Introduction
Breast carcinoma is the predominant cancer in women
worldwide (1). Despite advances in
treatment, metastasis, tumor recurrence and drug resistance are
currently the main challenges in breast cancer management (2), with metastasis occurring in almost
50% of the patients post-therapy (3).
miRNAs are 18–25 nucleotide non-coding RNAs that
control gene expression by degradation of mRNA or inhibiting the
translation of transcribed RNA into proteins (4,5).
Aberrant miRNA expression has been found to underlie several
cancers, including breast cancer (reviewed in ref. 6). miRNAs are known to regulate breast
cancer progression, by acting as promoters or inhibitors of
specific processes, including epithelial-mesenchymal transition
(EMT), angiogenesis, stemness of cancer stem cells, invasion,
metastasis and chemoresistance (3,6).
miRNA-93 (miR-93), a member of the pro-oncogenic
miR-106b-25 cluster (comprising miR-106b, miR-93 and miR-25) is
overexpressed in several cancers including breast cancer (7), and belongs to the miR-17 family of
miRNAs based on sequence similarity (8). The expression of miR-93 was shown to
be significantly increased in triple-negative breast cancer (TNBC)
patients when compared to normal tissues or non-TNBC tissues, and
associated with lymph node metastasis, TNM grade and Ki-67 staining
(9), suggesting that miR-93
controls proliferation and metastasis. Furthermore, the expression
of miR-93 was found to be increased in ER- or PR- breast cancer
patients when compared to hormonal receptor-positive breast cancer
patients (10). However, studies
have also revealed that miR-93 has contradictory roles in
inhibiting breast cancer metastasis, by regulating the
proliferation and differentiation of breast cancer stem cells
(11). Nonetheless, the exact
mechanism of miR-93 or its gene targets that mediate breast cancer
metastasis remain largely unknown.
We examined the role of miR-93 in MDA-MB-231 breast
cancer cells, a TNBC cell line. Overexpression of miR-93 decreased
cell migration and invasion, while, inhibition of miR-93 elicited
the opposite effects in MDA-MB-231 cells. WNK lysine deficient
protein kinase 1 (WNK1), one of the targets of miR-93
identified by TargetScan prediction, was verified as a putative
target by the luciferase assay. Furthermore, we show that
siRNA-mediated silencing of WNK1, resulted in decreased
invasive ability of these cells, suggesting that miR-93 mediated
changes in cell invasion was possibly via WNK1. Taken together, our
results unravel a novel relationship between miR-93, WNK1 and
metastasis that have potential implications in breast
carcinogenesis.
Materials and methods
Cell culture
MDA-MB-231 cells (HTB-26) were purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA) and were
grown in RPMI medium containing 10% fetal bovine serum (FBS).
Transfection of siRNA
MDA-MB-231 cells were seeded at a density of
2.5×105 or 3×104 cells/well in 6-well plates
or 24-well plates, respectively. Cells were subsequently
transfected with ON-TARGETplus SMARTpool siRNA targeting WNK1 and
non-targeting siRNA using DharmaFECT (GE Dharmacon. Pittsburgh, PA,
USA). The siRNA complexes were prepared in serum-free RPMI and made
up to a final concentration of 20 nM. The medium was replaced 24 h
post-transfection, and the transfected cells were cultured for 48
or 72 h as indicated.
Transfection of miRNA
Cells seeded at the same density as above, were
reverse transfected with hsa-miR-93-5p mimic/negative control
(Ambion, Austin, TX, USA) or LNA-hsa-miR-93-5p/scramble (Exiqon,
Vedbaek, Denmark) and Lipofectamine RNAiMAX (mimics) or
Lipofectamine 2000 (inhibitor). The miRNA complexes were prepared
in Opti-MEM and made up to a final concentration of 30 nM. The
medium was replaced with RPMI containing 10% FBS 6 h
post-transfection, and grown for 48 or 72 h as indicated.
RNA isolation and cDNA conversion
Total RNA 48 h post-transfection was isolated using
either RNAeasy kit or miRNAeasy kit (which included miRNA) (Qiagen,
Hilden, Germany). The isolated RNA was quantified on a
spectrophotometer (NanoDrop) and converted into cDNA. Briefly, 1 μg
of RNA was converted into cDNA using the SuperScript III
first-strand synthesis system (Invitrogen, Carlsbad, CA, USA) for
gene expression analysis. For analyzing miRNA expression, 20 ng of
RNA was converted to cDNA using the Universal cDNA synthesis kit
(Exiqon).
qPCR
The gene primers used in this study are summarized
in Table I. Gene expression levels
were quantified by real-time RT-PCR using the Fast SYBR-Green
Master Mix (Applied Biosystems, Foster City, CA, USA) in 96-well
MicroAmp Fast Optical plates (Applied Biosystems) on a 7900HT Fast
real-time PCR system (Applied Biosystems). Relative gene expression
was determined by the 2−ΔΔCt method using GAPDH
as the control (12).
 | Table IThe primers used for qPCR. |
Table I
The primers used for qPCR.
| Gene | Forward primer | Reverse primer |
|---|
| GAPDH |
GAAGGTGAAGGTCGGAGTCAACG |
TGCCATGGGTGGAATCATATTGG |
| JAK1 |
ACGAGTGTCTAGGGATGGCT |
CGCATCCTGGTGAGAAGGTT |
| STAT3 |
CTGTGGGAAGAATCACGCCT |
ACATCCTGAAGGTGCTGCTC |
| EZH1 |
TTCCTGCTCCAATGCCTCAG |
GTGCTTCCACTACGCAGAGT |
| HMGA2 |
CAGGAAGCAGCAGCAAGAAC |
AGGCAACATTGACCTGAGCA |
| TGFBR2 |
CTCATGGAGTTCAGCGAGCA |
GCAGCTCTGTGTTGTGGTTG |
| CDH1 |
ACAGCACGTACACAGCCCTA |
GCAGAAGTGTCCCTGTTCCAG |
| CLDN1 |
AAGACGATGAGGTGCAGAAGA |
ATTCGTACCTGGCATTGACTG |
| CLDN3 |
CAACACCATTATCCGGGACT |
CAACACCATTATCCGGGACT |
miRNA expression was quantified by real-time RT-PCR
with miRNA primer hsa-miR-93-5p or control primer U6 (Exiqon) and
ExiLENT™ SYBR-Green Master Mix (Exiqon). The rest of the procedure
was as described above.
Growth curve analysis using Alamar
blue
Growth of transfected cells was monitored using the
Alamar blue assay. Briefly, addition of the Alamar blue reagent
(Invitrogen) to culture medium at a ratio of 1:10 was carried out
24 h post-transfection. Cells were then incubated for 3 h at 37°C
and 5% CO2. Fluorescence intensity was then measured at
570 nm (excitation) and 585 nm (emission) wavelengths on a
microplate reader (SpectraMax; Molecular Devices, Sunnyvale, CA,
USA). Subsequently, cells were replenished with fresh medium and
the assay was repeated at 48 and 72 h.
Migration and invasion assays
Cells were harvested 48 h post-transfection. Cells
(2×104) were then seeded in 200 μl of serum-free RPMI
medium into the upper chamber of hydrated polycarbonate membrane
insets with 8 μm pores (Corning, Corning, NY, USA) for migration
assay (18 h) or into hydrated matrigel invasion chambers (BD
Biosciences, San Jose, CA, USA) for the invasion assay (20 h).
Subsequently, cells were fixed with 100% methanol followed by
staining with crystal violet (0.5% w/v). To determine the number of
cells that had migrated or invaded, images from the center and four
peripheral fields on the membrane were captured using a Nikon
SMZ1500 stereomicroscope at ×10 magnification and counted.
Cell adhesion assay
96-well plates were coated with 20 μg/ml collagen
type 1 (Invitrogen) overnight at 4°C. Subsequently, wells were
blocked with 1% BSA for 1 h after washing in phosphate buffered
saline (PBS). Cells (5×104) (48 h post-transfection)
were then seeded/well (in duplicates) in 100 μl of RPMI with 10%
FBS, and allowed to adhere for 40 min at 37°C with 5%
CO2. Following incubation, non-adherent cells in only
one replicate were removed by washing with PBS. MTS reagent was
then mixed with serum-free RPMI at a ratio of 1:5, and the wells
were replaced with this MTS reagent mixture and incubated for a
further 2 h. Following incubation, the OD (absorbance) was read at
590 nm and the percentage of adherent cells was calculated by the
formula: [OD of washed well/OD of non-washed well] × 100.
TargetScan prediction
miR-93 gene targets were predicted by the TargetScan
human database (release 7.1) using default parameters (http://www.targetscan.org).
3′UTR plasmid luciferase assay
Cells (3×104) were plated in 24-well
plates and incubated overnight. The next day, 30 nM of negative
control mimic or miR-93-5p mimic was co-transfected with 0.3 μg of
WNK1 plasmid (GeneCopoeia, Rockville, MD, USA) using Lipofectamine
2000. After 24 h, transfected cells were segregated and re-seeded.
After another 24 h post-incubation, the luciferase assay was
performed using the Luc-Pair duo luciferase assay kit
(GeneCopoeia). The luminescence was read using a spectrophotometer
and firefly luciferase was normalized to Renilla
luciferase.
Western blot analysis
Protein was extracted at 72 h post-transfection
using the RIPA buffer (Pierce, Waltham, MA, USA) and quantified by
the Bradford method (Bio-Rad Laboratories, Hercules, CA, USA). Each
sample containing 30 μg of protein was denatured at 95°C for 5 min
and separated on a 4–20% Mini-Protean TGX precast gel (Bio-Rad
Laboratories). Proteins were transferred onto a PVDF membrane,
which was blocked with 5% non-fat milk for 1 h at room temperature
and washed well, before incubation with rabbit polyclonal anti-WNK1
antibody (1:1000; Abcam, Cambridge, UK) overnight at 4°C. The next
day, secondary anti-rabbit HRP conjugated antibody (Pierce) was
added to the blots and incubated for 1 h at room temperature.
Subsequently, development of the blot was carried out using the
SuperSignal West Pico chemiluminescence susbtrate (Thermo Fisher
Scientific, Waltham, MA, USA) on an automatic film processor. The
bands were quantified on a densitometer (Bio-Rad Laboratories)
using Quantity One software. To ensure that equal amounts of
protein were loaded into each well, the blot was stripped and
re-probed with anti-β-actin antibody (Sigma-Aldrich, St. Louis, MO,
USA) to detect β-actin, the housekeeping protein.
Statistical analysis
The statistical analysis was performed with GraphPad
Prism5 software. Experiments were carried out in triplicates and
repeated at least two independent times. Unpaired, two tailed
Student’s t-test was used for analysis of data except the growth
curves which were analysed by two-way ANOVA. Results are
represented as mean ± SD and were considered significant at
P<0.05.
Results
Overexpression of miR-93 alters cell
migration, invasion and adhesion
Metastasis involves spread of cancer cells from the
primary tumor site to distant organs by invading adjacent tissues
and extravasating into the circulation (13). We evaluated the effect of miR-93
overexpression on migration, invasion and adhesion, since they are
major factors that define the metastatic nature of the cancer
cells. We also assessed the cell proliferation as it is a hallmark
of cancer.
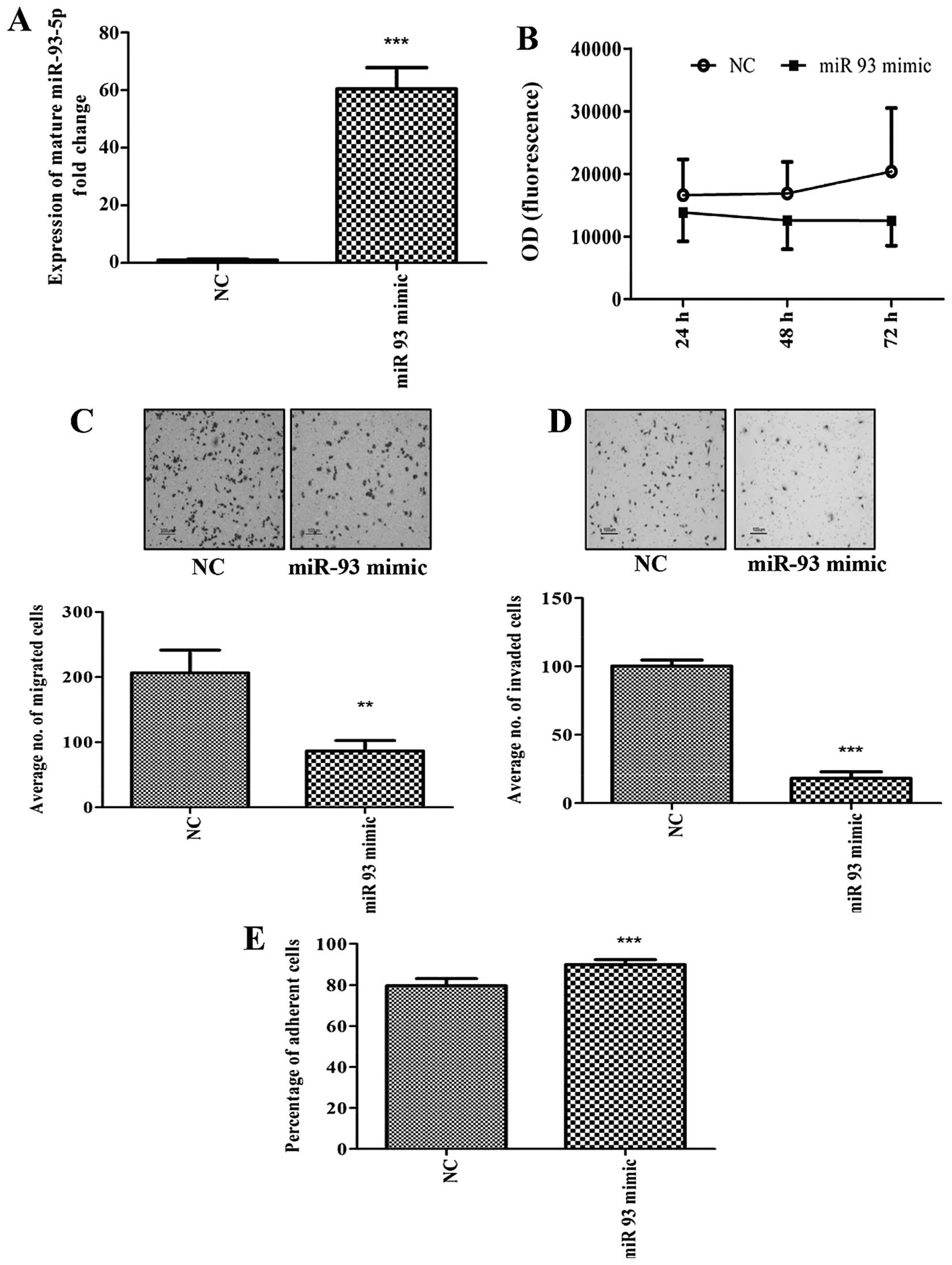
We first overexpressed miR-93 in MDA-MB-231 using
miR-93-5p mimics. The expression of miR-93-5p following
transfection was found to be increased by 60-fold (Fig. 1A). Although overexpression of
miR-93-5p in MDA-MB-231 cells did not alter cell proliferation
(Fig. 1B), decreased cell
migration (Fig. 1C) and cell
invasion (Fig. 1D), with increased
adhesion of cells to collagen type 1 (Fig. 1E) was observed. In addition,
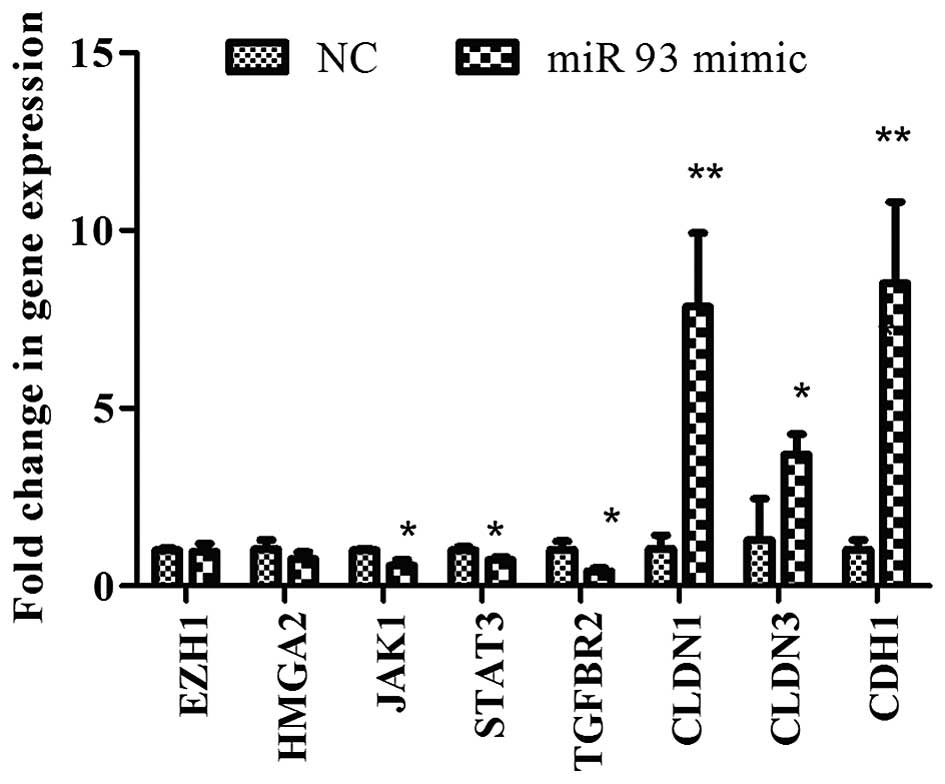
overexpression of miR-93 decreased the expression of stem cell
genes, JAK1 and STAT3 and TGFBR2, but
increased the CLDN1, CLDN3 and CDH1 mRNA
expression (Fig. 2).
miR-93 knockdown enhances cell migration
and invasion
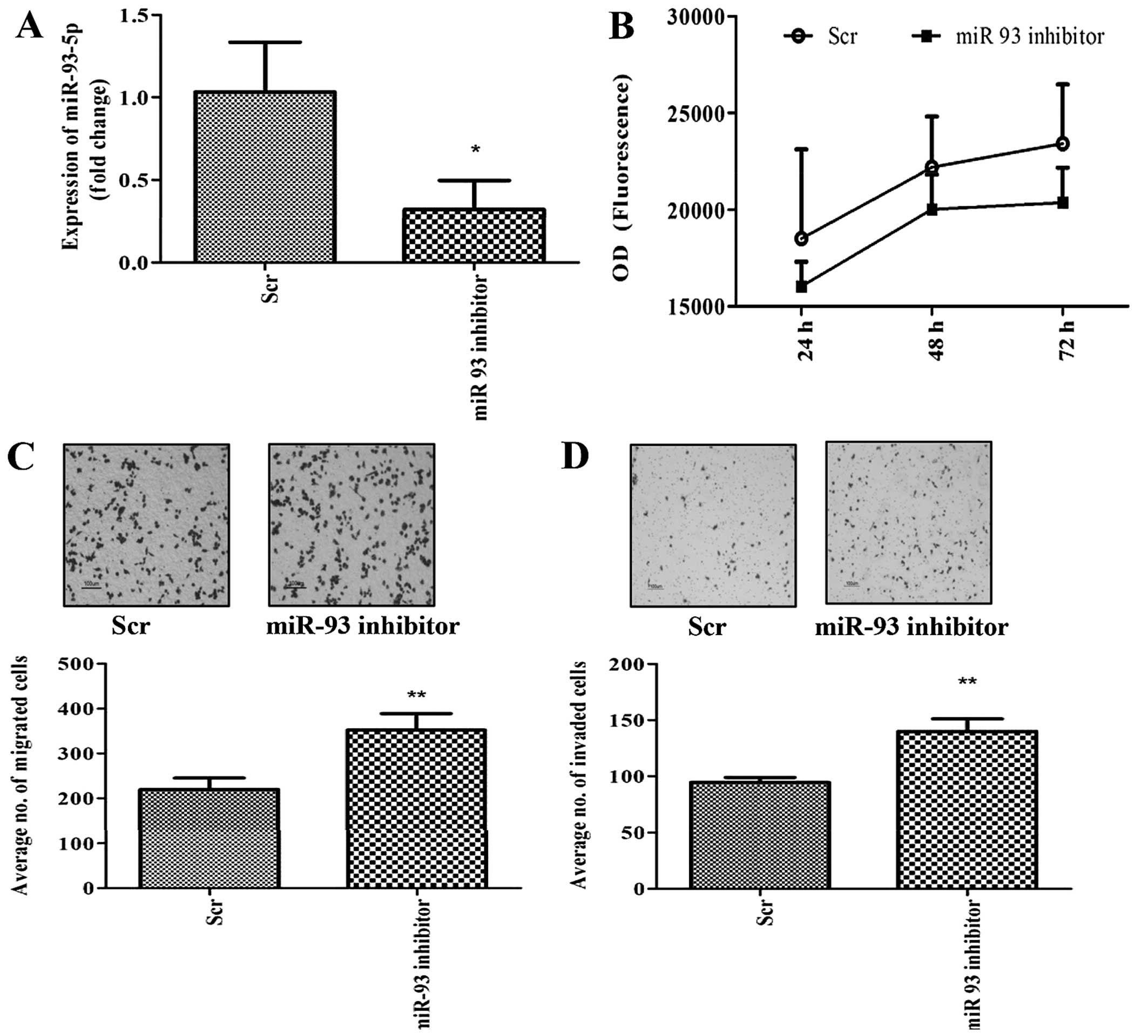
Given that overexpression of miR-93 decreased cell
migration and invasion, we inhibited miR-93-5p in MDA-MB-231 cells
in order to assess whether this effect could be reversed. Knockdown
of miR-93-5p was achieved by transfection of LNA-miR-93-5p
inhibitor into the cells and the knockdown efficiency was estimated
by qPCR to be ~68% (Fig. 3A). As
expected, inhibition of miR-93 had no effect on cell growth
(Fig. 3B), but increased cell
migration (Fig. 3C) and invasion
(Fig. 3D) compared to scrambled
transfected cells (controls).
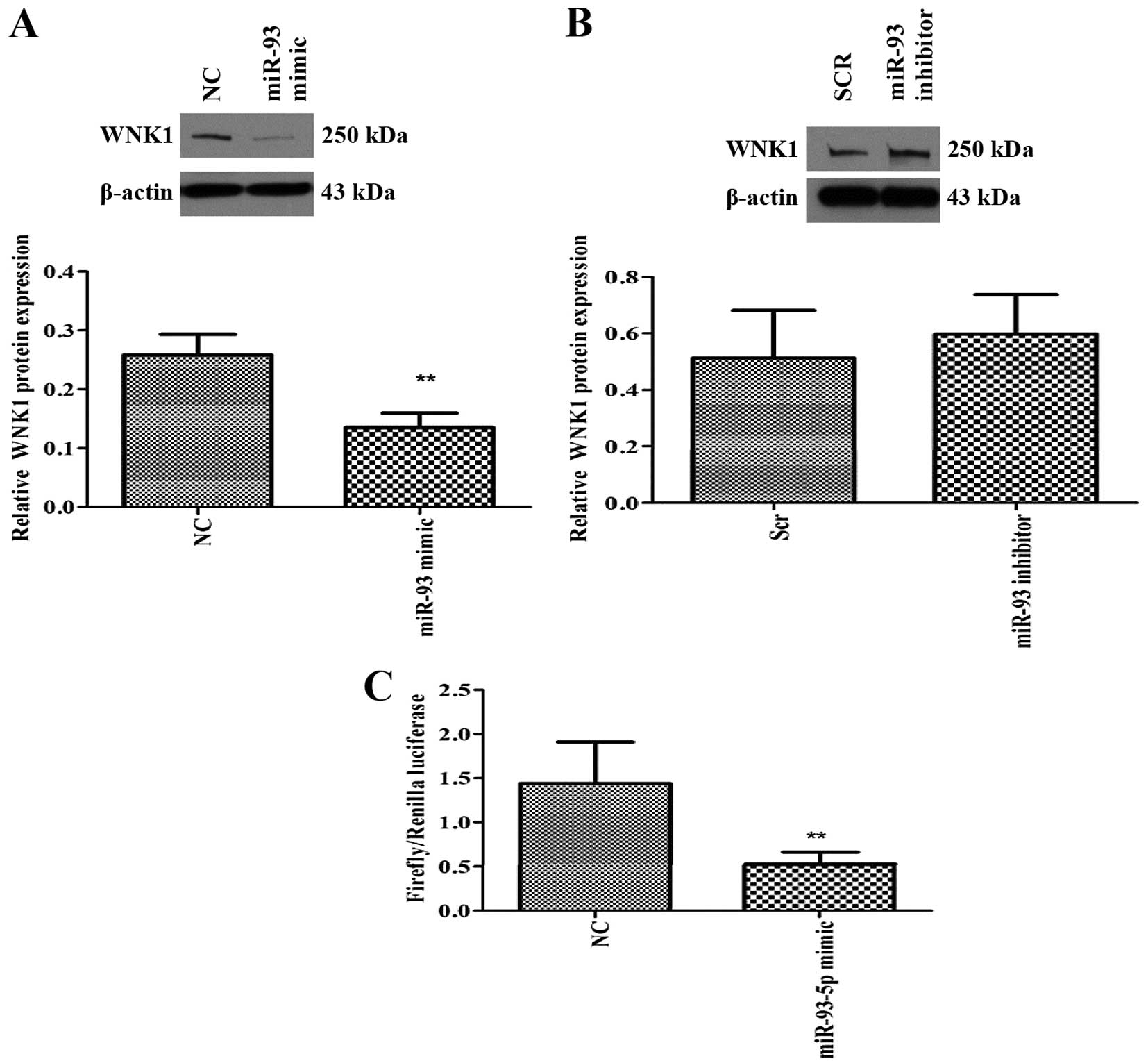
WNK1 is a target of miR-93-5p
miRNA-mRNA target prediction (TargetScan) revealed
that several members of the miR106b~25 and miR-17 family including
miR-93, could target two protein kinases, namely WNK1 and
WNK3. Since WNK1 is ubiquitously expressed (14) compared to WNK3, we examined
the effect of miR-93 on WNK1 expression. Overexpression of
miR-93-5p significantly decreased the expression of WNK1 protein
(Fig. 4A), suggesting that WNK1
may be a direct or indirect target of miR-93. On the contrary,
inhibition of miR-93-5p resulted in a modest increase in WNK1
protein expression (Fig. 4B). In
order to determine if WNK1 is a putative target of miR-93, we
performed 3′UTR luciferase assay, which confirmed that miR-93 binds
directly to the 3′UTR of WNK1 gene and inhibited its
expression (Fig. 4C).
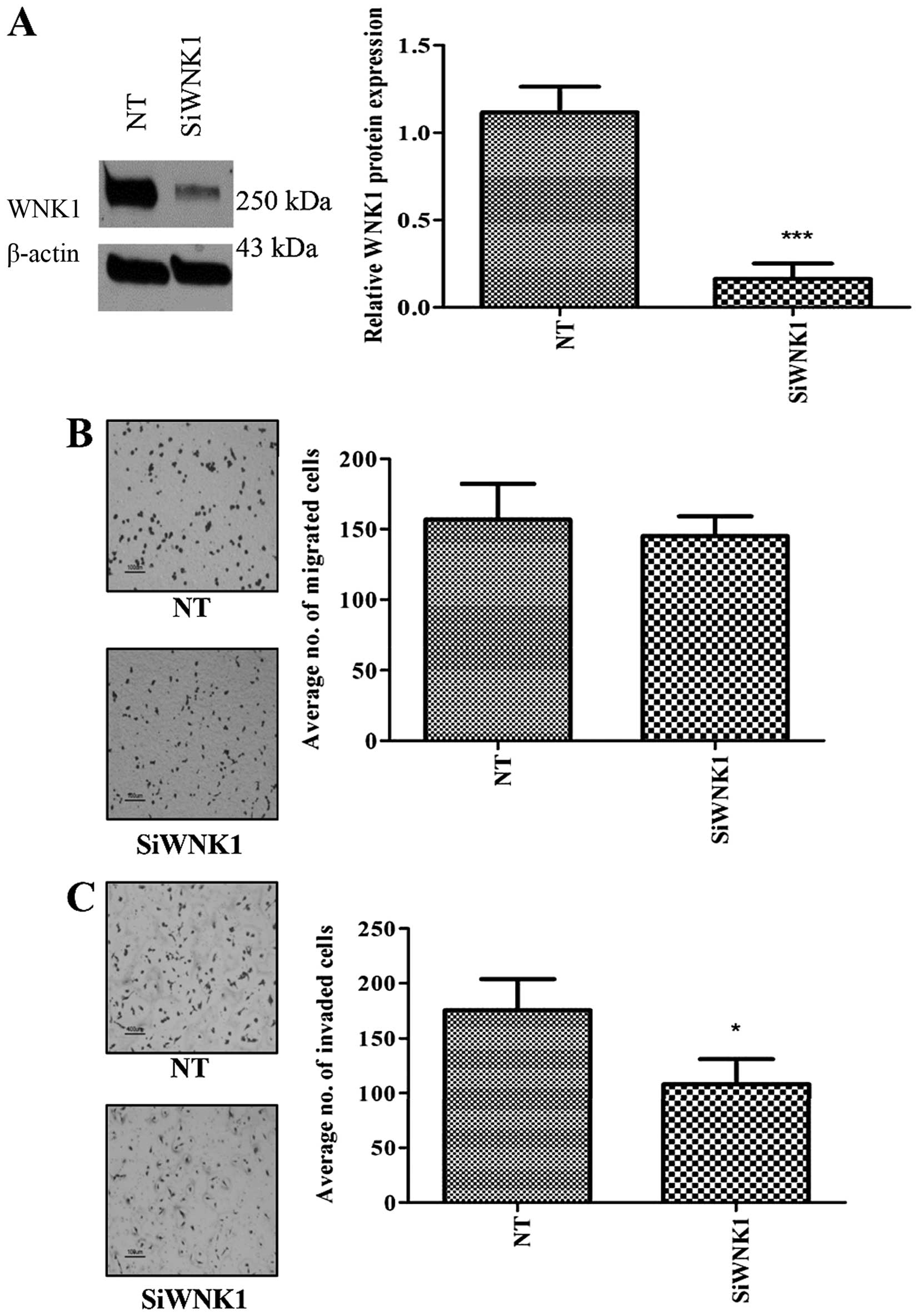
WNK1 knockdown decreased cell migration
and invasion
In order to evaluate the effect of WNK1 depletion in
MDA-MB-231, we inhibited WNK1 expression using siRNA and
performed cell migration and invasion assays. Although
siRNA-mediated silencing of the WNK1 gene induced no
alteration in cell proliferation (Fig.
5A) or migration (Fig. 5B), a
significant reduction in the invasive ability of the cells was
observed (Fig. 5C).
Discussion
miRNAs are known to function as tumor suppressors or
oncogenes that regulate pathogenesis and progression in several
cancers, including breast cancer (15). Thus, miRNAs and/or their targets
may serve as novel anticancer targets for therapeutic intervention.
In the present study, overexpression of miR-93 was observed to
decrease migration, invasion and increase adhesion in parallel,
suggesting that miR-93 functions as a tumor suppressor and a
negative regulator of metastasis in MDA-MB-231 cells. On the other
hand, inhibition of miR-93 in MDA-MB-231 cells increased cell
migratory capability and invasive potential, further supporting the
notion that miR-93 and its gene targets are involved in inhibiting
metastasis. Our results are consistent with Liu et al
(11), who reported that
overexpression of miR-93 inhibited cell invasion while inhibition
of miR-93 promoted invasion in SUM159 cells, another claudin low
TNBC cell line. In addition, the same authors found that induction
of miR-93 in SUM159 cells inhibited metastasis in NOD/SCID mice,
while it promoted tumor growth in MCF-7 cells suggesting that the
role of miR-93 was both cell line- and differentiation state
specific. Nonetheless, the role of miR-93 in MDA-MB-231 breast
cancer cells has not been reported previously.
Induction of EMT has been shown to increase
characteristics of stem/progenitor cells (16) and high miR-93 (and miR-106b)
expression has been found to be associated with stem cell-related
genes (17) and EMT-related genes
(18) in breast cancer, suggesting
that miR-93 and miR-106b regulate these two processes. MDA-MB-231
cells are known to contain a higher percentage of EMT-like
CD44+/CD24− cancer stem cells that are
associated with their increased malignant and metastatic phenotype
(19). However, in the present
study, miR-93 overexpression in MDA-MB-231 cells was associated
with decreased expression of stem cell-related genes (JAK1
and STAT3) and TGFBR2 (TGFβ signaling), and increased
expression of epithelial markers (CDH1, CLDN1 and
CLDN3), suggesting its involvement in the MET process as
reported earlier by Liu et al (11). Furthermore, miR-93 is known to
regulate MET during re-programming of fibroblasts to IPS cells via
downregulation of its target TGFBR2, (20) suggesting that miR-93 may be
critical for MET in various scenarios. Moreover, miR-93 (and miR-17
family) has been observed to regulate differentiation of stem cells
during embryonic development in mice, via downregulating the
expression of STAT3 (21),
suggesting that miR-93 regulates differentiation of stem cells
through STAT3 in different cell types.
In breast cancer, miR-93 has been identified as a
basal sub-type specific miRNA by a meta-analysis involving three
independent studies (6). However,
another recent meta-analysis has also revealed that the miR-17
family of miRNAs that consists of 6 miRNAs (miR-17-5p, miR-20a,
miR-20b, miR-106a, miR-106b and miR-93) inhibit metastasis of
basal-like tumors by repressing genes involved in EMT (22). The authors showed that
overexpression of miR-17-5p suppresses breast cancer metastasis by
inhibiting the expression of pro-metastatic genes. In addition,
several studies have shown that in breast cancer, miR-17-5p is a
tumor suppressor and inhibits proliferation (23), and that miR-17/20 has an
anti-invasive role (24). Since
miR-93 shares the same seed sequence with miR-17, it is possible
that miR-93 inhibits breast cancer metastasis via the same gene
targets.
Published literature suggests that miR-93 can act as
a tumor suppressor or an oncogene depending on the tumor type, and
thus, has contradictory roles in promoting or inhibiting
metastasis. In colon cancer, overexpression of miR-93 has been
shown to suppress the proliferation and colony forming ability of
colon cancer stem cells (25) and
also inhibit growth, migration, invasion and recurrence of
colorectal cancer (26,27), while miR-93 promotes proliferation,
migration and invasion of nasopharyngeal cancer (28). Nevertheless, what remains
consistent are that the gene targets of the miRNAs from the
miR-106b~25 cluster and miR-17 family across several cancers (for
example, E2F1, CCNB1, p21, BIM, TGFBR2 are regulated by miR-93 in
breast, colorectal, gastric and nasopharyngeal cancers among
others) (26,29–31),
suggesting that the miRNA expression is differentially regulated
(inhibited or overexpressed) in cancer, resulting in upregulation
or downregulation of its targets (that are oncogenes or tumor
suppressors respectively), which are critical for cancer
progression.
Humans contain four different WNK genes
(WNK1-4), among which only WNK1 is ubiquitously
expressed in all tissues (14).
Loss of WNK1 during development has been shown to result in
embryonic lethality in mice (32)
and zebrafish embryos (33),
suggesting that WNK1 is critical for development. Among the WNKs,
WNK1 is known to interact with diverse signaling pathways such as
Smad/Tgfb (34), Erk5/MAPK
(35) and PI-3K pathways (36) to regulate cell proliferation,
survival, angiogenesis and metastasis, suggesting that WNK1 has
important roles in tumorigenesis. Studies have shown that WNK1, is
an important protein kinase that is required for mitosis and
abscission (37), migration and
invasion of neural tumor cells via ganglioside GD3 (38), migration of glioma cells (39) and migration, angiogenesis and EMT
in endothelial cells (40).
Furthermore, WNK1 has been shown to regulate Slug, Zeb1 and
β-catenin in endothelial cells (40), with the expression levels of Slug
increasing in the presence of WNK1. Slug has well-known roles in
tumor invasion (41) and thus,
provides an important link between WNK1 and metastasis.
In addition, silencing of WNK1 in a mouse progenitor
cell line inhibited differentiation of the cells into neuronal and
glial lineage, and upregulated the expression of stem
cells/progenitor marker nestin (42). Recently, inhibition of ERK5 or
inhibition of genes that phosphorylate and activate ERK5, such as
MAP3K2 and WNK1 has been shown to reduce tumor growth and
metastasis in prostate cancer in vivo (43). Furthermore, depletion of AKT/WNK1
has been shown to revert EMT and inhibit cell migration in lung
cancer cells (44). Together,
these studies also highlight the importance of WNK1 in the
regulation of differentiation, tumor growth and metastasis.
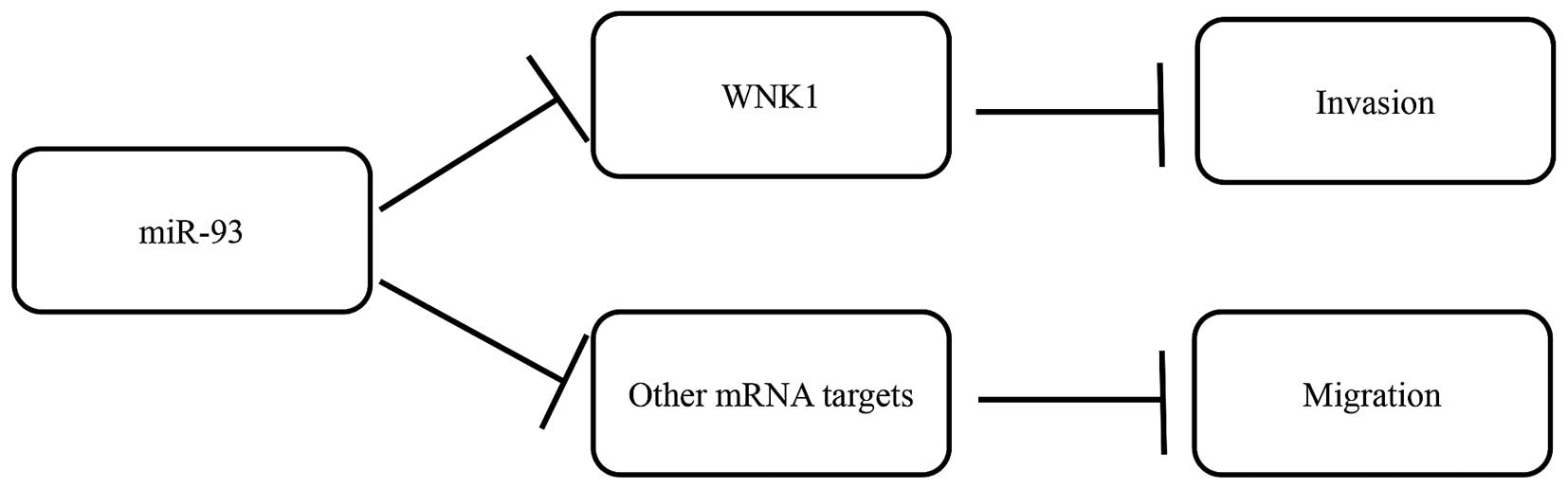
While miR-93 has several gene targets, we postulate
that the effects on migration and invasion following miR-93
overexpression were possibly mediated via decreasing WNK1
(identified from target prediction). Hence, we inhibited WNK1
expression using siRNA in the MDA-MB-231 cells and observed
decreased invasive ability, but not cell migration, suggesting that
the effects of miR-93 overexpression in reducing cell invasion was
mediated via decreasing the expression of WNK1
post-transcriptionally (Fig. 6).
It would appear that decrease in migration observed after miR-93
overexpression was not brought about by WNK1, but possibly through
other miR-93 gene targets that remain to be elucidated.
In summary, we have demonstrated that overexpression
of miR-93 decrease cell migration and invasion in MDA-MB-231 breast
cancer cells in vitro. We have identified WNK1 as a novel
target of miR-93 that mediates cell invasion. Further in
vivo studies are required to ascertain the
miR-93-WNK1-metastasis cascade that has potential implications in
breast cancer therapy.
Acknowledgements
The present study was supported by the Ministry of
Education grant (MOE2013-T2-1-129). Jia Pei Lim is a recipient of
the Ong Hin Tiang Scholarship in Cancer Research.
References
|
1
|
Bombonati A and Sgroi DC: The molecular
pathology of breast cancer progression. J Pathol. 223:307–317.
2011. View Article : Google Scholar :
|
|
2
|
André F and Zielinski CC: Optimal
strategies for the treatment of metastatic triple-negative breast
cancer with currently approved agents. Ann Oncol. 23(Suppl 6):
vi46–vi51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin Y, Liu AY, Fan C, Zheng H, Li Y, Zhang
C, Wu S, Yu D, Huang Z, Liu F, et al: MicroRNA-33b inhibits breast
cancer metastasis by targeting HMGA2, SALL4 and Twist1. Sci Rep.
5:99952015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhong X, Coukos G and Zhang L: miRNAs in
human cancer. Methods Mol Biol. 822:295–306. 2012. View Article : Google Scholar
|
|
6
|
van Schooneveld E, Wildiers H, Vergote I,
Vermeulen PB, Dirix LY and Van Laere SJ: Dysregulation of microRNAs
in breast cancer and their potential role as prognostic and
predictive biomarkers in patient management. Breast Cancer Res.
17:212015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang L, Du WW, Yang W, Rutnam ZJ, Peng C,
Li H, O’Malley YQ, Askeland RW, Sugg S, Liu M, et al: MiR-93
enhances angiogenesis and metastasis by targeting LATS2. Cell
Cycle. 11:4352–4365. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith AL, Iwanaga R, Drasin DJ, Micalizzi
DS, Vartuli RL, Tan AC and Ford HL: The miR-106b-25 cluster targets
Smad7, activates TGF-β signaling, and induces EMT and tumor
initiating cell characteristics downstream of Six1 in human breast
cancer. Oncogene. 31:5162–5171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu J, Xu J, Wu Y, Chen Q, Zheng W, Lu X,
Zhou C and Jiao D: Identification of microRNA-93 as a functional
dysregulated miRNA in triple-negative breast cancer. Tumour Biol.
36:251–258. 2015. View Article : Google Scholar
|
|
10
|
Kolacinska A, Morawiec J, Pawlowska Z,
Szemraj J, Szymanska B, Malachowska B, Morawiec Z,
Morawiec-Sztandera A, Pakula L, Kubiak R, et al: Association of
microRNA-93, 190, 200b and receptor status in core biopsies from
stage III breast cancer patients. DNA Cell Biol. 33:624–629. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu S, Patel SH, Ginestier C, Ibarra I,
Martin-Trevino R, Bai S, McDermott SP, Shang L, Ke J, Ou SJ, et al:
MicroRNA93 regulates proliferation and differentiation of normal
and malignant breast stem cells. PLoS Genet. 8:e10027512012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
13
|
Kang Y and Massagué J:
Epithelial-mesenchymal transitions: Twist in development and
metastasis. Cell. 118:277–279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moniz S and Jordan P: Emerging roles for
WNK kinases in cancer. Cell Mol Life Sci. 67:1265–1276. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W and Luo YP: MicroRNAs in breast
cancer: Oncogene and tumor suppressors with clinical potential. J
Zhejiang Univ Sci B. 16:18–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong DJ, Liu H, Ridky TW, Cassarino D,
Segal E and Chang HY: Module map of stem cell genes guides creation
of epithelial cancer stem cells. Cell Stem Cell. 2:333–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarrió D, Rodriguez-Pinilla SM, Hardisson
D, Cano A, Moreno-Bueno G and Palacios J: Epithelial-mesenchymal
transition in breast cancer relates to the basal-like phenotype.
Cancer Res. 68:989–997. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuperwasser C, Dessain S, Bierbaum BE,
Garnet D, Sperandio K, Gauvin GP, Naber SP, Weinberg RA and
Rosenblatt M: A mouse model of human breast cancer metastasis to
human bone. Cancer Res. 65:6130–6138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Yang CS, Nakashima K and Rana TM:
Small RNA-mediated regulation of iPS cell generation. EMBO J.
30:823–834. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Foshay KM and Gallicano GI: miR-17 family
miRNAs are expressed during early mammalian development and
regulate stem cell differentiation. Dev Biol. 326:431–443. 2009.
View Article : Google Scholar
|
|
22
|
Fan M, Sethuraman A, Brown M, Sun W and
Pfeffer LM: Systematic analysis of metastasis-associated genes
identifies miR-17-5p as a metastatic suppressor of basal-like
breast cancer. Breast Cancer Res Treat. 146:487–502. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hossain A, Kuo MT and Saunders GF:
Mir-17-5p regulates breast cancer cell proliferation by inhibiting
translation of AIB1 mRNA. Mol Cell Biol. 26:8191–8201. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Z, Willmarth NE, Zhou J, Katiyar S,
Wang M, Liu Y, McCue PA, Quong AA, Lisanti MP and Pestell RG:
microRNA 17/20 inhibits cellular invasion and tumor metastasis in
breast cancer by heterotypic signaling. Proc Natl Acad Sci USA.
107:8231–8236. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu XF, Zou J, Bao ZJ and Dong J: miR-93
suppresses proliferation and colony formation of human colon cancer
stem cells. World J Gastroenterol. 17:4711–4717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang IP, Tsai HL, Hou MF, Chen KC, Tsai
PC, Huang SW, Chou WW, Wang JY and Juo SH: MicroRNA-93 inhibits
tumor growth and early relapse of human colorectal cancer by
affecting genes involved in the cell cycle. Carcinogenesis.
33:1522–1530. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Q, Zou Z, Zou C, Zhang Q, Huang R,
Guan X, Li Q, Han Z, Wang D, Wei H, et al: MicroRNA-93 suppress
colorectal cancer development via Wnt/β-catenin pathway
downregulating. Tumour Biol. 36:1701–1710. 2015. View Article : Google Scholar
|
|
28
|
Xu YF, Mao YP, Li YQ, Ren XY, He QM, Tang
XR, Sun Y, Liu N and Ma J: MicroRNA-93 promotes cell growth and
invasion in nasopharyngeal carcinoma by targeting disabled
homolog-2. Cancer Lett. 363:146–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H and Yan X: Cantharidin modulates
the E2F1/MCM7-miR-106b-93/p21-PTEN signaling axis in MCF-7 breast
cancer cells. Oncol Lett. 10:2849–2855. 2015.
|
|
30
|
Petrocca F, Vecchione A and Croce CM:
Emerging role of miR-106b-25/miR-17-92 clusters in the control of
transforming growth factor beta signaling. Cancer Res.
68:8191–8194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lyu X, Fang W, Cai L, Zheng H, Ye Y, Zhang
L, Li J, Peng H, Cho WC, Wang E, et al: TGFβR2 is a major target of
miR-93 in nasopharyngeal carcinoma aggressiveness. Mol Cancer.
13:512014. View Article : Google Scholar
|
|
32
|
Xie J, Wu T, Xu K, Huang IK, Cleaver O and
Huang CL: Endothelial-specific expression of WNK1 kinase is
essential for angiogenesis and heart development in mice. Am J
Pathol. 175:1315–1327. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lai JG, Tsai SM, Tu HC, Chen WC, Kou FJ,
Lu JW, Wang HD, Huang CL and Yuh CH: Zebrafish WNK lysine deficient
protein kinase 1 (wnk1) affects angiogenesis associated with VEGF
signaling. PLoS One. 9:e1061292014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee BH, Chen W, Stippec S and Cobb MH:
Biological cross-talk between WNK1 and the transforming growth
factor beta-Smad signaling pathway. J Biol Chem. 282:17985–17996.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilson FH, Disse-Nicodème S, Choate KA,
Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV,
Lipkin GW, Achard JM, et al: Human hypertension caused by mutations
in WNK kinases. Science. 293:1107–1112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ,
Lee BH, English JM, Ortega B, Huang CL and Cobb MH: WNK1 activates
SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci
USA. 102:10315–10320. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tu SW, Bugde A, Luby-Phelps K and Cobb MH:
WNK1 is required for mitosis and abscission. Proc Natl Acad Sci
USA. 108:1385–1390. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeng G, Gao L and Yu RK: Reduced cell
migration, tumor growth and experimental metastasis of rat F-11
cells whose expression of GD3-synthase is suppressed. Int J Cancer.
88:53–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu W, Begum G, Pointer K, Clark PA, Yang
SS, Lin SH, Kahle KT, Kuo JS and Sun D: WNK1-OSR1 kinase-mediated
phospho-activation of Na+-K+-2Cl−
cotransporter facilitates glioma migration. Mol Cancer. 13:312014.
View Article : Google Scholar
|
|
40
|
Dbouk HA, Weil LM, Perera GK, Dellinger
MT, Pearson G, Brekken RA and Cobb MH: Actions of the protein
kinase WNK1 on endothelial cells are differentially mediated by its
substrate kinases OSR1 and SPAK. Proc Natl Acad Sci USA.
111:15999–16004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alves CC, Carneiro F, Hoefler H and Becker
KF: Role of the epithelial-mesenchymal transition regulator Slug in
primary human cancers. Front Biosci (Landmark Ed). 14:3035–3050.
2009. View Article : Google Scholar
|
|
42
|
Sun X, Gao L, Yu RK and Zeng G:
Down-regulation of WNK1 protein kinase in neural progenitor cells
suppresses cell proliferation and migration. J Neurochem.
99:1114–1121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fulford L, Milewski D, Ustiyan V,
Ravishankar N, Cai Y, Le T, Masineni S, Kasper S, Aronow B,
Kalinichenko VV, et al: The transcription factor FOXF1 promotes
prostate cancer by stimulating the mitogen-activated protein kinase
ERK5. Sci Signal. 9:ra482016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hsu YL, Hung JY, Chiang SY, Jian SF, Wu
CY, Lin YS, Tsai YM, Chou SH, Tsai MJ and Kuo PL: Lung
cancer-derived galectin-1 contributes to cancer associated
fibroblast-mediated cancer progression and immune suppression
through TDO2/kynurenine axis. Oncotarget. 7:27584–27598.
2016.PubMed/NCBI
|