Radiotherapy is an effective strategy for the
treatment of many kinds of cancer to kill or control the malignant
tumor cells. However, its benefits have been limited due to
radiation resistance. It is imperative to identify new targets and
develop cytotoxic drugs to increase radiosensitivity.
Radiosensitization has traditionally been performed with agents
known to induce apoptosis. However, recent studies demonstrate
autophagy induced by radiation also plays an important role in
cancer cell fate decision, particularly in solid tumors (1). Autophagy is an evolutionarily
conserved catabolic process that delivers cellular constituents,
including damaged or superfluous organelles and long-lived proteins
to lysosomes for degradation and recycling, thereby regulating
cellular homeostasis. The key steps of autophagy include
initiation, phagophore nucleation and elongation, fusion and
degradation. Autophagy initiation begins with the membrane
distention from either the ER or Golgi complex, followed by
formation of autophagosome, a cup shaped double-membrane structure
which sequesters cellular components. Subsequently, autophagosome
fuses with the lysosome to form an autolysosome which is the site
for degradation of the sequestered cargo by the action of the
lysosomal hydrolytic enzymes (2).
More than 40 autophagy-related proteins have been
identified by large-scale genetic screening in yeast and various
mammalian species (3). The core
pathway of mammalian autophagy comprises at least four molecular
components, including: i) ULK complex (comprising ULK1, ATG13,
ATG101 and FIP200), and plays a key role in the induction of
autophagy. Autophagy inducers cause dephosphorylation of ULK1; the
ULK1 complex then dissociates from the mTORC1 complex, which leads
to increasing activity of the BECN1 complex through phosphorylation
of AMBRA1 and BECN1 (Beclin 1, autophagy related); ii) core
PtdIns3K complex (comprising PIK3C3, PIK3R4/Vps15 and BECN1); BECN1
is a component of core PtdIns3K complex, which participates in
phagophore nucleation and elongation. Phosphorylation BECN1
recruits ATG14, AMBRA1 or UVRAG to the PtdIns3K complexes to
promote autophagy (4); iii) ATG9
and VMP1, two transmembrane proteins, are critical in leading to
autophagosome formation. The biogenesis of the phagophore is
thought to initiate at the phagophore assembly site (PAS). Most of
the ATG proteins transiently reside at the PAS, although the
ultrastructure of this site and the interactions among the ATG
proteins during phagophore formation are not known (5). ULK1 complex assembles at the PAS
where autophagy is initiated and recruits ATG9-vesicles in order to
initiate the formation of autophagosomes (6). VMP1 induced by autophagy stimulus
interacts with the BECN1 BH3 domain partitioning BECN1 to the
autophagic pathway. This interaction promotes the recruitment of
the autophagy-specific PtdIns3K complex to the PAS and activates
the PtdIns3K complex, which generates the PtdIns3p. PtdIns3p is
necessary to recruit the rest of the ATG machinery, leading to
autophagosome formation (7); iv)
ATG12 and ATG 8/LC3, are two ubiquitin-like protein conjugation
systems essential for autophagosome formation. Ubiquitin-like
molecule ATG12 is activated and transferred to the E2-like
conjugating enzyme ATG10 by E1-like enzyme ATG7, ultimately
attached to ATG5 4, 6, 7. In addition, ATG7 and the E2-like enzyme
ATG3 promote the LC3 (ATG8 in yeast) is conjugated to the lipid
phosphatidylethanolamine (8).
Elongation of the phagophore membrane is dependent on the ATG12 and
LC3 conjugation systems (9). Most
of these core autophagy pathway components are directly controlled
by cellular stress signals (10,11).
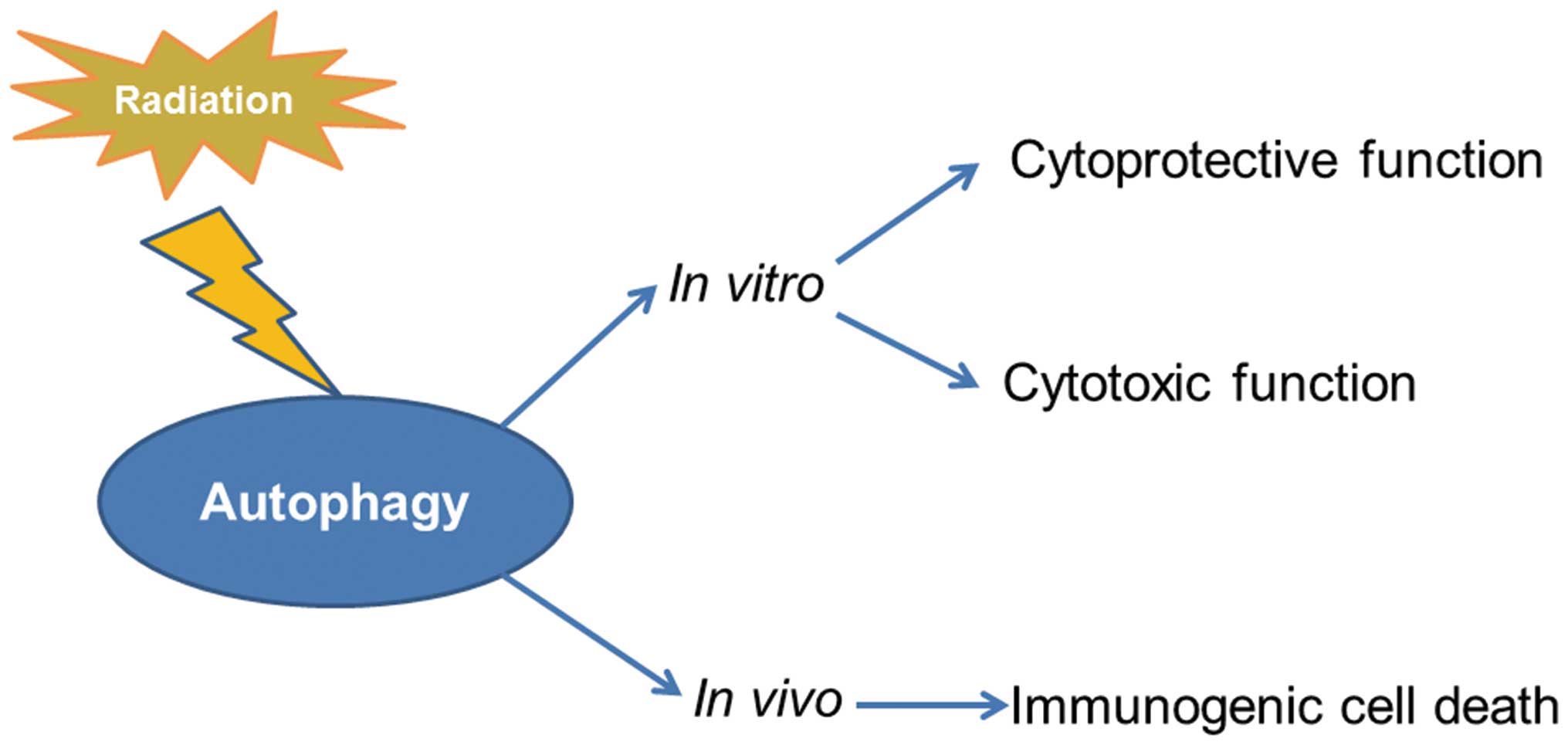
Autophagy induced by radiation play bi-directional
effects in cell fate decision whether cells survive or die depends
on severity and duration of this phenomenon (12). When the stress is mild, autophagy
can degrade and recycle damaged or unwanted cellular constituents
in autophagolysosomal vesicles to provide additional energy supply
during stress, which has an essential effect in quality control of
organelles and cellular adaptation to stress (13). However, several reports demonstrate
that various drugs in combination with radiation promote autophagic
cell death rather than apoptosis significantly contributing to the
antitumor effects of radiosensitizing treatment or radiotherapy in
glioma (14). Hence, autophagy
induced by radiation also functions as a pro-death mechanism.
Besides dual activity of autophagy on tumor cell fate in
vitro, recently in vivo studies demonstrate that
irradiation-induced autophagy exerts a crucial activity on tumor
clearance by the immune system. Autophagy induced by radiation
contributes to the release of cell death-associated danger signals
ATP and HMGB1 that trigger antitumor host immune responses.
Furthermore, autophagy inhibition reduces radioresponses in
vivo due to deficient immunogenic signaling (15,16).
The initiation signal of autophagy induced by
radiation have not been completely elucidated, although biochemical
analysis performed during the last few years has identified several
proteins in DNA damage repair signaling pathway or oxidative stress
signaling pathway participated in modulation of autophagy.
Clarifying crosstalk between autophagy and intracellular radiation
response is beneficial to provide novel targets for exploiting
novel radiosensitizers to enhance the effects of anticancer
therapies for cancer patient.
A large amount of recent studies have shown that
tumor resistance to radiation therapy is often associated with the
upregulation of autophagy in many kinds of tumor cell lines, such
as colon cancer cells, prostate cancer cells, malignant glioma
cells, nasopharyngeal carcinoma cells, and breast cancer cells
(17,18). The putative cytoprotective function
of autophagy induced by radiation is generally considered to
reflect the capacity of the cell to eliminate toxic species such as
free radicals and damaged and unwanted proteins or organelles to
generate energy and metabolic precursors (19). Due to the cytoprotective function
of autophagy, autophagy inhibitors (such as chloroquine,
bafilomycin, 3-methyl adenine, or ammonium chloride) and genetic
silencing or knockdown of autophagy-associated genes (such as
BECN1, ATG 5, 7 or 12) have the potential to be exploited to
increase tumor cell radiosensitivity, usually via the promotion of
apoptosis (15).
Moreover, some studies reported that autophagy
enhanced the anticancer effects of radiotherapy on patients with
oral squamous cell carcinoma and glioblastoma cells (20,21).
Vitamin D and its analogs such as EB 1089 could enhance the
response to radiation in breast cancer and non-small cell lung
cancer through the promotion of a cytotoxic form of autophagy
(22,23). Hence, autophagy is also recognized
as having the potential to contribute to cell killing in response
to radiation. This kind of cell death was defined as autophagic
cell death or type II apoptosis (24). Autophagic cell death can serve as a
part of backup cell death form when stress-induced apoptosis is
blocked with broad spectrum caspase inhibitor, z-VAD-Fmk, autophagy
can provide an alternative cell death mechanism (25). Moreover, autophagy is important to
the radiation-induced senescence. Inhibition of autophagy results
in a switch from radiation-induced senescence to apoptosis in
breast cancer cells (26).
Radiation injury to DNA is caused directly or
indirectly and is known to be repaired immediately (32). ATM, a primary sensor of DNA damage
after exposure to radiation, plays a crucial role in initiating
autophagy (33). In response to
genotoxic stress, DNA damage is detected by MNR (comprising MRE11,
NBS1 and Rad50 proteins) and RPA (human replication protein A)
complexes which act as sensors and recruit ATM and ATR
(ataxia-telangiectasia and RAD3 related) to the site of the lesion,
these kinases are activated by DNA lesions and direct the DDR
pathways through phosphorylation of downstream targets (34). The activation of ATM/ATR triggers
phosphorylation of its downstream targets such as p53, mTOR and
FOXO3a, which could trigger radiation-induced autophagy (35).
p53, a critical component of the cellular reaction
to radiation, integrates DNA damage signals and
radiation-responsive autophagy (36). In unstressed cells, p53 is
maintained at low levels by the action of MDM2, an oncogenic E3
ligase, which is essential for both degradation and nuclear export
of p53. Following irradiation p53 tends to be rapidly stabilized by
reversible post-translational modifications (37). The function of p53 in response to
radiation is ATM-dependent. Activation of ATM results from the DNA
DSBs, which in turn phosphorylates p53 at Ser15 and Ser20. Whereas,
p53 and MDM2 are phosphorylated by ATM directly at Ser395. Both
phosphorylated p53 at Ser15 and Ser20 and phosphorylated MDM2
interrupt the binding of MDM2 with p53, and protect p53 from
ubiquitination and degradation. At the same time p53 levels are
increased, and majority of p53 translocates to the nucleus. As an
important transfection factor, p53 promotes autophagy by
transactivating its target genes that involved the diversity of
radiation responsive pathways in mammalian cells (38). One mechanism for p53-induced
autophagy is through activation of energy sensor AMPK pathway and
inhibition of the mTOR pathway. Another mechanism of p53-induced
autophagy is mediated by transactivating multiple genes with
proautophagic roles, including DRAM, PTEN (an inhibitor of the
PI3K/AKT signaling pathway), IGF-BP3, DAPK-1 ARF, JNK, Sestrin1/2,
IGF-BP3 and PUMA (39–41). Recently, Cui et al, verified
radiation induced autophagic cell death via the p53/DRAM signaling
pathway in breast cancer cells (42).
mTOR, an atypical serine/threonine kinase, can
modify downstream molecules and inhibits autophagy. As an
evolutionarily conserved kinase, mTOR functions largely as the
catalytic subunit of two distinct protein kinase complexes the
mTORC1 (comprising mTOR, mLST8 and Raptor) which is a rapamycin
sensitive complex (mTORC1) and the mTORC2 (comprising mTOR, mLST8
and Rictor), which is a rapamycin insensitive complex. Raptor and
Rictor serve as scaffolding proteins to regulate the assembly,
localization and substrate binding of mTORC1 and mTORC2,
respectively (43). Previous
studies have reported that altered mTORC1 signaling pathways were
mostly found in many human cancers, whereas mTORC2 was less
correlated (44). Once mTORC1 is
activated in DNA damage repair signaling pathway, mTORC1-mediated
phosphorylation prevents activation of ULK1 and its interacting
partner ATG13 as well as AMBRA1, the key link between the ULK1 and
BECN1 complex. In addition, the activation of mTORC1 prevents
AMPK-mediated phosphorylation which is required for full activation
of ULK1, which is a serine/threonine kinase that plays a key role
in initiator of autophagosome formation in mammalian systems
(45,46). The blockade of the mTORC1 can
activate the ULK1 complex and promote the interaction with the
PtdIns3K complex which is necessary for the formation of new
autophagosomes (47). Several
studies have reported on the positive effects of mTOR inhibitor on
sensitizing cancers to radiation therapy and chemotherapy in lung
cancer, pancreatic carcinoma cells and esophageal carcinoma cells
(48–51).
Under normal conditions, FOXO3a is attached to DNA.
The carboxyl-terminal domain (amino acids 616–623) of FOXO3a binds
to the FAT domain of ATM, (amino acids 1960–2566), which has been
postulated as a protein-binding domain, activation has been shown
to depend on its autophosphorylation at Ser 1981 contained in the
FAT domain (52). In response to
DNA damage induced by radiation the transcriptional activity and
protein expression level of FOXO3a increase. Furthermore, radiation
can induce the nuclear translocation of FOXO3a in Saos2 cells
(53). FOXO3a localization within
the nuclear phospho-ATM (Ser1981) foci in irradiated cells is
affected by its posttranslational modification (phospho-Thr32)
(54). Nuclear transcription
factor FOXO3a detaches from DNA to interact with ATM and activated
ATM via phosphorylation, which can trigger autophagy as describe
above. Additionally, FOXO3a regulates transcription of
autophagy-related genes, including LC3 or BNIP3 (55).
PARP-1 is another protein of DDR involved in the
regulation of autophagy. PARP-1 is a member of a family of 18 such
proteins which are known to bind to DNA and function in DNA damage
repair. Irradiation is known to induce DNA damage and activate
PARP-1. The important role of PARP-1 in radiation response and the
efficacy of PARP-1 inhibitors as radiosensitizers have been
investigated for more than 30 years (56). Following DNA binding, The PARP-1
enzymatic activity is triggered caused by depletion of ATP and
activation of AMPK. The PARP-1 activation can not only lead to the
inhibition of mTOR which induces autophagy but also promotes
HMGB1-mediated autophagy (57,58).
Chen, et al reported that radiation activates PARP-1 which
regulates autophagy via the activation of AMPK or inhibition mTOR
in CNE-2 human nasopha ryngeal carcinoma cells (59,60).
SIRT1, a member of the mammalian sirtuin family,
plays an important role in autophagy. The mechanism of SIRT1 in
regulating this process is due to its ability to deacetylate
histones and non-histone proteins, such as p53, FOXO3a and H4K16
(lysine 16 on histone H4) in response to stresses and DNA damage
(61). SIRT1 interacts with p53
and affects the transcriptional activity of p53 which can be,
directly or indirectly, involved in DDR. SIRT1 regulates autophagy
by both epigenetic and posttranslational mechanisms (62). SIRT1 regulates autophagy gene H4K16
(lysine 16 on histone H4) expression through histone deacetylation.
H4K16 deacetylation inhibits the transcription of genes involved in
the early and late steps of autophagy in multiple cell types,
resulting in decreased autophagic flux. Moreover, SIRT1 indirectly
regulates autophagy by deacetylation of FOXO3a, leading to
increased expression of autophagy-related genes, including BNIP3,
which are critical for autophagy induction.
Growing evidence has suggested that mitochondria may
also be an important extranuclear target in mediating the cytotoxic
effects of radiation (63,64). Mitochondria are essential
organelles which perform multiple functions. During the process of
tricarboxylic acid cycle and oxidative phosphorylation,
mitochondria utilize oxygen to generate ATP from organic fuel
molecules using the electrochemical gradient generated across the
inner of two membranes by the electron transport chain, but in the
process also produce ROS (65).
Radiation promote the accumulation of ROS, which acts
straightforwardly by direct modifications of biological molecules
like proteins, lipids and nucleic acids and leads to mitochondrial
dysfunction post-irradiation and causes an energy imbalance.
Once the AMP/ATP ratio increases, AMPK, a genuine
sensor of the energetic state of the cell, was activated. AMPK
which directly responds to the so-called adenylate energy charge as
the enzyme is activated by very low increases of AMP levels (and,
to certain extent, of ADP), and deactivated by ATP, and could be
activated by upstream kinases. Phospho-active AMPK activates
autophagy to restore the correct adenylate energy charge. At the
molecular level, active AMPK stimulates autophagy by means of at
least four distinct mechanisms. These include: i) phosphorylation
of the mTORC1 inhibitor, TSC2 at Ser1387, which induces RHEB GTPase
activity; RHEB-GDP strongly inhibits the activity of mTOR and
activate autophagy, and does so both in vitro and in
vivo (66); ii)
phosphorylation of ULK1 at Ser317 and Ser777. ULK1 is then free to
interact with and to be phosphorylated by AMPK (67); iii) AMPK regulates autophagy by
phosphorylating PIK3C3 and BECN1 at Thr388, S90 and S93; iv) AMPK
regulates autophagy not only by promoting formation of the
PIK3C3-BECN1 complex, but also by inhibiting the interaction of
BECN1 and BCL2 to release more available BECN1 to form PtdIns3K
complexes (68).
As previously indicated ER is another target of
radiation, radiation initially induces ROS production, which would
activate ER membrane sensors of ER stress in IEC-6 cells and breast
cancer MDA-MB-231 and MCF-7 cells (76,77).
ER stress induced by radiation triggers signal transduction
pathways, known as unfolded protein response (UPR) also through the
induction of DNA damage (78,79).
ER stress and UPR related genes are required for stress induced
autophagy. The last few years have evidenced that the ER stress and
autophagy processes are closely related as some of the signaling
routes activated during the ER stress response are involved in
stimulating autophagy.
Although the main signaling pathway of ER stress
that is activated following irradiation is still a debatable one;
some recent evidence suggests that PERK-eIF2a and/or IRE1a may
serve as the main executing pathways of ER stress in irradiation
scenarios (80–82). A link between autophagy and the ER
stress has been further substantiated by the PERK-eIF2α pathway
which is essential for autophagy induction after ER stress. PERK
phosphorylates the eukaryotic initiation factor 2α (eIF2α) on
residue serine 51, which then initiates a cascade of events that
decreases the overload of misfolded proteins, thereby alleviating
ER stress (83). eIF2α
phosphorylation stimulates the selective translation of the ATF4
transcription factor (although general translation is shut off) and
CHOP (a transcription factor induced by ATF4), which were shown to
transcriptionally regulate more than a dozen ATG genes. These genes
are necessary for sustained autophagy (84). PERK activated by oxidative stress
can also directly inhibit mTOR or indirectly inhibits mTOR via
activation of PARP1, which can induce autophagy (85). In addition, IRE1 was activated in
response to a variety of cellular stressors after exposure to
radiation. In mammalian cells, IRE1-JNK pathway was required for
autophagy activation after ER stress (86,87).
Activation of IRE1 and JNK causes Bcl-2 phosphorylation,
dissociation of BECN1, activation of the PI3K complex and induces
autophagy. Moreover, dysregulated autophagy may also trigger the
IRE1 activity with concomitant activation of UPR, thereby dampening
excessive autophagy triggered via the PERK/eIF2α pathway and
pointing to a plausible feedback mechanism in the control of UPR
signaling (88).
ROS is a family of highly reactive molecules which
includes free oxygen radicals, like superoxide anion
(O2•−), hydroxyl radical (OH•),
and non-radical oxygen derivatives, like the stable hydrogen
peroxide (H2O2). ROS are unstable molecules
which have the capacity to readily convert to many different viable
and active forms. The superoxide radicals react to form other ROS,
namely, hydrogen peroxides and hydroxyl radicals, and interconvert
with RNS, which generate effects similar to ROS (89). Radiation exposure has been
intimately linked to increased reactive ROS production and
persistent oxidative stress in cells. Accumulating data implicate
accumulation of ROS from radiolysis of water molecules, damage
mitochondria and ER regulation of autophagy in oxidative stress
response (66,90). Although multiple studies reported
that accumulation of ROS regulated autophagy by pathways such as:
i) oxidization of ATG4 leading to accumulation of autophagosomes;
ii) activation of the AMPK signaling cascade inducing the
initiation of autophagy through the ULK1 complex; iii) disruption
of BECN1-BCL-2 interaction leading to the initiation of autophagy;
or iv) alteration of mitochondria homeostasis leading to mitophagy
activation (75). Furthermore,
reactive oxygen/nitrogen species (ROS/RNS) generated in the context
of radiation exposure are essential activators of cytoplasmic
signaling cascades such as p38 MAPK, JNK, HIF-1α, which play
essential roles in the regulation of autophagy (91).
p38 MAPK and JNK are two of the major MAPK
subfamilies, which are serine/threonine kinases that mediate
responses to various extracellular stimuli. Once activated in
response to various stresses, p38 MAPK and JNK lead to
phosphorylation-dependent activation of other kinases and
transcription factors and play a crucial role in the modulation of
autophagy (92). It has been
previously reported that disruption of ROS clearance activates the
p38 MAPK pathway which acts as a key regulator in ROS-mediated
autophagy (93). The activation of
p38 MAPK was in part responsible for the downregulation of
phosphorylated mTOR and the subsequent activation of autophagy
(94). p38 MAPK depletion is also
found to regulate the trafficking of ATG9, a multi-spanning
membrane protein that is essential for autophagy (95). Fractionated radiation activated p38
MAPK which is involved in radiation-induced stabilization of HIF-1α
which play an important role in switching autophagy. Consistently,
inhibition of p38 MAPK also effectively attenuated
radiation-induced stabilization of HIF-1α (96).
After exposure to radiation, the expression of
HIF-1α increased, then HIF-1α translocated into the nucleus. In the
nucleus, HIF-1α bound to the hypoxia response element in its target
promoter and promoted the transcription of its target (97). HIF-1α is a subunit of HIF1 which
consists of a regulatory HIF-1α subunit and the constitutively
expressed HIF-1β subunit, both are members of the basic
helix-loop-helix and PER-ARNT-SIM families of transcription factors
(98). Pervious opinion considered
that while HIF-1β is constitutively expressed, HIF-1α is an oxygen
sensitive subunit and its expression is induced under hypoxic
conditions (99). Accumulating
evidence has recently revealed that HIF-1α is activated in cancer
cells not only under hypoxic conditions, but also in the presence
of oxygen when the following conditions are satisfied. In addition
to post-translational mechanisms, regulation at the level of
transcription and translation initiation is also important for the
activation of HIF-1α under normoxic conditions (100). Koshikawa et al reported
that ROS generated in mitochondria upregulated the transcription of
the HIF-1α gene via the PI3K-Akt/PKC/ HDAC pathway, leading to the
accumulation and activation of HIF-1α in tumor cells (101). ROS upregulates HIF-1α
transcription by activating non-hypoxic factors in a
redox-sensitive manner (102).
HIF-1α induces autophagy by a mechanism involving the upregulated
expression of its target genes BNIP3 and its homologue NIX and the
dissociation of the BECN1-BCL2 complex, leading to release of
BECN1, which is capable of triggering autophagy (103,104).
Radiation activates the cell surface glycohydrolases
which play a crucial role in the production of ceramide at the
plasma membrane. Ceramide is localized within lipid rafts in the
plasma membrane, which are specialized membrane microdomains that
regulate various signaling pathways (105). Ceramide is also a central
molecule of sphingolipid metabolism and involved in the regulation
of autophagy at various levels (106,107). Ceramide downregulates the
expression of amino acid and nutrient transporters in the context
of starvation, a state that induces survival autophagy by reducing
mTOR signaling pathway or activating AMPK (108,109). Further evidence indicated that
ceramide increases BECN1 expression by activating JNK kinase, which
in turn activates c-Jun, a known transcription factor for BECN1
expression (110). Ceramide
accumulation also can induce ER stress, mTOR inhibition via TRB3
(tribbles homologue 3), which triggered lethal autophagy (111–113). Moreover, ceramide can be
generated by neutral sphingomyelinase in mitochondria, which is a
late event upon the cell irradiation following the early phase of
ceramide accumulation at the cell plasma membrane (106). Ceramide accumulation in the
mitochondria can lead to ceramide stress-induced mitochondrial
fragmentation, and decrease in ATP production (114). These important mitochondrial
parameter changes can induce mitophagy (115).
Cellular response to radiation-induced stress is
generally mediated through the production of the second messenger,
in-flux of Ca2+ (90,116,117). These radiation-induced changes in
Ca2+ concentration can be elevations, oscillations or
single transient changes within minutes to days after exposure to
radiation. Radiation regulates intracellular Ca2+
concentration through several different pathways. Firstly,
radiation can induce the accumulation of ceramide, which can
regulate influx of extracellular Ca2+ at plasma membrane
channel level (106). Secondly,
radiation can activate PLC, the main enzyme localized at the plasma
membrane and producing IP3 and consequently inducing
Ca2+ release from ER (90,118). Several studies in mammalian cells
and yeast models have demonstrated a potential role for
mitochondrial parameters regulated by Ca2+ signals in
modulating and/or triggering mitophagy (115,119). Ca2+ release events
from the ER, the major intracellular Ca2+ -storage
organelle that have an immediate effect on the physiological
function of mitochondria and lysosomes (120). Ca2+ released from the
ER activates members of the PKC family. In mammals, PKCθ, a member
of the PKC family, is a novel factor that mediates
Ca2+-dependent induction of autophagy in response to ER
stress. Increased Ca2+ concentrations induce PKCθ
phosporylation and its localization to cytosolic LC3 puncta
(121). In addition, the
CAMKK2-PRKAA-mTOR pathway is an important signaling pathway for
Ca2+ -induced autophagy, which is also a cascade with
established roles in multiple cellular processes including ribosome
biogenesis and transcription in addition to cell motility and
metabolism (119). In addition
elevation of intracellular Ca2+ can activate AMPK, which
plays a role in Ca2+ -mediated signal transduction
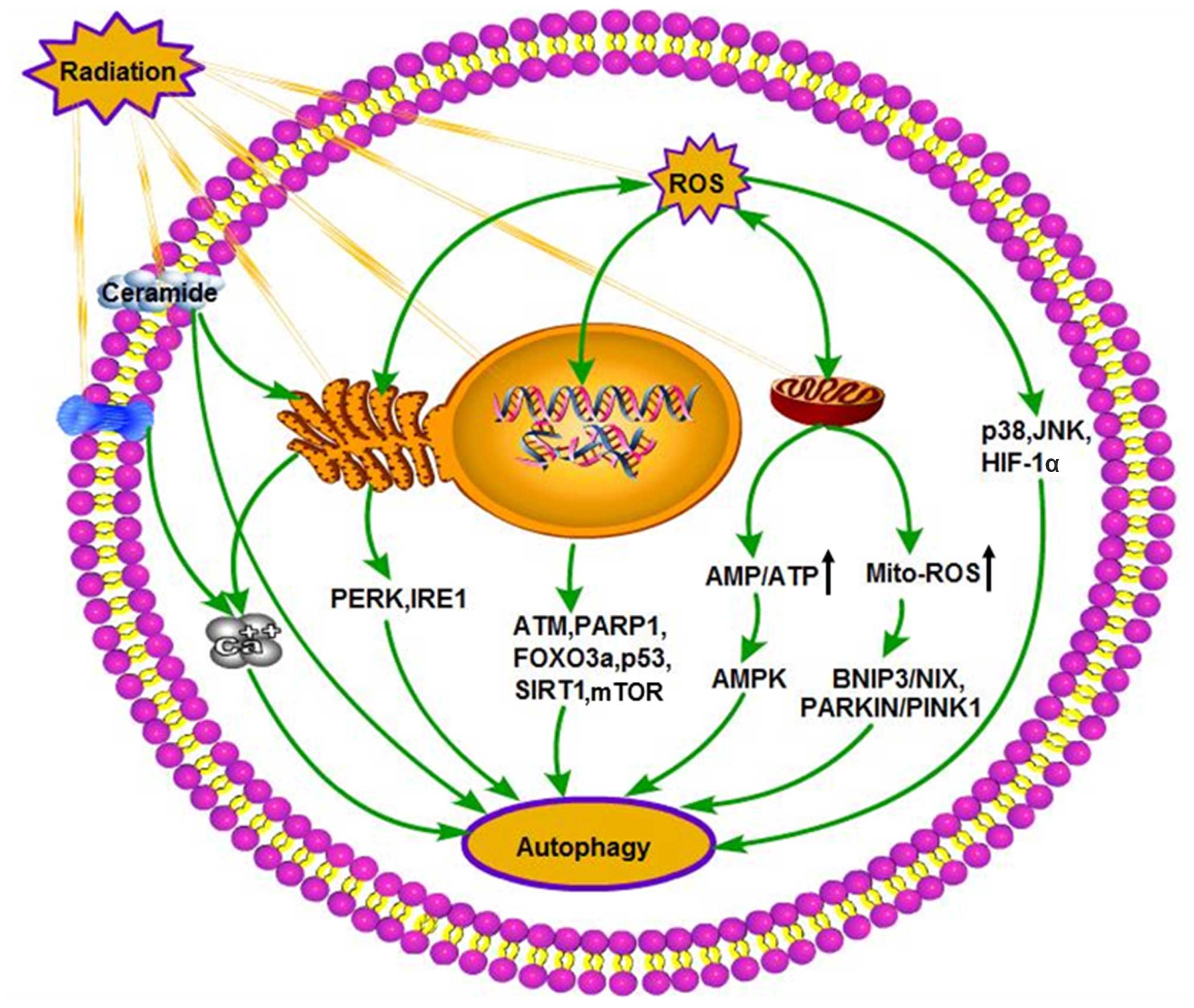
pathways (122,123). An overview of the major
intracellular autophagic triggers induced by radiation is shown in
Fig. 2.
Various preclinical models revealed that autophagy
is activated in irradiated tumor cells and that ATG expression
patterns are upregulated following irradiation. Inhibition of
autophagy genes such as ATG5 alone or a mixture of BECN1, ATG3,
ATG4b, and ATG5 together leads to the strongest, and significant
sensitizing effect found in radiation (124). Recent in vitro and in
vivo studies in preclinical models suggested that modulation of
autophagy can be used as a therapeutic modality to enhance the
efficacy of radiation therapy. Currently, multiple clinical trials
have been initiated combined with autophagy inhibitors, such as
hydroxy chloroquine and chloroquine, which associated with
increased sensitivity to radiation (125,126). However, the role of autophagy in
the resistance of cancer cells to radiation therapy remains
controversial. One logical outcome of this argument is that
chloroquine and hydroxychloroquine are unlikely to be appropriate
drugs for this purpose, because of the fact that patients with
malaria are able to endure treatment with these drugs for years,
which suggests that the doses of chloroquine and hydroxychloroquine
that are used effectively for malaria treatment may not actually be
acting to inhibit autophagy. Another logical outcome is that
various studies indicated that the sensitivity of tumor cells to
radiation could be enhanced by co-treatment with rapamycin, an
autophagy promoter, in various kinds of tumor cells (44,50,127).
|
1
|
Jaboin JJ, Shinohara ET, Moretti L, Yang
ES, Kaminski JM and Lu B: The role of mTOR inhibition in augmenting
radiation induced autophagy. Technol Cancer Res Treat. 6:443–447.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Periyasamy P, Guo ML and Buch S: Cocaine
induces astrocytosis through ER stress-mediated activation of
autophagy. Autophagy. 12:1310–1329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Codogno P: Shining light on autophagy. Nat
Rev Mol Cell Biol. 15:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JM, Jung CH, Seo M, Otto NM, Grunwald
D, Kim KH, Moriarity B, Kim YM, Starker C, Nho RS, et al: The ULK1
complex mediates MTORC1 signaling to the autophagy initiation
machinery via binding and phosphorylating ATG14. Autophagy.
12:547–564. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao Z, Delorme-Axford E, Backues SK and
Klionsky DJ: Atg41/Icy2 regulates autophagosome formation.
Autophagy. 11:2288–2299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng Y, Backues SK, Baba M, Heo JM, Harper
JW and Klionsky DJ: Phosphorylation of Atg9 regulates movement to
the phagophore assembly site and the rate of autophagosome
formation. Autophagy. 12:648–658. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molejon MI, Ropolo A and Vaccaro MI: VMP1
is a new player in the regulation of the autophagy-specific
phosphatidylinositol 3-kinase complex activation. Autophagy.
9:933–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murrow L, Malhotra R and Debnath J:
ATG12-ATG3 interacts with Alix to promote basal autophagic flux and
late endosome function. Nat Cell Biol. 17:300–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perluigi M, Di Domenico F and Butterfield
DA: mTOR signaling in aging and neurodegeneration: At the crossroad
between metabolism dysfunction and impairment of autophagy.
Neurobiol Dis. 84:39–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Z and Klionsky DJ: Mammalian
autophagy: Core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar :
|
|
11
|
Kroemer G, Mariño G and Levine B:
Autophagy and the integrated stress response. Mol Cell. 40:280–293.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalby KN, Tekedereli I, Lopez-Berestein G
and Ozpolat B: Targeting the prodeath and prosurvival functions of
autophagy as novel therapeutic strategies in cancer. Autophagy.
6:322–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito H, Daido S, Kanzawa T, Kondo S and
Kondo Y: Radiation-induced autophagy is associated with LC3 and its
inhibition sensitizes malignant glioma cells. Int J Oncol.
26:1401–1410. 2005.PubMed/NCBI
|
|
14
|
Li X, Cen Y, Cai Y, Liu T, Liu H, Cao G,
Liu D, Li B, Peng W, Zou J, et al: TLR9-ERK-mTOR signaling is
critical for autophagic cell death induced by CpG
oligodeoxynucleotide 107 combined with irradiation in glioma cells.
Sci Rep. 6:271042016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ko A, Kanehisa A, Martins I, Senovilla L,
Chargari C, Dugue D, Mariño G, Kepp O, Michaud M, Perfettini JL, et
al: Autophagy inhibition radiosensitizes in vitro, yet reduces
radioresponses in vivo due to deficient immunogenic signalling.
Cell Death Differ. 21:92–99. 2014. View Article : Google Scholar
|
|
16
|
Kepp O, Senovilla L, Vitale I, Vacchelli
E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N,
et al: Consensus guidelines for the detection of immunogenic cell
death. OncoImmunology. 3:e9556912014. View Article : Google Scholar
|
|
17
|
Mo N, Lu YK, Xie WM, Liu Y, Zhou WX, Wang
HX, Nong L, Jia YX, Tan AH, Chen Y, et al: Inhibition of autophagy
enhances the radiosensitivity of nasopharyngeal carcinoma by
reducing Rad51 expression. Oncol Rep. 32:1905–1912. 2014.PubMed/NCBI
|
|
18
|
Sun Q, Liu T, Yuan Y, Guo Z, Xie G, Du S,
Lin X, Xu Z, Liu M, Wang W, et al: MiR-200c inhibits autophagy and
enhances radiosensitivity in breast cancer cells by targeting
UBQLN1. Int J Cancer. 136:1003–1012. 2015. View Article : Google Scholar
|
|
19
|
Yang Y, Yang Y, Yang X, Zhu H, Guo Q, Chen
X, Zhang H, Cheng H and Sun X: Autophagy and its function in
radiosensitivity. Tumour Biol. 36:4079–4087. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu SY, Liu YW, Wang YK, Lin TH, Li YZ,
Chen SH and Lee YR: Ionizing radiation induces autophagy in human
oral squamous cell carcinoma. J BUON. 19:137–144. 2014.PubMed/NCBI
|
|
21
|
Saglar E, Unlu S, Babalioglu I, Gokce SC
and Mergen H: Assessment of ER stress and autophagy induced by
ionizing radiation in both radiotherapy patients and ex vivo
irradiated samples. J Biochem Mol Toxicol. 28:413–417. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bristol ML, Di X, Beckman MJ, Wilson EN,
Henderson SC, Maiti A, Fan Z and Gewirtz DA: Dual functions of
autophagy in the response of breast tumor cells to radiation:
Cytoprotective autophagy with radiation alone and cytotoxic
autophagy in radio-sensitization by vitamin D 3. Autophagy.
8:739–753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma K, Goehe RW, Di X, Hicks MA II,
Torti SV, Torti FM, Harada H and Gewirtz DA: A novel cytostatic
form of autophagy in sensitization of non-small cell lung cancer
cells to radiation by vitamin D and the vitamin D analog, EB 1089.
Autophagy. 10:2346–2361. 2014. View Article : Google Scholar
|
|
24
|
Gewirtz DA, Hilliker ML and Wilson EN:
Promotion of autophagy as a mechanism for radiation sensitization
of breast tumor cells. Radiother Oncol. 92:323–328. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma K, Le N, Alotaibi M and Gewirtz DA:
Cytotoxic autophagy in cancer therapy. Int J Mol Sci.
15:10034–10051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang YH, Yang PM, Chuah QY, Lee YJ, Hsieh
YF, Peng CW and Chiu SJ: Autophagy promotes radiation-induced
senescence but inhibits bystander effects in human breast cancer
cells. Autophagy. 10:1212–1228. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Golden EB, Pellicciotta I, Demaria S,
Barcellos-Hoff MH and Formenti SC: The convergence of radiation and
immunogenic cell death signaling pathways. Front Oncol. 2:882012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saitoh T and Akira S: Regulation of innate
immune responses by autophagy-related proteins. J Cell Biol.
189:925–935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Y, Martins I, Ma Y, Kepp O, Galluzzi
L and Kroemer G: Autophagy-dependent ATP release from dying cells
via lysosomal exocytosis. Autophagy. 9:1624–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Michaud M, Martins I, Sukkurwala AQ,
Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot
G, et al: Autophagy-dependent anticancer immune responses induced
by chemotherapeutic agents in mice. Science. 334:1573–1577. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ratikan JA, Sayre JW and Schaue D:
Chloroquine engages the immune system to eradicate irradiated
breast tumors in mice. Int J Radiat Oncol Biol Phys. 87:761–768.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Havaki S, Kotsinas A, Chronopoulos E,
Kletsas D, Georgakilas A and Gorgoulis VG: The role of oxidative
DNA damage in radiation induced bystander effect. Cancer Lett.
356:43–51. 2015. View Article : Google Scholar
|
|
33
|
Sengupta S and Harris CC: p53: traffic cop
at the crossroads of DNA repair and recombination. Nat Rev Mol Cell
Biol. 6:44–55. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith J, Tho LM, Xu N and Gillespie DA:
The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and
cancer. Adv Cancer Res. 108:73–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang N, Jia L, Liu Y, Liang B, Kong D,
Yan M, Ma S and Liu X: ATM pathway is essential for ionizing
radiation-induced autophagy. Cell Signal. 25:2530–2539. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jen KY and Cheung VG: Identification of
novel p53 target genes in ionizing radiation response. Cancer Res.
65:7666–7673. 2005.PubMed/NCBI
|
|
37
|
Li M, Brooks CL, Wu-Baer F, Chen D, Baer R
and Gu W: Mono-versus polyubiquitination: Differential control of
p53 fate by Mdm2. Science. 302:1972–1975. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fei P and El-Deiry WS: P53 and radiation
responses. Oncogene. 22:5774–5783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang J, Di J, Cao H, Bai J and Zheng J:
p53-mediated autophagic regulation: A prospective strategy for
cancer therapy. Cancer Lett. 363:101–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zheng R, Yao Q, Du S, Ren C, Sun Q, Xu Z,
Lin X and Yuan Y: The status of p53 in cancer cells affects the
role of autophagy in tumor radiosensitisation. J BUON. 19:336–341.
2014.PubMed/NCBI
|
|
41
|
Feng Z, Zhang H, Levine AJ and Jin S: The
coordinate regulation of the p53 and mTOR pathways in cells. Proc
Natl Acad Sci USA. 102:8204–8209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cui L, Song Z, Liang B, Jia L, Ma S and
Liu X: Radiation induces autophagic cell death via the p53/DRAM
signaling pathway in breast cancer cells. Oncol Rep. 35:3639–3647.
2016.PubMed/NCBI
|
|
43
|
Xu K, Liu P and Wei W: mTOR signaling in
tumorigenesis. Biochim Biophys Acta. 1846:638–654. 2014.PubMed/NCBI
|
|
44
|
Zheng H, Wang M, Wu J, Wang ZM, Nan HJ and
Sun H: Inhibition of mTOR enhances radiosensitivity of lung cancer
cells and protects normal lung cells against radiation. Biochem
Cell Biol. 94:213–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Egan DF, Shackelford DB, Mihaylova MM,
Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor
R, et al: Phosphorylation of ULK1 (hATG1) by AMP-activated protein
kinase connects energy sensing to mitophagy. Science. 331:456–461.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim J, Kundu M, Viollet B and Guan KL:
AMPK and mTOR regulate autophagy through direct phosphorylation of
Ulk1. Nat Cell Biol. 13:132–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hönscheid P, Datta K and Muders MH:
Autophagy: Detection, regulation and its role in cancer and therapy
response. Int J Radiat Biol. 90:628–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nagata Y, Takahashi A, Ohnishi K, Ota I,
Ohnishi T, Tojo T and Taniguchi S: Effect of rapamycin, an mTOR
inhibitor, on radiation sensitivity of lung cancer cells having
different p53 gene status. Int J Oncol. 37:1001–1010. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dai ZJ, Gao J, Kang HF, Ma YG, Ma XB, Lu
WF, Lin S, Ma HB, Wang XJ and Wu WY: Targeted inhibition of
mammalian target of rapamycin (mTOR) enhances radiosensitivity in
pancreatic carcinoma cells. Drug Des Devel Ther. 7:149–159. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang D, Xiang J, Gu Y, Xu W, Xu H, Zu M,
Pei D and Zheng J: Inhibition of mammalian target of rapamycin by
rapamycin increases the radiosensitivity of esophageal carcinoma
Eca109 cells. Oncol Lett. 8:575–581. 2014.PubMed/NCBI
|
|
51
|
Ushijima H, Suzuki Y, Oike T, Komachi M,
Yoshimoto Y, Ando K, Okonogi N, Sato H, Noda SE, Saito J, et al:
Radiosensitization effect of an mTOR inhibitor, temsirolimus, on
lung adenocarcinoma A549 cells under normoxic and hypoxic
conditions. J Radiat Res (Tokyo). 56:663–668. 2015. View Article : Google Scholar
|
|
52
|
Tsai WB, Chung YM, Takahashi Y, Xu Z and
Hu MC: Functional interaction between FOXO3a and ATM regulates DNA
damage response. Nat Cell Biol. 10:460–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yang JY, Xia W and Hu MC: Ionizing
radiation activates expression of FOXO3a, Fas ligand, and Bim, and
induces cell apoptosis. Int J Oncol. 29:643–648. 2006.PubMed/NCBI
|
|
54
|
Tarrade S, Bhardwaj T, Flegal M, Bertrand
L, Velegzhaninov I, Moskalev A and Klokov D: Histone H2AX is
involved in FoxO3a-mediated transcriptional responses to ionizing
radiation to maintain genome stability. Int J Mol Sci.
16:29996–30014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tran H, Brunet A, Grenier JM, Datta SR,
Fornace AJ Jr, DiStefano PS, Chiang LW and Greenberg ME: DNA repair
pathway stimulated by the forkhead transcription factor FOXO3a
through the Gadd45 protein. Science. 296:530–534. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cho EA, Kim EJ, Kwak SJ and Juhnn YS: cAMP
signaling inhibits radiation-induced ATM phosphorylation leading to
the augmentation of apoptosis in human lung cancer cells. Mol
Cancer. 13:362014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rodríguez-Vargas JM, Ruiz-Magaña MJ,
Ruiz-Ruiz C, Majuelos-Melguizo J, Peralta-Leal A, Rodríguez MI,
Muñoz-Gámez JA, de Almodóvar MR, Siles E, Rivas AL, et al:
ROS-induced DNA damage and PARP-1 are required for optimal
induction of starvation-induced autophagy. Cell Res. 22:1181–1198.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang M, Liu L, Xie M, Sun X, Yu Y, Kang R,
Yang L, Zhu S, Cao L and Tang D: Poly-ADP-ribosylation of HMGB1
regulates TNFSF10/TRAIL resistance through autophagy. Autophagy.
11:214–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bridges KA, Toniatti C, Buser CA, Liu H,
Buchholz TA and Meyn RE: Niraparib (MK-4827), a novel
poly(ADP-Ribose) polymerase inhibitor, radiosensitizes human lung
and breast cancer cells. Oncotarget. 5:5076–5086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Chen ZT, Zhao W, Qu S, Li L, Lu XD, Su F,
Liang ZG, Guo SY and Zhu XD: PARP-1 promotes autophagy via the
AMPK/mTOR pathway in CNE-2 human nasopharyngeal carcinoma cells
following ionizing radiation, while inhibition of autophagy
contributes to the radiation sensitization of CNE-2 cells. Mol Med
Rep. 12:1868–1876. 2015.PubMed/NCBI
|
|
61
|
Xie Y, Zhang J, Ye S, He M, Ren R, Yuan D
and Shao C: SirT1 regulates radiosensitivity of hepatoma cells
differently under normoxic and hypoxic conditions. Cancer Sci.
103:1238–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lapierre LR, Kumsta C, Sandri M, Ballabio
A and Hansen M: Transcriptional and epigenetic regulation of
autophagy in aging. Autophagy. 11:867–880. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang B, Davidson MM, Zhou H, Wang C,
Walker WF and Hei TK: Cytoplasmic irradiation results in
mitochondrial dysfunction and DRP1-dependent mitochondrial fission.
Cancer Res. 73:6700–6710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kam WW and Banati RB: Effects of ionizing
radiation on mitochondria. Free Radic Biol Med. 65:607–619. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shadel GS and Horvath TL: Mitochondrial
ROS signaling in organismal homeostasis. Cell. 163:560–569. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin WJ and Kuang HY: Oxidative stress
induces autophagy in response to multiple noxious stimuli in
retinal ganglion cells. Autophagy. 10:1692–1701. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Filomeni G, De Zio D and Cecconi F:
Oxidative stress and autophagy: The clash between damage and
metabolic needs. Cell Death Differ. 22:377–388. 2015. View Article : Google Scholar :
|
|
68
|
Zhang D, Wang W, Sun X, Xu D, Wang C,
Zhang Q, Wang H, Luo W, Chen Y, Chen H, et al: AMPK regulates
autophagy by phosphorylating BECN1 at Threonine 388. Autophagy.
12:1447–1459. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shimura T, Kobayashi J, Komatsu K and
Kunugita N: Severe mitochondrial damage associated with low-dose
radiation sensitivity in ATM- and NBS1-deficient cells. Cell Cycle.
15:1099–1107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shimura T, Sasatani M, Kamiya K, Kawai H,
Inaba Y and Kunugita N: Mitochondrial reactive oxygen species
perturb AKT/cyclin D1 cell cycle signaling via oxidative
inactivation of PP2A in lowdose irradiated human fibroblasts.
Oncotarget. 7:3559–3570. 2016.
|
|
71
|
Garg AD, Dudek AM, Ferreira GB, Verfaillie
T, Vandenabeele P, Krysko DV, Mathieu C and Agostinis P:
ROS-induced autophagy in cancer cells assists in evasion from
determinants of immunogenic cell death. Autophagy. 9:1292–1307.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Clerkin JS, Naughton R, Quiney C and
Cotter TG: Mechanisms of ROS modulated cell survival during
carcinogenesis. Cancer Lett. 266:30–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Datta K, Suman S and Fornace AJ Jr:
Radiation persistently promoted oxidative stress, activated mTOR
via PI3K/Akt, and downregulated autophagy pathway in mouse
intestine. Int J Biochem Cell Biol. 57:167–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Narendra DP, Jin SM, Tanaka A, Suen DF,
Gautier CA, Shen J, Cookson MR and Youle RJ: PINK1 is selectively
stabilized on impaired mitochondria to activate Parkin. PLoS Biol.
8:e10002982010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Poillet-Perez L, Despouy G,
Delage-Mourroux R and Boyer-Guittaut M: Interplay between ROS and
autophagy in cancer cells, from tumor initiation to cancer therapy.
Redox Biol. 4:184–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang B, Wang Y, Pang X, Su Y, Ai G and
Wang T: ER stress induced by ionising radiation in IEC-6 cells. Int
J Radiat Biol. 86:429–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li F, Zheng X, Liu Y, Li P, Liu X, Ye F,
Zhao T, Wu Q, Jin X and Li Q: Different roles of CHOP and JNK in
mediating radiation-induced autophagy and apoptosis in breast
cancer cells. Radiat Res. 185:539–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chiu HW, Fang WH, Chen YL, Wu MD, Yuan GF,
Ho SY and Wang YJ: Monascuspiloin enhances the radiation
sensitivity of human prostate cancer cells by stimulating
endoplasmic reticulum stress and inducing autophagy. PLoS One.
7:e404622012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chiu HW, Yeh YL, Wang YC, Huang WJ, Ho SY,
Lin P and Wang YJ: Combination of the novel histone deacetylase
inhibitor YCW1 and radiation induces autophagic cell death through
the downregulation of BNIP3 in triple-negative breast cancer cells
in vitro and in an orthotopic mouse model. Mol Cancer. 15:462016.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yang Z, Xu Y, Xu L, Maccauro G, Rossi B,
Chen Y, Li H, Zhang J, Sun H, Yang Y, et al: Regulation of
autophagy via PERK-eIF2α effectively relieve the radiation myelitis
induced by iodine-125. PLoS One. 8:e768192013. View Article : Google Scholar
|
|
81
|
Kim KW, Moretti L, Mitchell LR, Jung DK
and Lu B: Endoplasmic reticulum stress mediates radiation-induced
autophagy by perk-eIF2alpha in caspase-3/7-deficient cells.
Oncogene. 29:3241–3251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kim EJ, Lee YJ, Kang S and Lim YB:
Ionizing radiation activates PERK/eIF2α/ATF4 signaling via ER
stress-independent pathway in human vascular endothelial cells. Int
J Radiat Biol. 90:306–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Harding HP, Zhang Y, Zeng H, Novoa I, Lu
PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al: An
integrated stress response regulates amino acid metabolism and
resistance to oxidative stress. Mol Cell. 11:619–633. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Milani M, Rzymski T, Mellor HR, Pike L,
Bottini A, Generali D and Harris AL: The role of ATF4 stabilization
and autophagy in resistance of breast cancer cells treated with
Bortezomib. Cancer Res. 69:4415–4423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Huang Q, Wu YT, Tan HL, Ong CN and Shen
HM: A novel function of poly(ADP-ribose) polymerase-1 in modulation
of autophagy and necrosis under oxidative stress. Cell Death
Differ. 16:264–277. 2009. View Article : Google Scholar
|
|
86
|
Kyriakis JM, Banerjee P, Nikolakaki E, Dai
T, Rubie EA, Ahmad MF, Avruch J and Woodgett JR: The
stress-activated protein kinase subfamily of c-Jun kinases. Nature.
369:156–160. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Urano F, Wang X, Bertolotti A, Zhang Y,
Chung P, Harding HP and Ron D: Coupling of stress in the ER to
activation of JNK protein kinases by transmembrane protein kinase
IRE1. Science. 287:664–666. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Senft D and Ronai ZA: UPR, autophagy, and
mitochondria crosstalk underlies the ER stress response. Trends
Biochem Sci. 40:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Davalli P, Mitic T, Caporali A, Lauriola A
and D’Arca D: ROS, cell senescence, and novel molecular mechanisms
in aging and age-related diseases. Oxid Med Cell Longev.
2016:35651272016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Corre I, Niaudet C and Paris F: Plasma
membrane signaling induced by ionizing radiation. Mutat Res.
704:61–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu Y, Cui Y, Shi M, Zhang Q, Wang Q and
Chen X: Deferoxamine promotes MDA-MB-231 cell migration and
invasion through increased ROS-dependent HIF-1α accumulation. Cell
Physiol Biochem. 33:1036–1046. 2014. View Article : Google Scholar
|
|
92
|
Sridharan S, Jain K and Basu A: Regulation
of autophagy by kinases. Cancers (Basel). 3:2630–2654. 2011.
View Article : Google Scholar
|
|
93
|
Bode JG, Ehlting C and Häussinger D: The
macrophage response towards LPS and its control through the
p38(MAPK)-STAT3 axis. Cell Signal. 24:1185–1194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Tang G, Yue Z, Talloczy Z, Hagemann T, Cho
W, Messing A, Sulzer DL and Goldman JE: Autophagy induced by
Alexander disease-mutant GFAP accumulation is regulated by p38/MAPK
and mTOR signaling pathways. Hum Mol Genet. 17:1540–1555. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lien SC, Chang SF, Lee PL, Wei SY, Chang
MD, Chang JY and Chiu JJ: Mechanical regulation of cancer cell
apoptosis and autophagy: Roles of bone morphogenetic protein
receptor, Smad1/5, and p38 MAPK. Biochim Biophys Acta.
1833:3124–3133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kim YH, Yoo KC, Cui YH, Uddin N, Lim EJ,
Kim MJ, Nam SY, Kim IG, Suh Y and Lee SJ: Radiation promotes
malignant progression of glioma cells through HIF-1alpha
stabilization. Cancer Lett. 354:132–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gu Q, He Y, Ji J, Yao Y, Shen W, Luo J,
Zhu W, Cao H, Geng Y, Xu J, et al: Hypoxia-inducible factor 1α
(HIF-1α) and reactive oxygen species (ROS) mediates
radiation-induced invasiveness through the SDF-1α/CXCR4 pathway in
non-small cell lung carcinoma cells. Oncotarget. 6:10893–10907.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li P, Shi J, He Q, Hu Q, Wang YY, Zhang
LJ, Chan WT and Chen WX: Streptococcus pneumoniae induces autophagy
through the inhibition of the PI3K-I/Akt/mTOR pathway and ROS
hypergeneration in A549 cells. PLoS One. 10:e01227532015.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxiainducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar
|
|
100
|
Harada H: Hypoxia-inducible factor
1-mediated characteristic features of cancer cells for tumor
radioresistance. J Radiat Res (Tokyo). 57(Suppl 1): i99–i105. 2016.
View Article : Google Scholar
|
|
101
|
Koshikawa N, Hayashi J, Nakagawara A and
Takenaga K: Reactive oxygen species-generating mitochondrial DNA
mutation up-regulates hypoxia-inducible factor-1alpha gene
transcription via phosphatidylinositol 3-kinase-Akt/protein kinase
C/histone deacetylase pathway. J Biol Chem. 284:33185–33194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bonello S, Zähringer C, BelAiba RS,
Djordjevic T, Hess J, Michiels C, Kietzmann T and Görlach A:
Reactive oxygen species activate the HIF-1alpha promoter via a
functional NFkappaB site. Arterioscler Thromb Vasc Biol.
27:755–761. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Noman MZ, Janji B, Berchem G, Mami-Chouaib
F and Chouaib S: Hypoxia-induced autophagy: A new player in cancer
immunotherapy? Autophagy. 8:704–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bellot G, Garcia-Medina R, Gounon P,
Chiche J, Roux D, Pouysségur J and Mazure NM: Hypoxia-induced
autophagy is mediated through hypoxia-inducible factor induction of
BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol.
29:2570–2581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tirodkar TS and Voelkel-Johnson C:
Sphingolipids in apoptosis. Exp Oncol. 34:231–242. 2012.PubMed/NCBI
|
|
106
|
Aureli M, Murdica V, Loberto N, Samarani
M, Prinetti A, Bassi R and Sonnino S: Exploring the link between
ceramide and ionizing radiation. Glycoconj J. 31:449–459. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Young MM, Kester M and Wang HG:
Sphingolipids: Regulators of crosstalk between apoptosis and
autophagy. J Lipid Res. 54:5–19. 2013. View Article : Google Scholar :
|
|
108
|
Edinger AL: Starvation in the midst of
plenty: Making sense of ceramide-induced autophagy by analysing
nutrient transporter expression. Biochem Soc Trans. 37:253–258.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Peralta ER and Edinger AL:
Ceramide-induced starvation triggers homeostatic autophagy.
Autophagy. 5:407–409. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Li DD, Wang LL, Deng R, Tang J, Shen Y,
Guo JF, Wang Y, Xia LP, Feng GK, Liu QQ, et al: The pivotal role of
c-Jun NH2-terminal kinase-mediated Beclin 1 expression during
anticancer agents-induced autophagy in cancer cells. Oncogene.
28:886–898. 2009. View Article : Google Scholar
|
|
111
|
Jiang W and Ogretmen B: Autophagy paradox
and ceramide. Biochim Biophys Acta. 1841:783–792. 2014. View Article : Google Scholar :
|
|
112
|
Dany M and Ogretmen B: Ceramide induced
mitophagy and tumor suppression. Biochim Biophys Acta.
1853B:2834–2845. 2015. View Article : Google Scholar
|
|
113
|
Salazar M, Carracedo A, Salanueva IJ,
Hernández-Tiedra S, Lorente M, Egia A, Vázquez P, Blázquez C,
Torres S, García S, et al: Cannabinoid action induces
autophagy-mediated cell death through stimulation of ER stress in
human glioma cells. J Clin Invest. 119:1359–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Sentelle RD, Senkal CE, Jiang W, Ponnusamy
S, Gencer S, Selvam SP, Ramshesh VK, Peterson YK, Lemasters JJ,
Szulc ZM, et al: Ceramide targets autophagosomes to mitochondria
and induces lethal mitophagy. Nat Chem Biol. 8:831–838. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rimessi A, Bonora M, Marchi S, Patergnani
S, Marobbio CM, Lasorsa FM and Pinton P: Perturbed mitochondrial
Ca2+ signals as causes or consequences of mitophagy
induction. Autophagy. 9:1677–1686. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Voehringer DW, Story MD, O’Neil RG and
Meyn RE: Modulating Ca2+ in radiation-induced apoptosis
suppresses DNA fragmentation but does not enhance clonogenic
survival. Int J Radiat Biol. 71:237–243. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Teshima K, Yamamoto A, Yamaoka K, Honda Y,
Honda S, Sasaki T and Kojima S: Involvement of calcium ion in
elevation of mRNA for gamma-glutamylcysteine synthetase (gamma-GCS)
induced by low-dose gamma-rays. Int J Radiat Biol. 76:1631–1639.
2000. View Article : Google Scholar
|
|
118
|
Todd DG, Mikkelsen RB, Rorrer WK, Valerie
K and Schmidt-Ullrich RK: Ionizing radiation stimulates existing
signal transduction pathways involving the activation of epidermal
growth factor receptor and ERBB-3, and changes of intracellular
calcium in A431 human squamous carcinoma cells. J Recept Signal
Transduct Res. 19:885–908. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
East DA and Campanella M: Ca2+
in quality control: An unresolved riddle critical to autophagy and
mitophagy. Autophagy. 9:1710–1719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
La Rovere RM, Roest G, Bultynck G and
Parys JB: Intracellular Ca(2+) signaling and
Ca(2+) microdomains in the control of cell survival,
apoptosis and autophagy. Cell Calcium. 60:74–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sakaki K, Wu J and Kaufman RJ: Protein
kinase Ctheta is required for autophagy in response to stress in
the endoplasmic reticulum. J Biol Chem. 283:15370–15380. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Woods A, Dickerson K, Heath R, Hong SP,
Momcilovic M, Johnstone SR, Carlson M and Carling D:
Ca2+ /calmodulin-dependent protein kinase kinase-beta
acts upstream of AMP-activated protein kinase in mammalian cells.
Cell Metab. 2:21–33. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Zhang J, Chiu J, Zhang H, Qi T, Tang Q, Ma
K, Lu H and Li G: Autophagic cell death induced by resveratrol
depends on the Ca(2+)/AMPK/mTOR pathway in A549 cells.
Biochem Pharmacol. 86:317–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Rosenfeld MR, Ye X, Supko JG, Desideri S,
Grossman SA, Brem S, Mikkelson T, Wang D, Chang YC, Hu J, et al: A
phase I/II trial of hydroxychloroquine in conjunction with
radiation therapy and concurrent and adjuvant temozolomide in
patients with newly diagnosed glioblastoma multiforme. Autophagy.
10:1359–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Rojas-Puentes LL, Gonzalez-Pinedo M,
Crismatt A, Ortega-Gomez A, Gamboa-Vignolle C, Nuñez-Gomez R,
Dorantes-Gallareta Y, Arce-Salinas C and Arrieta O: Phase II
randomized, double-blind, placebo-controlled study of wholebrain
irradiation with concomitant chloroquine for brain metastases.
Radiat Oncol. 8:2092013. View Article : Google Scholar
|
|
127
|
Chen YH, Wei MF, Wang CW, Lee HW, Pan SL,
Gao M, Kuo SH, Cheng AL and Teng CM: Dual phosphoinositide
3-kinase/mammalian target of rapamycin inhibitor is an effective
radiosensitizer for colorectal cancer. Cancer Lett. 357:582–590.
2015. View Article : Google Scholar
|
|
128
|
Ozpolat B and Benbrook DM: Targeting
autophagy in cancer management - strategies and developments.
Cancer Manag Res. 7:291–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Liang DH, El-Zein R and Dave B: Autophagy
inhibition to increase radiosensitization in breast cancer. J Nucl
Med Radiat Ther. 6:62015. View Article : Google Scholar
|