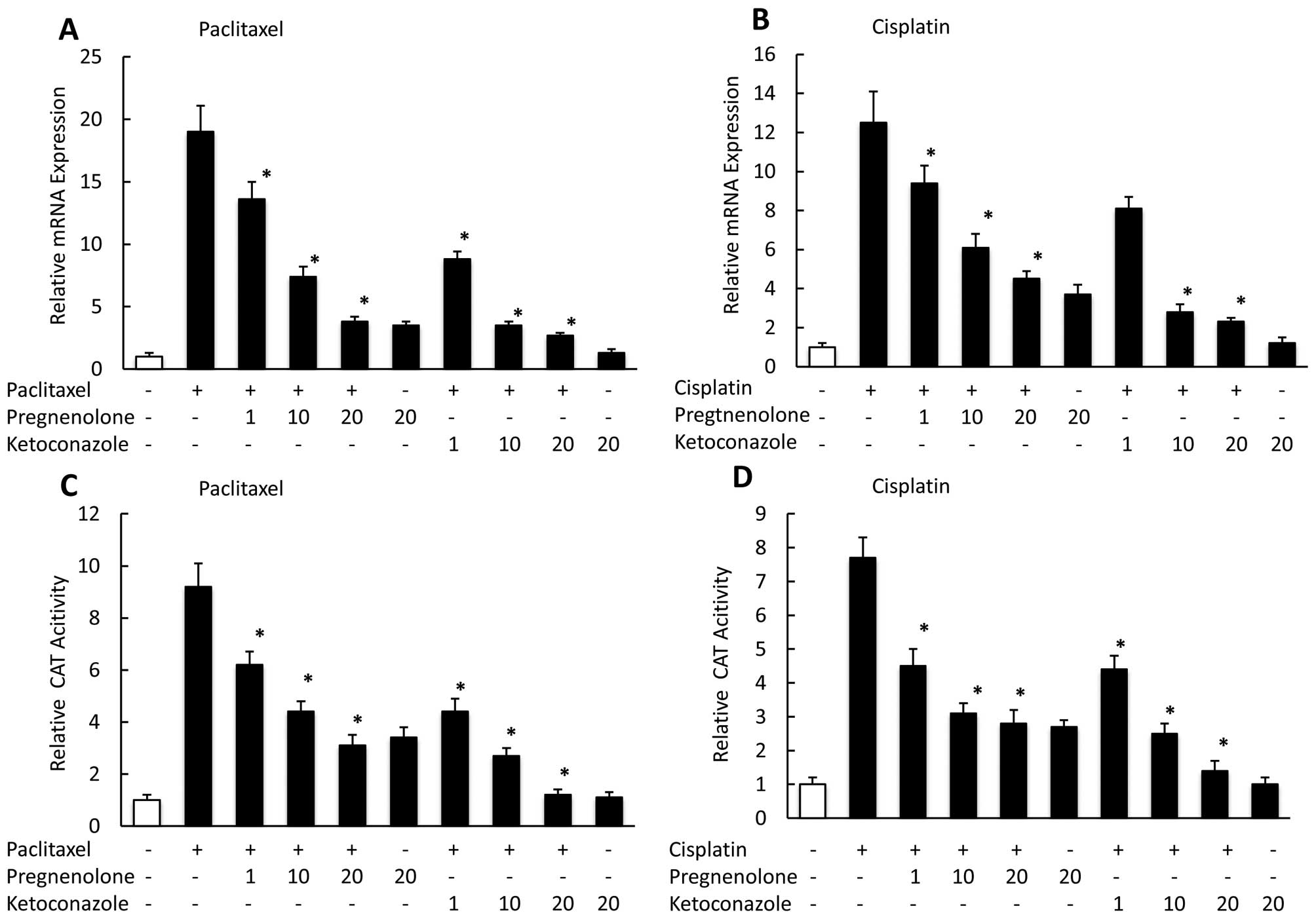
Effect of combining paclitaxel or
cisplatin with PXR ligand or antagonist on transcription of
PXR-related genes
To determine the effect of treating CaOV-3 cells
with anticancer drugs in combination with the PXR agonist
pregnenolone, or the PXR antagonist ketoconazole on the PXR target
genes, MDR1 and CYP3A4, we used real-time PCR to assess their mRNA
expression levels at 24 h. We observed pregnenolone moderately
suppressed the MDR1 mRNA expression enhanced by paclitaxel or
cisplatin, whereas ketoconazole had a significant negative effect
on the MDR1 expression enhanced by paclitaxel (Fig. 4A) or cisplatin (Fig. 4B) in a dose-dependent manner. We
also examined the effect on PXR-mediated transcription of combining
paclitaxel or cisplatin with a PXR ligand or antagonist.
Pregnenolone suppressed PXR-mediated transac-tivation by paclitaxel
or cisplatin, whereas ketoconazole more strongly reduced
transcription in the presence of paclitaxel (Fig. 4C) and cisplatin (Fig. 4D) in a dose-dependent manner.
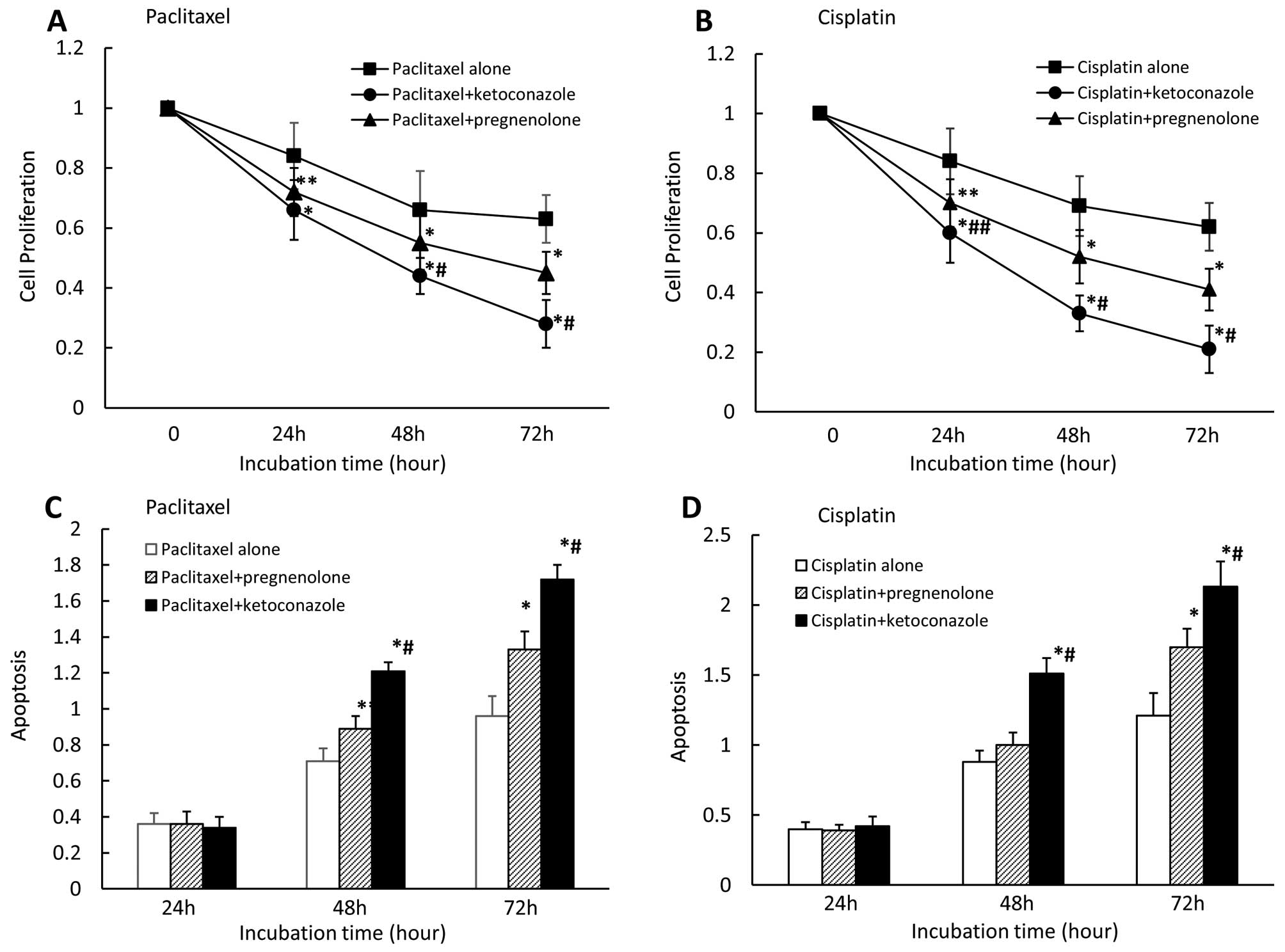
Effect of combining paclitaxel or
cisplatin with PXR ligand or antagonist on cell proliferation and
apoptosis in CaOV-3 cells
We also examined the effects of combining the
anticancer paclitaxel or cisplatin with the PXR ligand
pregnenolone, or the PXR antagonist ketoconazole on cell
proliferation and apoptosis. We found that pregnenolone
(moderately) and ketoconazole (strongly) enhanced cell growth
inhibition in the presence of paclitaxel (Fig. 5A) or cisplatin (Fig. 5B) after 24, 48 and 72 h. Cell
growth inhibition significantly differed between pregnenolone and
ketoconazole in the presence of paclitaxel for 48 and 72 h and
cisplatin for 24, 48 and 72 h. We also observed that pregnenolone
(moderately) and ketoconazole (strongly) enhanced apoptosis in the
presence of paclitaxel (Fig. 5C)
or cisplatin (Fig. 5D) after 48
and 72 h for either agent. Apoptosis rates significantly differed
between pregnenolone and ketoconazole in the presence of either
paclitaxel or cisplatin for 48 and 72 h for either agent.
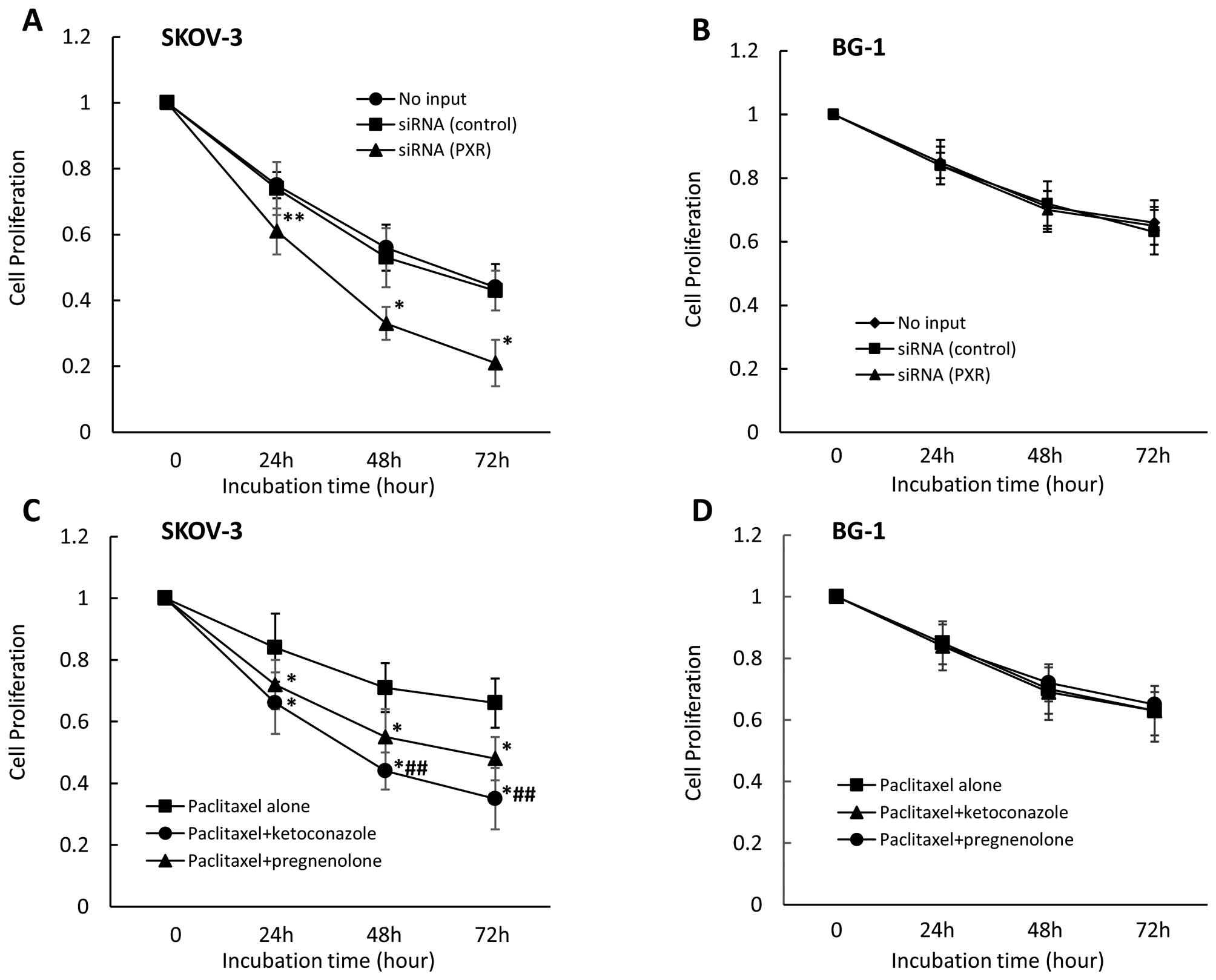
Effect of PXR siRNA and PXR ligand or
antagonist on cell proliferation in other ovarian cell lines
treated with paclitaxel or cisplatin
We also examined the effect of PXR downregulation on
cell proliferation in other ovarian cancer cell lines. We observed
that PXR downregulation significantly enhanced cell growth
inhibition in the presence of paclitaxel in SKOV-3 cells, which
strongly express PXR (Fig. 6A) but
not in BG-1 cells which do not express PXR (Fig. 6B). Cell growth inhibition did not
differ between control siRNA-treated and untransfected cells. In
examination of the effect on cell proliferation of combining,
anticancer drugs with pregnenolone or ketoconazole, pregnenolone
(moderately) and ketoconazole (more strongly) enhanced cell growth
inhibition in the presence of paclitaxel in SKOV-3 cells (Fig. 6C), but not in BG-1 cells (Fig. 6D). Cell growth inhibition in the
presence of paclitaxel for 48 and 72 h significantly differed
between pregnenolone- and ketoconazole-treated SKOV-3 cells.
Effect of PXR and CAR siRNA on MDR1
expression, cell proliferation, and apoptosis in CaOV-3 cells
Finally, we examined the effect of downregulating
PXR and CAR on MDR1 expression, cell proliferation, and apoptosis
in the presence of paclitaxel in CaOV-3 cells. We observed that the
downregulation of both PXR and CAR expression completely blocked
the MDR1 expression enhanced by paclitaxel; PXR or CAR inhibited
MDR1 expression significantly but not completely (Fig. 7A). MDR1 expression significantly
differed between cells with downregulated PXR and those with
down-regulated CAR in the presence of paclitaxel or cisplatin. Use
of siRNA to interfere with both PXR and CAR significantly enhanced
cell growth inhibition in the presence of paclitaxel for 24, 48 and
72 h compared with that of either knocked-down PXR or CAR alone
(Fig. 7B). In the presence of
paclitaxel for 48 and 72 h, downregulating both PXR and CAR (i.e.,
in cells with both PXR and CAR siRNA transfections) significantly
enhanced apoptosis compared with cells transfected with either PXR
or CAR siRNA alone (Fig. 7C). Cell
growth inhibition and apoptosis significantly differed between
cells transfected with PXR siRNA and those transfected with CAR
siRNA in the presence of paclitaxel (Fig. 7C).
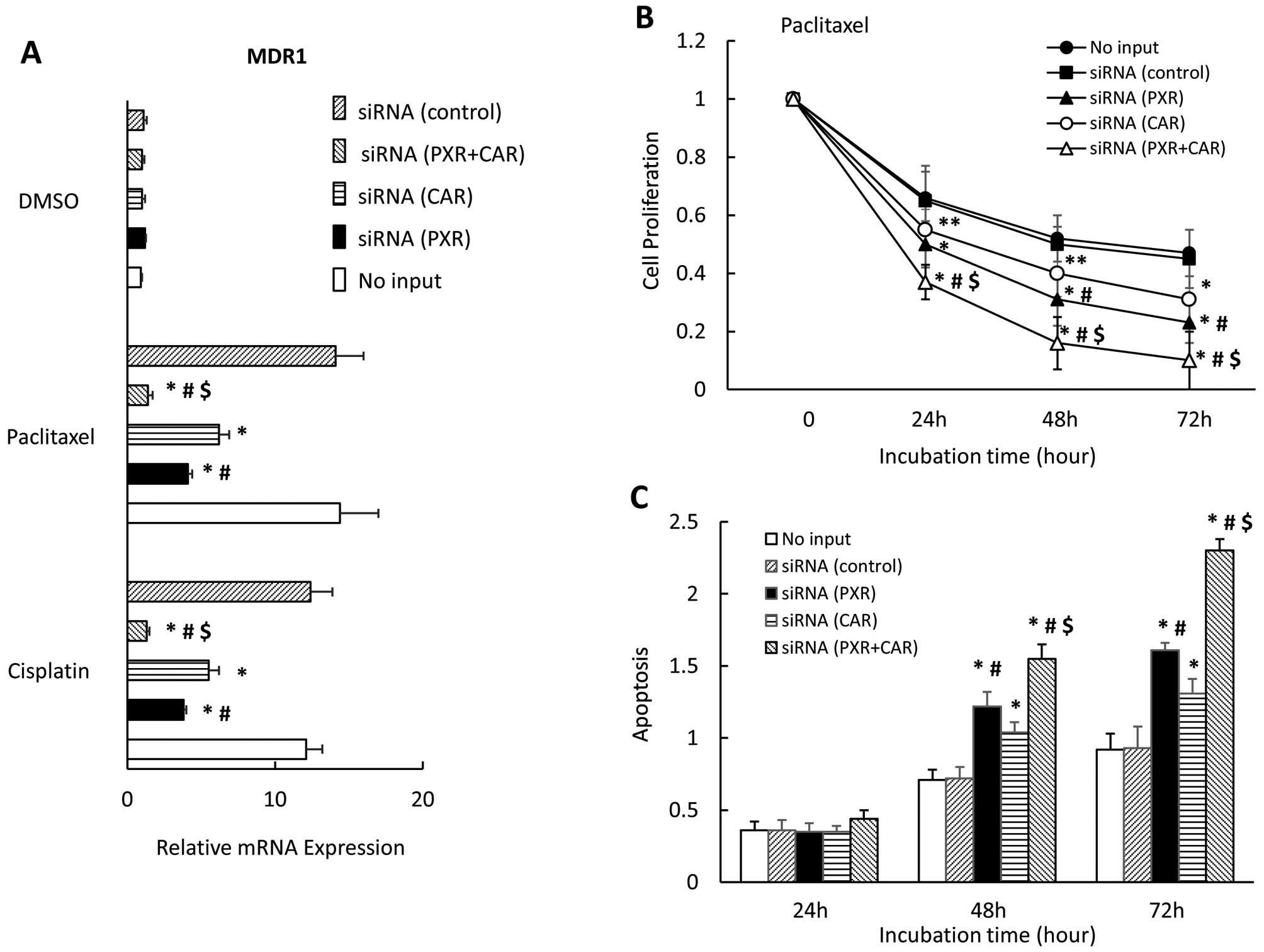 | Figure 7Effect of PXR- and CAR
siRNA on MDR1 expression, cell proliferation and apoptosis in
CaOV-3 cells. CaOV-3 cells were transfected with PXR, CAR or
control siRNA and treated with paclitaxel or DMSO for 36 h.
MDR1 mRNA expression was analyzed by quantitative real-time
PCR. Each experiment was performed in triplicate 4 times (internal
control, 18S; negative control, DMSO). Change in gene expression
was calculated as ratio of target gene to internal control (A).
CaOV-3 cells were transfected with PXR-, CAR- or control
siRNA or no siRNA, and were seeded and incubated with paclitaxel
for 0, 24, 48 or 72 h (B). TUNEL assay of apoptotic CaOV-3 cells
transfected with PXR-, CAR- or control siRNA, or no siRNA
and treated with paclitaxel for 36 h (C). Results show mean ± SD of
5 independent experiments (*P<0.01;
**P<0.05 vs. control siRNA group,
#P<0.01 vs. CAR siRNA, $P<0.01 vs. PXR
siRNA). |
Discussion
We investigated whether inhibition of the
PXR-mediated pathway could affect cytotoxicity of the anticancer
drugs, paclitaxel and/or cisplatin, in several ovarian cancer cell
lines including CaOV-3, SKOV-3, and BG-1 cells, to examine the
possible effect of PXR on augmentation of drug sensitivity and
overcoming drug resistance. We observed that phthalate and
pregnenolone had significant effects on the PXR-CYP3A4 pathway,
whereas the PXR-MDR1 pathway was significantly increased in the
presence of paclitaxel or cisplatin. We also observed that
downregulating PXR strongly inhibited the augmentation of MDR1
expression and PXR-mediated transcription by PXR ligands, and
significantly enhanced the cell growth inhibition and apoptosis in
the presence of paclitaxel or cisplatin. In addition, we found that
pregnenolone moderately and ketoconazole strongly suppressed the
augmented MDR1 expression and PXR-mediated transactivation by
paclitaxel or cisplatin, and enhanced cell-growth inhibition and
apoptosis in the presence of paclitaxel or cisplatin. Cell growth
inhibition and apoptosis were significantly enhanced in the cells
transfected with PXR siRNA compared with those transfected
with CAR siRNA in the presence of paclitaxel.
We previously demonstrated that PXR ligands enhance
PXR-mediated transcription in a ligand- and promoter-dependent
fashion, which in turn differentially regulate expression of
individual PXR targets, such as CYP3A4 and MDR1, in endometrial
cancer cells (29). We also
observed that steroids and endocrine-disrupting chemicals enhanced
CYP3A4 expression, whereas the anticancer agents, paclitaxel or
cisplatin had positive effects on MDR1 expression in endometrial
cancer cells (29). In this study,
we observed that pregnenolone and phthalate mainly enhanced the
CYP3A4 pathway, including mRNA expression and PXR-mediated
transcription, but paclitaxel and cisplatin had stronger effects on
the MDR1 pathway in ovarian cancer cells. This implies two
different ligands and promoters, which differentially regulate
expression of individual PXR targets, such as CYP3A4 and MDR1, in
ovarian cancer cell lines.
MDR1 was originally identified because its
overexpression in cultured cancer cells was associated with an
acquired cross-resistance to multiple anticancer drugs, and it has
been shown to be an ATP-dependent efflux pump of hydrophobic
anticancer drugs (7). Clinical
paclitaxel resistance is often associated with MDR1 overexpression;
in vitro paclitaxel resistance typically occurs with
overexpression of the MDR1 gene (6). However, several clinical trials have
attempted to alter P-glycoprotein activity and thus improve
clinical outcomes using verapamil and dexamethasone; most of these
studies showed no clear-cut success (9–11).
An earlier report showed that siRNA targeted to MDR1 could
sensitize paclitaxel-resistant ovarian cancer cells in vitro
(35), which suggests that siRNA
treatment may present a new approach for treating MDR1-mediated
drug resistance. Here, we showed that the mRNA levels of
CYP3A4 and MDR1 were strongly inhibited in cells
transfected with PXR siRNA, in the presence of PXR ligands,
pregnenolone, phthalate, paclitaxel, and cisplatin, compared with
cells treated with control siRNA and untransfected cells. In
addition, we observed no PXR ligand that enhanced PXR-mediated
transactivation through MDR1 and CYP3A4 promoters in
cells transfected with PXR siRNA. These data suggest that
the PXR-CYP3A4 and PXR-MDR1 pathways are blocked by downregulated
PXR in ovarian cancer cells. Next, we found that downregulating PXR
expression significantly enhanced cell-growth inhibition and
apoptosis in the presence of the anticancer agents, paclitaxel and
cisplatin, which indicates that downregulating PXR in ovarian
cancer might alter its response to anticancer agents.
Pregnenolone is an endogenous steroid hormone, known
as a ligand for PXR (36,37). It has a strong positive effect on
the CYP3A4 pathway, compared with its effect on MDR1. The
combination of cisplatin or paclitaxel with pregnenolone suppressed
the MDR1 expression induced by anticancer agents in a
dose-dependent manner. Because pregnenolone also suppressed
PXR-mediated transcription through the MDR1 promoter in the
presence of paclitaxel or cisplatin, this suppression mediated by
PXR, and the binding of pregnenolone to PXR might inhibit the
PXR-mediated activation by paclitaxel and cisplatin. Ketoconazole,
an antifungal drug, is reported to inhibit the PXR activation by
binding to PXR (38) and to be a
PXR antagonist, an inhibitor of both PXR-mediated drug metabolism
and the MDR1 pathway in HepG2 cells (39). In this study, we showed that
ketoconazole strongly inhibited augmentation of the MDR1 pathway by
paclitaxel or cisplatin in dose-dependent manner. These data imply
that pregnenolone partially suppressed the MDR1 pathway because
pregnenolone weakly enhanced the MDR1 pathway through PXR. In
contrast, ketoconazole strongly inhibited the MDR1 pathway because
ketoconazole had no positive effect on PXR-mediated genes. The
different inhibitory effects on the MDR1 pathway might have caused
the different effects of pregnenolone and ketoconazole on cell
growth inhibition and apoptosis in the presence of paclitaxel or
cisplatin.
In this study, we observed PXR downregulation to
suppress PXR-mediated transactivation through the MDR
promoter, but it did not completely inhibit the paclitaxel or
cisplatin-augmented MDR1 expression. Moreover, ketoconazole, a PXR
antagonist, could not completely block the paclitaxel or
cisplatin-enhanced MDR1 expression; and RNA interference of both
PXR and CAR completely abolished MDR1 overexpression
and enhanced cell growth inhibition and apoptosis in the presence
of paclitaxel compared with interference of either PXR or CAR
alone, which further indicates that both PXR-and CAR-mediated
pathways affect drug resistance. We also observed that stronger
effect of PXR downregulation on cell growth inhibition and
apoptosis compared with CAR downregulation in the presence
of paclitaxel. PXR and CAR reportedly share several ligands
including paclitaxel and cisplatin, and several target genes
including MDR1 (21,22,29,30).
Moreover, activation of phase I drug metabolism enzymes (such as
CYP3A4) by PXR and/or CAR might affect the mechanism for drug
resistance (40). Thus, the roles
of xenobiotic receptors such as PXR and CAR in drug resistance for
anticancer agents warrant further study.
In conclusion, inhibition of PXR-mediated pathways
could augment sensitivity, or even overcome resistance, to
anticancer agents in the treatment of ovarian cancer, suggesting a
novel means for ovarian cancer patients, especially for those that
have resistance to anticancer agents.
Acknowledgements
This study was supported in part by a research grant
from the Ministry of Education, Culture, Sports, Science and
Technology of Japan (no. 25462558).
References
|
1
|
Rubin SC, Randall TC, Armstrong KA, Chi DS
and Hoskins WJ: Ten-year follow-up of ovarian cancer patients after
second-look laparotomy with negative findings. Obstet Gynecol.
93:21–24. 1999.PubMed/NCBI
|
|
2
|
Armstrong DK: Relapsed ovarian cancer:
Challenges and management strategies for a chronic disease.
Oncologist. 7(Suppl 5): 20–28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
National Institute for Health and Care
Excellence. Guidance on the use of paclitaxel in the treatment of
ovarian cancer. http://www.nice.org.uk/guidance/ta55.
Accessed January 22, 2003
|
|
4
|
McGuire WP, Hoskins WJ, Brady MF, Kucera
PR, Partridge EE, Look KY, Clarke-Pearson DL and Davidson M:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Piccart MJ, Bertelsen K, James K, Cassidy
J, Mangioni C, Simonsen E, Stuart G, Kaye S, Vergote I, Blom R, et
al: Randomized intergroup trial of cisplatin-paclitaxel versus
cisplatin-cyclophosphamide in women with advanced epithelial
ovarian cancer: Three-year results. J Natl Cancer Inst. 92:699–708.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsuruo T, Naito M, Tomida A, Fujita N,
Mashima T, Sakamoto H and Haga N: Molecular targeting therapy of
cancer: Drug resistance, apoptosis and survival signal. Cancer Sci.
94:15–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE
and Gottesman MM: P-glycoprotein: From genomics to mechanism.
Oncogene. 22:7468–7485. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ling V: Multidrug resistance: Molecular
mechanisms and clinical relevance. Cancer Chemother Pharmacol.
40(Suppl): S3–S8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dalton WS, Crowley JJ, Salmon SS, Grogan
TM, Laufman LR, Weiss GR and Bonnet JD: A phase III randomized
study of oral verapamil as a chemosensitizer to reverse drug
resistance in patients with refractory myeloma. A Southwest
Oncology Group study. Cancer. 75:815–820. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sonneveld P, Suciu S, Weijermans P, Beksac
M, Neuwirtova R, Solbu G, Lokhorst H, van der Lelie J, Dohner H,
Gerhartz H, et al; European Organization for Research and Treatment
of Cancer (EORTC); Leukaemia Cooperative Group (LCG); Dutch
Haemato-Oncology Cooperative Study Group (HOVON). Cyclosporin A
combined with vincristine, doxorubicin and dexamethasone (VAD)
compared with VAD alone in patients with advanced refractory
multiple myeloma: An EORTC-HOVON randomized phase III study
(06914). Br J Haematol. 115:895–902. 2001. View Article : Google Scholar
|
|
11
|
Leonard GD, Fojo T and Bates SE: The role
of ABC transporters in clinical practice. Oncologist. 8:411–424.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kliewer SA, Moore JT, Wade L, Staudinger
JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM,
Zetterström RH, et al: An orphan nuclear receptor activated by
pregnanes defines a novel steroid signaling pathway. Cell.
92:73–82. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lehmann JM, McKee DD, Watson MA, Willson
TM, Moore JT and Kliewer SA: The human orphan nuclear receptor PXR
is activated by compounds that regulate CYP3A4 gene expression and
cause drug interactions. J Clin Invest. 102:1016–1023. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bertilsson G, Heidrich J, Svensson K,
Asman M, Jendeberg L, Sydow-Bäckman M, Ohlsson R, Postlind H,
Blomquist P and Berkenstam A: Identification of a human nuclear
receptor defines a new signaling pathway for CYP3A induction. Proc
Natl Acad Sci USA. 95:12208–12213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, LeCulyse E, Liu L, Hu M, Matoney
L, Zhu W and Yan B: Rat pregnane X receptor: Molecular cloning,
tissue distribution, and xenobiotic regulation. Arch Biochem
Biophys. 368:14–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blumberg B, Sabbagh W Jr, Juguilon H,
Bolado J Jr, van Meter CM, Ong ES and Evans RM: SXR, a novel
steroid and xenobiotic-sensing nuclear receptor. Genes Dev.
12:3195–3205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pascussi JM, Jounaidi Y, Drocourt L,
Domergue J, Balabaud C, Maurel P and Vilarem MJ: Evidence for the
presence of a functional pregnane X receptor response element in
the CYP3A7 promoter gene. Biochem Biophys Res Commun. 260:377–381.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schuetz EG, Brimer C and Schuetz JD:
Environmental xenobiotics and the antihormones cyproterone acetate
and spironolactone use the nuclear hormone pregnenolone X receptor
to activate the CYP3A23 hormone response element. Mol Pharmacol.
54:1113–1117. 1998.PubMed/NCBI
|
|
19
|
de Wildt SN, Kearns GL, Leeder JS and van
den Anker JN: Cytochrome P450 3A: Ontogeny and drug disposition.
Clin Pharmacokinet. 37:485–505. 1999. View Article : Google Scholar
|
|
20
|
Ketter TA, Flockhart DA, Post RM, Denicoff
K, Pazzaglia PJ, Marangell LB, George MS and Callahan AM: The
emerging role of cytochrome P450 3A in psychopharmacology. J Clin
Psychopharmacol. 15:387–398. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Synold TW, Dussault I and Forman BM: The
orphan nuclear receptor SXR coordinately regulates drug metabolism
and efflux. Nat Med. 7:584–590. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Geick A, Eichelbaum M and Burk O: Nuclear
receptor response elements mediate induction of intestinal MDR1 by
rifampin. J Biol Chem. 276:14581–14587. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kliewer SA, Goodwin B and Willson TM: The
nuclear pregnane X receptor: A key regulator of xenobiotic
metabolism. Endocr Rev. 23:687–702. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hartley DP, Dai X, Yabut J, Chu X, Cheng
O, Zhang T, He YD, Roberts C, Ulrich R, Evers R, et al:
Identification of potential pharmacological and toxicological
targets differentiating structural analogs by a combination of
transcriptional profiling and promoter analysis in LS-180 and
Caco-2 adenocarcinoma cell lines. Pharmacogenet Genomics.
16:579–599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guzelian J, Barwick JL, Hunter L, Phang
TL, Quattrochi LC and Guzelian PS: Identification of genes
controlled by the pregnane X receptor by microarray analysis of
mRNAs from pregnenolone 16alpha-carbonitrile-treated rats. Toxicol
Sci. 94:379–387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masuyama H, Nakatsukasa H, Takamoto N and
Hiramatsu Y: Down-regulation of pregnane X receptor contributes to
cell growth inhibition and apoptosis by anticancer agents in
endometrial cancer cells. Mol Pharmacol. 72:1045–1053. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gupta D, Venkatesh M, Wang H, Kim S, Sinz
M, Goldberg GL, Whitney K, Longley C and Mani S: Expanding the
roles for pregnane X receptor in cancer: Proliferation and drug
resistance in ovarian cancer. Clin Cancer Res. 14:5332–5340. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yue X, Akahira J, Utsunomiya H, Miki Y,
Takahashi N, Niikura H, Ito K, Sasano H, Okamura K and Yaegashi N:
Steroid and xenobiotic receptor (SXR) as a possible prognostic
marker in epithelial ovarian cancer. Pathol Int. 60:400–406. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Masuyama H, Suwaki N, Tateishi Y,
Nakatsukasa H, Segawa T and Hiramatsu Y: The pregnane X receptor
regulates gene expression in a ligand- and promoter-selective
fashion. Mol Endocrinol. 19:1170–1180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Masuyama H, Nobumoto E, Zhang G
and Hiramatsu Y: The inhibition of constitutive androstane
receptor-mediated pathway enhances the effects of anticancer agents
in ovarian cancer cells. Biochem Pharmacol. 90:356–366. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie W and Evans RM: Orphan nuclear
receptors: The exotics of xenobiotics. J Biol Chem.
276:37739–37742. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qatanani M and Moore DD: CAR, the
continuously advancing receptor, in drug metabolism and disease.
Curr Drug Metab. 6:329–339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Petru E, Sevin BU, Perras J, Boike G,
Ramos R, Nguyen H and Averette HE: Comparative chemosensitivity
profiles in four human ovarian carcinoma cell lines measuring ATP
bioluminescence. Gynecol Oncol. 38:155–160. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith JA, Ngo H, Martin MC and Wolf JK: An
evaluation of cytotoxicity of the taxane and platinum agents
combination treatment in a panel of human ovarian carcinoma cell
lines. Gynecol Oncol. 98:141–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Duan Z, Brakora KA and Seiden MV:
Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small
interfering RNA and reversal of paclitaxel resistance in human
ovarian cancer cells. Mol Cancer Ther. 3:833–838. 2004.PubMed/NCBI
|
|
36
|
Masuyama H, Hiramatsu Y, Kunitomi M, Kudo
T and MacDonald PN: Endocrine disrupting chemicals, phthalic acid
and nonylphenol, activate Pregnane X receptor-mediated
transcription. Mol Endocrinol. 14:421–428. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masuyama H, Hiramatsu Y, Mizutani Y,
Inoshita H and Kudo T: The expression of pregnane X receptor and
its target gene, cytochrome P450 3A1, in perinatal mouse. Mol Cell
Endocrinol. 172:47–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang H, Huang H, Li H, Teotico DG, Sinz M,
Baker SD, Staudinger J, Kalpana G, Redinbo MR and Mani S: Activated
pregnenolone X-receptor is a target for ketoconazole and its
analogs. Clin Cancer Res. 13:2488–2495. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang H, Wang H, Sinz M, Zoeckler M,
Staudinger J, Redinbo MR, Teotico DG, Locker J, Kalpana GV and Mani
S: Inhibition of drug metabolism by blocking the activation of
nuclear receptors by ketoconazole. Oncogene. 26:258–268. 2007.
View Article : Google Scholar
|
|
40
|
Chen Y, Tang Y, Guo C, Wang J, Boral D and
Nie D: Nuclear receptors in the multidrug resistance through the
regulation of drug-metabolizing enzymes and drug transporters.
Biochem Pharmacol. 83:1112–1126. 2012. View Article : Google Scholar : PubMed/NCBI
|