Introduction
During cancer progression, tumor cells actively
interact with the tumor microenvironment, including extracellular
matrices (ECMs), cytokines, growth factors, and neighboring cells,
such as endothelial cells, macrophages, fibroblasts, and
neutrophils, through which they acquire the capacity for migration,
invasion, metastasis, and angiogenesis (1). Metastatic spread of tumors after
surgical excision increases the risk of mortality in cancer
patients and remains a major obstacle for the complete treatment of
malignant tumors. During metastasis, tumor cells undergo a
multistep process known as the metastatic cascade, which includes
disconnection from ECMs, degradation of the surrounding tissues,
entrance into the circulation, migration and arrival at secondary
sites, extravasation, and establishment of secondary metastatic
foci (2,3). Several regulatory factors are
overexpressed in tumors as well as the surrounding stromal cells
and promote the metastatic process. Among them, matrix
metalloproteinases (MMPs), zinc-dependent endopeptidases, are
regarded as the most important effectors, and they collectively
degrade diverse substrates in the extracellular milieu (4–6).
Based on their specificity for substrates, MMPs can be classified
as gelatinases, collagenases, stromelysins, and membrane-type MMPs.
Elevated expression and activity of MMPs in primary tumors and/or
plasma are positively correlated with rapid progression and high
incidence of metastasis in diverse types of cancer as well as
shorter survival time. In addition to their proteolytic activities,
MMPs contribute to tumor cell proliferation by modulating the
bioavailability of growth factors and to the formation of
tumor-associated vasculature, which promotes tumor dissemination,
by degrading the vascular basement membrane and remodeling ECMs
(7,8). Therefore, MMPs are considered
valuable diagnostic markers as well as potential therapeutic
targets for managing malignant tumors.
Angiogenesis plays pivotal roles in the development
of malignant tumors at multiple levels and is controlled by the
balance of endogenous stimulators and inhibitors that are secreted
by tumor cells and host cells, including endothelial cells,
fibroblasts, stromal cells, and immune cells, in response to tumor
cells (9,10). Initially, tumors are avascular
masses that depend on the pre-existing vasculature in their
microenvironment. However, when tumor cells grow beyond the extent
of passive diffusion for oxygen and nutrient, they become hypoxic.
Hypoxia causes an imbalance between the production of pro- and
anti-angiogenic factors, which is known as the angiogenic switch,
via overexpression and stabilization of hypoxia-inducible factor
(HIF)-1α, leading to enhancement of blood vessel formation
(11–13). Newly formed blood vessels within
hypoxic tumors act as a path to supply sufficient oxygen and
nutrients, eliminate waste products, and migrate and infiltrate to
the circulation, thus permitting rapid growth and metastasis.
Therefore, targeting tumor-induced angiogenesis has been an
important strategy for cancer therapy.
Gardenia jasminoides Ellis (GJE, family
Rubiaceae) has long been used in Asian countries as a traditional
herbal medicine for the treatment of inflammation, hepatic
diseases, hypertension, edema, jaundice, and headache (14,15).
The ripe fruit of GJE, Gardeniae Fructus (GF, Chinese name,
Zhi Zi), has been used in traditional medicine and has various
pharmacological effects, such as anti-oxidant, anti-inflammatory,
anti-thrombotic, anti-hyperlipidemic, anti-viral, anti-convulsive,
anti-hyperuricemic, neuroprotective, and anxiolytic activities
(16–20). In addition, stir-baked GF extract
was reported to inhibit MMP-16 activity and alter the cellular
morphology of HT1080 human fibrosarcoma cells with no cytotoxicity
(21). However, studies on the
anti-metastatic and anti-angiogenic effects of baked GF and its
underlying mechanisms have not been performed.
In this study, we aimed to determine whether ethanol
extract of baked GF (EBGF) can control malignant tumor cells by
inhibition of metastasis and angiogenesis using in vitro
assays, ex vivo rat aortic ring assays, and an in
vivo pulmonary metastasis model. Furthermore, we elucidated the
underlying anti-metastatic and anti-angiogenic activities of EBGF
in detail.
Materials and methods
Preparation of EBGF
Baked G. Fructus was purchased from
Yeongcheon Oriental Herbal Market (Yeongcheon, Korea) and stored in
the herbal bank of the Korean Medicine (KM) Application Center. To
prepare EBGF, 30 g of baked G. Fructus was crushed to a
powder, soaked in 300 ml of 70% ethanol, and then extracted by
shaking at 100 rpm for 24 h at 40°C. After filtering the extract
using a testing sieve (150 μm, Retsch Haan, Germany), the sample
was lyophilized and then stored in a desiccator at −20°C. The total
collected EBGF was 9.11 g, and the yield was 30.36%.
Cells
HT1080 cells (human fibrosarcoma) and B16F10 cells
(murine melanoma) were obtained from the Korean Cell Line Bank
(KCLB, Seoul, Korea) and maintained in RPMI-1640 or DMEM
supplemented with 10% FBS (Biotechnics Research Inc., Lake Forest,
CA, USA) and 100 U/ml penicillin/100 μg/ml streptomycin (Cellgro,
Manassas, VA, USA) in 5% CO2 at 37°C. Human umbilical
vein endothelial cells (HUVECs) obtained from Innopharmascreen
(Asan, Korea) were maintained in endothelial cell growth medium-2
(EGM-2; Promocell, Heidelberg, Germany) and used at passage
3–8.
Cell viability and colony formation
assays
After cells (5×103/well/96-well plates)
were treated with or without the specified concentrations of EBGF
for 48 h, cell viability was assessed using the Cell Counting Kit-8
(CCK-8; Dojindo Laboratories, Kumamoto, Japan). To evaluate colony
formation, cells (5×102/well/12-well plates) were
incubated in the presence or absence of the indicated
concentrations of EBGF. After 7 days, colonies were stained with
0.2% crystal violet/20% methanol (w/v) solution for 30 min, and the
visible colonies were counted.
Determination of MMP-9, MMP-13, and uPA
mRNA levels
Total RNA from each sample was isolated using an RNA
extraction solution (BioAssay Co., Daejeon, Korea) and reverse
transcribed to cDNA using a 1st Strand cDNA synthesis kit (BioAssay
Co.) according to the manufacturer’s protocol. cDNA aliquots were
amplified by polymerase chain reaction (PCR) using the following
primers: hMMP-9, 5′-TCTTCCCTGGAGACTGAGAA-3′ and
5′-GGCAAGTCTTCCGAGTAGTTT-3′, hMMP-13, 5′-GGCAAACTTGACGATAACACC-3′
and 5′-GCCCATCAAATGGGTAGAAGT-3′, huPA, 5′-AGGGCAGCACTGTGAAATAGA-3′
and 5′-TCTTGGACAAGCAGCTTTAGG-3′, β-actin,
5′-ATGAAGATCCTGACCGAGCGT-3′ and 5′-AACGCAGCTCAGTAACAGTCCG-3′.
Preparation of conditioned medium
(CM)
Cells were treated with the indicated concentrations
of EBGF in complete media for 24 h, washed twice with 0.5% FBS
media, and then incubated for 24 h in 0.5% FBS media. Culture media
were harvested and centrifuged at 12,000 rpm for 15 min at 4°C to
remove cell debris, and the supernatants were collected as the
CM.
MMP and uPA activity assays
MMP and uPA activity in the CM of HT1080 cells was
determined using MMP Activity assay kit (Fluorometric-Green, cat.
no. ab112146, Abcam, Cambridge, MA, USA) and uPA Activity assay kit
(Colorimetric, cat. no. ECM600, Chemicon, Billerica, MA, USA),
respectively, according to the manufacturer’s protocol. In brief,
for MMP activity assays, 25 μl of CM was incubated with an equal
volume of 2 mM APMA (4-aminophenylmercuric acid) working solution
for 15 min and was then mixed with 50 μl of the broad spectrum MMP
fluorogenic peptide substrate solution in 96-well black plates
(SPL, Kyounggi-do, Korea). After 1, 2 and 3 h, green fluorescence
intensity was measured in a SpectraMaxi3 microplate reader
(Molecular Devices, Sunnyvale, CA, USA) set at Ex/Em 490/525 nm.
For uPA activity assays, 160 μl of diluted CM was mixed with 20 μl
of assay buffer and subsequently incubated with 20 μl of the
chromogenic substrate in 96-well plates at 37°C for 30 min to 24 h.
The optical density was read at 405 nm in a SpectraMaxi3 microplate
reader, and the relative activity was calculated from a standard
curve.
Assessment of migration and invasion
activities
The migration ability of the cells was assessed by a
wound migration assay and Transwell migration assay as described
previously (22). For invasion
assays, Transwell chambers coated with 30 μl of diluted Matrigel
(BD Biosciences, Bedford, MA, USA) as the intervening barrier were
used.
Western blot analyses
For the extraction of whole cell lysates and
nuclear/cytosolic fractions, M-PER Mammalian Protein Extraction
reagent and NE-PER Nuclear and Cytosolic Extraction reagent (Thermo
Scientific, Rockford, IL, USA) were used, respectively. Western
blotting was performed as described previously (23), and proteins were visualized with a
Bio-Rad Clarity™ Western ECL Substrate and ChemiDoc™ Touch Imaging
System (Bio-Rad, Hercules, CA, USA). Antibodies against p-IκBα,
t-IκBα, NF-κBp65, p-Akt, t-Akt, p-mTOR, tubulin, and TBP were
obtained from Cell Signaling Technology (Danvers, MA, USA), and an
anti-HIF-1α antibody was obtained from BD Biosciences.
Immunocytochemistry
After cells were seeded in 35-mm glass bottom dishes
(SPL Lifesciences, Korea), they were treated with EBGF for 12 h and
then stimulated with PMA (5 nM) for 30 min or incubated under
hypoxic conditions (1% O2) for 6 h for the nuclear
translocation NF-κBp65 or HIF-1α analysis, respectively. After
washing with cold PBS three times, cells were subjected to
immunocytochemistry as previously described (24). Alexa 488- or Alexa 568-conjugated
goat anti-mouse IgG was used as a secondary antibody. After
counterstaining with DAPI, cells were analyzed under a fluorescence
microscope (Nikon Eclipse Ti).
Microvessel sprouting assays
Aortic rings were prepared from rat dorsal thoracic
aortas as previously described (25). Rat aortic rings were cultured on
Matrigel with EGM-2 for 3 days to initiate vessel outgrowth and
were then further incubated with EBGF-treated or untreated HT1080
CM for 5 days. Microvessel sprouting was observed daily and imaged
using a phase-contrast inverted microscope.
Tube formation assays
Endothelial cell capillary-like tube formation was
assessed using a Cultrex in vitro angiogenesis assay kit
(Trevigen, Gaithersburg, MD, USA) according to the manufacturer’s
protocol. In brief, basement membrane extract (BME) was coated on
pre-chilled 96-well culture plates (50 μl/well) and polymerized for
30 min at 37°C. HUVECs (5×104) suspended in 100 μl
EBGF-treated or untreated HT1080 CM were added to each well and
incubated for 4–12 h at 37°C. Tube formation was visualized under a
phase contrast inverted microscope.
Proteome profiler antibody arrays
The expression profile of 55
angiogenesis/invasion-related proteins in the EBGF-treated or
untreated CM was determined using a Proteome Profiler™ Human
Angiogenesis Array kit (R&D Systems, Minneapolis, MN, USA)
according to the manufacturer’s protocol. Blots were visualized
using Bio-Rad Clarity Western ECL substrate and a ChemiDoc Touch
Imaging system. For preparation of CM, HT1080 cells were incubated
with or without EBGF (500 μg/ml) for 24 h in complete medium,
washed twice with 0.5% FBS medium, and then incubated under hypoxic
conditions (1% O2) for an additional 24 h in 0.5% FBS
medium.
In vivo pulmonary metastasis
experiment
Pulmonary metastasis in C57BL/6J mice was induced by
intravenous injection of B16F10 cells (2×105 cells/200
μl of PBS) via the tail vein. After injection, mice were randomly
divided into 3 groups (n=5 per group) and administered saline or
EBGF daily at 50 and 100 mg/kg during the experiment. After the
mice were sacrificed, the lungs were removed and weighed. After
fixation with Bouin’s solution (Sigma), metastatic black colonies
on the lung surface were macroscopically counted. All animal
experiments were approved by the Animal Care and Use Committee of
the Korea Institute of Oriental Medicine (KIOM, Daejeon, Korea;
reference nos. #14-054 and #16-003) and were performed in
accordance with their guidelines.
Statistical analyses
Data are expressed as the mean±standard deviation
(SD). Statistical significance was assessed using Student’s t-test
and one-way ANOVA with GraphPad Prism software (GraphPad Prism
Software Inc., CA, USA). A p-value <0.05 was considered to be
statistically significant.
Results
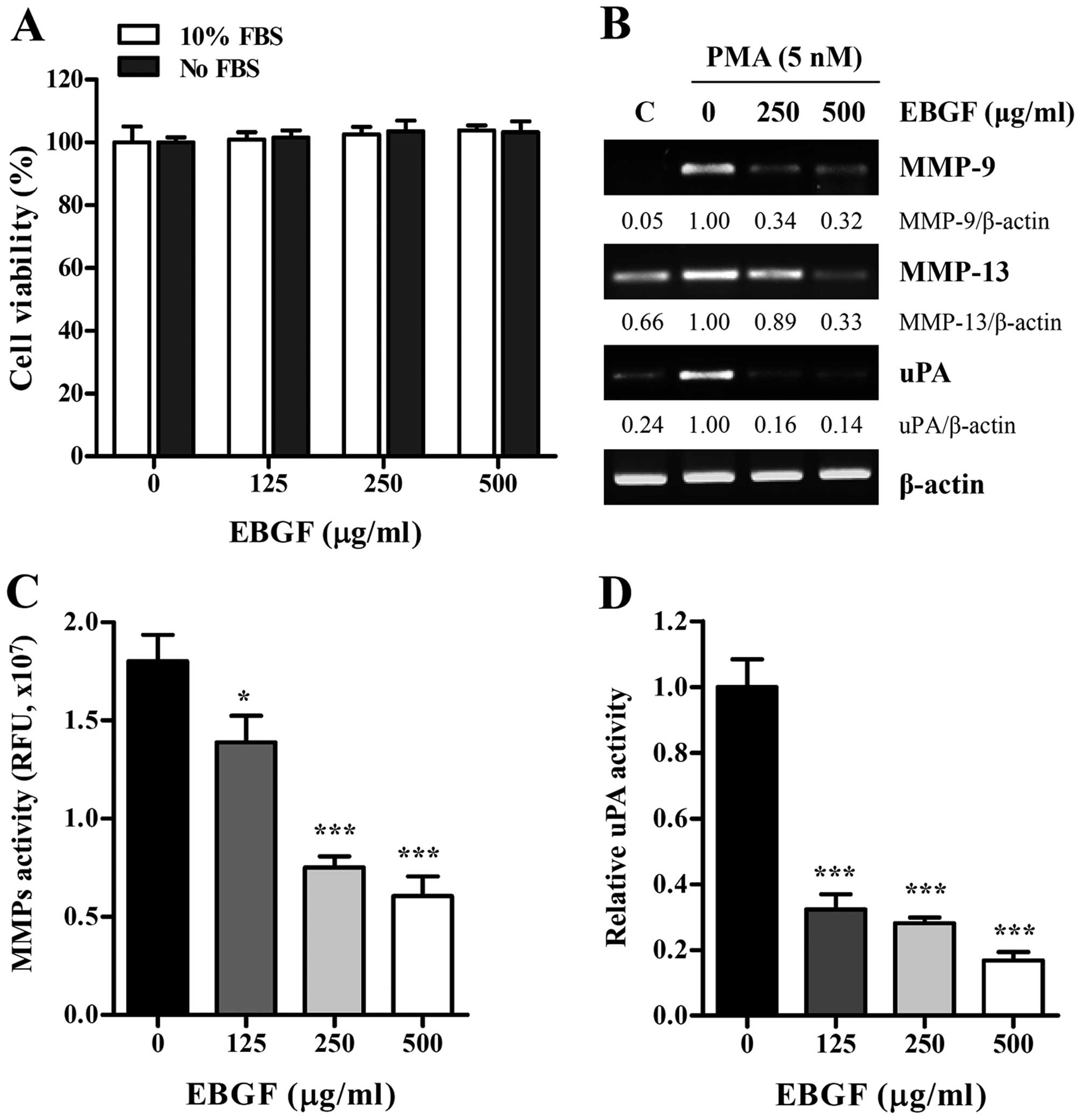
EBGF at non-cytotoxic concentrations
reduces MMP and uPA activities in HT1080 cells
Prior to the assessment of anti-metastatic activity
of EBGF, we first examined its cytotoxicity in HT1080 cells using a
CCK assay. As shown in Fig. 1A,
EBGF at concentrations up to 500 μg/ml had no cytotoxic effects in
the presence or absence of FBS; thus, we treated HT1080 cells with
EBGF at the maximum concentration of 500 μg/ml in all subsequent
experiments. Because the expression and activation of MMPs and uPA
play central roles in promoting cancer metastasis, we initially
examined the effect of EBGF on the transcriptional levels of MMPs
and uPA by RT-PCR. Under resting conditions, HT1080 cells expressed
low levels of MMP-9, MMP-13, and uPA, but PMA stimulation strongly
elevated their expression. In contrast, EBGF treatment almost
completely blocked the PMA-induced increase in the levels of MMP-9,
MMP-13, and uPA (Fig. 1B).
Analysis of proteolytic activities using EBGF-treated or untreated
CM revealed that EBGF dramatically inhibited the MMP and uPA
activities in HT1080 cells in a dose-dependent manner (MMP
activity; F=76.95, p<0.0001, uPA activity; F=167.2, p<0.0001,
one-way ANOVA) (Fig. 1C and
D).
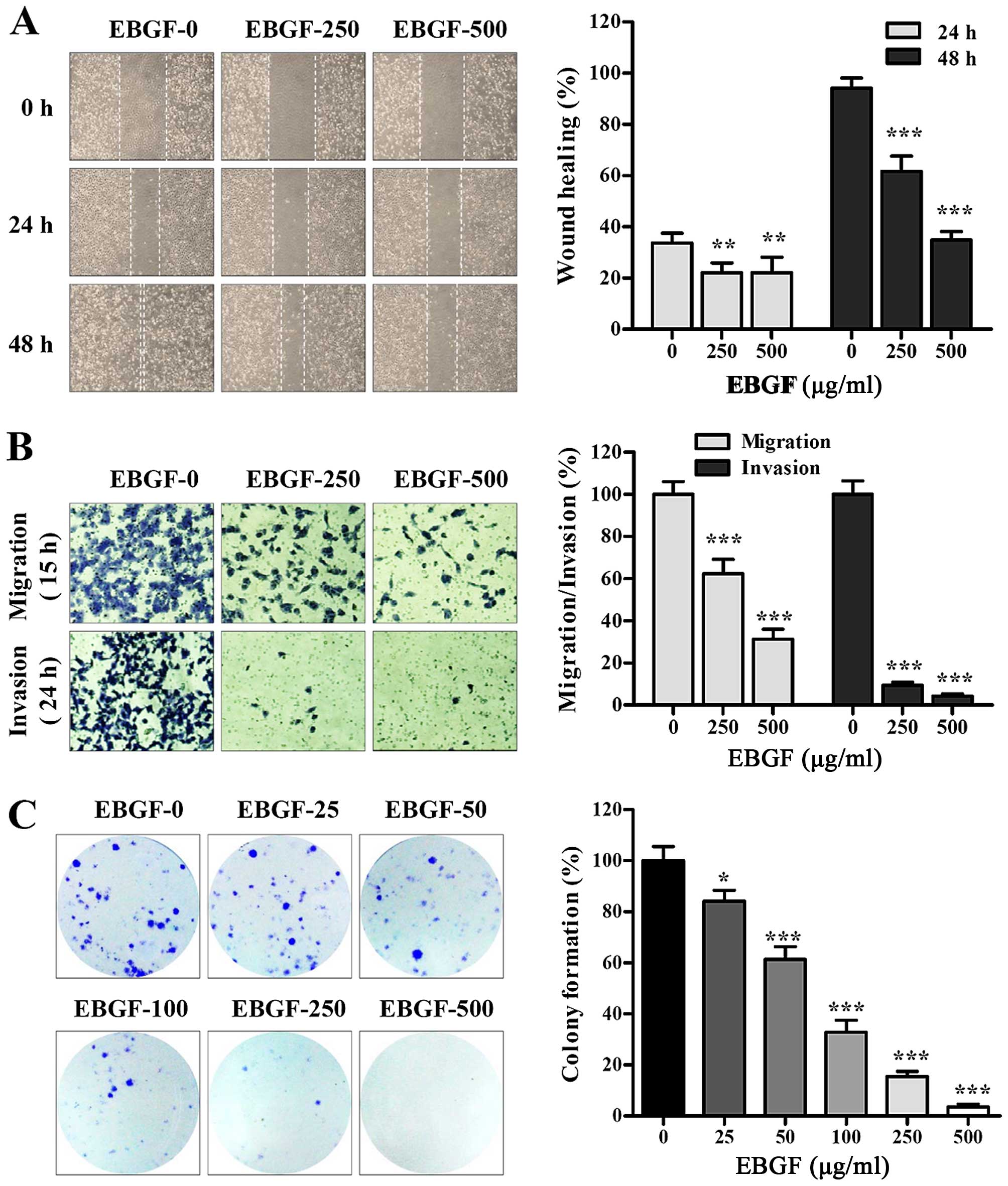
EBGF suppresses metastasis of HT1080
cells
To investigate the effects of EBGF on migration and
invasion in vitro, we first assessed the ability of the
cells to migrate across a wound region. Untreated HT1080 control
cells migrated successfully, leading to ~33.7 and 94.2% healing at
24 and 48 h, respectively. However, EBGF treatment at 250 and 500
μg/ml for 48 h significantly inhibited wound migration to ~65.1 and
36.9% of that of the control cells, respectively (24 h; F=10.2,
p=0.0026, 48 h; F=212.7, p<0.0001, one-way ANOVA) (Fig. 2A). In a Transwell assay, EBGF
markedly inhibited serum-induced migration and invasion compared to
that of the control cells, showing reductions of ~70 and 95% at 500
μg/ml, respectively (migration; F=170.1, p<0.0001, invasion;
F=996.9, p<0.0001, one-way ANOVA) (Fig. 2B). In addition, the colony
formation ability was significantly reduced by EBGF treatment in a
dose-dependent manner (F=260.7, p<0.0001, one-way ANOVA)
(Fig. 2C). These results indicate
that EBGF exhibits potent anti-metastatic activity via suppression
of MMP and uPA activities.
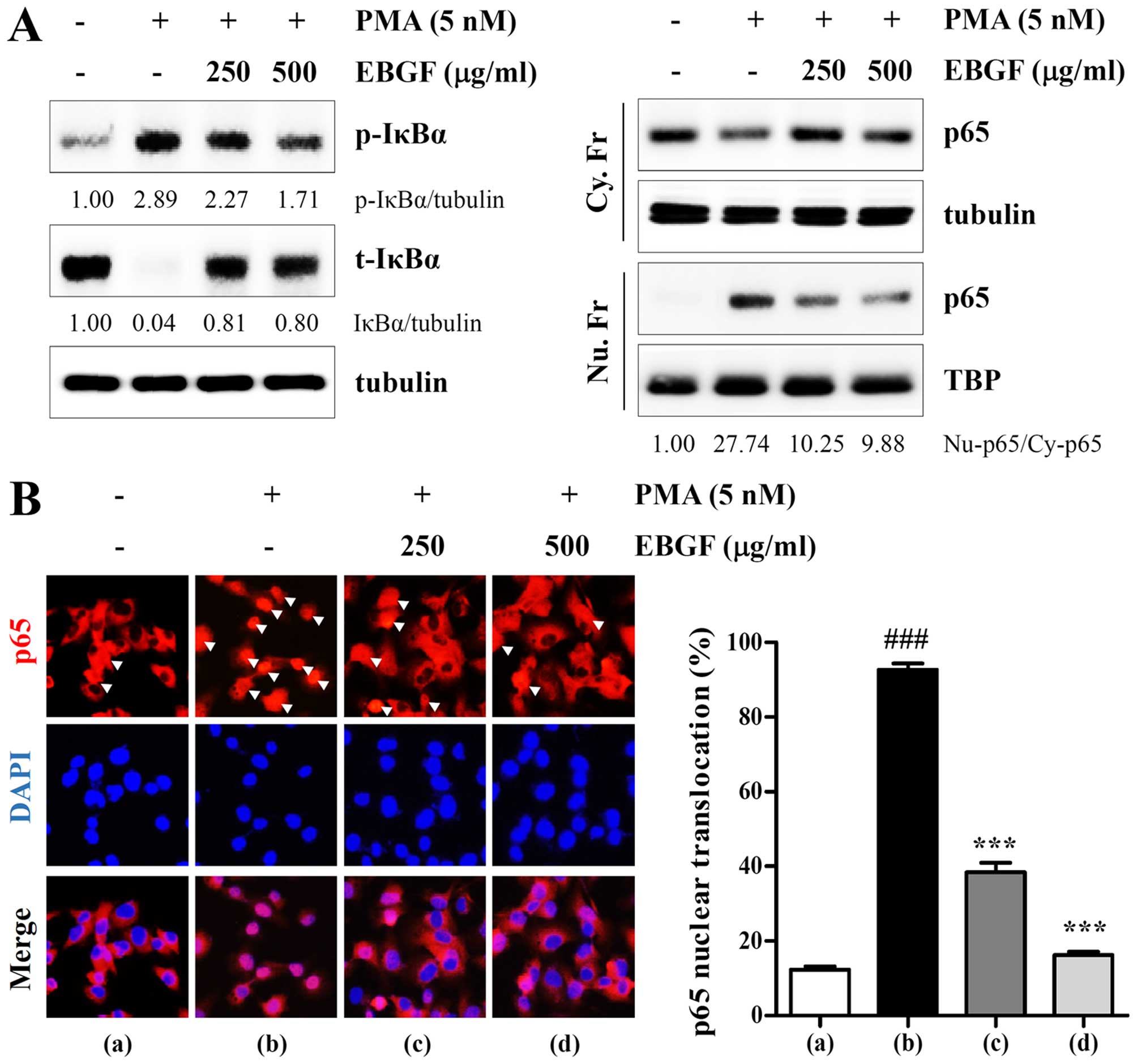
EBGF blocks PMA-induced NF-κB activation
in HT1080 cells
To further elucidate the mechanisms by which EBGF
suppresses metastatic potential, we examined whether EBFG prevents
the activation of the transcription factor NF-κB, which is involved
in the regulation of several proteolytic enzymes and invasion of
cancer cells. NF-κB activation occurs via translocation of the Rel
family from the cytosol to the nucleus, preceded by the
phosphorylation and degradation of IκBα by IκB kinase. As shown in
Fig. 3A, using western blotting,
we confirmed that PMA stimulation increased phosphorylation and
degradation of IκBα, accompanied by increases in the nuclear
protein level of NF-κB subunit p65. However, the PMA-induced
increase in the p-IκBα/IκBα ratio as well as p65 nuclear
translocation was significantly decreased in EBGF-treated HT1080
cells compared with that of control cells, indicating that
PMA-induced NF-κB activation was efficiently blocked by EBGF.
Similarly, immunocytochemistry analysis showed that EBGF
substantially inhibited PMA-induced p65 nuclear translocation by
approximately 83.8% compared with that of the untreated control
cells (F=449.1, p<0.0001, one-way ANOVA) (Fig. 3B). Taken together, these results
suggest that EBGF inhibits the metastatic potential of HT1080 cells
by reducing MMP and uPA activities via suppression of NF-κB
activation.
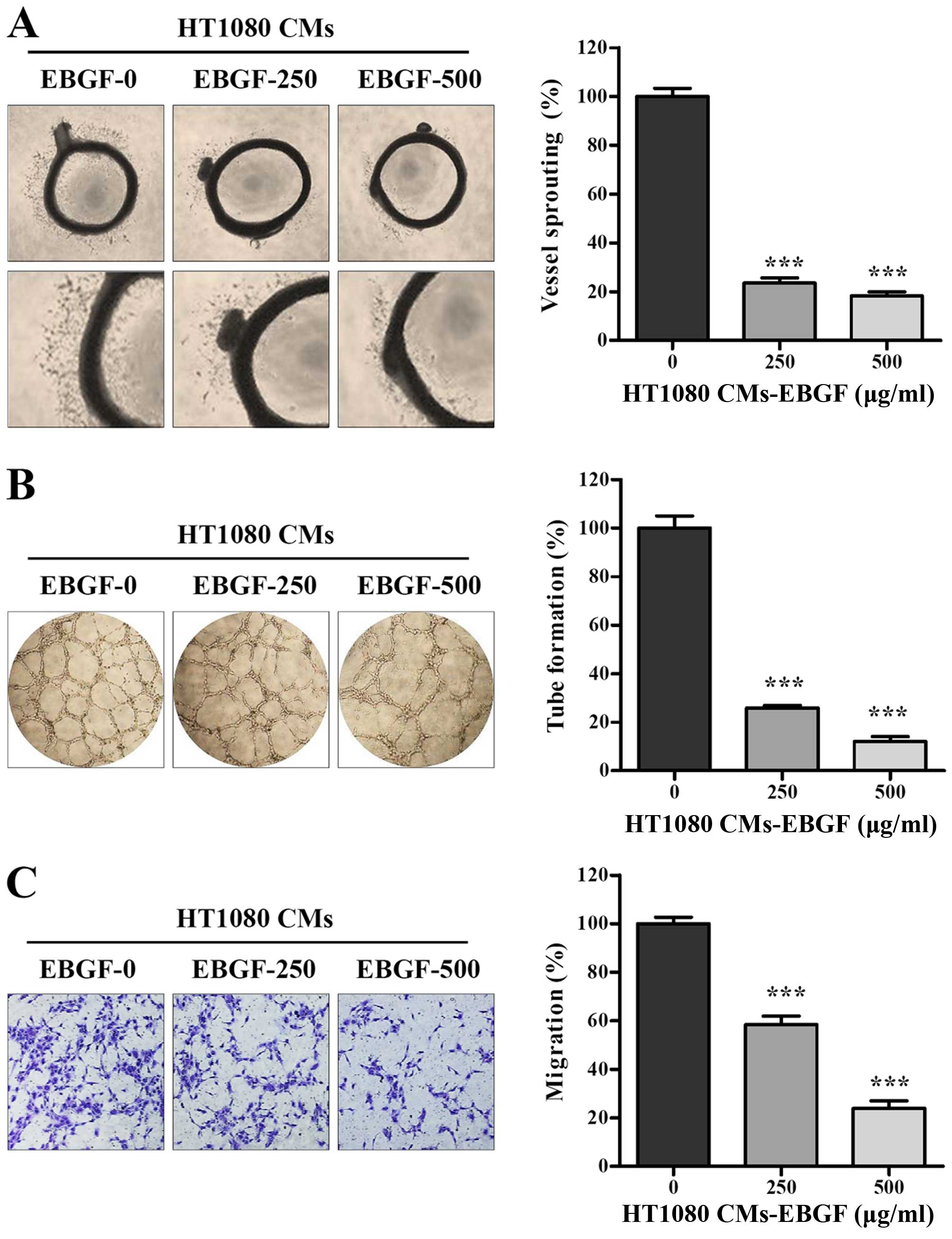
EBGF-treated CM of HT1080 cells
suppresses angiogenesis in vitro and ex vivo
In the tumor microenvironment, tumor cells produce
several pro-angiogenic factors in response to stimuli such as
hypoxia and inflammation and promote angiogenesis, which is
essential for sustained growth and metastasis. Consistent with
previous studies, we confirmed that CM from HT1080 triggered ex
vivo microvessel sprouting from rat aortic rings and induced
robust tube formation as well as migration of endothelial cells
in vitro (Fig. 4).
EBGF-treated CM had strong inhibitory effects on microvessel
formation around aortic rings in a dose-dependent manner, leading
to ~80% inhibition at 500 μg/ml compared with that of the control
CM (F=331.4, p<0.0001, one-way ANOVA) (Fig. 4A). In addition, EBGF-treated CM did
not induce a complete tube-like network of HUVECs and resulted in
lower tube formation than that of the control CM (F=281.9,
p<0.0001, one-way ANOVA) (Fig.
4B). The migration of HUVECs across the Transwell with
EBGF-treated CM showed reductions of ~40 and 75% at 250 and 500
μg/ml compared with that of the control CM, respectively (F=145.7,
p<0.0001, one-way ANOVA) (Fig.
4C). These results collectively indicate that EBGF efficiently
antagonizes tumor-induced angiogenesis.
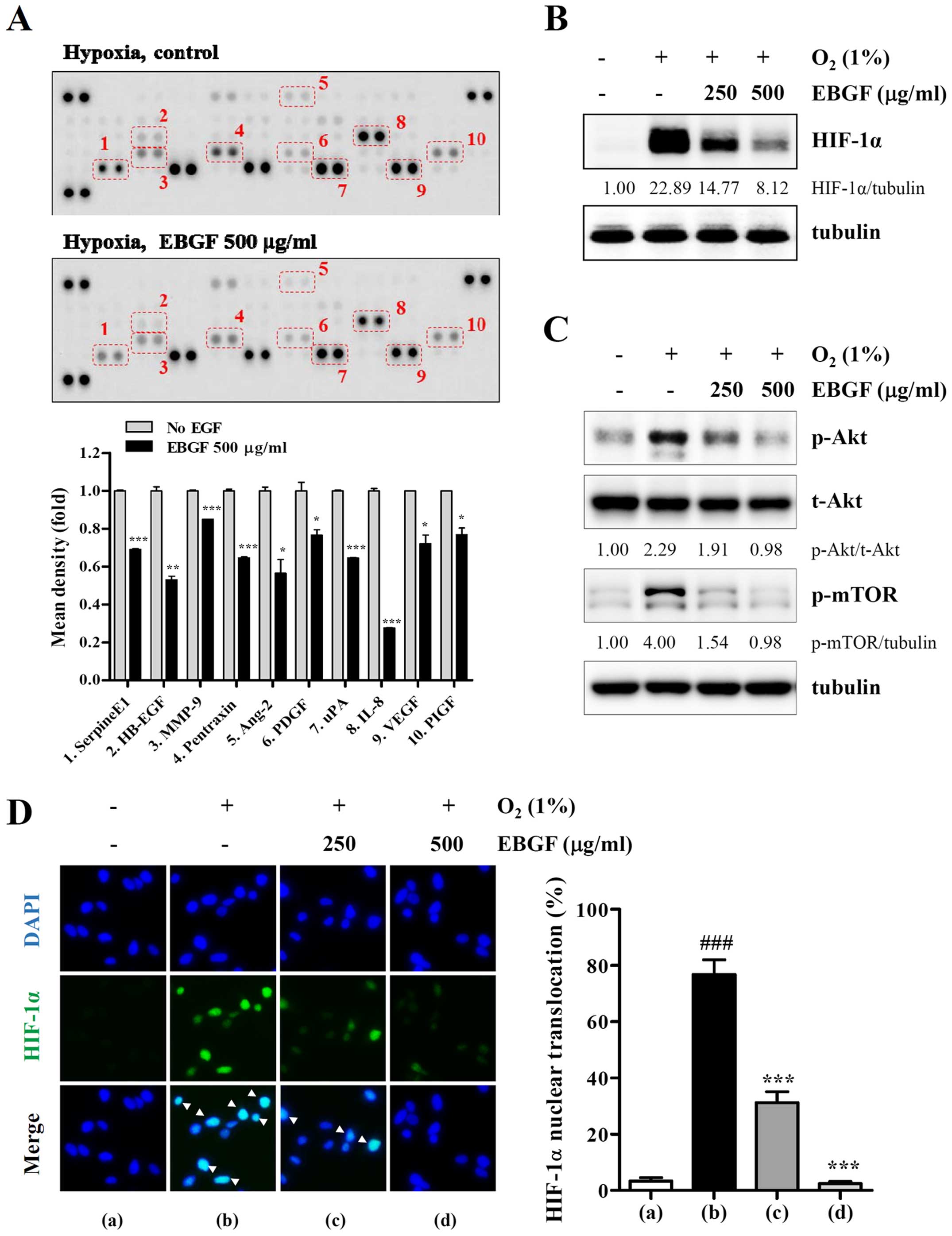
EBGF decreases levels of
angiogenesis-associated proteins under hypoxic conditions via
suppression of the HIF-1α pathway
Angiogenesis is essential for tumor growth and
metastasis and depends on the release of pro-angiogenic factors by
tumor cells and the surrounding cells. Because EBGF had inhibitory
effects on tumor-induced angiogenesis, levels of pro-angiogenic
factors in the control CM and EBGF-treated CM were analyzed using a
Human Angiogenesis Proteome Profiler array. As shown in Fig. 5A, EBGF treatment under hypoxic
conditions significantly decreased the production of pro-angiogenic
factors, including serpine E1, EGF, MMP-9, pentraxin, Ang-2, PDGF,
uPA, IL-8, VEGF, and PIGF. Under hypoxic conditions, HIF-1α is a
key transcriptional regulator for the production of
angiogenesis-related proteins. Consistent with the inhibitory
effects on tumor-induced angiogenesis, EBGF strongly suppressed
hypoxia-induced HIF-1α accumulation and Akt/mTOR phosphorylation
(Fig. 5B and 5C). In addition,
cells with nuclear HIF-1α under hypoxic conditions were
significantly decreased by EBGF treatment in a dose-dependent
manner (F=492.2, p<0.0001, one-way ANOVA) (Fig. 5D). These results indicate that EBGF
reduced the production of angiogenesis-related proteins via
suppression of the HIF-1α pathway.
EBGF administration suppresses in vivo
pulmonary metastasis of B16F10 cells with no side effects
To assess the anti-metastatic activity of EBGF in
vivo, we examined the extent of pulmonary colonization of
intravenously injected B16F10 cells in saline- and EBGF-treated
C57BL/6J mice. In saline-treated control mice, B16F10 cells
efficiently metastasized to the lungs and formed many colonies
(217.8±44.3), while EBGF administration at 50 and 100 mg/kg reduced
the number of colonies to 122.2±16.4 and 91.8±24.9, respectively
(F=22.78, p<0.0001, one-way ANOVA) (Fig. 6A). In addition, lung weight was
also significantly decreased in EBGF-treated mice compared with
that of control mice, which corresponded to the degree of pulmonary
colonization of tumor cells, and resulted in an ~60% reduction
(F=49.20, p<0.0001, one-way ANOVA) (Fig. 6B). During the experiments, the
differences in the body weight between control mice and
EBGF-administered mice were insignificant (Fig. 6C). To further confirm the safety of
EBGF in vivo, body weight and organ weight were measured
after daily administration of EBGF at 50 and 100 mg/kg or saline to
normal mice with no tumors for 21 days. As shown in Fig. 6D, EBGF-treated mice were not
affected in terms of organ weight and body weight and showed
similar levels compared with those of control mice, indicating that
EBGF suppressed pulmonary metastasis of B16F10 cells without
causing toxicity.
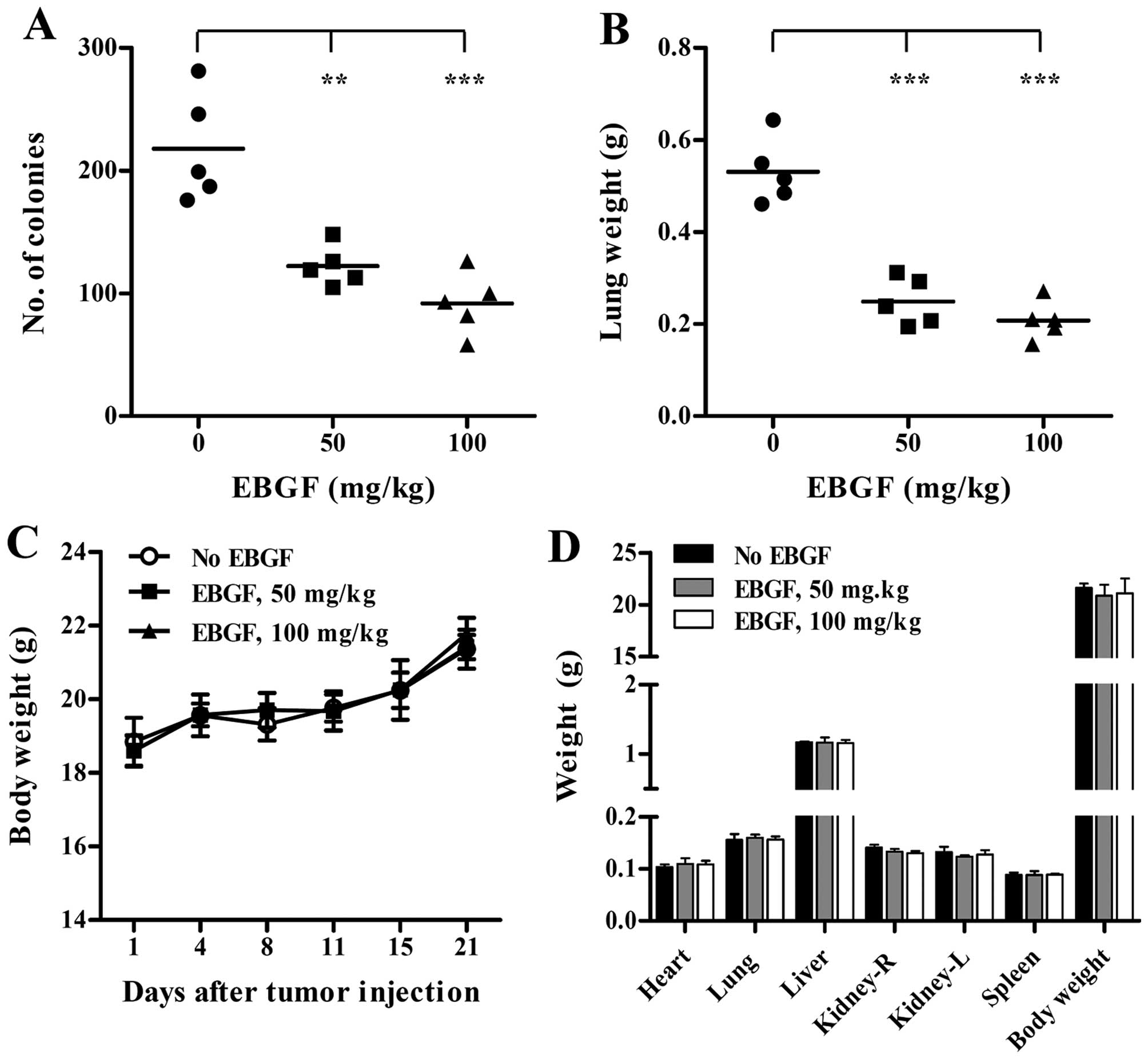 | Figure 6EBGF administration suppresses in
vivo pulmonary metastasis of B16F10 cells with no adverse
effects. (A and B) After intravenous injection of B16F10 cells
(2×105/200 μl PBS/mouse), mice were randomly divided
into 3 groups and orally administered saline or 50 and 100 mg/kg of
EBGF daily for 21 days. On day 21, metastasized colonies on the
lung surface were counted, and the lungs were weighed. Bars
represent the mean value of each group (n=5 per group). Statistical
significance was determined using Student’s t-test.
**p<0.01 and ***p<0.001 vs. saline. (C)
During the experiments, body weights were measured on days 1, 4, 8,
11, 15 and 21. (D) Each group of mice (n=3 per group) was orally
administered saline, 50 and 100 mg/kg of EBGF daily for 21 days.
After sacrifice, body weight and weight of organs were measured.
Data are expressed as the mean ± SD. |
Discussion
Formation of new blood vessels in tumor masses is an
important step in tumor progression and metastasis. Without
angiogenesis, solid tumors cannot grow larger than a few
millimeters and cannot form metastatic foci in distant organs;
thus, targeting the vasculature of tumors is recognized as a
promising strategy for cancer therapy (26,27).
Tumor cells can release positive regulators of angiogenesis,
mobilize an angiogenic stimulator from ECMs, and recruit host cells
that produce angiogenic proteins, leading to the enhancement of EC
proliferation, migration, and cell-cell and cell-ECM adhesion.
Among pro-angiogenic proteins, VEGF is overexpressed in most
cancers and is closely associated with increased microvessel
density of tumors and poor prognosis in cancer patients. In
addition, VEGF is upregulated in tumor masses under hypoxic
conditions via the HIF-1α pathway and promotes transition from
dormant avascularized tumors to outgrowing vascularized ones
(28–31). Therefore, drugs targeting VEGF
production and the VEGF-mediated signal transduction pathway have
been developed and evaluated in a large number of clinical trials
for cancer therapy. Neutralizing antibodies to VEGF, such as
bevacizumab, have been approved by the US FDA and used in
combination with chemotherapy to treat metastatic colorectal
cancer, non-small cell lung cancer, renal cell carcinoma, ovarian
cancer, and others. Tyrosine kinase inhibitors targeting VEGFR,
such as sunitinib and sorafenib, also have been approved for
treating hepatocellular carcinoma, renal cell carcinoma, and others
(27).
Despite their high efficacy, these anti-angiogenic
treatments exhibited several limitations, including narrow
adaptation, side effects, and resistance, and failed to induce a
long-term response in the majority of patients. Thus, alternative
approaches to provide better outcomes, fewer side effects, and more
benefits are needed. Phytochemicals including curcumin,
resveratrol, genistein, and sulforaphane have been shown to target
numerous angiogenesis mediators and have anti-angiogenic effects in
various cancers (32). In
addition, herbal drugs, such as Cordyceps militaris,
Patrinia scabiosaefolia, Ocimum gratissimum,
Salvia officinalis, Eupatorium fortunei, and Anisi
stellate fructus, have also been reported to have anti-angiogenic
activities (22,33–36).
Interestingly, administration of Cordyceps militaris or
ethanol extract of Patrinia scabiosaefolia (EEPS)
efficiently reduced tumor volumes in colorectal cancer or melanoma
via suppression of tumor angiogenesis, strongly supporting the role
of angiogenesis in tumor progression (33,34).
In a previous study, crocetin, a major component of
GF, strongly suppressed TPA-induced skin carcinogenesis via
reduction of TPA-induced nuclear proto-oncogenes, such as c-jun,
c-fos, and c-myc, in mouse epidermis (37). In addition, stir-baked GF extract
inhibited the branching structure of HT1080 cells in a
three-dimensional (3-D) culture and suppressed MMP-16 activity
against DQ-gelatin (21), but it
did not affect cell viability. Geniposide and genipin are known to
be major components of GF and are responsible for various
pharmacological effects, including hepatoprotective,
neuroprotective, anti-oxidative, anti-inflammatory, and
anti-thrombotic activities. Genipin induced apoptosis in HepG3
hepatocarcinoma cells, PC-3 prostate cancer cells, and HeLa
cervical cancer cells (38). In
addition, genipin reduced MMP-2 activity via upregulation of TIMP-1
and p38 activation, leading to suppression of the metastatic
potential of human hepatocarcinoma cells at non-toxic doses
(39). Ethanol extracts of GF and
its n-butanol fraction were previously reported to possess potent
anti-angiogenic activity in the chick embryo chorioallantonic
membrane (CAM) assay (40).
Geniposide had potent anti-angiogenic activity and inhibited the
growth of transformed NIH3T3 cells (41).
In this study, we demonstrated that EBGF at
non-toxic doses dramatically reduced the PMA-induced increase in
the expression of MMP-9, MMP-13, and uPA in HT1080 cells via
suppression of NF-κB activation (Figs.
1 and 3); consequently, wound
migration, colony formation, and migration/invasion through
Matrigel in a Transwell system were relatively lower in
EBGF-treated cells than those in untreated control cells (Fig. 2). In addition, we observed that
HT1080 control CM promoted sprouting of microvessels from aortic
rings and capillary-like tube formation and migration of HUVECs,
while EBGF-treated HT1080 CM weakly induced these angiogenic
changes, indicating that EBGF suppressed the production of
pro-angiogenic factors from HT1080 cells (Fig. 4). Under hypoxic conditions, HT1080
cells released pro-angiogenic factors, including serpine E1, EGF,
MMP-9, pentraxin, Ang-2, PDGF, uPA, IL-8, VEGF, and PIGF, which
stimulate microvascular EC proliferation, enhance EC migration and
sprouting, inhibit EC apoptosis, and increase EC permeability. In
contrast, EBGF-treated HT1080 cells produced these pro-angiogenic
factors to a lesser extent compared with those of control HT1080
cells via suppression of the HIF-1α/Akt/mTOR pathway, supporting
the anti-angiogenic potential of EBGF (Fig. 5). We also observed that daily oral
administration of EBGF effectively suppressed pulmonary metastasis
of B16F10 melanoma cells in C57BL/6 mice, and no adverse reactions,
including loss of body weight and changes in organ weight, were
observed (Fig. 6). In conclusion,
this study demonstrates the anti-metastatic and anti-angiogenic
activities of EBGF in detail, and our data suggest that EBGF can be
used as a safe and potent herbal medicine for treating patients
with metastatic malignant tumors.
Acknowledgements
This study has been supported by the Grant K16281
awarded to Korea Institute of Oriental Medicine (KIOM) from
Ministry of Science, ICT and Future Planning (MSIP), Republic of
Korea.
References
|
1
|
Kumar S and Weaver VM: Mechanics,
malignancy, and metastasis: The force journey of a tumor cell.
Cancer Metastasis Rev. 28:113–127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis - correlation in invasive
breast carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar
|
|
5
|
Stetler-Stevenson WG and Yu AE: Proteases
in invasion: Matrix metalloproteinases. Semin Cancer Biol.
11:143–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
John A and Tuszynski G: The role of matrix
metalloproteinases in tumor angiogenesis and tumor metastasis.
Pathol Oncol Res. 7:14–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
8
|
Stetler-Stevenson WG: Matrix
metalloproteinases in angiogenesis: A moving target for therapeutic
intervention. J Clin Invest. 103:1237–1241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gupta MK and Qin RY: Mechanism and its
regulation of tumor-induced angiogenesis. World J Gastroenterol.
9:1144–1155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nussenbaum F and Herman IM: Tumor
angiogenesis: Insights and innovations. J Oncol. 2010:1326412010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Papetti M and Herman IM: Mechanisms of
normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol.
282:C947–C970. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baeriswyl V and Christofori G: The
angiogenic switch in carcinogenesis. Semin Cancer Biol. 19:329–337.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hida K, Kawamoto T, Ohga N, Akiyama K,
Hida Y and Shindoh M: Altered angiogenesis in the tumor
microenvironment. Pathol Int. 61:630–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aburada M, Sasaki H and Harada M:
Pharmacological studies of Gardeniae fructus. II. Contribution of
the constituent crude drugs to choleretic activity of ‘Inchinko-to’
in rats (author’s transl). Yakugaku Zasshi. 96:147–153. 1976.(In
Japanese). PubMed/NCBI
|
|
15
|
Miyasita S: A historical study of Chinese
drugs for the treatment of Jaundice. Am J Chin Med Gard City N Y.
4:239–243. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koo HJ, Song YS, Kim HJ, Lee YH, Hong SM,
Kim SJ, Kim BC, Jin C, Lim CJ and Park EH: Antiinflammatory effects
of genipin, an active principle of gardenia. Eur J Pharmacol.
495:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin YJ, Lai CC, Lai CH, Sue SC, Lin CW,
Hung CH, Lin TH, Hsu WY, Huang SM, Hung YL, et al: Inhibition of
enterovirus 71 infections and viral IRES activity by Fructus
gardeniae and geniposide. Eur J Med Chem. 62:206–213. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toriizuka K, Kamiki H, Ohmura NY, Fujii M,
Hori Y, Fukumura M, Hirai Y, Isoda S, Nemoto Y and Ida Y:
Anxiolytic effect of Gardeniae Fructus-extract containing active
ingredient from Kamishoyosan (KSS), a Japanese traditional Kampo
medicine. Life Sci. 77:3010–3020. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu QH, Zhu JX, Ji J, Wei LL, Miao MX and
Ji H: Fructus Gardenia extract ameliorates oxonate-induced
hyperuricemia with renal dysfunction in mice by regulating organic
ion transporters and mOIT3. Molecules. 18:8976–8993. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang HY, Liu H, Yang M and Wei SF:
Antithrombotic activities of aqueous extract from Gardenia
jasminoides and its main constituent. Pharm Biol. 51:221–225. 2013.
View Article : Google Scholar
|
|
21
|
Yang JG, Shen YH, Hong Y, Jin FH, Zhao SH,
Wang MC, Shi XJ and Fang XX: Stir-baked Fructus gardeniae (L.)
extracts inhibit matrix metalloproteinases and alter cell
morphology. J Ethnopharmacol. 117:285–289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim A, Im M and Ma JY: Anisi stellati
fructus extract attenuates the in vitro and in vivo metastatic and
angiogenic potential of malignant cancer cells by downregulating
proteolytic activity and pro-angiogenic factors. Int J Oncol.
45:1937–1948. 2014.PubMed/NCBI
|
|
23
|
Kim A, Im M, Yim NH, Kim T and Ma JY: A
novel herbal medicine, KIOM-C, induces autophagic and apoptotic
cell death mediated by activation of JNK and reactive oxygen
species in HT1080 human fibrosarcoma cells. PLoS One. 9:e987032014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim A, Im M, Gu MJ and Ma JY: Citrus
unshiu peel extract alleviates cancer-induced weight loss in mice
bearing CT-26 adenocarcinoma. Sci Rep. 6:242142016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim A, Im M, Yim NH and Ma JY: Reduction
of metastatic and angiogenic potency of malignant cancer by
Eupatorium fortunei via suppression of MMP-9 activity and VEGF
production. Sci Rep. 4:69942014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Albini A, Tosetti F, Li VW, Noonan DM and
Li WW: Cancer prevention by targeting angiogenesis. Nat Rev Clin
Oncol. 9:498–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jayson GC, Kerbel R, Ellis LM and Harris
AL: Antiangiogenic therapy in oncology: Current status and future
directions. Lancet. 388:518–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abdelrahim M, Konduri S, Basha R, Philip
PA and Baker CH: Angiogenesis: An update and potential drug
approaches (Review). Int J Oncol. 36:5–18. 2010.
|
|
29
|
Lin SC, Liao WL, Lee JC and Tsai SJ:
Hypoxia-regulated gene network in drug resistance and cancer
progression. Exp Biol Med (Maywood). 239:779–792. 2014. View Article : Google Scholar
|
|
30
|
Liao D, Corle C, Seagroves TN and Johnson
RS: Hypoxia-inducible factor-1alpha is a key regulator of
metastasis in a transgenic model of cancer initiation and
progression. Cancer Res. 67:563–572. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou J, Schmid T, Schnitzer S and Brüne B:
Tumor hypoxia and cancer progression. Cancer Lett. 237:10–21. 2006.
View Article : Google Scholar
|
|
32
|
Wang Z, Dabrosin C, Yin X, Fuster MM,
Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B,
Ribatti D, et al: Broad targeting of angiogenesis for cancer
prevention and therapy. Semin Cancer Biol. 35(Suppl): S224–S243.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen L, Liu L, Ye L, Shen A, Chen Y,
Sferra TJ and Peng J: Patrinia scabiosaefolia inhibits colorectal
cancer growth through suppression of tumor angiogenesis. Oncol Rep.
30:1439–1443. 2013.PubMed/NCBI
|
|
34
|
Ruma IM, Putranto EW, Kondo E, Watanabe R,
Saito K, Inoue Y, Yamamoto K, Nakata S, Kaihata M, Murata H, et al:
Extract of Cordyceps militaris inhibits angiogenesis and suppresses
tumor growth of human malignant melanoma cells. Int J Oncol.
45:209–218. 2014.PubMed/NCBI
|
|
35
|
Nangia-Makker P, Tait L, Shekhar MP,
Palomino E, Hogan V, Piechocki MP, Funasaka T and Raz A: Inhibition
of breast tumor growth and angiogenesis by a medicinal herb: Ocimum
gratissimum. Int J Cancer. 121:884–894. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Keshavarz M, Mostafaie A, Mansouri K,
Bidmeshkipour A, Motlagh HR and Parvaneh S: In vitro and ex vivo
antiangiogenic activity of Salvia officinalis. Phytother Res.
24:1526–1531. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hsu JD, Chou FP, Lee MJ, Chiang HC, Lin
YL, Shiow SJ and Wang CJ: Suppression of the TPA-induced expression
of nuclear-protooncogenes in mouse epidermis by crocetin via
antioxidant activity. Anticancer Res. 19B:4221–4227. 1999.
|
|
38
|
Cao H, Feng Q, Xu W, Li X, Kang Z, Ren Y
and Du L: Genipin induced apoptosis associated with activation of
the c-Jun NH2-terminal kinase and p53 protein in HeLa cells. Biol
Pharm Bull. 33:1343–1348. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang N, Zhu M, Tsao SW, Man K, Zhang Z and
Feng Y: Up-regulation of TIMP-1 by genipin inhibits MMP-2
activities and suppresses the metastatic potential of human
hepatocellular carcinoma. PLoS One. 7:e463182012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park EH, Joo MH, Kim SH and Lim CJ:
Antiangiogenic activity of Gardenia jasminoides fruit. Phytother
Res. 17:961–962. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koo HJ, Lee S, Shin KH, Kim BC, Lim CJ and
Park EH: Geniposide, an anti-angiogenic compound from the fruits of
Gardenia jasminoides. Planta Med. 70:467–469. 2004. View Article : Google Scholar : PubMed/NCBI
|