Introduction
Genes involved in the thyroid differentiation are
essentially thyroid transcription factor-1 (TTF-1) and human
paired box-8 (PAX-8). Both TTF-1 and PAX-8
control the expression of thyroglobulin (Tg),
thyroperoxidase (TPO), thyroid-stimulating hormone receptor
(TSHr) and sodium/iodide symporter (NIS) by binding
to the promoters of these genes (1).
TTF-1 (also known as NKX2-1,
T/EBP or TITF-1) is commonly expressed in thyroid
gland and the central nervous system (2). It is considered as a marker of
differentiation in thyroid and lung carcinomas and has been widely
used to discern the tumours of thyroid and lung origin, in the
patients with metastatic disease (3). Moreover, it is also a useful
immunohistochemical marker in the diagnosis of these cancers
(4,5). TTF-1 mRNA has been detected in
papillary thyroid carcinomas (PTC) but not in anaplastic cancers;
therefore, TTF-1 would be a marker to distinguish between
these two types of thyroid neoplasms (6,7).
Regarding its prognosis, TTF-1 may be increased in PTC with
aggressive clinical course (8). It
is also reported that high or low expression of TTF-1
compared to normal levels is a marker of worse prognosis in lung
adenocarcinoma; suggesting its importance in disease progression
(9). Notably, NKX2-1
encoding TTF-1 has been described as a double-edged sword
gene due to its dual function, with both pro- and anti-oncogenic
activities in lung cancer, depending on the co-existing oncogenic
events and differentiation status (10–13).
PAX-8 is a transcription factor expressed in
thyrocytes and renal cells (14).
It was found to be important in the regulation of organogenesis of
kidney and Müllerian system and expressed in several tumours
including thyroid, kidney and ovarian carcinomas (14,15).
It is also considered to be a diagnostic marker for renal,
endometrial and ovarian cancers (16) but inconsistent results are reported
concerning its expression in thyroid carcinomas. In fact,
PAX-8 was found to be expressed in differentiated thyroid
tumours [PTC and follicular TC (FTC)] while, discrepancies were
described in results for anaplastic thyroid carcinomas (6,17).
Hence, the relevance of PAX-8 in thyroid carcinogenesis
still remains to be investigated.
Several authors pointed out the key roles of both
TTF-1 and PAX-8 in the differentiation of thyrocytes.
Presta et al (18)
postulated that the induction of PAX-8 induces
re-differentiation of undifferentiated thyroid cancer cells
(18). Mu et al (19) showed that co-expression of
TTF-1 and PAX-8 in thyroid tumour cell lines, derived
from papillary or follicular cancers and infected with recombinant
adenoviruses (AdTTF-1 and AdPAX-8), leads to accumulation of
TPO and Tg and organification and intracellular
retention of iodine. Interestingly, transient expression of
TTF-1 and PAX-8 in mouse embryonic stem cells leads
to the development of functional follicular cells able to organize
iodine (20). Therefore,
TTF-1 and PAX-8 act together to promote the
generation and differentiation of functional thyroid tissue.
Hence, the critical importance of TTF-1 and
PAX-8 in thyroid organogenesis and their function directs
the attention to their expression status which could be modulated
during tumorigenesis by gene mutations, post-transcriptional
regulations or by epigenetic modifications (21,22).
The use of histone deacetylase inhibitors [HDACi; trichostatin A
(TSA), depsipeptide (DEPSI), sodium butyrate (NaB), suberoylanilide
hydroxamic acid (SAHA), LBH589 or valproic acid (VPA)] or
demethylating agents [5′azacytidine (5′-AZA), generally used in the
treatment of malignant hemopathies] showed dissimilar effects on
expression of TTF-1 or PAX-8 (23–27).
We noted only slight discrepancy in the published results, even if
the authors used the same cell line and similar molecule, thus,
making it quite difficult to conclude about the usefulness of these
epigenetic modulators in cell differentiation and anticancer
activity. Moreover, Kondo et al (28) correlated the absence of
TTF-1 expression with methylated status of TTF-1 in
several differentiated and undifferentiated thyroid carcinoma cell
lines. They also showed a positive correlation between acetylation
of histone H3-Lys9 and the absence of TTF-1 expression
(28) and thus, concluded that DNA
demethylating agents could restore TTF-1 gene expression in
thyroid carcinoma cell lines. Our team showed that other molecules
would impact on cell differentiation through expression of
TTF-1. We reported that TTF-1 is tightly regulated
through Wnt/β-catenin signaling pathway in PTC cells by several
mechanisms including transcriptional regulation mediated by
β-catenin-binding to a TCF/LEF-responsive element in the
TTF-1 promoter and by post-transcriptional modifications
influencing TTF-1 mRNA and protein expressions (29). We speculated that the
administration of GSK-3β inhibitors such as lithium chloride (LiCl)
or other epigenetic TTF-1 modulators could stimulate
TTF-1 expression and as a consequence, promote
differentiation of thyroid cells in pathologies where TTF-1
expression is low or absent (30).
This hypothesis was recently validated when LiCl was shown to
improve the effi-cacy of treatment for thyroid carcinoma by
radioiodine (31). Moreover,
bortezomib (Velcade®), a proteasome inhibitor used in
multiple myeloma, showed antineoplastic effects on anaplastic
thyroid carcinoma-derived cell lines and regulates TTF-1 and
PAX-8 expressions (32).
Furthermore, it has been described to modulate histone acetylation
(33).
Thus, in the present study, we hypothesized that
both TTF-1 and PAX-8 are tightly regulated and their
over- or underexpression could influence the tissue
differentiation. Therefore, we first investigated the role of
TTF-1 and PAX-8 in proliferation and tumorigenicity,
then we analyzed the efficiency of several pharmacological
molecules able to modulate TTF-1 and PAX-8
expressions and studied their outcome in apoptosis.
Materials and methods
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), Opti-MEM,
Roswell Park Memorial Institute medium (RPMI), fetal calf serum
(FCS), Lipofectamine 2000™ (1 mg/ml), propidium iodide (PI) kit and
PCR primers were purchased from Life Technologies™ (Saint Aubin,
France). Annexin-V Fluos kit was purchased from Roche
(Neuilly-sur-Seine, France). BD Matrigel™ (Basement Membrane Matrix
Growth Factor Reduced) was purchased from BD Biosciences (Le Pont
de Claix, France). Water was purified by Milli-Q system (Millipore,
Saint Quentin en Yvelines, France). The chemicals used in the
present study were of highest analytical grade.
Cell lines and cell culture
TPC-1, a human PTC cell line and ARO cells derived
from anaplastic thyroid carcinoma were kindly provided by Dr C.
Dupuy (Gustave Roussy, Villejuif, France). BHP
10-3SCmice cells have the same genetic profile as TPC-1
cell line and were kindly provided by Dr G. Clayman (MD Anderson
Cancer Center, Houston, TX, USA). TPC-1 and BHP 10-3 were grown in
DMEM and ARO in RPMI medium. DMEM and RPMI were supplemented with
10% fetal calf serum (FCS), penicillin (100 U/ml) and streptomycin
(10 μg/ml) and maintained at 37°C in an atmosphere of 5%
CO2 and 95% humidity. Cells were systematically tested
by PCR analysis to be free of mycoplasma.
Establishment of clones stably expressing
TTF-1 and PAX-8
Plasmids used in the present study are human
expression vectors (cDNA TTF-1 and cDNA PAX-8) cloned in pcDNA3
(34,35). pcDNA3.1-TTF-1 and
pcDNA3.1-PAX-8 plasmids containing ampicillin and neomycin
resistance genes were a generous gift from Dr M. Polak (INSERM UMR
S1016 CNRS, UMR 8104 Institut Cochin, Paris, France). The pcDNA3
empty vector was used as a control.
To obtain the cell lines with TTF-1 or
PAX-8 stable expressions, TPC-1, BHP 10-3 and ARO cells were
seeded in 6-well plates and transfected with pcDNA-TTF-1 or
with pcDNA-PAX-8 plasmids. In order to control the
transfection effects, the three cell lines were also stably
transfected with pcDNA3 empty vector. Transfections were realized
by Lipofectamine 2000 as recommended by the supplier. Briefly, 6 μg
of plasmid and 7.5 μl of Lipofectamine 2000 (1 μg/μl) were mixed in
2 ml of serum free Opti-MEM culture medium. After 6 h of incubation
at 37°C, the medium was replaced with complete DMEM culture medium
containing FCS. Then, after 48 h of incubation, cell colonies were
collected and expanded in the same growth medium. The selective
pressure by neomycin (88 μM) was maintained in cell culture during
three weeks.
Pharmacological treatments
Cells were seeded in 6-well plates at a
concentration of 4×105 cells/well in 2 ml of DMEM. After
24 h, cells are treated by one of the pharmacological agents:
trichostatin A (TSA; 300 nM or 1 μM), lithium chloride (LiCl; 20
mM), valproic acid (VPA; 3 mM), 5′-azacitidin (5′-AZA; 500 nM or 1
μM) or bortezomib (BOR; 100 nM). Cells were incubated for 48 h at
37°C in cell culture medium for hydrophilic (LiCl, VPA and 5′-AZA)
or in ethanol (EtOH 100%) for hydrophobic (TSA and BOR) molecules.
A calibration curve was performed by spectrophotometry to ensure
the final TSA and BOR concentrations after dissolution. Each
condition was tested in duplicate in three independent
experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA extraction and RT-qPCR were performed to
compare TTF-1 and PAX-8 mRNA levels for: i) basal
expression of wild-type cell lines; ii) WT cell lines vs. their
stably trans-fected clones; and iii) treated cells with
pharmacological molecules vs. untreated cells. RNA extraction was
performed from 5×106 collected cells using RNeasy Mini
kit (Qiagen, Courtaboeuf, France) as previously described (36). RNA purity and quantity were
evaluated by NanoDrop® ND-1000 spectrophotometry
(spectrophotometer; Thermo Fisher Scientific, Wilmington, DE, USA).
First-strand cDNA was generated with M-MLV RT from Life
Technologies and real-time PCR (qPCR) was carried out with
StepOnePlus PCR System (AB Applied Biosystems, Villebon-sur-Yvette,
France) using GoTaq® qPCR Master Mix (Promega,
Charbonnières-les-Bains, France) according to the manufacturer’s
instructions. The following primers were used to amplify the target
genes: i) TTF-1 forward (F), 5′-CGCGTTTAGACCAAGGAAC-3′ and
TTF-1 reverse (R), 5′-GAGTGTGCCCAGAGTGAAG-3′; ii) PAX-8 (F),
5′-AGGTGGTGGAGAAGATTGG-3′ and PAX-8 (R),
5′-ATAGGGAGGTTGAATGGTTG-3′. Gene expression was determined by
quantification-comparative 2−ΔΔCt method (37) and normalized to GAPDH levels
using these sequences: [GAPDH (F),
5′-ATCCCATCACCATCTTCCAG-3′ and GAPDH (R),
5′-CCATCACGCCACAGTTTCC-3′]. Results are expressed as relative mRNA
levels comparing the clones or treated cells to WT cell lines and
represent at least three independent experiments realized in
duplicate.
Immunoblotting
Cells were lysed and total proteins were extracted
using mammalian protein extraction reagent (M-PER; Thermo Fisher
Scientific, Rockford, IL, USA) in the presence of a protease
inhibitor cocktail (Roche, Neuilly-sur-Seine, France) and
quantified by Bio-RAD assay at 570 nm (36,38).
The amount of 30 μg of each sample were heated at 70°C for 10 min
with 1X sample reducing buffer (NuPAGE sample reducing agent;
Invitrogen) and 1X sample loading buffer (NuPAGE LDS sample agent;
Invitrogen). Samples were then loaded on 10% polyacrylamide gel
(NuPAGE Bis-Tris mini gels 10%; Life Technologies). Proteins were
transferred on nitrocellulose membranes using iBlot™ Dry blotting
system (Invitrogen). After saturation with either I-Block reagent
(Tropix, Inc., Bedford, MA, USA) or 10% BSA (bovine serum albumin)
solution, membranes were incubated overnight at 4°C under agitation
with one of the following primary antibodies: monoclonal rabbit
TTF-1 (1:1,000, ab133737; Abcam Biochemicals, Paris, France);
monoclonal rabbit PAX-8 (1:1,000, ab53490; Abcam Biochemicals).
β-actin-HRP (horseradish peroxidase) was used as an internal
control (1:1,000; Sigma-Aldrich Chemicals Co., Saint Quentin
Fallavier, France). Blots were then washed and incubated with
corresponding secondary antibodies: anti-rabbit-AP (alkaline
phosphatase 1:20,000; Tropix) or anti-rabbit-HRP (1:3,000; Cell
Signaling Technology, Saint Quentin en Yvelines, France). Bands
were revealed with CDP-Star Chemiluminescence reagent (alkaline
phosphatase system; Perkin Elmer, Courtaboeuf, France) or by
enhanced chemiluminescence reagent (HRP system, Clarity™; Bio-Rad
Laboratories, Marnes-la-Coquette, France). Experiments were
repeated at least 3 times.
Doubling time and cell cycle
analysis
In order to establish the doubling time of WT cell
lines compared to their stably transfected clones by TTF-1
or PAX-8, 104 cells/well were seeded in 200 μl
culture medium in 96-well plates. Cells were then incubated in the
IncuCyte™ (Essen Instruments, Inc., Ann Arbor, MI, USA); that
allows a non-invasive automated method to monitor viable cell
growth and proliferation in culture. Each well was scanned by
camera fitted in IncuCyte™ at 4-h intervals for three days, with 4
images per well. Thereby, time lapse cell confluence was obtained
for each cell type and their doubling time was calculated according
to an exponential regression equation, given as: y =
axebx, via the formula: t1/2 = ln(2)/b.
For cell cycle analysis, TPC-1, BHP 10-3 and ARO and
their corresponding transfected clones were collected and incubated
with DNA staining buffer (50 μg/ml PI in 0.1% sodium citrate, 0.1%
Triton X-100, 100 μg/ml RNase A) in the dark, for 30 min at room
temperature. Samples were then analysed by flow cytometry (Accuri
C6 flow cytometer; BD Biosciences, San Jose, CA, USA). The results
are of at least three independent experiments and represent the
percentage of cells distributed in the cell cycle phases, in the
stable clones vs. their corresponding WT cell lines or in treated
cells vs. untreated ones.
Cell migration assays
Scratch test was performed to evaluate the effects
of stable transfection either with TTF-1 or PAX-8
genes on cell migration, as previously described (39). Briefly, 100 μl BD Matrigel™ was
plated in 96-well ImageLock™ cell migration plates (Essen
BioScience Inc., Ann Arbor, MI, USA) 24 h before seeding of TPC-1,
BHP 10-3 and ARO cells and their corresponding stably transfected
clones. Cells (1×104/well) were then plated and when
reached 90% confluence, the monolayer was scratched with a 96-pin
WoundMaker (Essen BioScience). The cells were maintained in fresh
culture medium until complete wound confluence and the cell
mobility was monitored by IncuCyte™ every 4 h by ‘scratch wound’
scan type. Results are presented as scratch wound width in function
of time.
Annexin V apoptosis assay
Apoptosis was determined by flow cytometric analysis
using Annexin V kit. Each cell line was seeded in 6-well plates
(3.5×105 cells/well) in 2 ml medium and
pharmacologically treated with EtOH (20 μl), TSA (300 nM or 1 μM),
LiCl (20 mM), VPA (3 mM), BOR (100 nM) or 5′-AZA (500 nM or 1 μM).
After 48 h of incubation, cells were collected and centrifuged and
the pellets were stained with Annexin V Fluos kit (Roche,
Neuilly-sur-Seine, France) according to the manufacturer’s
instructions. Experiments were performed in triplicate of
independent experiments and data represent percentage of apoptotic
cells compared to non-treated cells, normalized to EtOH
condition.
Animal studies and tumorigenicity
tests
All animal experiments and the use of cell lines
were approved by the institutional Ethics Committee of Animal
Experimentation (CEEA) and research council (Integrated Research
Cancer Institute in Villejuif; IRCIV), registered in the French
Ministry of Higher Education and Research (Ministère de
l’Enseignement Supérieur et de la Recherche; MESR) under the
authorization number CEEA IRCIV/IGR no. 26: 94-226, no. 2011-09 and
carried out according to French laws under the conditions
established by the European Community (Directive 2010/63/UE).
Six-week-old nude nu/nu and five-week-old NSG (NOD/SCID Gamma)
female mice were purchased from the Animal Facility of the
Institute Gustave Roussy and housed in a sterilised laminar flow
caging system. Water and bedding were given ad libitum and
autoclaved before being put in the cages. All efforts were made to
minimize animal sufferance and animals were sacrificed by
CO2 inhalation at the end of the experiments, before the
collection of tumours.
Nude mice were used to test the tumorigenicity of
BHP 10-3 and ARO cell lines and their respective clones. Since,
TPC-1 cells are reported as non-tumorigenic in nude mice (40), NSG mice were used for this purpose.
All cell lines and their corresponding clones diluted in 100 μl PBS
were injected subcutaneously into the flank of mice (n=5/group) at
a rate of 2.0×106 cells/mouse for BHP 10-3 and ARO or
107 cells/mouse for TPC-1. Mice were monitored every two
or three days for tumour growth and then sacrificed when tumours
reached a volume of 1,000 mm3. Tumours were immediately
frozen in liquid nitrogen for western blot analysis.
Protein extractions from tumours and
western blot experiments
Tumours were ground, total proteins were extracted
and western blots were performed as described above, to evaluate
expression of the TTF-1 and PAX-8 protein.
Statistical analysis
The data are presented as mean ± SD (standard
deviation). By using GraphPad Prism 4 software, Mann-Witney test
was employed to compare between two groups of treatments and
Kruskal-Wallis test was performed to compare among multiple
treatments. Non-parametric analysis of longitudinal data in
factorial experiments was performed to compare the treatments in
vivo, using ‘nparLD’ package from ‘R’ software. P<0.05 was
considered as the statistically significant level.
Results
TTF-1 and PAX-8 have different expression
profiles in wild-type cell lines
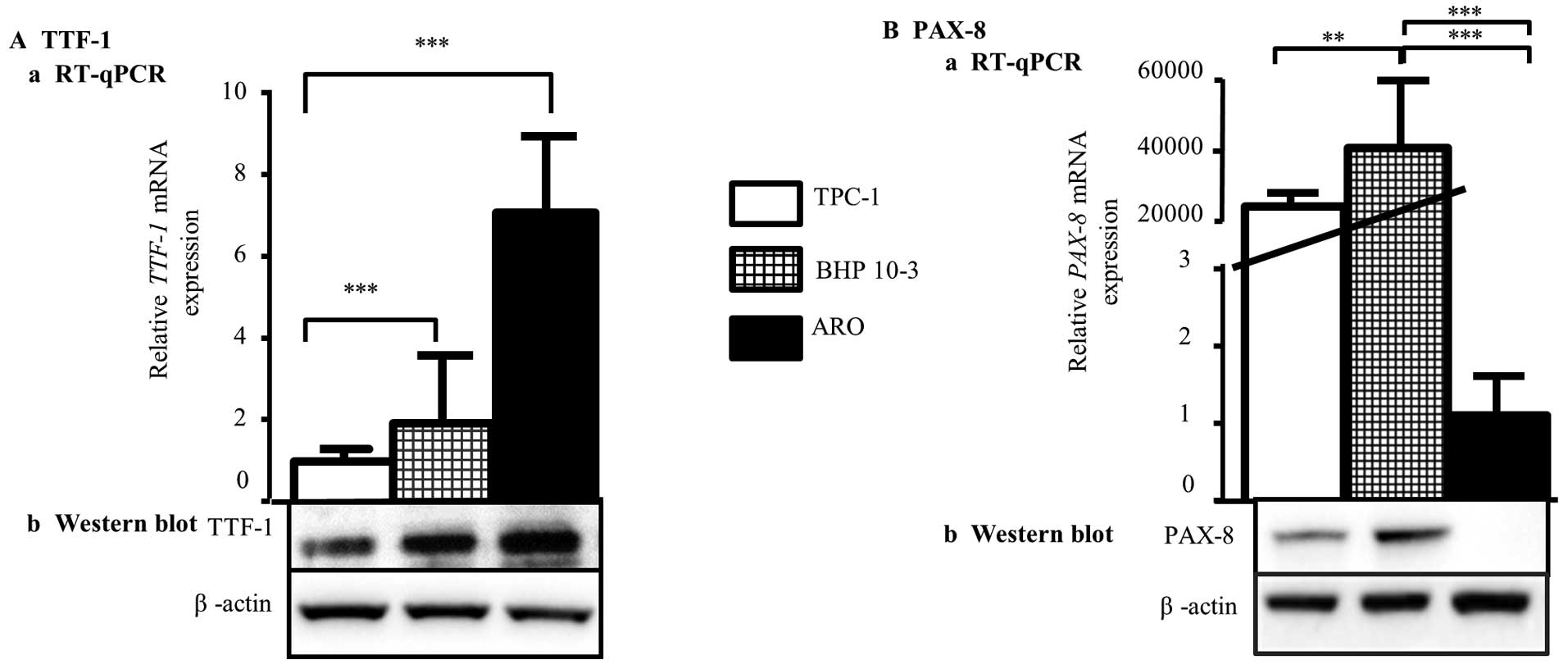
First, we tested expression of the TTF-1 and PAX-8
in TPC-1, BHP 10-3 and ARO cell lines by RT-qPCR (Fig. 1A) and western blot analysis
(Fig. 1B). Regarding TTF-1 basal
expression, our results showed that TTF-1 is more expressed in ARO
compared to TPC-1 and BHP 10-3 cell lines, both at mRNA (Fig. 1A-a) and protein (Fig. 1B-a) levels. Concerning PAX-8, mRNA
and protein were found to be highly expressed in BHP 10-3 cells
compared to TPC-1 cells whereas it was detected slightly in
anaplastic ARO cell line (Fig. 1A-b
and B-b). These results confirmed the thyroid origin of the
three cell lines, and the different expression profiles of
TTF-1 and PAX-8 genes.
TTF-1 upregulates PAX-8 in thyroid
carcinoma cell lines
TPC-1, BHP 10-3 and ARO cell lines were stably
transfected with pcDNA plasmids containing either TTF-1 or PAX-8
genes or with pcDNA3 empty vector. After 3 weeks of neomycin
selection, the resulting clones were collected and characterized by
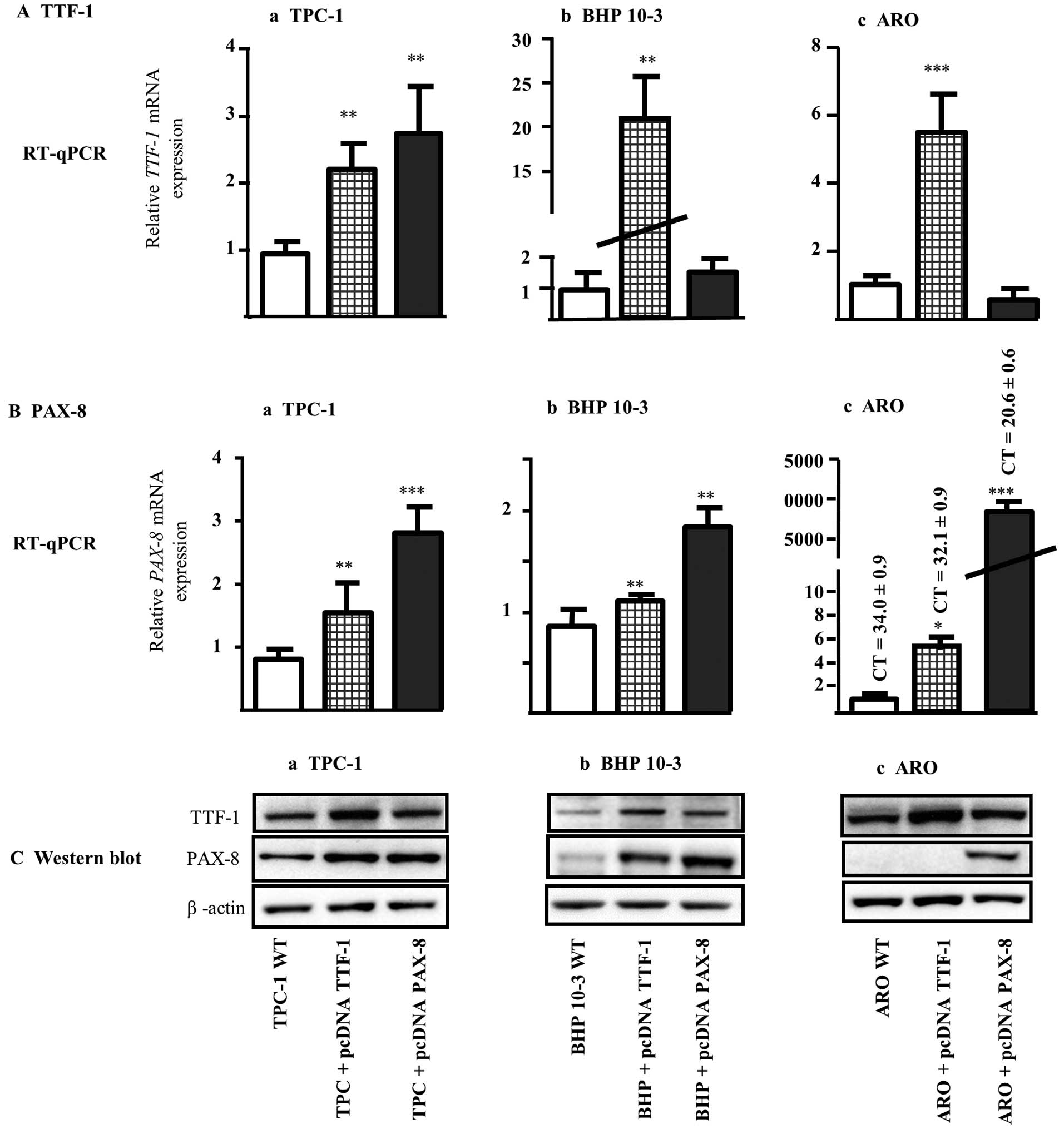
RT-qPCR and western blot analysis.
Firstly, we assessed the effects of pcDNA3 empty
vector on the expression of TTF-1 and PAX-8 mRNA by
RT-qPCR. We found that TTF-1 mRNA level was not affected by
pcDNA3 stable transfection in either TPC-1 or ARO cells. Concerning
BHP 10-3 cells, we noted a decrease in mRNA TTF-1 expression
that will not interfere with our following experiments, since no
induction in TTF-1 transcription factor was observed. Also,
for PAX-8, the stable transfection did not affect the mRNA
levels of the three cell lines tested (data not shown).
Then, clones derived from each cell line transfected
with pcDNA plasmids containing either TTF-1 or PAX-8
genes were tested for the expression profiles of TTF-1 and PAX-8
mRNA and their corresponding proteins (Fig. 2). The relative mRNA TTF-1
level in transfected clones with pcDNA TTF-1 for TPC-1, BHP 10-3
and ARO cells was ~2.5-, 20- and 5-fold, respectively more
important compared to their corresponding wild type cell lines
(Fig. 2A-a-c; RT-qPCR). The
upregulation of TTF-1 mRNA levels was paralleled by a
similar increase in TTF-1 protein in all pcDNA TTF-1 derived clones
(Fig. 2C-a-c; western blot
analysis, upper panels). Similarly, the relative mRNA PAX-8
expression was respectively 3-, 2- and 8000-fold higher in TPC-1,
BHP 10-3 and ARO clones transfected with pcDNA PAX-8 vector
(Fig. 2B-a-c; RT-qPCR) and
paralleled with higher PAX-8 protein level (Fig. 2C-a-c; western blot analysis,
mid-panels). It should be noticed that the strong increase in
PAX-8 level found in ARO clone transfected with pcDNA PAX-8
could be a consequence of low mRNA levels at basal state in ARO WT
cells (ct=34±0.9) (Fig. 2B-c,
RT-qPCR and 2C-c, western blot analysis).
Therefore, in the three cell lines tested, TTF-1
induction is able to enhance mRNA and protein PAX-8 contents except
when the basal state of PAX-8 is absent or too low (Fig. 2B-c and 2C-c). Taking together our
results showed that the upregulation of TTF-1 is able to induce
PAX-8 expression.
Upregulation of TTF-1 acts more
effectively on cell doubling time and cell cycle than PAX-8
Both cell doubling time and cell cycle of the clones
were compared to their corresponding WT cells. In the three clones
stably transfected with pcDNA TTF-1, our results showed that the
doubling time calculated according to the confluence was
significantly increased compared to their counterpart WT cells
(Table I). The pcDNA PAX-8
transfection had no effects on doubling time of TPC-1 and ARO
derived clones but significantly increased the growth of BHP 10-3
clone; this might be due to high basal expression of PAX-8
in BHP 10-3 WT cells compared to TPC-1 and ARO cells (Fig. 1B and C). Moreover, it should also
be noted that the growth characteristics of each WT cell line is
different, thus, making it difficult to compare their growth rate
(TPC-1 and BHP 10-3 spread along the surface of the support while
ARO grows in clusters).
 | Table IDoubling time and cell cycle
progression of TPC-1, BHP 10-3 and ARO cells and their
corresponding clones transfected with pcDNA TTF-1 or pcDNA
PAX-8. |
Table I
Doubling time and cell cycle
progression of TPC-1, BHP 10-3 and ARO cells and their
corresponding clones transfected with pcDNA TTF-1 or pcDNA
PAX-8.
| Doubling time | Cell cycle |
|---|
|
|
|
|---|
| Cell lines and
clones | Mean ± SD (h) | G0/G1 (%) | S (%) | G2/M (%) |
|---|
| TPC-1 WT |
25.00±4.61 |
80.86±4.15 |
8.20±2.95 |
10.82±1.60 |
| TPC-1 + pcDNA3 | NT | 80.2±13.15 | 5.45±1.48 | 13.90±13.44 |
| TPC-1 + pcDNA
TTF-1 |
43.12±5.76c |
93.70±2.63c |
3.34±2.28a |
2.30±0.57c |
| TPC-1 + pcDNA
PAX-8 | 25.23±2.87 | 85.02±4.62 | 6.32±3.30 | 8.48±1.77 |
| BHP 10-3 WT |
47.12±5.95 |
74.63±3.33 |
17.10±1.67 |
12.65±3.31 |
| BHP 10-3 +
pcDNA3 | NT | 77.95±0.78 | 12.00±1.93 | 15.65±1.06 |
| BHP 10-3 + pcDNA
TTF-1 |
57.75±5.57b | 77.93±2.74 |
11.00±1.41b | 11.05±2.20 |
| BHP 10-3 + pcDNA
PAX-8 |
33.36±3.68c | 58.30±13.23 | 18.9±7.3 | 18.98±6.92 |
| ARO WT |
28.73±2.14 |
54.00±2.26 |
32.90±0.28 |
14.20±1.41 |
| ARO + pcDNA3 | NT | 59.25±0.49 | 29.30±1.41 | 11.05±2.05 |
| ARO + pcDNA
TTF-1 |
47.13±3.24c |
61.70±0.42a |
23.55±2.76a | 15.75±1.91 |
| ARO + pcDNA
PAX-8 | 25.74±5.54 | 56.55±2.90 |
22.15±0.35c |
22.10±1.98a |
These results were further supported by cell cycle
studies, wherein we observed that TTF-1 induction triggered
an increase in cell percentage at G0/G1 phase, up to significant
level for TPC-1 and ARO clones (Table
I). Concerning the empty vector, the results of cell cycle
studies did not show any difference between WT cells and the
corresponding cell clones transfected with pcDNA3 alone (Table I).
These results suggest that in the tested cell lines,
only TTF-1 could have anti-proliferative effects by delaying
cell growth and halting cell cycle in G0/G1 phase.
A high basal state expression of PAX-8
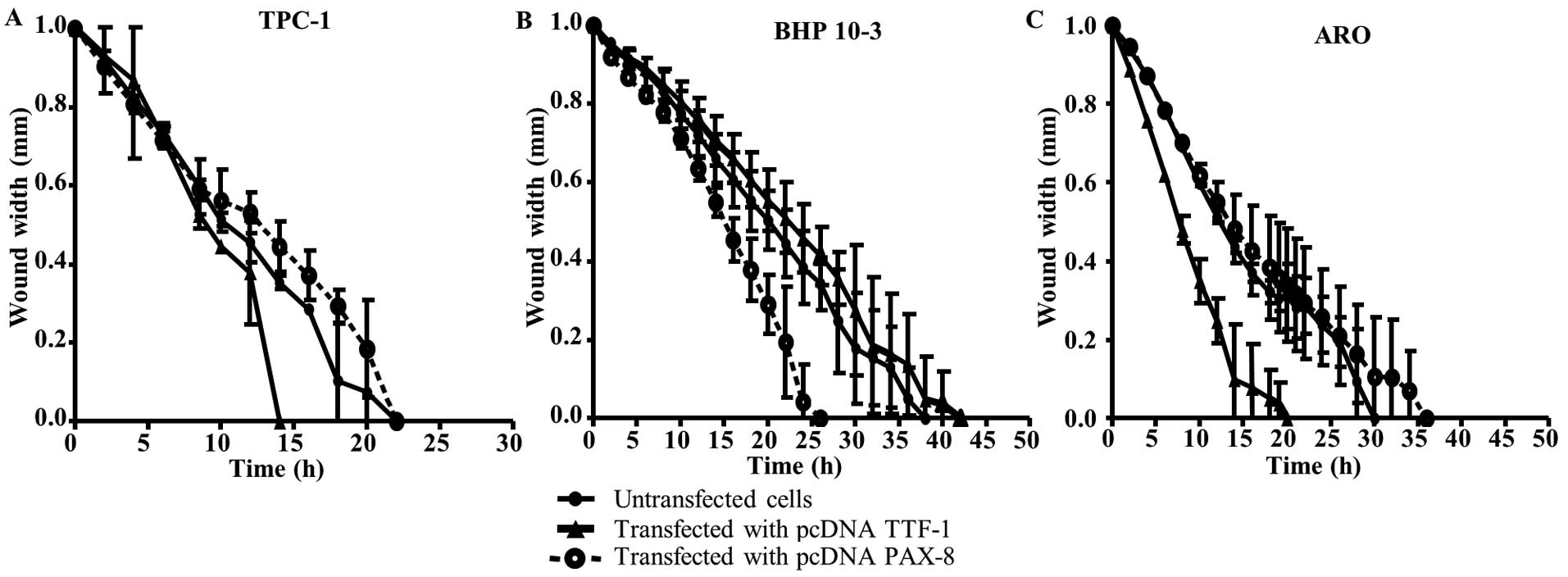
influences the migration ability
Wild-type cell lines and their corresponding clones
were assessed for their migration ability. We observed that TTF-1
transfection leads to increased cell migration in clones derived
from TPC-1 and ARO WT cell lines (respectively for clones vs. WT:
14 vs. 22 h for TPC-1 and 14 vs. 30 h for ARO; Fig. 3A and C, respectively); no effects
were observed for BHP 10−3 cells. While in this latter
cell line, PAX-8 transfection enhanced the migration of its
corresponding clone (respectively for clone vs. WT: 24 vs. 35 h;
Fig. 3B). However, PAX-8
transfection did not affect the migration of TPC-1 and ARO cells.
Herein, each transcription factor also seems to have an impact on
the migration behavior that appeared to be more influenced when a
high PAX-8 basal state level was detected.
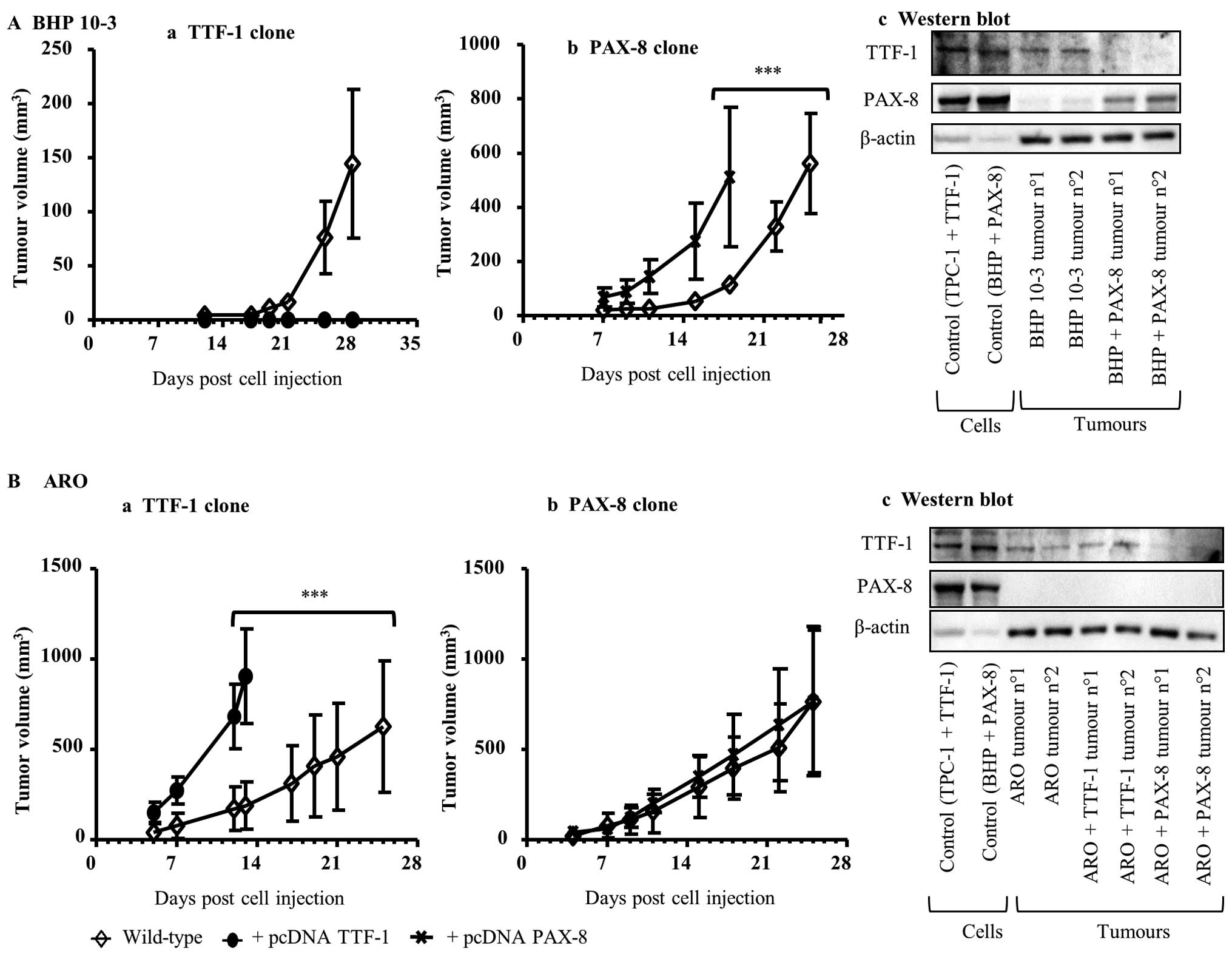
TTF-1 expression mainly influences
tumorigenicity
According to our previous results, we postulated
that the induction of TTF-1 or PAX-8 could influence
the tumorigenic potential. Concerning TPC-1 cells, known to be
non-tumorigenic in nude mice (40), the enhancement in the expression of
both transcription factors did not result in tumour formation in
NSG mice, known to be more immuno-depressed than nude mice,
injected with TPC-1 WT or with TPC-1 + pcDNA TTF-1 or TPC-1 + pcDNA
PAX-8 clones (data not shown).
Regarding BHP 10-3 cell line, its injection in nude
mice resulted in tumour development. This observation is in
accordance with previous results showing that BHP 10-3 cells are
tumorigenic in immuno-depressed mice (41). Notably, when BHP 10-3 + pcDNA TTF-1
clone highly expressing TTF-1 was injected in nude mice, no
tumours developed (Fig. 4A-a).
Contrastingly, the inoculation of BHP 10-3 + pcDNA PAX-8 clone in
nude mice increased tumour growth (Fig. 4A-b). These results were further
confirmed by western blot analysis. Tumours collected at the end of
the experiments and analyzed for TTF-1 and of PAX-8 protein
contents showed an increase in PAX-8 levels in tumours derived from
BHP 10-3 + pcDNA PAX-8 clone compared to BHP 10-3 WT tumours.
Whereas, the TTF-1 protein contents were less expressed in the same
samples (Fig. 4A-c).
In ARO cell line, also known to be tumorigenic
(40), TTF-1 induction (ARO
+ pcDNA TTF-1) increased tumour growth (Fig. 4B-a), whereas PAX-8
upregulation (ARO + pcDNA PAX-8) did not influence tumorigenicity
(Fig. 4B-b). Herein, western blot
analysis showed that TTF-1 protein pattern seems to be slightly
increased in the ARO + pcDNA TTF-1 derived clone. Concerning
PAX-8, the protein was not expressed either in ARO derived
clones (pcDNA TTF-1 and pcDNA PAX-8) or in the corresponding WT
tumours (Fig. 4B-c).
Taken together, these results demonstrated that the
basal state of TTF-1 or PAX-8 influences
tumorigenicity. Moreover, TTF-1 seems to influence tumour
development more than PAX-8 expression.
TTF-1 is more sensitive to epigenetic
modulators than PAX-8
Then, the ability of some pharmacological agents to
modulate expression of the TTF-1 and PAX-8 in TPC-1,
BHP 10-3 and ARO cell lines was studied. It was found that
TTF-1 expression seems to be more affected than PAX-8
by most of the molecules studied (Table II).
 | Table IIEffects of pharmacological molecules
on expression of TTF-1 and PAX-8 in TPC-1, BHP10-3 and ARO thyroid
cell lines. |
Table II
Effects of pharmacological molecules
on expression of TTF-1 and PAX-8 in TPC-1, BHP10-3 and ARO thyroid
cell lines.
| TPC-1 | BHP 10-3 | ARO |
|---|
|
|
|
|
|---|
| TTF-1 | PAX-8 | TTF-1 | PAX-8 | TTF-1 | PAX-8 |
|---|
| EtOH 1% | 1.00±0.04 | 1.00±0.06 | 1.00±0.08 | 1.00±0.07 | 1.00±0.04 | 1.00±0.10 |
| TSA 0.3 μM | 1.12±0.54 |
1.56±0.28a | 0.95±0.16 |
0.71±0.10a |
2.80±0.37b | 1.09±0.10 |
| TSA 1 μM |
2.66±0.46a |
5.98±1.57b |
4.13±1.96a |
1.21±0.08a |
17.65±0.46c | 1.16±0.02 |
| VPA 3 mM |
5.20±1.47a | 1.35±0.20 |
1.99±0.22b |
1.55±0.05a |
2.70±0.53a |
5.23±1.24b |
| 5′AZA 0.5 μM |
0.30±0.11b |
0.31±0.13c |
0.40±0.10b |
0.56±0.17a |
14.92±1.37b |
2.56±0.60a |
| 5′AZA 1.0 μM |
0.27±0.09b |
0.30±0.09c |
0.36±0.09a | 0.72±0.32 |
15.25±0.56b |
3.27±0.08c |
| LiCl 10 mM |
3.54±1.11a |
2.37±1.32a | 0.65±0.18 | 0.97±0.08 |
0.23±0.05b | 0.76±0.15 |
| BOR 100 nM |
4.37±1.39a | 1.82±0.66 |
1.81±0.55a | 0.91±0.09 |
0.46±0.13b |
0.51±0.09a |
In TPC-1 cells, all molecules except 5′-AZA
increased TTF-1 expression whereas, only LiCl and TSA
significantly induced the PAX-8 level (Table II).
Regarding BHP 10-3 cells, all molecules enhanced
TTF-1 expression except for GSK-3β inhibitor (LiCl) and 5′-AZA
which inhibited it. PAX-8 expression was slightly induced
only by VPA and reduced by both TSA and 5′-AZA (Table II).
In ARO cell line, only epigenetic modulators (HDACi
and demethylating agents) significantly increased TTF-1
expression, while LiCl and BOR lead to its inhibition (Table II). Concerning PAX-8, only
VPA and 5′-AZA significantly increased its expression while BOR
significantly decreased PAX-8 level (Table II).
Epigenetic modulators provoke
apoptosis
Flow cytometric analysis performed on TPC-1, BHP
10-3 and ARO cell lines showed that the used molecules could
stimulate apoptosis (Table III).
Regarding TPC-1 cell line, all the tested molecules induced early
and late apoptosis (Table III).
Notably, for BHP 10-3 cells, only HDACi were able to provoke early
apoptosis while all the other molecules induced late apoptosis
(Table III). Concerning ARO cell
line, all the tested molecules induced early apoptosis however,
only HDACi and BOR triggered late apoptosis (Table III).
 | Table IIIEffects of epigenetic modulators on
apoptosis of TPC-1, BHP 10-3 and ARO cell lines. |
Table III
Effects of epigenetic modulators on
apoptosis of TPC-1, BHP 10-3 and ARO cell lines.
| TPC-1 | BHP 10-3 | ARO |
|---|
|
|
|
|
|---|
| EA | LA | EA | LA | EA | LA |
|---|
| EtOH 1% | 1.00±0.03 | 1.00±0.03 | 1.00±0.02 | 1.00±0.02 | 1.00±0.03 | 1.00±0.02 |
| TSA 0.3 μM |
1.42±0.02c |
1.50±0.02a |
1.18±0.02c | 1.00±0.02 |
2.02±0.02b |
1.10±0.03c |
| TSA 1 μM |
1.84±0.01c |
1.51±0.01b |
1.32±0.01c |
0.66±0.01c |
2.21±0.15b |
2.33±0.08c |
| VPA 3 mM |
1.10±0.01b |
5.00±0.01c |
1.10±0.01a | 1.00±0.01 |
1.10±0.01b |
1.43±0.07b |
| 5′-AZA 0.5 μM |
1.48±0.01b |
4.00±0.01c | 0.88±0.02 |
2.50±0.02b |
1.87±0.03c | 0.84±0.04 |
| 5′-AZA 1.0 μM |
1.55±0.01b |
3.00±0.02c | 1.02±0.01 |
2.50±0.02b |
1.53±0.06c | 1.13±0.07 |
| LiCl 10 mM |
1.40±0.05c |
4.00±0.05c | 0.89±0.05 |
2.00±0.05b |
1.29±0.05b | 0.91±0.04 |
| BOR 100 nM |
2.22±0.04c |
2.00±0.04b | 1.00±0.04 |
3.70±0.04b |
2.22±0.20b |
2.04±0.05b |
Discussion
TTF-1 and PAX-8 are the major
transcription factors involved in the development and
differentiation of thyroid gland and play a key role in regulating
the expressions of thyroid genes. We hypothesized that an
enhancement of expression of TTF-1 and PAX-8 could
affect both aggressiveness and tumorigenic properties. This
hypothesis was evaluated in three models of thyroid carcinoma, two
cell lines derived from PTC (TPC-1, BHP 10-3) and one from
anaplastic carcinoma (ARO cell line).
In these models, we studied the basal state of
TTF-1 and PAX-8 expression. Our results are in
accordance with previous studies showing the presence of both
transcription factors in TPC-1 and BHP 10-3 cells (30,42).
Concerning ARO anaplastic cell line, only TTF-1 was
detected, thus providing once more evidence of its thyroid origin
(43,44). Moreover, our results showed that
the basal state of the expression of TTF-1 and PAX-8
is a major parameter that must always be taken into consideration
and could explain the divergence of results among various studies
concerning the role of these factors (6,10,11,17).
In fact, we found that the upregulation of either TTF-1 or
PAX-8 could affect tumorigenic behavior independently of the
histological origin of the cell lines used. We also confirmed
previous observations showing that the overexpression of
TTF-1 induces PAX-8 expression (45–47).
The functional studies depicted the importance of
TTF-1 in the regulation of cell cycle, migration and
tumorigenicity. TTF-1 overexpression led to anti-proliferative
effects reflected by an increase in doubling time and raised cell
percentage in G0/G1 phase leading to a decrease in cell migration
and tumorigenic potential. Contrastingly, this inspection was not
confirmed in ARO cells, wherein the upregulation of TTF-1
led to an increase in cell migration and tumorigenicity. We
hypothesized that the migratory and consequently the tumorigenic
potential of enhanced TTF-1 level are dependent on its
background expression within the cell lines. Thus, the
‘TTF-1 paradox’ is again highlighted (10,48).
In the same context, functional studies of PAX-8 depicted
that its basal state plays a key role in proliferation and
tumorigenicity which are enhanced when PAX-8 is highly expressed in
tumor cells.
Taken together, our observations converge to the
findings that the induction of TTF-1 and PAX-8 and
their consequences on tumour growth depends on their basal states.
Over-expression of one of these factors beyond a certain
‘threshold’ level could lead to pro-tumorigenic effects. Therefore,
the modulation of these genes seems to be important to re-establish
the differentiation balance.
To improve this hypothesis, we modulated the
expression of TTF-1 and PAX-8 by several pharmacological agents. In
the case of TTF-1, our results support the observations reported in
the literature showing that TTF-1 is mainly regulated by
epigenetic mechanisms such as methylation and acetylation but also
by inhibitors of GSK-3β and of proteasome (28,30).
Whereas, PAX-8 generally appeared to be less sensitive to
different treatments; suggesting that other molecular mechanisms
might also be involved in its regulation. Interestingly,
demethylating agent (5′-AZA) was capable of inducing PAX-8
only in ARO cell line, emphasizing that PAX-8 regulation in
anaplastic thyroid tumours is different from PTC.
The evaluation of these molecules on apoptosis
showed that all the used molecules triggered early and/or late
apoptosis or in some cases both. It is further important to
highlight the fact that, even though some agents did not modulate
gene expression, they succeeded in triggering apoptosis. Indeed,
these pharmacological agents have a large spectrum and a wide range
of targets (21,49,50).
Epigenetic modulators such as HDACi or demethylating agents (TSA,
VPA and 5′-AZA) used in our experiments are known to act directly
on DNA conformation and therefore, on the regulation of several
genes to induce inhibition of proliferation, cell cycle arrest,
apoptosis and even senescence (49). In the same way, BOR or LiCl also
act on several signaling pathways (51,52).
Taken together, our results suggest that different
pathways could be involved in regulation of TTF-1 and
PAX-8. Therefore, the response to various treatments could
be different according to the genetic background of the cell line
studied. Nevertheless, we can speculate that an excessive induction
of both transcription factors could lead to pro-tumorigenic effects
e.g. treatment with 5′-AZA in anaplastic carcinomas could have
opposite unintended effects. Indeed, 5′-AZA showed to increase,
in vitro, iodine uptake and the expression of NIS (53) and has been tested in phase-I
clinical trials. The positive effects of its use after clinical
trials have not yet been published (https://clinicaltrials.gov/ct2/show/NCT00004062?term=azacytidine+thyroid&rank=1).
In conclusion, the present study improved our
understandings regarding the role of thyroid transcription factors
in tumorigenesis. We showed that both genetic and pharmacological
approaches are able to induce expression of TTF-1 and
PAX-8. Thus, in future, it will be quite helpful to
systematically take into account the basal state of these
transcription factors while exploring tumour development. Moreover,
it will also be important to consider TTF-1 and PAX-8
as part of a duo. These findings could open new therapeutic
perspectives for the treatment of thyroid carcinomas.
Acknowledgements
We thank Professor Karim Benihoud and Dr Corinne
Dupuy for their support and constructive discussions during the
execution of the present study.
References
|
1
|
Civitareale D, Lonigro R, Sinclair AJ and
Di Lauro R: A thyroid-specific nuclear protein essential for
tissue-specific expression of the thyroglobulin promoter. EMBO J.
8:2537–2542. 1989.PubMed/NCBI
|
|
2
|
Guazzi S, Price M, De Felice M, Damante G,
Mattei MG and DiLauro R: Thyroid nuclear factor 1 (TTF-1) contains
a homeodomain and displays a novel DNA binding specificity. EMBO J.
9:3631–3639. 1990.PubMed/NCBI
|
|
3
|
Ordóñez NG: Value of thyroid transcription
factor-1 immunostaining in distinguishing small cell lung
carcinomas from other small cell carcinomas. Am J Surg Pathol.
24:1217–1223. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Wall NR, Kocher K, Duclos N,
Fabbro D, Neuberg D, Griffin JD, Shi Y and Gilliland DG: Stable
expression of small interfering RNA sensitizes TEL-PDGFbetaR to
inhibition with imatinib or rapamycin. J Clin Invest.
113:1784–1791. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ziad A, Ruchala M, Breborowicz J, Gembicki
M, Sowinski J and Grzymislawski M: Immunoexpression of TTF-1 and
Ki-67 in a coexistent anaplastic and follicular thyroid cancer with
rare long-life surviving. Folia Histochem Cytobiol. 46:461–464.
2008.
|
|
6
|
Zhang P, Zuo H, Nakamura Y, Nakamura M,
Wakasa T and Kakudo K: Immunohistochemical analysis of
thyroid-specific transcription factors in thyroid tumors. Pathol
Int. 56:240–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ordóñez NG: Thyroid transcription factor-1
is a marker of lung and thyroid carcinomas. Adv Anat Pathol.
7:123–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fenton CL, Patel A, Burch HB, Tuttle RM
and Francis GL: Nuclear localization of thyroid transcription
factor-1 correlates with serum thyrotropin activity and may be
increased in differentiated thyroid carcinomas with aggressive
clinical course. Ann Clin Lab Sci. 31:245–252. 2001.PubMed/NCBI
|
|
9
|
Barletta JA, Perner S, Iafrate AJ, Yeap
BY, Weir BA, Johnson LA, Johnson BE, Meyerson M, Rubin MA, Travis
WD, et al: Clinical significance of TTF-1 protein expression and
TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med.
13(8B): 1977–1986. 2009. View Article : Google Scholar
|
|
10
|
Yamaguchi T, Hosono Y, Yanagisawa K and
Takahashi T: NKX2-1/TTF-1: An enigmatic oncogene that functions as
a double-edged sword for cancer cell survival and progression.
Cancer Cell. 23:718–723. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mu D: The complexity of thyroid
transcription factor 1 with both pro- and anti-oncogenic
activities. J Biol Chem. 288:24992–25000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang DH, Sholl LM, Rojas-Rudilla V, Hall
DL, Shivdasani P, Garcia EP, MacConaill LE, Vivero M, Hornick JL,
Kuo FC, et al: KRAS and NKX2-1 mutations in invasive mucinous
adenocarcinoma of the lung. J Thorac Oncol. 11:496–503. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takanashi Y, Tajima S, Hayakawa T,
Neyatani H and Funai K: KRAS mutation-positive bronchial surface
epithelium (BSE)-type lung adenocarcinoma with strong expression of
TTF-1: A case providing a further insight as for the role of TTF-1
in the oncogenesis. Int J Clin Exp Pathol. 8:15338–15343. 2015.
|
|
14
|
Hu Y, Hartmann A, Stoehr C, Zhang S, Wang
M, Tacha D, Montironi R, Lopez-Beltran A and Cheng L: PAX8 is
expressed in the majority of renal epithelial neoplasms: An
immunohistochemical study of 223 cases using a mouse monoclonal
antibody. J Clin Pathol. 65:254–256. 2012. View Article : Google Scholar
|
|
15
|
Laury AR, Perets R, Piao H, Krane JF,
Barletta JA, French C, Chirieac LR, Lis R, Loda M, Hornick JL, et
al: A comprehensive analysis of PAX8 expression in human epithelial
tumors. Am J Surg Pathol. 35:816–826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ordóñez NG: Value of PAX 8 immunostaining
in tumor diagnosis: A review and update. Adv Anat Pathol.
19:140–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nonaka D, Tang Y, Chiriboga L, Rivera M
and Ghossein R: Diagnostic utility of thyroid transcription factors
Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol.
21:192–200. 2008.
|
|
18
|
Presta I, Arturi F, Ferretti E, Mattei T,
Scarpelli D, Tosi E, Scipioni A, Celano M, Gulino A, Filetti S, et
al: Recovery of NIS expression in thyroid cancer cells by
overexpression of Pax8 gene. BMC Cancer. 5:802005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mu D, Huang R, Li S, Ma X, Lou C and Kuang
A: Combining transfer of TTF-1 and Pax-8 gene: A potential strategy
to promote radioiodine therapy of thyroid carcinoma. Cancer Gene
Ther. 19:402–411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Antonica F, Kasprzyk DF, Opitz R, Iacovino
M, Liao XH, Dumitrescu AM, Refetoff S, Peremans K, Manto M, Kyba M,
et al: Generation of functional thyroid from embryonic stem cells.
Nature. 491:66–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dokmanovic M, Clarke C and Marks PA:
Histone deacetylase inhibitors: Overview and perspectives. Mol
Cancer Res. 5:981–989. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slingerland M, Guchelaar HJ and Gelderblom
H: Histone deacetylase inhibitors: An overview of the clinical
studies in solid tumors. Anticancer Drugs. 25:140–149. 2014.
View Article : Google Scholar
|
|
23
|
Fortunati N, Catalano MG, Arena K,
Brignardello E, Piovesan A and Boccuzzi G: Valproic acid induces
the expression of the Na+/I− symporter and
iodine uptake in poorly differentiated thyroid cancer cells. J Clin
Endocrinol Metab. 89:1006–1009. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furuya F, Shimura H, Suzuki H, Taki K,
Ohta K, Haraguchi K, Onaya T, Endo T and Kobayashi T: Histone
deacetylase inhibitors restore radioiodide uptake and retention in
poorly differentiated and anaplastic thyroid cancer cells by
expression of the sodium/iodide symporter thyroperoxidase and
thyroglobulin. Endocrinology. 145:2865–2875. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pugliese M, Fortunati N, Germano A, Asioli
S, Marano F, Palestini N, Frairia R, Boccuzzi G and Catalano MG:
Histone deacetylase inhibition affects sodium iodide symporter
expression and induces 131I cytotoxicity in anaplastic thyroid
cancer cells. Thyroid. 23:838–846. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Puppin C, D’Aurizio F, D’Elia AV,
Cesaratto L, Tell G, Russo D, Filetti S, Ferretti E, Tosi E, Mattei
T, et al: Effects of histone acetylation on sodium iodide symporter
promoter and expression of thyroid-specific transcription factors.
Endocrinology. 146:3967–3974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vivaldi A, Miasaki FY, Ciampi R, Agate L,
Collecchi P, Capodanno A, Pinchera A and Elisei R:
Re-differentiation of thyroid carcinoma cell lines treated with
5-Aza-2′-deoxycytidine and retinoic acid. Mol Cell Endocrinol.
307:142–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kondo T, Nakazawa T, Ma D, Niu D,
Mochizuki K, Kawasaki T, Nakamura N, Yamane T, Kobayashi M and
Katoh R: Epigenetic silencing of TTF-1/NKX2-1 through DNA
hypermethylation and histone H3 modulation in thyroid carcinomas.
Lab Invest. 89:791–799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Raouane M, Desmaele D, Gilbert-Sirieix M,
Gueutin C, Zouhiri F, Bourgaux C, Lepeltier E, Gref R, Ben Salah R,
Clayman G, et al: Synthesis, characterization, and in vivo delivery
of siRNA-squalene nanoparticles targeting fusion oncogene in
papillary thyroid carcinoma. J Med Chem. 54:4067–4076. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gilbert-Sirieix M, Makoukji J, Kimura S,
Talbot M, Caillou B, Massaad C and Massaad-Massade L: Wnt/β-catenin
signaling pathway is a direct enhancer of thyroid transcription
factor-1 in human papillary thyroid carcinoma cells. PLoS One.
6:e222802011. View Article : Google Scholar
|
|
31
|
Yamazaki CA, Padovani RP, Biscolla RP,
Ikejiri ES, Marchetti RR, Castiglioni ML, Matsumura LK, Maciel RM
and Furlanetto RP: Lithium as an adjuvant in the postoperative
ablation of remnant tissue in low-risk thyroid carcinoma. Thyroid.
22:1002–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Altmann A, Markert A, Askoxylakis V,
Schöning T, Jesenofsky R, Eisenhut M and Haberkorn U: Antitumor
effects of proteasome inhibition in anaplastic thyroid carcinoma. J
Nucl Med. 53:1764–1771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliva J, Dedes J, Li J, French SW and
Bardag-Gorce F: Epigenetics of proteasome inhibition in the liver
of rats fed ethanol chronically. World J Gastroenterol. 15:705–712.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pohlenz J, Dumitrescu A, Zundel D, Martiné
U, Schönberger W, Koo E, Weiss RE, Cohen RN, Kimura S and Refetoff
S: Partial deficiency of thyroid transcription factor 1 produces
predominantly neurological defects in humans and mice. J Clin
Invest. 109:469–473. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vilain C, Rydlewski C, Duprez L, Heinrichs
C, Abramowicz M, Malvaux P, Renneboog B, Parma J, Costagliola S and
Vassart G: Autosomal dominant transmission of congenital thyroid
hypoplasia due to loss-of-function mutation of PAX8. J Clin
Endocrinol Metab. 86:234–238. 2001.PubMed/NCBI
|
|
36
|
Ali HM, Maksimenko A, Urbinati G, Chapuis
H, Raouane M, Desmaële D, Yasuhiro H, Harashima H, Couvreur P and
Massaad-Massade L: Effects of silencing the RET/PTC1 oncogene in
papillary thyroid carcinoma by siRNA-squalene nanoparticles with
and without fusogenic companion GALA-cholesterol. Thyroid.
24:327–338. 2014. View Article : Google Scholar
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
38
|
Urbinati G, Ali HM, Rousseau Q, Chapuis H,
Desmaële D, Couvreur P and Massaad-Massade L: Antineoplastic
effects of siRNA against TMPRSS2-ERG junction oncogene in prostate
cancer. PLoS One. 10:e01252772015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ali HM, Urbinati G, Chapuis H, Desmaele D,
Bertrand JR, Couvreur P and Massaad-Massade L: Effects of siRNA on
RET/PTC3 junction oncogene in papillary thyroid carcinoma: From
molecular and cellular studies to preclinical investigations. PLoS
One. 9:e959642014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ouyang B, Knauf JA, Smith EP, Zhang L,
Ramsey T, Yusuff N, Batt D and Fagin JA: Inhibitors of Raf kinase
activity block growth of thyroid cancer cells with RET/PTC or BRAF
mutations in vitro and in vivo. Clin Cancer Res. 12:1785–1793.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ahn SH, Henderson Y, Kang Y, Chattopadhyay
C, Holton P, Wang M, Briggs K and Clayman GL: An orthotopic model
of papillary thyroid carcinoma in athymic nude mice. Arch
Otolaryngol Head Neck Surg. 134:190–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
van Staveren WC, Solís DW, Delys L, Duprez
L, Andry G, Franc B, Thomas G, Libert F, Dumont JE, Detours V, et
al: Human thyroid tumor cell lines derived from different tumor
types present a common dedifferentiated phenotype. Cancer Res.
67:8113–8120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chen ST, Shieh HY, Lin JD, Chang KS and
Lin KH: Overexpression of thyroid hormone receptor beta1 is
associated with thyrotropin receptor gene expression and
proliferation in a human thyroid carcinoma cell line. J Endocrinol.
165:379–389. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zito G, Richiusa P, Bommarito A, Carissimi
E, Russo L, Coppola A, Zerilli M, Rodolico V, Criscimanna A, Amato
M, et al: In vitro identification and characterization of
CD133(pos) cancer stem-like cells in anaplastic thyroid carcinoma
cell lines. PLoS One. 3:e35442008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Christophe-Hobertus C, Lefort A, Libert F
and Christophe D: Functional inactivation of thyroid transcription
factor-1 in PCCl3 thyroid cells. Mol Cell Endocrinol. 358:36–45.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miccadei S, De Leo R, Zammarchi E, Natali
PG and Civitareale D: The synergistic activity of thyroid
transcription factor 1 and Pax 8 relies on the promoter/enhancer
interplay. Mol Endocrinol. 16:837–846. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nitsch R, Di Dato V, di Gennaro A, de
Cristofaro T, Abbondante S, De Felice M, Zannini M and Di Lauro R:
Comparative genomics reveals a functional thyroid-specific element
in the far upstream region of the PAX8 gene. BMC Genomics.
11:3062010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gilbert-Sirieix M and Massaad-Massade L:
TTF-1: Neither angel nor demon. Med Sci (Paris). 27:183–186.
2011.(In French). View Article : Google Scholar
|
|
49
|
Giannini G, Cabri W, Fattorusso C and
Rodriquez M: Histone deacetylase inhibitors in the treatment of
cancer: Overview and perspectives. Future Med Chem. 4:1439–1460.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li KK, Li F, Li QS, Yang K and Jin B: DNA
methylation as a target of epigenetic therapeutics in cancer.
Anticancer Agents Med Chem. 13:242–247. 2013. View Article : Google Scholar
|
|
51
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Almond JB and Cohen GM: The proteasome: A
novel target for cancer chemotherapy. Leukemia. 16:433–443. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Provenzano MJ, Fitzgerald MP, Krager K and
Domann FE: Increased iodine uptake in thyroid carcinoma after
treatment with sodium butyrate. Otolaryngol Head Neck Surg.
137:722–728. 2007. View Article : Google Scholar : PubMed/NCBI
|