Introduction
Pancreatic cancer is a fatal malignant tumor that is
often diagnosed at an advanced stage, and accompanied by invasion
and metastasis (1). The metastasis
of pancreatic cancer involves complex molecular mechanisms and
hence, an accurate understanding of the mechanisms of pancreatic
cancer metastasis is the prerequisite to an effective treatment for
this disease.
Tumor metastasis refers to a multistep process
involving tumor cell migration from the primary site to a distant
site. This process includes: local invasion, intravasation,
transportation, extravasation, and colonization (2). A study has shown that the
re-induction of epithelial-mesenchymal transition (EMT) plays an
important role in tumor metastasis (3). Although EMT is a critical enabler of
metastasis in tumors with an epithelial origin, triggering EMT does
not indicate that cells have entered an irreversible transition
process (4). Thiery proposed a
theory of two-step tumor metastasis, which states that the invasion
and systemic spread of primary epithelial tumors are achieved by
activating EMT (3). Three cell
phenotypes exists during the process of EMT, specifically
epithelial phenotype (E), mesenchymal phenotype (M) and partial EMT
state (P) which is an intermediate phenotype having both epithelial
and mesenchymal features (5,6). The
tumor cells eventually form metastatic foci through a reverse
process, transit back to epithelial phenotype, when disseminated to
a distant site.
Currently, the exact mechanisms underlying key
events for the spatiotemporal regulation of EMT-MET have not been
elucidated (7). In pancreatic
carcinomas, CAFs can compose up to 80% of the tumor mass (8). Also, in this study CAFs were found to
secrete factors that enhance epithelial tumor cell proliferation
and mutagenesis as well as angiogenesis (8). Literature has confirmed that
activated cancer associated fibroblasts (CAF) can affect the
promotion of tumor progression by EMT through the hedgehog (HH)
pathway (9).
We speculated that the state of fibroblasts may play
an important role in the EMT-CTC (circulating tumor cell)-MET
homing microenvironment. Therefore, our study utilized a
three-dimensional co-culture to simulate the malignant interstitial
tumor microenvironment, and observed the morphological changes of
tumor cells during EMT using confocal laser scanning microscopy,
RT-PCR, and western blotting. This study aims to comprehend the
mechanism of pancreatic cancer metastasis from the perspective of
the tumor microenvironment, and provide a new basis for therapeutic
research.
Materials and methods
Materials
RIPA cracking liquid kits were obtained from
Beyotime Biotechnology (Shanghai China). The DMEM culture medium
and fetal calf serum were purchased from the Hyclone (USA).
Transwell chambers were purchased from Millipore (Shanghai, China).
Matrigel and One-Step RT-PCR kit were obtained from BD Biosciences
(NJ, USA). E-cadherin, vimentin, Gli1, Snail and β-actin antibodies
were purchased from the Santa Cruz Biotechnology (Santa Cruz, CA,
USA). PTCH specific blocker were purchased from the Sigma Co. Ltd.,
Shanghai, China.
Cell cultures and treatments
The human pancreatic cancer cell lines (BxPc-3,
Panc-1; obtained from the American Tissue Type Collection, USA)
were maintained in Dulbecco's modified Eagle's medium (Gibco, USA)
supplemented with penicillin (100 U/ml), streptomycin (100
µg/ml), 0.1 mM non-essential amino acids, 0.2 mM glutamine,
1 mM pyruvate, and 10% heat-inactivated fetal bovine serum and
incubated in 5% CO2 humidified atmosphere at 37°C. Cells
were grown to 80% confluency. In the invasion and migration
experiments, the cells were cultured in Dulbecco's modified Eagle's
medium without fetal bovine serum.
CAF cell separation, culture, and
purification
Normal fibroblast (NF), CAF and hepatic stellate
(HSF) cells were derived from patients with pancreatic cancer and
hepatic trauma from the Second Affiliated Hospital of Xi'an
Jiaotong University. All patients were newly diagnosed and had not
received any relevant treatment prior to surgery. Informed consent
was obtained from all patients before taking specimens. Fibroblast
isolation was conducted as described previously (10). First, the tissue was trimmed to
1×1×1 mm and washed gently with PBS three times (5 min each). Next,
the tissues were washed once with the medium and placed in fresh
cell cultural medium containing 15% fetal calf serum, 2 mM
L-glutamine, and 10% double-antibody. The tissue was cut with a
sterile scalpel blade and sections of cells were gently scraped
with a blunt blade. The cells were cultured in an incubator for 3–5
days at 37°C and 5% CO2. The medium was replaced once
and every 3 days afterwards; after 14 days, the cells fully covered
the petri dish. When the cell density reached 80–90%, the cells
were digested with trypsin and regenerated at a rate of 1:3. The
CAFs and NFs used in the experiment were the 3rd and 5th
generations of cells cultured in vitro, respectively, and
showed no obvious aging phenotype.
Medium preparation of pancreatic CAFs,
NFs and HSFs
CAFs were added to 6-well plates at a density of
1.5×105/ml and rinsed with PBS after 24 h. The medium
was replaced with serum-free medium and cultured for 48 h, after
which the culture broth was collected, centrifuged to remove the
cells and debris, and the supernatant obtained was the CAF
conditioned medium. These samples were stored at 4°C. The
conditioned medium of NFs and HSFs was collected in the same
manner.
Indirect co-culture model of CAFs and
pancreatic cancer cells
The pancreatic cancer cells BxPc-3 and Panc-1 were
added to Petri dishes at a density of 1.5×105/ml; after
24 h, CAF-CM was added, and the cells were cultured for 48 h. Cells
in PBS or serum-free medium were used as controls. An inverted
phase contrast microscope was used to observe the morphology and
growth of pancreatic cancer cells in each Petri dish. Proteins were
extracted from the cells. The co-culture of HSFs, NFs and
pancreatic cancer cells was performed in the same manner.
Cell migration experiment
Cell migration capability was evaluated in a scratch
test. BxPc-3 (10×105) and Panc-1 cells were seeded in
1.5 ml media in each well into a 24-well plate. The cells were
grown to a confluent layer (48 h), and then a scratch was made in
each well by using a pipette tip. Subsequently, the cells were
washed gently with PBS, and then culture broth of CAF, NF and F
were added to the wells. Starting picture was taken at time-point
0. The cells were then incubated at 37°C in a 5% CO2
atmosphere, and new pictures were taken after 24 h. The 24 h
time-point was chosen to decrease the potential impact of
proliferation on the closing of the scratch. Image-Pro Plus 5.0
from NIH was used to standardize and present the results.
Cell invasion experiment
Cell invasion was examined using Transwell assays,
Following incubation for 48 h, 3×104 cells were
transferred to the top of the Matrigel-coated invasion chambers (BD
Biosciences, San Jose, CA, USA) in serum-free DMEM. DMEM containing
10% FBS was added to the lower chamber. After 24 h, the
non-invading cells were removed, and the invading cells were fixed
using 95% ethanol, stained with 0.1% crystal violet and images were
captured at ×100 magnification under an inverted phase contrast
microscope (Olympus CKX31/41; Olympus, Tokyo, Japan). The
experiments were repeated three times independently.
RT-PCR
Total RNA was extracted from the cells using TRIzol
reagent. A total of 2 µg RNA was reversed transcribed into
first-strand cDNA using the Revert Aid First Strand cDNA Synthesis
kit. PCR primer sequences were as follows: E-cadherin forward,
5′-CAATGGTGTCCATGTGAACA-3′; reverse, 5′-CCTCCTACCCTCCTGTTCG-3′.
Vimentin forward, 5′-CGCTTCGCCAACTACAT-3′; reverse,
5′-AGGGCATCCACTTCACAG-3′. β-actin forward,
5′-ATCGTGCGTGACATTAAGGAGAAG-3′; reverse,
5′-AGGAAGGAAGGCTGGAAGAGTG-3′. Gli1 forward,
5′-ATAGTGAGCCATGCTGTCTCC-3′; reverse, 5′-TCTCTCTGGCTGCTCCATAACC-3′.
Snail1 forward, 5′-AAGGATCTCCAGGCTCGAAAG-3′; reverse,
5′-GCTTCGGATGTGCATCTTGA-3′. The PCR conditions were as follows: an
initial reaction at 42°C for 1 h was used for cDNA synthesis,
followed by denaturation at 94°C for 5 min and 22 cycles of the
following reactions: 94°C for 30 sec, 55°C for 30 sec and 72°C for
30 sec. After the last cycle, the reaction was amplified at 72°C
for 10 min. The housekeeping gene β-actin was used as an internal
reference.
Western blotting
A total of 5×105 cells in the logarithmic
growth phase was added to 0.5 ml of pre-chilled cell lysis buffer
and incubated on ice for 30 min. After centrifugation, the
supernatant was collected and the protein contents were measured.
The proteins were separated by 10% SDS-PAGE and blotted onto a
nitrocellulose membrane by semi-dry transfer. Next, the membrane
was immersed in TBST containing 5% skim milk for blocking followed
by overnight incubation with the primary antibody at 4°C. The
following day, the membrane was incubated with the secondary
antibody conjugated to horseradish peroxidase at 1:2,000 dilution
(Santa Cruz) at room temperature for 2 h, and then an enhanced
chemiluminescence kit (Amersham Pharmacia Biotech, Amersham, UK)
was used for staining. The membrane was photographed and the
results were analyzed.
Statistical analysis
Each experiment was repeated at least three times.
The data were expressed as mean ± SD and analyzed using the
Student's t-test and one-way ANOVA. P<0.05 indicates a
statistically significant difference.
Results
Preliminary identification of pancreas
CAFs, NFs and HSFs
After 10 days, cell morphology was observed using an
inverted phase contrast microscope, HSFs, NFs and CAFs showed a
spindle shape (Fig. 1). The CAFs
were spindle- or fusiform-shaped, had inconsistent sizes, showed
dense growth, and were disorderly arranged. For NFs and HSFs, the
cells showed multiple flat stellate shapes with similar cell sizes,
were arranged in the same direction, and had a radially outward
appearance (Fig. 1). TEM
observations showed that CAFs had large cell nuclei, were evenly
colored, and contained one or two nucleoli. In the cytoplasm, a
large number of rough endoplasmic reticulum, mitochondria, and
bundles of parallel subcapsular filaments were observed. For NFs
and HSFs, the cells had an irregular cell nucleus, were rich in
rough endoplasmic reticulum in the cytoplasm, and had no filaments
(Fig. 1). Immunohistochemical
results of CAFs showed that vimentin and α-smooth muscle actin
expression was positive. In NFs and HSFs, vimentin showed positive
expression, while α-smooth muscle actin expression was negative
(Fig. 1C and D).
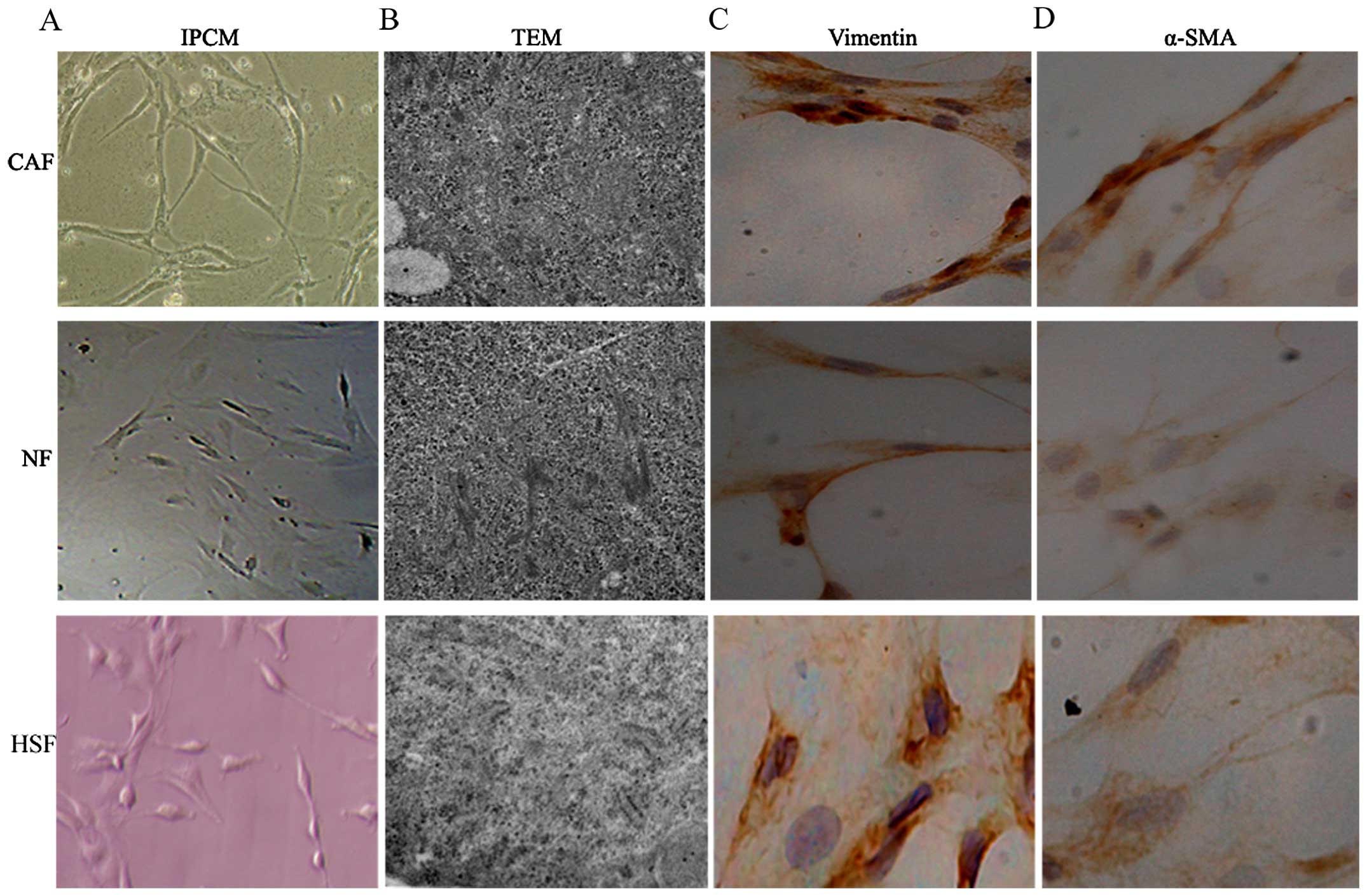 | Figure 1Preliminary identification of pancreas
CAFs, NFs and HSFs. HSFs, NFs and CAFs showed a spindle shape. (A)
The CAFs were spindle or fusiform shaped, had inconsistent sizes,
showed dense growth, and were disorderly arranged. For NFs and
HSFs, the cells showed multiple flat stellate shapes with similar
cell sizes, were arranged in the same direction, and had a radially
outward appearance. (B) TEM observations showed that CAFs had large
cell nuclei, were evenly colored, and contained one or two
nucleoli. In the cytoplasm, a large number of rough endoplasmic
reticulum, mitochondria, and bundles of parallel subcapsular
filaments were observed. For NFs and HSFs, the cells had an
irregular cell nucleus, were rich in rough endoplasmic reticulum in
the cytoplasm, and had no filaments. (C and D) Immunohistochemical
results of CAFs showed that vimentin and α-smooth muscle actin
expression was positive. In NFs and HSFs, vimentin showed positive
expression, while α-smooth muscle actin expression was
negative. |
Changes of partial EMT in co-cultured
pancreatic cancer cells
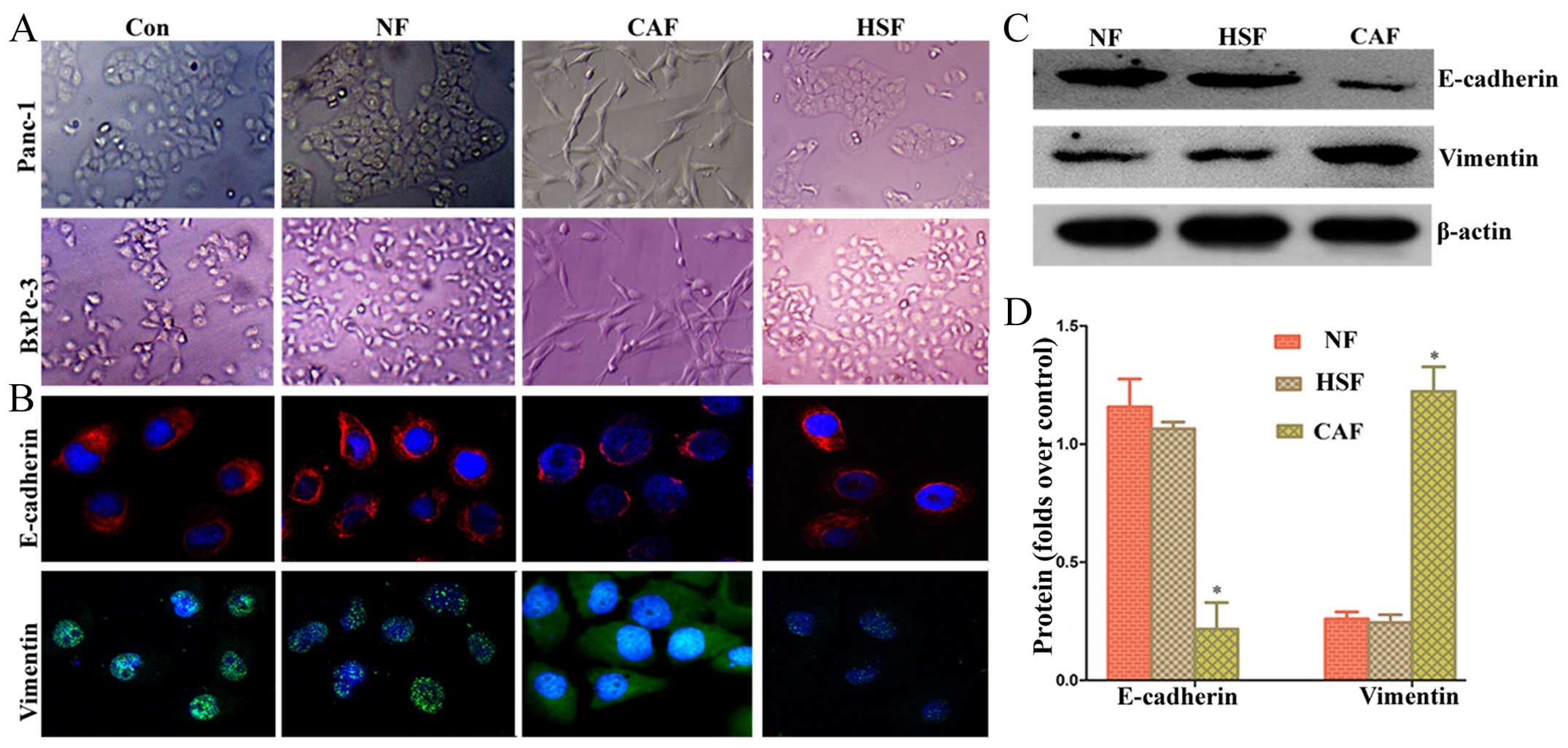
To determine whether co-culture of pancreatic cancer
cells with CAF promotes EMT, we first observed their morphology
under conventional light microscopy. We found that after indirect
co-culture with CAF, pancreatic cancer cells changed significantly
from rounded to spindle-shaped or fusiform cells with a chaotic,
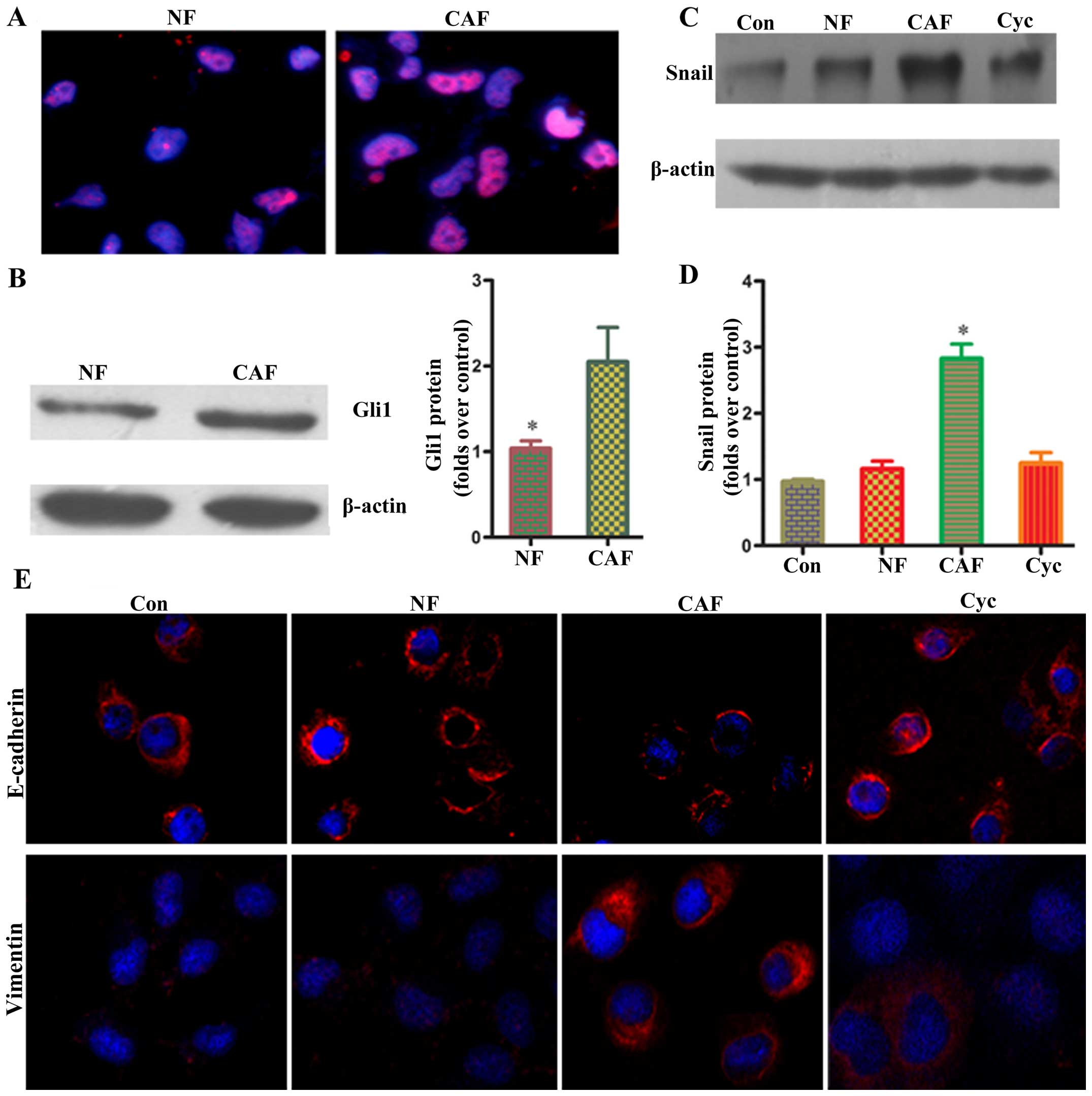
non-directional arrangement, and radial appearance (Fig. 2A). Confocal laser scanning
microscopy was carried out to assess the fluorescent intensity of
Panc-1 cells. The results showed that pancreatic cancer cells
co-cultured with CAF had a higher vimentin fluorescent intensity
than the NF/HSF co-culture group and single culture group, whereas
the E-cadherin fluorescent intensity was lower than the NF/HSF
co-culture group and single culture group (Fig. 2B). Western blotting results showed
that pancreatic cancer cells in the CAF co-culture group had a
higher protein expression of vimentin than the NF/HSF co-culture
group, whereas the protein expression of E-cadherin was lower than
that in the NF/HSF co-culture group (Fig. 2C and D). The detection results of
BxPc-3 cells were consistent with Panc-1 cells, suggesting that
activated CAF can enhance EMT changes in pancreatic cancer cells,
while EMT could not occur after co-culturing with quiescent
HSF.
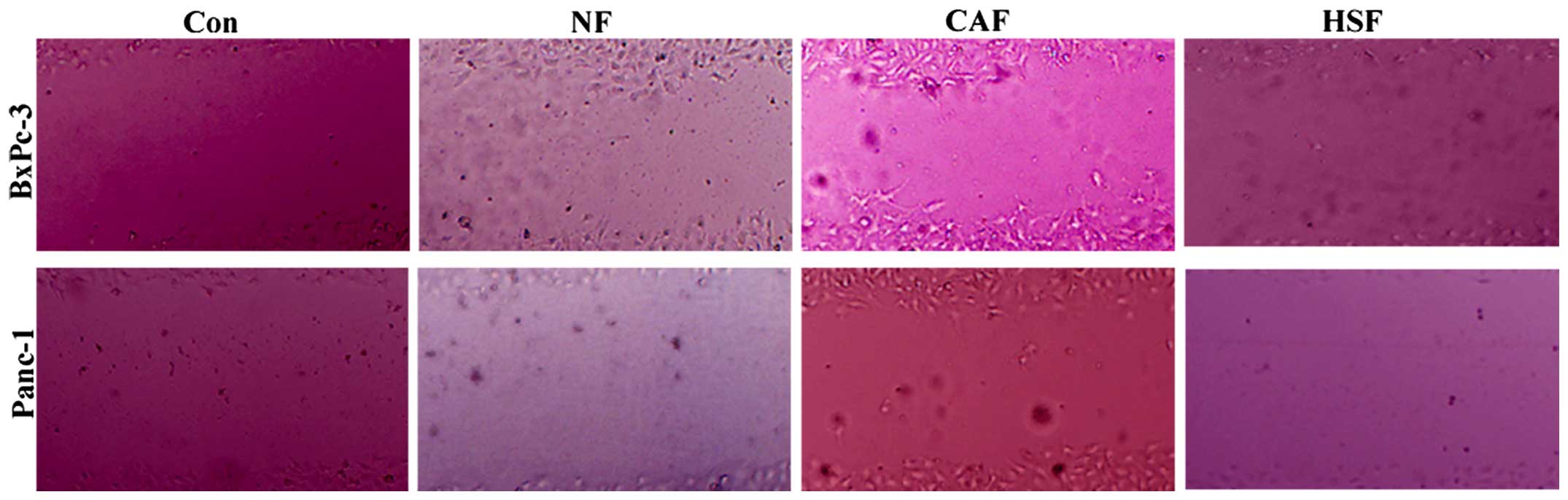
Changes in the migration capacity of
co-cultured pancreatic cancer cells
To clarify whether CAF-induced partial EMT in
pancreatic cancer cells can promote the pancreatic tumor migration,
an indirect co-culture model was employed, where BxPc-3 and Panc-1
cells were treated with CAF-conditioned medium (CM) for 48 h,
followed by a scratch assay to examine the effects of CAF on the
migration of pancreatic cancer cells. After 48 h of treatment,
single culture or NF/HSF co-cultured BxPc-3 and Panc-1 cells
exhibited weaker migratory capacity than the CAF co-culture group
(Fig. 3). Results showed that
CAF-induced partial EMT in pancreatic cancer cells enhancing
pancreatic cancer cell migration; however, co-culture with
quiescent HSF was unable to promote the migration of cancer
cells.
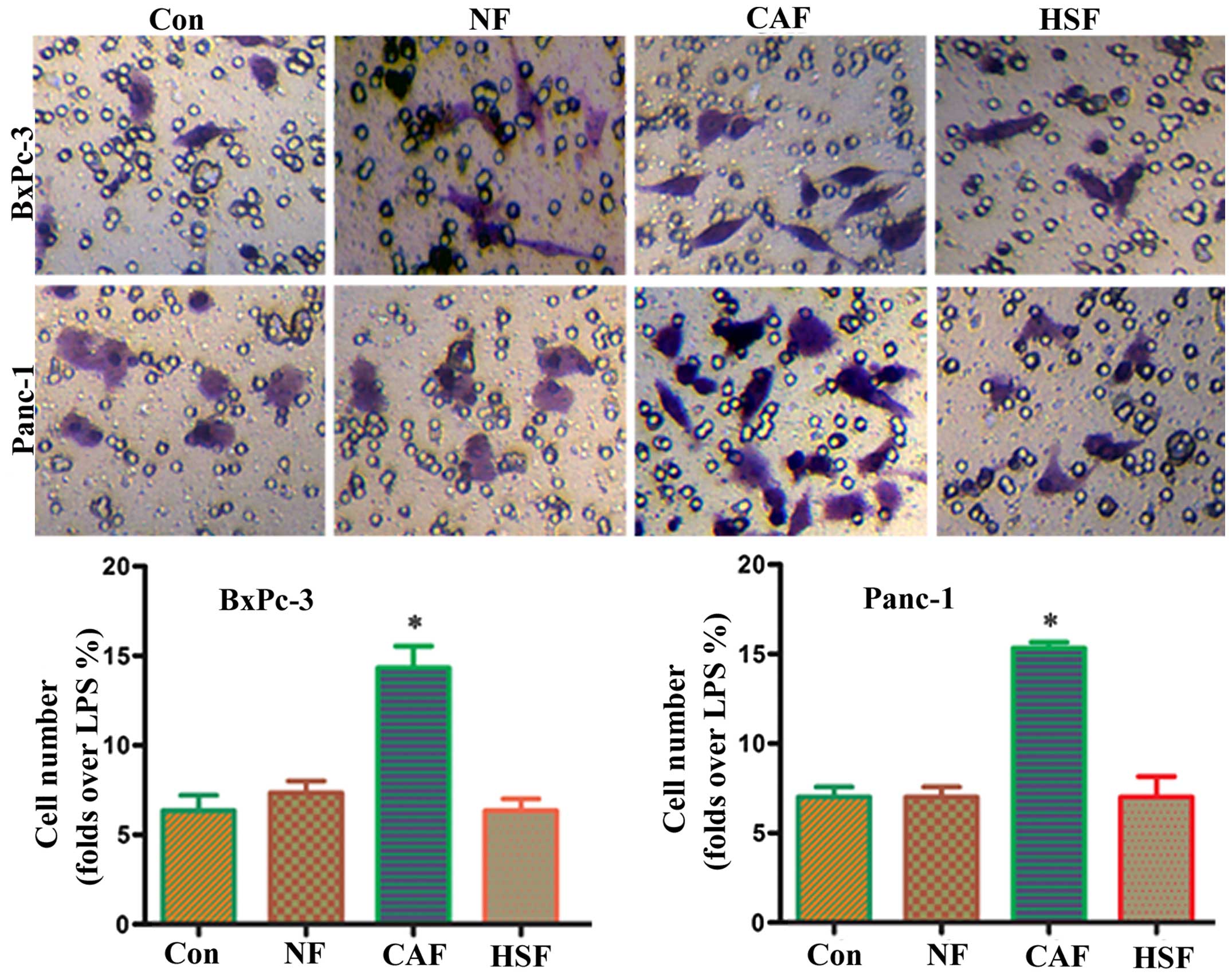
Changes in the invasive capacity of
co-cultured pancreatic cancer cells
To determine whether CAF-induced partial EMT in
pancreatic cancer cells was able to promote the invasive capacity
of pancreatic cancer cells, an indirect co-culture model was
employed, followed by a Transwell migration assay using an NF-CM
induction group and PBS alone as a control. Results showed that the
CAF-CM treatment group had a significantly higher number of
migrated cells than the PBS or NF/HSF-CM induction group (Fig. 4). This suggests that CAF-induced
partial EMT in pancreatic cancer cells enhancing the invasive
capacity of pancreatic cancer cells, whereas co-culture with
quiescent HSF was unable to promote the invasive capacity.
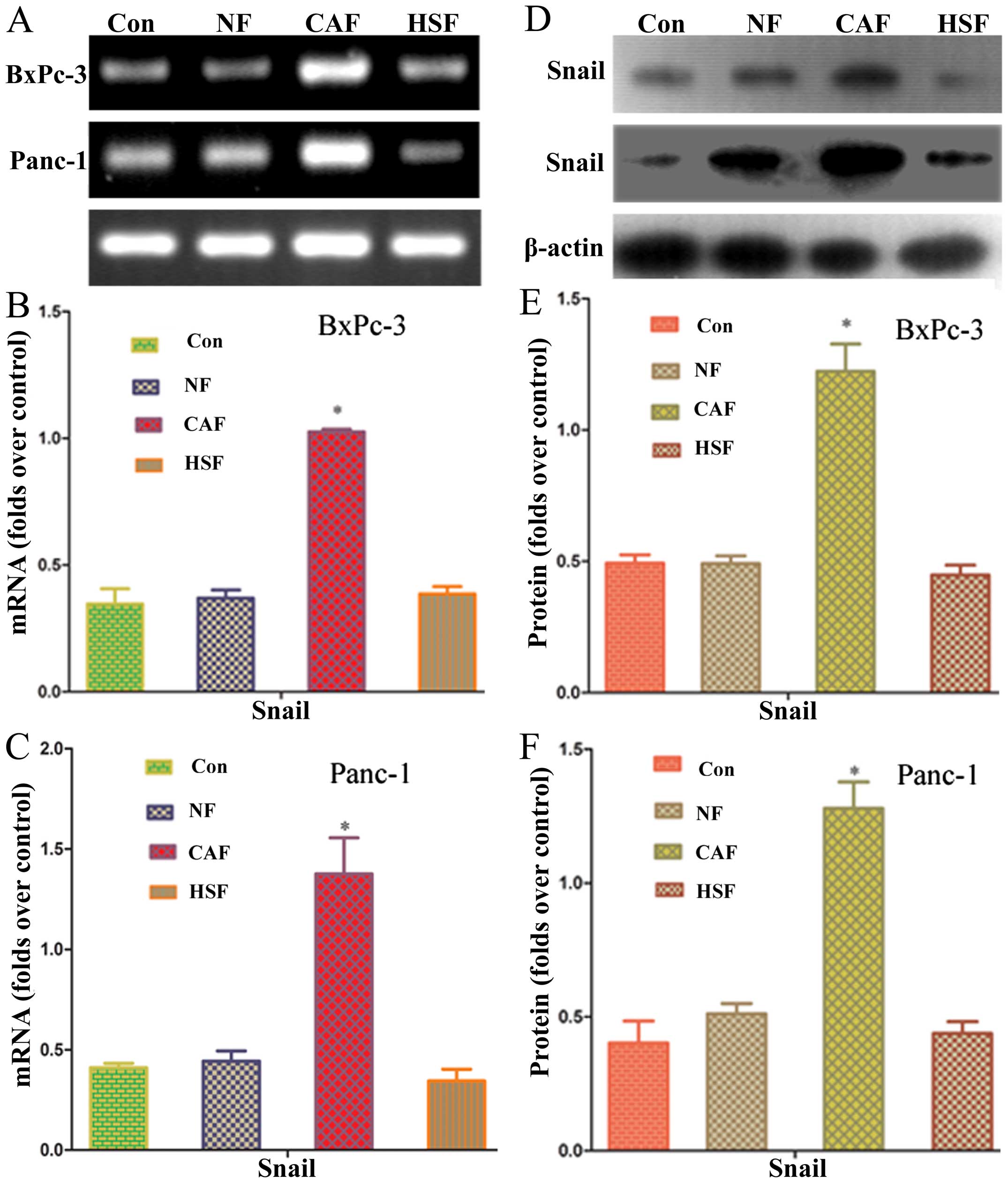
Effects of CAF on Snail mRNA and protein
in pancreatic cancer
Snail has been reported to regulate EMT marker,
E-cadherin, which is a transcription factor upstream of vimentin
expression (11). In order to
clarify whether pancreatic cancer cells co-cultured with CAF
affected Snail expression, RT-PCR and western blotting were used to
determine Snail mRNA and protein expressions, respectively. PCR
results showed that the CAF co-culture group had a significantly
higher level of Snail mRNA than the NF/HSF co-culture group or
single culture group (P<0.05) (Fig.
5). Western blotting results showed that the level of Snail
protein expression was significantly upregulated as compared with
the NF/HSF co-culture group and single culture group (P<0.05)
(Fig. 5). These results further
confirm that Snail expression in pancreatic cancer cells was
enhanced after co-culture with CAF.
CAF regulates Snail mRNA and protein via
the HH pathway in pancreatic cancer cells
Gli1 have been reported to be terminal transcription
regulators of the HH pathway, and play a very important role in the
activation of CAF (12). Activated
CAF can promote tumor progression via paracrine action. In order to
clarify whether the upregulation of Snail in pancreatic cancer
cells after co-culture is related to the HH pathway in CAF, changes
of Gli1 immunofluorescence in CAF were determined using confocal
laser scanning microscopy. Results showed that CAF had a
significantly higher Gli1 fluorescent intensity than the NF group
(Fig. 6). Western blot analysis
showed that Gli1 protein levels were significantly upregulated as
compared with the NF group (P<0.05) (Fig. 6). These results further confirm
that the HH pathway was activated in CAF. To verify whether the HH
pathway in CAF was related to EMT in pancreatic cancer cells, CAF
was pretreated with an HH-specific blocker (cyclopamine) for 24 h;
the supernatant was then obtained and cultured with pancreatic
cancer cells. Then, Snail protein expression was determined by
western blotting. The results showed that Snail protein expression
in the cyclopamine group was significantly decreased as compared
with the CAF group, and the corresponding expression of E-cadherin
and vimentin showed significant differences as compared with the
CAF group. The above results confirm that activating the HH
signaling pathway in CAF may trigger Snail transcription factors in
pancreatic cancer cells via paracrine action, thereby enhancing EMT
in pancreatic cancer cells.
Discussion
In tumor metastasis, EMT promotes cellular migration
and invasion to break through the basement membrane (4). It also facilitates the entry of tumor
cells into blood circulation, thereby forming CTCs, which promote
angiogenesis and intravasation (13,14).
Three cell phenotypes exist during the process of EMT, specifically
epithelial phenotype (E), mesenchymal phenotype (M) and partial EMT
state (P) having both epithelial and mesenchymal features (5). E-cadherin is considered a guardian of
epithelial state in various cell types, while vimentin is a
canonical molecular marker of EMT events (15). The EMT core transcription factors,
Snail1 and Snail2, are able to inhibit the transcription of
E-cadherin by directly binding to E-boxes on the E-cadherin
promoter (2,15). Our study found that activation of
Snail transcription factors enhanced EMT, upregulated vimentin
expression, and downregulated E-cadherin expression in pancreatic
cancer cells, while also enhancing the invasive capacity of
pancreatic cancer cells. Our results were consistent with previous
studies (11,16).
The occurrence of partial EMT is often not initiated
by core tumor cell transcription factors, but instead by the tumor
microenvironment (15). In
pancreatic carcinomas, CAFs can compose up to 80% of the tumor mass
(8). CAFs can secrete cytokines
and growth factors (such as TGF-β, HGF, FGF, NGF IGF) to enhance
epithelial cells migration (17).
Various EMT-mediating signaling pathways are cell- or
tissue-specific, and interference of EMT may require synergism of
multiple signaling pathways, including TGF-β, HH, Wnt, and Notch
pathways (15). In particular, the
TGF-β pathway is a major pathway that mediates EMT (18). Previous literature has indicated
that TGF-β and BMPs induce core EMT transcription factors,
Snail1/2, Zeb1/2, and Twist1 (19,20).
Fibroblasts in the tumor microenvironment are an important source
of TGF-β for tumor cells (21).
The HH signaling pathway is the key pathway for the development of
various tumors by suppressing E-cadherin expression through
interactions with Wnt, EGF/FGF, and TGF-β signaling pathways,
thereby inducing the onset of EMT and further promoting metastasis
(22). The transcription factor of
the HH signaling pathway is Gli1, which has a zinc-finger motif. In
the absence of HH signaling, Gli1 is hydrolyzed into 75-kDa
fragments that enter the nucleus and inhibit HH-responsive genes
(23). Degradation of Gli1 is
inhibited when HH binds to PTCH (23). Full-length Gli1 enters the nucleus
and induces the expression of its target genes (23). The HH signaling pathway is closely
associated with partial EMT (24).
For instance, overexpression of Gli1 by transient transfection of
rabbit kidney epithelial cells (RK3E) with low Gli1 expression
showed that Snail mRNA expression was upregulated, suggesting that
HH signaling can induce Snail upregulation (25). The HH signaling pathway can
directly upregulate the Notch ligand, JAG2 (26). The binding of JAG2 to Notch
activates the Notch signaling pathway that regulates Snail
expression (27). The HH signaling
pathway may also induce TGF-β signaling that further activates
MAP3K7 (28). The IKK-NF-κB
signaling pathway upregulates the expression of Snail2 (29). Ma et al reported that
activated HH in CAF can promote the invasion and metastasis of
tumor cells via paracrine action (30). Our results showed that HH in CAF
can regulate Snail expression in tumor cells through paracrine
action and control partial EMT in cancer cells, which were
consistent with results of previous studies. Most importantly, it
was found that HH signaling in interstitial cells activates partial
EMT in adjacent cancer cells, and this mechanism may provide new
perspectives in tumor-microenvironment interaction.
The colonization and metastasis of CTCs require the
process of P to E transition (2).
This process, together with the changes in the microenvironment, is
closely associated with the changes in the expression of relevant
genes at the molecular level. The liver is a common target for the
metastasis of pancreatic cancer (31). Our study showed that after an
indirect co-culture with HSF, BxPc-3, and Panc-1, cells had
significantly higher E-cadherin expression and reduced vimentin
expression as compared with the CAF co-culture group, with
corresponding reduction in migratory and invasive capacities. PCR
and western blotting results showed that both Snail mRNA and
protein expression levels in cancer cells were decreased. This
suggested that the removal of paracrine action in CAF may be
involved in the P to E transition of tumor cells, thus enhancing
the colonization capacity of tumor cells.
In this study, we demonstrated that activated CAF in
the tumor microenvironment may activate the Snail transcription
factor in cancer cells through the paracrine action of the HH
signaling pathway. This, therefore, leads to the enhancement of
partial EMT in pancreatic cancer cells, upregulation of vimentin
expression, downregulation of E-cadherin expression, and
enhancement of the invasive capacity of pancreatic cancer cells. We
hypothesize that, after CTCs have entered a new homing environment,
the paracrine action of normal non-activated fibroblasts
downregulate the Gli1-Snail transcriptional regulation axis,
leading to P-to-E transition and the formation of new metastatic
foci. The metastatic lesion activates CAF, generate partial EMT,
which leads to a new cycle, thereby promoting the development of
tumors (31,32). These results indicate that the
activation of interstitial fibroblasts may be the key to the
spatiotemporal regulation of EMT. The reverse process of EMT could
become a new preventive approach for the recurrence of tumor cells.
Improving our understanding of the molecular regulation of the
dynamic EMT process in tumor metastasis may provide us with a more
effective way to eradicate tumor metastasis.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China, NSFC (no. 81402583); Natural Science
Foundation of Shaanxi Province (no. 2014JQ4165); Xi'an Jiaotong
University Education Foundation, XJTUEF (no xjj2014077); and the
Hospital Fund of the Second Affiliated Hospital of the Health
Science Center, Xi'an Jiaotong University [no. RC (XM)201402)].
References
|
1
|
Winter JM, Cameron JL, Campbell KA, Arnold
MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS,
et al: 1423 pancreaticoduodenectomies for pancreatic cancer: A
single-institution experience. J Gastrointest Surg. 10:1199–1210;
discussion 1210–1191. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsai JH and Yang J: Epithelial-mesenchymal
plasticity in carcinoma metastasis. Genes Dev. 27:2192–2206. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: Insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jordan NV, Johnson GL and Abell AN:
Tracking the intermediate stages of epithelial-mesenchymal
transition in epithelial stem cells and cancer. Cell Cycle.
10:2865–2873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Herreros AG, Peiró S, Nassour M and
Savagner P: Snail family regulation and epithelial mesenchymal
transitions in breast cancer progression. J Mammary Gland Biol
Neoplasia. 15:135–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chui MH: Insights into cancer metastasis
from a clinicopathologic perspective: Epithelial-mesenchymal
transition is not a necessary step. Int J Cancer. 132:1487–1495.
2013. View Article : Google Scholar
|
|
8
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of Hedgehog signaling
enhances delivery of chemotherapy in a mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walter K, Omura N, Hong SM, Griffith M,
Vincent A, Borges M and Goggins M: Overexpression of smoothened
activates the sonic hedgehog signaling pathway in pancreatic
cancer-associated fibroblasts. Clin Cancer Res. 16:1781–1789. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walter K, Omura N, Hong SM, Griffith M and
Goggins M: Pancreatic cancer associated fibroblasts display normal
allelo-types. Cancer Biol Ther. 7:882–888. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rimkus TK, Carpenter RL, Qasem S, Chan M
and Lo HW: Targeting the sonic hedgehog signaling pathway: Review
of smoothened and GLI inhibitors. Cancers (Basel). 8:82016.
View Article : Google Scholar
|
|
13
|
Kallergi G, Papadaki MA, Politaki E,
Mavroudis D, Georgoulias V and Agelaki S: Epithelial to mesenchymal
transition markers expressed in circulating tumour cells of early
and metastatic breast cancer patients. Breast Cancer Res.
13:R592011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ota I, Li XY, Hu Y and Weiss SJ: Induction
of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration
program in cancer cells by Snail1. Proc Natl Acad Sci USA.
106:20318–20323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batlle E, Sancho E, Francí C, Domínguez D,
Monfar M, Baulida J and García De Herreros A: The transcription
factor snail is a repressor of E-cadherin gene expression in
epithelial tumour cells. Nat Cell Biol. 2:84–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gascard P and Tlsty TD:
Carcinoma-associated fibroblasts: Orchestrating the composition of
malignancy. Genes Dev. 30:1002–1019. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katsuno Y, Lamouille S and Derynck R:
TGF-β signaling and epithelial-mesenchymal transition in cancer
progression. Curr Opin Oncol. 25:76–84. 2013. View Article : Google Scholar
|
|
19
|
Eckert MA, Lwin TM, Chang AT, Kim J, Danis
E, Ohno-Machado L and Yang J: Twist1-induced invadopodia formation
promotes tumor metastasis. Cancer Cell. 19:372–386. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu FL, Mo EP, Yang L, Du J, Wang HS,
Zhang H, Kurihara H, Xu J and Cai SH: Autophagy is involved in
TGF-β1-induced protective mechanisms and formation of
cancer-associated fibroblasts phenotype in tumor microenvironment.
Oncotarget. 7:4122–4141. 2016.
|
|
22
|
Zhang J, Tian XJ and Xing J: Signal
transduction pathways of EMT induced by TGF-β, SHH, and WNT and
their crosstalks. J Clin Med. 5:52016. View Article : Google Scholar
|
|
23
|
Carpenter RL and Lo HW: Hedgehog pathway
and GLI1 isoforms in human cancer. Discov Med. 13:105–113.
2012.PubMed/NCBI
|
|
24
|
Palle K, Mani C, Tripathi K and Athar M:
Aberrant GLI1 Activation in DNA damage response, carcinogenesis and
chemo-resistance. Cancers (Basel). 7:2330–2351. 2015. View Article : Google Scholar
|
|
25
|
Li X, Deng W, Nail CD, Bailey SK, Kraus
MH, Ruppert JM and Lobo-Ruppert SM: Snail induction is an early
response to Gli1 that determines the efficiency of epithelial
transformation. Oncogene. 25:609–621. 2006.
|
|
26
|
Visbal AP, LaMarca HL, Villanueva H,
Toneff MJ, Li Y, Rosen JM and Lewis MT: Altered differentiation and
paracrine stimulation of mammary epithelial cell proliferation by
conditionally activated Smoothened. Dev Biol. 352:116–127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katoh Y and Katoh M: Hedgehog target
genes: Mechanisms of carcinogenesis induced by aberrant hedgehog
signaling activation. Curr Mol Med. 9:873–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perrot CY, Javelaud D and Mauviel A:
Overlapping activities of TGF-β and Hedgehog signaling in cancer:
Therapeutic targets for cancer treatment. Pharmacol Ther.
137:183–199. 2013. View Article : Google Scholar
|
|
29
|
Ren X, Wang F, Ji B and Gao C: TLR7
agonist induced repression of hepatocellular carcinoma via the
TLR7-IKK-NF-κB-IL6 signaling pathway. Oncol Lett. 11:2965–2970.
2016.PubMed/NCBI
|
|
30
|
Ma J, Cheng J, Gong Y, Tian L and Huang Q:
Downregulation of Wnt signaling by sonic hedgehog activation
promotes repopulation of human tumor cell lines. Dis Model Mech.
8:385–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shi H, Li J and Fu D: Process of hepatic
metastasis from pancreatic cancer: Biology with clinical
significance. J Cancer Res Clin Oncol. 142:1137–1161. 2016.
View Article : Google Scholar
|
|
32
|
Pantel K and Brakenhoff RH: Dissecting the
metastatic cascade. Nat Rev Cancer. 4:448–456. 2004. View Article : Google Scholar : PubMed/NCBI
|