Introduction
Hepatocellular carcinoma (HCC) is a common and
aggressive human malignancy, and it is currently the second most
common cause of cancer-related death worldwide (1,2).
Despite great efforts to improve patient outcomes through advances
in diagnostic and therapeutic modalities (3), the 5-year survival of HCC is still
<50% (2). Metastasis is the
most deadly and least understood aspect of cancer and one of the
primary reasons for the high mortality of HCC (4). Increasing evidence indicates that the
epithelial-to-mesenchymal transition (EMT), a process by which
epithelial cells lose polarity, gain migratory and invasive
properties and are converted to a mesenchymal phenotype (5–7), is
an initial and critical step in HCC tumor metastasis (8). We have previously reported that EMT
is involved in early disease recurrence and is associated with the
IL-6 pathway in HCC (9).
EMT contributes to tissue repair and organ fibrosis
as well as promotes cancer progression (10,11)
and the generation of cells with stem cell-like properties
(12). However, a mechanistic
understanding of how specific transcription factors induce EMT is
still lacking. Thus, uncovering the signaling pathways through
which transcription factors regulate EMT is of significant
interest, as this information could have broad biological
significance. The Wnt proteins are a family of transcription
factors that play critical roles in cell proliferation, migration
and invasion (13) by binding to
and activating one or more of the ten known Frizzled (Fzd)
receptors (14). Many previous
studies have demonstrated activation of canonical Wnt/β-catenin
signaling during EMT (15–17). In addition, a study assessing
pharmacologic and genetic perturbations has revealed that Fzd2
drives EMT and cell migration through a previously unrecognized,
non-canonical pathway that involves molecules such as Fyn and Stat3
(13).
In the present study, expression analyses and in
vitro functional analyses were conducted to identify the
EMT-inducing factors that significantly influence the biological
behavior of HCC. Surgical specimens were also obtained from HCC
patients to verify the in vitro findings. Furthermore, the
role of the non-canonical Wnt pathway in HCC EMT was analyzed in
vitro using HCC cell lines, as well as in vivo.
Materials and methods
Cell lines and culture
Fifteen human HCC cell lines [HepG2, HuH-7, HuH-2,
Hep3B, SNU182, PLC/PRF/5(P5), HLE, SNU398, SNU449, SNU387, HLF,
SNU475, HuH-1, Focus, and SK-Hep] were utilized in the present
study. Several human hepatoma cell lines (SNU182, SNU398, SNU449,
SNU387 and SNU475) were obtained from the American Type Culture
Collection (ATCC, Rockville, MD, USA). In addition, the hepatoma
cell lines SK-Hep, P5, HepG2, and Hep3B were kindly provided by
Barrie Bode (Northern Illinois University, DeKalb, IL, USA), HuH-7
was contributed by Jake Liang (NIDDK, National Institutes of
Health, Bethesda, MD, USA), Focus was provided by Jack Wands (Brown
University, Providence, RI, USA), and HLE, HLF, HuH-1 and HuH-2
were obtained from Rikon. All cell lines were propagated in
RPMI-1640 medium (Life Technologies Corp., Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Life Technologies Corp.).
Cultures were incubated at 37°C/5% CO2.
Patients and specimens
Cancerous tissues and surrounding non-cancerous
hepatic parenchyma were obtained from 100 primary HCC patients who
underwent resection at Nagoya University Hospital during the period
from May 1994 to December 2003. The study was approved by the
Ethics Committee, and informed consent was obtained from all
patients. The mean follow-up period was 51.3±40.5 months.
Real-time polymerase chain reaction
Total RNA isolated from primary HCC tissues and
corresponding non-cancerous tissues was used to generate cDNA,
which was amplified using PCR primers specific for E-cadherin,
vimentin, Fzd2 and GAPDH. PCR amplification consisted of an initial
denaturation step at 94°C for 5 min, followed by 30 cycles at 94°C
for 15 sec, 60°C for 15 sec and 72°C for 12 sec.
RNA expression was determined by real-time
quantitative PCR. Real-time detection of the emission intensity of
SYBR Green was performed with an ABI PRISM 7000 Sequence Detector
(Perkin-Elmer Applied Biosystems, Foster City, CA, USA).
Quantitative PCR was performed at least three times for each
sample, and a no-template negative control was used.
The EMT status of each patient's tumor was
determined from E-cadherin and vimentin mRNA expression as follows:
vimentin/E-cadherin <2 = epithelial (E); and vimentin/E-cadherin
≥2 = mesenchymal (M).
Fzd2 expression in each patient's tumor was
classified according to the mRNA expression in cancerous tissue
versus that in non-cancerous tissue as follows: Fzd2 expression in
cancerous tissue/Fzd2 expression in non-cancerous tissue <2 =
Fzd2 low-expression group; and Fzd2 expression in cancerous
tissue/Fzd2 expression in non-cancerous tissue ≥2 = Fzd2
high-expression group.
Western blot analysis
Cell lysates were prepared and loaded onto 4–12%
gels to separate proteins by SDS-PAGE. The proteins were then
transferred to polyvinylidene difluoride membranes. The membranes
were blocked with phosphate-buffered saline-Tween containing 5%
(w/v) non-fat milk for 2 h at room temperature with shaking and
then incubated overnight at 4°C with a primary antibody (rat
anti-Frizzled2 diluted 1:1,000; R&S Systems, #MAB1307).
Fzd2 short interfering RNA
transfection
HLF cells were seeded in 6-well plates
(2×105 cells/well) and transfected the next day with
either 30 nM predesigned short interfering RNA (siRNA) targeting
Fzd2 or control siRNA (GE Healthcare, Buckinghamshire, UK). After
72 h, the Fzd2 protein and mRNA levels were analyzed by western
blotting and real-time PCR, respectively.
Cell proliferation, migration, and
invasion assays
HLF cells were seeded in 96-well plates
(5×103 cells/well) and transfected the following day
with Fzd2 or control siRNA. Cell proliferation was evaluated using
premix WST-1 reagent (Takara Bio, Japan), and absorbance was
measured at 440 nm.
Migratory ability was assessed by wound healing
assays, which were performed using the culture insert method (Ibidi
GmbH, Germany). When the cell layer was confluent at 24 h after
transfection, the culture insert was removed. The area of migration
was measured using ImageJ software (Wayne Rasband, National
Institute of Health, USA) at 24 h after removal of the insert.
Cell invasion was assessed using Matrigel
invasion chambers
HLF cells were seeded into 6-well plates
(5×104 cells/well) and transfected the following day
with Fzd2 or control siRNA. At 48 h post-transfection, the cells
were plated in transwell chambers (BD Bioscience) pre-coated with
Matrigel Invasion Chamber medium. After approximately 24 h,
non-invading cells were removed, and invasive cells attached to the
lower surface of the membrane were stained. The number of invasive
cells was determined from five randomly selected fields of
view.
Statistical analysis
Between-group differences were evaluated using
Fisher's exact test or the χ2 test. In addition, the
recurrence-free and overall survival rates were calculated using
the Kaplan-Meier method, and differences between survival curves
were analyzed using the log-rank test. Independent prognostic
factors were analyzed using Cox proportional hazards regression
models. The data are presented as the mean ± SD, and a P-value of
<0.05 was considered statistically significant. Analysis was
conducted using JMP version 11 software (JMP, SAS Institute, Cary,
NC, USA).
Results
E-cadherin, vimentin and EMT-inducing
factors in human HCC cell lines
E-cadherin and vimentin mRNA expression was measured
by real-time PCR in 15 HCC cell lines to determine the extent of
EMT. Six of the cell lines (HepG2, HuH7, HuH2, Hep3B, SNU-182, and
P5) were classified as epithelial, and nine (HLE, SNU-398, SNU-449,
SNU-387, HLF, SNU-475, HuH1, Focus, and SK-Hep) were classified as
mesenchymal based on their E-cadherin and vimentin expression.
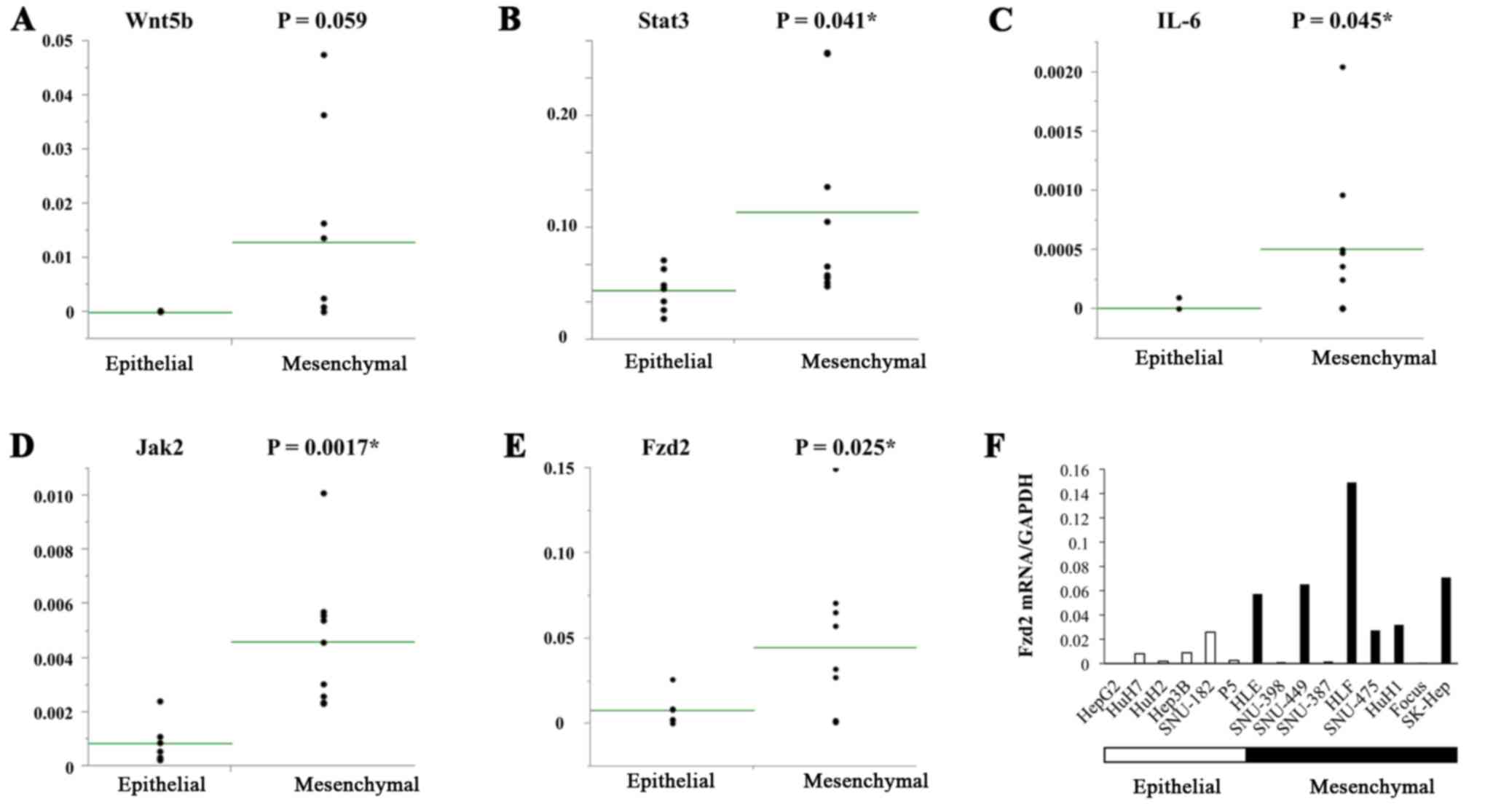
The levels of EMT-inducing factors (Wnt5b, Stat3,
IL-6, Jak2, and Fzd2) were also measured in the HCC cell lines, and
their associations with EMT were examined. Several of these factors
(Stat3, IL-6, Jak2, and Fzd2) were expressed at high levels in the
mesenchymal HCC cell lines (Fig.
1A–E). In particular, Fzd2 was highly expressed in 4 of the 9
mesenchymal cell lines (HLF, SK-Hep, SNU-449 and HLE), but it was
not expressed in any of the 6 epithelial cell lines (Fig. 1F).
Impact of Fzd2 expression on
proliferation, migration and invasion
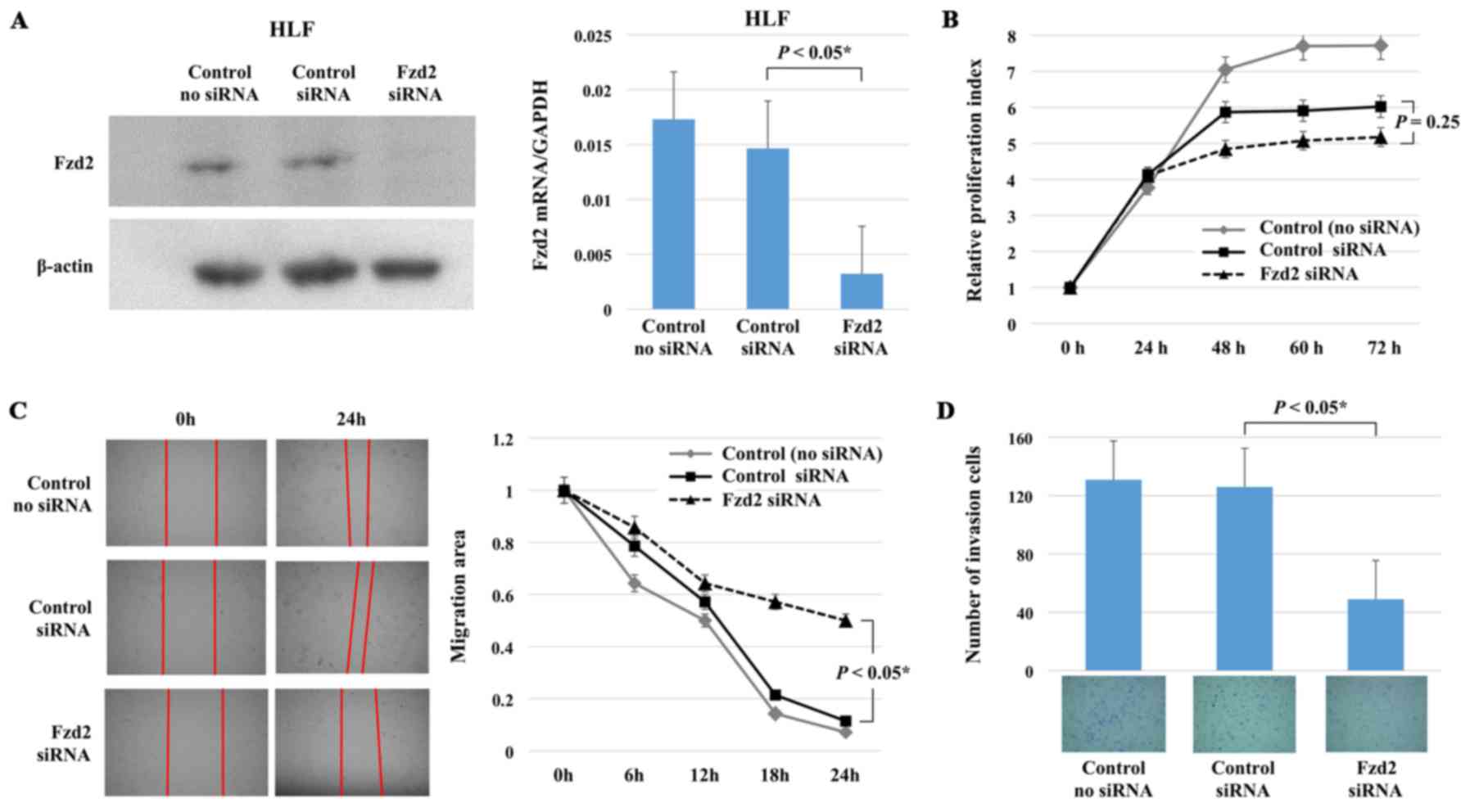
To further verify the relationship of Fzd2 with EMT
in HCC, Fzd2 siRNA was transfected into mesenchymal HLF cells, and
the impacts on cell proliferation, migration, and invasion were
evaluated. Fzd2 mRNA and protein expression was significantly
inhibited in the HLF cells following treatment with Fzd2 siRNA
(Fig. 2A).
Next, the proliferation, migration and invasiveness
of HLF cells treated with Fzd2 siRNA were examined, and the results
were compared with those for HLF cells treated with control siRNA.
Cell proliferation was not affected by Fzd2 inhibition; however,
cell migration and invasiveness were significantly reduced
(Fig. 2B–D). These results
demonstrated that although Fzd2 knockdown failed to reduce
proliferation, it inhibited migration and invasion, indicative of
reduced EMT.
Clinical implication of Fzd2 status in
HCC patients
Subsequently, we measured Fzd2 mRNA expression in
cancerous and surrounding non-cancerous tissues from 100 HCC
patients using real-time PCR. The Fzd2 status was then determined
based on the cancerous tissue to non-cancerous tissue ratio, as
described in Materials and methods. The patients were classified
into an Fzd2 low-expression group (64 patients) or Fzd2
high-expression group (36 patients). Similarly, E-cadherin and
vimentin expression was measured by real-time PCR in the cancerous
and surrounding non-cancerous tissues from the same patients. The
EMT status was then determined based on the vimentin to E-cadherin
ratio (V/E ratio), and the patients were classified into an
epithelial group (61 patients) or mesenchymal group (39
patients).
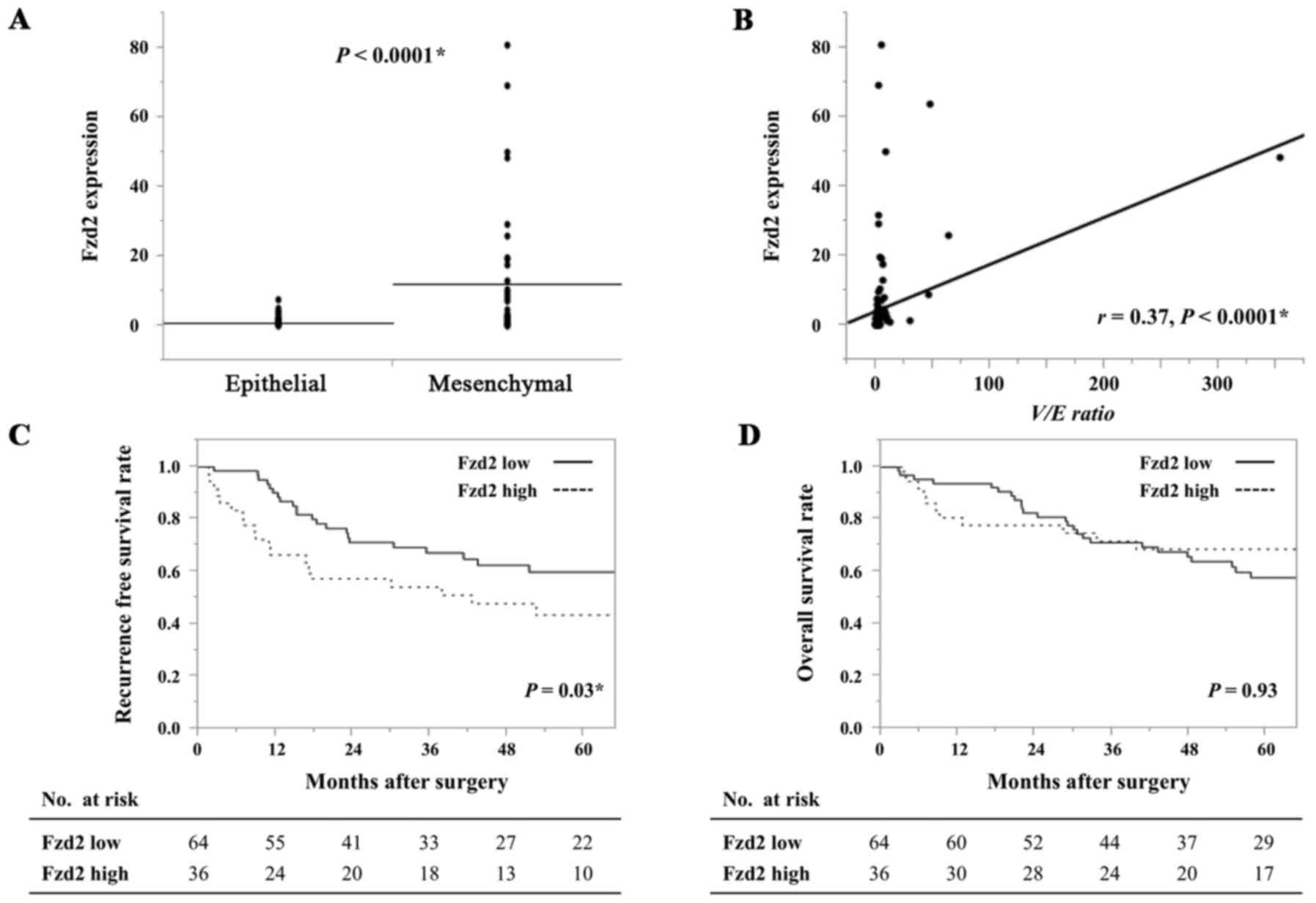
Examination of the relationship between Fzd2 and EMT
revealed that Fzd2 expression was significantly higher in the
mesenchymal group than in the epithelial group (P<0.0001)
(Fig. 3A). Additionally, a
significant correlation was detected between the V/E ratio and Fzd2
expression (r=0.37, P<0.0001) (Fig.
3B). However, no significant correlation was observed between
the Fzd2 status and any other clinicopathological parameter
(Table I). These results further
indicated that Fzd2 expression was an essential mediator of EMT in
HCC.
 | Table ICorrelation between Fzd2 expression
and clinicopathological features. |
Table I
Correlation between Fzd2 expression
and clinicopathological features.
| Characteristic | Fzd2 low (n=64) | Fzd2 high (n=36) | P-value |
|---|
| Age (>65/≤64) | 32/32 | 20/16 | 0.74 |
| Male/female | 51/13 | 32/4 | 0.25 |
| Virus
(HBV/HCV/others) | 9/42/13 | 6/23/7 | 0.53 |
| Histologic type of
tumor | | | |
|
Mod/Well/Poor/others | 43/7/4/10 | 24/6/0/6 | 0.41 |
| Tumor size (cm) | | | |
| >3/≤3 | 38/26 | 23/13 | 0.48 |
| Tumor
multiplicity | | | |
|
Solitary/multiple | 50/14 | 22/13 | 0.14 |
| Pattern of tumor
growth | | | |
|
Expansive/infiltrative | 53/11 | 30/6 | 0.60 |
| Formation of
fibrous capsule | | | |
|
Present/absent | 48/16 | 20/16 | 0.23 |
| Septal
formation | | | |
|
Present/absent | 44/20 | 20/16 | 0.36 |
| Vascular
invasion | | | |
|
Present/absent | 15/49 | 12/24 | 0.46 |
| AFP level
(ng/ml) | | | |
| >20/≤20 | 37/27 | 20/16 | 0.98 |
| Pugh-Child's
classification | | | |
| A/B | 58/6 | 34/1 | 0.62 |
| Pathological
stage | | | |
| III/III IVa | 42/22 | 19/17 | 0.31 |
| EMT status | | | |
|
Epithelial/mesenchymal | 51/13 | 10/26 | <0.0001 |
Prognosis of HCC patients according to
the status of EMT and Fzd2
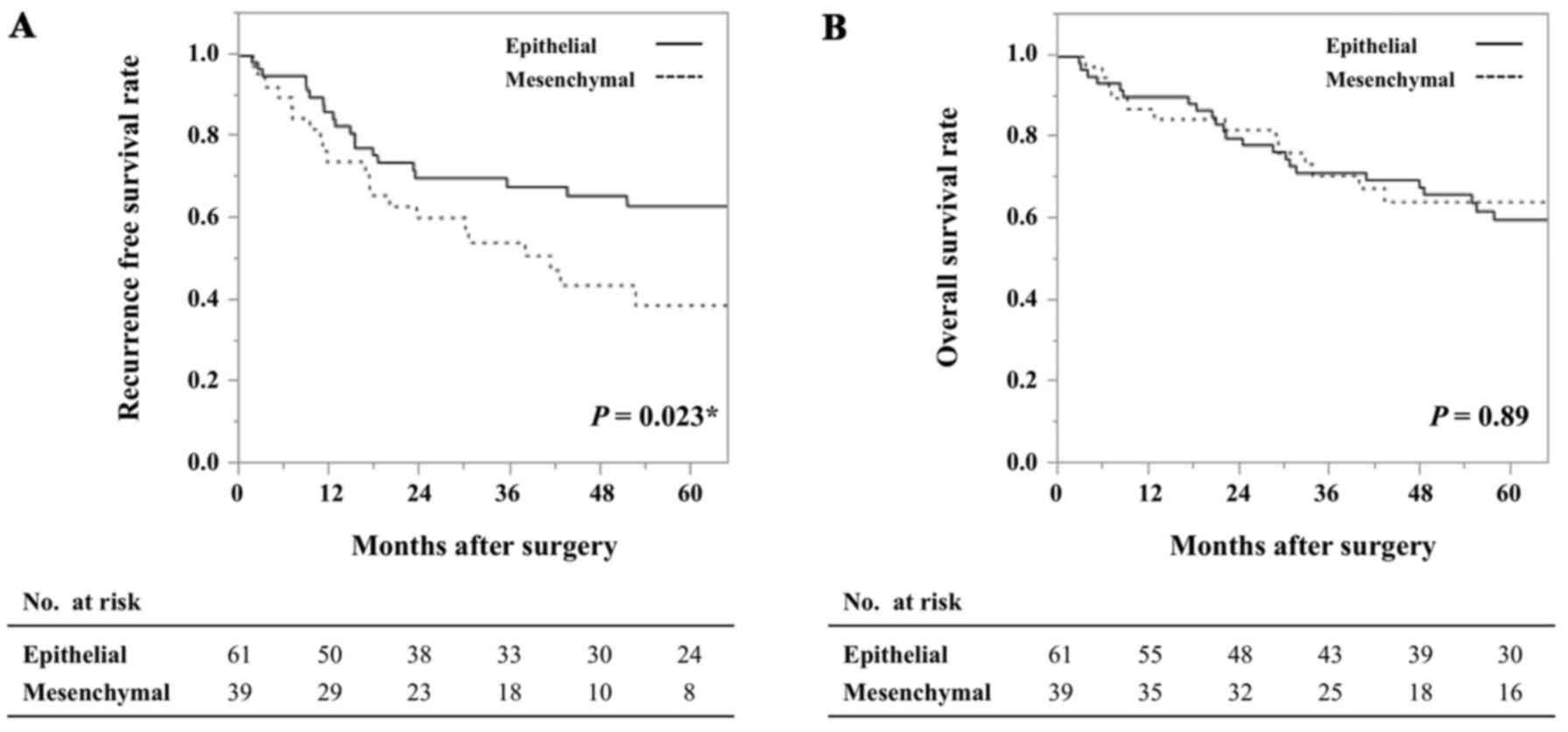
Analysis of survival based on the EMT status
revealed no difference in overall survival (P=0.89) but a
significant difference in recurrence-free survival between the
epithelial and mesenchymal groups (P=0.023), indicating that the
patients with a mesenchymal tumor were more prone to experiencing
earlier recurrence than those with an epithelial tumor (Fig. 4).
Similarly, analysis of survival based on the Fzd2
status revealed a significant difference in recurrence-free
survival (P=0.03) (Fig. 3C) but no
difference in overall survival (P=0.93) (Fig. 3D). Thus, the HCC patients in the
Fzd2 high-expression group more frequently experienced earlier
recurrence than those in the Fzd2 low-expression group.
Univariate and multivariate analyses of
clinicopathological factors for HCC recurrence
Clinicopathological factors were also analyzed as
predictive factors for recurrence. Univariate analysis showed that
male gender was associated with early recurrence, and this
association was significant for the patients with high Fzd2
expression (P=0.036). Multivariate analysis also revealed that high
Fzd2 expression was more strongly associated with earlier
recurrence than low Fzd2 expression (P=0.043) (Table II).
 | Table IIUnivariate and multivariate analysis
for predictors of recurrence-free survival. |
Table II
Univariate and multivariate analysis
for predictors of recurrence-free survival.
| Predictors | Univariate analysis
| Multivariate
analysis
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥65 vs
<64) | 0.74
(0.40–1.32) | 0.31 | | |
| Gender (male vs
female) | 2.22
(0.96–6.46) | 0.064 | 2.15
(0.92–6.27) | 0.077 |
| Tumor size (3
cm) | 1.43
(0.76–2.87) | 0.28 | | |
| Histological
type | 1.69
(0.40–4.74) | 0.42 | | |
| Tumor
multiplicity | 1.57
(0.80–2.97) | 0.18 | | |
| Pattern of tumor
growth | 1.37
(0.58–2.85) | 0.44 | | |
| Formation of
fibrous capsule | 0.94
(0.47–2.02) | 0.87 | | |
| Septal
formation | 1.53
(0.76–3.41) | 0.24 | | |
| Vascular
invasion | 1.74
(0.88–3.38) | 0.13 | | |
| AFP | 1.14
(0.61–2.19) | 0.68 | | |
| Child-Pugh | 0.58
(0.094–1.90) | 0.42 | | |
| Pathological
stage | 1.30
(0.68–2.43) | 0.42 | | |
| Fzd2 status
(high) | 1.89
(1.04–3.38) | 0.036a | 1.85
(1.02–3.31) | 0.043a |
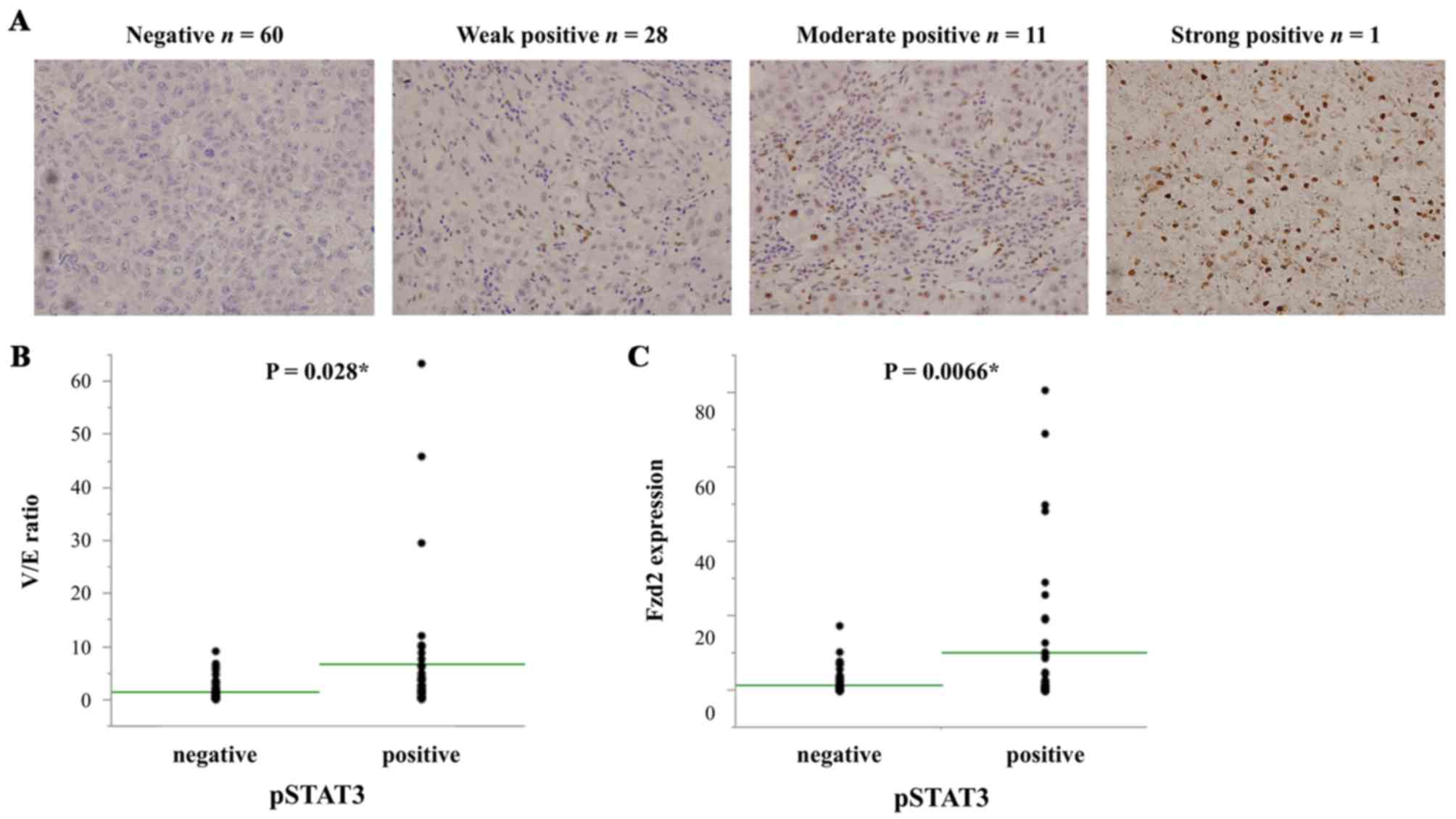
Immunohistochemical analysis of pSTAT3
and the correlations of its expression with the status of EMT and
Fzd2 in HCC patients
Immunohistochemical staining for pSTAT3 revealed
that it was localized to the nuclei of HCC cells. Negative pSTAT3
staining was observed in 60 out of 100 patients, whereas weak,
moderate and strong positive staining were observed in 28, 11 and 1
patients, respectively (Fig. 5A).
The patients with positive pSTAT3 staining had a significantly
higher V/E ratio than those with negative staining (P=0.0028)
(Fig. 5B). In addition, they had
significantly higher Fzd2 expression (P=0.0066) (Fig. 5C). These results revealed that
pSTAT3 expression was significantly correlated with EMT and the
Fzd2 status.
Discussion
The invasiveness and metastasis of many cancers,
including HCC, represent major obstacles for cancer treatment and
are associated with poor survival. In this regard, EMT is thought
to be an important biological process that plays critical roles in
tumor cell invasion and metastasis, and it has been actively
investigated in association with HCC (18–20).
Thus, studies examining the mechanism underlying EMT and
identifying novel targets for controlling HCC invasiveness and
metastasis are urgently needed.
Several growth factors, including TGFβ, Wnt, EGF,
and HGF, have been shown to trigger EMT both during embryonic
development and in normal and transformed cell lines (10). In particular, Wnt5 ligands have
been reported to be overexpressed in mesenchymal cells derived from
late-stage HCC tumors. Furthermore, Fzd2 has been shown to be
overexpressed in late-stage HCC and lung cancer, and its
overexpression has been reported to be correlated with poor patient
survival (13). In the present
study, the correlation among Fzd2 expression, EMT, and patient
prognosis was investigated in resected HCC clinical samples. The
results revealed that high Fzd2 expression was strongly correlated
with mesenchymal-type tumors among the HCC patients. In addition,
the status of EMT and Fzd2 was significantly associated with
recurrence-free survival. These data further support the notion
that Fzd2 has important roles in the process of EMT. Thus, Fzd2 was
demonstrated to play an important role in inducing EMT in HCC, and
it could be a novel predictor of early recurrence after HCC
resection.
In recent years, constitutive activation of the
Stat3 signaling pathway has been detected in a variety of human
tumors, and Stat3 activation has frequently been reported in
association with tumor invasiveness and metastasis (21–23).
In our study, pStat3 expression was detected in ≤40% of the
surgically resected HCC specimens by immunohistochemical analysis,
and its expression was strongly correlated with the status of Fzd2
and EMT. These findings suggest that Stat3 phosphorylation is
involved in the Fzd2-dependent EMT and cell migration. With regard
to cell proliferation, migration, and invasiveness in HCC, Fzd2
knockdown or treatment with an anti-Fzd2 antibody has been reported
to result in reduced cell migration and invasion and inhibition of
tumor growth and metastasis in a mouse xenograft model (13). In addition, shRNA-Fzd2 has been
shown to suppress cell proliferation in HLF and PLC/PRF/5 cells
(24). However, our results showed
that the migration and invasiveness of HLF cells were significantly
inhibited by treatment with Fzd2 siRNA, whereas proliferation was
only marginally affected. Taken together, these results indicate
that the signaling pathway regulating cell proliferation might also
be influenced by mechanisms independent of Wnt5/Fzd2.
EMT is known to generate cells with properties of
stem cells (12). We have
previously reported that the IL-6 pathway is associated with HCC
EMT (9), and recent reports have
demonstrated that IL-6 promotes Stat3 activation and expression in
stem cells in HCC (25,26). Interestingly, Janus kinase, which
also activates Stat3, is required for IL-6- but not for
Wnt5/Fzd2-mediated activation of Stat3 (13). In general, Stat3 is known to
function downstream of both Wnt5/Fzd2 and the IL-6/Jak2 signaling
pathway. Although additional studies are needed to clarify these
findings, we hypothesize that Wnt5/Fzd2 might be more closely
associated with migration and invasion, whereas IL-6/Jak2 might
regulate stemness and proliferation. In any case, at least two
pathways are involved in the process of EMT, and combined treatment
with an anti-Fzd2 antibody and IL-6R antibody might more
effectively suppress Stat3 activation than the blockade of either
pathway alone in the treatment of HCC (27).
In conclusion, Fzd2 regulates EMT and cell migration
and invasion in HCC, and it might be a novel predictor of
recurrence in HCC. Furthermore, Stat3 might be controlled by both
Wnt5/Fzd2 and the IL-6/Jak2 signaling pathway and play an important
role in EMT.
Abbreviations:
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
HCC
|
hepatocellular carcinoma
|
|
Fzd2
|
Frizzled 2
|
|
mRNA
|
messenger RNA
|
|
siRNA
|
short interfering RNA
|
|
HBV
|
hepatitis B virus
|
|
HCV
|
hepatitis C virus
|
|
well
|
well-differentiated adenocarcinoma
|
|
mod
|
moderately differentiated
adenocarcinoma
|
|
poor
|
poorly differentiated
adenocarcinoma
|
|
AFP
|
α-fetoprotein
|
References
|
1
|
Poon D, Anderson BO, Chen LT, Tanaka K,
Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al Asian
Oncology Summit: Management of hepatocellular carcinoma in Asia:
Consensus statement from the Asian Oncology Summit 2009. Lancet
Oncol. 10:1111–1118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGlynn KA, Petrick JL and London WT:
Global epidemiology of hepatocellular carcinoma: An emphasis on
demographic and regional variability. Clin Liver Dis. 19:223–238.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thorgeirsson SS and Grisham JW: Molecular
pathogenesis of human hepatocellular carcinoma. Nat Genet.
31:339–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson EW, Newgreen DF and Tarin D:
Carcinoma invasion and metastasis: A role for
epithelial-mesenchymal transition? Cancer Res. 65:5991–5995;
discussion 5995. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Zijl F, Krupitza G and Mikulits W:
Initial steps of metastasis: Cell invasion and endothelial
transmigration. Mutat Res. 728:23–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamada S, Okumura N, Wei L, Fuchs BC,
Fujii T, Sugimoto H, Nomoto S, Takeda S, Tanabe KK and Kodera Y:
Epithelial to mesenchymal transition is associated with shorter
disease-free survival in hepatocellular carcinoma. Ann Surg Oncol.
21:3882–3890. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Micalizzi DS, Farabaugh SM and Ford HL:
Epithelial-mesenchymal transition in cancer: Parallels between
normal development and tumor progression. J Mammary Gland Biol
Neoplasia. 15:117–134. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gujral TS, Chan M, Peshkin L, Sorger PK,
Kirschner MW and MacBeath G: A noncanonical Frizzled2 pathway
regulates epithelial-mesenchymal transition and metastasis. Cell.
159:844–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Willert K, Brown JD, Danenberg E, Duncan
AW, Weissman IL, Reya T, Yates JR III and Nusse R: Wnt proteins are
lipid-modified and can act as stem cell growth factors. Nature.
423:448–452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deka J, Wiedemann N, Anderle P,
Murphy-Seiler F, Bultinck J, Eyckerman S, Stehle JC, André S,
Vilain N, Zilian O, et al: Bcl9/Bcl9l are critical for Wnt-mediated
regulation of stem cell traits in colon epithelium and
adenocarcinomas. Cancer Res. 70:6619–6628. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta S, Iljin K, Sara H, Mpindi JP,
Mirtti T, Vainio P, Rantala J, Alanen K, Nees M and Kallioniemi O:
FZD4 as a mediator of ERG oncogene-induced WNT signaling and
epithelial-to-mesenchymal transition in human prostate cancer
cells. Cancer Res. 70:6735–6745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu ZQ, Brabletz T, Fearon E, Willis AL, Hu
CY, Li XY and Weiss SJ: Canonical Wnt suppressor, Axin2, promotes
colon carcinoma oncogenic activity. Proc Natl Acad Sci USA.
109:11312–11317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok
WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL, et al: Twist
overexpression correlates with hepatocellular carcinoma metastasis
through induction of epithelial-mesenchymal transition. Clin Cancer
Res. 12:5369–5376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giannelli G, Bergamini C, Fransvea E,
Sgarra C and Antonaci S: Laminin-5 with transforming growth
factor-β1 induces epithelial to mesenchymal transition in
hepatocellular carcinoma. Gastroenterology. 129:1375–1383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee TK, Man K, Poon RT, Lo CM, Yuen AP, Ng
IO, Ng KT, Leonard W and Fan ST: Signal transducers and activators
of transcription 5b activation enhances hepatocellular carcinoma
aggressiveness through induction of epithelial-mesenchymal
transition. Cancer Res. 66:9948–9956. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen CL, Hsieh FC, Lieblein JC, Brown J,
Chan C, Wallace JA, Cheng G, Hall BM and Lin J: Stat3 activation in
human endometrial and cervical cancers. Br J Cancer. 96:591–599.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kusaba T, Nakayama T, Yamazumi K, Yakata
Y, Yoshizaki A, Nagayasu T and Sekine I: Expression of p-STAT3 in
human colorectal adenocarcinoma and adenoma; correlation with
clinicopathological factors. J Clin Pathol. 58:833–838. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suiqing C, Min Z and Lirong C:
Overexpression of phosphorylated-STAT3 correlated with the invasion
and metastasis of cutaneous squamous cell carcinoma. J Dermatol.
32:354–360. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: Suppression of hepatocellular
carcinoma cell proliferation by short hairpin RNA of frizzled 2
with Sonazoid-enhanced irradiation. Int J Oncol. 48:123–129.
2016.
|
|
25
|
Won C, Kim BH, Yi EH, Choi KJ, Kim EK,
Jeong JM, Lee JH, Jang JJ, Yoon JH, Jeong WI, et al: Signal
transducer and activator of transcription 3-mediated CD133
up-regulation contributes to promotion of hepatocellular carcinoma.
Hepatology. 62:1160–1173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wan S, Zhao E, Kryczek I, Vatan L,
Sadovskaya A, Ludema G, Simeone DM, Zou W and Welling TH:
Tumor-associated macrophages produce interleukin 6 and signal via
STAT3 to promote expansion of human hepatocellular carcinoma stem
cells. Gastroenterology. 147:1393–1404. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghoshal S, Fuchs BC and Tanabe KK: STAT3
is a key transcriptional regulator of cancer stem cell marker CD133
in HCC. Hepatobiliary Surg Nutr. 5:201–203. 2016. View Article : Google Scholar : PubMed/NCBI
|