Introduction
Breast cancer is the most common malignancy and
second leading cause of cancer death in women, accounting for 29%
of all female cancers and 15% of all cancer deaths (1). The main cause of death in breast
cancer patients is metastasis (1).
Therefore, preventing or controlling the molecular events that lead
to tumour metastasis is a major goal in breast cancer research and
treatment design. Metastases occur when cancer cells from a primary
tumour site spread to distant organs and form new tumours.
Metastasis is a multistep process that includes migration and
invasion, intravasation, arrest and extravasation, and metastatic
colonization (2). Cancer cell
migration is induced by various signalling molecules, including
transforming growth factor-β (TGF-β), integrin, receptor tyrosine
kinase, and reactive oxygen species (ROS).
The role of ROS in triggering signalling pathways
for cell migration and invasion has been well-established (3,4).
ROS, particularly superoxide and peroxide, are constantly generated
inside cells by specific enzyme complexes, such as NADPH oxidase
(NOX) and nitric oxide synthases (NOS); they are also generated as
by-products of oxidation-reduction reactivations, including those
underlying mitochondrial respiration (5). In transformed epithelial cells,
constitutively activated mitogenic pathways increase intracellular
ROS directly or by activating metabolic pathways (6). Additionally, endogenous ROS can be
generated by a variety of sources. Mitochondria are major sites of
ROS generation in mammalian cells, and mitochondrial ROS (mitoROS)
functions as a signalling molecule that stimulates cell
proliferation and motility (7).
Targeting mitochondria to reduce ROS is an emerging strategy for
treating cancer. Goh et al used transgenic mice expressing
the mitochondrial catalase and mouse mammary tumour virus-polyoma
middle T oncoprotein (MMTV-PyMT) and demonstrated that targeting
mitoROS could inhibit tumour progression and prevent metastasis
(8).
NecroX-5 is a derivative of the NecroX series of
compounds, all of which are mitoROS scavengers (9–11).
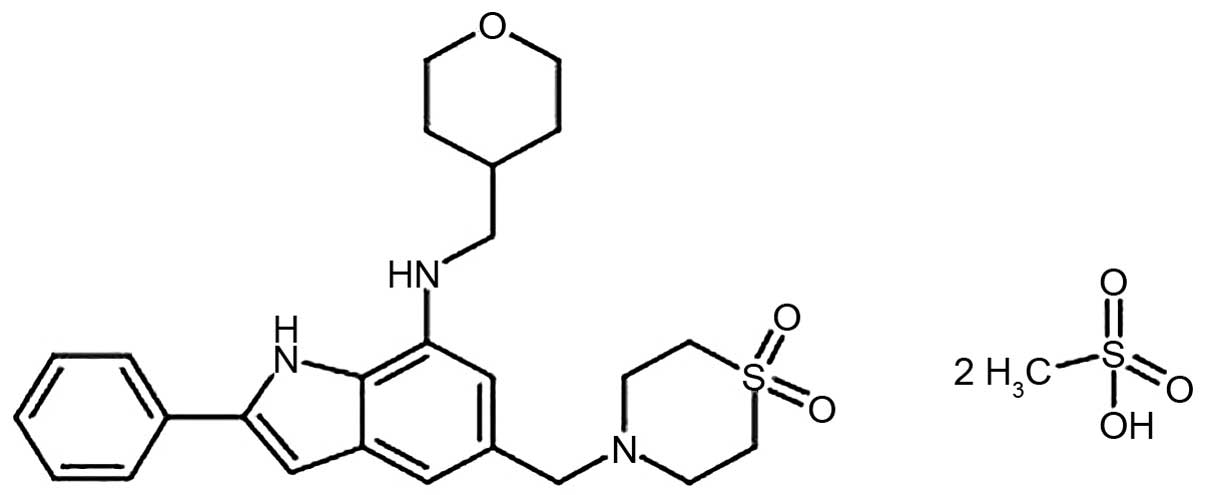
The chemical composition of NecroX-5 is
C25H31N3O3S•2CH4O3S
with a molecular weight of 645.83 g/mol (Fig. 1). The therapeutic aim of NecroX is
to protect cells from necrotic damage caused by CCl4-induced
hepatotoxicity, gentamicin-induced ototoxicity, and ischaemic
injury in the liver and heart (9–12).
However, the anti-tumourigenic effects of NecroX-5 have not been
explored to date. In this study, we evaluated whether NecroX-5 can
inhibit the metastasis of breast cancer cells and sought to
elucidate the molecular mechanisms of this inhibition.
Materials and methods
Reagents and cell lines
DNase and collagenase (Sigma-Aldrich, St. Louis, MO,
USA) were dissolved in Dulbecco's modified Eagle's medium (DMEM).
NecroX-5 (LG Life Sciences, Ltd., Seoul, Korea), Mitoquinone
(MitoQ; BioTrend, Switzerland), 2′,7′-dichlorodihydrofluorescein
diacetate (H2DCFDA), MitoSOX-red, Fluo4-AM (Thermo
Fisher Scientific, Waltham, MA, USA), MK-2206 (Biovision, Palo
Alto, CA, USA), and bepridil (bepridil hydrochloride;
Sigma-Aldrich) were dissolved in dimethyl sulphoxide (DMSO) for
each condition and dose. Mouse breast cancer 4T1 and human breast
cancer HCC70, MDA-MB-231 and MDA-MB-453 cells were purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA). The
TUBO-P2J cell line was established from a metastatic lung nodule
that had spontaneously developed during a mechanistic study
involving an anti-neu antibody in TUBO-bearing mice (13). Cells were grown in DMEM of RPMI
with 10% fetal bovine serum and 1% penicillin, and cultured at 37°C
with 5% CO2.
Animal study and lung colony assay
Six-week-old female BALB/c mice were obtained from
Orient Bio (Taejun, Korea). Tumours were established by the
subcutaneous injection of 2×105 TUBO-P2J cells into the
backs of the mice. When the tumour volume reached 100–150
mm3 in the treatment group, NecroX-5 was dissolved in
phosphate-buffered saline (PBS) and administered via
intraperitoneal injection (2.5 mg/kg/day). The same volume of PBS
was injected into the control group. Tumour volumes were measured
along three orthogonal axes (x, y and z) and calculated as tumour
volume = (xyz)/2. All animal procedures were performed in
accordance with the animal experimental guidelines set by the
Institutional Animal Care and Use Committee of the INJE University
College of Medicine (protocol no. 2013-018). Lung metastasis was
evaluated using a lung colony assay on day 23 following tumour
implantation. Single cell suspensions were prepared from the lung
tissue via enzyme digestion using a mixture of DNase (0.1 mg/ml)
and collagenase (4 mg/ml) and seeded in 48-well culture plates in a
4-fold serial dilution. The plates were incubated in complete DMEM
with 500 μg/ml of G418 (Sigma-Aldrich) and stained between
days 10 and 14 with crystal violet (0.1% crystal violet
acetate).
Migration assay
Breast cancer cell migration was evaluated with
24-well Transwell plates (Falcon, Bedford, MA, USA). The
appropriate number of cells was added to the upper chamber and
incubated for 6 or 24 h. The upper surface of the membrane was
wiped with a cotton-tipped applicator to remove residual cells.
Cells in the bottom compartment were fixed and stained with
hematoxylin and eosin (H&E). Cells in four randomly selected
fields at ×200 magnification were counted.
ROS measurements
Cytoplasmic ROS (cytoROS) was measured with
H2DCFDA. Cells treated with Necrox-5 and MK-2206 for 1 h
were harvested with trypsin (0.025%) and stained with 10 μM
H2DCFDA for 30 min at 37°C. Fluorescence intensity was
measured using a FACSCanto II flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). MitoROS was measured with MitoSOX-red.
Cells were treated with NecroX-5, MK-2206 and bepridil for 1 h in
96-well black microplates (Corning, Inc., Corning, NY, USA) and
loaded with 5 μM MitoSOX-red. MitoROS levels were detected
with a fluorescence reader (Molecular Devices, Sunnyvale, CA, USA)
at 510/580 nm.
Western blot assay
Whole-cell lysates were prepared in
radio-immunoprecipitation assay (RIPA) buffer (Thermo Fisher
Scientific) on ice with an added phosphatase inhibitor cocktail
(PhosSTOP, cat no. 04906845001; Roche, Basel, Switzerland). The
protein concentrations for each sample were determined using the
bicinchoninic acid (BCA) protein assay kit (Thermo Fisher
Scientific) according to the manufacturer's directions. Equal
amounts of protein (40 μg) were resolved by electrophoresis
on 10% sodium dodecyl sulphate (SDS) polyacrylamide gels and
transferred to nitrocellulose membranes. The membranes were blocked
with 5% non-fat dry milk in TBS and incubated at room temperature
for 2 h with primary antibodies pAKT t308 (monoclonal, rabbit,
dilution used 1:1,000, cat no. 2965), pAKT s473 (monoclonal,
rabbit, dilution used 1:1,000, cat no. 4058), AKT (polyclonal,
rabbit, dilution used 1:2,000, cat no. 9272), PI3K p85 (polyclonal,
rabbit, dilution used 1:1,000, cat no. 4292), and pPI3K p110α
(polyclonal, rabbit, dilution used 1:1,000, cat no. 4255) (Cell
Signaling Technology). One hour with secondary antibodies were
diluted 1:2,000 and incubated for 1 h (IgG HRP-linked, anti-rabbit
and anti-mouse antibodies, cat no. 7074 and 7076, respectively;
Cell Signaling Technology). The results were visualized using
enhanced chemiluminescent (ECL) detection reagents (Millipore,
Darmstadt, Germany).
Ca2+ measurements
The effects of NecroX-5 on intracellular calcium
levels were analysed with Fluo-4 AM and a confocal microscope (LSM
700; Carl Zeiss, Oberkochen, Germany). Briefly, harvested cells
were washed twice with PBS and incubated at 37°C with 5 μM
Fluo-4 AM (excitation/emission, 494/506 nm). After washing twice
with PBS, the stained cells were placed in a perfusion chamber at
room temperature. Fluorescence intensity was measured every 30 sec
using a confocal microscope with ZEN 2009 software. Regions of
interest in the cells were selected to monitor changes in
fluorescence intensity over time, and background was identified as
an area without cells. Fluorescence intensity in cells treated for
10 min with normal tyrode solution [143.0 mM NaCl, 5.4 mM KCl, 1.8
mM CaCl2, 0.5 mM MgCl2, 5.5 mM glucose, and
5.0 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid [HEPES,
pH 7.4)] was used as the baseline value (F0). Fluorescence
intensity during NecroX-5 perfusion (10 μM) was recorded
every 30 sec for 20 min, and peak values (F1) were analysed
relative to the baseline value (F1/F0) after subtracting the
autofluorescence in the absence of any fluorescent dye. The
temperature was set to 23±1°C while recording. To measure calcium
influx, intracellular free Ca2+ was depleted with
ethylene glycol tetraacetic acid (EGTA, 3 mM) and thapsigargin (TG,
2 μM). Ca2+ influx was induced by the subsequent
addition of 2 mM Ca2+ with or without NecroX-5 and
measured with a fluorescence reader (Molecular Devices) at 485/520
nm.
Results
NecroX-5 reduces breast cancer cell
metastasis by inhibiting cell migration
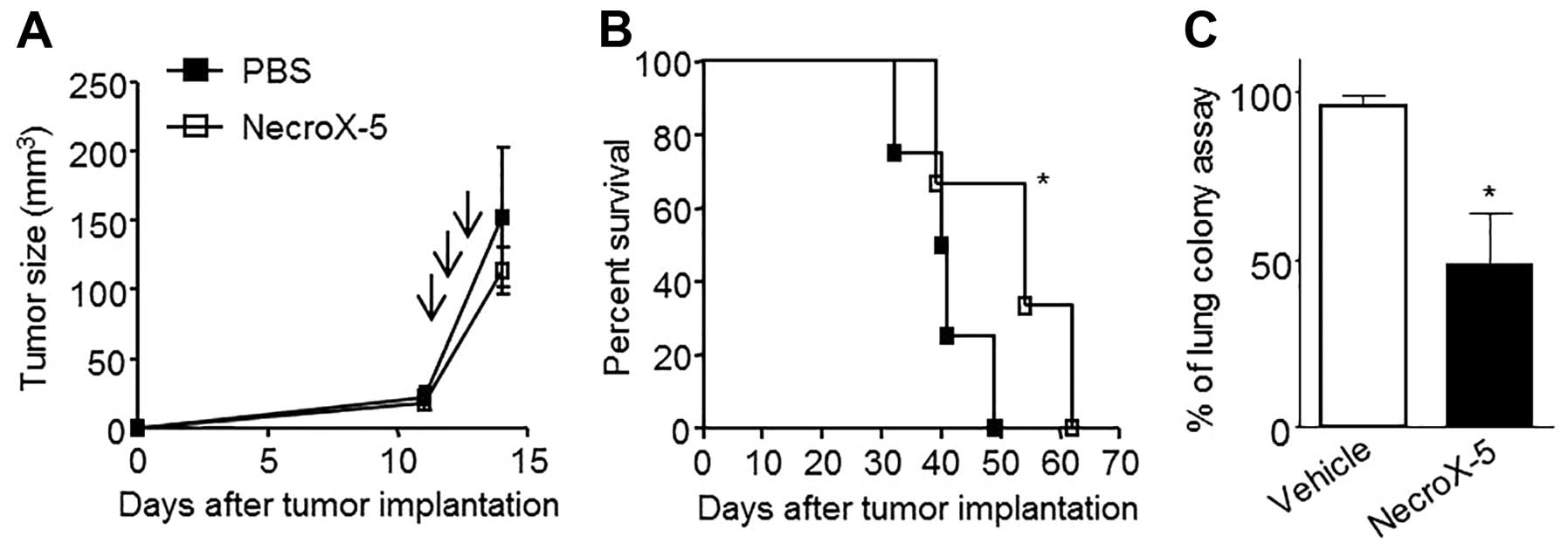
To test the anticancer effects of NecroX-5, TUBO-P2J
tumour-bearing mice were treated three times with 500 μg of
NecroX-5 every other day. NecroX-5 treatment did not inhibit tumour
growth (Fig. 2A), however, it was
able to significantly extend the mean survival from 40.5 to 55 days
(p<0.05) (Fig. 2B). The number
of cells that metastasized to the lung was also significantly
reduced by 60% (Fig. 2C). These
data suggested that NecroX-5 inhibited the metastasis of TUBO-P2J
cells, without inhibiting the tumour growth. To determine whether
the anti-metastatic effect of NecroX-5 was related to an inhibition
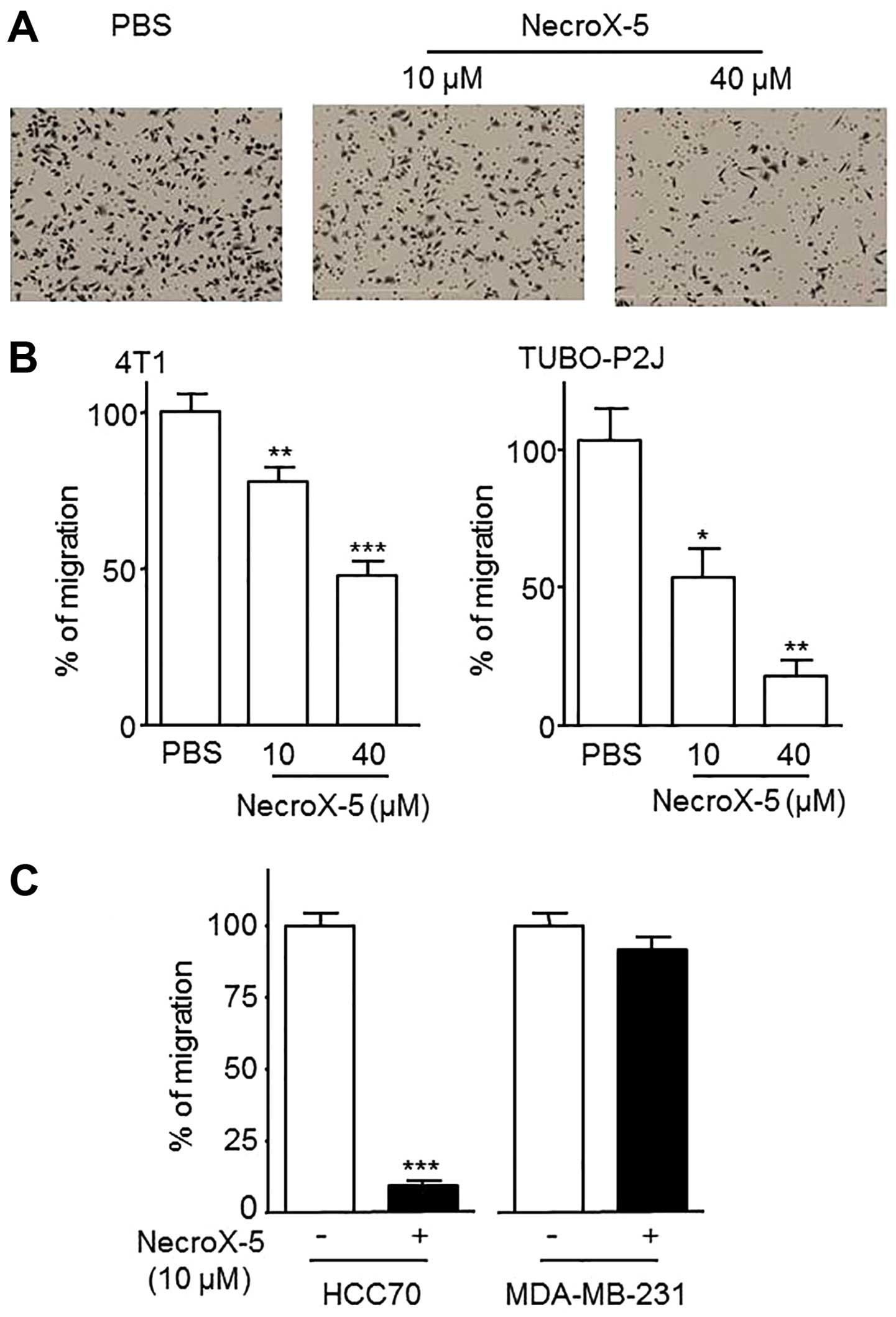
of cell migration, we performed a Transwell migration assay.
NecroX-5 significantly decreased the migration of the mouse breast
cancer cell lines 4T1 and TUBO-P2J, in a dose-dependent manner
(Fig. 3A and B). However, NecroX-5
had a different effect on the human breast cancer cells. While it
reduced the number of migrated cells in HCC70, it had no effect in
MDA-MB-231 (Fig. 3C).
NecroX-5 indirectly reduces mitoROS
levels
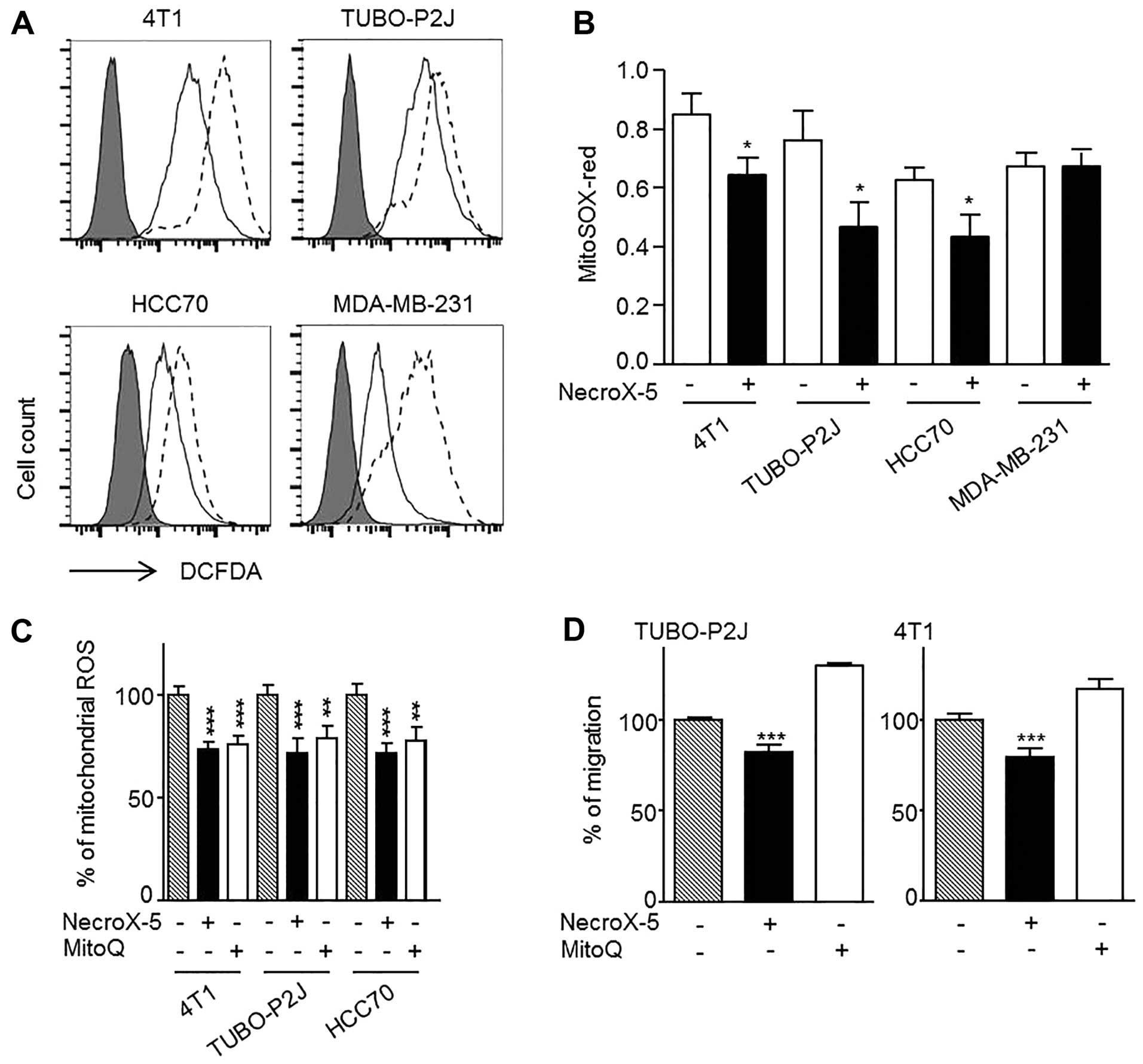
To determine whether the observed decrease in cancer
cell migration was related to the ROS scavenging activity of
NecroX-5, the levels of cytoROS and mitoROS were measured with
H2DCFDA and MitoSOX-red staining, respectively. The
intensity of H2DCFDA decreased following NecroX-5
treatment in all the breast cancer cells tested (Fig. 4A). Interestingly, MDA-MB-231 cells
exhibited the most dramatic decrease in H2DCFDA
intensity. Similarly, MitoSOX-red intensities significantly
decreased in 4T1, TUBO-P2J and HCC70 cells, but appeared unchanged
in the MDA-MB-231 cells, which were not susceptible to NecroX-5
(Fig. 4B). Next, we utilized MitoQ
to test whether the observed decrease in migration was modulated by
the reduction in mitoROS levels. MitoQ had a similar effect on
mitoROS levels as NecroX-5 (Fig.
4C), however, it did not inhibit cell migration (Fig. 4D). These data suggested that the
reduction in mitoROS caused by NecroX-5 was not a direct cause of
cell migration inhibition.
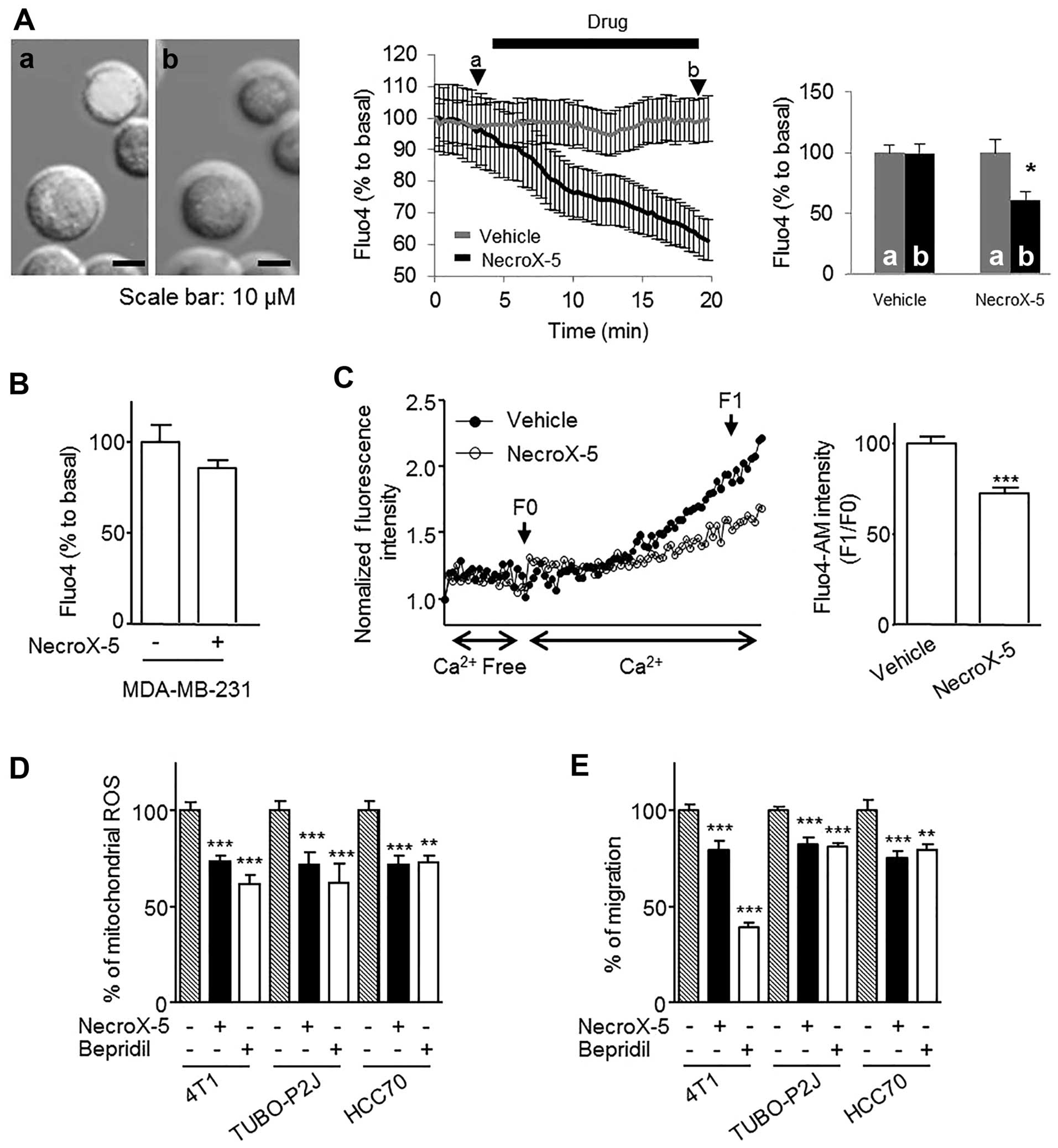
NecroX-5 reduces intracellular calcium
concentrations by limiting calcium influx, which decreases mitoROS
levels and inhibited cell migration
NecroX-5 can reduce mitochondrial calcium
(Ca2+) concentrations, which is important for mitoROS
regulation and cell migration (10,14,15).
Accordingly, we evaluated changes in intracellular free calcium
concentrations using Fluo-4 AM staining. In the HCC70 cells,
NecroX-5 decreased intracellular free Ca2+ by 50% within
20 min (Fig. 5A) but had no effect
in MDA-MB-231 (Fig. 5B). To
determine whether the decrease in Intracellular Ca2+ was
mediated by a decrease in Ca2+ influx, 4T1 cells were
treated with TG and ethylene glycol tetraacetic acid to chelate
Ca2+ and its subsequent influx was followed.
NecroX-5-treated cells significantly decreased the Ca2+
influx (Fig. 5C). To test if the
decrease in Intracellular Ca2+ was related to the
biological effect of NecroX-5, we utilized the calcium influx
blocker bepridil. Bepridil treatment significantly reduced mitoROS
levels (Fig. 5D) and decreased the
migratory ability of 4T1, TUBO-P2J and HCC70 cells (Fig. 5E). These data suggest that the
effect of NecroX-5 on cancer cell migration and mitoROS levels is
mediated by a reduction in Intracellular Ca2+
concentrations.
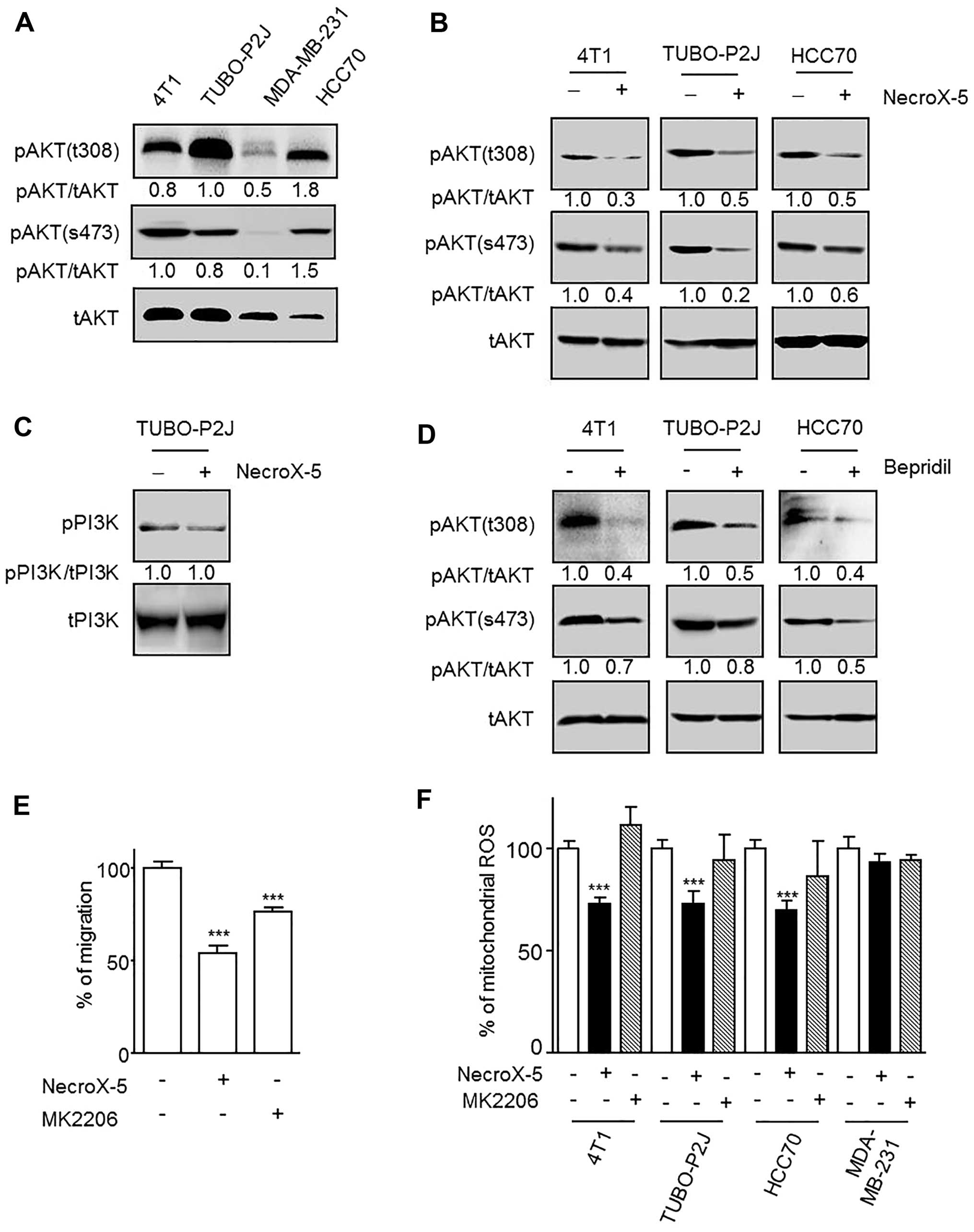
NecroX-5 effect on cell migration is
mediated by AKT inhibition
One of the differences between the NecroX-5
responsive and non-responsive cell lines was the basal activity of
AKT. 4T1, TUBO-P2J and HCC70 responded to NecroX-5 and demonstrated
full AKT activation (phosphorylation at Thr308 and Ser473), whereas
MDA-MB-231 did not respond to NecroX-5 or display AKT activation
(Fig. 6A). Additionally, treatment
with 10 μM NecroX-5 significantly reduced the AKT activation
levels in all of the responsive cell lines (Fig. 6B). Unlike the downregulation of
AKT, pPI3K levels were not changed by NecroX-5 (Fig. 6C). Furthermore, we found this
PI3K-independent AKT inhibition was also inducible by bepridil
(Fig. 6D). We used an AKT specific
inhibitor (MK-2206) to test if AKT inhibition mediated the
reduction in cell migration and mitoROS levels. The migratory
ability of 4T1 cells was found to be significantly reduced upon
treatment with 2.5 μM MK-2206 (Fig. 6E). In contrast, MK-2206 did not
reduce mitoROS levels in any of the tested cells (Fig. 6F).
Discussion
This study evaluated the inhibitory effects of
NecroX-5 on breast cancer cell metastasis in order to elucidate the
NecroX-5 mechanism of action. We demonstrated the inhibitory
effects of NecroX-5 on lung metastasis and its ability to extend
mouse survival significantly. Additionally, Transwell migration
assays revealed that NecroX-5 inhibits cell migration.
Interestingly, NecroX-5 did not inhibit migration in any of the
breast cancer cell lines tested, leading us to conclude that there
were differences in how the cell lines responded to the inhibitory
mechanisms of NecroX-5.
Cell migration and invasion are the first steps of
metastasis. A growing body of evidence suggests that ROS plays
important roles in cell migration and invasion (3). Sources of intracellular ROS include
the mitochondrial electron transport chain (ETC), phagocytic and
non-phagocytic NOX, xanthine oxidases, cyclooxygenases (COX), and
lipoxygenases (LOX) (16).
However, it is currently unclear which sources are more important
for tumour metastasis. In this study, cytoROS and mitoROS were
separately evaluated with H2DCFDA and MitoSOX-red,
respectively. NecroX-5 medicated inhibition of cell migration
correlated with the reduced levels of mitoROS, but not cytoROS.
This suggested that mitoROS levels are an important target for
controlling tumour metastasis. However, other studies have
suggested that the inhibition of cell migration and metastasis
correlated with reduced cytoROS levels (8,17–19).
These differences could be due to differences in experiment
settings. Others evaluated migration under specific conditions,
whereas we assessed intrinsic migration without any stimulation.
Alternatively, NecroX-5 may also inhibit migration by means other
than the reduction of mitoROS levels that we identified in this
study. Additionally, we concluded that NecroX-5 reduced mitoROS
levels indirectly because they remained unchanged in MDA-MB-231
after treatment. Furthermore, experiments with MitoQ demonstrate
that reduced mitoROS levels by NecroX-5 may not inhibit cell
migration.
MitoROS levels are directly regulated by multiple
inputs, including Sirt3, Forkhead box O transcription factors
(FOXOs), immunoreceptors, PI3K-AKT, hypoxia, cytokine stimulation,
calcium influx, mitophagy and uncoupling proteins (UCPs) (14). Among these inputs, we evaluated
changes in the intracellular Ca2+ concentrations because
it modulates both ROS generation and clearance (20,21).
Moreover, calcium is a critical regulator of cell migration
(15,22–27).
NecroX-5 has been shown to reduce mitochondrial calcium
concentrations (10); our results
demonstrate that NecroX-5 significantly reduced intracellular
Ca2+ in NecroX-5-responsive cells (HCC70 and 4T1), but
not in unresponsive cells (MDA-MB-231). intracellular
Ca2+ levels are determined by a balance between 'on'
reactions that introduce Ca2+ into the cytoplasm and
'off' reactions that signal its removal through a coordinated
effort of buffers, pumps, and exchangers (28). The 'on' reactions involves calcium
influx from the extracellular space and calcium release from
intracellular storage in various organelles, including the
endoplasmic/sarcoplasmic reticulum (ER/SR), lysosomes, and
mitochondria (15). Although we
could not rule out other mechanisms that participate in
Ca2+ homeostasis, we were able to demonstrate that
NecroX-5 reduced Ca2+ influx from the extracellular
space. Blocking experiments with bepridil demonstrated that
NecroX-5′s mechanism of action is to reduce the Ca2+
influx, thereby mediating the reduction of mitoROS levels and the
inhibition of cell migration.
We also evaluated the PI3K/AKT pathway because it
has been shown to promote cell survival and increase proliferation
and motility (29,30). Also NecroX-5 inhibits both cell
proliferation and migration in HCC70 cells, and NecroX-5 responsive
cell lines exhibited full activation of AKT at baseline (Thr308 and
Ser473), while unresponsive cells did not. Western blot analysis
revealed that NecroX-5 inhibited AKT in a PI3K-independent and
intracellular calcium-dependent manner. In a previous study, AKT
was shown to be regulated by calcium/calmodulin in a
PI3K-independent manner (31,32).
Thus, it is possible that AKT downregulation by NecroX-5 may be
mediated by calmodulin inactivation in response to intracellular
calcium reduction.
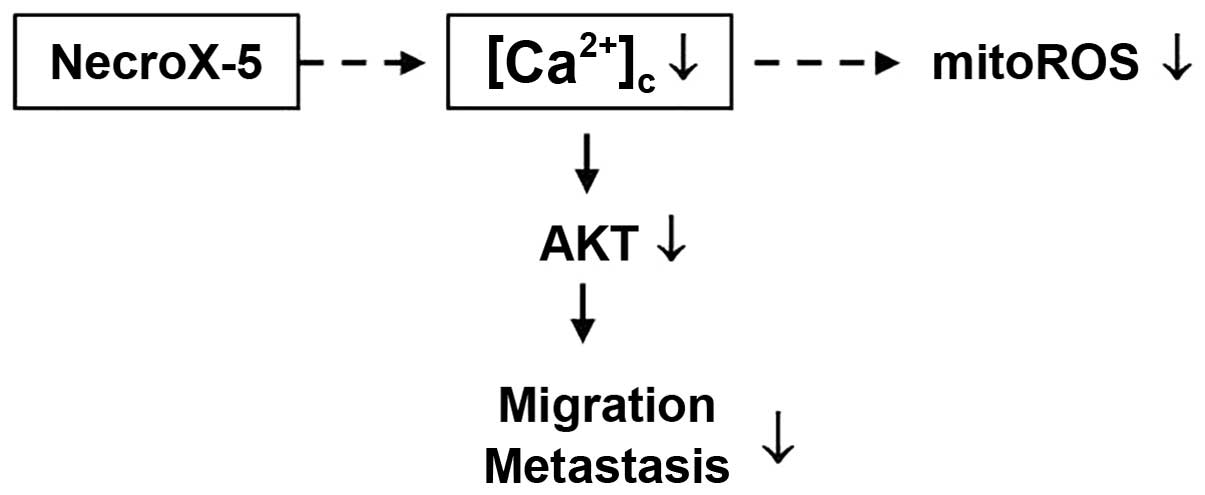
In conclusion, this study introduced NecroX-5 as a
novel anticancer drug that inhibits tumour cell metastasis through
the Ca2+-dependent modulation of AKT signalling
(Fig. 7). Additionally, the
downregulation of AKT by blocking calcium influx may be the
mechanism by which NecroX-5 inhibits migration. Finally, we found
that mitoROS reduction was not related to AKT downregulation
(Fig. 7). Future studies should
attempt to elucidate the mechanisms by which intracellular calcium
concentrations decrease, identify target channels, and evaluate the
effects of calcium release in the context of NecroX-5
treatment.
Acknowledgments
This study was supported by the National Research
Foundation of Korea (NRF) and funding granted by the Ministry of
Education of Korea (2010-0020224) and by the Ministry of Science,
and Future Planning (R13-2007-023-00000-0).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurd TR, DeGennaro M and Lehmann R: Redox
regulation of cell migration and adhesion. Trends Cell Biol.
22:107–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tochhawng L, Deng S, Pervaiz S and Yap CT:
Redox regulation of cancer cell migration and invasion.
Mitochondrion. 13:246–253. 2013. View Article : Google Scholar
|
|
5
|
Pani G, Galeotti T and Chiarugi P:
Metastasis: Cancer cell's escape from oxidative stress. Cancer
Metastasis Rev. 29:351–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Halliwell B: Oxidative stress and cancer:
Have we moved forward? Biochem J. 401:1–11. 2007. View Article : Google Scholar
|
|
7
|
Storz P: Reactive oxygen species in tumor
progression. Front Biosci. 10:1881–1896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goh J, Enns L, Fatemie S, Hopkins H,
Morton J, Pettan-Brewer C and Ladiges W: Mitochondrial targeted
catalase suppresses invasive breast cancer in mice. BMC Cancer.
11:1912011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim HJ, Koo SY, Ahn BH, Park O, Park DH,
Seo DO, Won JH, Yim HJ, Kwak HS, Park HS, et al: NecroX as a novel
class of mitochondrial reactive oxygen species and ONOO−
scavenger. Arch Pharm Res. 33:1813–1823. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thu VT, Kim HK, Long T, Lee SR, Hanh TM,
Ko TH, Heo HJ, Kim N, Kim SH, Ko KS, et al: NecroX-5 prevents
hypoxia/reoxygenation injury by inhibiting the mitochondrial
calcium uniporter. Cardiovasc Res. 94:342–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park MK, Lee BD, Chae SW, Chi J, Kwon SK
and Song JJ: Protective effect of NecroX, a novel necroptosis
inhibitor, on gentamicin-induced ototoxicity. Int J Pediatr
Otorhinolaryngol. 76:1265–1269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi JM, Park KM, Kim SH, Hwang DW, Chon
SH, Lee JH, Lee SY and Lee YJ: Effect of necrosis modulator
necrox-7 on hepatic ischemia-reperfusion injury in beagle dogs.
Transplant Proc. 42:3414–3421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song H, Kim TO, Ma SY, Park JH, Choi JH,
Kim JH, Kang MS, Bae SK, Kim KH, Kim TH, et al: Intratumoral
heterogeneity impacts the response to anti-neu antibody therapy.
BMC Cancer. 14:6472014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sena LA and Chandel NS: Physiological
roles of mitochondrial reactive oxygen species. Mol Cell.
48:158–167. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prevarskaya N, Skryma R and Shuba Y:
Calcium in tumour metastasis: New roles for known actors. Nat Rev
Cancer. 11:609–618. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gloire G, Legrand-Poels S and Piette J:
NF-kappaB activation by reactive oxygen species: Fifteen years
later. Biochem Pharmacol. 72:1493–1505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alexandrova AY, Kopnin PB, Vasiliev JM and
Kopnin BP: ROS up-regulation mediates Ras-induced changes of cell
morphology and motility. Exp Cell Res. 312:2066–2073. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishikawa K, Takenaga K, Akimoto M,
Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y and Hayashi
J: ROS-generating mitochondrial DNA mutations can regulate tumor
cell metastasis. Science. 320:661–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishikawa M: Reactive oxygen species in
tumor metastasis. Cancer Lett. 266:53–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yan Y, Wei CL, Zhang WR, Cheng HP and Liu
J: Cross-talk between calcium and reactive oxygen species
signaling. Acta Pharmacol Sin. 27:821–826. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang DX and Gutterman DD: Mitochondrial
reactive oxygen species-mediated signaling in endothelial cells. Am
J Physiol Heart Circ Physiol. 292:H2023–H2031. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pettit EJ and Fay FS: Cytosolic free
calcium and the cytoskeleton in the control of leukocyte
chemotaxis. Physiol Rev. 78:949–967. 1998.PubMed/NCBI
|
|
23
|
Roderick HL and Cook SJ: Ca2+
signalling checkpoints in cancer: Remodelling Ca2+ for
cancer cell proliferation and survival. Nat Rev Cancer. 8:361–375.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clark K, Langeslag M, van Leeuwen B, Ran
L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K and van Leeuwen
FN: TRPM7, a novel regulator of actomyosin contractility and cell
adhesion. EMBO J. 25:290–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waning J, Vriens J, Owsianik G, Stuwe L,
Mally S, Fabian A, Frippiat C, Nilius B and Schwab A: A novel
function of capsaicin-sensitive TRPV1 channels: Involvement in cell
migration. Cell Calcium. 42:17–25. 2007. View Article : Google Scholar
|
|
26
|
Hewavitharana T, Deng X, Soboloff J and
Gill DL: role of STIM and Orai proteins in the store-operated
calcium signaling pathway. Cell Calcium. 42:173–182. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang S, Zhang JJ and Huang XY: Orail and
STIM1 are critical for breast tumor cell migration and metastasis.
Cancer Cell. 15:124–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Berridge MJ, Bootman MD and Roderick HL:
Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev
Mol Cell Biol. 4:517–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sullivan LB and Chandel NS: Mitochondrial
reactive oxygen species and cancer. Cancer Metab. 2:172014.
View Article : Google Scholar
|
|
31
|
Coticchia CM, Revankar CM, Deb TB, Dickson
RB and Johnson MD: Calmodulin modulates Akt activity in human
breast cancer cell lines. Breast Cancer Res Treat. 115:545–560.
2009. View Article : Google Scholar
|
|
32
|
Deb TB, Coticchia CM and Dickson RB:
Calmodulin-mediated activation of Akt regulates survival of
c-Myc-overexpressing mouse mammary carcinoma cells. J Biol Chem.
279:38903–38911. 2004. View Article : Google Scholar : PubMed/NCBI
|