Introduction
Breast cancer is the most common form of cancer in
women. According to the World Cancer Report, there are more than
1.6 million new cases annually in women (1). In China, breast cancer was estimated
to account for 15% of all newly diagnosed cancers in women in 2015
and is thought to be the leading cause of cancer death in women
under the age of 45 years (2).
Although the overall mortality rate has declined due to the greater
efficacy of therapeutic interventions, recurrence and metastasis
are still major challenges in tackling the disease (3). Therefore, investigations of
progression-related molecular mechanisms will significantly benefit
targeted breast cancer therapy and improve disease outcomes.
RYBP (RING1- and YY1-binding protein) has been shown
to interact with RING1, RNF2 and YY1 (4), and is a member of the polycomb (PcG)
group of proteins (5). It has been
shown to be downregulated in several types of tumors (6–8).
Overexpression of RYBP using an adenovirus-based system has been
shown to decrease cell proliferation in various cancer cell lines,
including U2OS, SAOS2, A549, TOV112 and TOV21 (9). In a study of integrative genomic
profiling of human prostate cancer, RYBP was implicated as a
potential cooperative tumor suppressor (10). Regarding its role in prognosis, an
integrative analysis of gene expression in cervical cancer
indicated that patients with low expression levels of RYBP have
shorter progression-free survival rates (11). Recently, downregulation of RYBP has
been found to decrease cell sensitivity to chemotherapy, and is
associated with poor prognosis in patients with non-small cell lung
cancer (NSCLC) and hepatocellular carcinoma (HCC) (8,12).
However, whether RYBP plays a role in breast cancer progression
remains unclear.
Recent studies have demonstrated that REST-003, a
non-coding RNA, is expressed at low levels in weakly invasive
breast cancer cells but at elevated levels in highly invasive
cancer cells, and REST-003 expression is controlled by an
uncharacterized serine/arginine repeat-related protein (SRRM3)
(13). The research revealed that
REST-003 and SRRM3 played an important role in regulating cell
invasiveness and emphasized their potential novel therapeutic value
in breast cancer.
In the present study, we hypothesized that RYBP may
function as a novel molecular target in breast cancer therapy. We
investigated the expression level of RYBP and explored the effect
of RYBP overexpression on tumor growth and metastasis in both
breast cancer cell lines and in a nude mouse model. We also
analyzed the potential molecular mechanism involved in this
process.
Materials and methods
Cell culture
Normal 76N and tumor SK-BR-3, ZR-75-1, T47D,
MDA-MB-231 and MCF-7 breast cells were used in this study. All
cells were purchased from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). Cells were
maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing
10% fetal bovine serum (10% DMEM) and cultured in 5% CO2
at 37°C.
Patients and samples
Thirty-three paired primary tumors and matched
peritumoral tissues of breast cancer samples were collected from
patients who underwent surgical resection in the First Affiliated
Hospital of Sun Yat-sen University, Guangzhou, China, between
January 2014 and January 2016. Patients diagnosed with distant
metastasis were excluded.
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted from pretreated cells using
TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to
the manufacturer’s protocol. The RNA samples were
reverse-transcribed into cDNA using the PrimeScript™ RT reagent kit
(Takara Co., Ltd., Tokyo, Japan). The resulting cDNAs were
amplified with GoTaq® qPCR Master Mix (Promega, Madison,
WI, USA) following standard procedures, and the experiment was
performed on a MiniOpticon™ real-time PCR detection instrument
(Bio-Rad Laboratories, Hercules, CA, USA). The primers used in this
study are shown in Table I.
 | Table IPrimers used in the present
study. |
Table I
Primers used in the present
study.
| Primer name | Sequence
(5′-3′) |
|---|
| H-REST F |
GAACTCATACAGGAGAACGCC |
| H-REST R |
GAACTGCCGTGGGTTCACA |
| H-REST003 F |
AGTGTCGGGGCGACTCCCG |
| H-REST003 R |
GGCATTCCTAACTGAAATAGG |
| H-SRRM3 F |
TCCTGGAGCTCCAGCCGCTCGCCC |
| H-SRRM3 R |
CTCAGAGTGCCTTGCGCGGCCCTCG |
| H-RYBP-F |
CGCAGACGAAGGGTTTTGG |
| HRYBP-R |
GAATTGATCCGAGGTTTTCTGGT |
| H-GAPDH-F |
GAGTCAACGGATTTGGTCGT |
| H-GAPDH-R |
GACAAGCTTCCCGTTCTCAG |
Immunohistochemistry (IHC)
Breast cancer tissues and the paired peritumoral
tissues were fixed in 10% formalin and embedded in paraffin. The
blocks were cut into 4-mm sections and then dewaxed and hydrated.
After endogenous peroxidase was blocked with 3% hydrogen peroxide,
the sections were incubated with RYBP primary antibody (HPA053357;
Sigma-Aldrich, Schnelldorf, Germany) and diluted 1:500 at 4°C
overnight. Subsequently, the sections were washed to remove unbound
antibody and incubated with the secondary antibody (sc-2055,
1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room
temperature for 1 h. The sections were visualized using the
Vectastain ABC Peroxidase Elite kit (Vector Laboratories,
Burlingame, CA, USA) and 3,3′-diaminobenzidine (DAB)
tetrahydrochloride (Sigma-Aldrich) as a substrate.
Western blot analysis
Fresh breast cancer tissues and paired peritumoral
tissues were washed with phosphate-buffered saline (PBS) and stored
at −80°C after surgical resection. Cell lysates were prepared using
the NucBuster™ Protein Extraction kit (Novagen, Darmstadt, Germany)
according to the manufacturer’s instructions. The protein
concentration was quantified using the Bradford assay, separated by
10% SDS-PAGE electrophoresis and transferred to PVDF membranes. The
PVDF membranes were blocked with 5% skim milk powder for 1 h at
room temperature and then incubated with primary antibody against
RYBP (1:400 dilution), E-cadherin (sc-7870, 1:2,000 dilution; Santa
Cruz Biotechnology), snail (sc-393172, 1:200 dilution; Santa Cruz
Biotechnology), cyclin A (ab38, 1:1,000 dilution; Abcam, Cambridge,
MA, USA) and cyclin B1 (ab32053, 1:2,500 dilution; Abcam) at 4°C
overnight. The samples were then incubated with the secondary
antibody (sc-2006, 1:1,000 dilution; Santa Cruz Biotechnology) at
room temperature for 1 h, and the blots were visualized using
chemiluminescence (ECL; Forevergen Biosciences Center, Guangzhou,
China). GAPDH (sc-365062, 1:1,000 dilution; Santa Cruz
Biotechnology) was used as a reference.
Stable cell line generation and
transfection
RYBP (Gene ID: 23429) was cloned into the
EcoRI and XbaI sites of the LV-003 lentivirus vector
(Forevergen Biosciences Center). The resulting lentivirus vector
was co-transfected with packaging vectors into HEK-293T cells, and
the cell supernatant was then collected after 48 h of transfection
and subjected to ultracentrifugation at 25,000 rpm for 1.5 h. The
lentivirus was then re-suspended in PBS, and 5–10 μg/ml of
polybrene was added to the RYBP lentivirus for MDA-MB-231 and
SK-BR-3 cell infection. After a 48-h incubation, the cells were
cultured in 10% DMEM with 2 μg/ml puromycin for 72 h to generate
MDA-MB-231-RYBP and SK-BR-3-RYBP cells. The cell cycle
distribution, cell migration and cell invasion of MDA-MB-231-RYBP
and SK-BR-3-RYBP cells were then analyzed to evaluate cell growth
and metastasis.
RYBP (Gene ID: 23429) and SRRM3 (Gene ID: 222183)
was cloned into the EcoRI and XbaI sites of the pCMV
vector. The si-SMMR3 (5′-AGAAGAAGAGUGUGAAGAAUU-3′) and si-REST003
(5′-GCAGCAGCACGGAGGAAUU-3′) were purchased from Shanghai
GenePharma, Co., Ltd., (Shanghai, China). Cells were seeded into
6-well plates 12 h prior to transfection. A final concentration of
100 nM of siRNA (Shanghai GenePharma) and 2 μg of plasmid were
transfected into the cells according to standard procedures. After
48 h of culture, the cells were collected for mRNA expression and
cell metastasis analysis.
Clonogenic assay of cells in vitro
After treatment, the cells were seeded into 6-well
plates at a density of 1.5×105 cells/well and cultured
in 10% DMEM. The cell colonies were fixed with glutaraldehyde (6.0%
v/v), stained with crystal violet (0.5% w/v) and counted after 15
days. Clone formation ratios were calculated as colonies/total
cells. A colony was defined as consisting of at least 50 cells
(14). The experiments were
repeated three times.
MTS assay
Cell viability was measured using the MTS
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]
assay. The cells were cultured in 96-well flat-bottomed plates at a
density of 1×103 cells/well 24 h prior to the MTS assay.
The cells were then stained with MTS reagent in 10% DMEM for 4 h
and subjected to a microplate reader (Diatek) to determine the OD
value of each well at a wavelength of 490 nm. Three biological
replicates were evaluated.
Flow cytometry
Stable RYBP-overexpressing cell lines
(MDA-MB-231-RYBP and SK-BR-3-RYBP) and their corresponding cells
(MDA-MB-231-NC and SK-BR-3-NC) were fixed with 1 ml of 70% ice-cold
ethanol overnight at 4°C. The cells were washed twice with PBS and
centrifuged for 10 min at 1,000 rpm; the cell pellets were then
stained with 50 μg/ml of propidium iodide and 100 U/ml of RNase A,
and incubated in PBS for 30 min at room temperature in the dark. A
flow cytometer (BD Biosciences, San Jose, CA, USA) with CellQuest
software was used to analyze the cell cycle distribution, and the
proportions of cells in different phases of the cell cycle were
determined based on the DNA content.
Transwell assay
Cell migration and invasion assays were performed
using the Transwell assay (Transwell; Cambridge, MA, USA) (15). The cells were cultured in the upper
compartment of the Transwell at a density of 2×104
cells/50 μl/well. The cells in the upper compartment migrated to
the lower compartment through an 8 μm membrane (for the migration
assay) or a Matrigel-coated membrane (for the invasion assay)
(Becton-Dickinson, Bedford, MA, USA). Cells in the upper
compartment were cultured in serum-free DMEM, while 10% DMEM was
added to the lower compartment. After 48 h of cell culture, the
cells in the upper compartment were removed, and the
migrated/invasive cells in the lower compartment were fixed in
methanol and stained with 10% Giemsa solution (Sigma-Aldrich). The
cell numbers in four high-power fields were counted under a
microscope. Three biological replicates were evaluated.
Nude mouse model
For tumor xenograft model establishment, 4- to
6-weeks-old BALB/c nude mice were obtained from the Animal Center
Laboratory of Sun-Yat sen University. The Institute Research
Medical Ethics Committee of Sun Yat-sen University granted approval
for this study. Approximately 5×106 MDA-MB-231-RYBP,
SK-BR-3-RYBP, and their control cells (MDA-MB-231-NC and
SK-BR-3-NC) were subcutaneously injected into each group
(n=5/group) (16). The tumor
volume and general physical condition of the mice were monitored
every 6 days. The tumor volume was calculated as the
width2 × length/2. After 42 days of modification, the
mice were sacrificed and the xenograft tumors were removed. For the
nude mouse model of breast cancer metastasis to the lung,
5×106 MDA-MB-231-RYBP, SK-BR-3-RYBP and their control
cells (MDA-MB-231-NC and SK-BR-3-NC) were injected into the mammary
fat pad of mice as previously described (17) (n=5 per group). Six weeks after the
cell implantation, when signs of disease (weight loss and decreased
grooming) were observed in the mice, the lungs of each mouse were
dissected, and meta-static foci in the lungs were counted.
Hematoxylin and eosin (H&E) staining was also performed to
facilitate review.
Ethical standards
All human studies were approved by Sun Yat-sen
University. All human studies were performed in accordance with the
1975 Helsinki Declaration and its subsequent amendments. All
subjects signed an informed consent form prior to inclusion in the
study.
Statistical analysis
The SPSS 18.0 software (IBM, Armonk, NY, USA) was
used for the statistical analysis. The results are presented as the
mean ± SD. The Student’s t-test method was used to analyze
differences between the two groups. For the disease-free survival
(DSF) analysis, the expression data for RYBP mRNA z-Scores (RNA Seq
V2 RSEM) from 350 cases were downloaded from The Cancer Genome
Atlas (TCGA) dataset [Breast Invasive Carcinoma (TCGA, Cell 2015)]
and analyzed using GraphPad Prism v.6 (Graphpad Software Inc., La
Jolla, CA, USA). DSF curves were generated using the Kaplan-Meier
method, and the log-rank Chi-square test was used to analyze the
significance of the correlation between patients with high and low
RYBP expression. P<0.05 was considered a statistically
significant difference.
Results
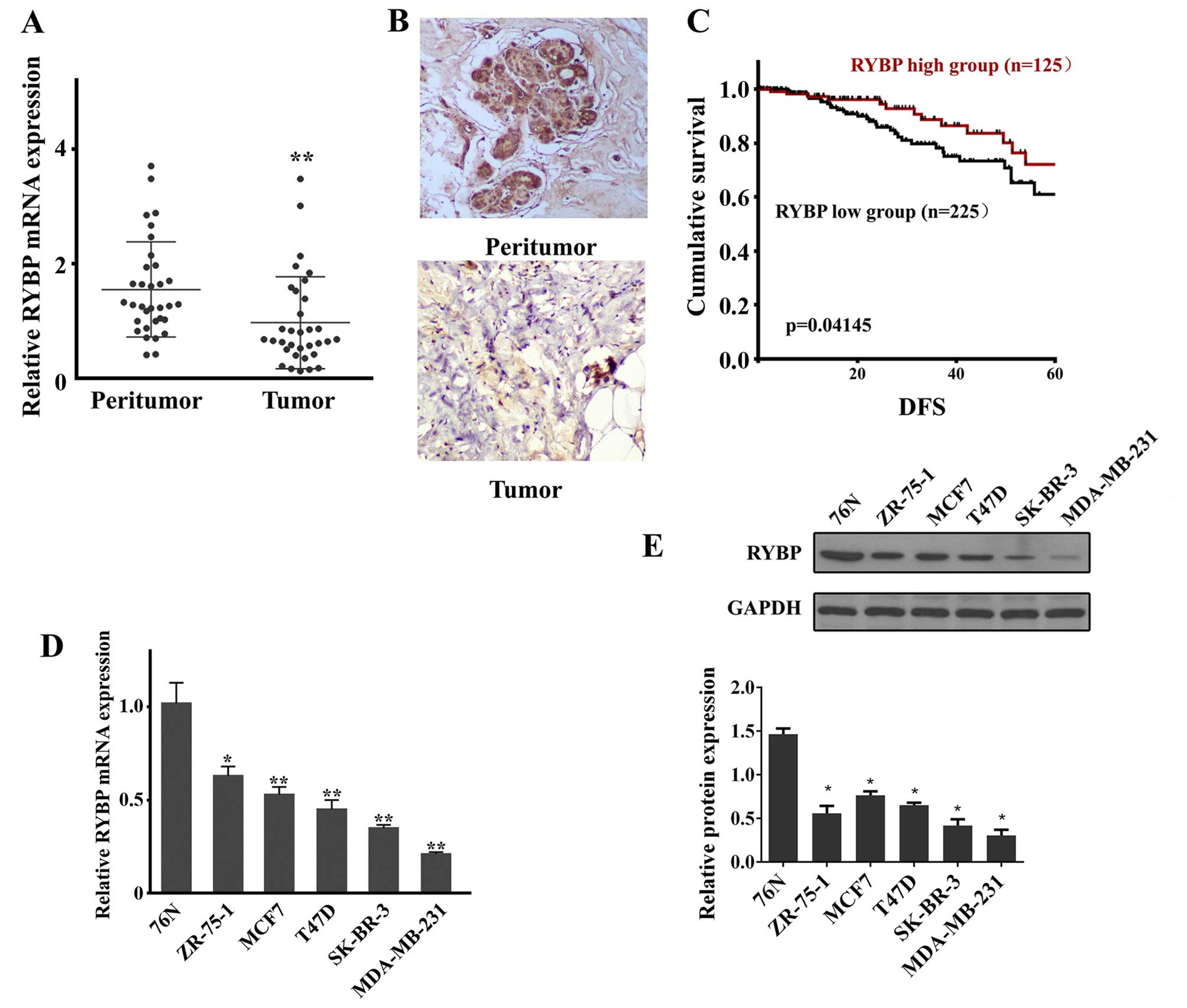
RYBP is downregulated in clinical samples
of breast cancer patients and cell lines and is associated with
poor survival in breast cancer patients
To determine whether the expression of RYBP was
altered in breast cancer tumors, qPCR and western blot analysis
were conducted. As shown in Fig.
1, both RYBP mRNA (Fig. 1A)
and protein (Fig. 1B) were
downregulated in the tumor tissues compared with the peritumoral
tissues of breast cancer patients.
To assess whether RYBP expression is associated with
longer survival in breast cancer patients, DFS was determined in
the TCGA dataset breast cancer patient cohort. The survival
analysis showed that DFS differed between patients with high and
low RYBP expression. Patients with high RYBP expression had
significantly longer DFS than those with low RYBP expression
(Fig. 1C).
The expression levels of RYBP mRNA (Fig. 1D) and protein (Fig. 1E) were also evaluated in normal 76N
and tumor SK-BR-3, ZR-75-1, T47D, MDA-MB-231 and MCF-7 breast
cells. As shown in Fig. 1D and E,
the qPCR and western blot results showed that RYBP was
downregulated in all analyzed breast tumor cells. Of these cells,
SK-BR-3 and MDA-MB-231 cells exhibited the first and second lowest
expression, and were therefore selected for subsequent analysis of
RYBP function.
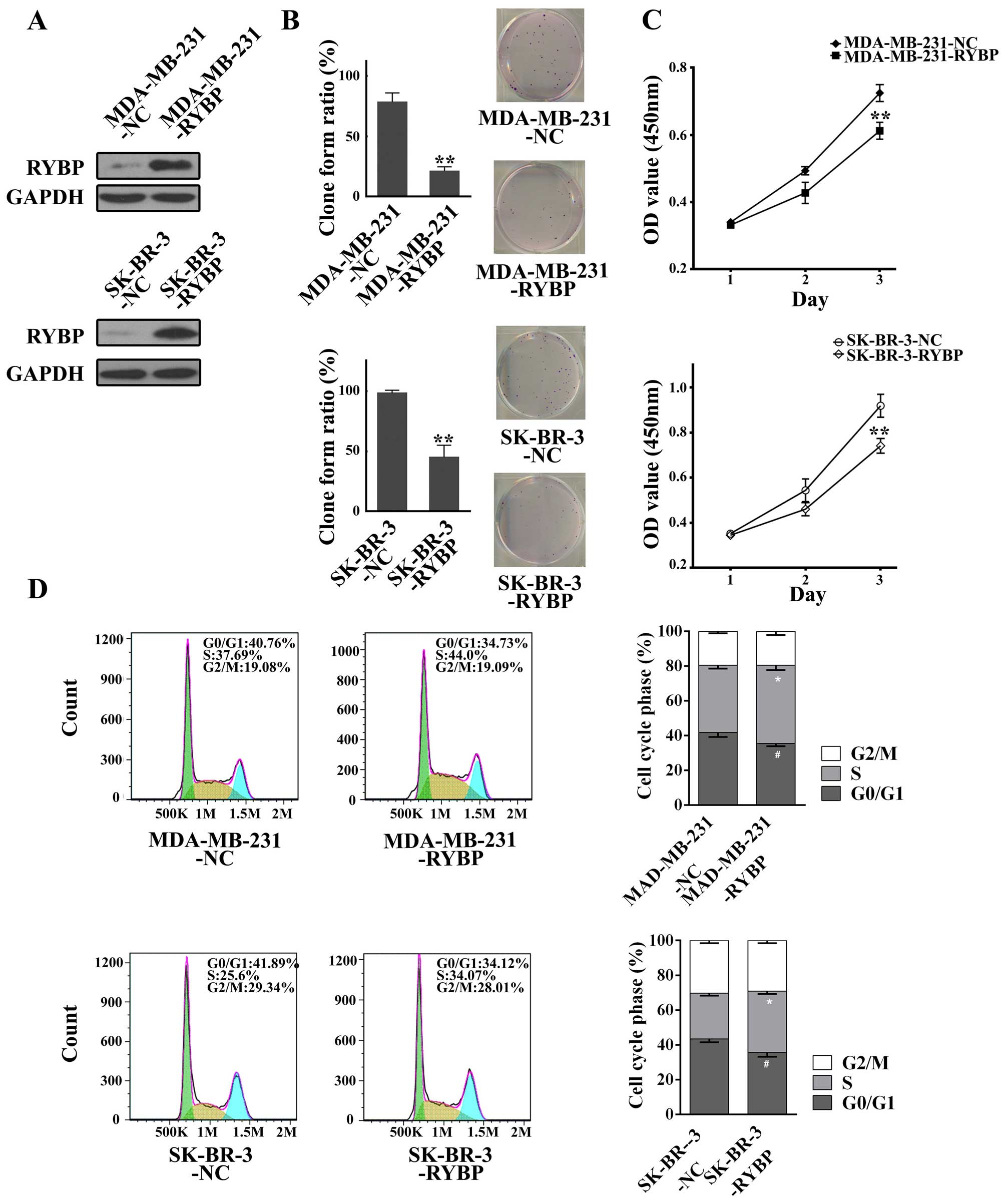
Overexpression of RYBP suppresses cell
growth and metastasis in MDA-MB-231 and SK-BR-3
The differential expression of RYBP between tumorous
and peritumor breast cancer tissues led us to investigate its role
in breast cancer development and metastasis. Because RYBP was
significantly downregulated in SK-BR-3 and MDA-MB-231 cells, we
applied lentiviral-mediated overexpression of RYBP in these two
tumor cell lines. As shown in Fig.
2A, lentivirus-mediated stable RYBP overexpressing cell lines,
MDA-MB-231-RYBP and SK-BR-3-RYBP, were established, as evidenced by
the upregulation of RYBP and apparent in the western blots compared
with their corresponding negative controls of MDA-MB-231-NC and
SK-BR-3-NC cells. Cell proliferation was then determined using
clonogenic and MTS assays. The clonogenic assay of cells showed
that RYBP-overexpressing cells (MDA-MB-231-RYBP and SK-BR-3-RYBP)
had a lower rate of clone formation compared with NC cells
(Fig. 2B). The MTS assay also
revealed consistently decreasing OD values in MDA-MB-231-RYBP and
SK-BR-3-RYBP cells compared with negative control cells (Fig. 2C). Furthermore, flow cytometric
analysis of the cell cycle distribution revealed a higher
proportion of S-phase cells in MDA-MB-231-RYBP compared with
MDA-MB-231-NC cells. A higher proportion of S-phase cells was also
observed in SK-BR-3-RYBP cells (Fig.
2D).
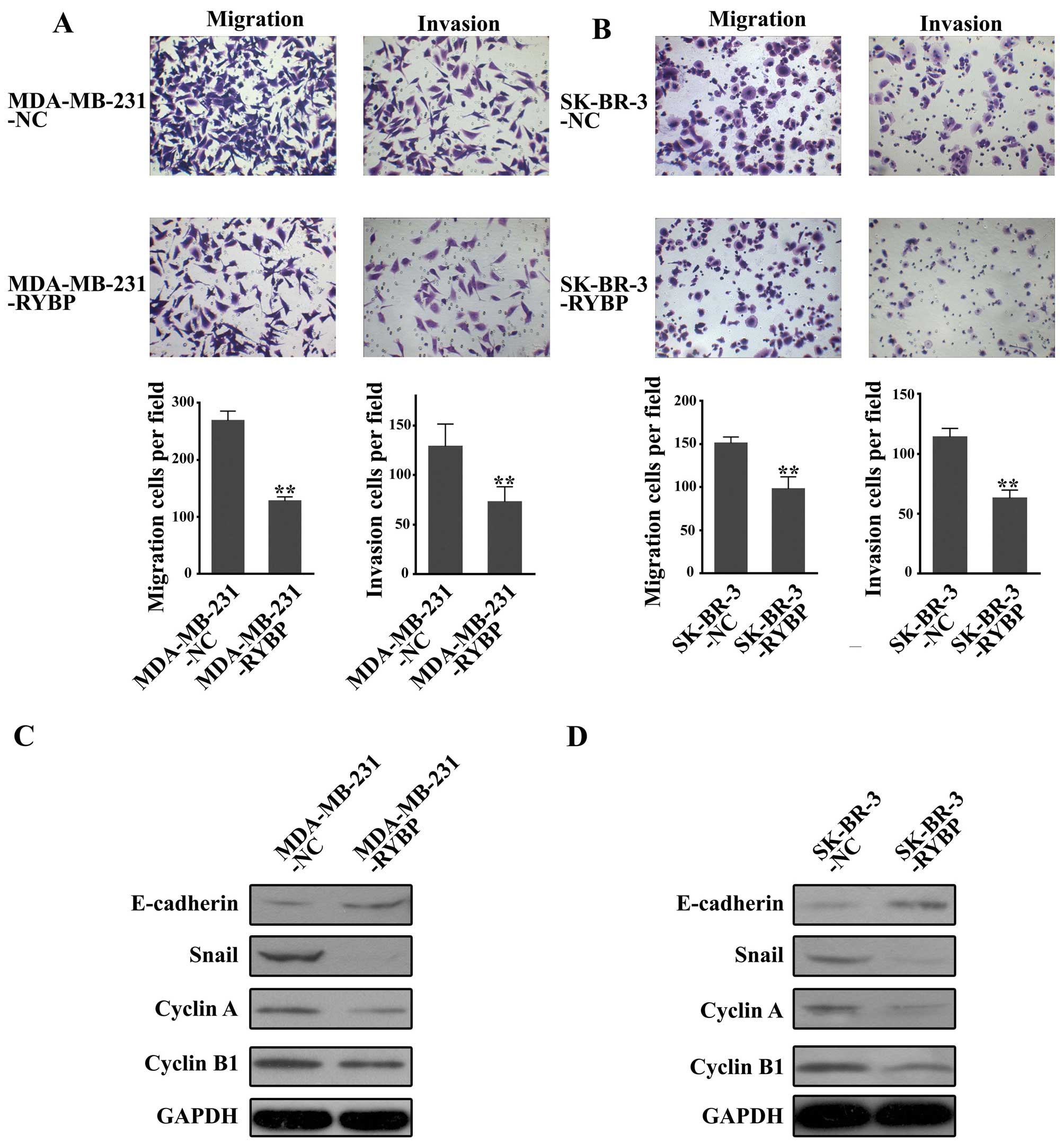
Transwell assays were performed to evaluate the
effects of RYBP overexpression on cell migration and invasive
ability in breast cancer cells. As shown in Fig. 3A and B, the number of migrated
cells and invasive cells in MDA-MB-231-RYBP was significantly lower
than that in MDA-MB-231-NC. Similar results were observed in
SK-BR-3-RYBP cells.
Western blot analysis of proliferation-related
proteins (cyclin A and cyclin B1) and EMT-related markers
(E-cadherin and snail) revealed that E-cadherin was upregulated
while snail, cyclin A and cyclin B1 were down-regulated in
MDA-MB-231-RYBP and SK-BR-3-RYBP cells (Fig. 3C and D).
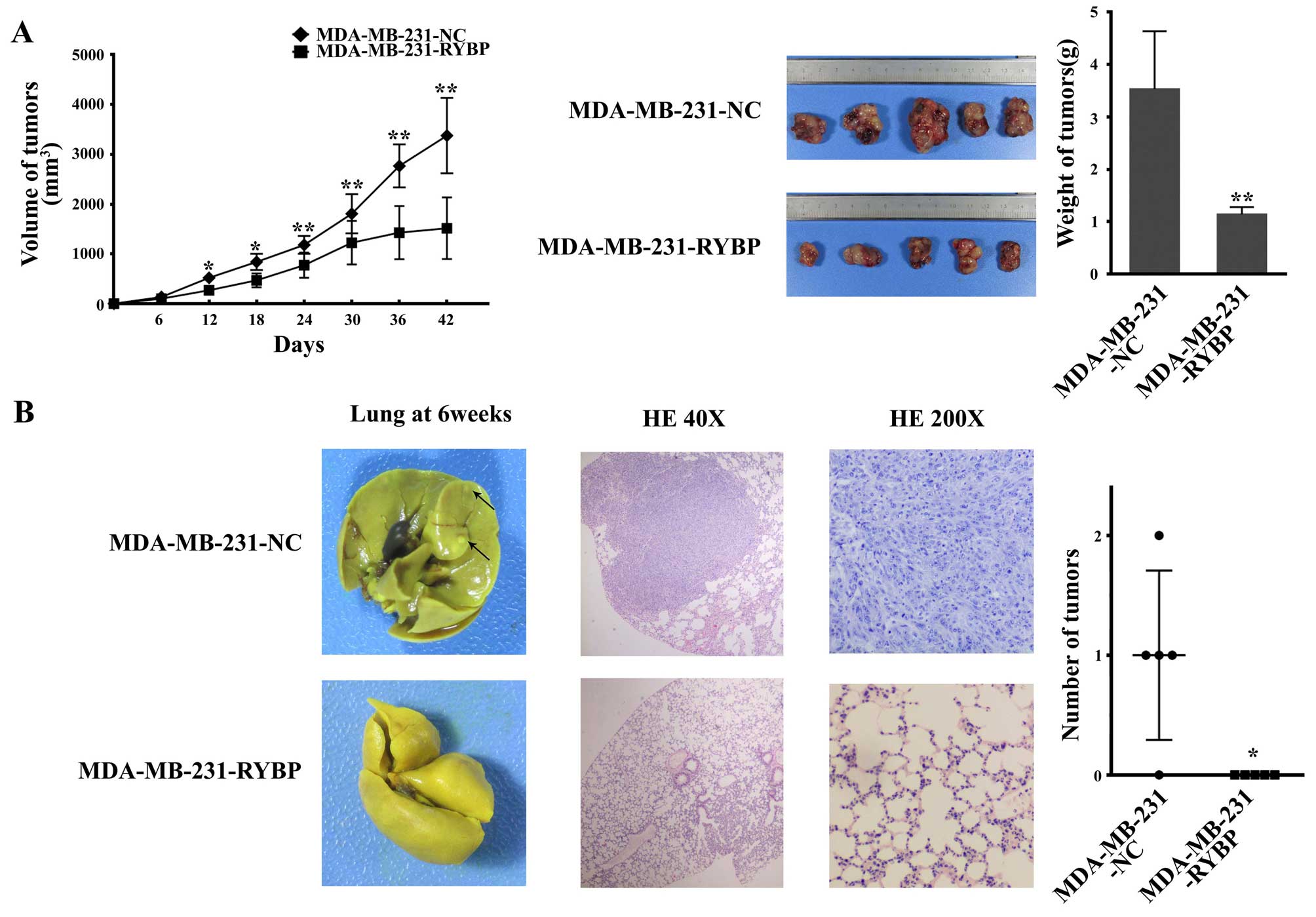
Overexpression of RYBP suppresses cell
growth and metastasis of MDA-MB-231 and SK-BR-3 cells in nude
mice
Cell growth and metastasis were further analyzed in
nude mice. MDA-MB-231-RYBP and MDA-MB-231-NC cells were xenografted
subcutaneously into nude mice. Twelve days after subcutaneous
implantation, the nude mice bearing MDA-MB-231-RYBP tumors showed a
significantly lower tumor volume compared with the mice with
MDA-MB-231-NC cell implantation. A smaller tumor size and lower
tumor weight were also observed in the nude mice with subcutaneous
MDA-MB-231-RYBP implantation after 42 days (Fig. 4A).
To assess the effect of RYBP on tumor metastasis
in vivo, MDA-MB-231-RYBP cells and negative control cells
(MDA-MB-231-NC) were injected into the mammary fat pads of mice to
mimic the metastatic process in breast cancer patients. Six weeks
after cell implantation, the cells metastasized to the lungs.
Metastatic foci were observed in the lungs of MDA-MB-231-NC-treated
nude mice. However, barely any metastatic foci were observed in the
lungs of MDA-MB-231-RYBP-treated nude mice. H&E staining
revealed a mass of tumor cells in the lungs of MDA-MB-231-NC mice,
whereas reduced metastases were observed in the lungs of
MDA-MB-231-RYBP-treated nude mice (Fig. 4B).
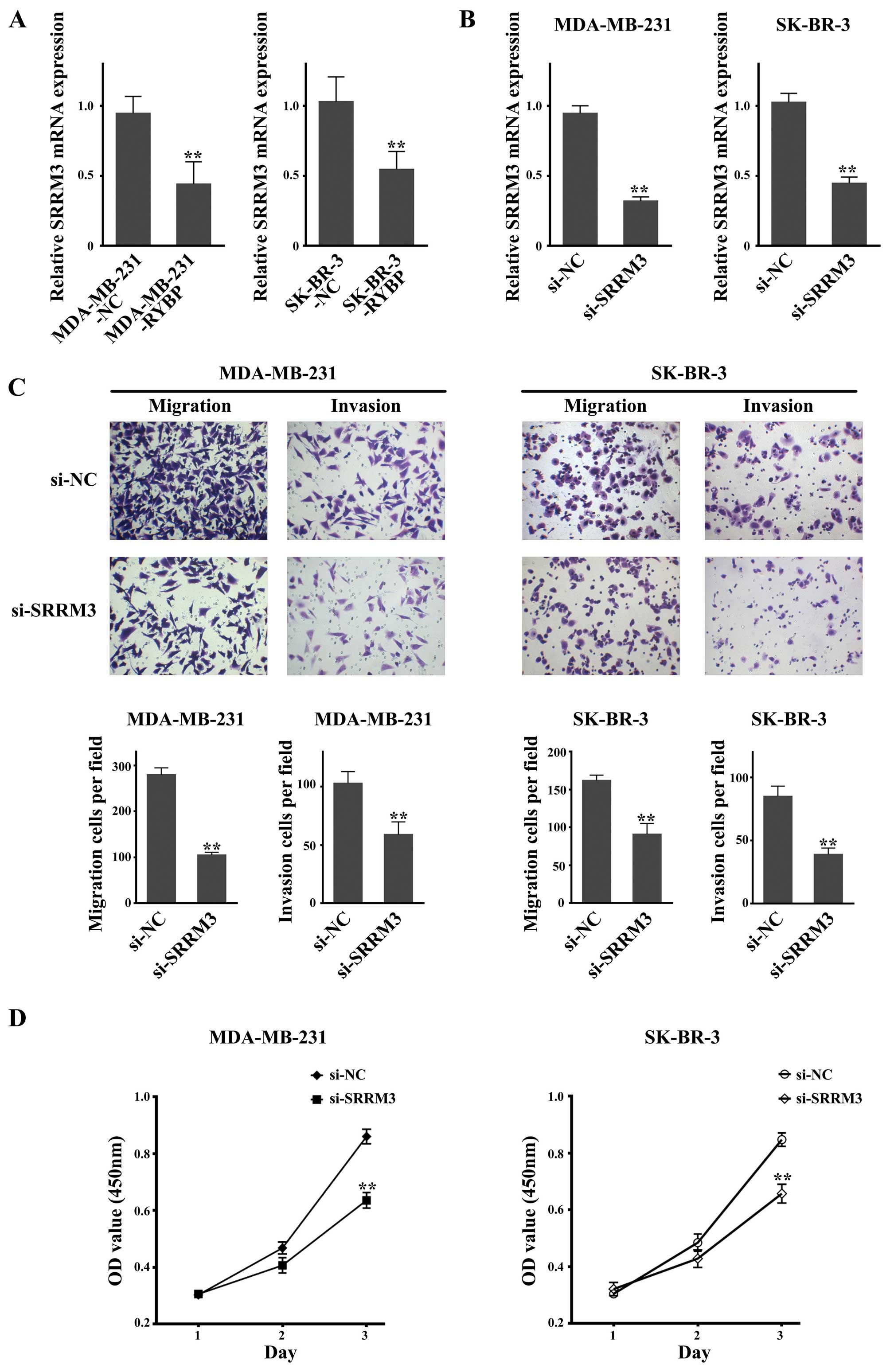
Knockdown of SRRM3, which is
downregulated after RYBP overexpression, suppresses cell growth and
metastasis in MDA-MB-231 and SK-BR-3 cells
SRRM3 has been reported to suppress MDA-MB-231
Matrigel invasiveness (13), and
therefore its role was investigated in the present study. As shown
in Fig. 5A, the qPCR results
showed that SRRM3 was downregulated in both
RYBP-overexpressing cell lines (MDA-MB-231-RYBP and SK-BR-3-RYBP).
The Transwell assay showed that knockdown of SRRM3 mRNA
expression by siRNA in MDA-MB-231 and SK-BR-3 cells resulted in a
significant reduction of the quantity of migrated and invasive
(Fig. 5B and C). Cell growth
inhibition was observed using the MTS assay. The OD values were
significantly lower in the si-SRRM3-treated MDA-MB-231 and
SK-BR-3 cells compared with the si-NC-treated cells (Fig. 5D).
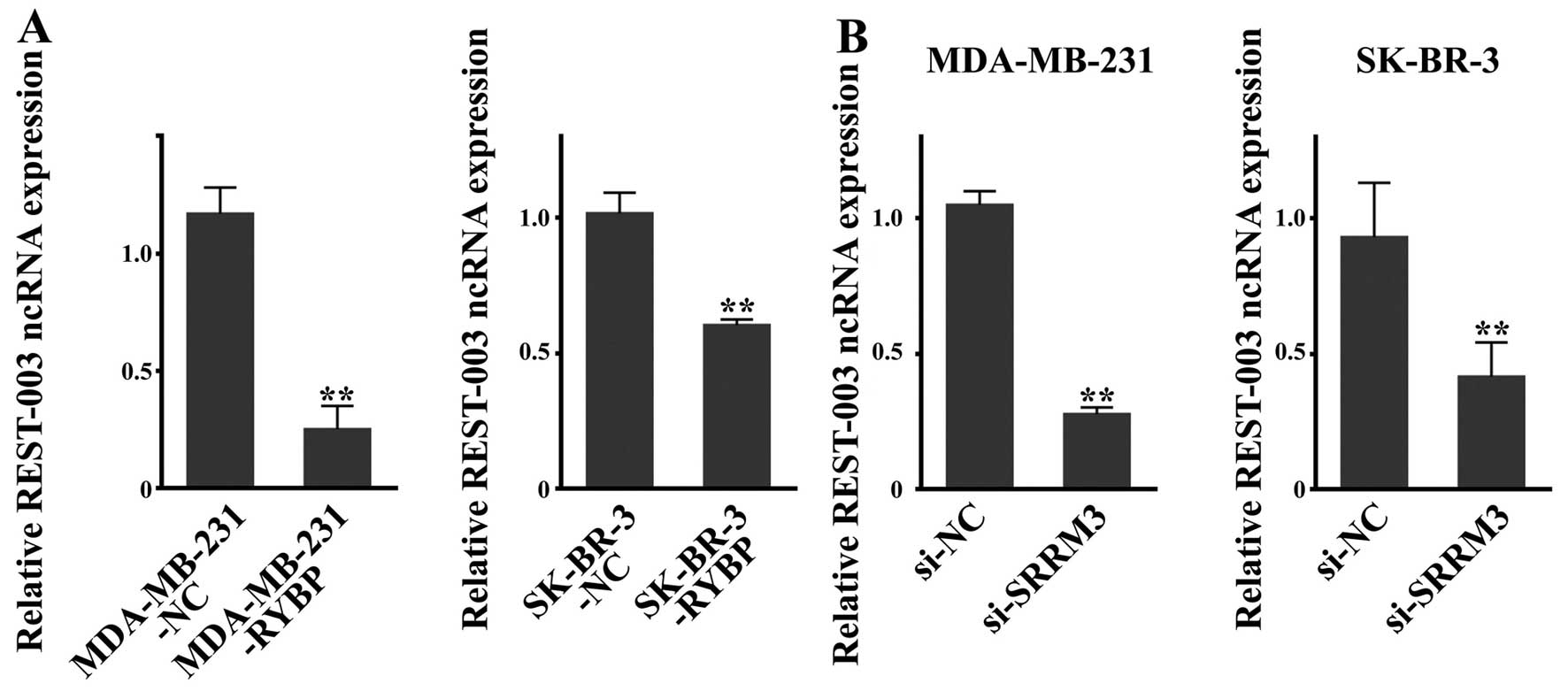
REST-003 ncRNA levels are downregulated
in RYBP-overexpressing and SRRM3-inhibited cells
REST-003 ncRNA is thought to be regulated by SRRM3
(13). Determination of REST-003
ncRNA expression by qRT-PCR revealed that REST-003 ncRNA was
downregulated in MDA-MB-231-RYBP and SK-BR-3-RYBP cells (Fig. 6A). Because SRRM3
downregulation was also observed after RYBP overexpression, as
mentioned above, the expression of REST-003 ncRNA was subjected to
further analysis in si-SRRM3 cells. As shown in Fig. 6B, REST-003 ncRNA was significantly
reduced in the si-SRRM3 cells of MDA-MB-231 and SK-BR-3
cells when compared with the negative control si-NC cells.
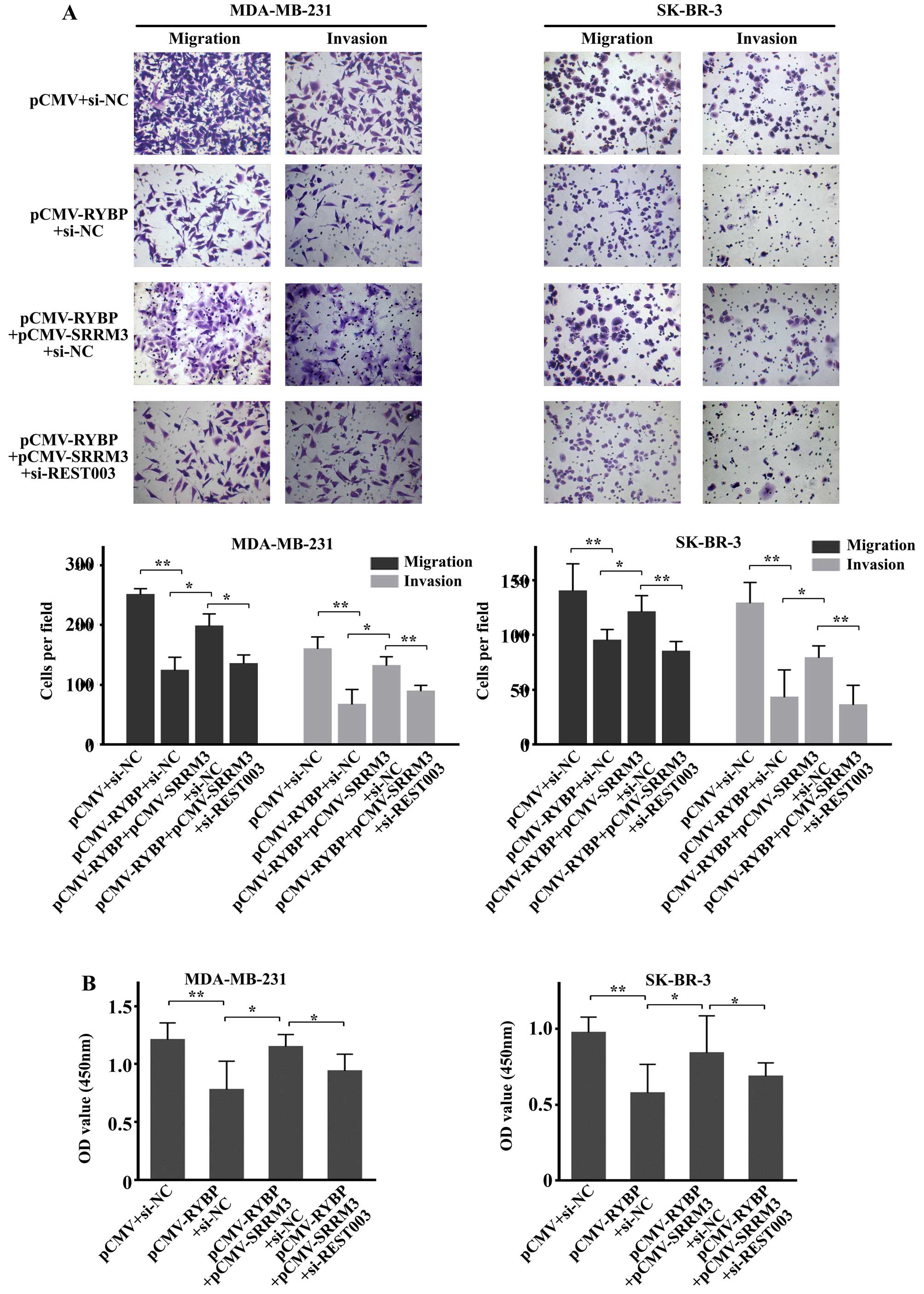
RYBP overexpression suppresses cell
migration, invasion and growth by downregulating
SRRM3/REST-003
To further explore the molecular mechanisms of RYBP,
SRRM3 and REST-003 in breast cancer, we constructed an RYBP and
SRRM3-overexpressing plasmid (PCMV-RYBP and PCMV-SRRM3). We also
downregulated REST003 expression in the cells that were transfected
with PCMV-RYBP and PCMV-SRRM3. The results revealed that
overexpression of RYBP suppressed the migration and invasion of
tumor cells (MDA-MB-231 and SK-BR-3), and increased tumor cell
migration and invasion was observed in the
PCMV-RYBP+PCMV-SRRM3+siNC group compared with the PCMV-RYBP+siNC
group (Fig. 7A). Furthermore, cell
migration and invasion were suppressed in the
pCMV-RYBP+pCMV-SRRM3+siREST003 group compared with the
pCMV-RYBP+pCMV-SRRM3+siNC group (Fig.
7A). The MTT assay showed similar results. A higher OD value
was obtained for MDA-MB-231 and SK-BR-3 cells in the
PCMV-RYBP+PCMV-SRRM3+siNC group compared with the pCMV-RYBP+siNC
group, and a lower value was determined for the
pCMV-RYBP+pCMV-SRRM3+siREST003 group (Fig. 7B). These results demonstrated that
RYBP could influence the migration and growth of breast cancer
cells by downregulating SRRM3/REST003. This molecular mechanism
plays an important role in the function of breast cancer cells.
Discussion
RYBP belongs to the PcG family of proteins and is
considered to function as a transcriptional suppressor via
epigenetic modification of chromatin (18). Downregulated expression of RYBP has
been reported in HCC cell lines and clinical samples. In the
present study, we enhanced our knowledge of the expression of RYBP
in breast cancer and demonstrated that RYBP is downregulated in
breast cancer cell lines (SK-BR-3, ZR-75-1, T47D, MDA-MB-231 and
MCF-7), as well as in clinical breast cancer samples.
To date, the inhibitory role of RYBP in tumor
progression has only been confirmed in HCC and NSCLC. Low
expression levels of RYBP have been shown to correlate with a poor
prognosis in patients with HCC and NSCLC and are also associated
with shorter survival durations in patients with cervical cancer
who received chemoradiotherapy (8,11,12).
By overexpressing RYBP, we observed suppressed cell proliferation,
migration and invasion in two breast cancer cell lines (MDA-MB-231
and SK-BR3). Our results for the nude mouse xenograft tumor
experiment as well as our assessment of metastatic tumor foci,
showed that RYBP suppressed MDA-MB-231 cell growth and metastasis
in vivo. Survival analysis of breast cancer patients with
low or high RYBP expression provided additional support for the
contention that high RYBP expression correlates with longer 5-year
survival rates in breast cancer patients. Taken together, these
animal model and survival studies suggest that increased RYBP
expression plays a demonstrable role in the repression of tumor
growth and metastasis in breast cancer.
Analysis of the cell cycle distribution by flow
cytometry revealed that cells were arrested at the S phase in the
RYBP-overexpressing groups, which suggests that S-phase arrest
contributes to the observed cell growth inhibition by RYBP. Cyclin
A levels normally peak in G2/M-phase of the cell cycle. Cyclin B1
has been reported to be involved in regulating the initiation of
mitosis, and increased expression of cyclin B1 has been suggested
to be a negative prognostic marker of proliferation in breast
cancer (19–21). Downregulation of cyclin A and
cyclin B1 expression was confirmed by western blot analysis, and
these results suggested that RYBP disrupted the cell cycle and
inhibited proliferation.
Epithelial-mesenchymal transition (EMT) has been
found to regulate breast cancer cell metastasis. EMT converts
epithelial cells into migratory cells that are able to traverse the
extracellular matrix. This process results in a loss of epithelial
cell traits and a gain of the properties of mesenchymal cells
(22), leading to the
establishment of distant metastases (23,24).
E-cadherin and Snail are important markers of the EMT process.
Research has showed that a central event in EMT is the
downregulation of E-cadherin expression, which leads to a loss of
cell-to-cell contact (25).
Additionally, the transcription factor Snail has been reported to
be a major suppressor of E-cadherin in many tumor types. Increased
Snail expression leads to more aggressive tumor features, which
enhances tumor cell migration and invasion (26,27).
Our evaluation of E-cadherin and snail1 revealed upregulated
E-cadherin expression and reduced activation of snail1 in
RYBP-overexpressing breast cancer cells, suggesting a potential
role for RYBP in reducing invasive and migrating phenotypes in
breast cancer cells. Thus, E-cadherin and snail1 may be involved in
reversing the EMT process.
The mRNA abundance of SRRM3 was decreased in
response to RYBP overexpression. Reduced cell growth and migration
as well as invasive ability were also observed in
RYBP-overexpressing MDA-MB-231 and SK-BR-3 cells (13), and our results confirmed the
ability of SRRM3 to promote cell invasion and cell migration in
breast cancer. Moreover, because SRRM3 was downregulated in
RYBP-overexpressing cells, we constructed an SRRM3-overexpressing
plasmid (pCMV-SRRM3) and transfected it into the
RYBP-overexpressing cells lines. The results revealed increased
migration and growth of cancer cells, suggesting that RYBP may
regulate SRRM3 and thus inhibit breast cancer cell metastasis.
An alternatively spliced RE1-silencing transcription
(REST) factor, REST-003, which is regulated by SRRM3, has been
shown to be positively associated with breast cancer cell invasion
(13). We found a reduced mRNA
abundance of REST-003 in response to RYBP overexpression.
Downregulation of SRRM3 resulted in a decrease in REST-003 ncRNA,
consistent with the results of a previous study (13). We also observed suppressed
migration and growth in response to REST-003 downregulation in
cells transfected with pCMV-RYBP and pCMV-SRRM3 compared with the
pCMV-RYBP+pCMV-SRRM+siREST003 group. Thus, cell migration and
growth were promoted following the upregulation of REST-003 by
SRRM3 overexpression, and this process could be suppressed by the
downregulation of REST-003. These results suggest that REST-003
ncRNA is mediated by SRRM3, and that SRRM3 is positioned downstream
of RYBP.
In conclusion, in the present study, we focused on
the role of RYBP in breast cancer. In addition to the findings
related to HCC and NSCLC showing that RYBP inhibits tumor
progression, this study provides novel evidence that RYBP inhibits
tumor growth and metastasis in breast cancer. Furthermore, our
results suggest that SRRM3 and REST-003 lie downstream of RYBP. In
future research, we will continue to explore the precise molecular
mechanisms of RYBP, SRRM3, REST-003, and other potential molecules
that may be involved in this process. We would also like to explore
the epigenetic changes induced by overexpression of RYBP in breast
cancer cells. Our findings support the notion that RYBP may offer a
potential therapeutic target in breast cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (81372821).
Abbreviations:
|
RYBP
|
RING1 and YY1 binding protein
|
|
PcG
|
polycomb
|
|
NSCLC
|
non-small cell lung cancer
|
|
HCC
|
hepatocellular carcinoma
|
|
DFS
|
disease-free survival
|
|
TCGA
|
The Cancer Genome Atlas
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SRRM3
|
serine/arginine repeat-related
protein
|
|
REST
|
RE1-silencing transcription
|
References
|
1
|
Stewart BW and Kleihues P: World cancer
report. World Cancer Rep. 45:12–351. 2003.
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siddiqui JA, Singh A, Chagtoo M, Singh N,
Godbole MM and Chakravarti B: Phytochemicals for breast cancer
therapy: Current status and future implications. Curr Cancer Drug
Targets. 15:116–135. 2015. View Article : Google Scholar
|
|
4
|
García E, Marcos-Gutiérrez C, del Mar
Lorente M, Moreno JC and Vidal M: RYBP, a new repressor protein
that interacts with components of the mammalian Polycomb complex,
and with the transcription factor YY1. EMBO J. 18:3404–3418. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang W, Qin JJ, Voruganti S, Nag S, Zhou J
and Zhang R; Polycomb Group. Polycomb Group (PcG) proteins and
human cancers: Multifaceted functions and therapeutic implications.
Med Res Rev. 35:1220–1267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen D, Zhang J, Li M, Rayburn ER, Wang H
and Zhang R: RYBP stabilizes p53 by modulating MDM2. EMBO Rep.
10:166–172. 2009. View Article : Google Scholar :
|
|
7
|
Sánchez-Beato M, Sánchez E,
González-Carreró J, Morente M, Díez A, Sánchez-Verde L, Martín MC,
Cigudosa JC, Vidal M and Piris MA: Variability in the expression of
polycomb proteins in different normal and tumoral tissues. A pilot
study using tissue microarrays. Mod Pathol. 19:684–694. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Cheng J, Qin JJ, Voruganti S, Nag
S, Fan J, Gao Q and Zhang R: RYBP expression is associated with
better survival of patients with hepatocellular carcinoma (HCC) and
responsiveness to chemotherapy of HCC cells in vitro and in vivo.
Oncotarget. 5:11604–11619. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Novak RL and Phillips AC:
Adenoviral-mediated Rybp expression promotes tumor cell-specific
apoptosis. Cancer Gene Ther. 15:713–722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taylor BS, Schultz N, Hieronymus H,
Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva
B, et al: Integrative genomic profiling of human prostate cancer.
Cancer Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lando M, Holden M, Bergersen LC, Svendsrud
DH, Stokke T, Sundfør K, Glad IK, Kristensen GB and Lyng H: Gene
dosage, expression, and ontology analysis identifies driver genes
in the carcinogenesis and chemoradioresistance of cervical cancer.
PLoS Genet. 5:e10007192009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voruganti S, Xu F, Qin JJ, Guo Y, Sarkar
S, Gao M, Zheng Z, Wang MH, Zhou J, Qian B, et al: RYBP predicts
survival of patients with non-small cell lung cancer and regulates
tumor cell growth and the response to chemotherapy. Cancer Lett.
369:386–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee NS, Evgrafov OV, Souaiaia T, Bonyad A,
Herstein J, Lee JY, Kim J, Ning Y, Sixto M, Weitz AC, et al:
Non-coding RNAs derived from an alternatively spliced REST
transcript (REST-003) regulate breast cancer invasiveness. Sci Rep.
5:112072015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Franken NA, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar
|
|
15
|
Kramer N, Walzl A, Unger C, Rosner M,
Krupitza G, Hengstschläger M and Dolznig H: In vitro cell migration
and invasion assays. Mutat Res. 752:10–24. 2013. View Article : Google Scholar
|
|
16
|
Lee YJ, Lin WL, Chen NF, Chuang SK and
Tseng TH: Demethylwedelolactone derivatives inhibit invasive growth
in vitro and lung metastasis of MDA-MB-231 breast cancer cells in
nude mice. Eur J Med Chem. 56:361–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suetsugu A, Honma K, Saji S, Moriwaki H,
Ochiya T and Hoffman RM: Imaging exosome transfer from breast
cancer cells to stroma at metastatic sites in orthotopic nude-mouse
models. Adv Drug Deliv Rev. 65:383–390. 2013. View Article : Google Scholar
|
|
18
|
Sparmann A and van Lohuizen M: Polycomb
silencers control cell fate, development and cancer. Nat Rev
Cancer. 6:846–856. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giacinti C and Giordano A and Giordano A:
RB and cell cycle progression. Oncogene. 25:5220–5227. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koliadi A, Nilsson C, Holmqvist M,
Holmberg L, de La Torre M, Wärnberg F and Fjällskog ML: Cyclin B is
an immunohistochemical proliferation marker which can predict for
breast cancer death in low-risk node negative breast cancer. Acta
Oncol. 49:816–820. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niméus-Malmström E, Koliadi A, Ahlin C,
Holmqvist M, Holmberg L, Amini RM, Jirström K, Wärnberg F,
Blomqvist C, Fernö M, et al: Cyclin B1 is a prognostic
proliferation marker with a high reproducibility in a
population-based lymph node negative breast cancer cohort. Int J
Cancer. 127:961–967. 2010.
|
|
22
|
Foroni C, Broggini M, Generali D and Damia
G: Epithelial-mesenchymal transition and breast cancer: Role,
molecular mechanisms and clinical impact. Cancer Treat Rev.
38:689–697. 2012. View Article : Google Scholar
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Palena C, Roselli M, Litzinger MT, Ferroni
P, Costarelli L, Spila A, Cavaliere F, Huang B, Fernando RI,
Hamilton DH, et al: Overexpression of the EMT driver brachyury in
breast carcinomas: Association with poor prognosis. J Natl Cancer
Inst. 106:1062014. View Article : Google Scholar
|
|
25
|
Perl AK, Wilgenbus P, Dahl U, Semb H and
Christofori G: A causal role for E-cadherin in the transition from
adenoma to carcinoma. Nature. 392:190–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiao W, Miyazaki K and Kitajima Y: Inverse
correlation between E-cadherin and Snail expression in
hepatocellular carcinoma cell lines in vitro and in vivo. Br J
Cancer. 86:98–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rosivatz E, Becker KF, Kremmer E, Schott
C, Blechschmidt K, Höfler H and Sarbia M: Expression and nuclear
localization of Snail, an E-cadherin repressor, in adenocarcinomas
of the upper gastrointestinal tract. Virchows Arch. 448:277–287.
2006. View Article : Google Scholar
|