Introduction
Although the incidence of gastric cancer (GC) has
been declining worldwide over the past few decades, the reported
frequency of GC-related mortality in 2008 was still the fourth
highest in males and fifth highest in females (1). In Japan, GC is one of the most common
causes of death, despite advances in diagnosis and treatment.
Particularly, unresectable or recurrent GC is associated with an
extremely poor prognosis even when treated with novel therapeutic
agents, including taxanes (paclitaxel and docetaxel), irinotecan,
S-1, oxaliplatin and capecitabine, which are known to be
efficacious in gastric cancer (2–7). A
multi-center randomized controlled trial (SPIRITS trial) performed
in Japan reported that the median overall survival and
progression-free survival in patients with advanced GC treated with
S-1 plus cisplatin were significantly longer in those treated with
S-1 alone (8). Therefore, the
Gastric Cancer Treatment Guidelines 2010 issued by the Japanese
Gastric Cancer Association recommended the S-1 plus cisplatin
combination regimen as the standard first-line treatment for
unresectable and recurrent GC (9).
However, even with this treatment, the median overall survival was
13 months, and the progression-free survival time was 6 months,
suggesting the need for novel therapeutic modalities. Recently,
novel molecular targeted therapies, such as trastuzumab and
ramucirumab, have shown additional therapeutic effects (10,11);
however, their survival benefits are limited.
After identification of tumor associated antigens,
such as the MAGE family in 1991, cancer immunotherapy has become a
promising approach to fight cancer with minimum toxicity (12,13).
Recently, several clinical trials using peptide vaccine therapy
targeting cancer-specific antigen peptides have been performed in
the world and suggested improvement in patient survival (14–16).
We identified novel cancer-testis antigens that
showed specific overexpression in GC tissues using the genome-wide
cDNA microarray method. Forkhead box protein M1 (FoxM1) is a member
of the Forkhead family of transcription factors (17,18).
FoxM1 plays important roles in the cell cycle by regulating both
the transition from the G1 to S phase and progression to mitosis
(18–20). Recently, FoxM1 has been linked to
tumorigenesis and progression of several types of malignancies.
Overexpression of FoxM1 has been observed in various cancers of the
liver, breast, prostate, brain, cervix, colon and lung (21–27).
We also showed that FoxM1 was overexpressed in GC and its
overexpression was a significant prognostic factor and had an
association with chemo-resistance in GC (28). Upregulated lung cancer 10 (URLC10),
KIF20 and DEPDC1, which have been used for cancer vaccine therapy
as oncogenic peptides (29–31),
were also confirmed to show over-expression in GC. A vaccination
with a peptide derived from vascular endothelial growth factor
receptor-1 (VEGFR-1) has also been reported to show cytotoxicity
for tumors as an anti-angiogenic cancer vaccine (32).
In the present study, multiple therapeutic peptide
vaccines consisting of 4 cancer-testis antigens (FoxM1, URLC10,
KIF20 and DEPDC1) and one anti-angiogenic peptide, i.e., VEGFR1,
were administered to unresectable and recurrent GC patients who
showed resistance to the standard chemotherapy and their efficacy
and safety were assessed.
Materials and methods
Patient eligibility
Patients diagnosed with gastric adenocarcinoma that
was considered unresectable or who had recurrent disease and failed
to respond to the standard therapy were enrolled in this trial at
the Department of Gastroenterological Surgery, Osaka University
Hospital or the Department of Surgery, Osaka Medical Center for
Cancer and Cardiovascular Diseases. The following were the other
main inclusion criteria: i) Eastern Cooperative Oncology (ECOG)
performance status of 0 or 1; ii) age between 20 years and 84
years; iii) adequate bone-marrow, cardiac, pulmonary, hepatic and
renal functions including leukocyte count
2,000–10,000/mm3, platelet count
>70,000/mm3, hemoglobin level >8.0 g/dl, aspartate
aminotransferase and alanine aminotransferase <100 U/l, total
bilirubin <1.5, and creatinine <1.5 times the institutional
normal upper limits; iv) life expectancy >3 months; v) no
therapy in 4 weeks prior to the initiation of this study; and vi)
signed informed consent. The main exclusion criteria were: i) the
presence of another serious disease such as uncontrolled diabetes,
hepatic disorder, cardiac disease, or hemorrhage/bleeding; ii)
pregnant or breast-feeding woman; iii) patients who planned to
become pregnant during the study period; iv) symptomatic infectious
disease; v) need for concurrent treatment with steroids or
immunosuppressive agents; vi) uncontrolled other malignant disease;
vii) unhealed wound; viii) intestinal obstruction or interstitial
pneumonia; and ix) decision of unsuitableness by the principal
investigator or physician in charge.
Study design
The present study was a phase II open-label,
non-randomized cancer vaccine trial for unresectable or recurrent
GC in patients who had failed to respond to the standard therapy in
an exploratory setting. All enrolled patients received the
vaccination without study personnel knowing the patient's HLA-A
status and the HLA-A genotypes were key-opened at the analysis
point. The HLA genotype information was held by an evaluation
committee, and both patients and investigators were blinded to the
results until completion of the study. The HLA-A*2402 restricted
epitope peptide cocktail containing peptides for FoxM1, URLC10,
KIF20, DEPDC1 and VFGFR1 each at a dose of 1 mg were prepared in
incomplete Freund's adjuvant (Montanide ISA-51VG; Seppic, Paris,
France) and injected subcutaneously weekly in the inguinal region
of the patients. One treatment cycle consisted of four injections
on days 1, 8, 15 and 22. The primary endpoints were the safety of
the peptide vaccination and overall survival. The secondary
endpoints were clinical responses and immunological responses.
Toxicities were assessed by the Common Terminology Criteria for
Adverse Events version 4.0 (CTCAE ver4.0). To assess the clinical
responses, computed tomography imaging was performed within a month
before starting the first cycle and within 2 weeks after every two
cycles. Every measurable region such as liver, lung or lymph node
metastasis was evaluated by the Response Evaluation Criteria in
Solid Tumors (RECIST) (33). The
overall survival, which was measured in days from the first
vaccination to death, was analyzed by the Kaplan-Meier method.
Immunological monitoring was performed with an enzyme-linked
immunospot (ELISPOT) assay using in vitro culturing of
lymphocytes derived from peripheral blood at pre- and
post-vaccination periods as described below.
This trial was approved by the Ethics Committees of
both the Osaka University and Osaka Medical Center for Cancer and
Cardiovascular Diseases, registered at UMIN (http://www.umin.ac.jp; Trial registration ID:
UMIN000004389), and carried out in accordance with the Helsinki
declaration on experimentation on human subjects.
Peptides
HLA-A*2402-restricted CMV peptide (QYDP-VAALF),
FOXM1-262 (IYTWIEDHF), URLC10-177 (RYCNLEGPPI) (34), DEPDC1-294 (EYYELFVNI) (35), KIF20A-66 (KVYLRVRPLL) (36) and GMP-graded VEGFR1-1084 peptide
(SYGVLLWEIF) (32) were
synthesized by the American Peptide Co. (Sunnyvale, CA, USA) per a
standard solid-phase synthesis method and purified by
reversed-phase high-performance liquid chromatography (HPLC). The
purity (>90%) and identity of the peptides were determined by
analytical HPLC and mass spectrometry, respectively.
Treatment protocol
A mixture of 1 mg each of FOXM1-262, URLC10-177,
DEPDC1-294, KIF20A-66 and VEGFR1-1084 were emulsified together with
1 ml of incomplete Freund's adjuvant and injected subcutaneously at
inguinal regions from side to side every week 4 times in one cycle.
Toxicities, clinical responses and peptide-specific immunological
responses within 2 cycles were evaluated.
Isolation and stock of peripheral blood
mononuclear cells
Peripheral blood cells were obtained from patients
at the end of every cycle of the treatment. Peripheral blood
mononuclear cells (PBMCs) were isolated immediately with a
Ficoll-Paque Plus density gradient solution (GE Healthcare, Little
Chalfont, UK), suspended in Cell Banker (Juji Field, Inc., Tokyo,
japan) and frozen and stored in liquid nitrogen.
Enzyme-linked immunospot (ELISPOT)
assay
To assess the specific CTL response, an ELISPOT
assay was performed following in vitro expansion. Frozen
PBMCs derived from the same patient were thawed at the same time,
and their viability was confirmed to be >90%. PBMCs
(5×105/ml) were cultured with 10 µg/ml of the
respective peptide and 100 IU/ml of IL-2 (Novartis, Emeryville, CA,
USA) at 37°C. The peptide was added to the culture at day 0 and day
7 (final concentration 10 µg/ml) and cells were harvested
after two weeks. Following CD4+ cell depletion with a
Dynal CD4-positive isolation kit (Invitrogen, Carlsbad, CA, USA)
the cells were used as responder cells in the ELISPOT assay. The
IFN-γ ELISPOT assay was performed using a Human IFN-γ ELISPOT PLUS
kit (Mabtech, Inc., Cincinnati, OH, USA) per the instructions
supplied by the manufacturer. Briefly, HLA-A*2402-positive
B-lymphoblast TISI cells (IHWG Cell and Gene Bank, Seattle, WA,
USA) were incubated with 20 µg/ml of FOXM1-262, URLC10-177,
DEPDC1-294, KIF20A-66 or VEGFR1-1084 peptides overnight, and then
the residual peptide in the media was washed out to prepare
peptide-pulsed TISI cells as the stimulator cells. Prepared
CD4-cells were cultured with peptide-pulsed TISI cells
(2×104 cells/well) at 1/1, 1/2, 1/4 and 1/8 mixture
ratios of responder cells and stimulator cells (R/S ratio) on
96-well plates (Millipore, Bedford, MA, USA) at 37°C overnight.
Non-peptide-pulsed TISI cells were used as negative control
stimulator cells. All ELISPOT assays were performed in triplicate.
The plates were analyzed with an automated ELISPOT reader,
ImmunoSPOT S4 (Cellular Technology, Ltd., Cleveland, OH, USA) and
ImmunoSpot Professional Software version 5.0 (Cellular Technology).
The number of peptide-specific spots was calculated by subtracting
the spot number in the control well from the spot numbers in wells
with peptide-pulsed TISI cells. The CTL response was considered
positive when the average of the peptide-specific spot numbers of
three wells was >15/well and a significant difference
(P<0.05) was demonstrated between the average spot numbers. The
sensitivity of our ELISPOT assay was periodically estimated as
approximately average by the ELISPOT panel of the Cancer
Immunotherapy Consortium (CIC).
Statistical analysis
Statistical analysis was performed using Student's
t-test and Fisher's exact test. Overall survival (OS) curves were
estimated using the Kaplan-Meier methodology and compared by the
log-rank test. P<0.05 were considered significant. All
statistical analyses were performed with JMP 8.0.2 software (SAS
Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Thirty-five patients were enrolled in this trial
between November 2010 and March 2012. The study database was locked
on March 31, 2013 and the genotype of HLA-A was key-opened.
Table I shows the patient
characteristics at study entry. They included 21 males and 14
females. Six patients had unresectable gastric cancer and the
remaining 29 had recurrent disease after surgery. Twenty-four
(68.6%) had HLA-A*2402 [24(+)] and the remaining 11 (31.4%) were
negative for HLA-A*2402 [24(−)]. The patients received at least one
vaccination injection (average 13.3 times, from 2 to 48). The
backgrounds of the patients were not significantly different
between 24(+) and 24(−) including age, gender, performance status
and prior therapy, as shown in Table
I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Total
(n=35) | With
A2402
(n=24) | Without
A2402
(n=11) |
|---|
| Age (years) | 64 (35–81) | 64 (34–81) | 65 (37–76) |
| Gender
(male/female) | 21/14 | 14/10 | 7/4 |
| Performance status
(0/1) | 0/35 | 0/24 | 0/11 |
| Pre-treatment | | | |
| Surgery (+/−) | 29/6 | 20/4 | 9/2 |
| S-1 (+/−) | 30/5 | 22/2 | 8/3 |
| Cisplatin
(+/−) | 23/12 | 16/8 | 7/4 |
| CPT-11 (+/−) | 28/7 | 19/5 | 9/2 |
| Taxanes (+/−) | 27/8 | 20/4 | 7/4 |
| Others (+/−) | 10/25 | 8/16 | 2/9 |
Toxicity
Table II lists the
adverse effects recorded during the vaccination therapy. The
therapy was well-tolerated without any severe adverse events
associated with the therapy except for 4 patients in the 24(+)
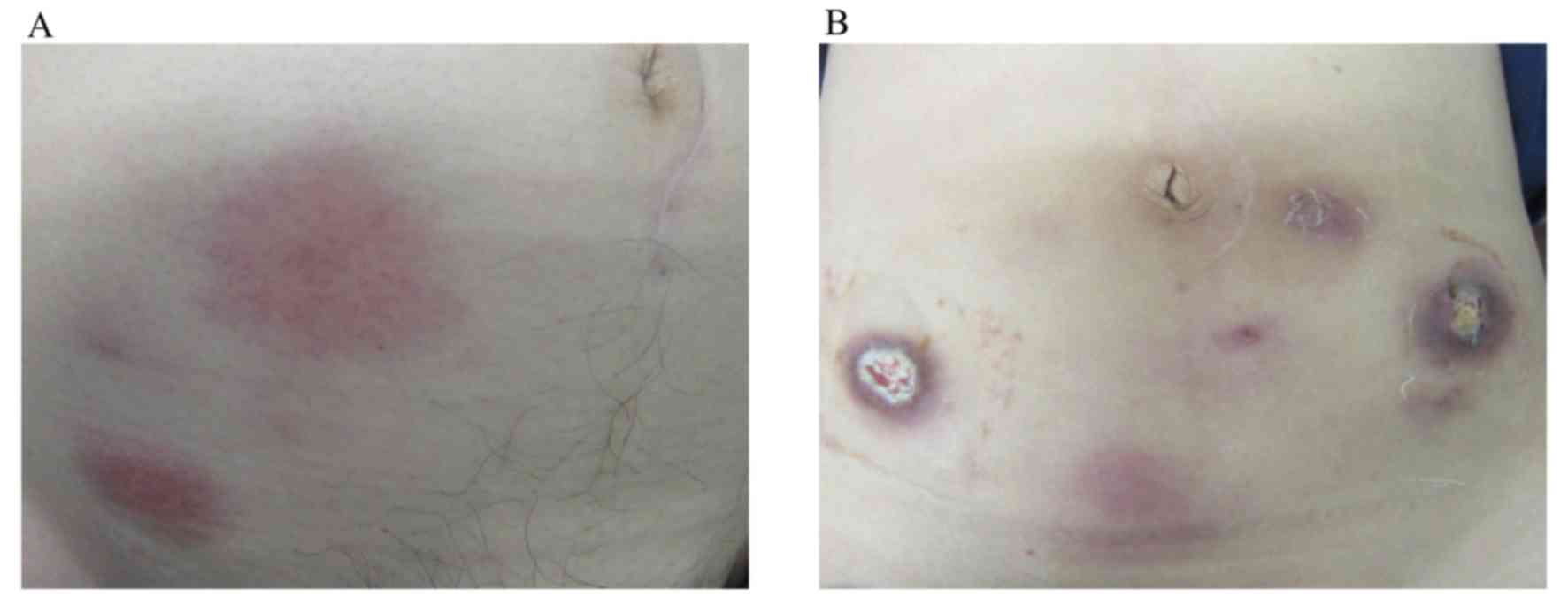
group who showed grade 3 injection-site reactions. Representative
injection-site reactions are shown in Fig. 1. The grade 2 skin reaction is shown
in Fig. 1A and the grade 3 skin
ulceration is shown in Fig. 1B.
One patient suffered grade 4 anemia due to bleeding of a
progressive gastric tumor. Grade 3 AST/ALT elevation was observed
in 2 patients, and grade 3 creatinine elevation was found in one
patient, which could have been caused by disease progression.
 | Table IIToxicity profile. |
Table II
Toxicity profile.
| With
A2402
(n=24)
Grade
| Without
A2402
(n=11)
Grade
|
|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
|---|
| Injection-site
reaction | 12 | 0 | 4 | / | 4 | 0 | 0 | / |
| Leukopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
|
Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anorexia | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Increase in
AST/ALT | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| Increase in
creatine | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Fever | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| Flu-like
symptoms | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Clinical responses
Of 35 patients, 22 cases continued more than 2
cycles (8 weeks) and had the computed tomography (CT) scan for
evaluation of disease status after induction of vaccination
therapy. The clinical responses were classified as partial response
(PR) in 0 patients (0%), stable disease (SD) in 10 patients (45%)
and progressive disease (PD) in 12 patients (55%). The remaining 13
patients did not have a post-therapeutic CT scan because the study
was stopped within 2 cycles due to disease progression (Table III).
 | Table IIIClinical and immunological
outcomes. |
Table III
Clinical and immunological
outcomes.
| Factors | Responses | No. of patients
(%) |
|---|
| Objective
response | SD/PD/NE | 10/12/13 |
| Local skin
reaction | +/− | 18/17 |
| CTL response
(n=20) | | |
| URLC10 | +/− | 18 (90%)/2 |
| DEPDC1 | +/− | 12 (60%)/8 |
| KIF20A | +/− | 12 (60%)/8 |
| FOXM1 | +/− | 20 (100%)/0 |
| VEGFR1 | +/− | 11 (55%)/9 |
Immunological monitoring
Twenty patients with A24(+) received at least one
course of the vaccination and were subjected to immunological
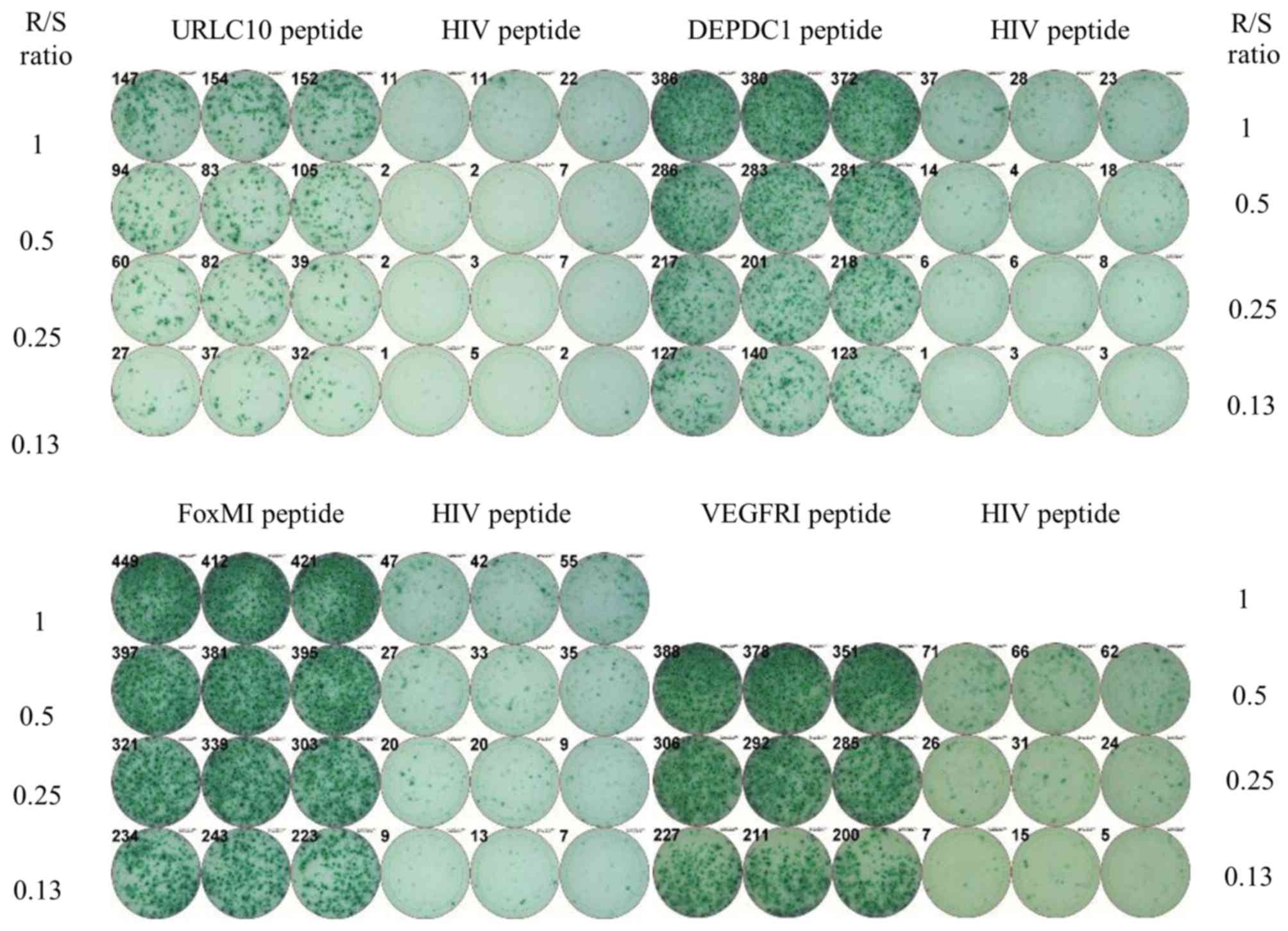
analysis with peripheral blood. A representative ELISPOT assay is
shown in Fig. 2. Patient 24 showed
substantial T cell responses specific to the URLC10, DEPDC1, FoxM1
and VEGFR1 peptides in comparison to the irrelevant peptide. The
positive CTL responses specific for URLC10, DEPDC1, KIF20A, FOXM1
and VEGFR1 were observed in 90, 60, 60, 100 and 55% of the
patients, respectively. All patients showed CTL response specific
to multiple antigen peptides (more than one).
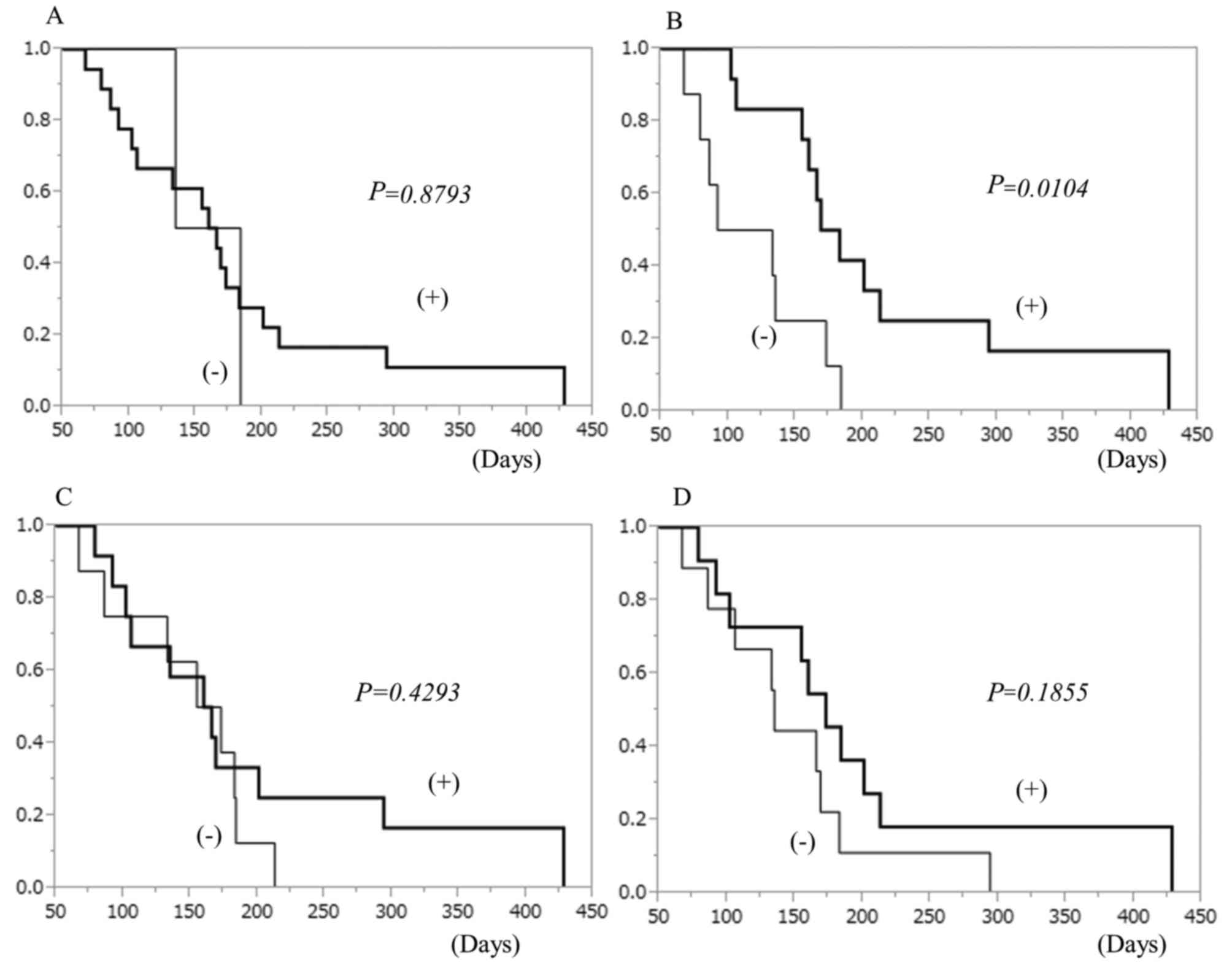
Survival analysis
Patients who showed CTL response had a tendency
toward better survival than those who showed no response,
especially to the DEPDC1 peptide (Fig.
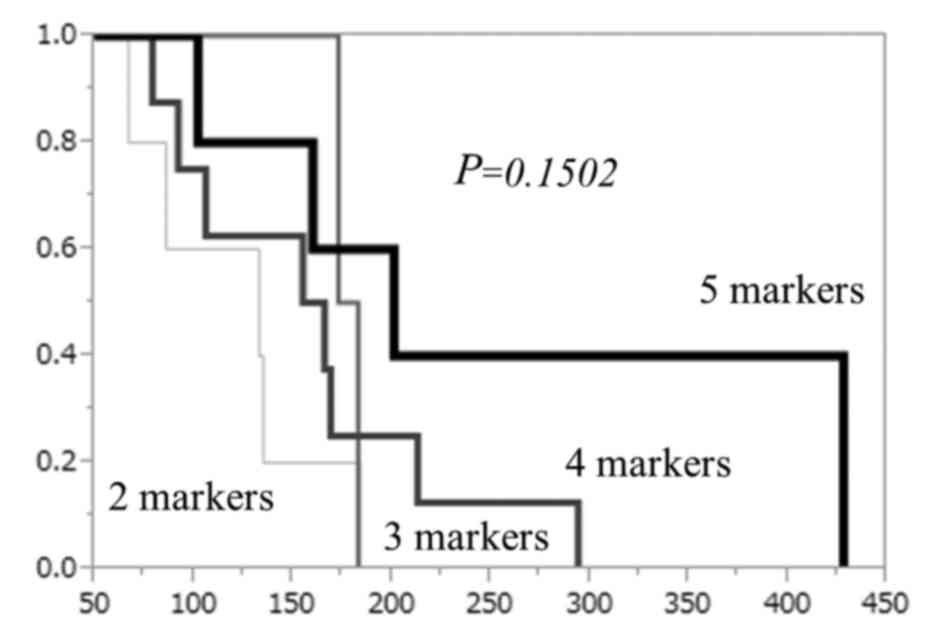
3). The overall survival tended to be better when the number of
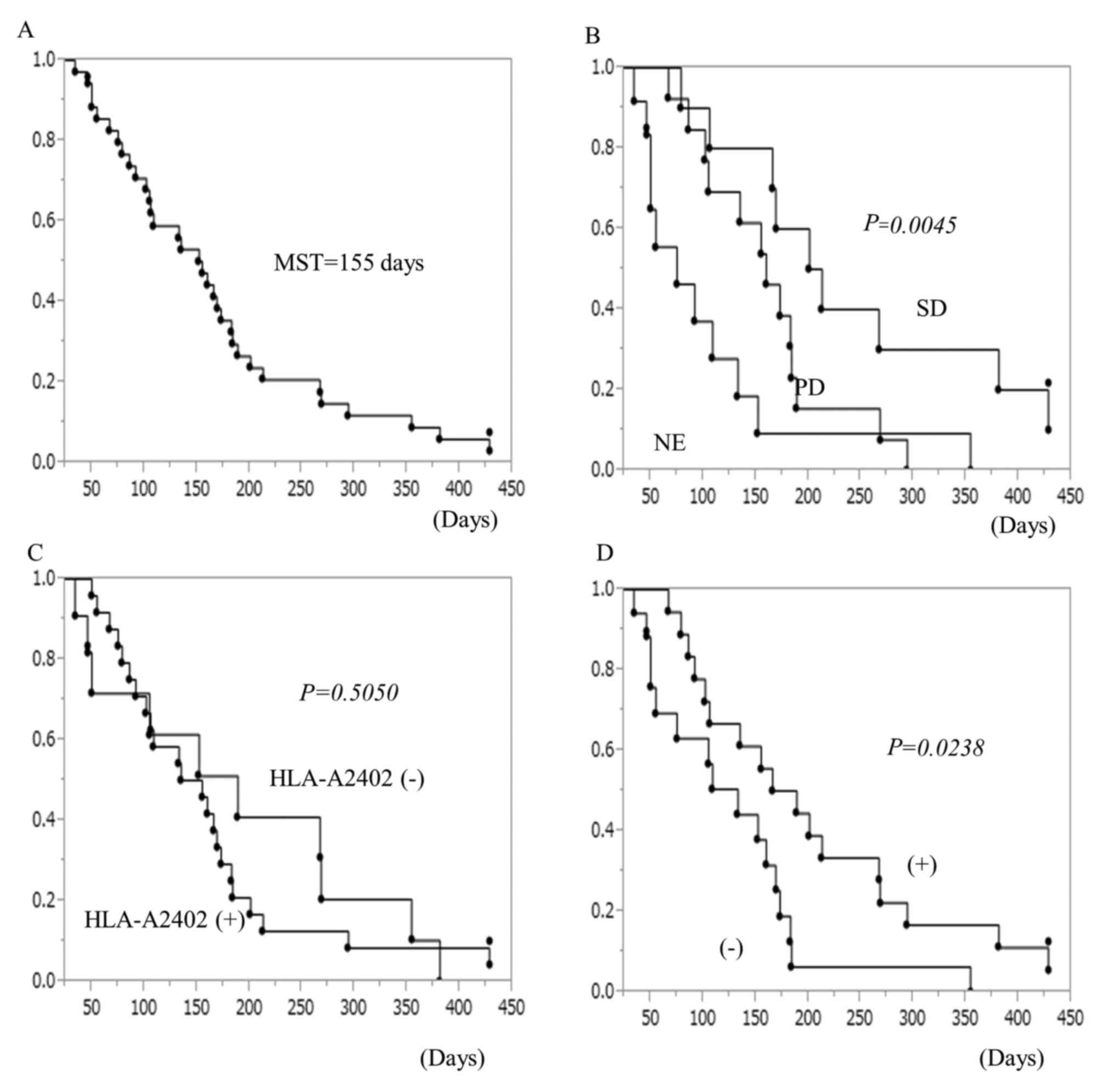
the peptides that induced CTL responses was higher (Fig. 4). The overall survival curve of all
patients is shown in Fig. 5A. The
median survival time was 155 days. The association between clinical
effects classified by RECIST criteria and survival duration are
shown in Fig. 5B. Patients whose
tumors showed stable disease after 2 cycles of vaccine therapy had
significantly better prognosis than other patients. The survival
curves depending on HLA-A type are shown in Fig. 5C. There was no significant
difference between patients with HLA-A2402 and those with other
types. Local skin reactions were observed in 18 patients (Table III). Patients who suffered local
skin reactions due to vaccine injections showed significantly
better prognosis than those without skin reactions (Fig. 5D).
Discussion
In the present study, we developed a cancer vaccine
therapy with multiple peptides specific for GC and we applied it in
advanced GC patients who had failed to respond to the standard
therapy as a monotherapy. Thirty-five patients were enrolled in
this trial; 24 (69%) patients had HLA-A2402, and the remaining 11
did not have it, which was information that was key-opened at the
end of the present study. The differences between the cases with
HLA-A2402 and those without were not significant in this study,
which might indicate that cancer vaccine treatment with multiple
peptide antigens did not provide clinical benefit to advanced GC
patients. However, in the A24(+) group, the patients that had a CTL
response to a specific peptide, especially DEPDC1, had a better
prognosis. Furthermore, patients that had a local skin reaction had
a significantly better prognosis than those without local skin
reactions. These results might indicate an association between the
vaccination-induced immune response and patient prognosis.
According to the present study, the cancer
vaccination using a combination of multiple peptides (DEPDC1,
FoxM1, KIF20, URLC10 and VEGFR1) were well tolerated by advanced GC
patients who had failed to respond to standard therapy.
Furthermore, specific cytotoxic T cells for these five peptide
antigens were frequently observed in the peripheral blood of
patients after vaccinations, and patients who showed the CTL
induction tended to have a better prognosis than those with no CTL
induction. First, we chose four cancer antigens suitable for GC
because of the following preferable characteristics: frequent and
homogeneous expression in tumor tissues, cancer-specific expression
and high immunogenicity. FoxM1 is a well-studied molecule
associated with cancer development, and we have reported that its
overexpression makes it worth consideration as a prognostic marker
in GC (28). DEPDC1, KIF20 and
URLC10 were also reported as cancer-specific antigens and have been
applied in peptide vaccination therapy (29–31,34–36).
An anti-angiogenic vaccine targeting VEGFR-1 was also widely
studied in patients with advanced solid tumors (29,32).
Previously, we performed a phase II clinical trial with the
combination therapy of chemotherapy and peptide vaccine therapy
using VEGFR-1 and VEGFR-2 (37).
In this trial, the combination therapy was well tolerated and high
frequent CTL induction specific for anti-angiogenic peptides was
observed despite the combined chemotherapy.
Kono et al (38) performed a clinical study of cancer
vaccine treatment with HLA-A24-restricted multi-epitope peptides
(TTK, LY6K and IMP3) as monotherapy for 60 advanced esophageal
cancer patients. They showed that, although the overall survival
between A24(+) and A24(−) groups was not significantly different,
the progression-free survival in the A24(+) group was significantly
better than that in the A24(−) group. In the A24(+) group, the
specific CTL response to multiple peptides could improve overall
survival of esophageal cancer patients. They concluded that cancer
vaccine treatment with multiple peptides as a monotherapy can be a
promising therapy for patients with advanced esophageal cancer who
had failed to respond to standard therapy. Although we used a
HLA-A24-restricted peptide vaccine, the survival benefit in A24(+)
patients was not observed in the present study. We speculated that
this was due to the number of enrolled patients, 35 was small and
only 22 (63%) of the enrolled patients continued until at least two
cycles (8 times) of vaccines were complete. The remaining 13
patients discontinued vaccines due to disease progression because
we enrolled patients with far-advanced diseases, who showed
resistance to multiple regimens of chemotherapy. The US FDA
published guidance for therapeutic cancer vaccines (39) that indicated that the appearance of
a clinical effect in cancer vaccine therapy may be delayed compared
to chemotherapy due to the mechanism of immune responses, and
longer observation periods may be needed to evaluate the clinical
effects. It is hard to expect clinical benefits for patients after
multiple chemotherapy regimens due to very poor immune system
status. They recommended that cancer vaccine treatment was more
suitable for cancer patients as an adjuvant therapy after curative
surgery.
In conclusion, peptide vaccine therapy using a
mixture of five peptides was found to be safe and could induce
specific T cell responses in patients with advanced GC. The
survival benefit of peptide vaccine monotherapy may not have been
shown for patients with far advanced GC in this preliminary study,
and further studies are needed to confirm these results.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ajani JA, Fairweather J, Dumas P, Patt YZ,
Pazdur R and Mansfield PF: Phase II study of Taxol in patients with
advanced gastric carcinoma. Cancer J Sci Am. 4:269–274.
1998.PubMed/NCBI
|
|
3
|
Einzig AI, Neuberg D, Remick SC, Karp DD,
O'Dwyer PJ, Stewart JA and Benson AB III: Phase II trial of
docetaxel (Taxotere) in patients with adenocarcinoma of the upper
gastrointestinal tract previously untreated with cytotoxic
chemotherapy: The Eastern Cooperative Oncology Group (ECOG) results
of protocol E1293. Med Oncol. 13:87–93. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Futatsuki K, Wakui A, Nakao I, Sakata Y,
Kambe M, Shimada Y, Yoshino M, Taguchi T and Ogawa N: Late phase II
study of irinotecan hydrochloride (CPT-11) in advanced gastric
cancer. CPT-11 Gastrointestinal Cancer Study Group. Gan To Kagaku
Ryoho. 21:1033–1038. 1994.In Japanese. PubMed/NCBI
|
|
5
|
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi
K, Mitachi Y and Taguchi T: Late phase II study of novel oral
fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1
M otastat potassium) in advanced gastric cancer patients. Eur J
Cancer. 34:1715–1720. 1998. View Article : Google Scholar
|
|
6
|
Taguchi T, Tsukagoshi S, Furue H, Niitani
H and Noda K: Phase I clinical study of oxaliplatin. Gan To Kagaku
Ryoho. 25:1899–1907. 1998.In Japanese. PubMed/NCBI
|
|
7
|
Liu C, Sun Q, Hang X, Zhong B and Wang D:
Multicenter phase II study of capecitabine plus oxaliplatin as a
first-line therapy in Chinese patients with advanced gastric
cancer. Anticancer Drugs. 19:825–831. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomized controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al RAINBOW Study Group: Ramucirumab plus paclitaxel versus placebo
plus paclitaxel in patients with previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boon T, De Plaen E, Lurquin C, Van den
Eynde B, van der Bruggen P, Traversari C, Amar-Costesec A and Van
Pel A: Identification of tumour rejection antigens recognized by T
lymphocytes. Cancer Surv. 13:23–37. 1992.PubMed/NCBI
|
|
13
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: Moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al IMPACT Study Investigators: Sipuleucel-T immunotherapy
for castration-resistant prostate cancer. N Engl J Med.
363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwartzentruber DJ, Lawson DH, Richards
JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K,
Pockaj B, et al: gp100 peptide vaccine and interleukin-2 in
patients with advanced melanoma. N Engl J Med. 364:2119–2127. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weber JS, O'Day S, Urba W, Powderly J,
Nichol G, Yellin M, Snively J and Hersh E: Phase I/II study of
ipilimumab for patients with metastatic melanoma. J Clin Oncol.
26:5950–5956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teh MT, Wong ST, Neill GW, Ghali LR,
Philpott MP and Quinn AG: FOXM1 is a downstream target of Gli1 in
basal cell carcinomas. Cancer Res. 62:4773–4780. 2002.PubMed/NCBI
|
|
18
|
Wierstra I and Alves J: FOXM1, a typical
proliferation-associated transcription factor. Biol Chem.
388:1257–1274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang IC, Chen YJ, Hughes D, Petrovic V,
Major ML, Park HJ, Tan Y, Ackerson T and Costa RH: Forkhead box M1
regulates the transcriptional network of genes essential for
mitotic progression and genes encoding the SCF (Skp2-Cks1)
ubiquitin ligase. Mol Cell Biol. 25:10875–10894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laoukili J, Kooistra MR, Brás A, Kauw J,
Kerkhoven RM, Morrison A, Clevers H and Medema RH: FoxM1 is
required for execution of the mitotic programme and chromosome
stability. Nat Cell Biol. 7:126–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalinichenko VV, Major ML, Wang X,
Petrovic V, Kuechle J, Yoder HM, Dennewitz MB, Shin B, Datta A,
Raychaudhuri P, et al: Foxm1b transcription factor is essential for
development of hepatocellular carcinomas and is negatively
regulated by the p19ARF tumor suppressor. Genes Dev. 18:830–850.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wonsey DR and Follettie MT: Loss of the
forkhead transcription factor FoxM1 causes centrosome amplification
and mitotic catastrophe. Cancer Res. 65:5181–5189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kalin TV, Wang IC, Ackerson TJ, Major ML,
Detrisac CJ, Kalinichenko VV, Lyubimov A and Costa RH: Increased
levels of the FoxM1 transcription factor accelerate development and
progression of prostate carcinomas in both TRAMP and LADY
transgenic mice. Cancer Res. 66:1712–1720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu M, Dai B, Kang SH, Ban K, Huang FJ,
Lang FF, Aldape KD, Xie TX, Pelloski CE, Xie K, et al: FoxM1B is
overexpressed in human glioblastomas and critically regulates the
tumorigenicity of glioma cells. Cancer Res. 66:3593–3602. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan DW, Yu SY, Chiu PM, Yao KM, Liu VW,
Cheung AN and Ngan HY: Overexpression of FOXM1 transcription factor
is associated with cervical cancer progression and pathogenesis. J
Pathol. 215:245–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uddin S, Ahmed M, Hussain A, Abubaker J,
Al-Sanea N, Abduljabbar A, Ashari LH, Alhomoud S, Al-Dayel F, Jehan
Z, et al: Genome-wide expression analysis of Middle Eastern
colorectal cancer reveals FOXM1 as a novel target for cancer
therapy. Am J Pathol. 178:537–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim IM, Ackerson T, Ramakrishna S,
Tretiakova M, Wang IC, Kalin TV, Major ML, Gusarova GA, Yoder HM,
Costa RH, et al: The Forkhead Box m1 transcription factor
stimulates the proliferation of tumor cells during development of
lung cancer. Cancer Res. 66:2153–2161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okada K, Fujiwara Y, Takahashi T, Nakamura
Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Mori
M, et al: Overexpression of forkhead box M1 transcription factor
(FOXM1) is a potential prognostic marker and enhances
chemo-resistance for docetaxel in gastric cancer. Ann Surg Oncol.
20:1035–1043. 2013. View Article : Google Scholar
|
|
29
|
Higashihara Y, Kato J, Nagahara A, Izumi
K, Konishi M, Kodani T, Serizawa N, Osada T and Watanabe S: Phase I
clinical trial of peptide vaccination with URLC10 and VEGFR1
epitope peptides in patients with advanced gastric cancer. Int J
Oncol. 44:662–668. 2014.PubMed/NCBI
|
|
30
|
Murahashi M, Hijikata Y, Yamada K, Tanaka
Y, Kishimoto J, Inoue H, Marumoto T, Takahashi A, Okazaki T and
Takeda K: Phase I clinical trial of a five-peptide cancer vaccine
combined with cyclophosphamide in advanced solid tumors. Clin
Immunol. 166–167. 48–58. 2016.
|
|
31
|
Suzuki N, Hazama S, Ueno T, Matsui H,
Shindo Y, Iida M, Yoshimura K, Yoshino S, Takeda K and Oka M: A
phase I clinical trial of vaccination with KIF20A-derived peptide
in combination with gemcitabine for patients with advanced
pancreatic cancer. J Immunother. 37:36–42. 2014. View Article : Google Scholar
|
|
32
|
Yoshimura K, Minami T, Nozawa M and Uemura
H: Phase I clinical trial of human vascular endothelial growth
factor receptor 1 peptide vaccines for patients with metastatic
renal cell carcinoma. Br J Cancer. 108:1260–1266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. European Organization for
Research and Treatment of Cancer, National Cancer Institute of the
United States, National Cancer Institute of Canada. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iinuma H, Fukushima R, Inaba T, Tamura J,
Inoue T, Ogawa E, Horikawa M, Ikeda Y, Matsutani N, Takeda K, et
al: Phase I clinical study of multiple epitope peptide vaccine
combined with chemoradiation therapy in esophageal cancer patients.
J Transl Med. 12:842014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Obara W, Ohsawa R, Kanehira M, Takata R,
Tsunoda T, Yoshida K, Takeda K, Katagiri T, Nakamura Y and Fujioka
T: Cancer peptide vaccine therapy developed from oncoantigens
identified through genome-wide expression profile analysis for
bladder cancer. Jpn J Clin Oncol. 42:591–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Asahara S, Takeda K, Yamao K, Maguchi H
and Yamaue H: Phase I/II clinical trial using HLA-A24-restricted
peptide vaccine derived from KIF20A for patients with advanced
pancreatic cancer. J Transl Med. 11:2912013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Masuzawa T, Fujiwara Y, Okada K, Nakamura
A, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Osawa
R, et al: Phase I/II study of S-1 plus cisplatin combined with
peptide vaccines for human vascular endothelial growth factor
receptor 1 and 2 in patients with advanced gastric cancer. Int J
Oncol. 41:1297–1304. 2012.PubMed/NCBI
|
|
38
|
Kono K, Iinuma H, Akutsu Y, Tanaka H,
Hayashi N, Uchikado Y, Noguchi T, Fujii H, Okinaka K, Fukushima R,
et al: Multicenter, phase II clinical trial of cancer vaccination
for advanced esophageal cancer with three peptides derived from
novel cancer-testis antigens. J Transl Med. 10:1412012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Finn OJ, Khleif SN and Herberman RB: The
FDA guidance on therapeutic cancer vaccines: The need for revision
to include preventive cancer vaccines or for a new guidance
dedicated to them. Cancer Prev Res (Phila). 8:1011–1016. 2015.
View Article : Google Scholar
|