Introduction
Esophageal squamous cell carcinoma (ESCC) is a
highly aggressive malignancy with a significant mortality rate
(1,2). Despite improvements in the surgical
management of ESCC and development of useful chemotherapies and
chemoradio-therapies (CRTs), the prognosis of patients with
advanced disease remains poor (3–5).
Furthermore, the identification of novel therapeutic targets is
essential for individual curative adjuvant or neoadjuvant
therapies.
Stathmin 1 (STMN1) is a major cytosolic
phosphoprotein that regulates microtubule dynamics by preventing
tubulin polymerization and promoting microtubule destabilization
(6). STMN1 is highly expressed in
multiple human malignancies and is therefore also known as
oncoprotein 18. STMN1 expression correlates with tumor progression
and poor prognosis in various cancers (7–17)
including ESCC (18). These
studies indicated that STMN1 is a fundamental cancer-associated
gene and a potential target for the diagnosis and treatment of
cancers, including ESCC. However, the exact significance of STMN1
in tumor progression and therapeutic resistance has not yet been
elucidated in ESCC patients treated with CRT.
The expression of STMN1 is known to be functionally
linked to chemosensitivity to microtubule-stabilizing agents such
as taxanes, which are widely used anticancer agents. Inhibiting
STMN1 expression enhances chemosensitivity to paclitaxel in
osteosarcoma cells (19) and ESCCs
(20) in vitro, while the
overexpression of STMN1 decreases breast cancer cell sensitivity to
paclitaxel and vinblastine (7). In
Japan, docetaxel is considered a key drug in ESCC chemotherapy
along with cisplatin plus 5-fluorouracil (5-FU) (21,22).
In addition, radiation therapy with or without chemotherapy has
been established as one of the more effective treatments for ESCC
(23) and STMN1 was reported to
increase the radiation resistance in lung cancer cells (24). The identification of novel
predictive factors for therapeutic responses to docetaxel and
radiation is essential for ESCC management because drug resistance
leads to poor clinical outcomes.
The aim of the present study was to evaluate the
prognostic significance of STMN1 expression in ESCC by examining
the associations of tumor expression levels with various
clinicopathological parameters, particularly 5-year
disease-specific and overall survival. Moreover, we evaluated the
role of STMN1 in chemoradiation resistance in biopsied ESCC tissues
and ESCC cell lines.
Materials and methods
Patients and samples
To evaluate the clinicopathological significance of
STMN1 in ESCC, surgical specimens were obtained from 172 ESCC
patients (158 men and 14 women; 40–83 years old; mean age, 63.4
years) who had undergone potentially curative surgery at the Gunma
University Department of General Surgical Science between 1997 and
2010. All patients provided written informed consent. None had
received radiotherapy or chemotherapy prior to surgery; no patient
displayed hematogenic metastases at the time of surgery. The median
follow-up period for survivors was 32.6 (0.7–128) months. The
pathological features of the specimens were classified based on the
6th edition of the TNM classification of the International Union
against Cancer (25).
For examination of the association of STMN1
expression with chemoresistance to docetaxel in vivo,
pretreatment biopsy specimens were obtained from 15 patients (12
men and 3 women; 55–76 years old; mean age, 65.3 years) who had
received neoadjuvant CRT, including a docetaxel protocol, followed
by tumor resection surgery at the Gunma University Department of
general Surgical Science between 2005 and 2008. For docetaxel
combination CRT, external radiotherapy was delivered by a 2-field
technique using a 10-MV photon beam at 2 Gy per fraction per day, 5
fractions per week, for a total of 40 Gy. Concurrent chemotherapy
consisted of 7 mg/m2 docetaxel intravenously
administered for 1 h before radiotherapy on days 1, 8, 15 and 22.
Treatment, toxicity, and response evaluation details were
previously described (26).
Immunohistochemistry
Prior to the analysis, resected surgical specimens
and biopsy specimens were fixed with 10% formaldehyde, embedded in
paraffin blocks, cut into 4-μm thick sections and mounted
onto glass slides. STMN1 expression was assessed by
immunohistochemistry using a standard streptavidin-biotin
peroxidase complex method as previously described (27). Each 4-μm section was then
incubated at 4°C overnight with mouse anti-STMN1 monoclonal
antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted
1:50 in phosphate-buffered saline (PBS) containing 1% bovine serum
albumin (BSA) and primary mouse anti-Ki-67 monoclonal antibody
(clone MIB-1; 1:40 dilution; Dako, Glostrup, Denmark). For negative
controls, the specific primary antibody was replaced with PBS.
Immunolabeling was visualized using the chromogen
3,3′-diaminobenzidine tetrahydrochlo-ride applied as a 0.02%
solution containing 0.005% hydrogen peroxide in 50 mM ammonium
acetate-citrate acid buffer (pH 6.0). Sections were then lightly
counterstained in Mayer's hematoxylin and mounted.
Evaluation of immunostaining
For assessing STMN1 immunoreactivity, the product of
cytoplasmic intensity and quantity was calculated for each section
as previously described (28). The
intensity was graded as 0, negative, 1, low, 2, medium, or 3, high,
and the quantity as 0, no expression, 1, positivity in <1% of
cells, 2, positivity in 1–9%, 3, positivity in 10–50%, or 4,
positivity in >50%. The ESCC patients were divided into two
groups according to the mean STMN1 immunoreactivity score of the
surgical specimens. The labeling index was calculated as the
percentage of nuclear-stained cells for each section on the basis
of ~1,000 tumor cell nuclei and was counted at the site with the
maximum number of positive nuclei in the whole tumor. The ESCC
patients were classified as high or low based on the ROC-derived
Ki-67 labeling index cut-off value.
Evaluation of therapeutic response to
docetaxel combination CRT in surgically resected specimens
The pathological evaluation of surgically resected
tumor samples from patients receiving CRT (including docetaxel) was
conducted by two experienced pathologists who were blinded to the
clinical responses. The histopathological response to neoadjuvant
treatment was divided into four grades (0, 1, 2 and 3) according to
the guidelines of the Japan Esophageal Society (29). The pathological viability of the
residual tumor cells was assessed as follows: grade 3, histological
fibrosis with or without inflammation extending through the
different layers of the esophageal wall, but no viable residual
tumor cells; grade 2, less than one-third of residual tumor cells
were viable; grade 1, more than one-third of residual tumor cells
were viable; and grade 0, no therapeutic effect.
Cell lines
Four human ESCC cell lines (TE-1, TE-8, TE-15 and
KYSE140) and a non-malignant human esophageal epithelial cell line
(HET1A) were used to examine the associations of STMN1 expression
level with both docetaxel and radiation sensitivity in
vitro. All ESCC cell lines were cultured in RPMI-1640 medium
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal
bovine serum (FBS) and antibiotics (100 U/ml penicillin and 100
μg/ml streptomycin) and cultured in a humidified 5%
Co2 incubator at 37°C. These cells were not
cross-contaminated with other cell lines by STR-PCR.
siRNA and transfections
An STMN1-specific siRNA (Bonac Corp., Kurume, Japan)
was mixed with 0.4% lipofectamine RNAiMAX (Invitrogen, Carlsbad,
CA, USA) in 6-well flat-bottom microtiter plates and incubated.
TE-8 and KYSE140 ESCCs in 2 ml of RPMI-1640 medium were then seeded
on microtiter plates and incubated in a humidified 5%
Co2 incubator at 37°C for 24 h before analysis.
Knockdown was confirmed at the protein level by western blot
analysis.
Western blot analysis
Total protein was extracted using PRo-PREP protein
extraction solution (Intron Biotechnology, Inc., Kyungki-Do,
Korea). Total protein was separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using
Bis-Tris gels and was transferred to membranes. After membranes
were blocked with 5% skim milk, STMN1 protein was detected by
immunoblotting with anti-STMN1 rabbit polyclonal antibodies (Cell
Signaling Technology, Danvers, MA, USA) and vascular endothelial
growth factor a (VEGF) with an anti-VEGF rabbit monoclonal antibody
(Abcam, Cambridge, UK) diluted 1:1,000. An anti-HSC70 antibody
(1:500; Santa Cruz Biotechnology) served as a gel loading control.
Specific signals were detected using the ECL Prime Western blotting
detection system (GE Healthcare, Chicago, IL, USA) and quantified
by an ImageQuant LAS 4000 instrument (GE Healthcare).
Docetaxel sensitivity assay
The WST-8 test from the Cell Counting kit-8 (CCK-8;
Dojindo laboratories, Kumamoto, Japan) was used to evaluate the
docetaxel sensitivity of tumor cells. At 48 h after transfection,
TE-8 and KYSE140 cells were seeded into 96-well plates at
1×104 cells/well in 100 μl of culture medium for
24 h prior to drug exposure. After 24 h of pre-incubation, cells
were treated with various concentrations of docetaxel for 24 h.
Following drug exposure, 10 μl of WST-8 reagent solution was
added to each well, and cells were incubated for an additional 2 h
at 37°C in an incubator. Cell viability was estimated by the
optical density values at 450 nm, with the reference wavelength set
at 650 nm.
Radiation sensitivity assay
Radiation sensitivity was evaluated by the colony
formation assay. TE8 cells (400 cells/well) treated with siRNAs
were seeded in 6-well plates. After 24 h of pre-incubation, cells
were X-irradiated at 0, 2, 4, 6, or 8 Gy and incubated at 37°C for
10 days to form colonies. Cells were fixed and stained with 2%
crystal violet in 100% ethanol. Colonies were manually counted. The
fraction of cells that survived treatment was calculated relative
to non-treated controls.
Statistical analysis
Group differences in means and proportions were
evaluated using the Student's t-test, the Chi-square test, or
repeated-measures ANOVA as appropriate. Kaplan-Meier survival
curves were generated, and statistical significance was determined
using the log-rank test. Univariate and multivariate survival
analyses were conducted using the Cox proportional hazards
regression model. All statistical analyses were performed using JMP
software (SAS Institute, Inc., Cary, NC, USA).
Results
Association of STMN1 expression with
clinicopathological features of ESCC
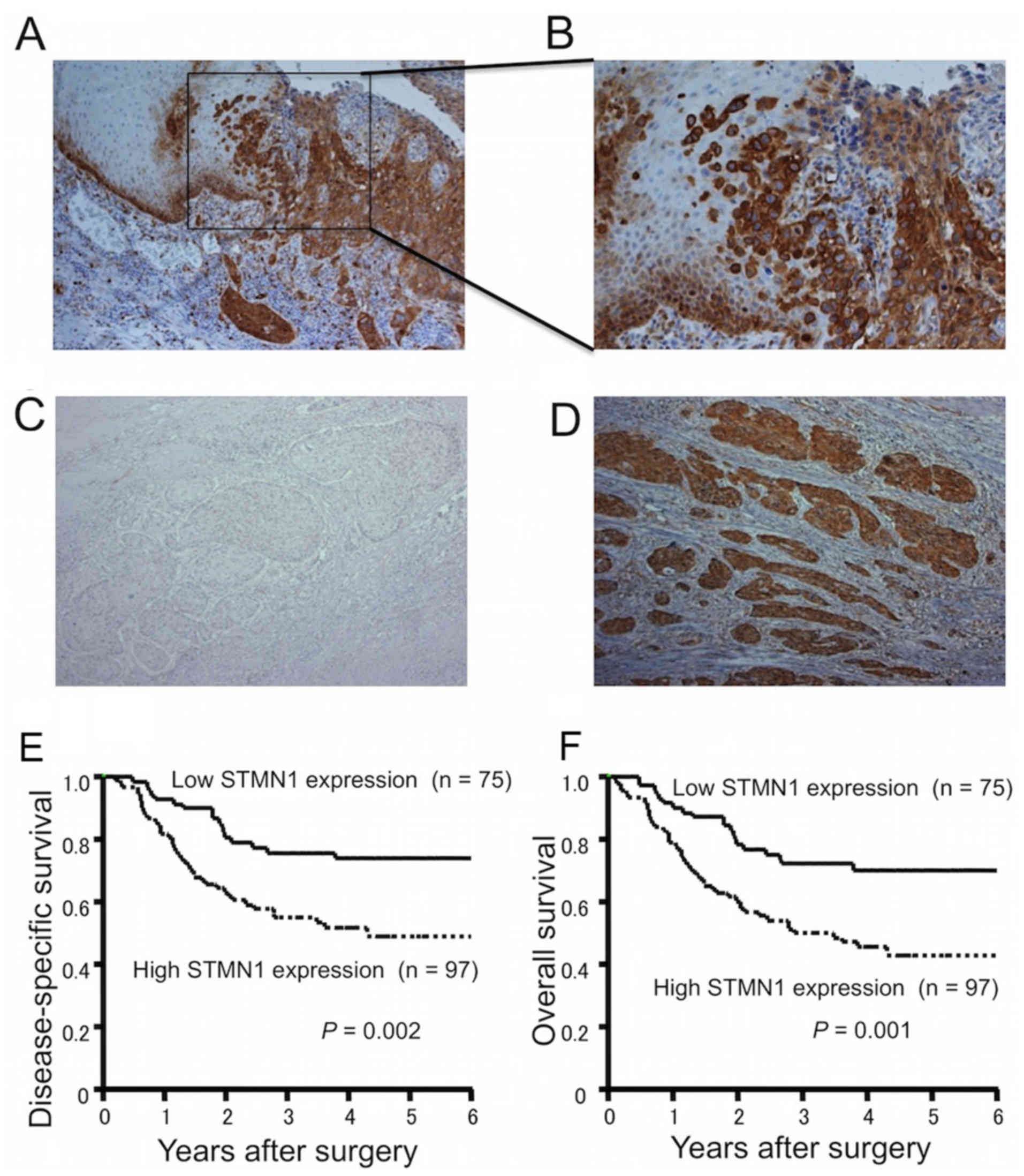
The immunoexpression of STMN1 was stronger in the
marginal regions of primary ESCCs than in the main tumor or
surrounding normal tissues (Fig. 1A
and B). When classified according to the mean STMN1
immunoreactivity score, 75 ESCC samples (43.6%) were defined as
having low STMN1 expression (Fig.
1C), while 97 ESCC samples (56.4%) were defined as having high
STMN1 expression (Fig. 1D).
STMN1 expression in relation to nine patient
clinico-pathological characteristics is listed in Table I. High STMN1 expression was
significantly associated with progression of tumor depth (P=0.001),
lymph node metastasis (P=0.007), lymphatic invasion (P<0.001),
venous invasion (P<0.001), tumor stage (P<0.001) and
proliferation maker Ki-67 labeling index (P=0.0039).
 | Table IAssociation of stathmin 1 (STMN1)
expression with clinicopathological features in 172 esophageal
squamous cell carcinoma (ESCC) patients. |
Table I
Association of stathmin 1 (STMN1)
expression with clinicopathological features in 172 esophageal
squamous cell carcinoma (ESCC) patients.
| Factors | STMN1 expression
| P-value |
|---|
Low
(n=75) | High
(n=97) |
|---|
| Gender |
| Male | 69 | 89 | 0.953 |
| Female | 6 | 8 | |
Age
(years)
(mean ± SD) | 62.0±8.8 | 64.4±7.6 | 0.084 |
| Tumor depth |
| T1 | 43 | 29 | 0.001a |
| T2 | 6 | 14 | |
| T3 | 23 | 53 | |
| T4 | 3 | 1 | |
| Lymph node
metastasis |
| N0 | 36 | 27 | 0.007a |
| N1 | 39 | 70 | |
| Distant
metastasis |
| M0 | 65 | 75 | 0.114 |
| M1 | 10 | 22 | |
| Lymphatic
invasion |
| Negative | 21 | 7 | <0.001a |
| Positive | 54 | 90 | |
| Venous
invasion |
| Negative | 34 | 14 | <0.001a |
| Positive | 41 | 83 | |
| Stage |
| I | 30 | 11 | <0.001a |
| II | 19 | 35 | |
| III | 16 | 29 | |
| IV | 10 | 22 | |
Ki-67 labeling
index
(mean ± SD) | 35.8±18.4 | 45.8±17.5 | 0.0039a |
STMN1 expression and postoperative
survival in ESCC patients
The 5-year disease-specific survival rate was
significantly reduced in ESCC patients with high STMN1 expression
compared to those with low STMN1 expression (49.5 vs. 73.9%,
respectively; P=0.002; Fig. 1E).
The 5-year overall survival rate was also significantly reduced in
ESCC patients with high STMN1 expression compared to those with low
STMN1 expression (43.6 vs. 70.2%, respectively; P=0.001; Fig. 1F). In univariate analysis, high
STMN1 expression was a significant prognostic factor for poor
survival (P=0.002; Table II).
Multivariate analysis of the six factors significant in univariate
analysis showed that high STMN1 expression was an independent risk
factor for poor 5-year disease-specific survival (RR=1.339, 95% CI,
1.016–1.804, P=0.038; Table
II).
 | Table IIUnivariate and multivariate analysis
of clinicopathological factors affecting 5-year disease-specific
survival rate following surgery in 172 patients with ESCC. |
Table II
Univariate and multivariate analysis
of clinicopathological factors affecting 5-year disease-specific
survival rate following surgery in 172 patients with ESCC.
| Clinicopathological
variables | Univariate analysis
| Multivariate
analysis
|
|---|
| RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| Gender
(Male/female) | 0.844 | 0.499–1.266 | 0.445 | – | – | – |
| Age (<63/>63
years) | 0.923 | 0.696–1.219 | 0.574 | – | – | – |
| Depth of tumor
invasion (T1-2/T3-4) | 2.090 | 1.602–2.795 | <0.001a | 1.548 | 1.171–2.096 | 0.002a |
| Lymph node
metastasis (N0/N1) | 2.667 | 1.826–4.295 | <0.001a | 1.817 | 1.190–3.037 | 0.004a |
| Distant metastasis
(M0/M1) | 1.856 | 1.446–2.365 | <0.001a | 1.369 | 1.036–1.797 | 0.027a |
| Lymphatic invasion
(Negative/positive) | 4.188 | 1.969–17.60 | <0.001a | 0.970 | 0.318–4.625 | 0.962 |
| Venous invasion
(Negative/positive) | 2.330 | 1.594–3.752 | <0.001a | 2.003 | 0.977–5.844 | 0.060 |
| Ki67 labeling index
(Low/high) | 1.809 | 0.982–3.55 | 0.0574 | – | – | – |
| STMN1 expression
(Low/high) | 1.538 | 1.170–2.066 | 0.002a | 1.339 | 1.016–1.804 | 0.038a |
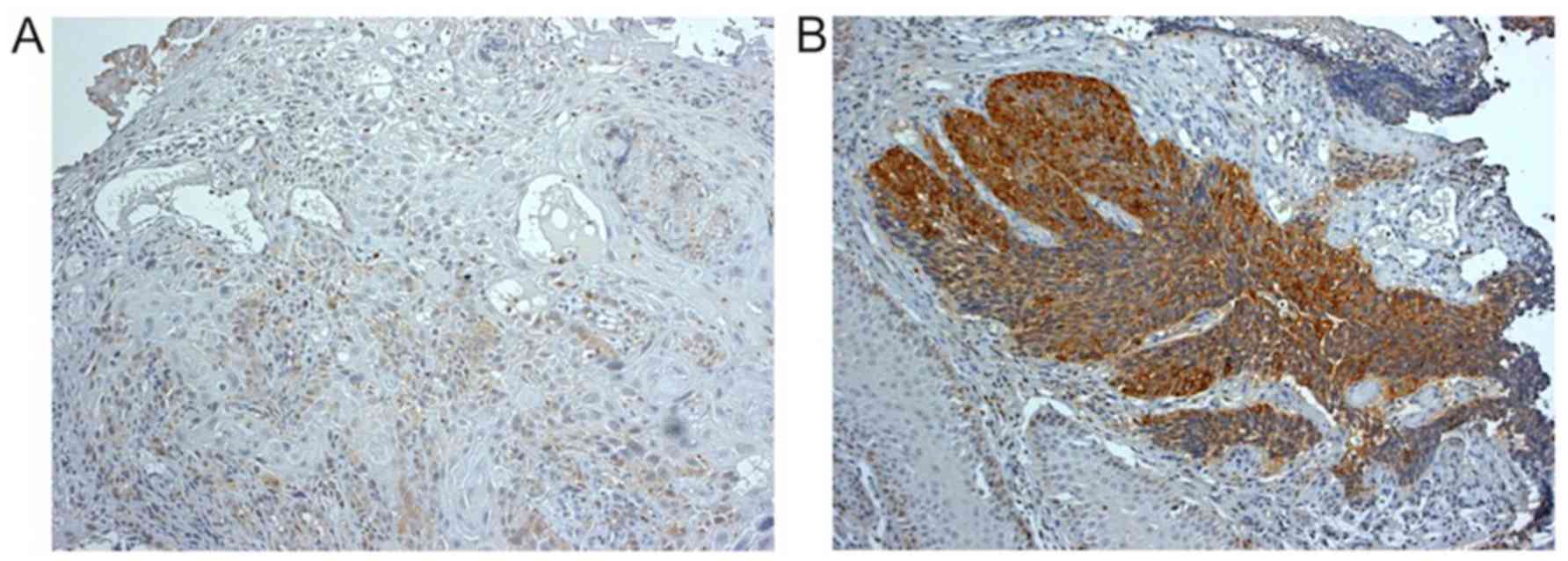
Inverse association of pretreatment STMN1
expression in ESCC with efficacy of docetaxel combination CRT
STMN1 expression was investigated by
immunohistochemistry in 15 biopsy samples obtained before the
docetaxel combination CRT. According to the mean STMN1
immunoreactivity score, six ESCC samples (40.0%) were classified as
having low STMN1 expression (Fig.
2A) and nine (60.0%) as having high STMN1 expression (Fig. 2B). The histological response to
neoadjuvant CRT as evaluated from the surgically resected specimens
was grade 3 in three patients, grade 2 in six patients, and grade 1
in six patients. Examination of the association of STMN1 expression
in biopsy samples with histological response of surgically resected
specimens revealed that STMN1 expression was negatively associated
with the therapeutic efficacy of docetaxel combination CRT
(P=0.029; Table III). Six
patients had T3 stage and 9 had T4 stage before CRT treatment.
After treated by preoperative CRT, 6 patients had grade 1
pathological response, 6 had grade 2 and 3 had grade 3.
 | Table IIISTMN1 expression in pretreatment
biopsy specimens was inversely associated with the therapeutic
efficacy of docetaxel combination CRT. |
Table III
STMN1 expression in pretreatment
biopsy specimens was inversely associated with the therapeutic
efficacy of docetaxel combination CRT.
| STMN1 | Pathological grade
1 | Pathological grade
2, 3 | P-value |
|---|
| Low expression | 0 | 6 | 0.029 |
| High
expression | 6 | 3 | |
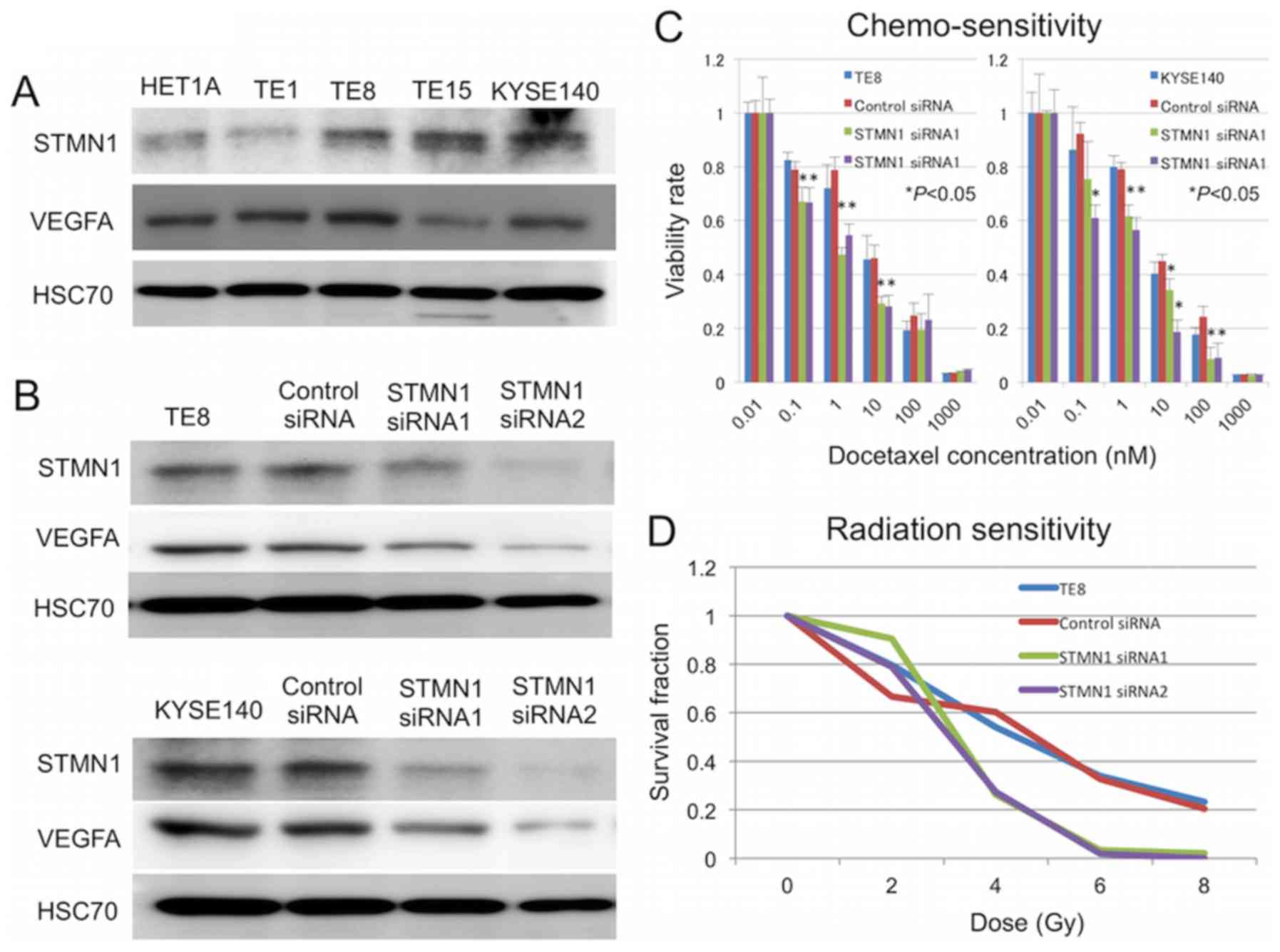
Inhibition of STMN1 expression sensitizes
ESCCs to docetaxel in vitro
Several reports have identified STMN1 as a marker
for resistance against taxane agents, including in esophageal
cancer (7,19,20,30).
To determine whether the silencing of STMN1 can increase
sensitivity to docetaxel in vitro, TE-8 and KYSE140 cells
were transfected with STMN1-specific siRNA, and sensitivity to
docetaxel treatment was determined by cell viability assays.
Western blotting confirmed a reduction in STMN1 protein expression
by 72 h after transfection (Fig.
3B). Cells transfected with STMN1 siRNA showed significantly
higher sensitivity to docetaxel treatment than untreated control
cells and control siRNA-transfected cells (P<0.05; Fig. 3C). The expression of the
therapeutic resistance inducible gene VEGF was also lower in
STMN1-suppressed cells than control cells (Fig. 3B). Moreover, STMN1 knockdown
enhanced the radiation sensitivity of TE-8 and KYSE140 cells
(Fig. 3D).
Discussion
High STMN1 expression was strongly associated with
ESCC progression and nuclear Ki-67 accumulation and was an
independent prognostic factor for reduced survival in our ESCC
cohort without preoperative adjuvant therapies. Moreover, high
STMN1 expression in pre-treatment biopsy samples was related to
reduced preoperative CRT response. In vitro analysis
suggested that STMN1 regulates sensitivity to docetaxel and
radiation as well as the induction of the CRT-resistance factor
VEGF in ESCCs.
Metastasis resulting from tumor invasiveness is the
main cause of death in ESCC patients. In the present study, we
immunohistochemically examined STMN1 expression and found that
STMN1 was significantly associated with tumor depth, lymph node
metastasis, lymphatic invasion, and venous invasion. In a previous
study, STMN1 was shown to stimulate cell motility through the
extracellular matrix in vitro and to increase the metastatic
potential of sarcoma in vivo (31). In addition, Li et al
(32) reported that STMN1 promotes
epithelial-to-mesenchymal transition by regulating microtubule
dynamics. Epithelial-to-mesenchymal transition is thought to be
required for the acquisition of metastatic behavior in carcinoma
cells, including ESCCs (33).
Moreover, STMN1 expression has been linked to PI3K activity and has
been suggested as a marker for the activated PI3K signaling pathway
(34). Therefore, STMN1 may exert
oncogenic functions and contribute to tumor invasiveness. The
present study is in agreement with these previous reports
demonstrating that STMN1 plays an important role in tumor
metastasis and can predict poor prognosis. These findings suggest
that STMN1 is a target for new anti-metastatic drugs for ESCC.
Radiation sensitivity increased in STMN1-suppressed
cells compared to that in control cells. Radiation resistance in
cancer cells is regulated by multiple factors, including hypoxia,
receptor tyrosine kinase/akt, inflammation, DNA damage repair,
adhesion pathway and developmental pathway (35,36).
Among these factors, we focused on the hypoxia-inducible factor 1,
α subunit (HIF-1α)/VEGF pathway because Yoshie et al
(37) reported that STMN1
knockdown can suppress this radiation resistance pathway and
angiogenesis in human endometrial and endothelial cells, but not in
cancer cells. In this study, we confirmed the suppression of VEGF
by STMN1 knockdown in ESCCs and found positive associations of
STMN1 expression with both venous invasion and lymphatic invasion
in surgical ESCC samples. These data are consistent with previous
results in non-cancerous cells. Therefore, VEGF suppression by
STMN1 knockdown may overcome radiation resistance in ESCC. To the
best of our knowledge, this study is the first suggesting that
STMN1 can be used as a predictive biomarker for the response to
docetaxel combination CRT in clinical ESCC samples.
In conclusion, the present study revealed that high
STMN1 expression was a significant factor predicting poor prognosis
in ESCC patients. Poor outcomes may result from diminished response
to docetaxel combination CRT in ESCCs overexpressing STMN1. The
assessment of STMN1 expression in pretreatment biopsy specimens may
be a novel strategy to predict ESCC aggression and treatment
response against CRT. Our results also strongly suggest that STMN1
is a promising molecular target to overcome CRT resistance in
ESCC.
Abbreviations:
|
ESCC
|
esophageal squamous cell carcinoma
|
|
CRTs
|
chemoradiotherapies
|
|
STMN1
|
stathmin 1
|
|
5-FU
|
5-fluorouracil
|
|
VEGF
|
vascular endothelial growth factor
a
|
|
HIF-1α
|
hypoxia-inducible factor 1 alpha
subunit
|
Acknowledgments
The present study was supported by grants-in-aid for
Scientific Research from the Japan Society for the Promotion of
Science (JSPS) (Grant nos. 26461969, 15K10129 and 15K10085).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gebski V, Burmeister B, Smithers BM, Foo
K, Zalcberg J and Simes J; Australasian Gastro-Intestinal Trials
group: Survival benefits from neoadjuvant chemoradiotherapy or
chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet
Oncol. 8:226–234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mariette C, Piessen G and Triboulet JP:
Therapeutic strategies in oesophageal carcinoma: Role of surgery
and other modalities. Lancet Oncol. 8:545–553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al CROSS group:
Preoperative chemoradiotherapy for esophageal or junctional cancer.
N Engl J Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubin CI and Atweh GF: The role of
stathmin in the regulation of the cell cycle. J Cell Biochem.
93:242–250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alli E, Yang JM, Ford JM and Hait WN:
Reversal of stathmin-mediated resistance to paclitaxel and
vinblastine in human breast carcinoma cells. Mol Pharmacol.
71:1233–1240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Golouh R, Cufer T, Sadikov A, Nussdorfer
P, Usher PA, Brünner N, Schmitt M, Lesche R, Maier S, Timmermans M,
et al: The prognostic value of Stathmin-1, S100B2, and SYK proteins
in ER-positive primary breast cancer patients treated with adjuvant
tamoxifen monotherapy: an immunohistochemical study. Breast Cancer
Res Treat. 110:317–326. 2008. View Article : Google Scholar
|
|
9
|
Ghosh R, Gu G, Tillman E, Yuan J, Wang Y,
Fazli L, Rennie PS and Kasper S: Increased expression and
differential phosphorylation of stathmin may promote prostate
cancer progression. Prostate. 67:1038–1052. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xi W, Rui W, Fang L, Ke D, Ping G and
Hui-Zhong Z: Expression of stathmin/op18 as a significant
prognostic factor for cervical carcinoma patients. J Cancer Res
Clin Oncol. 135:837–846. 2009. View Article : Google Scholar
|
|
11
|
Kim JY, Harvard C, You L, Xu Z,
Kuchenbecker K, Baehner R and Jablons D: Stathmin is overexpressed
in malignant mesothelioma. Anticancer Res. 27:39–44.
2007.PubMed/NCBI
|
|
12
|
Jeon TY, Han ME, Lee YW, Lee YS, Kim GH,
Song GA, Hur GY, Kim JY, Kim HJ, Yoon S, et al: Overexpression of
stathmin1 in the diffuse type of gastric cancer and its roles in
proliferation and migration of gastric cancer cells. Br J Cancer.
102:710–718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang W, Tong JH, Chan AW, Lung RW, Chau
SL, Wong QW, Wong N, Yu J, Cheng AS and To KF: Stathmin1 plays
oncogenic role and is a target of microRNA-223 in gastric cancer.
PloS One. 7:e339192012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsieh SY, Huang SF, Yu MC, Yeh TS, Chen
TC, Lin YJ, Chang CJ, Sung CM, Lee YL and Hsu CY: Stathmin1
overexpression associated with polyploidy, tumor-cell invasion,
early recurrence, and poor prognosis in human hepatoma. Mol
Carcinog. 49:476–487. 2010.PubMed/NCBI
|
|
15
|
Trovik J, Wik E, Stefansson IM,
Marcickiewicz J, Tingulstad S, Staff AC, Njolstad TS; MoMaTec Study
Group; Vandenput I and Amant F: Stathmin overexpression identifies
high-risk patients and lymph node metastasis in endometrial cancer.
Clin Cancer Res. 17:3368–3377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng P, Liu YX, Chen L, Liu XH, Xiao ZQ,
Zhao L, Li GQ, Zhou J, Ding YQ and Li JM: Stathmin, a new target of
PRl-3 identified by proteomic methods, plays a key role in
progression and metastasis of colorectal cancer. J Proteome Res.
9:4897–4905. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin WC, Chen SC, Hu FC, Chueh SC, Pu YS,
Yu HJ and Huang KH: Expression of stathmin in localized upper
urinary tract urothelial carcinoma: Correlations with prognosis.
Urology. 74:1264–1269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akhtar J, Wang Z, Yu C, Li CS, Shi YL and
Liu HJ: STMN-1 is a potential marker of lymph node metastasis in
distal esophageal adenocarcinomas and silencing its expression can
reverse malignant phenotype of tumor cells. BMC Cancer. 14:282014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang R, Dong K, Lin F, Wang X, Gao P, Wei
SH, Cheng SY and Zhang HZ: Inhibiting proliferation and enhancing
chemosensitivity to taxanes in osteosarcoma cells by RNA
interference-mediated downregulation of stathmin expression. Mol
Med. 13:567–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng W, Xiaoyan X, Xuan Y, Xiangke L,
Zichang Y, Ran Z, Liuxing W and Qingxia F: Silencing
stathmin-modulating efficiency of chemotherapy for esophageal
squamous cell cancer with paclitaxel. Cancer Gene Ther. 22:115–121.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thallinger CM, Raderer M and Hejna M:
Esophageal cancer: A critical evaluation of systemic second-line
therapy. J Clin Oncol. 29:4709–4714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pasini F, De Manzoni G, Pedrazzani C,
Grandinetti A, Durante E, Gabbani M, Tomezzoli A, Griso C,
Guglielmi A, Pelosi G, et al: High pathological response rate in
locally advanced esophageal cancer after neoadjuvant combined
modality therapy: Dose finding of a weekly chemotherapy schedule
with protracted venous infusion of 5-fluorouracil and dose
escalation of cisplatin, docetaxel and concurrent radiotherapy. Ann
Oncol. 16:1133–1139. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okumura H, Uchikado Y, Setoyama T,
Matsumoto M, Owaki T, Ishigami S and Natsugoe S: Biomarkers for
predicting the response of esophageal squamous cell carcinoma to
neoadjuvant chemoradiation therapy. Surg Today. 44:421–428. 2014.
View Article : Google Scholar
|
|
24
|
Zhang X, Ji J, Yang Y, Zhang J and Shen L:
Stathmin1 increases radioresistance by enhancing autophagy in
non-small-cell lung cancer cells. Onco Targets Ther. 9:2565–2574.
2016.PubMed/NCBI
|
|
25
|
Sobin LH and Wittekind CW: TNM
Classification of Malignant Tumours. 6th edition. John Wiley &
Sons; Hoboken, NJ: 2002
|
|
26
|
Fukuchi M, Fukai Y, Sohda M, Miyazaki T,
Nakajima M, Inose T, Tanaka N, Tsukada K, Kato H and Kuwano H:
Expression of the prolyl isomerase Pin1 is a useful indicator of
sensitivity to chemoradiotherapy in advanced esophageal squamous
cell carcinoma. Oncol Rep. 21:853–859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Suzuki S, Miyazaki T, Tanaka N, Sakai M,
Sano A, Inose T, Sohda M, Nakajima M, Kato H and Kuwano H:
Prognostic significance of CD151 expression in esophageal squamous
cell carcinoma with aggressive cell proliferation and invasiveness.
Ann Surg Oncol. 18:888–893. 2011. View Article : Google Scholar
|
|
28
|
Singer S, Ehemann V, Brauckhoff A, Keith
M, Vreden S, Schirmacher P and Breuhahn K: Protumorigenic
overexpression of stathmin/op18 by gain-of-function mutation in p53
in human hepatocarcinogenesis. Hepatology. 46:759–768. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Japanese Society for Esophageal Diseases:
Guidelines for the Clinical and Pathological Studies on Carcinoma
of the Esophagus. 10th edition. Kanehara; 2007
|
|
30
|
Watanabe A, Suzuki H, Yokobori T, Altan B,
Kubo N, Araki K, Wada S, Mochida Y, Sasaki S, Kashiwabara K, et al:
Forkhead box protein C2 contributes to invasion and metastasis of
extrahepatic cholangiocarcinoma, resulting in a poor prognosis.
Cancer Sci. 104:1427–1432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Belletti B, Nicoloso MS, Schiappacassi M,
Berton S, Lovat F, Wolf K, Canzonieri V, D'andrea S, Zucchetto A,
Friedl P, et al: Stathmin activity influences sarcoma cell shape,
motility, and metastatic potential. Mol Biol Cell. 19:2003–2013.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li N, Jiang P, Du W, Wu Z, Li C, Qiao M,
Yang X and Wu M: Siva1 suppresses epithelial-mesenchymal transition
and metastasis of tumor cells by inhibiting stathmin and
stabilizing microtubules. Proc Natl Acad Sci USA. 108:12851–12856.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yokobori T, Suzuki S, Tanaka N, Inose T,
Sohda M, Sano A, Sakai M, Nakajima M, Miyazaki T, Kato H, et al:
MiR-150 is associated with poor prognosis in esophageal squamous
cell carcinoma via targeting the EMT inducer ZEB1. Cancer Sci.
104:48–54. 2013. View Article : Google Scholar
|
|
34
|
Salvesen HB, Carter SL, Mannelqvist M,
Dutt A, Getz G, Stefansson IM, Raeder MB, Sos ML, Engelsen IB,
Trovik J, et al: Integrated genomic profiling of endometrial
carcinoma associates aggressive tumors with indicators of PI3
kinase activation. Proc Natl Acad Sci USA. 106:4834–4839. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim BM and Hong Y, Lee S, Liu P, Lim JH,
Lee YH, Lee TH, Chang KT and Hong Y: Therapeutic implications for
overcoming radiation resistance in cancer therapy. Int J Mol Sci.
16:26880–26913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vaupel P, Thews O and Hoeckel M: Treatment
resistance of solid tumors: Role of hypoxia and anemia. Med Oncol.
18:243–259. 2001. View Article : Google Scholar
|
|
37
|
Yoshie M, Miyajima E, Kyo S and Tamura K:
Stathmin, a micro-tubule regulatory protein, is associated with
hypoxia-inducible factor-1alpha levels in human endometrial and
endothelial cells. Endocrinology. 150:2413–2418. 2009. View Article : Google Scholar : PubMed/NCBI
|