Introduction
Oral cancer is the most prevalent type of cancer in
the oral cavity and is associated with high mortality and poor
prognosis (1,2). Despite surgical treatment,
chemotherapy, or radiotherapy, the 5-year survival rate has
remained <50% in the past decades (3). Recurrence and distant metastases play
a crucial role in the morbidity and mortality associated with oral
cancer, and their mechanisms remain unclear (4). During progression to metastases,
tumor cells losing their adhesive ability become detached from the
primary tissue, invade the basement membrane, circulate via blood
vessels, and finally colonize a new location in the host. A growing
body of evidence suggests that the epithelial-to-mesenchymal
transition (EMT), which occurs normally during embryonic
development, tissue remodeling, and wound healing, is a critical
early event in metastasis (4–6). EMT
is the cellular and molecular process by which cell-to-cell
interactions and apicobasal polarity disappear and a mesenchymal
phenotype develops; these events are required for cell motility and
invasion of the basement membrane during metastasis (7,8).
Furthermore, researchers have shown that EMT is associated with the
dedifferentiation program that leads to malignant carcinoma,
because EMT is responsible for the ability of invasive cancer cells
to migrate and reach distant tissues (9).
Several research groups have studied cancer stem
cells (CSCs) during metastasis and found that these cells are
associated with tumor recurrence after treatment and with treatment
resistance (10,11). CSCs, as defined by the American
Association for Cancer Research, are a small minority of cells with
the capability of self-renewal and differentiation into the
heterogeneous lineages that proliferate extensively and constitute
the tumor mass (12,13). This minority of cancer cells is
believed to be responsible for formation of the bundle of a tumor
that consists of cells at varying stages of differentiation
(14). In this respect, the
hierarchy of a tumor is similar to the tissue from which it
originates, and CSCs are considered neoplastic counterparts of
normal stem cells. Thus, CSCs are expected to have a stem cell-like
phenotype (generally referred to as stemness). Furthermore, CSCs
have been found in several types of solid tumors, such as ovarian
(15), brain (16,17),
breast (18), prostate (19), and pancreatic cancers (11) as well as melanoma (20) and head and neck cancer (21–23).
A recent breakthrough in EMT research showed that
EMT can impart stem cell-like properties of CSCs to epithelial
cells. The acquisition of stem cell-like properties by cancer cells
is a crucial step for cancer progression and treatment resistance
(23). Some mechanisms underlying
the EMT-induced stem cell-like properties of cancer cells have been
uncovered. Epithelial cells undergoing EMT are characterized by the
downregulation of epithelial makers (such as cytokeratin; CK), a
loss of cell polarity, and intercellular adhesion molecules (for
instance E-cadherin and occludin), which is accompanied by the
upregulation of mesenchymal markers (vimentin, N-cadherin, and
fibronectin), and acquisition of fibroblast-like morphological
features with cytoskeleton reorganization. The loss of E-cadherin
and acquisition of N-cadherin are known as cadherin switching, a
major hallmark of EMT (24). The
role of adhesion molecules in EMT-induced stemness in CSCs remains
unclear.
Because CD133 (PROM1) is expressed by hematopoietic
progenitors, it aroused great interest regarding its potential as a
cell surface marker of CSCs (14).
The CD133 protein is a pentaspan cell surface receptor; neither its
ligand nor its secondary messengers are known. According to one
study, CD133 is expressed in combination with the
CD44+α2β1high phenotype in ~0.1% of cells in
a large number of samples of prostate tumors, irrespective of their
grade or metastatic state (25).
These cells are capable of self-renewal, proliferation, and
multilineage differentiation in vitro, with recapitulation
of the original tumor phenotype, consistent with CSC properties
(19,26). Olempska et al showed that
ABCG2 and CD133 are strongly coexpressed in two out of five human
pancreatic adenocarcinoma cell lines; thus, CD133 may also be a
putative CSC marker in the pancreas (27). CSCs are significantly enriched in
CD133+ subpopulations of human colon cancer cells and
hepatocellular carcinomas, according to the ability of these
CD133+ cells to self-renew, differentiate, and to form
colonies and proliferate in vitro and judging by their
ability to modify the original tumor phenotype when tumor-derived
CD133+ cells are transplanted subcutaneously into
immunodeficient mice (28).
Growing evidence suggests that EMT bestows carcinoma cells at the
tumor front with CSC-like properties and plays an important role in
formation of CSCs. Nevertheless, no one has studied the possible
association of EMT specification with CSC-like properties
associated with CD133 expression in primary cancer cells and
metastatic cells.
On the basis of the above studies, here, we
demonstrate in oral-cancer cells the molecular mechanisms that are
involved in the expression of CD133 by the cancer cells: the
mechanisms that induce EMT and lead to acquisition of CSC-like
properties by primary tumor cells and metastatic cells of head and
neck cancer. These results may point to new avenues of research
with clinical implications.
Materials and methods
Cell culture
Experiments were performed on two cell lines: YD15
and YD15M. YD15 cells were derived from mucoepidermoid carcinoma of
the tongue and YD15M cells from a lymph node metastasis from YD15.
Both cell lines were purchased from the Korean Cell Line Bank
(KCLB, Seoul, Korea; cat. nos. 60504 and 60505, respectively) and
maintained in the RPMI-1640 medium (Gibco, Daejeon, Korea)
supplemented with 10% heat-inactivated fetal bovine serum (Biomeda
Co., CA, USA) and 10% antibiotic-antimycotic solution (Welgene,
Daegu, Korea) at 37°C in a humidified atmosphere containing 5% of
CO2. The medium was replaced with a fresh one, and the
adherent cells were allowed to reach ~70% confluence. Then, the
cells were detached by means of a trypsin-ethylenediamine
tetraacetic acid solution (trypsin-EDTA: Gibco-BRL) and plated
again (subcultured) in 6-well plates for each experiment.
Western blotting
When the cells reached ~70% confluence, the medium
was removed, and the cells were washed twice with
phosphate-buffered saline (PBS, pH 7.4). Then, the cell lysate was
prepared in 200 ml of cold lysis buffer (1% NP-40, 50 mM Tris-HCl
pH 7.5, 150 mM NaCl, 0.02% sodium azide, 150 mg/ml
phenylmethylsulfonyl fluoride (PMSF), 2 mg/ml aprotinin, 20 mg/ml
leupeptin, and 1 mg/ml pepstatin A). Approximately 30 mg of protein
of the cell lysate was separated by sodium dodecyl sulfate
polyacrylamide gel electrophoresis (in a 10% gel) and transferred
to a polyvinylidene difluoride membrane (Amersham, Piscataway, NJ,
USA). Each membrane was blocked with a blocking solution containing
5% skim milk in Tris-buffered saline with Tween-20 (TBST; 2.42 g/l
Tris-HCl pH 7.6, 8 g/l NaCl, 0.1% Tween-20) for 0.5 h and rinsed
briefly in TBST. The membrane was incubated overnight at 4°C with
the appropriate primary antibody: an anti-E-cadherin antibody
(dilution 1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA),
anti-β-catenin (1:1,000, Santa Cruz Biotechnology), anti-vimentin
(1:1,000, Santa Cruz Biotechnology), anti-CD133 (1:500,
Mybiosource, San Diego, CA, USA), anti-PCNA (1:1,000, Cell
Signaling Technology, Beverley, MA, USA), anti-panCK (1:1000, Cell
Signaling Technology), anti-ALDHA1 (1:1,000, Cell Signaling
Technology), anti-OCT-4 (1:1,000, Cell Signaling Technology), or an
anti-NANOG antibody (1:1,000, Cell Signaling Technology). A mouse
monoclonal anti-β-actin antibody (1:2,500, Santa Cruz
Biotechnology) was used as the control. Finally, the membrane was
washed in TBST and the immunoreactivity of the proteins was
analyzed with an enhanced chemiluminescence detection kit (Santa
Cruz Biotechnology). The intensity of bands was quantified in
densitometric software ImageJ (http://rsb.info.nih.gov/ij).
Double immunofluorescence staining and
confocal microscopy
The immunofluorescence assay was performed as
described previously (29). Cells
were seeded onto glass Lab-Tek II Chamber Slides (Thermo Fisher
Scientific Inc., Loughborough, UK) and incubated for 1 day. The
growth medium was then removed, and cell monolayers were washed
three times with a 1% PBS solution and fixed with 3.5%
paraformaldehyde for 10 min at room temperature. The cells were
washed three times with PBS and permeabilized by Triton X-100
(0.2%, 10 min at room temperature). Non-specific binding sites were
blocked by incubation with PBS containing 1% BSA, for 30 min at
room temperature. The cells were washed three times with PBS before
incubation with the rabbit anti-vimentin antibody (1:200) followed
by a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit
IgG antibody (1:1,000) and a mouse anti-β-catenin antibody (1:200;
or a mouse anti-CD133 antibody, 1:200), with subsequent incubation
with a Texas Red (TR)-conjugated goat anti-mouse IgG antibody
(1:1,000). In some cases, the samples were incubated with the
rabbit anti-PCNA antibody (1:200) followed by the FITC-conjugated
goat anti-rabbit IgG antibody (1:1,000) and mouse anti-CD133
antibody (1:200) with subsequent incubation with the TR-conjugated
goat anti-mouse IgG antibody (1:1,000). The cells were
counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for
visualization of nuclear morphology. The stained cells were
analyzed under a confocal microscope (Carl Zeiss, Oberkochen,
Germany), at excitation wavelength 490 nm and emission wavelength
540 nm for FITC, excitation 540 nm and emission 650 nm for TR, and
excitation 360 nm and emission 450 nm for DAPI.
Flow cytometric assessment of protein
expression
Suspensions of YD15 and YD15M cells that were
detached with trypsin-EDTA were washed with PBS and fixed with 3.5%
paraformaldehyde for 10 min at room temperature. The cells were
washed three times with PBS and permeabilized by Triton X-100
(0.2%, 10 min at room temperature). Non-specific binding sites were
blocked by treatment with PBS containing 1% BSA, at room
temperature for 30 min. The cells were washed three times with PBS
before incubation with the rabbit anti-vimentin antibody (1:1,000)
followed by the FITC-conjugated goat anti-rabbit IgG antibody
(1:1,000) and mouse anti-CD133 antibody (1:1,000) with subsequent
incubation with the TR-conjugated goat anti-mouse IgG antibody
(1:1,000). In some cases, the samples were incubated with the
rabbit anti-PCNA antibody (1:1,000) followed by the FITC-conjugated
goat anti-rabbit IgG antibody (1:1,000) and mouse anti-CD133
antibody (1:1,000) with subsequent incubation with the
TR-conjugated goat anti-mouse IgG antibody (1:1,000). After the
incubation, the cells with the bound antibodies were washed twice
with washing buffer and analyzed by flow cytometry (Beckman
Coulter, CA, USA). Approximately 10,000 counts were accumulated for
each sample. Anti-IgG (isotype) antibodies were used to assess the
non-specific binding (background) in this assay.
Magnetic cell separation
Magnetic cell separation (MACS) of
vimentin+ cells was performed with EasySep™magnet
according to the manufacturer’s instructions (Stem Cell
Technologies, Durham, NC, USA). Briefly, YD15 and YD15M cells were
plated in 6-well plates. After they reached 70% confluence, the
cells were detached and washed twice in cold PBS. Dissociated cells
were stained with the rabbit anti-vimentin antibody (1:1,000)
followed by the FITC-conjugated goat anti-rabbit IgG antibody
(1:1,000) and were incubated for 30 min at 4°C. After incubation
with immunomagnetic microbeads for 30 min at 4°C, the cells were
washed in PBS containing 0.5% BSA and 2 mM EDTA, filtered through a
40-mm cell strainer. Then the cells were passed through the
magnetic cell separation device (Auto-Macs; Miltenyi Biotec) for
selection of vimentin positive cells.
Flow cytometric analysis of cell cycle
with propidium iodide (PI) staining
Cells were trypsinized to obtain a single-cell
suspension, harvested by centrifugation, and washed with PBS. After
fixation in ice-cold 70% ethanol at 4°C overnight, the cells were
collected and washed twice with PBS. The cells were then stained
with PI (3 μg/ml) for 15 min. After that, DNA from 104
of G0/S phase cells was quantified on the flow cytometer (Beckman
Coulter).
Flow cytometric assessment of ALDH
activity
This assay of tumor-derived cells was conducted
using the ALDEFLUOR kit (StemCell Technologies). In particular, the
cells were suspended in ALDEFLUOR assay buffer containing ALDH1
substrate (BODIPY-aminoacetaldehyde, 1 M per 106 cells)
and incubated for 30 min at 37°C. As a negative control, some
batches of the cells were incubated with a specific ALDH1
inhibitor, diethylaminobenzaldehyde (DEAB; 50 mM). After that, the
cells were washed twice with washing buffer and analyzed by flow
cytometry (Beckman Coulter). Approximately 10,000 counts were
accumulated for each sample.
Statistical analysis
The data were expressed as mean ± standard
deviation. All experiments were repeated three times, and all
calculations were performed in Microsoft Excel. For significance
testing, we used Student’s t-test (at P<0.05).
Results
The ability of primary cancer cells and
metastatic cells to undergo EMT
The metastatic cell line, YD15M, which was derived
from mucoepidermoid carcinoma, showed morphological features of
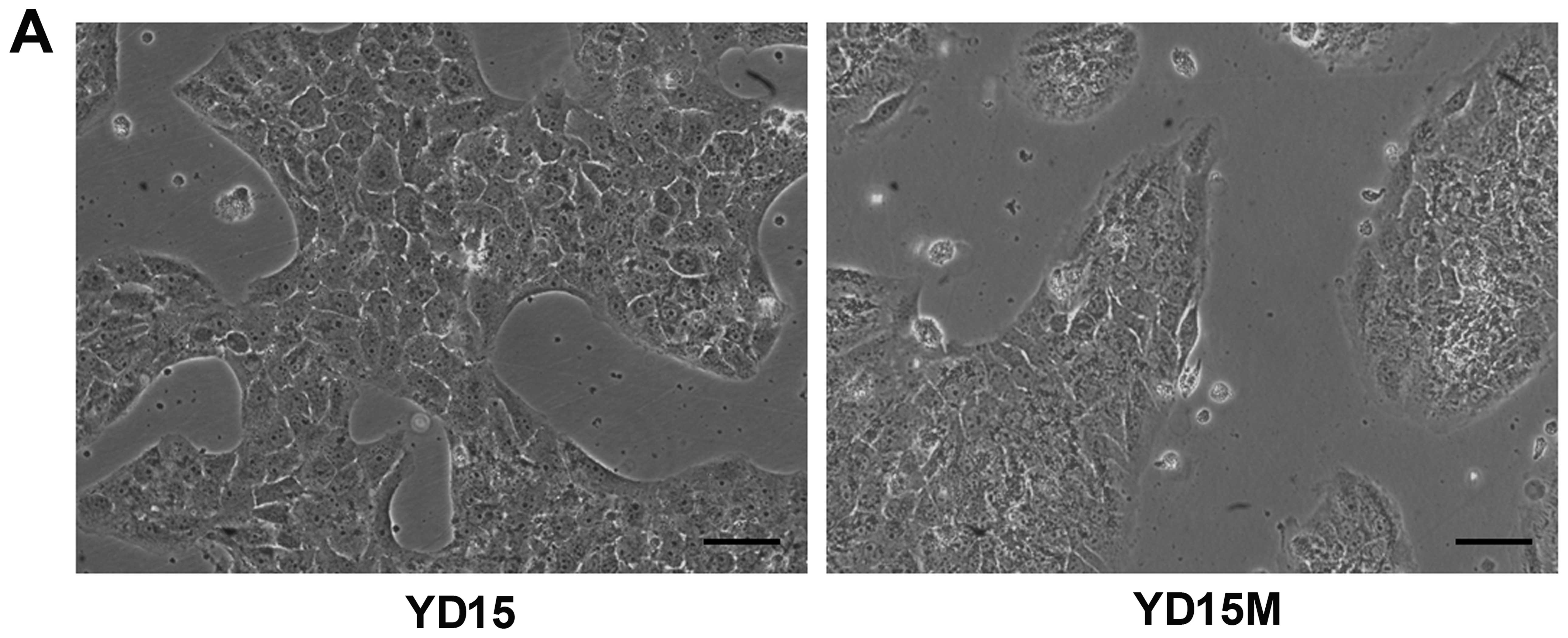
cells undergoing EMT (Fig. 1A).
The observed morphological changes in YD15M cells include switching
from a cuboid epithelial shape to a fibroblastic elongated
appearance, with colonies of irregular spherical shape. The protein
expression analysis of YD15M cells revealed a loss of the
epithelial markers E-cadherin and β-catenin; this was not the case
in YD15 cells (Fig. 1B).
Simultaneously, an increased level of the mesenchymal marker
vimentin was observed in YD15M cells. To verify these findings, we
used laser scanning confocal fluorescence microscopy to analyze the
expression of these epithelial and mesenchymal markers (Fig. 1C). As shown in the figure, YD15M
cells showed decreased protein expression of β-catenin (red) and
increased expression of vimentin (green). Moreover, a small
population of cells with nuclear localization of β-catenin was
observed among YD15M cells, but not among YD15 cells, indicating
that the metastatic YD15M cells were derived from YD15 cells in
association with EMT.
Relation of CD133 with vimentin
expression on primary cancer cells and metastatic cells
The protein expression levels of vimentin and CD133
were significantly higher in YD15M cells than in YD15 cells
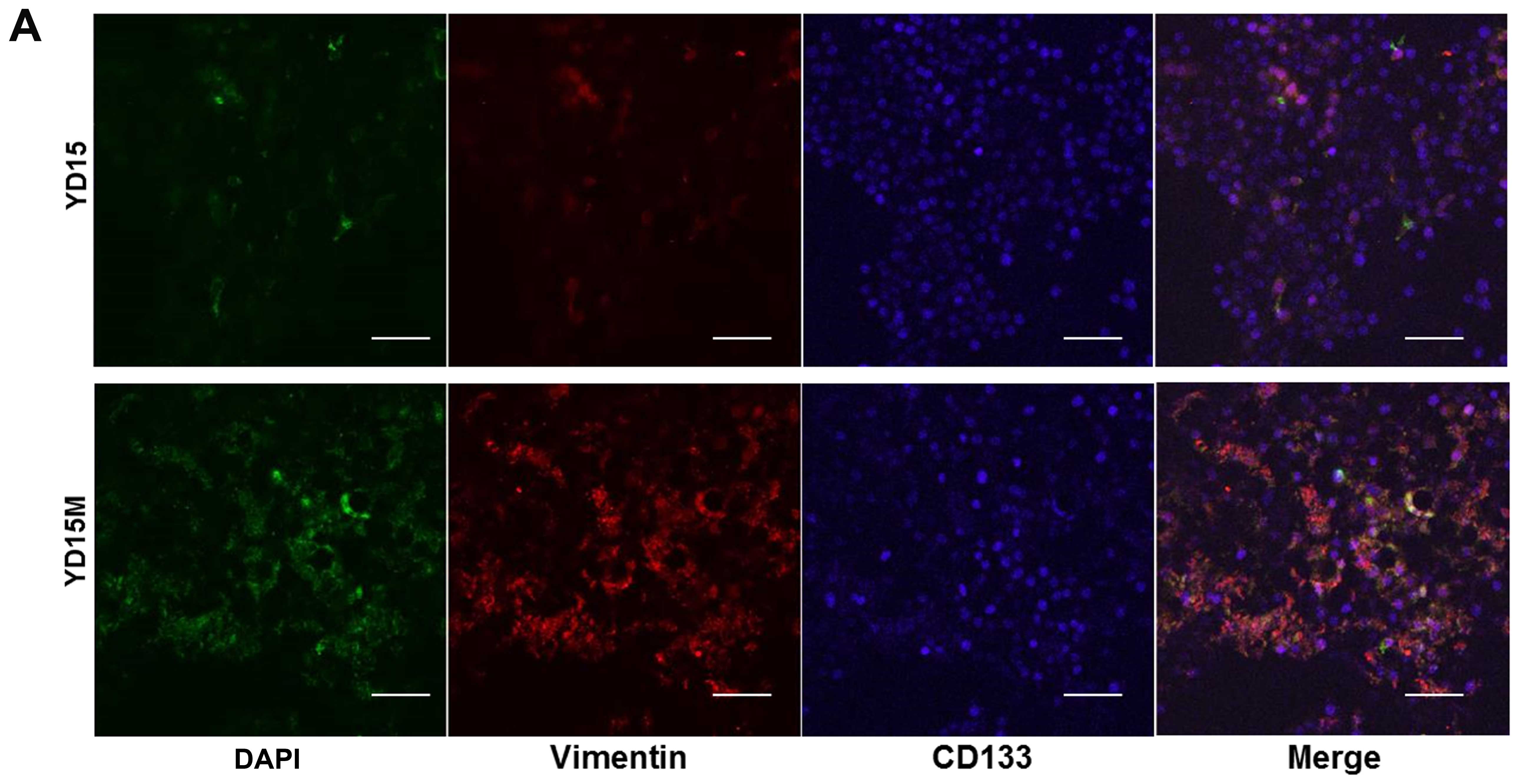
according to immunofluorescence assays (Fig. 2A). The percentage of cells
expressing CSC-specific protein CD133 was greater among YD15M cells
than among YD15 cells. The CD133 protein was observed mainly in the
cytoplasm and plasma membrane of the cells. To evaluate the
correlation of vimentin and CD133 expression in cells of the
primary and secondary tumors, we conducted dual-color flow
cytometric analysis. As evident in Fig. 2B, the subpopulation of
double-stained vimentin+CD133+ cells (gated
in sector B2 in the graphs) constituted 1.8% of YD15 cells. In
contrast, the percentage of cells in sector B2 among YD15M cells
was much greater: 16.9%; this result indicated that the metastasis
associated with the double-stained
CD133+vimentin+ cells showing features of
EMT. To test whether the metastatic cells gained CSC-like
properties, CD133 expression was assessed in vimentin+
cells during MACS (Fig. 2C). As
expected, the percentage of vimentin+CD133+
cells (gated in sector B2) was significantly greater (20.0%) among
YD15M cells than among YD15 cells (5.7%). This finding means that
the population of vimentin+ cells acquired some
properties of CSCs during metastasis.
The relation of proliferative
characteristics with CSC marker expression on primary cancer cells
and metastatic cells
We then tested whether the metastatic cells have the
proliferative properties corresponding to acquisition of CSC
characteristics. To this end, we quantified the expression of
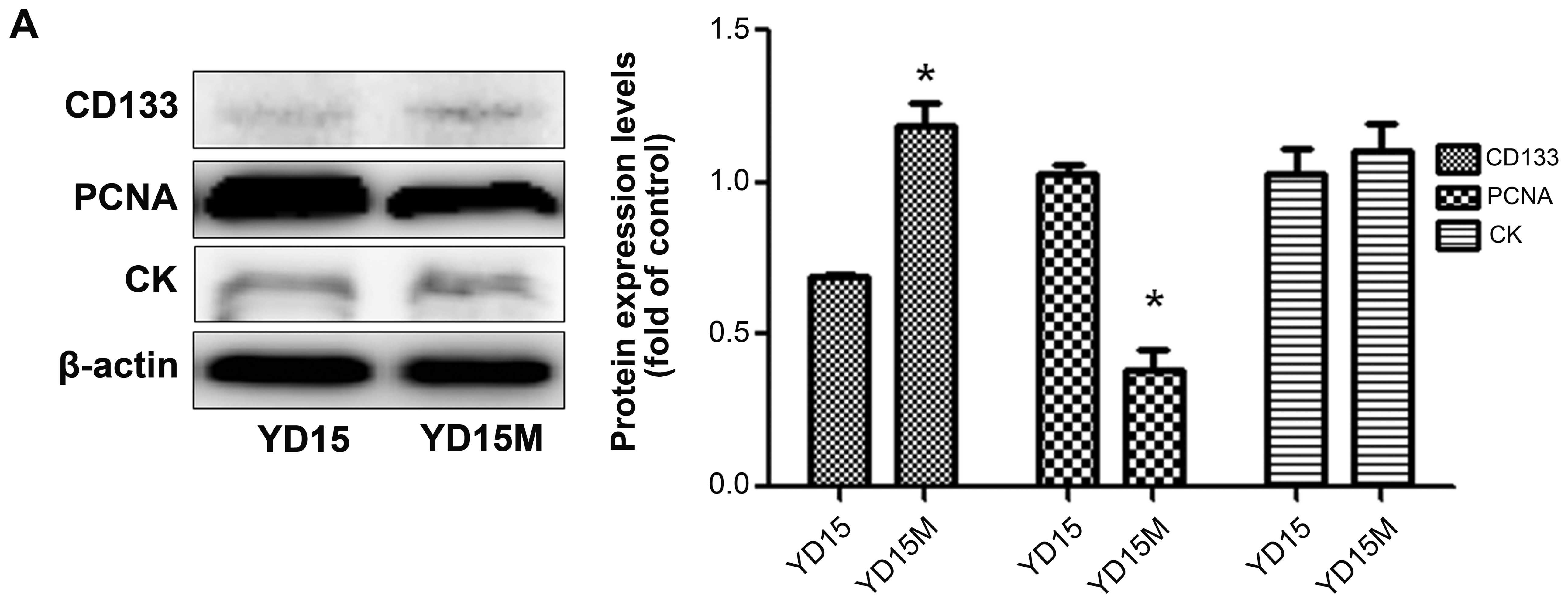
CD133, PCNA, and CK by western blotting (Fig. 3A). Downregulation of PCNA, but not
CD133, was observed in YD15M cells, in comparison with YD15 cells.
In addition, there was no significant difference in CK expression
between the two cell lines. In the dual-color flow cytometric
analysis for both PCNA and CD133, simultaneous strong expression of
PCNA and CD133 (gated in sector B2) could not detected among either
YD15 or YD15M cells (Fig. 3B). As
shown in the figure, the decreased expression of cell proliferation
marker PCNA (green) in YD15M cells was not accompanied by
downregulation of CD133 (red); this change corresponds to cells
with increased CSC-like properties (Fig. 3C). To confirm proliferation
indicators in YD15M cells (according to the PCNA western blotting),
we performed cell cycle analysis (Fig.
3D). The population of YD15M cells contained a higher
percentage of cells in the G0/G1 phase (91.9%) than did the
population of YD15 cells (61.15%). In contrast, the total
percentage of cells in the S phase was 1.1% among YD15M cells; this
figure is lower than that among YD15 cells (23.9%), confirming that
YD15M cells had weak proliferative characteristics. Thus, we
hypothesized that the weaker proliferation of the metastatic cell
line YD15M may correspond to CSC-like properties.
Stem cell-like properties of primary
cancer cells and metastatic cells
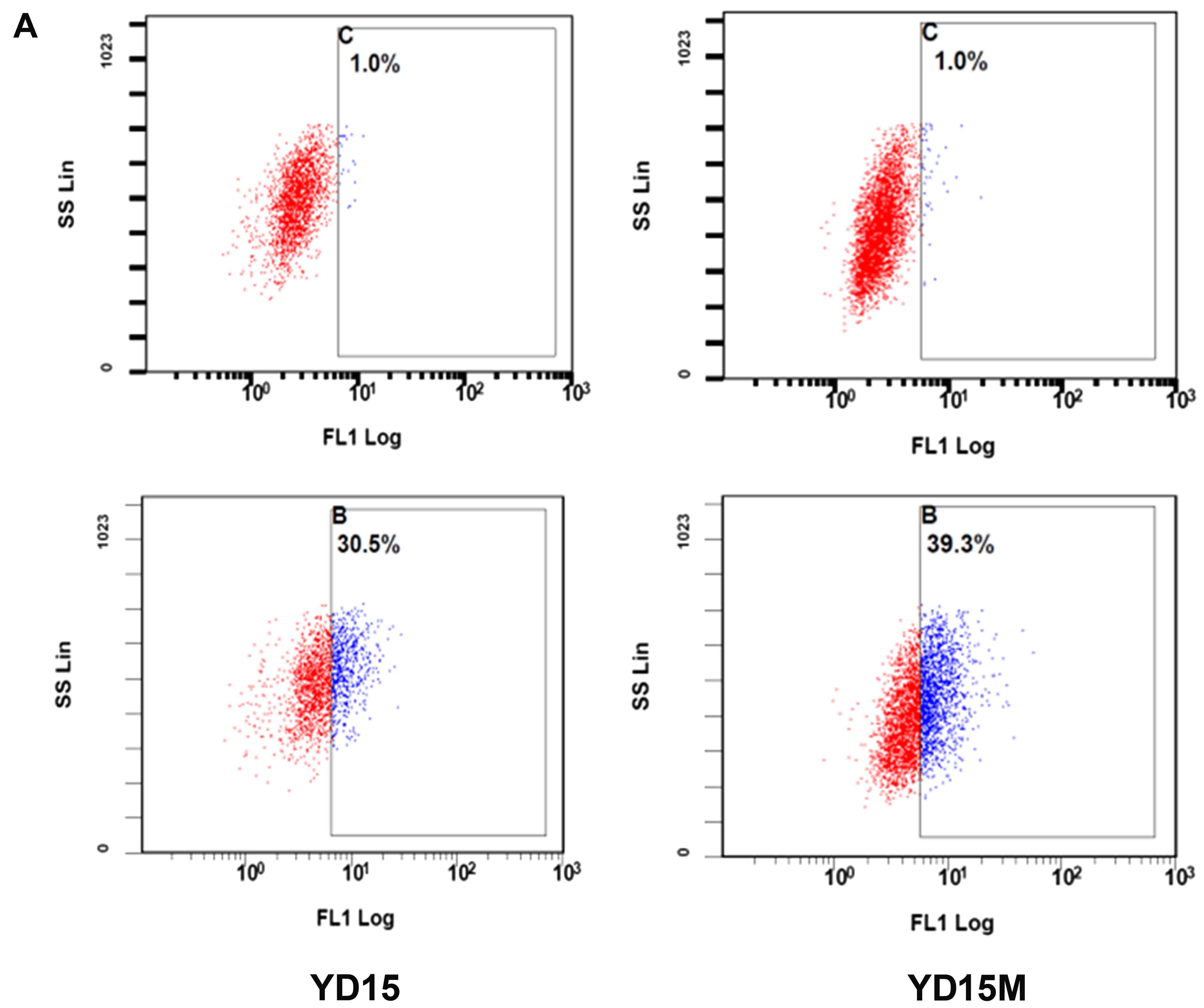
We utilized the ALDEFLUOR assay to assess the
presence and size of the subpopulation with ALDH enzymatic activity
among primary cancer cells and metastatic cells (Fig. 4A). The ALDEFLUOR-positive
subpopulation constituted 30.5% of YD15 cells. In contrast, it
constituted 39.3% of YD15M cells. Furthermore, protein levels of
ALDHA1, OCT4, and NANOG were higher in YD15M cells than in YD15
cells; in particular, OCT4 expression in YD15M cells was >5
times that in YD15 cells according to the western blot analysis
(Fig. 4B). The increased ALDH
activity and protein expression of ALDHA1, OCT4, and NANOG were
suggestive of acquisition of stem cell-like properties during
metastasis.
Discussion
Many patients with head and neck cancer die because
of metastasis and/or recurrence of the tumor (30). Nevertheless, the main events
underlying invasion and metastasis by cancer cells from the primary
tumor are still unclear in head and neck cancer, although the link
between EMT and metastasis was suggested recently (8,31).
One study suggested that progression of EMT endows cells with stem
cell-like traits and allows them to become more invasive and able
to migrate (32). Thus, we focused
on the association of EMT with CSCs in primary cancer cells and
metastatic cells; we tried to evaluate the relation between the
expression of EMT markers and stem cell-like properties after
metastasis.
EMT is an important step during the development of
metastases of epithelial carcinoma; previous research provided
in-depth understanding of the molecular mechanisms involved
(8). The loss of E-cadherin,
translocation of β-catenin to the nucleus, and induction of
vimentin are crucial events during EMT in cancer cells (32). In this study, cancer cells showed
morphological alterations from the typical cuboid epithelial
phenotype to the mesenchymal phenotype and showed mesenchyme-like
fibroblastic morphology accompanied by downregulation of the
epithelial marker E-cadherin and upregulation of vimentin in the
metastatic cell line YD15M. Partial nuclear localization of
β-catenin was also observed. These results suggest that EMT
processes take place during metastasis of mucoepidermoid carcinoma
cells, as was reported in another study (33).
The theory on CSCs is still evolving but has gained
momentum during the past five years; this theory can help to
explain some of the key characteristics of specific tumors,
including metastasis and the ability to invade (34). Recent studies support the notion
that a tumor can be initiated and maintained by a small
subpopulation of tumor cells that have stem cell-like properties,
and these highly tumorigenic cells within the bulk of the tumor are
considered CSCs (35).
In this study, we first sought to identify CSC-like
cells using the cell surface marker CD133 (prominin 1). CD133 is a
transmembrane glycoprotein (with five membrane-spanning domains)
and was originally identified in neuroepithelial stem cells.
CD133-expressing cancer cells with a stem cell-like phenotype are
believed to account for tumor recurrence (36). Moreover, CD133 was found to be a
common stemness marker and has been successfully used to isolate a
subpopulation of highly tumorigenic cells from various solid tumors
(37). In addition, CD133 is
expressed by healthy-tissue resident and hematopoietic stem cells
but is no longer detectable after differentiation. Similarly, CD133
is not expressed on cancer cells after their differentiation
(25).
In this study, we showed that EMT is associated with
acquisition of CSC properties. The protein levels of vimentin and
CD133 were significantly higher in the metastatic cells than in the
primary cancer cells. Furthermore, increased coexpression of
vimentin and CD133 was observed in the metastatic cells. This
result means that the population of vimentin+ cancer
cells acquired some properties of CSCs during metastasis.
In 2008, Mani et al reported that the
induction of EMT in immortalized human mammary epithelial cells
results in acquisition of mesenchymal traits and in expression of
stem cell markers (38). CSC-like
cells that are isolated either from mouse or human mammary glands
or from mammary carcinomas also express EMT markers (38). The acquisition of these stem
cell-like and tumorigenic characteristics is associated with EMT
induction, and those results are in agreement with this study.
These observations provide insights into the interplay between
epithelial-mesenchymal plasticity and stem cell-like properties
during metastasis and also cast doubt on the ‘inevitable’
association between EMT and CSCs. We believe that acquisition of
CSC-like properties is accompanied by EMT and may stimulate
migratory abilities of cancer cells.
Emerging evidence revealed a new characteristic of
CSCs: their resistance to conventional chemotherapeutic agents
because of the low proliferation rate (39). Here, we found that metastatic cells
showed significantly lower expression of PCNA (cell proliferation
marker) and a higher percentage of G0/G1 phase cells in the cell
cycle. For this reason, metastatic cells are thought to have a
slower proliferation rate, which may be one of CSC-like properties
(40).
The crucial role of the pluripotency-related
transcription factors (e.g., OCT4 and NANOG) in somatic cell
reprogramming has been extensively studied (41). Nevertheless, the association of
these factors with EMT in cancer cells is poorly understood.
Sporadic reports link a gene signature of pluripotency to EMT; for
example, most of these studies suggest that expression of
pluripotency-related genes is associated with the mesenchymal
phenotype and cancer invasiveness (42). In addition, some of these studies
show that higher activity of ALDH (a cytosolic enzyme that is
responsible for oxidation of intracellular aldehydes) can be used
to improve identification of colon CSC population with the
EpCAMhighCD44+ phenotype as well as
glioblastoma, retinoblastoma, and breast CSC populations (43,44).
In light of this study, it seems reasonable to hypothesize that
expression of ALDH1A1, OCT4, and NANOG (or the more general marker:
ALDH activity) may help to characterize CSCs during metastasis.
EMT was first recognized to be crucial for
embryogenesis and is assumed to be required for invasiveness and
metastasis of carcinoma cells because EMT promotes the loss of
contact inhibition and increases cell motility. Therefore, the EMT
pathway may be sufficient for acquisition of both invasiveness and
stem cell properties by cancer cells; these changes will allow for
establishment of a tumor at a distant site. We found that in
addition to morphological changes, reduced E-cadherin expression
and increased vimentin expression were associated with acquisition
of EMT properties. In addition, coexpression of vimentin and CD133
was observed in metastatic cells, along with decreased PCNA
expression, increased ALDH activity and upregulation of ALDHA1,
OCT4, NANOG: these are CSC-like properties. These data substantiate
the role of EMT and support the importance of CSCs during
metastasis; therefore, our results could facilitate the development
of a novel classification system and therapeutic strategies against
oral cancer.
Acknowledgements
This study was supported by a grant (no. A121228)
from the Korean Health Technology R&D Project, Ministry of
Health & Welfare, Republic of Korea and the National Research
Foundation of Korea grant funded by the Korean government (no.
2011-0030759).
References
|
1
|
Zhu LF, Hu Y, Yang CC, Xu XH, Ning TY,
Wang ZL, Ye JH and Liu LK: Snail overexpression induces an
epithelial to mesenchymal transition and cancer stem cell-like
properties in SCC9 cells. Lab Invest. 92:744–752. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun L, Diamond ME, Ottaviano AJ, Joseph
MJ, Ananthanarayan V and Munshi HG: Transforming growth factor-beta
1 promotes matrix metalloproteinase-9-mediated oral cancer invasion
through snail expression. Mol Cancer Res. 6:10–20. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Silverman S Jr: Demographics and
occurrence of oral and pharyngeal cancers. The outcomes, the
trends, the challenge. J Am Dent Assoc. 132(Suppl): S7–S11. 2001.
View Article : Google Scholar
|
|
4
|
Ramos DM, Dang D and Sadler S: The role of
the integrin alpha v beta6 in regulating the epithelial to
mesenchymal transition in oral cancer. Anticancer Res. 29:125–130.
2009.PubMed/NCBI
|
|
5
|
Crnic I and Christofori G: Novel
technologies and recent advances in metastasis research. Int J Dev
Biol. 48:573–581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Molloy T and van ’t Veer LJ: Recent
advances in metastasis research. Curr Opin Genet Dev. 18:35–41.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang CC, Zhu LF, Xu XH, Ning TY, Ye JH and
Liu LK: Membrane Type 1 matrix metalloproteinase induces an
epithelial to mesenchymal transition and cancer stem cell-like
properties in SCC9 cells. BMC Cancer. 13:1712013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Medema JP: Cancer stem cells: The
challenges ahead. Nat Cell Biol. 15:338–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Charafe-Jauffret E, Ginestier C, Iovino F,
Tarpin C, Diebel M, Esterni B, Houvenaeghel G, Extra JM, Bertucci
F, Jacquemier J, et al: Aldehyde dehydrogenase 1-positive cancer
stem cells mediate metastasis and poor clinical outcome in
inflammatory breast cancer. Clin Cancer Res. 16:45–55. 2010.
View Article : Google Scholar
|
|
11
|
Hermann PC, Huber SL, Herrler T, Aicher A,
Ellwart JW, Guba M, Bruns CJ and Heeschen C: Distinct populations
of cancer stem cells determine tumor growth and metastatic activity
in human pancreatic cancer. Cell Stem Cell. 1:313–323. 2007.
View Article : Google Scholar
|
|
12
|
Fan YL, Zheng M, Tang YL and Liang XH: A
new perspective of vasculogenic mimicry: EMT and cancer stem cells
(Review). Oncol Lett. 6:1174–1180. 2013.PubMed/NCBI
|
|
13
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Elsaba TM, Martinez-Pomares L, Robins AR,
Crook S, Seth R, Jackson D, McCart A, Silver AR, Tomlinson IP and
Ilyas M: The stem cell marker CD133 associates with enhanced colony
formation and cell motility in colorectal cancer. PLoS One.
5:e107142010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bapat SA, Mali AM, Koppikar CB and Kurrey
NK: Stem and progenitor-like cells contribute to the aggressive
behavior of human epithelial ovarian cancer. Cancer Res.
65:3025–3029. 2005.PubMed/NCBI
|
|
16
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, Geschwind DH, Bronner-Fraser M and Kornblum HI:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:15178–15183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
18
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Monroe MM, Anderson EC, Clayburgh DR and
Wong MH: Cancer stem cells in head and neck squamous cell
carcinoma. J Oncol. 2011:7627802011. View Article : Google Scholar
|
|
22
|
Davis SJ, Divi V, Owen JH, Bradford CR,
Carey TE, Papagerakis S and Prince ME: Metastatic potential of
cancer stem cells in head and neck squamous cell carcinoma. Arch
Otolaryngol Head Neck Surg. 136:1260–1266. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang CC, Hsu WH, Wang CC, Chou CH, Kuo
MY, Lin BR, Chen ST, Tai SK, Kuo ML and Yang MH: Connective tissue
growth factor activates pluripotency genes and
mesenchymal-epithelial transition in head and neck cancer cells.
Cancer Res. 73:4147–4157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nguyen PT, Kudo Y, Yoshida M, Iizuka S,
Ogawa I and Takata T: N-cadherin expression is correlated with
metastasis of spindle cell carcinoma of head and neck region. J
Oral Pathol Med. 40:77–82. 2011. View Article : Google Scholar
|
|
25
|
Mizrak D, Brittan M and Alison M: CD133:
Molecule of the moment. J Pathol. 214:3–9. 2008. View Article : Google Scholar
|
|
26
|
Neuzil J, Stantic M, Zobalova R, Chladova
J, Wang X, Prochazka L, Dong L, Andera L and Ralph SJ:
Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: What’s
in the name? Biochem Biophys Res Commun. 355:855–859. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olempska M, Eisenach PA, Ammerpohl O,
Ungefroren H, Fandrich F and Kalthoff H: Detection of tumor stem
cell markers in pancreatic carcinoma cell lines. Hepatobiliary
Pancreat Dis Int. 6:92–97. 2007.PubMed/NCBI
|
|
28
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110. 2007.
View Article : Google Scholar
|
|
29
|
Lim W, Kim J, Kim S, Karna S, Won J, Jeon
SM, Kim SY, Choi Y, Choi H and Kim O: Modulation of
lipopolysaccharide-induced NF-κB signaling pathway by 635 nm
irradiation via heat shock protein 27 in human gingival fibroblast
cells. Photochem Photobiol. 89:199–207. 2013. View Article : Google Scholar
|
|
30
|
Carvalho AL, Nishimoto IN, Califano JA and
Kowalski LP: Trends in incidence and prognosis for head and neck
cancer in the United States: A site-specific analysis of the SEER
database. Int J Cancer. 114:806–816. 2005. View Article : Google Scholar
|
|
31
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Al Saleh S, Sharaf LH and Luqmani YA:
Signalling pathways involved in endocrine resistance in breast
cancer and associations with epithelial to mesenchymal transition
(Review). Int J Oncol. 38:1197–1217. 2011.PubMed/NCBI
|
|
34
|
Pang LY, Bergkvist GT, Cervantes-Arias A,
Yool DA, Muirhead R and Argyle DJ: Identification of tumour
initiating cells in feline head and neck squamous cell carcinoma
and evidence for gefitinib induced epithelial to mesenchymal
transition. Vet J. 193:46–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou BB, Zhang H, Damelin M, Geles KG,
Grindley JC and Dirks PB: Tumour-initiating cells: Challenges and
opportunities for anticancer drug discovery. Nat Rev Drug Discov.
8:806–823. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Uchida N, Buck DW, He D, Reitsma MJ, Masek
M, Phan TV, Tsukamoto AS, Gage FH and Weissman IL: Direct isolation
of human central nervous system stem cells. Proc Natl Acad Sci USA.
97:14720–14725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iannolo G, Conticello C, Memeo L and De
Maria R: Apoptosis in normal and cancer stem cells. Crit Rev Oncol
Hematol. 66:42–51. 2008. View Article : Google Scholar
|
|
38
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang X, Yao Y, Zhu J, Jin K, Wang Y, Mao Y
and Zhou L: Differential proliferative index of cancer stem-like
cells in primary and recurrent medulloblastoma in human. Childs
Nerv Syst. 28:1869–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Anjomshoaa A, Nasri S, Humar B, McCall JL,
Chatterjee A, Yoon HS, McNoe L, Black MA and Reeve AE: Slow
proliferation as a biological feature of colorectal cancer
metastasis. Br J Cancer. 101:822–828. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bourguignon LY, Wong G, Earle C and Chen
L: Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes
miR-302 expression leading to self-renewal, clonal formation, and
cisplatin resistance in cancer stem cells from head and neck
squamous cell carcinoma. J Biol Chem. 287:32800–32824. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gangopadhyay S, Nandy A, Hor P and
Mukhopadhyay A: Breast cancer stem cells: A novel therapeutic
target. Clin Breast Cancer. 13:7–15. 2013. View Article : Google Scholar
|
|
43
|
Dalerba P, Dylla SJ, Park IK, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bar EE, Chaudhry A, Lin A, Fan X, Schreck
K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, et al:
Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like
cancer cells in glioblastoma. Stem Cells. 25:2524–2533. 2007.
View Article : Google Scholar : PubMed/NCBI
|