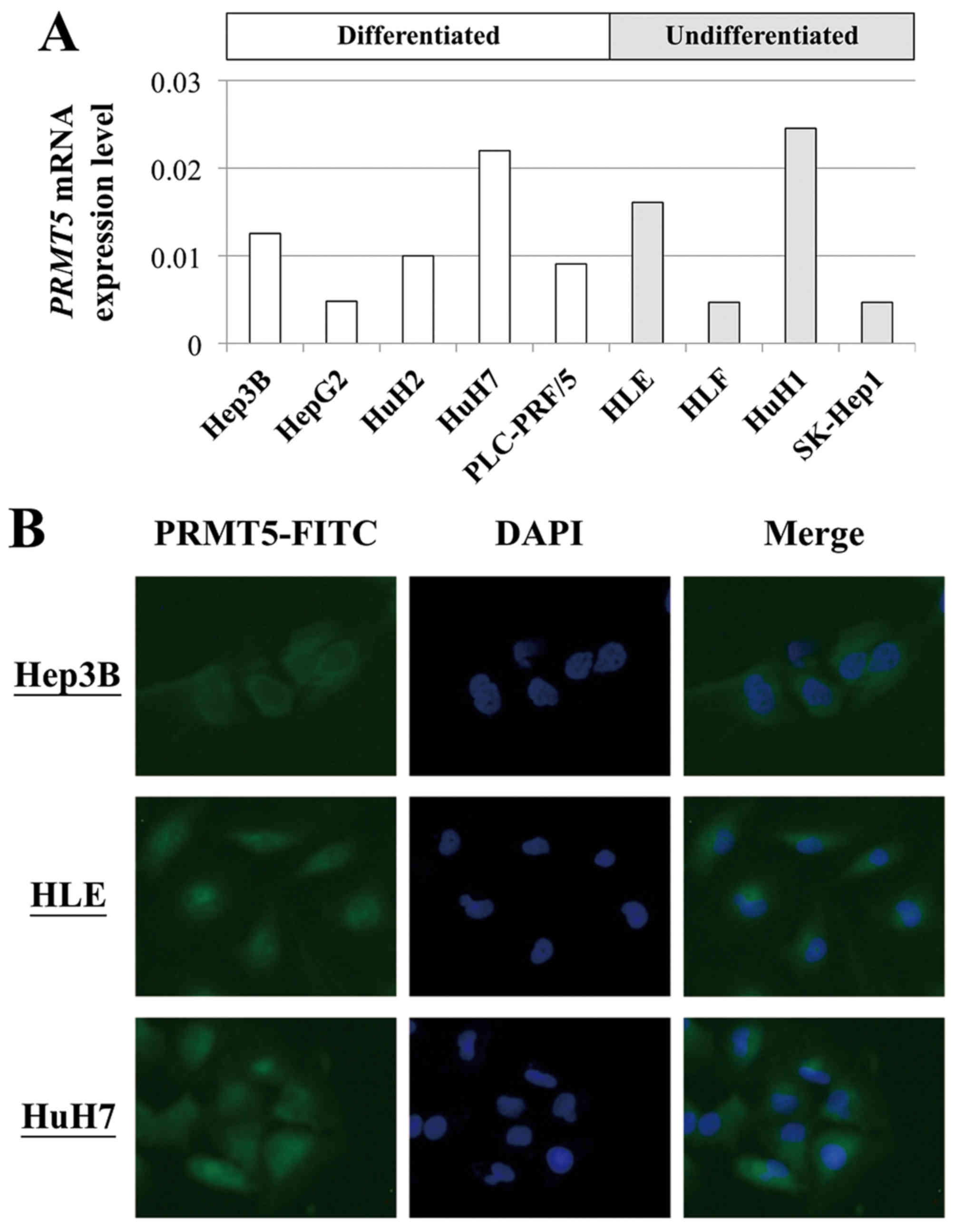
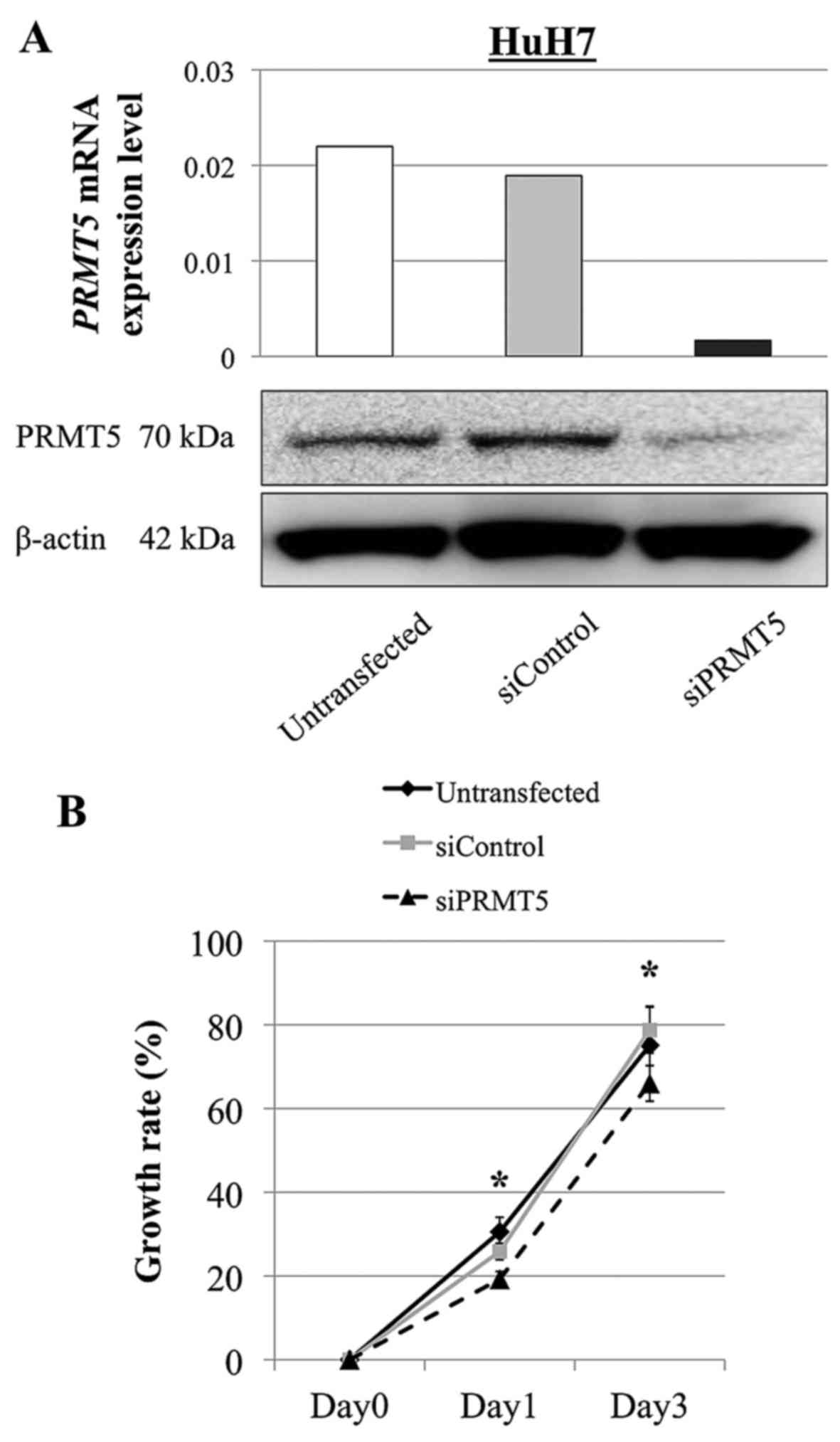
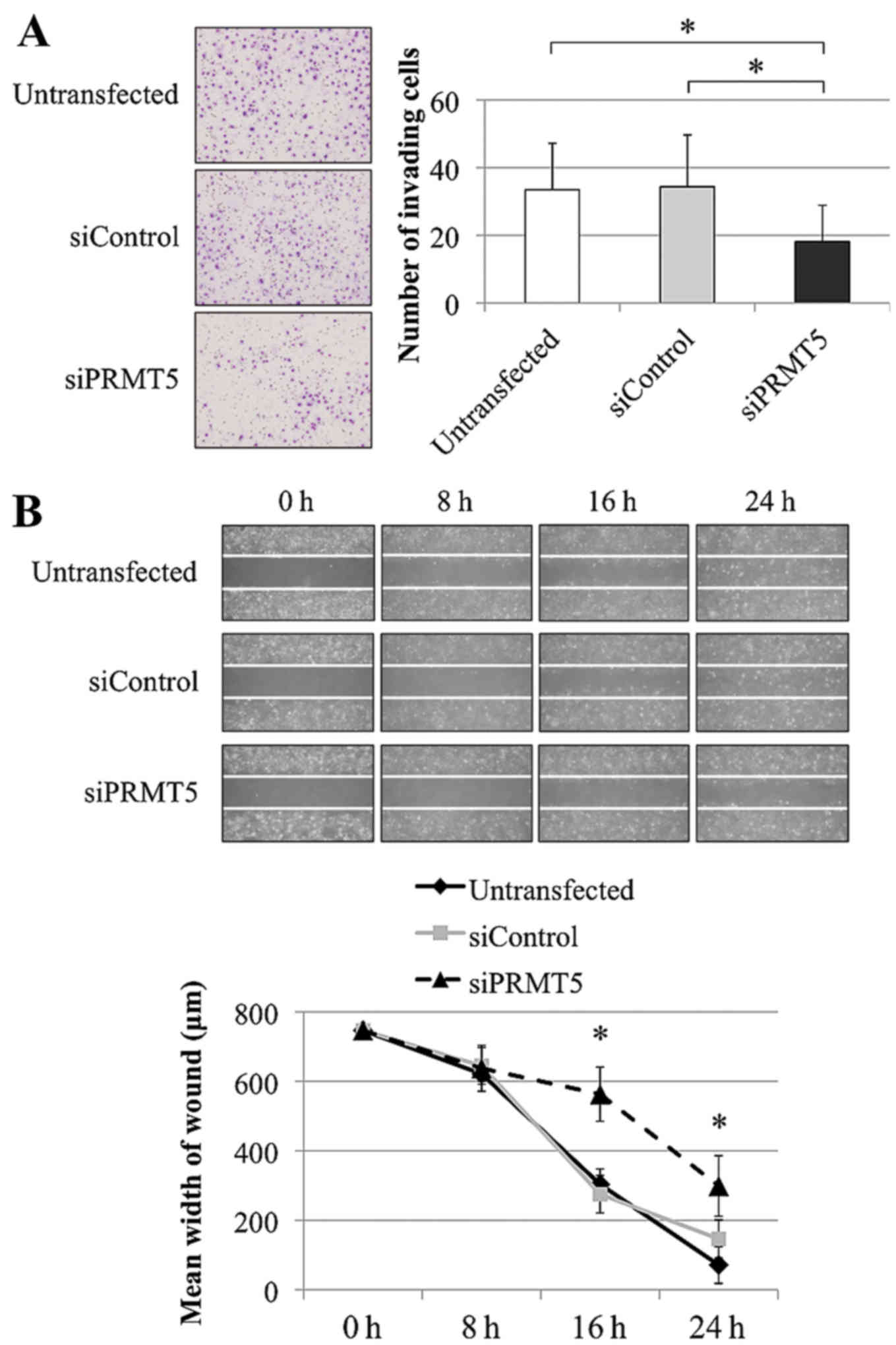
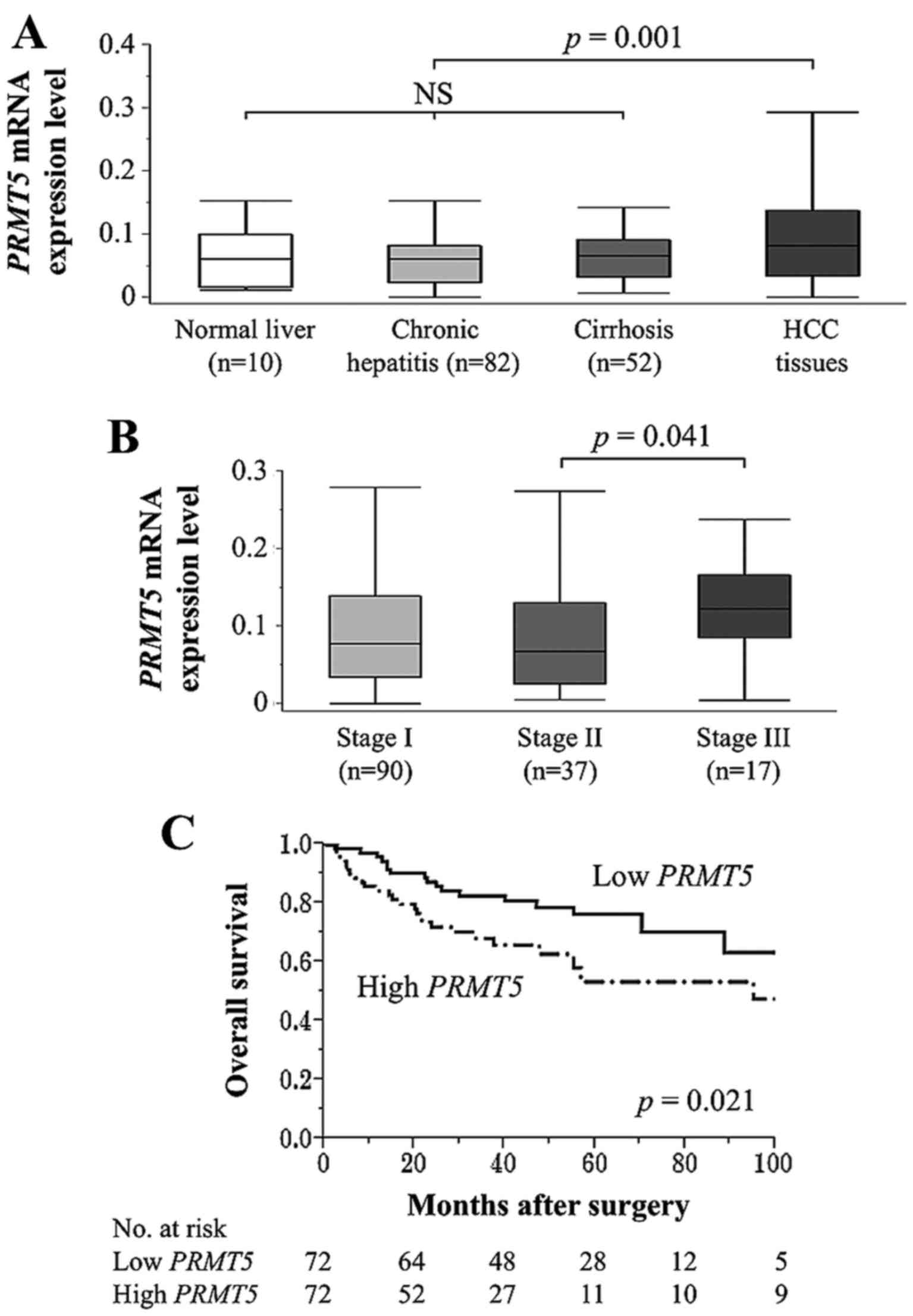
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanda M, Sugimoto H and Kodera Y: Genetic
and epigenetic aspects of initiation and progression of
hepatocellular carcinoma. World J Gastroenterol. 21:10584–10597.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar
|
|
5
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanda M, Sugimoto H, Nomoto S, Oya H,
Hibino S, Shimizu D, Takami H, Hashimoto R, Okamura Y, Yamada S, et
al: B cell translocation gene 1 serves as a novel prognostic
indicator of hepatocellular carcinoma. Int J Oncol. 46:641–648.
2015.
|
|
7
|
Stopa N, Krebs JE and Shechter D: The
PRMT5 arginine methyltransferase: Many roles in development, cancer
and beyond. Cell Mol Life Sci. 72:2041–2059. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka H, Hoshikawa Y, Oh-hara T, Koike S,
Naito M, Noda T, Arai H, Tsuruo T and Fujita N: PRMT5, a novel
TRAIL receptor-binding protein, inhibits TRAIL-induced apoptosis
via nuclear factor-kappaB activation. Mol Cancer Res. 7:557–569.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tee WW, Pardo M, Theunissen TW, Yu L,
Choudhary JS, Hajkova P and Surani MA: Prmt5 is essential for early
mouse development and acts in the cytoplasm to maintain ES cell
pluripotency. Genes Dev. 24:2772–2777. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicholas C, Yang J, Peters SB, Bill MA,
Baiocchi RA, Yan F, Sïf S, Tae S, Gaudio E, Wu X, et al: PRMT5 is
upregulated in malignant and metastatic melanoma and regulates
expression of MITF and p27(Kip1). PLoS One. 8:e747102013.
View Article : Google Scholar
|
|
11
|
Han X, Li R, Zhang W, Yang X, Wheeler CG,
Friedman GK, Province P, Ding Q, You Z, Fathallah-Shaykh HM, et al:
Expression of PRMT5 correlates with malignant grade in gliomas and
plays a pivotal role in tumor growth in vitro. J Neurooncol.
118:61–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Chitnis N, Nakagawa H, Kita Y,
Natsugoe S, Yang Y, Li Z, Wasik M, Klein-Szanto AJ, Rustgi AK, et
al: PRMT5 is required for lymphomagenesis triggered by multiple
oncogenic drivers. Cancer Discov. 5:288–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao X, Zhao S, Liu T, Liu Y, Liu Y and
Yang X: Overexpression of PRMT5 promotes tumor cell growth and is
associated with poor disease prognosis in epithelial ovarian
cancer. J Histochem Cytochem. 61:206–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ibrahim R, Matsubara D, Osman W, Morikawa
T, Goto A, Morita S, Ishikawa S, Aburatani H, Takai D, Nakajima J,
et al: Expression of PRMT5 in lung adenocarcinoma and its
significance in epithelial-mesenchymal transition. Hum Pathol.
45:1397–1405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Dong S, Li Z, Lu L, Zhang S, Chen
X, Cen X and Wu Y: Targeting protein arginine methyltransferase 5
inhibits human hepatocellular carcinoma growth via the
downregulation of beta-catenin. J Transl Med. 13:3492015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ezaka K, Kanda M, Sugimoto H, Shimizu D,
Oya H, Nomoto S, Sueoka S, Tanaka Y, Takami H, Hashimoto R, et al:
Reduced expression of adherens junctions associated protein 1
predicts recurrence of hepatocellular carcinoma after curative
hepatectomy. Ann Surg Oncol. 22(Suppl 3): S1499–S1507. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanda M, Nomoto S, Okamura Y, Hayashi M,
Hishida M, Fujii T, Nishikawa Y, Sugimoto H, Takeda S and Nakao A:
Promoter hypermethylation of fibulin 1 gene is associated with
tumor progression in hepatocellular carcinoma. Mol Carcinog.
50:571–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanda M, Nomoto S, Oya H, Takami H, Hibino
S, Hishida M, Suenaga M, Yamada S, Inokawa Y, Nishikawa Y, et al:
Downregulation of DENND2D by promoter hypermethylation is
associated with early recurrence of hepatocellular carcinoma. Int J
Oncol. 44:44–52. 2014.
|
|
19
|
Sobin LH, Gospodarowicz MK and Wittekind
CH: International Union Against Cancer: TNM Classification of
Malignant Tumors. 7th edition. Wiley-Blackwell; New York: 2009
|
|
20
|
Kanda M, Nomoto S, Oya H, Hashimoto R,
Takami H, Shimizu D, Sonohara F, Kobayashi D, Tanaka C, Yamada S,
et al: Decreased expression of prenyl diphosphate synthase subunit
2 correlates with reduced survival of patients with gastric cancer.
J Exp Clin Cancer Res. 33:882014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanda M, Nomoto S, Oya H, Shimizu D,
Takami H, Hibino S, Hashimoto R, Kobayashi D, Tanaka C, Yamada S,
et al: Dihydropyrimidinase-like 3 facilitates malignant behavior of
gastric cancer. J Exp Clin Cancer Res. 33:662014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanda M, Nomoto S, Okamura Y, Nishikawa Y,
Sugimoto H, Kanazumi N, Takeda S and Nakao A: Detection of
metallothionein 1G as a methylated tumor suppressor gene in human
hepatocellular carcinoma using a novel method of double combination
array analysis. Int J Oncol. 35:477–483. 2009.PubMed/NCBI
|
|
23
|
Kanda M, Shimizu D, Fujii T, Sueoka S,
Tanaka Y, Ezaka K, Takami H, Tanaka H, Hashimoto R, Iwata N, et al:
Function and diagnostic value of Anosmin-1 in gastric cancer
progression. Int J Cancer. 138:721–730. 2016. View Article : Google Scholar
|
|
24
|
Oya H, Kanda M, Sugimoto H, Shimizu D,
Takami H, Hibino S, Hashimoto R, Okamura Y, Yamada S, Fujii T, et
al: Dihydropyrimidinase-like 3 is a putative hepatocellular
carcinoma tumor suppressor. J Gastroenterol. 50:590–600. 2015.
View Article : Google Scholar
|
|
25
|
BLAST: Basic Local Alignment Search Tool.
http://blast.ncbi.nlm.nih.gov/Blast.cgi.
Accessed March 10, 2016.
|
|
26
|
Smil D, Eram MS, Li F, Kennedy S, Szewczyk
MM, Brown PJ, Barsyte-Lovejoy D, Arrowsmith CH, Vedadi M and
Schapira M: Discovery of a dual PRMT5-PRMT7 inhibitor. ACS Med Chem
Lett. 6:408–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan F, Alinari L, Lustberg ME, Martin LK,
Cordero-Nieves HM, Banasavadi-Siddegowda Y, Virk S, Barnholtz-Sloan
J, Bell EH, Wojton J, et al: Genetic validation of the protein
arginine methyltransferase PRMT5 as a candidate therapeutic target
in glioblastoma. Cancer Res. 74:1752–1765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim S, Günesdogan U, Zylicz JJ, Hackett
JA, Cougot D, Bao S, Lee C, Dietmann S, Allen GE, Sengupta R, et
al: PRMT5 protects genomic integrity during global DNA
demethylation in primordial germ cells and preimplantation embryos.
Mol Cell. 56:564–579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao J, Zhu Y, Nilsson M and Sundfeldt K:
TGF-β isoforms induce EMT independent migration of ovarian cancer
cells. Cancer Cell Int. 14:722014. View Article : Google Scholar
|
|
30
|
Cho EC, Zheng S, Munro S, Liu G, Carr SM,
Moehlenbrink J, Lu YC, Stimson L, Khan O, Konietzny R, et al:
Arginine methylation controls growth regulation by E2F-1. EMBO J.
31:1785–1797. 2012. View Article : Google Scholar : PubMed/NCBI
|