Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of
the leading malignant cancers of the head and neck and accounts for
~2.4% of newly diagnosed malignancies worldwide every year
(1,2). Despite the development of
conventional approaches of surgery, chemotherapy and radiotherapy,
the 5-year survival rate of patients with advanced tumor remains
poor, due to recurrence, metastasis and multiple drug resistance
(MDR) (3). To identify more
potential drugs and improve the survival and life quality of LSCC
patients, clarifying the mechanisms of LSCC growth, metastasis and
drug resistance remain an urgent need.
It is known that many solid tumors is significantly
stiffer than normal tissues because of the stiffer stroma formed
from extracellular matrix crosslinking and collagen deposition
(4–6). Recently studies reported that tumor
growth and metastasis are affected by both biochemical and physical
properties of the tumor microenvironment (4–8).
There is increasing evidence that the tumor mechanical force is a
critical factor of tumor initial, growth and metastasis. Schrader
et al showed that matrix stiffness regulates the
proliferation, dormancy and chemotherapeutic response of
hepatocellular carcinoma cells (9). Epithelial-mesenchymal transition
(EMT) is a critical program for tumor cell dissemination and
metastasis (10,11). High matrix stiffness could promote
the nuclear translocation of TWIST1, inducing EMT in breast cancer
and promoting tumor invasion and metastasis (12). In addition, soft matrix stiffness
is known to maintain self-renew of stem cell, such as embryonic
stem cell (ESC) and cancer stem cell (CSC) (13,14).
Previous studies have demonstrated that upregulation of stem
cell-associated genes such as Sox2, Oct3/4, CD133 and c-kit were
detected in B16 melanoma tumor cells cultured in soft substrate
(14). Moreover, Tajik et
al showed that mechanical force induced the transcription
upregulation through force-induced direct stretching of chromatin,
leading to a better understanding of how directional forces
influence the growth of cancer cells (15). However, the role of mechanical
factors in modulating the growth and progression of LSCC remain
poorly defined. In the present study, we employed a collagen-coated
polyacrylamide hydrogel system to understand how matrix stiffness
regulates the growth of LSCC. Although various cancer therapy
strategies have been greatly developed and improved, drug
resistance is still a challenging issue for patients. Here we
further explore the effect of matrix stiffness on the
chemosensitivity of LSCC.
Materials and methods
Cell culture
Human laryngeal squamous cell carcinoma Hep-2 cells
were obtained from China Center for Type Culture Collection (CCTCC,
Wuhan, China) and cultured according to the guidelines. Briefly,
Hep-2 cells were cultured in RPMI-1640 medium with 10% fetal bovine
serum (FBS), 100 U of penicillin and 100 mg/ml streptomycin.
Antibodies and reagents
Anti-human CD29 PE and anti-human Ki67 APC were
purchased from eBiosciences (La Jolla, CA, USA). Cyclin D1, cyclin
D3, p-FAK, FAK, p-Akt, Akt, p-STAT3, STAT3, p-Erk, Erk, Sox2, ABCG2
and β-actin antibodies were purchased from CST (Boston, MA, USA).
Annexin V Apoptosis Detection kit was from BD Pharmingen (San
Diego, CA, USA).
Polyacrylamide gel preparation
Polyacrylamide (PA) gels of variable stiffness were
prepared as previously described. Briefly, 25-mm glass coverslips
were soaked using 0.1 N NaOH and air dried, functionalized using
3-aminopropyltriethoxysilane (Sigma-Aldrich), washed in distilled
water and then soaked in 0.5% gluteraldehyde in PBS.
Acrylamide/bis-acrylamide gels polymerized between the
functionalized coverslip and a glass slide treated with a
hydrophobic silicon polymer (Rain-X™, SOPUS Products, Houston, TX,
USA). The gels were then washed twice with 50 mM HEPES and
incubated with 1 mM Sulfo-SANPAH (Thermo Scientific Pierce) in
HEPES buffer under UV light for 10 min. Excess Sulfo-SANPAH was
removed by extensive washing in 50 mM HEPES. A thin layer of
collagen-I was then crosslinked to the gels at 37°C overnight,
rinsed twice in 50 mM HEPES buffer, and sterilized. Gels were
soaked in serum-free culture media overnight before plating of
cells.
Apoptosis assays
Hep-2 cells were cultured in 1 or 8 kPa PA gels and
treated with cisplatin (5–20 µM) or 5-FU (5–20 µM)
for ≤36 h. In some cases, Hep-2 cells were transfected with Sox2 or
ABCG2 siRNA, and then cultured in 1 kPa gels and treated with
cisplatin or 5-fluorouracil (5-FU). Apoptosis was detected using
the Annexin V/propidium iodide kit (BD Pharmingen) by flow
cytometry.
CCK8 assay
CCK8 cell viability assay were performed according
to the manufacturer's instructions. Briefly, Hep-2 cells were
plated onto 1 or 8 kPa gels and cultured with 1.8 ml RPMI-1640
medium. Twelve hours later, cells were treated with a different
concentration of cisplatin or 5-FU. After 24 h of treatment, 200
µl WST-8 solution was added to each well, and the plates
were incubated for an additional 1.5 h at 37°C. Aliquot (200
µl) of the solution from each well was transferred into a
96-well culture plate. The absorbance of each plate at 450 nm
represented a direct correlation with the cell number in this
analysis, and was measured by a standard microplate reader (Bio-Tek
Instruments, Inc., Winooski, VT, USA).
Western blot analysis
Western blot analyses were performed following
standard procedures. Hep-2 cell lysate proteins were extracted and
analyzed by western blotting using anti-human-cyclin D1,
anti-human-cyclin D3, anti-human-p-FAK, anti-human-FAK,
anti-human-p-Akt, anti-human-Akt, anti-human-p-STAT3,
anti-human-STAT3, anti-human-p-Erk, anti-human-Erk,
anti-human-Sox2, anti-human-ABCG2 and anti-human-β-actin
antibodies, and then incubated with HRP-conjugated secondary
antibodies. Proteins were visual-ized by ECL western blotting
detection reagent (ECL kit; Pierce, Rockford, IL, USA).
Quantitative real-time PCR
Total RNA extracted from Hep-2 cells with TRIzol
reagent (Invitrogen) and reverse transcribed using a High-Capacity
RNA-to-cDNA kit (Applied Biosystems) according to standard
procedures. RT-PCR was performed in a Bio-Rad real-time PCR
detection system using SYBR Green Supermix (Bio-Rad, Carlsbad, CA,
USA) and relative quantification was performed using the ΔΔCt
method with β-actin as a reference. The primer sequences are shown
in Table I.
 | Table ISequences of primer used in real-time
PCR analysis. |
Table I
Sequences of primer used in real-time
PCR analysis.
| Gene name | Forward primer | Reverse primer |
|---|
| Sox2 |
GCCGAGTGGAAACTTTTGTCG |
GGCAGCGTGTACTTATCCTTCT |
| c-kit |
CGTTCTGCTCCTACTGCTTCG |
CCCACGCGGACTATTAAGTCT |
| Oct3/4 |
GGGAGATTGATAACTGGTGTGTT |
GTGTATATCCCAGGGTGATCCTC |
| Nanog |
TTTGTGGGCCTGAAGAAAACT |
AGGGCTGTCCTGAATAAGCAG |
| CD133 |
AGTCGGAAACTGGCAGATAGC |
GGTAGTGTTGTACTGGGCCAAT |
| nestin |
CTGCTACCCTTGAGACACCTG |
GCTCTGATCTCTGCATCTAC |
| Bmi1 |
CGTGTATTGTTCGTTACCTGGA |
CAGTAGTGGTCTGGTCTTGT |
| ActinB |
TGTTACCAACTGGGAAGACA |
GGGGTGTTGAAGGTCTCAAA |
Gene silencing experiments
siRNAs targeting human CD29, FAK or negative control
siRNAs was transfected into Hep-2 cells using Lipofectamine RNAiMAX
(Invitrogen) according to the manufacturer's instructions.
Twenty-four hours after trans-fection, Hep-2 cells were seeded on 8
kPa gels for additional 48-h culture and the expressions of Ki67
were analyzed. In some cases, Sox2, ABCG2 or negative control
siRNAs was transfected into Hep-2 cells. Twenty-four hours after
transfection, Hep-2 cells were seeded on 1 kPa gels and treated
with 10 µM cisplatin or 10 µM 5-FU. Apoptosis of
Hep-2 cells were analyzed by flow cytometry and CCK8 assay. All
siRNAs were siGenome pools (Dharmacon). Knockdown of the indicated
genes was verified by real-time PCR and western blot analysis.
Colony formation assay
Hep-2 cells were cultured for 48 h on either 1 or 8
kPa gels. Cells were then left untreated or treated with cisplatin
(10 µM) or 5-FU (10 µM) for 24 h. Then the medium was
changed and cultured for a further 48 h. Cells were trypsinized and
equal numbers re-plated at clonal density (10,000 cells/well) in
12-well plates in normal culture medium. After 10-day culture,
cells were fixed in 4% paraformaldehyde and stained with 0.5%
crystal violet.
Flow cytometry assay
For surface staining, Hep-2 cells were kept at 4°C
and stained with PE-conjugated anti-29 (eBioscience). For
intracellular staining, Hep-2 cells were first treated with
Fix/Perm solution and then stained with APC-conjugated anti-Ki67
(eBiosciences). Data were acquired on an BD FACSCanto II and
analyzed with FlowJo software.
Statistical analysis
Data are presented as mean ± SEM and assessed using
Student's t-test analysis. P-values <0.05 were considered
statistically significant. The analysis was conducted using the
Graphpad 6.0 software. All experiments were repeated at least three
times.
Results
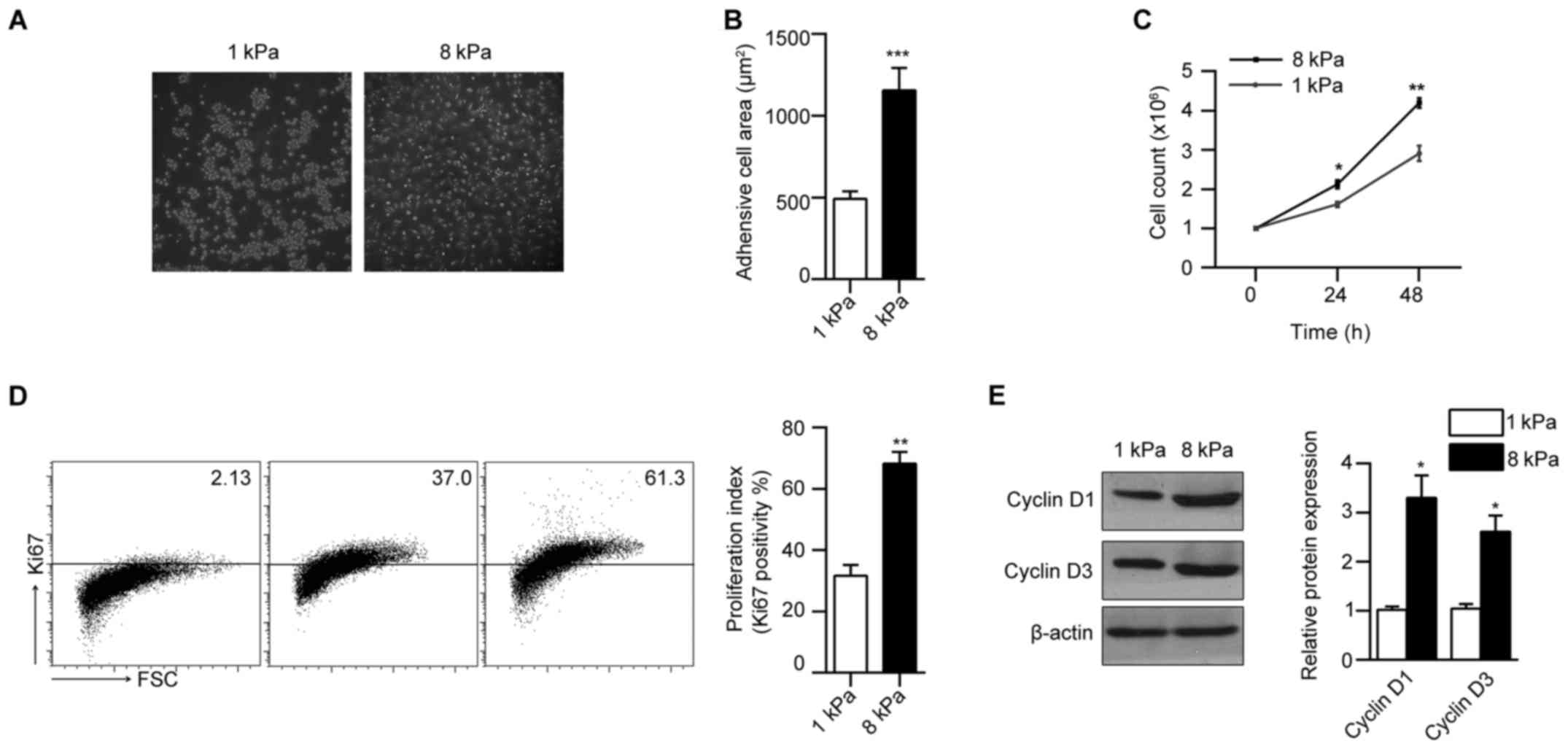
Matrix stiffness modulates Hep-2
laryngeal cancer cell morphology and proliferation
To investigate the influence of matrix stiffness on
the morphology and proliferation of Hep-2 laryngeal cancer cells,
we seeded cells on collagen coated polyacrylamide (PA) gel with
soft stiffness (1 kPa) and stiff stiffness (8 kPa). We found that
Hep-2 displayed drastically different morphologies in gels with
varying stiffness. After 24-h culture, cells maintained a rounded
shape on soft gels of 1 kPa and the cells cultured on 8 kPa
increased cell spreading along with increasing matrix stiffness
(Fig. 1A). Consistently, Hep-2
showed increased cell spreading area (Fig. 1B) along with increased matrix
stiffness. To test the effect of matrix stiffness on Hep-2
proliferation, we counted the cell numbers directly, and found an
increase in total cell number with increasing matrix stiffness
(Fig. 1C). We next found that the
frequency of Ki67-stained Hep-2 cells increased markedly with
increased matrix stiffness (Fig.
1D). Matrix stiffness had a corresponding effect on the
expression of cell cycle regulators of G1 progression. We observed
a strong reduction in the expression of cyclin D1 and cyclin D3 in
cells cultured on soft gel (Fig.
1E). These results suggest that morphology and proliferation of
Hep-2 were modulated by matrix stiffness.
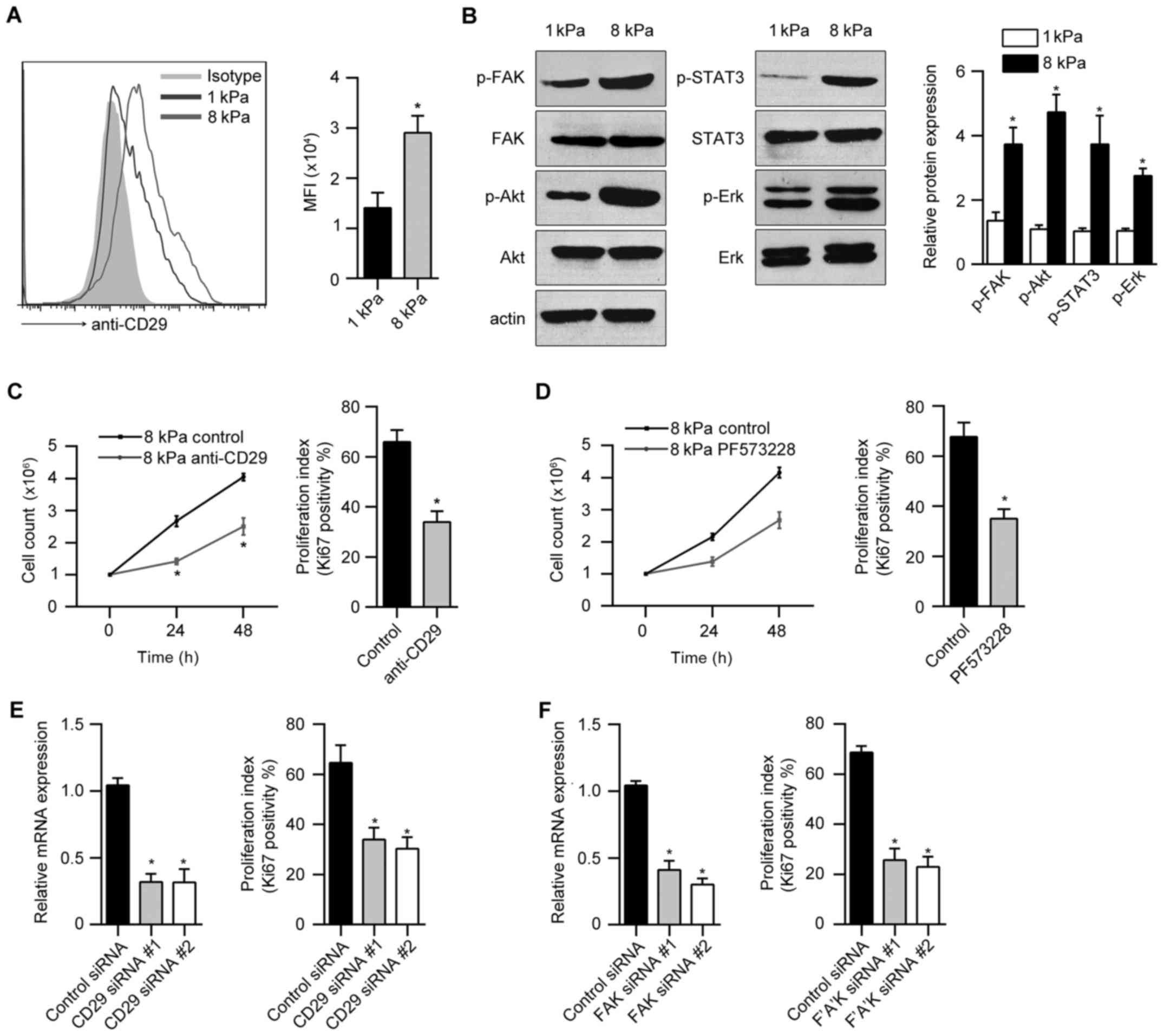
β1-integrin and phospho-FAK regulate the
stiffness-dependent proliferation of Hep-2 cells
Integrins and integrin-associated focal adhesions
are known to be important mediators of mechanotransduction.
Interestingly, we found that Hep-2 cells displayed an increase of
the β1-integrin (CD29) with increasing stiffness (Fig. 2A). Next, we analyzed the
stiffness-dependent activity of critical signaling pathways using
immunoblotting. We observed enhanced FAK, ERK, Akt and STAT3
phosphorylation in Hep-2 cell cultured on stiff (8 kPa) versus soft
(1 kPa) gels (Fig. 2B). To test
whether β1-integrin and phospho-FAK signal is responsible for the
stiffness-dependent proliferation of Hep-2 cells, we first blocked
the β1-integrin signal using a functional anti-CD29 blocking
antibody. Proliferation of Hep-2 cell cultured on 8 kPa gels was
significant reduced by treatment with anti-CD29 antibody relative
to relevant controls (Fig. 2C). To
investigate the effect of FAK activation on Hep-2 cell
proliferation, we treated Hep-2 cells cultured on 8 kPa gel with
the small molecular FAK inhibitor PF573228. FAK inhibitor markedly
inhibited the proliferation of Hep-2 cells (Fig. 2D). Furthermore, we used siRNA to
knock down β1-integrin or FAK expression in Hep-2 cells and
observed a significant reduction in cellular proliferation relative
to control siRNA transfection (Fig. 2E
and F).
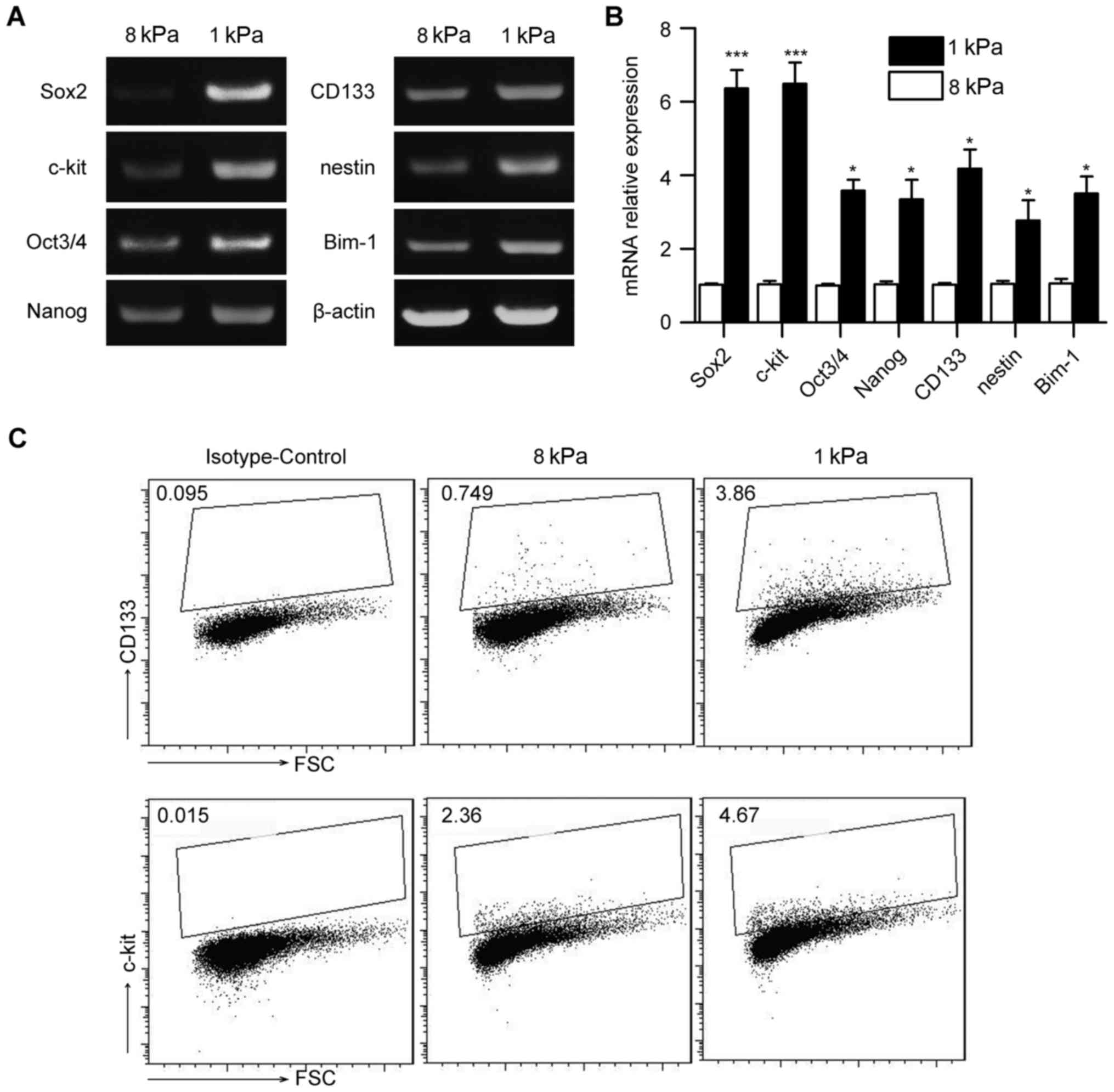
Matrix stiffness regulates stem
cell-associated genes in Hep-2 cells
Cancer cell cultured on soft stiffness is associated
with some features of cancer stem cells. To determine whether
matrix stiffness regulates stem-cell-associated genes in Hep-2
cells, we measured a panel of stem cell markers including Sox2,
Oct3/4, Nanog, CD133, nestin, Bmi-1 and c-kit using reverse
transcriptase-PCR and real-time PCR (Fig. 3A and B). Hep-2 cell cultured on
soft gel was associated with an increased stem cell-associated
genes. We further performed flow cytometric analyses for putative
cell-surface stem cell markers in Hep-2 cells cultured on soft and
stiff gels. Fig. 3C demonstrated
upregulation of cancer stem cell markers CD133 and c-kit in Hep-2
cells cultured on soft versus stiff gels. These results suggest
that matrix stiffness regulates stem cell-associated genes in Hep-2
cells.
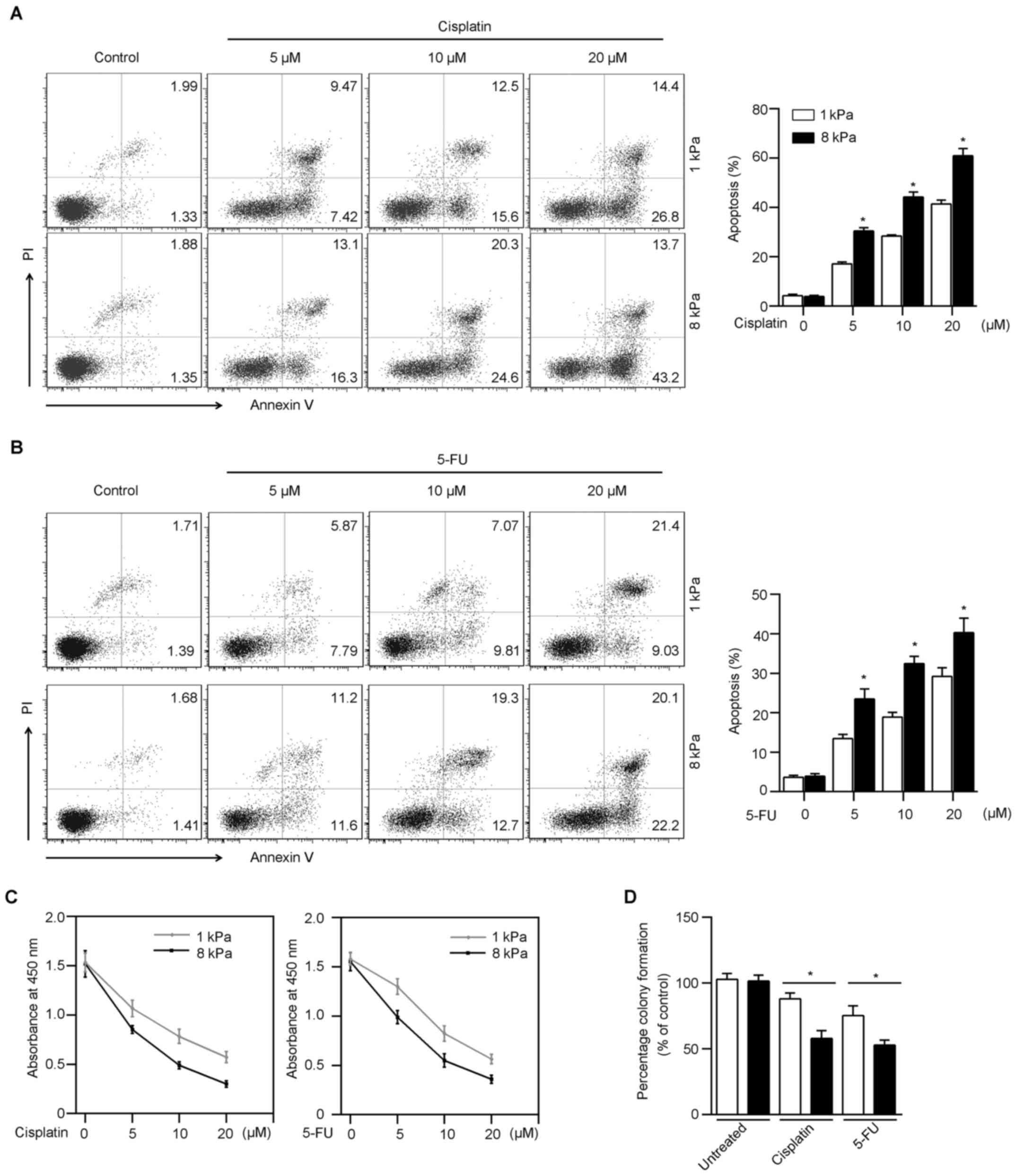
Matrix stiffness regulates
chemosensitivity of Hep-2
Cancer stem cells are known as resistant to
chemotherapeutic drug-induced apoptosis. To investigate whether
Hep2 cells cultured on soft gel are more drug-resistant, different
concentrations of cisplatin were added to the culture medium.
Following cisplatin treatment, an increased frequency of apoptotic
cells was induced in Hep-2 cells from stiff gels, compared those
from soft gels (Fig. 4A). To
confirm the validity of this finding, we used a second
chemotherapeutic agent, 5-fluorouracil (5-FU) to repeat the
experiment. Consistent with the result with cisplatin, Hep-2 cells
cultured on soft gels were more resistant to apoptosis induced by
5-FU (Fig. 4B). In addition, the
cell cytotoxicity of Hep-2 cells was detected by CCK-8 assay.
Fig. 4C showed that Hep-2 cells in
soft (1 kPa) gel were more resistant to chemo-drugs than in stiff
(8 kPa) gel. To investigate the influence of matrix stiffness on
the survival of Hep-2 tumor cells after chemotherapy, we performed
a colony formation assay. Following cisplatin or 5-FU treatment on
Hep-2 cells on soft (1 kPa) and stiff (8 kPa) gels, the surviving
Hep-2 cell population from soft gel formatted an increased colony
cultured on rigid 12-well plates (Fig.
4D). These results suggest that matrix stiffness regulates
chemosensitivity of Hep-2 cells, which is consistent with the
expression of stem cell-associated markers.
Sox2 is essential for the chemoresistance
of Hep-2 cells cultured in soft gels
Sox2 plays an essential role in the maintenance of
self-renewal of cancer stem cells. In addition, overexpression of
Sox2 promotes the migration and invasion in laryngeal squamous
cancer cells. To verify the possible correlation between Sox2
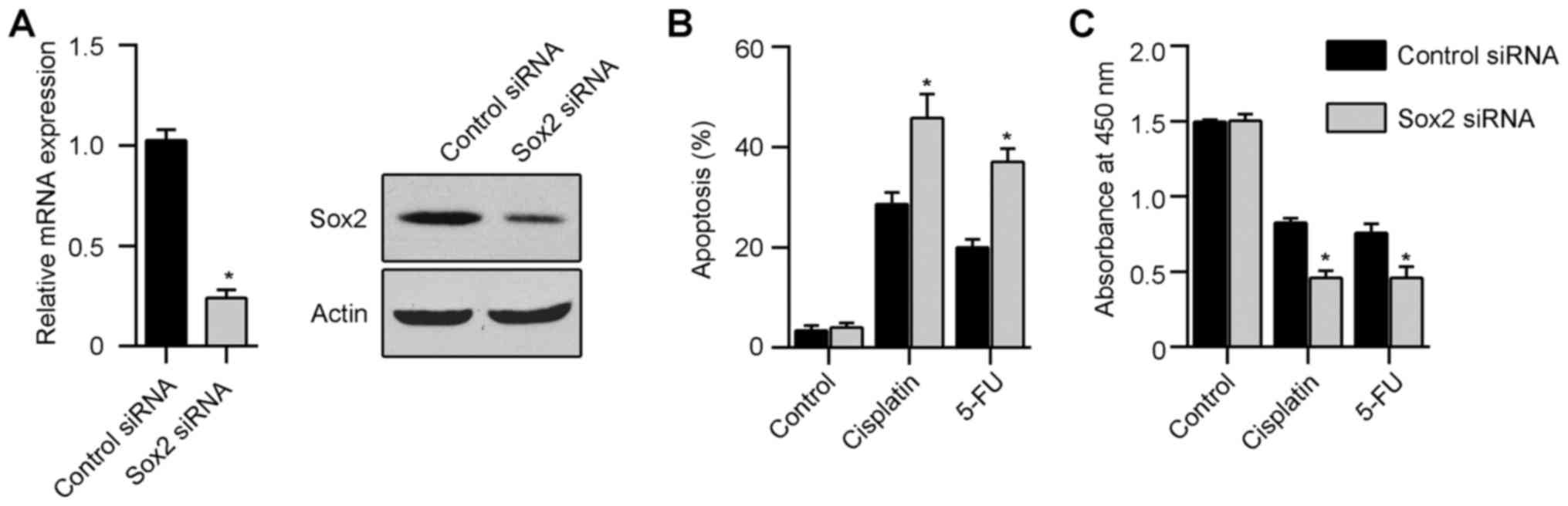
expression and chemoresistance of Hep-2 cells, we knocked down Sox2
in Hep-2 via siRNA interference and then cultured in 1 kPa gels.
Scrambled control siRNA treated cells were used as control. The
knockdown of Sox2 expression in Hep-2 cells was confirmed by
real-time PCR and western blot analysis (Fig. 5A). Thirty-six hours after culture
in 1 kPa gels, Hep-2 cells were treated with 10 µM
cisplatin. Flow cytometry and CCK8 assay showed that silencing of
Sox2 in Hep-2 cells on soft gel significantly decreased the
chemoresistance ability when compared with scrambled control
(Fig. 5B and C). Similarly, Sox2
siRNA decreased the chemoresistance ability of Hep-2 cells when
treated with 10 µM 5-fluorouracil (Fig. 5B and C).
Sox2 promotes chemoresistance of Hep-2
cells in soft stiffness via upregulating the expression of
ABCG2
ATP-binding cassette (ABC) transporters are
well-known to exclude a wide variety of chemotherapeutic drugs from
the cytoplasm, thereby leading to multidrug resistance. Among
various ABC transporters, ABCG2 is thought to play an important
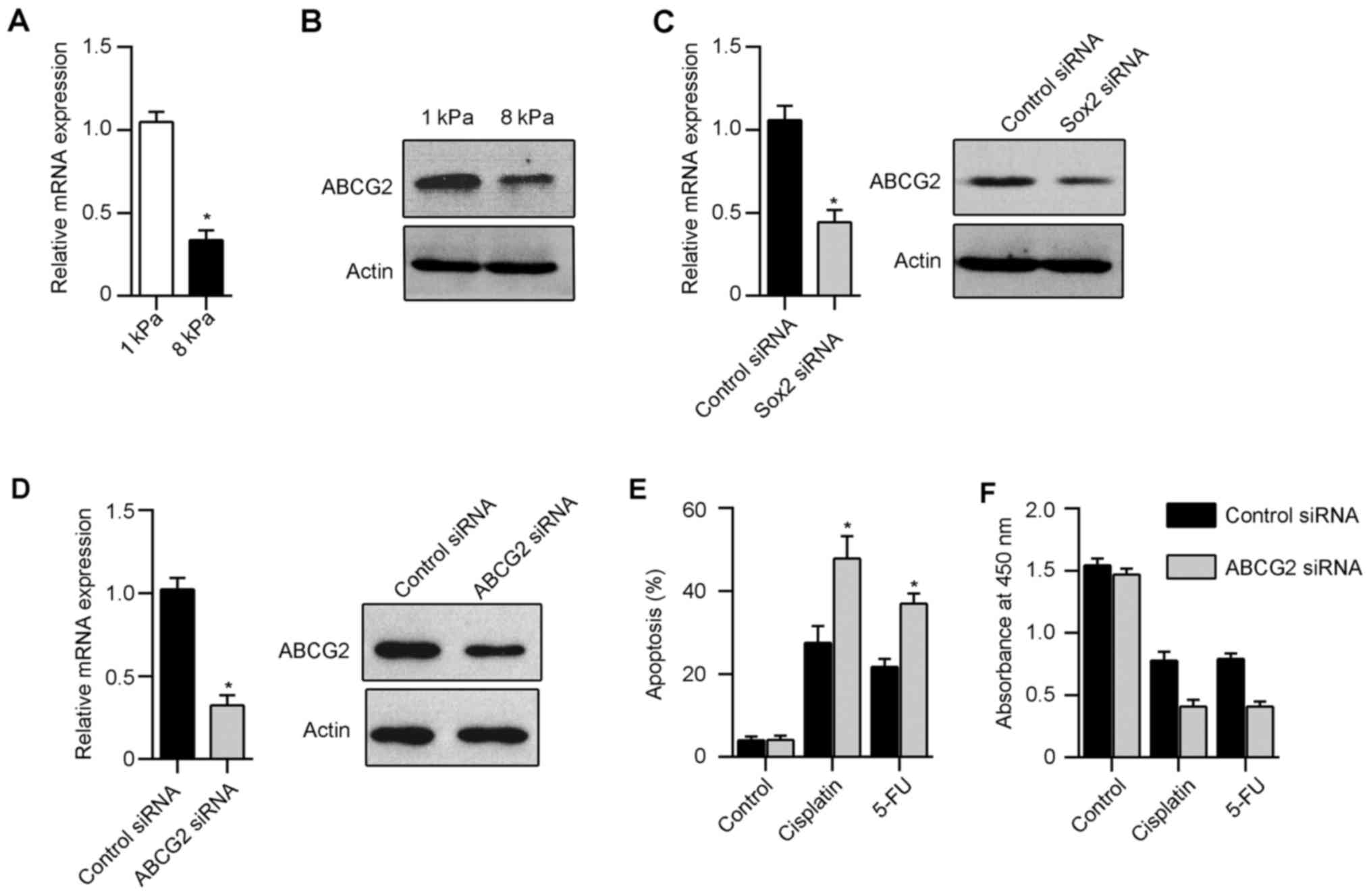
role in laryngeal squamous cancer cells. Interestingly, Hep-2 cells
cultured in soft gel expressed significant high level of ABCG2
compared with Hep-2 in stiff gel (Fig.
6A and B). In addition, silencing of Sox2 in Hep-2 cells
resulted in downregulation of ABCG2 compared with control cells
(Fig. 6C). Furthermore, we knocked
down ABCG2 in Hep-2 using siRNA and then cultured in 1 kPa gels
(Fig. 6D). Downregulation of ABCG2
in Hep-2 cells cultured in soft gels restored drug sensitivity
after cisplatin and 5-FU treatment (Fig. 6E and F). These data suggest that
Sox2 has a major role in drug resistance of Hep-2 cells in soft
gels by regulating the ABCG2 expression.
Discussion
Mechanical forces play an essential role in the
signal transduction at cell-matrix and cell-cell contacts,
modulating various cellular behaviors such as cell adhesion,
proliferation, migration, and differentiation (16–18).
In this study, we demonstrated that the stiffness of the external
matrix markedly alters the phenotype and behavior of laryngeal
squamous cell carcinoma cells in vitro. It has previously
been demonstrated that matrix stiffness can regulate proliferation
in glioma cells (19) and
hepatocellular carcinoma cells (9). Our data supported that stiff
substrate significantly promote the proliferation of Hep-2 cells,
verified by direct counting and Ki67 staining. In accordance with
these findings, we found that the expression of cyclin D1 and
cyclin D3 were upregulated in Hep-2 cells on 8 kPa gel. Focal
adhesion, an integrin-based adhesion complex, regulates cellular
behavior in various biological context by transferring external
force information in a bidirectional manner (20). Integrin-FAK signaling pathway is
responsible for mechanotransduction (21). Interestingly, we found that Hep-2
cells on stiff gels express higher level of β1-integrin.
Consistently, we have demonstrated that β1-integrin and FAK
activation regulate stiffness-dependent proliferation in Hep-2
cells. Treatment with anti-CD29 or FAK inhibitor PF573228, markedly
decreased the proliferation of Hep-2 cells on stiff gel.
Additionally, we observed an increase in the magnitude of ERK, Akt,
and STAT3 activation in Hep-2 cells cultured on stiff gels,
suggesting that increasing matrix stiffness promoted activity of
critical mitogenic signaling pathways.
Previous studies showed that soft matrix upregulate
stem-cell-associated genes in B16 or HCC cells. Most strikingly,
Liu et al showed that a few B16 cells cultured in soft
fibrin gel led to the formation of solid tumors in syngeneic or
severe combined immunodeficiency mice (14). In the present study, Hep-2 cells on
soft gel expressed higher level of stem cell markers (Sox2, Oct3/4,
Nanog, CD133, nestin, Bmi-1 and c-kit). However, the underlying
mechanisms regulating the expression of stem cell genes by
mechanical force needs to be further determined. A possible
mechanism elucidated by Tajik et al (15) is that externally mechanical force
induced the direct stretching of chromatin, leading to the
transcription upregulation.
It is known that cancer stem cells are more
resistant to chemotherapeutic drug-mediated cell death than
differentiated cancer cells (22,23).
Intriguingly, we demonstrated that Hep-2 cells on soft gel are more
resistant to chemotherapeutic drug than those from stiff gel, which
is consistent with previous study on HCC cells. A recent study
showed that Sox2 played a regulatory role in the growth, migration
and invasion of LSCC (24). We
showed that Sox2 is essential for the chemo-resistance of Hep-2
cells cultured in soft gels. Knockdown of Sox2 using siRNA
significant increased the drug-sensitivity of Hep-2 cells on soft
gels. ATP-binding cassette (ABC) transporter is known to exclude
drugs from cytoplasm to extracellular environment. Here, we provide
further evidence that Sox2 promote chemoresistance through
upregulating the expression of ABCG2, an ABC transporter which
plays an essential role in LSCC.
In conclusion, our study shows that increasing
matrix stiffness promotes the proliferation of Hep-2 cells, in
turn, Hep-2 cells cultured on soft substrate expressed higher level
of stem cell markers. Especially, the Sox2 upregulation in Hep-2
cells on soft gel induced the expression ABCG2, resulting in
resistance to chemotherapeutic drugs. Our present study provides a
new mechanism of the growth and behavior of LSCC. Thus, these
findings will provide a new platform for the future design of
anticancer drugs based on the biophysical properties of the tumor
site.
Acknowledgments
This study was supported by Natural Science
Foundation of Liaoning Province of China (no. 201202287).
References
|
1
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papadas TA, Alexopoulos EC, Mallis A,
Jelastopulu E, Mastronikolis NS and Goumas P: Survival after
laryngectomy: A review of 133 patients with laryngeal carcinoma.
Eur Arch Otorhinolaryngol. 267:1095–1101. 2010. View Article : Google Scholar
|
|
3
|
Chai LP, Wang ZF, Liang WY, Chen L, Chen
D, Wang AX and Zhang ZQ: In vitro and in vivo effect of 5-FC
combined gene therapy with TNF-α and CD suicide gene on human
laryngeal carcinoma cell line Hep-2. PLoS One. 8:e611362013.
View Article : Google Scholar
|
|
4
|
Paszek MJ, Zahir N, Johnson KR, Lakins JN,
Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M,
Boettiger D, et al: Tensional homeostasis and the malignant
phenotype. Cancer Cell. 8:241–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levental KR, Yu H, Kass L, Lakins JN,
Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et
al: Matrix crosslinking forces tumor progression by enhancing
integrin signaling. Cell. 139:891–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Samuel MS, Lopez JI, McGhee EJ, Croft DR,
Strachan D, Timpson P, Munro J, Schröder E, Zhou J, Brunton VG, et
al: Actomyosin-mediated cellular tension drives increased tissue
stiffness and β-catenin activation to induce epidermal hyperplasia
and tumor growth. Cancer Cell. 19:776–791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frantz C, Stewart KM and Weaver VM: The
extracellular matrix at a glance. J Cell Sci. 123:4195–4200. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schrader J, Gordon-Walker TT, Aucott RL,
van Deemter M, Quaas A, Walsh S, Benten D, Forbes SJ, Wells RG and
Iredale JP: Matrix stiffness modulates proliferation,
chemotherapeutic response, and dormancy in hepatocellular carcinoma
cells. Hepatology. 53:1192–1205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH,
Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, et al: Matrix
stiffness drives epithelial-mesenchymal transition and tumour
metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat
Cell Biol. 17:678–688. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chowdhury F, Li Y, Poh YC, Yokohama-Tamaki
T, Wang N and Tanaka TS: Soft substrates promote homogeneous
self-renewal of embryonic stem cells via downregulating cell-matrix
tractions. PLoS One. 5:e156552010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu J, Tan Y, Zhang H, Zhang Y, Xu P, Chen
J, Poh YC, Tang K, Wang N and Huang B: Soft fibrin gels promote
selection and growth of tumorigenic cells. Nat Mater. 11:734–741.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tajik A, Zhang Y, Wei F, Sun J, Jia Q,
Zhou W, Singh R, Khanna N, Belmont AS and Wang N: Transcription
upregulation via force-induced direct stretching of chromatin. Nat
Mater. 15:1287–1296. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaalouk DE and Lammerding J:
Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 10:63–73.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calvo F, Ege N, Grande-Garcia A, Hooper S,
Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary
E, Charras G, et al: Mechanotransduction and YAP-dependent matrix
remodelling is required for the generation and maintenance of
cancer-associated fibroblasts. Nat Cell Biol. 15:637–646. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Butcher DT, Alliston T and Weaver VM: A
tense situation: Forcing tumour progression. Nat Rev Cancer.
9:108–122. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang YL, Motte S and Kaufman LJ: Pore size
variable type I collagen gels and their interaction with glioma
cells. Biomaterials. 31:5678–5688. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanchanawong P, Shtengel G, Pasapera AM,
Ramko EB, Davidson MW, Hess HF and Waterman CM: Nanoscale
architecture of integrin-based cell adhesions. Nature. 468:580–584.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palazzo AF, Eng CH, Schlaepfer DD,
Marcantonio EE and Gundersen GG: Localized stabilization of
microtubules by integrin- and FAK-facilitated Rho signaling.
Science. 303:836–839. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cojoc M, Mäbert K, Muders MH and Dubrovska
A: A role for cancer stem cells in therapy resistance: Cellular and
molecular mechanisms. Semin Cancer Biol. 31:16–27. 2015. View Article : Google Scholar
|
|
24
|
Yang N, Hui L, Wang Y, Yang H and Jiang X:
SOX2 promotes the migration and invasion of laryngeal cancer cells
by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol Rep.
31:2651–2659. 2014.PubMed/NCBI
|