Introduction
SMAD4, a key regulator of TGF-β signaling, has a
critical role in cell growth, differentiation, migration and
apoptosis. Initially, SMAD4 was identified as a tumor
suppressor gene at a homozygous deleted region on human chromosome
18q21.1 in pancreatic ductal adenocarcinoma (1). SMAD4 inactivation at the gene or
protein expression level has been shown to be essential for the
progression of various tumors (2–7). It
is well known that SMAD4 functional inactivation occurs by loss of
heterozygosity (LOH) (6,8–10),
gene mutation (11–13), promoter hypermethylation (14), ubiquitin-mediated degradation
(15–17) and blocking nucleo-cytoplasmic
shuttling in many types of cancer (18–20).
Many studies have shown that the LOH or mutations of SMAD4
are associated with a poor prognosis in advanced gastric cancer
patients (21–23). We have also reported that SMAD4
expression is frequently downregulated in human gastric cancer by
SMAD4 LOH or partial promoter methylation, and its
alteration correlates with gastric cancer progression (24). However, this does not seem to be
enough to explain the functional loss of SMAD4. Our results
suggested a possibility of the downregulation of SMAD4 being
affected by another mechanism. Recent studies have analyzed the
genetic structure and function of the SMAD4 promoter region,
and predicted several interacting transcription factors, including
SP1, ETS1, NRF1 and HSF1 (25).
These results propose a strong potential that SMAD4 expression is
regulated by positive or negative transcription factors binding at
the SMAD4 promoter region. However, the function of
transcription factors for SMAD4 transcriptional activation is still
not completely understood.
Myeloid zinc finger 1 (MZF1/MZF1A/MZF1B/ZNF42) is a
member of SCAN-zinc finger (SCAN-ZF) transcription factor family
and has been mentioned in a number of cancers and cellular
functions. MZF1 is a bi-functional transcription factor that can
act as both a transcriptional repressor and activator, and is
involved in cellular differentiation, proliferation, migration and
apoptosis in various types of cancer (26). The mechanism of how MZF1 is
involved in cancer development, including its target molecules, is
still elusive. For example, MZF1 reduces tumor invasiveness through
transcriptional suppression of MMP2 (27) and IGF1R (28–30)
and transcriptional activation of TNFRSF10B (DR5) (31) and FPN (ferroportin) (32) in human solid tumors. Moreover, MZF1
interacts with the tumor suppressor LDOC1 and enhances its
apoptotic activity (33). However,
several reports have demonstrated that overexpression of MZF1
increases proliferation, migration, and metastasis through
regulation of its diverse target genes in cancer cells (34–36).
Therefore, there needs to be future work carried out that clarifies
and confirms the role of MZF1 in cancer.
In the present study, we identified MZF1 as a
putative transcription factor of SMAD4, and found that the
transcriptional level of SMAD4 is increased by MZF1. In addition,
we showed that MZF1 overexpression inhibits the migration of
gastric cancer cells, highlighting SMAD4 as a new target for the
tumor migration suppressor effect of MZF1 in gastric cancer cells,
and the present study suggests potential reasons for SMAD4
transcriptional repression during tumor progression. Furthermore,
our result support the notion that the MZF1-SMAD4 axis signaling
mechanism could be a potential target in the treatment of gastric
cancer.
Materials and methods
Cell culture
Human gastric carcinoma cells were obtained from the
Korean Cell Line Bank (KCLB; Seoul, Korea). All cell lines were
authenticated by short tandem repeat (STR) analysis at the
characterized cell line core facility at Abion, Inc., (Seoul,
Korea) during the study. The gastric carcinoma cells were
maintained in RPMI-1640 medium (HyClone Laboratories, Inc., Logan,
UT, USA), supplemented with 10% fetal bovine serum (FBS; HyClone
Laboratories) and 1% penicillin/streptomycin (HyClone
Laboratories). Cells were grown in a humidified atmosphere with 5%
CO2 at 37°C and routinely tested for mycoplasma
infection using Myco VALiD Mycoplasma PCR detection kit (Intron
Biotechnology, Gyeonggi-do, Korea).
Expression plasmid, si-RNA and
transfection
Cells were transfected with MZF1 expression plasmid
using FuGENE HD transfection reagent (Promega, Madison, WI, USA)
and then incubated for 24 h. si-SMAD4 duplexes were synthesized by
Invitrogen using the following sequence: 5′-GGU CAG CCA GCU ACU UAC
CAU CAU A-3′. si-MZF1 duplexes were synthesized by Cosmo Genetech
Co., Ltd., (Seoul, Korea) using the following sequence: 5′-CUA CUG
UAG GUG UCC AAU A-3′) (35).
Co-transfection of siRNA and plasmid was performed using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instruction.
Gene constructs
Luciferase reporter constructs were cloned using the
restriction map of the BAC729G3 bacterial artificial chromosome
(BAC) clone from the RPCI-11 human BAC library (Invitrogen), which
covers the alternative promoter region of SMAD4. The −1752
bp upstream region of the SMAD4 transcription start site and two
intra-gene region (+20 to +427 and +15422 to +16746) were subcloned
into the pGL3 basic vector (Promega). Deletion and mutant
constructs of putative SMAD4 promoters were generated by
PCR. For generation of point mutation, Pfu-DNA polymerase and DpnI
were used. All constructs were confirmed by sequencing.
Luciferase assay
After 24 h of transfection, cells were lysed with
luciferase assay buffer. Then, the luciferase activity was measured
using the Dual-luciferase reporter assay system according to the
manufacturer's instructions (Promega) and was followed by
luminescence measurement in a GENios Pro microplate reader (Tecan
Trading AG, Mannedorf, Switzerland).
Electrophoretic mobility shift assay
(EMSA) and chromatin immunoprecipitation (ChIP)
Nuclear extract of MKN74 cells were prepared by
Qproteome Nuclear Protein kit (Qiagen, Hilden, Germany) and
quantified by Pierce BCA assay kit (Thermo Fisher Scientific,
Waltham, MA, USA). DNA mobility shift assays were performed with
the following double-stranded oligonucleotides: MZF1 5′-CTC GGA GCG
GGA GGC GGG GGC AGC
CGG GAG AAA GG-3′. Two complementary oligonucleotides (1000 pmol of
each) were annealed and 5 pmol of the annealed oligonucleotides
were 5′-end-labeled with [γ-32P]-ATP (Amersham
Biosciences, Uppsala, Sweden) using T4 polynucleotide
kinase (New England Biolabs, Inc., Ipswich, MA, USA). Labeled
products were purified on a Sephadex G-25 column (Amersham
Biosciences). For antibody supershift analysis, nuclear extracts
were incubated for 30 min in binding buffer containing 7.5%
glycerol, 15 mM Tris-HCl, pH 7.5, 75 mM NaCl, 1.5 mM EDTA, pH 8.0,
1.5 mM dithiothreitol, 0.3% Nonidet P-40 and 1 µg of poly
(dI-dC), and then with the probe for 40 min at 37°C, and with 2
µg of antibodies overnight at 4°C or −20°C. The primary
antibodies used were as follows: His-probe (sc-8036) was purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and MZF1
(ab64866) was purchased from Abcam (Cambridge, UK). For the
competition assays, 100-fold excess amount of unlabeled competitor
was premixed with the radiolabeled probe before addition of the
binding mixture. DNA-protein complexes were resolved on 6%
non-denaturing PAGE gel at 250 V for 2.5 h. After separation, the
gels were dried and exposed to the phosphor screen. The relevant
protein-DNA probe complexes were analyzed by a BAS-1500 Image
Analyzer (Fujifilm, Tokyo, Japan). ChIP assays were performed using
the EZ-ChIP Kit (Millipore, Bedford, MA, USA) according to the
manufacturer's instructions. Immunoprecipitation was performed with
MZF1 antibody or rabbit IgG antibody (Santa Cruz Biotechnology).
DNA was analyzed by conventional PCR directed to specific regions
of the SMAD4 promoter and were amplified using the
respective forward and reverse primers: ChIP I (forward)
5′-CTCCCTCAAACAGGCCTTCGC-3′ and (reverse) 5′-CAG CTT TCC TTT CTC
CCG GCT-3′; ChIP II (forward) 5′-AGC CGG GAG AAA GGA AAG CTG-3′ and
(reverse) 5′-CCA AAC CGC TCC GTT ACC GCA-3′. PCR was performed for
30 cycles at 94°C (30 sec), 60°C (30 sec) and 72°C (30 sec).
Quantitative real-time (RT) PCR
Total RNA was extracted by TRIzol (Invitrogen) and
reversely transcribed to cDNA using the SuperScript II First-Strand
Synthesis system (Invitrogen). Following cDNA synthesis, qRT-PCR
was performed as described in a dual system LightCycler (Roche
Diagnostics) and the expression levels of target genes relative to
HPRT (control) were determined by a SYBR-Green-based comparative CT
method (relative fold change = 2−ΔΔCT). Primers used are
as follows: SMAD4 5′-TGG CCC AGG ATC AGT AGG T-3′ (forward) and
5′-CAT CAA CAC CAA TTC CAG CA-3′ (reverse); CTBP1 5′-ACT GCG TGA
CCC TGC ACT-3′ (forward) and 5′-GCC CCT TGT CTC ATC TGC-3′
(reverse). All primers were purchased from Cosmo Genetech Co., Ltd.
(Seoul, Korea).
Immunoblotting analysis
Whole cell lysates were prepared in RIPA buffer
supplemented with protease inhibitor cocktail (Roche Diagnostics).
Lysates were centrifuged at 4°C, 15,000 rpm for 20 min. Equal
amounts of protein samples were electrophoretically separated by
SDS-PAGE and transferred to nitrocellulose membrane. Membranes were
blocked in TBS-T (Tris-buffered saline with 0.05% Tween-20)
containing 5% non-fat dry milk and then incubated overnight at 4°C
with the primary antibodies [SMAD4 (sc-7966), MZF1, GAPDH (FL-335)]
diluted in the same buffer. GAPDH was used as loading control.
Membranes were washed with TBS-T and incubated with horseradish
peroxidase-conjugated secondary antibodies (Pierce, Rockford, IL,
USA). The results were visualized with ECL reaction.
Screening analysis of transcription
factor binding site (TFBS)
MatInspector (Genomatix Software GmbH, Munich,
Germany; http://www.genomatix.de) was used to
locate regulatory elements within the aforementioned core promoter
region, and the internet-based TFSEARCH: Searching TFBS program
(http://www.cbrc.jp/research/db/TFSEARCH.html) was used
to localize the putative transcription factor binding sites within
the 5′-flanking region of SMAD4. Alignment of human and
mouse promoter sequences was performed with NCBI's Ensemble
interface. Furthermore, mouse and chimpanzee SMAD4 promoter
sequences were compared with human genomic sequences for
conservation of the MZF1 binding motif.
Cell proliferation assay
Cell proliferation assays were performed using the
EZ-Cytox kit (Daeil Lab Service, Co., Ltd., Seoul, Korea) according
to the manufacturer's instructions. WST assays were performed as
previously described (37). Cell
were seeded at the density of 1×105 cells and
transfected with the expression vector.
Cell migration assay
Cell migration was analyzed using 24-well Transwell
plates with polycarbonate membranes (Corning Costar, Corning, Ny,
USA). For Transwell migration assay, cells were transfected with
expression vectors encoding wild-type (WT)-MZF1 or si-MZF1. Cells
were prepared 24 h post-transfection and then loaded into the upper
compartment. After incubation for 24 h at 37°C, the cell number was
detected with a GENios Pro microplate reader (Tecan Trading AG)
using 485/535 nm filter set as previously described. The migration
assay was performed in at least three independent experiments.
Values are expressed as percentages compared to the control. The
in vitro wound-healing assay was performed to examine the
migration on gastric cancer cells transfected with WT-MZF1 vectors
or si-MZF1. Transfected cells were grown on 96-well plates with
their respective culture media. After the growing cell layers had
reached confluence, wounds were prepared by a single scratch on the
monolayer using a wound maker and the wounded layers were washed
with phosphate-buffered saline (PBS) to remove the cell debris.
Cell plates were applied to the IncuCyte ZOOM (Essen BioScience,
Inc., Ann Arbor, MI, USA) and scanned every 1 h for 24 or 48 h. The
wound-healing assay was performed in triplicate in at least three
independent experiments.
Statistical analysis
The results were compared using one-way ANOVA
analysis followed by the Turkey's test for multiple comparisons.
Means were considered significant, at P<0.05. Statistical
analysis was performed using a GraphPad Prism package for personal
computers (GraphPad Software, Inc., San Diego, CA, USA). Results
were considered significant at P<0.05, P<0.01 or P<0.001.
All the data with error bars are presented as mean ± SD for at
least three independent experiments.
Results
MZF1 positively regulates SMAD4 promoter
activity and expression
To explore the transcriptional regulator candidates
of the SMAD4 promoter, we investigated the region from −1752
to +84 (1836 bp) to the transcription start site using
bioinformatic tools, such as MatInspector professional software and
the internet-based TFSEARCH database. In our previous study, we
identified the transcriptional start site of SMAD4 by
reverse transcription-PCR and nucleotide sequencing, and we
reported that hypermethylation of CpG site within this region might
be related to the transcriptional silencing of SMAD4 (37). Our previous results on the identity
of the transcription start site were consistent with the European
Molecular Biology Laboratory database (http://www.ensembl.org), despite of the presence of
several alternative transcripts encoding exon 1 and exon 2 of
SMAD4. In this analysis, we screened three transcription
factors, HSF1, RUNX1 (AML-1) and MZF1, which have a higher
likelihood of interaction than other transcription factor
candidates (Fig. 1A). According to
previous reports, we first performed a comparison analysis of
reported smad4 promoter regions in HEK293T and SNU638. HEK293T cell
was used as positive control (25). The basal promoter activity of these
three different SMAD4 promoter regions was investigated
using a vector constructs: Luc-1752 (−1752 to +84), Roth et
al (38) (+20 to +427) and
Minami et al (39) (+15422
to 16746). As shown in Fig. 1B,
luciferase activity of the −1752 to +84 (Luc-1752) construct was
nearly 80 to 150-fold increased compared with the pGL3 control,
whereas the other constructs did not have effect on SMAD4
promoter activity. Our data suggested that the −1752 to +84 region
is essential for basal SMAD4 promoter activity, we further
concentrated on the role of this region. The transcriptional
activity of these three transcription factors on the SMAD4
promoter region was investigated using a vector construct
'Luc-1752', a luciferase-conjugated SMAD4 promoter region.
As shown in Fig. 1C, transient
co-transfection of Luc-1752 and the MZF1 expression vector
exhibited over a 2.5-fold increase in the induction on
transcriptional activity of SMAD4 promoter. However,
co-transfection of Luc-1752 with HSF1 or AML-1 (RUNX1) did not
reveal an increase in the SMAD4 promoter activity. Next, we
analyzed SMAD4 and MZF1 expression in 13 gastric cancer cell lines.
We found that SMAD4 mRNA (r= 0.28, P= 0.002) and SMAD4
protein (r=0.35, P=0.38) levels showed a close correlation with
MZF1 protein level (Fig. 1D). In
particular, their expression was substantially decreased in
KATOIII, MKN28, MKN74, NCI-N87 and SNU5 cells, whereas MKN1,
SNU484, SNU620 and SNU668 had relatively high levels of their
expression. In addition, ectopic expression of MZF1 increased SMAD4
levels in MKN74 cells (low expresser), whereas si-MZF1 transfection
decreased SMAD4 levels in MKN1 cell (high expresser). MZF1 affects
SMAD4 expression at both the mRNA and protein levels in gastric
cancer cell lines (Fig. 1E). These
results suggest that SMAD4 is a novel target gene of the
transcription factor MZF1.
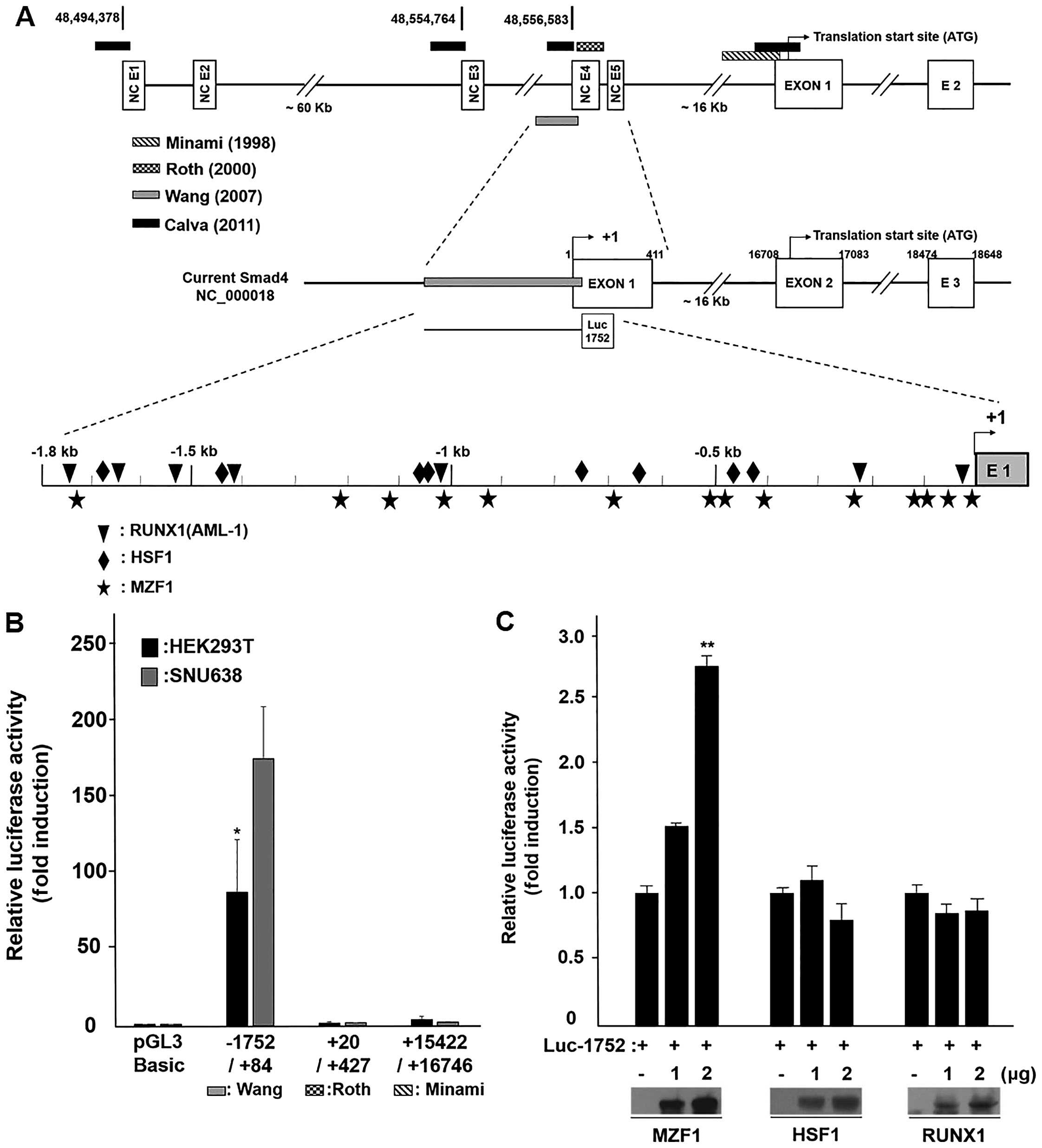 | Figure 1MZF1 positively regulates
SMAD4 promoter activity and expression. (A) Schematic
presentation of 5′-flanking region of human SMAD4 promoter
(top line) and current SMAD4 gene structure (middle line).
Black square, Calva et al (25); Dot, Roth et al (38); slash, Minami et al (39); Gray, Wang et al (24). Three transcription factors binding
site locus in the SMAD4 promoter and reporter construction
for luciferase assay. (B) The basal promoter activity of the three
different SMAD4 promoter regions. (C) Transcription factor
MZF1 activation of the SMAD4 promoter. Relative luciferase activity
was normalized by the wild-type Renilla luciferase activity.
Data represent the mean ± SD. *P<0.05,
**P<0.01 (Student's t-test). (D) qRT-PCR and
immunoblotting assays of SMAD4 and immunoblotting assay of MZF1 in
gastric cancer cell lines. SMAD4 mRNA and SMAD4 protein
levels showed a close correlation with MZF1 protein level: weak
correlation (+0.1 to +0.3), clear correlation (+0.3 to +0.7),
strong correlation (+0.7 to +1.0). (E) Cells were transfected with
WT-MZF1 or si-MZF1 and SMAD4 mRNA, and protein expression
was determined by qRT-PCR and immunoblotting assays. The qRT-PCR
values in D and E were normalized to the housekeeping gene CTBP1.
IB, immunoblot. *P<0.05, **P<0.01
(Student's t-test). |
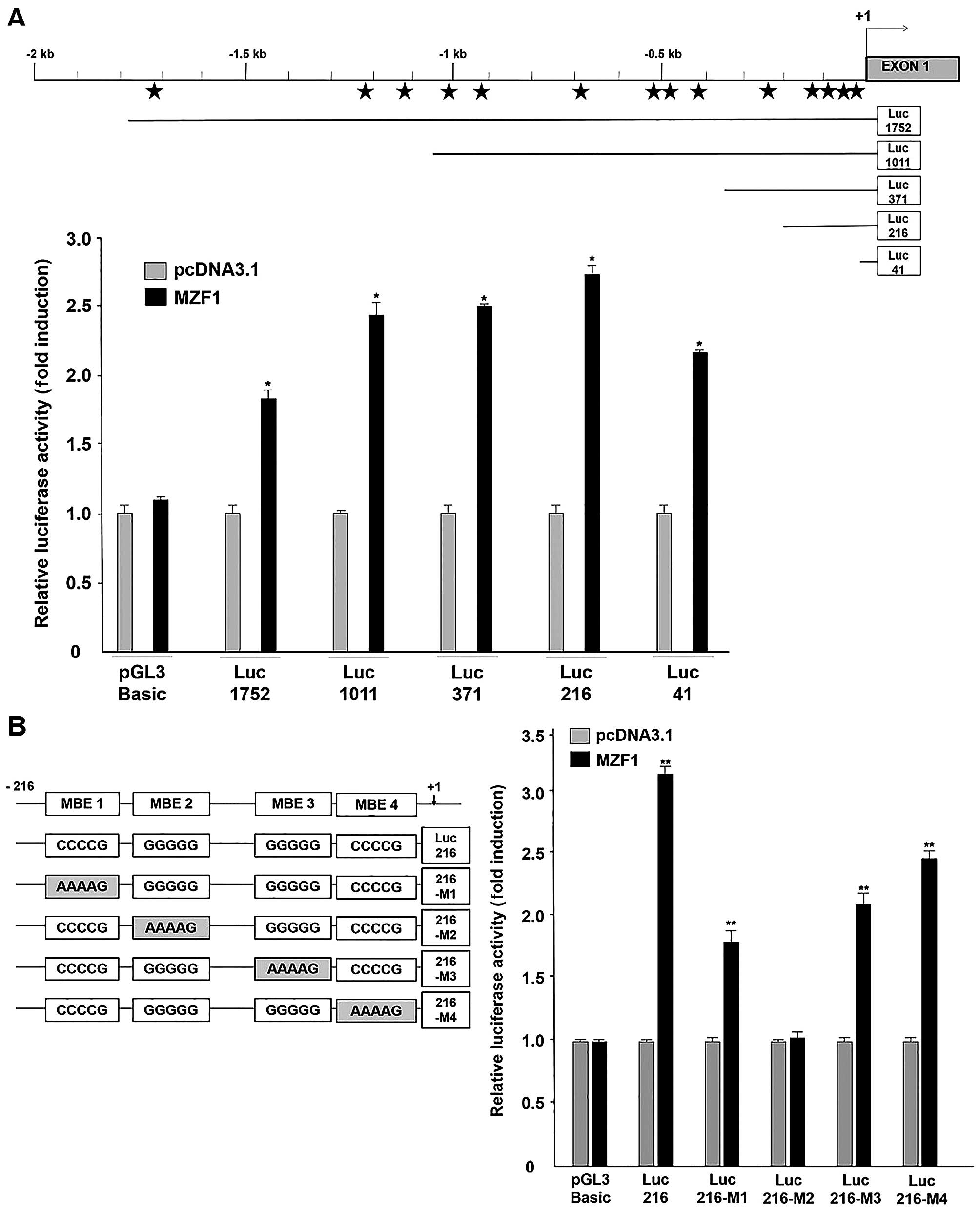
MZF1 directly binds to MEB2 (−80 to −77)
of SMAD4 promoter region
To analyze the core region enabling MZF1-mediated
transcriptional activation on the SMAD4 promoter, we
generated four constructs, each containing a partial deletion
mutant in the SMAD4 promoter, luc-1011, -371, -216 and -41
for luciferase assaying (Fig. 2A).
As shown in Fig. 2A, transient
co-transfection of the MZF1-expressing vector and each partial
deletion mutant construct exhibited induction in SMAD4
promoter activity from 1.7- to 3-fold. Among them, the Luc-216
construct showed the highest SMAD4 promoter activity under MZF1
overexpression. In further bioinformatic analysis, we identified
four putative MZF1-binding elements (MBE) on the Luc-216-containing
partially deleted SMAD4 promoter region (nucleotides −216 to
+84): MBE 1 (−102 to −99), MBE 2 (−80 to −77), MBE 3 (−52 to −49)
and MBE 4 (−12 to −9). To demonstrate essential MZF1-binding
element, we generated mutant reporter constructs containing mutated
MBE sequences (5′-AAAAG-3′) that disturb MZF1 binding. According to
the results (Fig. 2B, left panel),
mutation of MBE2 showed loss of promoter activity, whereas other
mutated MBEs exhibited over 1.5-fold increase in induction compared
to the negative control (Fig. 2B,
right panel). These results show that MZF1 could positively
regulate SMAD4 promoter activity and its critical binding
region might be MBE2 (−80 to −77) located from −216 to +84 in the
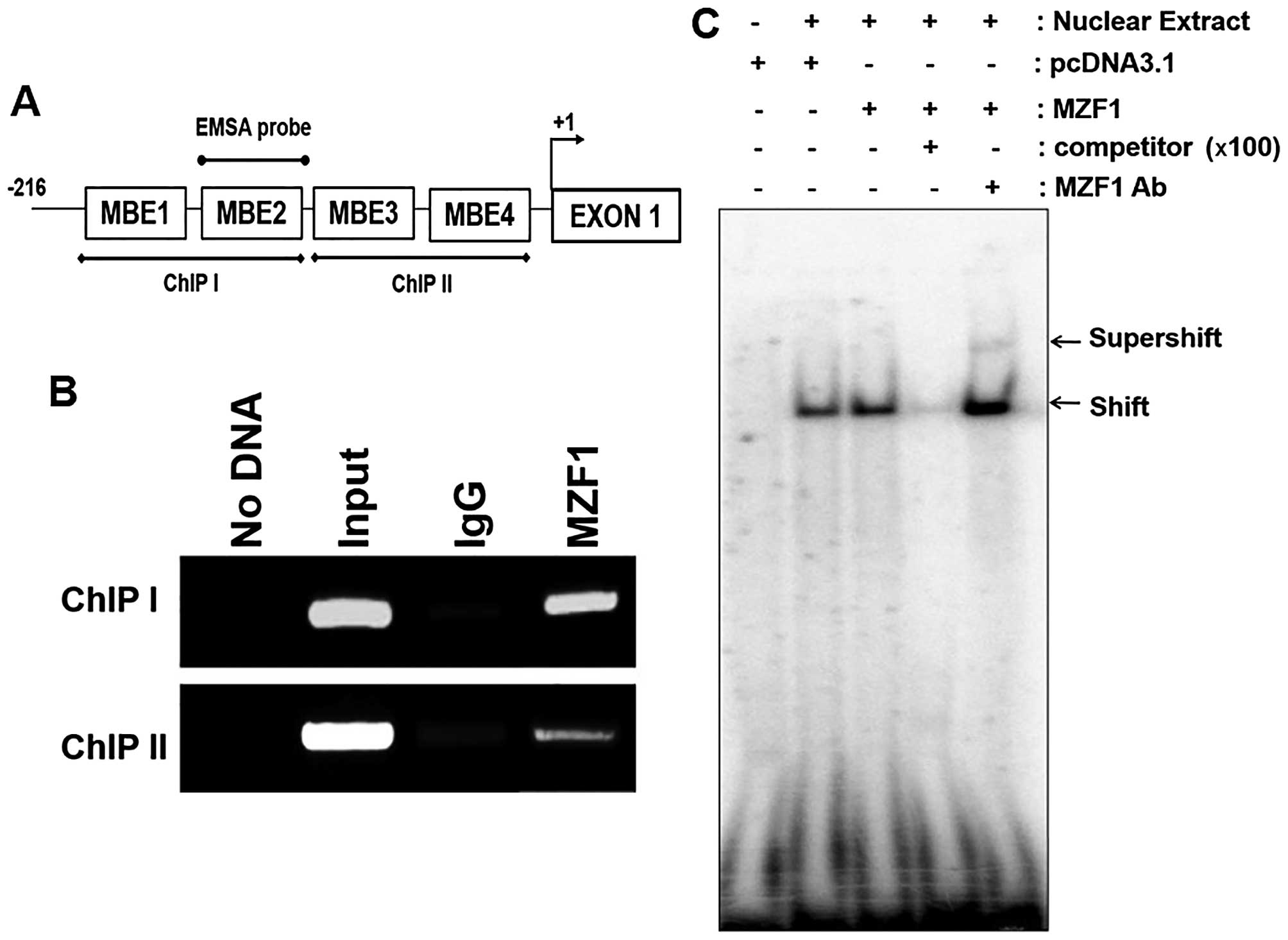
SMAD4 promoter. To examine the direct binding of MZF1 to the
SMAD4 promoter region, we performed ChIP assays and EMSAs.
For ChIP analysis, we produced ChIP I and ChIP II sequences
containing two independent MBE sites and we also designed, an EMSA
probe (Fig. 3A). In ChIP analysis,
MZF1 directly bound to the ChIP I sequence containing MBE1 and MBE2
sites (Fig. 3B). Moreover, EMSA
revealed that MZF1 specifically interacted with the EMSA probe
containing the MBE2 site (Fig. 3C)
and addition of an MZF1 antibody to the reaction mixture for EMSA
resulted in a supershifted band. These results indicate that MZF1
directly bind to MEB2 (−80 to −77) of SMAD4 promoter
region.
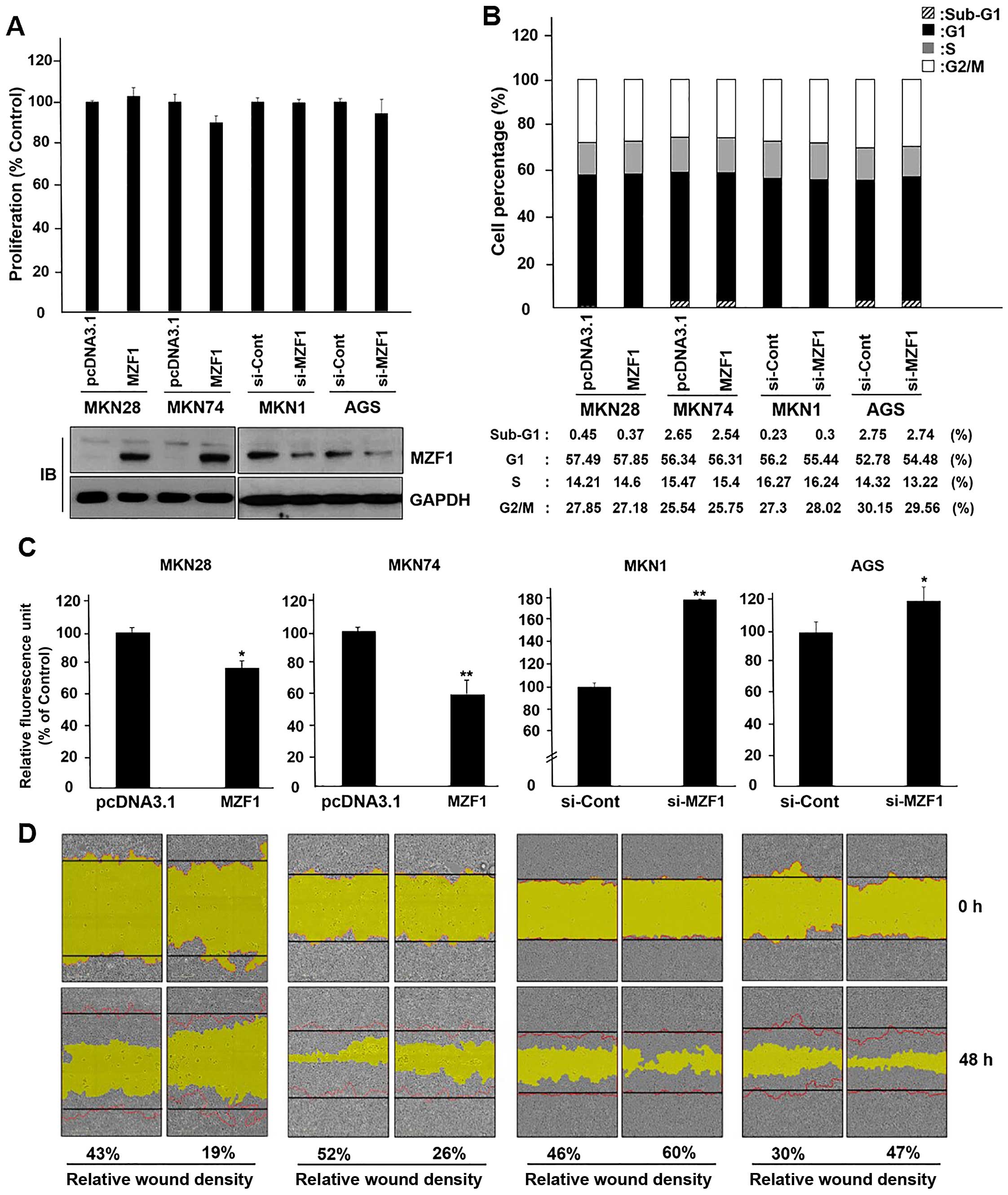
MZF1 inhibits migration of gastric cancer
cells
To elucidate the molecular function of MZF1 in
gastric cancer cells, we performed proliferation assay using WST
reagent and cell cycle analysis by flow cytometry after
overexpression or knockdown of MZF1. As shown in Fig. 4A, transient transfection of WT-MZF1
did not affect MKN28 and MKN74 cell proliferation and
siRNA-mediated knockdown of endogenous MZF1 neither affected MKN1
and AGS cell proliferation, nor did it affect the cell cycle change
of the 4 cell lines (Fig. 4B).
Next, we investigated whether MZF1 influences migration of gastric
cancer cells. We performed migration assay using the Transwell
migration assay and wound-healing assay. As a result, cell
migration was substantially decreased and increased by transfection
of WT-MZF1 and si-MZF1 (Fig. 4C and
D). These results support the idea that MZF1 overexpression
might negatively regulate migration, but does not have an effect on
cell proliferation and growth of gastric cancer cells.
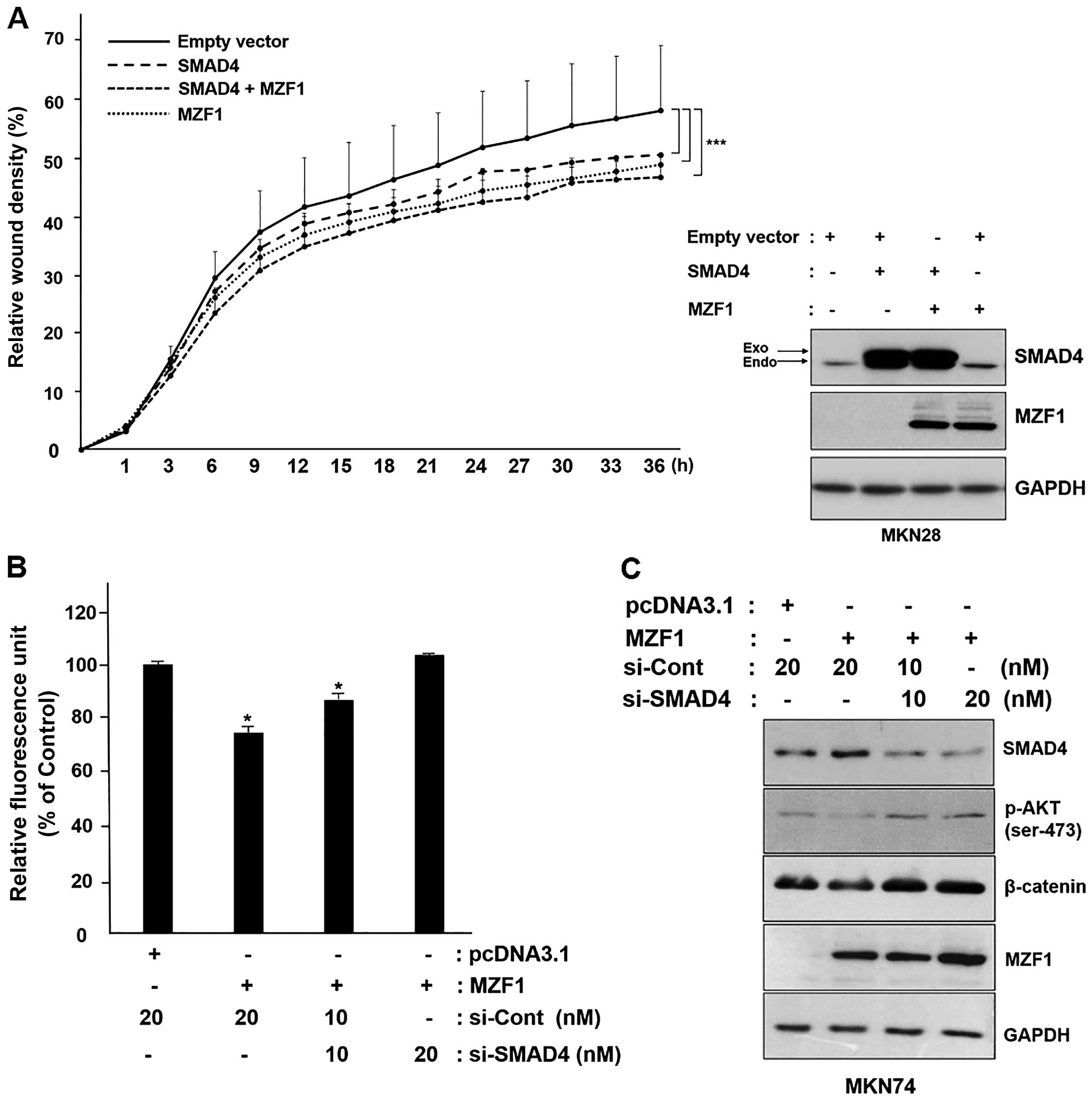
MZF1 suppresses cancer cell migration by
enhancing SMAD4 expression
According to previous reports, SMAD4 plays an
important role in the regulation of cancer cell migration, and
therefore, we analyzed whether MZF1 suppresses cancer cell
migration through regulation of SMAD4 expression. As shown in the
Fig. 5A, co-transfection of MZF1
and SMAD4 into MKN28 cells significantly exhibited suppressive
effect on cellular migration in the wound-healing assay. Consistent
with this, the inhibitory effect on migration by overexpression of
MZF1 and SMAD4 is reinforced due to MZF1-mediated increase in the
expression of SMAD4. We next examined whether silencing of SMAD4
expression inhibits MZF1-mediated cellular migration in human
gastric cancer MKN74 cells. Transient overexpression of MZF1
revealed over 30% reduction of MKN74 migration. In addition,
co-transfection of si-SMAD4 restored this MZF1-induced reduction of
migration in a dose-dependent manner (Fig. 5B). Likewise, the data from
immunoblotting analysis showed that a knockdown of SMAD4 expression
increased phosphorylation of AKT and expression of β-catenin, which
are key regulatory molecules in migration. As would be expected,
downregulation of AKT phosphorylation and suppression of β-catenin
expression was observed when MZF1 was overexpressed (Fig. 5C). In summary, our findings
indicated that MZF1 transcriptionally upregulates expression of
SMAD4, and this MZF1-mediated expression of SMAD4 act as a
suppressor of cancer migration in gastric cancer cells.
Discussion
Gastric cancer is one of the most commonly diagnosed
malignancies worldwide. Many risk factors have been associated with
the development of gastric cancer and the multifactorial pathogenic
mechanisms including gastric adenomas, polyps, and Helicobacter
pylori infection (23). SMAD4
is a multifunctional protein and its tumor suppressor effect has
been reported in many studies (40–42).
The loss of SMAD4 expression is an especially common feature in
human gastric cancer and is a critical event in the development and
progression of gastric cancer (22,23).
The well-known mechanisms of SMAD4 inactivation can
be divided into five groups: i) LOH is related to mechanisms of
SMAD4 inactivation. Previous studies have demonstrated that
deletion of SMAD4 frequently occurred in various cancers including
those of the brain (6), lung
(9), bladder (10) and colon (8). We have also reported that
SMAD4 LOH was detected in 20 of 70 (29%) gastric cancer
patients. Loss of SMAD4 expression occurred in 10 of 20 (50%)
LOH-positive cases. LOH of the SMAD4 locus was correlated
with loss of SMAD4 mRNA and SMAD4 protein expression in
gastric carcinoma and gastric cancer cells (24). ii) SMAD4 inactivation occurs by
SMAD4 mutation, which is frequently associated with
pancreatic (13), head and neck
(11) and colon (12) cancers. Mutational events of SMAD4
in various cancers were correlated with loss of SMAD4 expression,
which is associated with tumor malignant progression (1). However, SMAD4 mutation is an
infrequent occurrence. We have also reported that SMAD4 mutations
were not found in tissue samples of gastric carcinoma (24). iii) SMAD4 reduction is associated
with promoter hypermethylation. Previously, SMAD4 methylation has
been studied in colon and prostate cancer. The methylation-specific
PCR primers used in these studies were designed in the +20 to +427
region of the current SMAD4 transcription start site
(14,38). However, we were the first group to
confirm the location of the SMAD4 transcription start site
that is different from the previously reported SMAD4 promoter
region. We have reported that SMAD4 methylation is
correlated with loss of SMAD4 expression. However, SMAD4
promoter methylation was rare in gastric cancer progression
(24). iv) Functional loss of
SMAD4 occurs by ubiquitin-mediated degradation. For instance, SMAD4
is targeted for degradation through interactions with proteins
including Jun-activating binding protein 1 (JAB1) (16), SCFβ-TrCP-ubiquitin ligase complex
(15), and the carboxyl-terminus
of Hsc-70 interacting protein (CHIP) (17). v) SMAD4 is inactivated as a result
of interference in the nucleus-cytoplasm shuttle of SMAD4. SMAD4
shuttles continuously between the cytoplasm and the nucleus
(27). We have also reported that
nuclear expression, and not cytoplasmic expression, is strongly
correlated with the prognosis in gastric cancer (43). Nucleocytoplasmic shuttling is
specific for SMAD4-induced target gene regulation and the process
can involve R-SMADs. Previous studies indicated that the activation
of SMAD4 nuclear export signal (NES) depends on the nuclear
transport receptor CEM 1 (19). In
addition, some retention factors, such as microtubules (20), or nuclear import proteins, such as
ELF (embryonic liver fodrin) (18), could also be involved in the
subcellular distribution of SMAD4. Taken together, these factors
are highly significant for SMAD4 functional inactivation. However,
this does not seem to be enough to explain the functional loss of
SMAD4. Therefore, in this study, we focused on the regulation of
SMAD4 expression by transcription factors and searched for new
transcription factors based on the SMAD4 promoter region we
previously reported (24). In the
present study, we show that expression levels of MZF1 and SMAD4 are
low and extremely low, respectively, in cells including KATOIII,
MKN28, MKN74, NCI-N87 and SNU5. Moreover, protein expression of
MZF1 is correlated with SMAD4 mRNA (r=0.28, P= 0.002) and
SMAD4 protein expression (r= 0.35, P= 0.38) in gastric cancer cell
lines. Our previous studies showed that loss of SMAD4 expression
was caused by LOH (AGS, KATOIII, MKN28, MKN74, SNU5 and SNU216),
promoter methylation (NCI-N87) and mutation (SNU216). These
correlations were more increased when SNU216 cell with a
SMAD4 LOH and mutation were removed: SMAD4 mRNA and
MZF1 protein (r= 0.52, P= 0.003), SMAD4 protein and MZF1
protein (r=0.65, P=0.28). Our data suggested that SMAD4
inactivation may be caused by not only by LOH or promoter
methylation but also by abnormal expression of MZF1. This suggests
that MZF1 may contribute to mechanistic inactivation of SMAD4 in
gastric cancers. After investigation, we found that SMAD4
expression was increased by the transcription factor MZF1 in
gastric cancer cells, regulated through its direct binding to the
SMAD4 promoter region. This is the first study reporting a
positive transcriptional regulator for SMAD4. In a previous study,
it was shown that overexpression of MZF1 inhibits cancer cell
migration and tumorigenesis (27,44).
MZF1 is a multifunctional protein and its underlying molecular
mechanisms have not been fully clarified. Some studies have found
that the overexpression of MZF1 inhibits apoptosis and promotes
oncogenesis (45,46) showing that MZF1 might function as a
potential oncogene contributing to the development and progression
of human cancers (34). Although
the role of MZF1 in tumorigenesis is still controversial, we show
that overexpression of MZF1 inhibits gastric cancer cell migration
through decreased AKT and β-catenin expression. Interestingly,
SMAD4 is a well-known tumor migration suppressor; it elicits its
antitumor effects by blocking β-catenin signaling and SMAD4
directly suppresses the Wnt/β-catenin signaling activity in colon
cancer cells by decreasing β-catenin mRNA expression
(47). In addition, the loss of
SMAD4 activity activates the AKT pathway through upregulation of
anti-apoptotic proteins including BCL2, BCL2L2 (BCLW) and survivin
(48). Moreover, we found that
SMAD4 regulated the suppression of WNT/β-catenin signaling by
downregulating the oncoprotein AURKA in cancer (49) and contributed to the tumor
suppressor function of Tob1 including apoptosis and inhibiting
proliferation, migration, and invasion in gastric cancer cells
(37,50). Considering this fact, although both
MZF1 and SMAD4 have exhibited functions of migration suppression,
MZF1 likely inhibits cancer cell migration in conjunction with
SMAD4. In this study, we found that SMAD4 plays a critical role in
MZF1-inhibited gastric cancer cell migration. The migration
suppressor function of MZF1 is possible through regulation of SMAD4
expression. The present study provides new insight into the
molecular basis for the tumor suppressive effect of the MZF1-SMAD4
axis in gastric cancer cells. Taken together, SMAD4 is a novel
target of MZF1, which promotes the tumor suppressor function of
MZF1 in gastric tumorigenesis. This finding indicates that low
expression of MZF1 is linked to transcriptional repression of
SMAD4, at least in gastric cancer progression. We suggest that
MZF1-SMAD4 signaling may represent a new therapeutic target in
advanced human gastric cancer.
Acknowledgments
The present study was supported by a grant (nos.
NRF-2014R1A1A1008685 and NRF-2015R1C1A2A01055635) from the National
Research Foundation of Korea (NRF) by the Ministry of Science and
ICT & Future Planning. Also, we thank Dr Tara L. Sander
(Medical College of Wisconsin, Milwaukee, Wisconsin) for MZF1
(pcDNA 3.1-MZF1b-myc/His(−)) vector.
References
|
1
|
Miyaki M and Kuroki T: Role of Smad4
(DPC4) inactivation in human cancer. Biochem Biophys Res Commun.
306:799–804. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamada S, Fujii T, Kanda M, Sugimoto H,
Nomoto S and Kodera Y: Clinical significance of SMAD4 expression in
resectable pancreatic cancer: Correlation with tumor progression
and recurrence pattern. Cancer Res. 74(19 Suppl): 38312014.
View Article : Google Scholar
|
|
3
|
Sasaki S, Yamamoto H, Kaneto H, Ozeki I,
Adachi Y, Takagi H, Matsumoto T, Itoh H, Nagakawa T, Miyakawa H, et
al: Differential roles of alterations of p53, p16, and SMAD4
expression in the progression of intraductal papillary-mucinous
tumors of the pancreas. Oncol Rep. 10:21–25. 2003.
|
|
4
|
Mikami T, Ookawa K, Shimoyama T, Fukuda S,
Saito H and Munakata A: KAI1, CAR, and Smad4 expression in the
progression of colorectal tumor. J Gastroenterol. 36:465–469. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Horvath LG, Henshall SM, Kench JG, Turner
JJ, Golovsky D, Brenner PC, O'Neill GF, Kooner R, Stricker PD,
Grygiel JJ, et al: Loss of BMP2, Smad8, and Smad4 expression in
prostate cancer progression. Prostate. 59:234–242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He SM, Zhao ZW, Wang Y, Zhao JP, Wang L,
Hou F and Gao GD: Reduced expression of SMAD4 in gliomas correlates
with progression and survival of patients. J Exp Clin Cancer Res.
30:702011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Hattem A, Brosens L, de Leng W,
Morsink F, ten Kate FJ, Iacobuzio-Donahue CA, Giardiello FM and
Offerhaus J: SMAD4 Protein expression in polyps of juvenile
polyposis syndrome mirrors genetic status but does not reflect
neoplastic progression. Gastroenterology. 136:A452–A453. 2009.
View Article : Google Scholar
|
|
8
|
Yan P, Klingbiel D, Saridaki Z, Ceppa P,
Curto M, McKee TA, Roth A, Tejpar S, Delorenzi M, Bosman FT, et al:
Reduced expression of Smad4 is associated with poor survival in
colon cancer. Clin Cancer Res. 22:3037–3047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie M, He C and Wei S: Relationship
between expression of TGF-β1, Smad2, Smad4 and prognosis of
patients with resected non-small cell lung cancer. Zhongguo Fei Ai
Za Zhi. 18:543–548. 2015.In Chinese. PubMed/NCBI
|
|
10
|
Tang ZY, Yang LY, Zhang YJ, Peng KL and Qi
L: Smad4 and TGF-beta1 expression and clinical significance in
bladder transitional cell carcinoma. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 31:363–366. 2006.In Chinese. PubMed/NCBI
|
|
11
|
Qiu W, Schönleben F, Li X and Su GH:
Disruption of transforming growth factor beta-Smad signaling
pathway in head and neck squamous cell carcinoma as evidenced by
mutations of SMAD2 and SMAD4. Cancer Lett. 245:163–170. 2007.
View Article : Google Scholar
|
|
12
|
Miyaki M, Iijima T, Konishi M, Sakai K,
Ishii A, Yasuno M, Hishima T, Koike M, Shitara N, Iwama T, et al:
Higher frequency of Smad4 gene mutation in human colorectal cancer
with distant metastasis. Oncogene. 18:3098–3103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Blackford A, Serrano OK, Wolfgang CL,
Parmigiani G, Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ,
Eshleman JR, et al: SMAD4 gene mutations are associated with poor
prognosis in pancreatic cancer. Clin Cancer Res. 15:4674–4679.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aitchison AA, Veerakumarasivam A, Vias M,
Kumar R, Hamdy FC, Neal DE and Mills IG: Promoter methylation
correlates with reduced Smad4 expression in advanced prostate
cancer. Prostate. 68:661–674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan M, Tang Y, Tytler EM, Lu C, Jin B,
Vickers SM, Yang L, Shi X and Cao X: Smad4 protein stability is
regulated by ubiquitin ligase SCF beta-TrCP1. J Biol Chem.
279:14484–14487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan M and Cao X, Wu Y, Bai S, Wu L, Shi X,
Wang N and Cao X: Jab1 antagonizes TGF-beta signaling by inducing
Smad4 degradation. EMBO Rep. 3:171–176. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Xin H, Xu X, Huang M, Zhang X, Chen
Y, Zhang S, Fu XY and Chang Z: CHIP mediates degradation of Smad
proteins and potentially regulates Smad-induced transcription. Mol
Cell Biol. 24:856–864. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Y, Katuri V, Dillner A, Mishra B,
Deng CX and Mishra L: Disruption of transforming growth factor-beta
signaling in ELF beta-spectrin-deficient mice. Science.
299:574–577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inman GJ, Nicolás FJ and Hill CS:
Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of
TGF-beta receptor activity. Mol Cell. 10:283–294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong C, Li Z, Alvarez R Jr, Feng XH and
Goldschmidt-Clermont PJ: Microtubule binding to Smads may regulate
TGF beta activity. Mol Cell. 5:27–34. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wagner AD and Moehler M: Development of
targeted therapies in advanced gastric cancer: Promising
exploratory steps in a new era. Curr Opin Oncol. 21:381–385. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Powell SM, Harper JC, Hamilton SR,
Robinson CR and Cummings OW: Inactivation of Smad4 in gastric
carcinomas. Cancer Res. 57:4221–4224. 1997.PubMed/NCBI
|
|
23
|
Wu DM, Zhu HX, Zhao QH, Zhang ZZ, Wang SZ,
Wang ML, Gong WD, Tan M and Zhang ZD: Genetic variations in the
SMAD4 gene and gastric cancer susceptibility. World J
Gastroenterol. 16:5635–5641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang LH, Kim SH, Lee JH, Choi YL, Kim YC,
Park TS, Hong YC, Wu CF and Shin YK: Inactivation of SMAD4 tumor
suppressor gene during gastric carcinoma progression. Clin Cancer
Res. 13:102–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calva D, Dahdaleh FS, Woodfield G, Weigel
RJ, Carr JC, Chinnathambi S and Howe JR: Discovery of SMAD4
promoters, transcription factor binding sites and deletions in
juvenile polyposis patients. Nucleic Acids Res. 39:5369–5378. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eguchi T, Prince T, Wegiel B and
Calderwood SK: Role and regulation of myeloid zinc finger protein 1
in cancer. J Cell Biochem. 116:2146–2154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsai SJ, Hwang JM, Hsieh SC, Ying TH and
Hsieh YH: Overexpression of myeloid zinc finger 1 suppresses matrix
metalloproteinase-2 expression and reduces invasiveness of SiHa
human cervical cancer cells. Biochem Biophys Res Commun.
425:462–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
George SK, Vishwamitra D, Manshouri R, Shi
P and Amin HM: The ALK inhibitor ASP3026 eradicates
NPM-ALK+ T-cell anaplastic large-cell lymphoma in vitro
and in a systemic xenograft lymphoma model. Oncotarget.
5:5750–5763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vishwamitra D, Curry CV, Alkan S, Shi P
and Amin HM: Sumoylation sustains the stability of NPM-ALK
oncogenic protein and facilitates its nuclear accumulation in
T-cell anaplastic large-cell lymphoma. Blood. 124:35862014.
|
|
30
|
Vishwamitra D, Shi P, Wilson D, Manshouri
R, Vega F, Schlette EJ and Amin HM: Expression and effects of
inhibition of type I insulin-like growth factor receptor tyrosine
kinase in mantle cell lymphoma. Haematologica. 96:871–880. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horinaka M, Yoshida T, Tomosugi M, Yasuda
S, Sowa Y and Sakai T: Myeloid zinc finger 1 mediates sulindac
sulfide-induced upregulation of death receptor 5 of human colon
cancer cells. Sci Rep. 4:60002014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen Y, Zhang Z, Yang K, Du J, Xu Y and
Liu S: Myeloid zinc-finger 1 (MZF-1) suppresses prostate tumor
growth through enforcing ferroportin-conducted iron egress.
Oncogene. 34:3839–3847. 2015. View Article : Google Scholar
|
|
33
|
Inoue M, Takahashi K, Niide O, Shibata M,
Fukuzawa M and Ra C: LDOC1, a novel MZF-1-interacting protein,
induces apoptosis. FEBS Lett. 579:604–608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mudduluru G, Vajkoczy P and Allgayer H:
Myeloid zinc finger 1 induces migration, invasion, and in vivo
metastasis through Axl gene expression in solid cancer. Mol Cancer
Res. 8:159–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rafn B, Nielsen CF, Andersen SH,
Szyniarowski P, Corcelle-Termeau E, Valo E, Fehrenbacher N, Olsen
CJ, Daugaard M, Egebjerg C, et al: ErbB2-driven breast cancer cell
invasion depends on a complex signaling network activating myeloid
zinc finger-1-dependent cathepsin B expression. Mol Cell.
45:764–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan QW, Reed E, Zhong XS, Thornton K, Guo
Y and Yu JJ: MZF1 possesses a repressively regulatory function in
ERCC1 expression. Biochem Pharmacol. 71:761–771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kundu J, Wahab SM, Kundu JK, Choi YL,
Erkin OC, Lee HS, Park SG and Shin YK: Tob1 induces apoptosis and
inhibits proliferation, migration and invasion of gastric cancer
cells by activating Smad4 and inhibiting β-catenin signaling. Int J
Oncol. 41:839–848. 2012.PubMed/NCBI
|
|
38
|
Roth S, Laiho P, Salovaara R, Launonen V
and Aaltonen LA: No SMAD4 hypermethylation in colorectal cancer. Br
J Cancer. 83:1015–1019. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Minami R, Kitazawa R, Maeda S and Kitazawa
S: Analysis of 5′-flanking region of human Smad4 (DPC4) gene.
Biochim Biophys Acta. 1443:182–185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schwarte-Waldhoff I and Schmiegel W: Smad4
transcriptional pathways and angiogenesis. Int J Gastrointest
Cancer. 31:47–59. 2002. View Article : Google Scholar
|
|
41
|
Xia X, Wu W, Huang C, Cen G, Jiang T, Cao
J, Huang K and Qiu Z: SMAD4 and its role in pancreatic cancer.
Tumour Biol. 36:111–119. 2015. View Article : Google Scholar
|
|
42
|
Malkoski SP and Wang XJ: Two sides of the
story? Smad4 loss in pancreatic cancer versus head-and-neck cancer.
FEBS Lett. 586:1984–1992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SH, Lee SH, Choi YL, Wang LH, Park CK
and Shin YK: Extensive alteration in the expression profiles of
TGFB pathway signaling components and TP53 is observed along the
gastric dysplasia-carcinoma sequence. Histol Histopathol.
23:1439–1452. 2008.PubMed/NCBI
|
|
44
|
Vishwamitra D, Curry CV, Alkan S, Song YH,
Gallick GE, Kaseb AO, Shi P and Amin HM: The transcription factors
Ik-1 and MZF1 downregulate IGF-IR expression in NPM-ALK+
T-cell lymphoma. Mol Cancer. 14:532015. View Article : Google Scholar
|
|
45
|
Gaboli M, Kotsi PA, Gurrieri C, Cattoretti
G, Ronchetti S, Cordon-Cardo C, Broxmeyer HE, Hromas R and Pandolfi
PP: Mzf1 controls cell proliferation and tumorigenesis. Genes Dev.
15:1625–1630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Deng Y, Wang J, Wang G, Jin Y, Luo X, Xia
X, Gong J and Hu J: p55PIK transcriptionally activated by MZF1
promotes colorectal cancer cell proliferation. BioMed Res Int.
2013:8681312013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Tian X, Du H, Fu X, Li K, Li A and Zhang
Y: Smad4 restoration leads to a suppression of Wnt/beta-catenin
signaling activity and migration capacity in human colon carcinoma
cells. Biochem Biophys Res Commun. 380:478–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang B, Zhang B, Chen X, Bae S, Singh K,
Washington MK and Datta PK: Loss of Smad4 in colorectal cancer
induces resistance to 5-fluorouracil through activating Akt
pathway. Br J Cancer. 110:946–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jia L, Lee HS, Wu CF, Kundu J, Park SG,
Kim RN, Wang LH, Erkin ÖC, Choi JS, Chae SW, et al: SMAD4
suppresses AURKA-induced metastatic phenotypes via degradation of
AURKA in a TGFbeta-independent manner. Mol Cancer Res.
12:1779–1795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee HS, Kundu J, Kim RN and Shin YK:
Transducer of ERBB2.1 (TOB1) as a tumor suppressor: A mechanistic
perspective. Int J Mol Sci. 16:29815–29828. 2015. View Article : Google Scholar : PubMed/NCBI
|