Introduction
Cervical cancer is one of the most common cancers in
women worldwide and is especially prevalent in developing
countries. For example, ~98,900 new cases of cervical cancer and
30,500 deaths were reported in China in 2015 (1). Historically, surgery and radiotherapy
(RT) have been the two major treatments for invasive cervical
cancer. Most women with metastatic cervical cancer or local
recurrence after radiotherapy are candidates for palliative
chemotherapy. Radiotherapy is a pre- or postoperative adjuvant or
primary treatment in most locally advanced cervical cancers.
However, the resistance of tumor cells to radiation is a major
therapeutic problem.
Most normal tissues metabolize the 6-carbon glucose
into the 3-carbon pyruvate and then exploit the resulting energy in
the form of ATP via 'oxidative phosphorylation' (OXPHOS) in the
mitochondria. In contrast, cancer cells primarily use aerobic
glycolysis to convert glucose into lactic acid at a high rate to
support growth, even in the presence of oxygen. This metabolic
alteration is referred to as the 'Warburg effect' and it is this
energy metabolism that fuels tumor cell growth and division,
including chronic and often uncontrolled cell proliferation, and
may facilitate apoptosis resistance (2,3). A
critical player in this frequent cancer metabolism phenotype is the
mitochondrial-bound hexokinase 2 (HK2), the enzyme that catalyzes
the first rate-limiting step of the glycolytic pathway, where
glucose is phosphorylated to glucose-6-phosphate (G-6-P) with ATP
consumption (4). The relatively
high expression of HK2 in cancer cells is responsible for the
accelerated glucose flux (5) and
can distinguish malignant cells from the normal cells, and
contributes to tumor initiation, maintenance and metastasis
(6–8). Upon a key oncogenic AKT pathway
activation, HK2 translocates to the mitochondrial outer membrane,
where it interacts with the voltage-dependent anion channel (VDAC)
to help mitochondrion escape strong product inhibition by G-6-P and
obtain priority access to newly synthesized ATP (9–11).
Moreover, HK2 eventually inhibits caspase-9-dependent apoptosis, by
blocking the release of cytochrome c and interacting with
the permeability transition pore including VDAC1 and Bax (11–14).
Thus, HK2 not only improves the malignant cells' energy supply by
making them more dependent on the glycolytic metabolic profile and
more adaptive to survive in an anoxic environment, it also
immortalizes and protects malignant cells against apoptosis through
direct interaction with mitochondria.
The responses of malignant tumors to irradiation
vary in their respective resistance mechanisms. Radioresistance can
be affected by a lack of oxygen (15), cell cycle status (16), DNA damage and repair (17), apoptosis (18), growth factors and oncogenes
(19), stem cells (20), and other factors. Among these,
hypoxia-related radioresistance is the most important. The hypoxic
microenvironment can potentially serve as a protective sheath
against tumor damage (15,21–23).
Glycolysis, which is the main metabolic profile for tumor cells
according to Warburg effect, is also closely related with
radioresistance (24–27). Reports have proven that inhibition
of the Warburg effect enhances the radiosensitivity of cancers
(28–30). Some squamous cell carcinomas, such
as cervical squamous cell carcinoma, have proven to be the most
modifiable type of tumor cells by the manipulation of hypoxia in
practice as they are more likely to maintain colony formation
potential during long-term hypoxia. Although a number of recent
trials investigating hypoxic modifications have displayed
considerable efficacy, the effect has been too limited to raise a
broader interest in this field. Thus, in seeking a breakthrough in
providing an appropriate application for cervical cancer treatment
with superior irradiation sensitivity, we aimed to exploit hypoxic
glycolytic metabolism as a property unique to tumor cells, with a
focus on hexokinase 2 (HK2), the essential regulatory point of the
glycolysis pathway.
Materials and methods
Ethics
All applicable international and institutional
guidelines for the care and use of animals were followed. Animal
experiments were performed in strict accordance with the Guide for
the Care and Use of Laboratory Animals and were approved by the
Department of Laboratory Animal Science at Shanghai Jiao Tong
University School of Medicine. This report does not contain any
studies with human participants performed by any of the
authors.
TCGA data
Level 3 normalized counts of HK2 (RNA-Seq;
Illumina) data and cervical cancer clinical data were
downloaded from TCGA and analyzed in the R statistical environment.
Survival rates were calculated using the Kaplan-Meier method and
the log-rank test was used to compare the survival curves. The
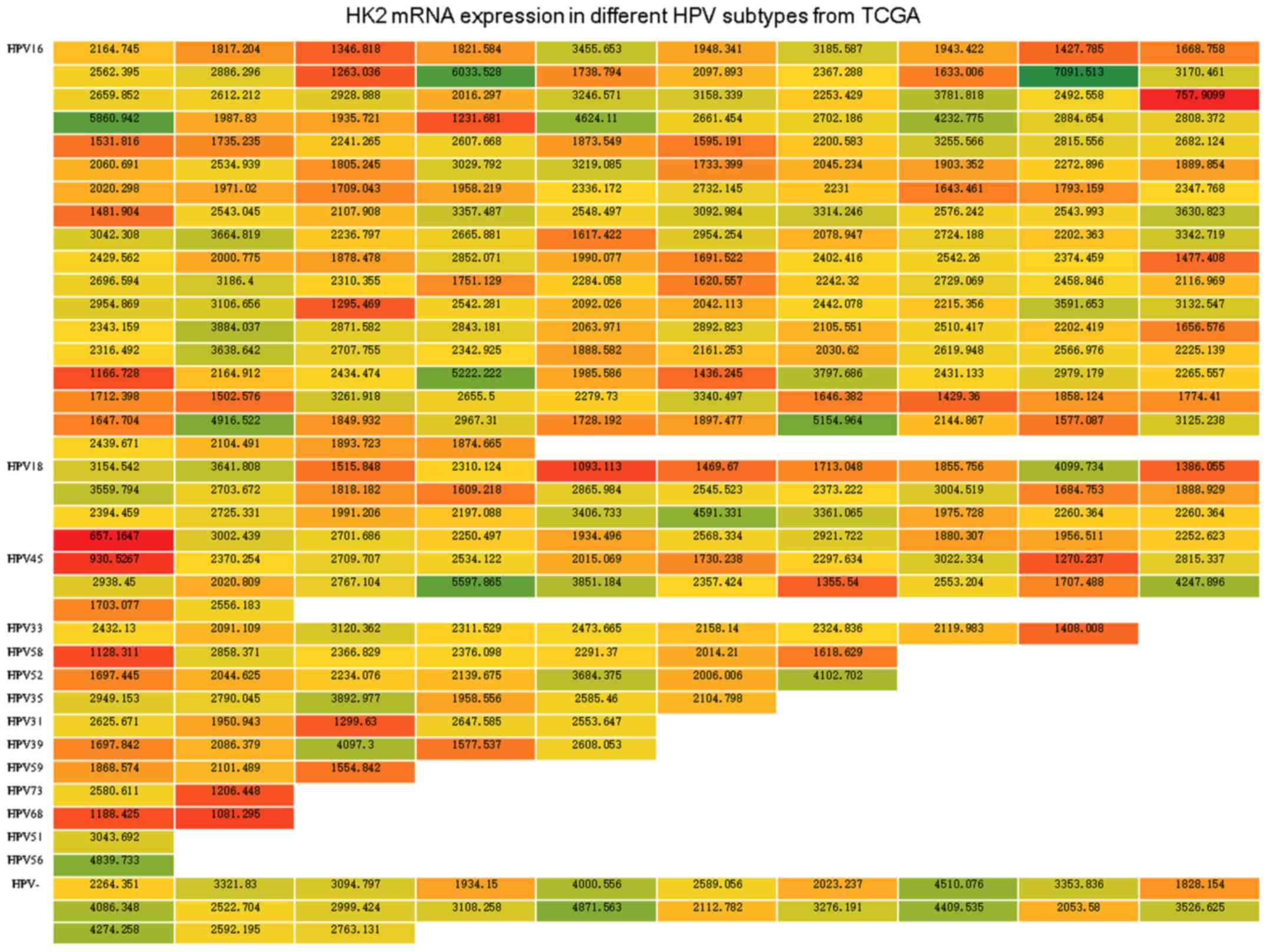
heatmap of the HK2-normalized counts from level 3 RNA-Seq TCGA data
was made by Excel: the green color is aligned to the largest
normalized count and the deepest red is aligned to the smallest
normalized count.
Cell lines and cell culture
The human cervical carcinoma lines HPV16(+) SiHa,
HPV18(+) HeLa, HPV18(+) SW756 and HPV(−) C33A were purchased from
ATCC and maintained in Dulbecco's modified Eagle's medium (DMEM)
F-12 1:1 medium (Gibco) with 10% fetal bovine serum (FBS; Gibco),
100 U/ml penicillin, sodium pyruvate and L-glutamine in a
humidified atmosphere of 5% CO2 at 37°C. The cell lines
were maintained in the laboratory of Dr S.F. Wu for eight months
and no further authentication was performed. Hypoxia environment
was made by treating cells with CoCl2 (Sigma, 150
μM/l) for 24 h (31–33).
Plasmid and lentivirus transfections
The overexpression plasmids, including
pCAG-myc-HPV16 E6, pCAG-myc-HPV16 E7, and pCAG-myc-blank, were
obtained from S.F. Wu, and the HK2 and HIF-1α shRNAs were purchased
from Shanghai GenePharma Co. Ltd. Plasmid transfection was
performed as previously described (34). Lentivirus-carrying small hairpin
RNAs (shRNA) targeting HK2 and HIF-1α were transfected into cells
at 60% confluence in 6-well plates for infection with polybrene (5
μg/ml; GenePharma). Medium was refreshed after 24 h of
transfection and the cells were incubated another 72 h before
analysis of mRNA or protein expression. The sequence used to
generate the shRNA targeting HK2 is 5′-GGGTGAAAGTAACGGACAATG-3′.
The sequence used to generate the shRNA targeting HIF-1α is
5′-GCCGAGGAAGAACTATGAACA-3′.
Western blot analysis and RT-PCR
For western blots, briefly, 60 μg of protein
was separated by SDS-PAGE and transferred onto polyvinylidene
fluoride (PVDF) membranes. Membranes were incubated with blocking
buffer for 2 h followed by incubation for 15 h with the following
primary antibodies: anti-PARP (diluted at 1:500; Cell Signaling
Technology, Beverley, MA, USA, #5625), anti-HPV16 E7 (diluted at
1:100; Bioss, Shanghai, China, #bs-10446R), anti-caspase 3 (diluted
at 1:500; Cell Signaling Technology, #9664), anti-Bcl2 (diluted at
1:500; Antibody Revolution, CA, USA, #ARH2043), anti-GAPDH
(Epitomics), anti-HK2 (diluted at 1:500; Aviva Systems Biology, CA,
USA, #ARP54303_P050), and anti-HIF-1α (diluted at 1:100; Boster,
Wuhan, China, #PB0245). An additional hour of incubation was
performed with the appropriate secondary antibody. For RT-PCR,
total RNA was extracted with TRIzol (Invitrogen). cDNA was
synthesized from 1 μg of total RNA according to the Takara
protocol. The genes of interest were amplified using appropriate
primers with 40 cycles. The primers sequences are listed in
Table I.
 | Table IPrimers used in this study. |
Table I
Primers used in this study.
| Primer | Sequence
(5′→3′) |
|---|
| HK2 F |
TGCTTGCCTACTTCTTCACG |
| HK2 R |
CATCTGGAGTGGACCTCACA |
| E6 F |
CGACCCAGAAAGTTACCACAGT |
| E6 R |
AATCCCGAAAAGCAAAGTCATA |
| E7 F |
GAGGAGGAAGATGAAATAGATGG |
| E7 R |
AACCGAAGCGTAGAGTCACAC |
| Glut1 F |
AATTTCATTGTGGGCATGTG |
| Glut1 R |
TCCTCGGGTGTCTTGTCACT |
| HIF-1α F |
GCAGCAACGACACAGAAACT |
| HIF-1α R |
GCAGGGTCAGCACTACTTCG |
| MGMT F |
TGGAGCTGTCTGGTTGTGAG |
| MGMTR |
GGGCTGCTAATTGCTGGTAA |
| GAPDH F |
AGAAGGCTGGGGCTCATTTG |
| GAPDH R |
AGGGGCCATCCACAGTCTTC |
| TFAM F |
CGTTTCTCCGAAGCATGTG |
| TFAM R |
TCCGCCCTATAAGCATCTTG |
| LDHA F |
AGCCCGATTCCGTTACCTA |
| LDHA R |
TGCTTGTGAACCTCTTTCCA |
| MIB F |
GCGATGCTTCCAACTTTAGG |
| MIB R |
TGCCCATTTACATCCACATC |
| mTOR F |
CCTCACAAGACATCGCTGAA |
| mTOR R |
GGATCTCCAGCTCTCCAAAGT |
Flow cytometric analysis of
apoptosis
For the apoptosis assay, the cells were trypsinized
and washed with fresh medium. Cells were then centrifuged for 3 min
at 1,500 rpm and the supernatant was discarded. The cell pellets
were resuspended in 1X binding buffer at 1–5×106/ml and
stained for 15 min using the Annexin V-PE/7AAD Apoptosis kit
(eBioscience, CA, USA), according to the manufacturer's
instructions. The number of apoptotic cells was analyzed by flow
cytometry (BD Accuri C6, USA).
Cell metabolism assays
Glycolytic rates were measured by calculating
extracellular acidification (ECAR) and oxygen consumption rates
(OCR) simultaneously in real-time using the Seahorse Biosciences
Extracellular Flux Analyzer. Cervical cancer cells
(4×105) were seeded into XF 24-well cell culture
micro-plates (Seahorse Biosciences) by BD Cell-Tak (BD Biosciences,
Oxfordshire, UK), and plates were incubated at 37°C for 1 h before
OCR and ECAR analysis. The experimental procedures included
monitoring the cells for oxygen consumption and lactic acid
production while injecting metabolic compounds into the media. The
compounds used were D-glucose (2 g/l), oligomycin (1 μM),
and 2-deoxyglu-cose (100 mM), which provided glycolysis-associated
ECAR, the maximum glycolytic capacity, and non-glycolytic ECAR,
respectively. Seahorse Biosciences assay media, which is an
unbuffered DMEM without glucose, pyruvate, or biocarbonate, was
used during experimentation. We adjusted the pH of the media before
each use with HCL and NaOH. Data are presented as extracellular
acidification rate (ECAR; mpol/min) for glycolysis and oxygen
consumption rate (OCR: pmol/min) for oxidative phosphorylation.
Each assay was performed in quadruplicate and representative data
from three independent experiments are shown.
Nude mouse xenograft models
Female athymic nude mice at 6 weeks of age were
purchased from the Shanghai Experimental Animal Center of the
Chinese Academy of Science. For xenograft tumor formation assays,
mice were randomly separated into two groups (five per group). SiHa
cells transfected with shNC or shHK2 were subcutaneously injected
into the two groups at a concentration of 1×107 cells
per mouse. We measured the tumor size every week for five weeks.
Then the mice were sacrificed, the tumors removed, fixed in
formalin, embedded in paraffin, and sectioned for IHC staining. The
tumor volume was calculated as follows: tumor volume
(mm3) = (longest diameter) × (shortest
diameter)2 × 0.5.
Statistical analysis
The differences between groups in protein levels
detected by western blots, RNA levels determined by RT-PCR, flow
cytometric analyses, cell metabolism assays, and in vivo
experiments were analyzed by Student's t-test. A two-sided test
with p<0.05 was considered statistically significant. All
statistical analyses were performed using SAS Release 8.02 (SAS
Institute Inc., Cary, NC, USA).
Results
Analysis of survival and HK2 expression
level from TCGA data
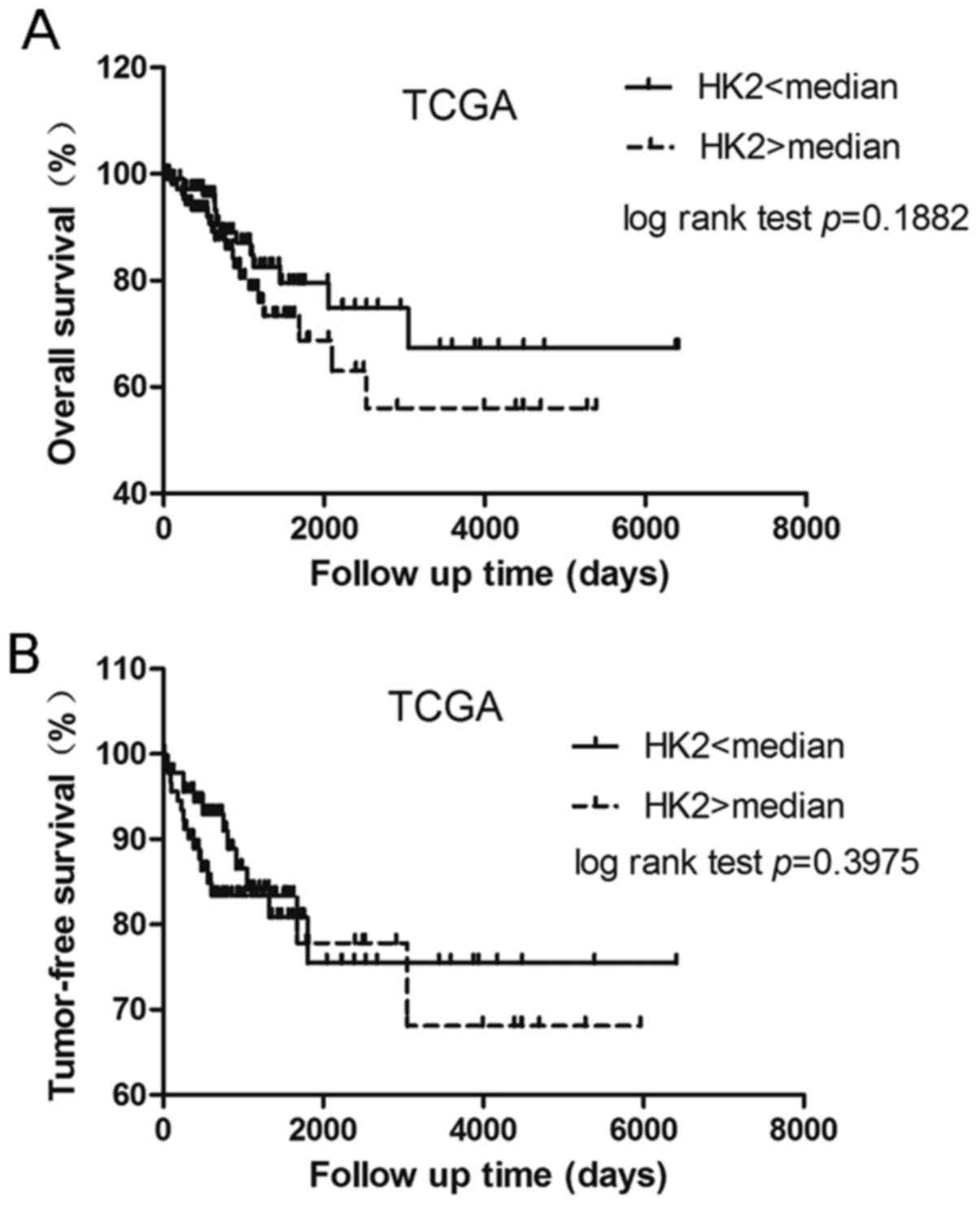
First we investigated the relationship between HK2
expression and the corresponding patient prognosis. For each
patient in the cervical cancer cohort (n=234), a normalized
RNA-Sequence count which stands for the mRNA expression of HK2, was
calculated from TCGA data. Normalized counts were dichotomized at
the median and the cohort was divided into two groups with
relatively low and high expression levels of HK2. Curves for
overall survival (OS) and tumor-free survival were plotted
according to the Kaplan-Meier method, with p-values determined by
the log-rank test. The difference between the two groups for
overall survival and tumor-free survival was not statistically
significant (Fig. 1). We also
organized the HK2 level in 3 normalized counts, which represent the
mRNA expression levels of HK2 from 307 cervical cancer specimens
from TCGA, into a heatmap according to infection with different HPV
subtypes (Fig. 2), we did not
observe an obvious connection between HK2 expression and each HPV
subtype infection.
The association between HPV16 E7 and HK2
expression
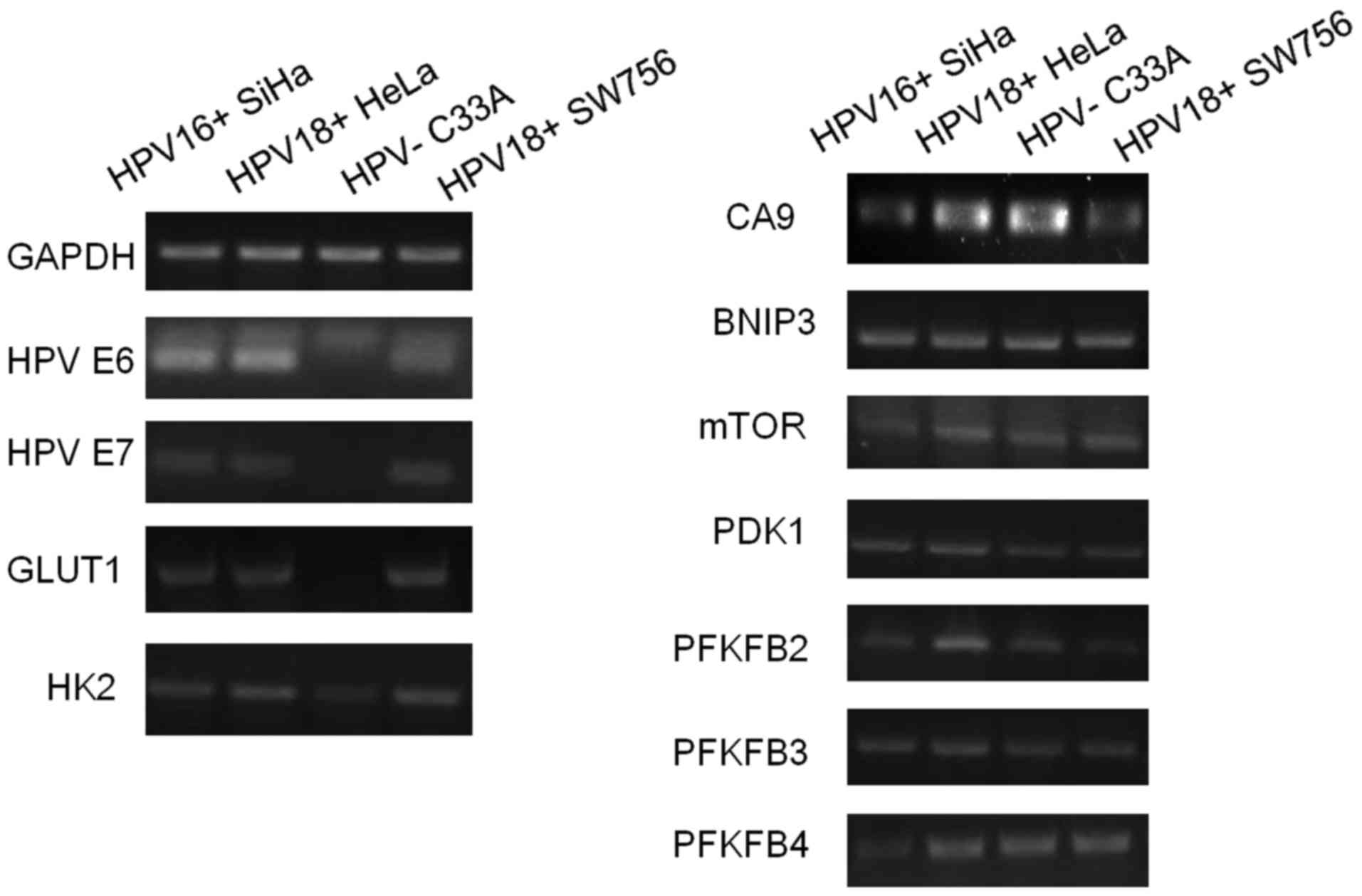
Using 4 different cervical cancer cell lines, we
sought to explore the expression levels of certain
metabolism-related genes and found that HK2 and Glut1 expression
levels were significantly weaker in HPV(−) cell lines, relative to
the three HPV(+) cells (Fig. 3).
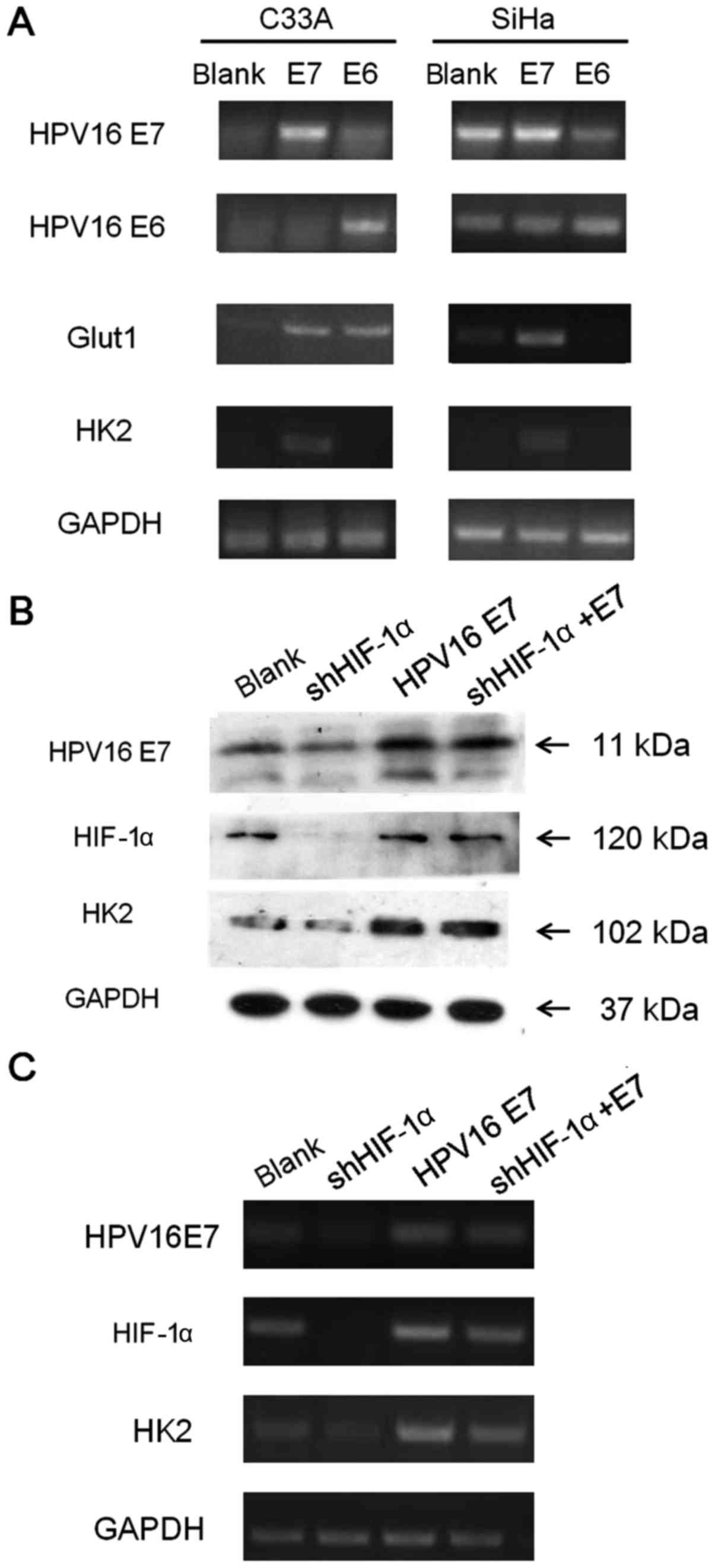
Next, we investigated whether HK2, Glut1 expression correlated with
the vital oncoproteins of HPV virus, E6 and E7. Our results
indicated that the E7 oncoprotein, but not E6, could enhance HK2
expression in both HPV(+) and HPV(−) cell lines at the RNA level
(Fig. 4A) and at the protein level
(Fig. 4B). E7 could also enhance
Glut1 mRNA expression in SiHa cell line, but the difference was not
obvious in C33A cell line. In order to explore whether the HPV16 E7
acceleration impact on HK2 involves the HIF-1α pathway, we knocked
down HIF-1α with shRNA. With the RT-PCR and western blotting
results in the SiHa cell line, we found that knock-down of HIF-1α
induced obvious attenuation of HK2, and HPV16 E7 could rescue HK2
expression when HIF-1α was knocked down, based on the observation
that the HK2 expression level in cells with both HIF-1α knock-down
and HPV16 E7 overexpression was much stronger than that in cells
with only HIF-1α knock-down (Fig. 4B
and C). Thus, we suspected that HPV16 E7 could promote the
expression of HK2 through a mechanism other than the HIF-1α
pathway.
The impact of HK2 on cervical cancer
cells in vitro
To determine the impact of HK2 expression on
cervical cancer cells, we attempted to knock-down HK2 in both the
HPV(+) SiHa cell line and the HPV(−) C33A cell line. As shown in
Fig. 5, in both normal and hypoxic
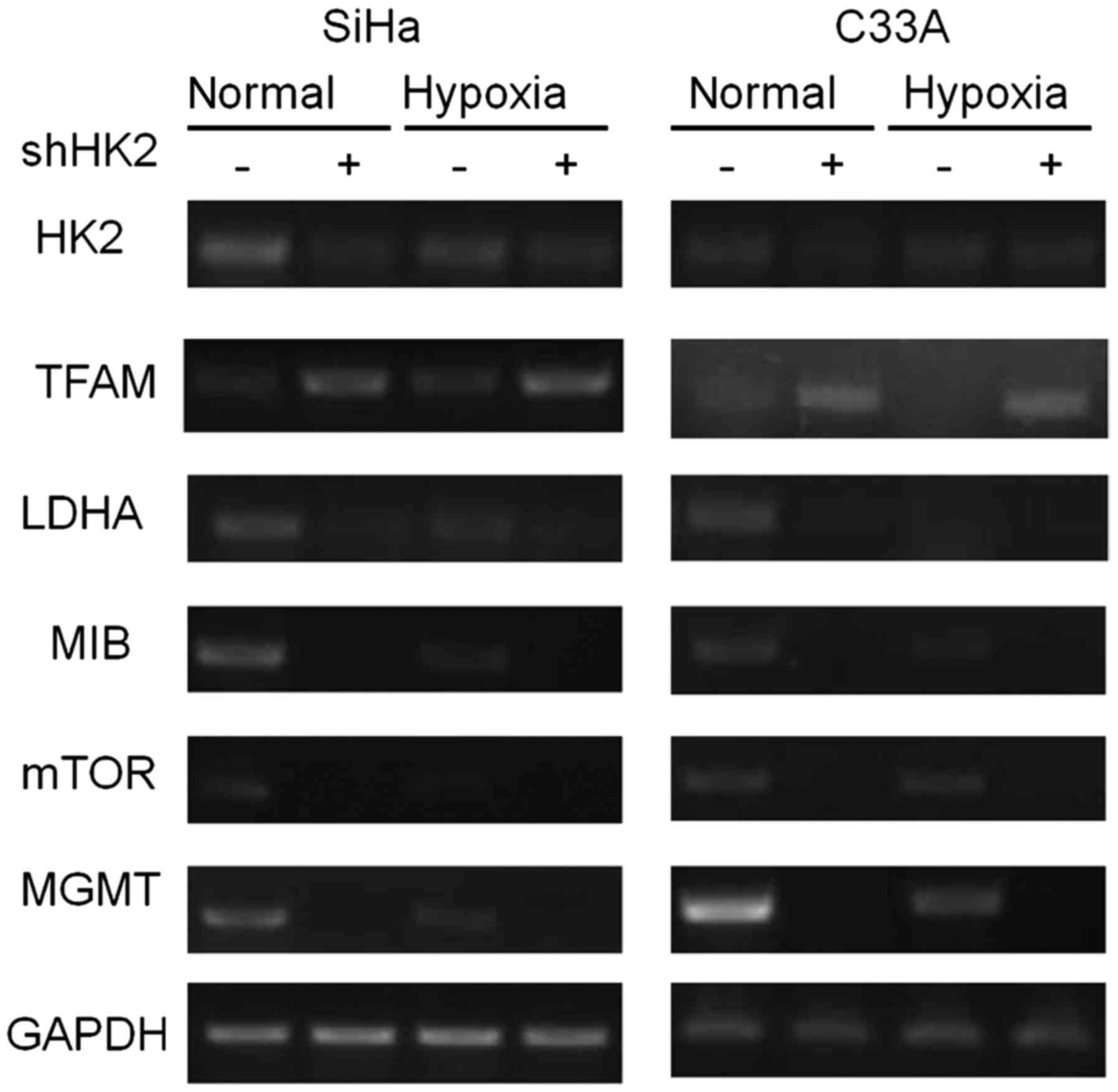
environments, knock-down of HK2 induced significant overexpression
of TFAM, which indicated a reinforcement of mitochondrial function,
as well as the down-regulation of LDHA, which indicated an ablation
of lactification ability. Moreover, knock-down of HK2 also
significantly abrogated the expression of MIB, mTOR and MGMT in
both normal and hypoxic environments (Fig. 5). Next, SRB analysis was used to
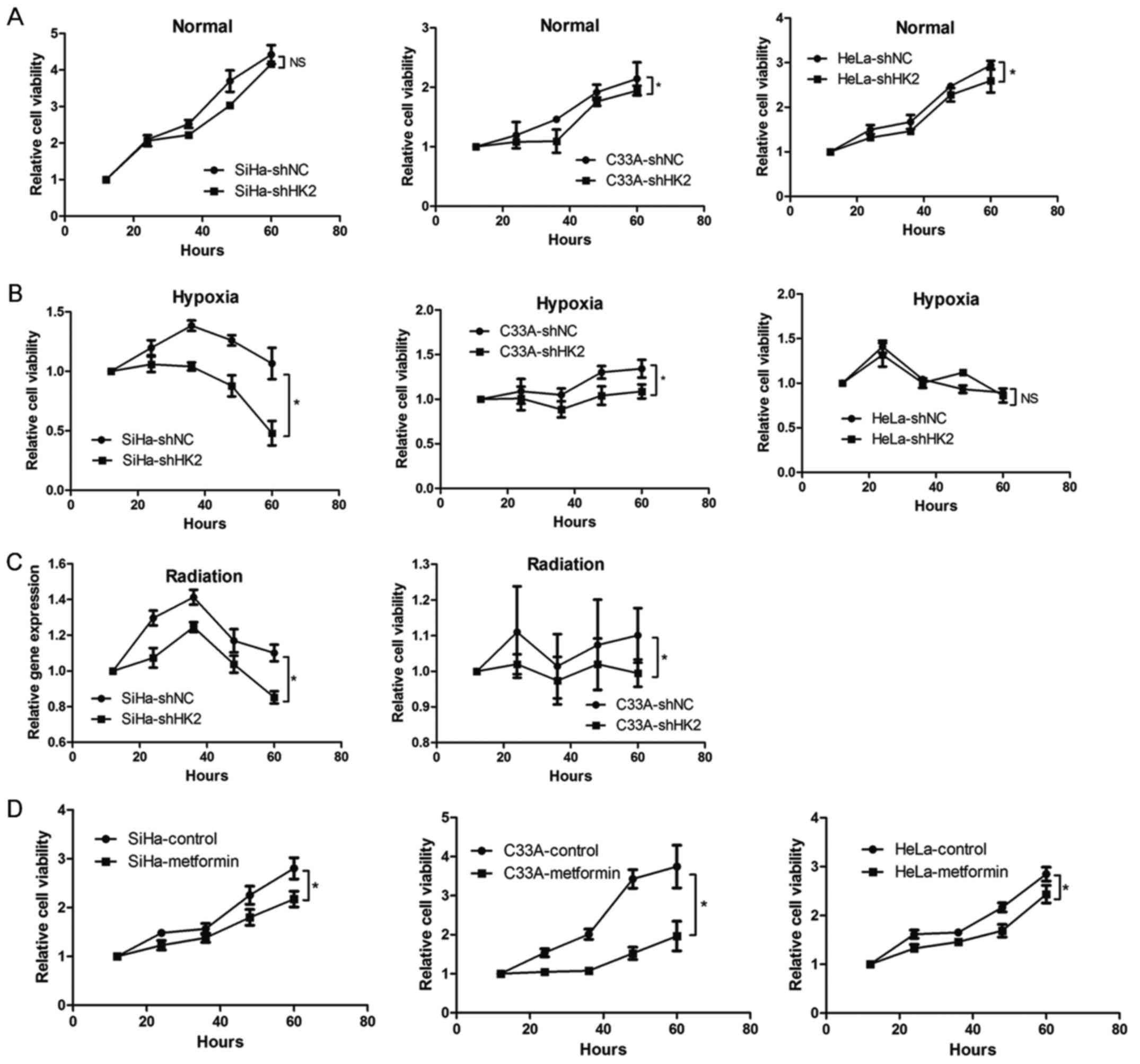
explore the impact of HK2 on proliferation ability of cervical
cancer cells. After knocking down HK2 expression, cervical cells
demonstrated significantly attenuated proliferation ability in
normal and hypoxic environments compared to shNC cells (Fig. 6A and B), with the exceptions of
SiHa cells in a normal environment and HeLa cells in a hypoxic
environment, even though the cervical cancer cells could not grow
vigorously after three days of incubation in the hypoxic
environment. In order to investigate the influence of HK2 on the
radiation sensitivity of cervical cancer cells, we compared their
proliferation abilities following irradiation exposure. We found
that 10 Gy-irradiated shHK2 SiHa and C33A cells exhibited much
greater proliferation-inhibiting effects compared to the 10
Gy-irradiated shNC group, even though cells did not grow vigorously
three days after irradiation (Fig.
6C). Next, we treated the cervical cancer cells with metformin,
which is reported to inhibit HK2 function (35–38),
and has been reported to impair glucose metabolism and tumor growth
in breast cancer (39). We found
that the proliferation of the metformin-exposed group was
significantly reduced compared to the control group (Fig. 6D). Therefore, these results
indicate that HK2 may exert a variety of impacts on cervical cancer
cells, including cell metabolism, the mTOR pathway and DNA damage.
Moreover, HK2 inhibition specifically attenuated the proliferation
of cervical cancer cells.
The effect of HK2 on cervical cancer cell
apoptosis in vitro
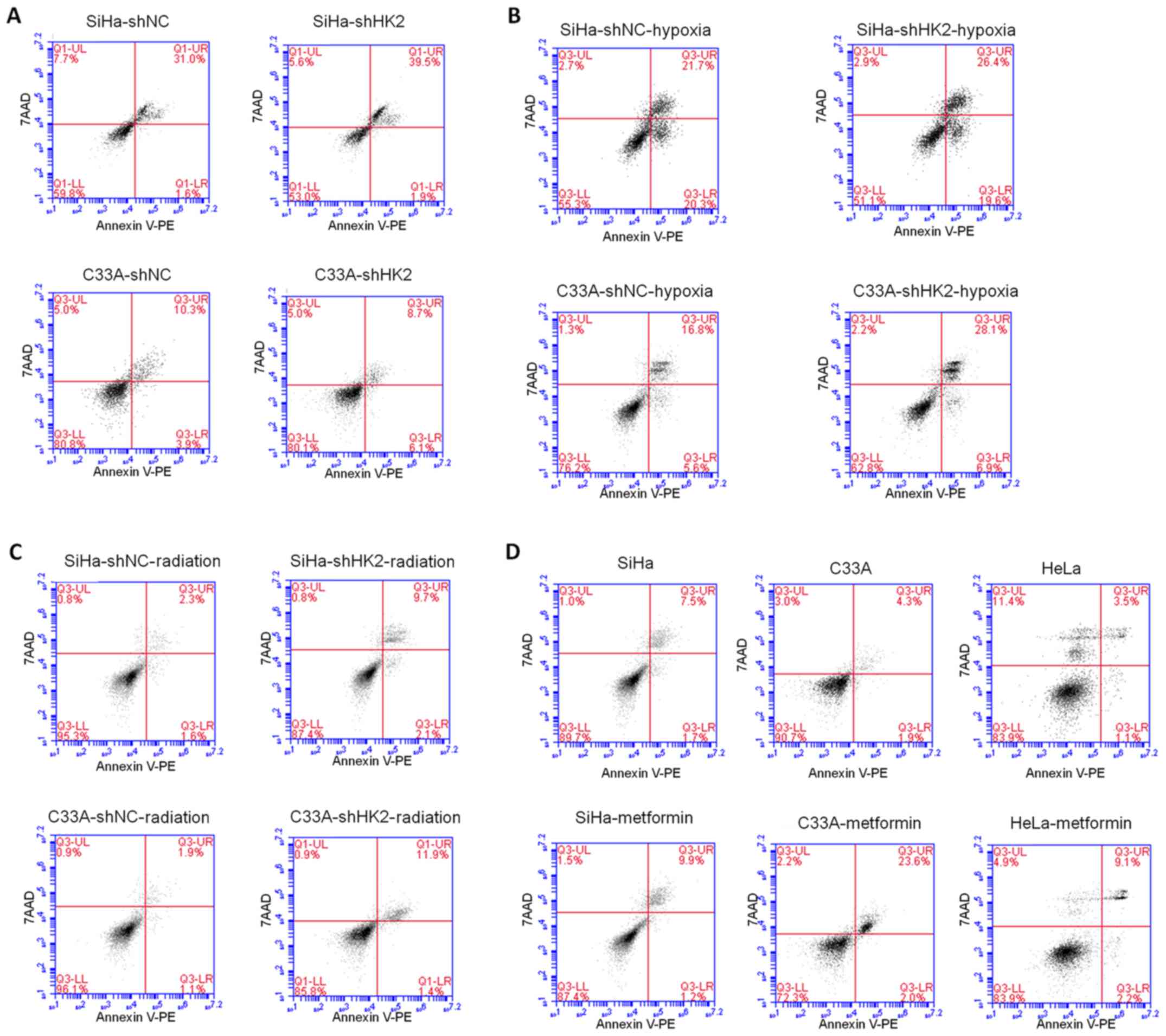
Next, the impact of HK2 on apoptosis in cervical
cancer cell lines in normal and hypoxic environments and after
radiation exposure was explored. Cell survival was assessed using
Annexin V-PE and 7AAD staining. In SiHa cells, the shHK2 group
demonstrated an increase in apoptosis, particularly in later
apoptotic events, compared to shNC control cells in normal and
hypoxic environments as well as after 10-Gy of irradiation
(Fig. 7A–C). In C33A cells, the
shHK2 group displayed an increase in apoptosis compared to shNC
control cells in earlier apoptosis in normal condition (Fig. 7A), and also in both earlier and
later apoptosis in the hypoxic environment and after 10 Gy of
irradiation (Fig. 7B and C).
Similarly, in SiHa, C33A and HeLa cells, metformin led to an
increase in apoptosis compared to control group cells, especially
in the C33A and HeLa cell lines (Fig.
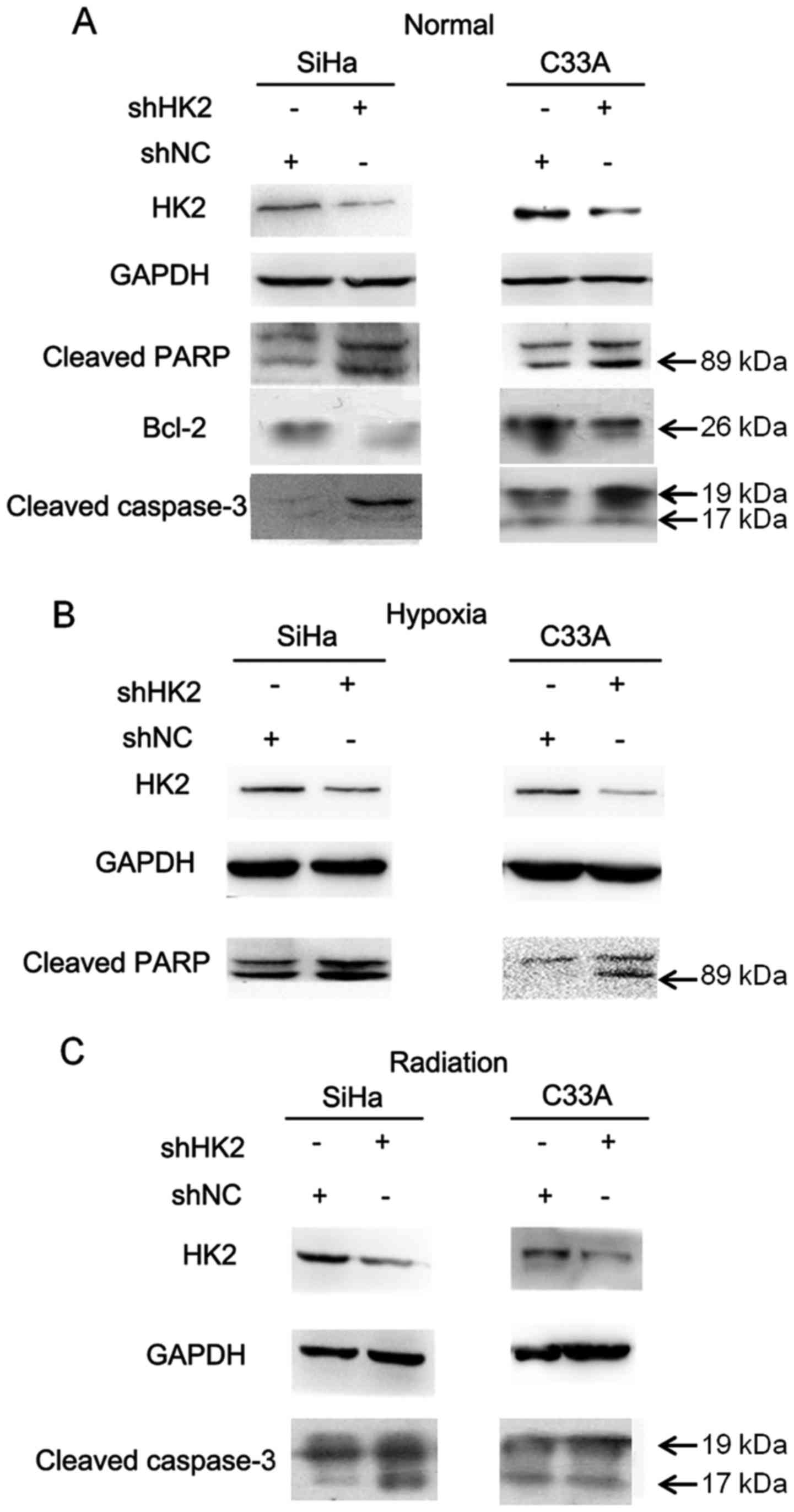
7D). To further explore the molecular mechanisms underlying the
anti-apoptotic role of HK2, we examined two biochemical markers of
apoptosis, polyadenosine diphosphate ribose polymerase (PARP) and
cleaved caspase-3, in the shHK2 and shNC stable cell lines. Cleaved
PARP and caspase-3 were detected at higher levels in the shHK2 cell
lines than in the shNC cell lines in normal environment (Fig. 8A). We also found higher expression
of cleaved PARP and cleaved caspase-3 in shHK2 cell lines than shNC
cell lines in the hypoxic environment and after irradiation,
respectively (Fig. 8B and C). The
B-cell lymphoma (Bcl) family of proteins is known to be closely
associated with apoptosis. As shown in Fig. 8A, a significant decrease in the
anti-apoptotic protein Bcl-2 was observed in shHK2 cells relative
to shNC cells. Taken together, inhibition of HK2 promoted the
apoptotic potential of cervical cancer cells.
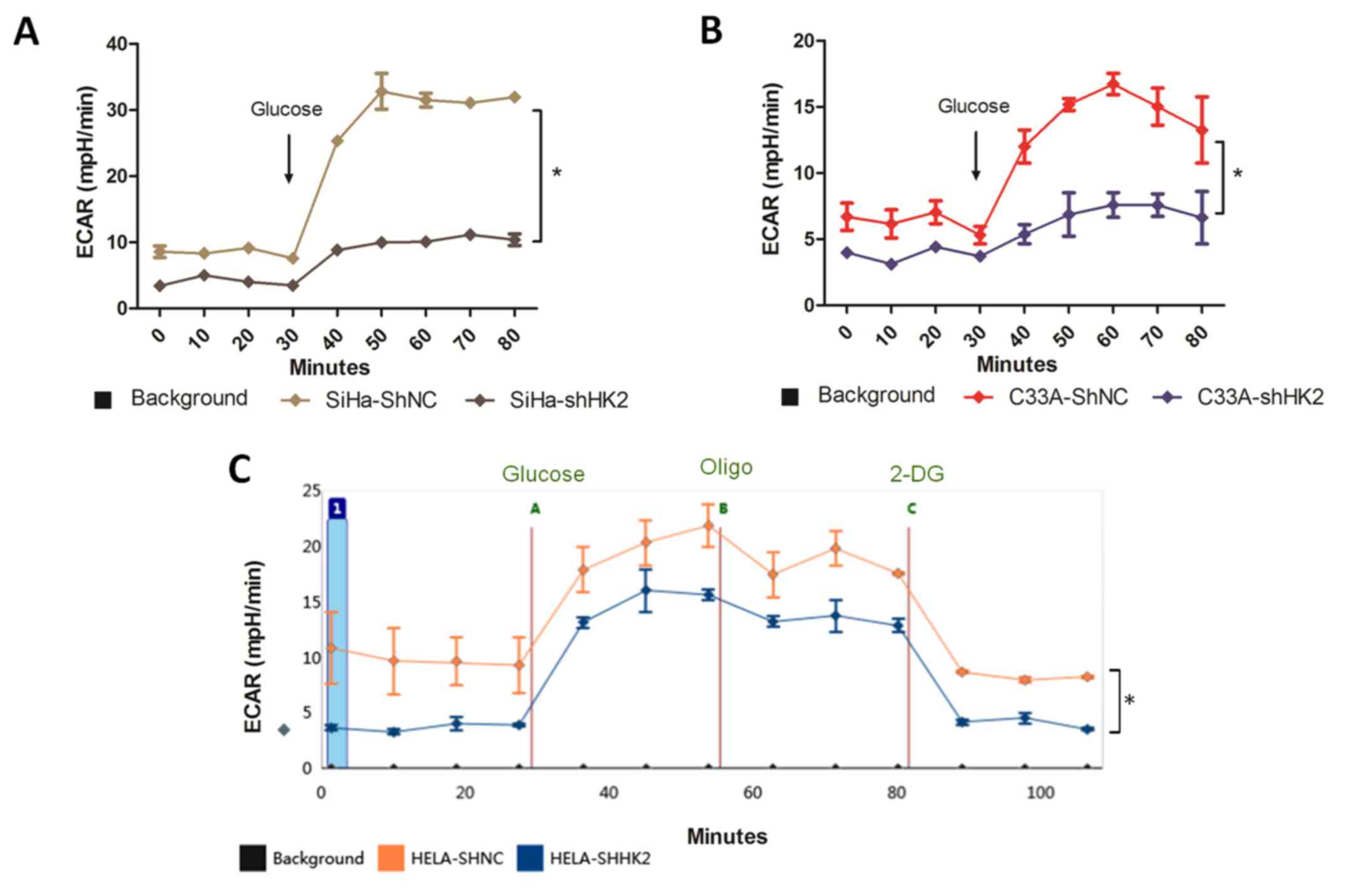
Metabolic changes in cervical cancer
cells
Since HK2 serves as the most critical enzyme
regulating glycolysis, we investigated the metabolic profile of
cervical cancer cells using the XF analyzer. In all three shNC cell
lines, the ECAR was nearly two times higher than that of the
respective shHK2 cell line under glucose starvation conditions,
indicating that HK2 knock-down resulted in a strong inhibition of
glycolysis in all cell lines tested (Fig. 9A–C). Glycolytic ECAR was measured
immediately after the injection of glucose. Glucose addition
usually stimulated glycolysis, but there was a noticeable
inhibition of glycolysis in the shHK2 group compared to shNC group,
indicating that the shHK2 group completely lacked glycolytic
flexibility upon glucose exposure (Fig. 9). By treating the cells with
oligomycin, which reduces mitochon drial respiration and maximizes
glycolytic ATP production, we calculated the complete cellular
glycolytic capacity. We observed more reserved glycolytic capacity
in HeLa-shNC than in HeLa-shHK2 cells (Fig. 9C). The addition of 2-deoxy-glucose
(2-DG), an inhibitor of glycolysis, was intended to ensure that the
ECAR measured was a result of glycolytic metabolism, and we
confirmed that the ECAR returned to non-glycolytic levels in both
shNC and shHK2 HeLa cell lines after 2-DG treatment (Fig. 9C). Basal measurements of the
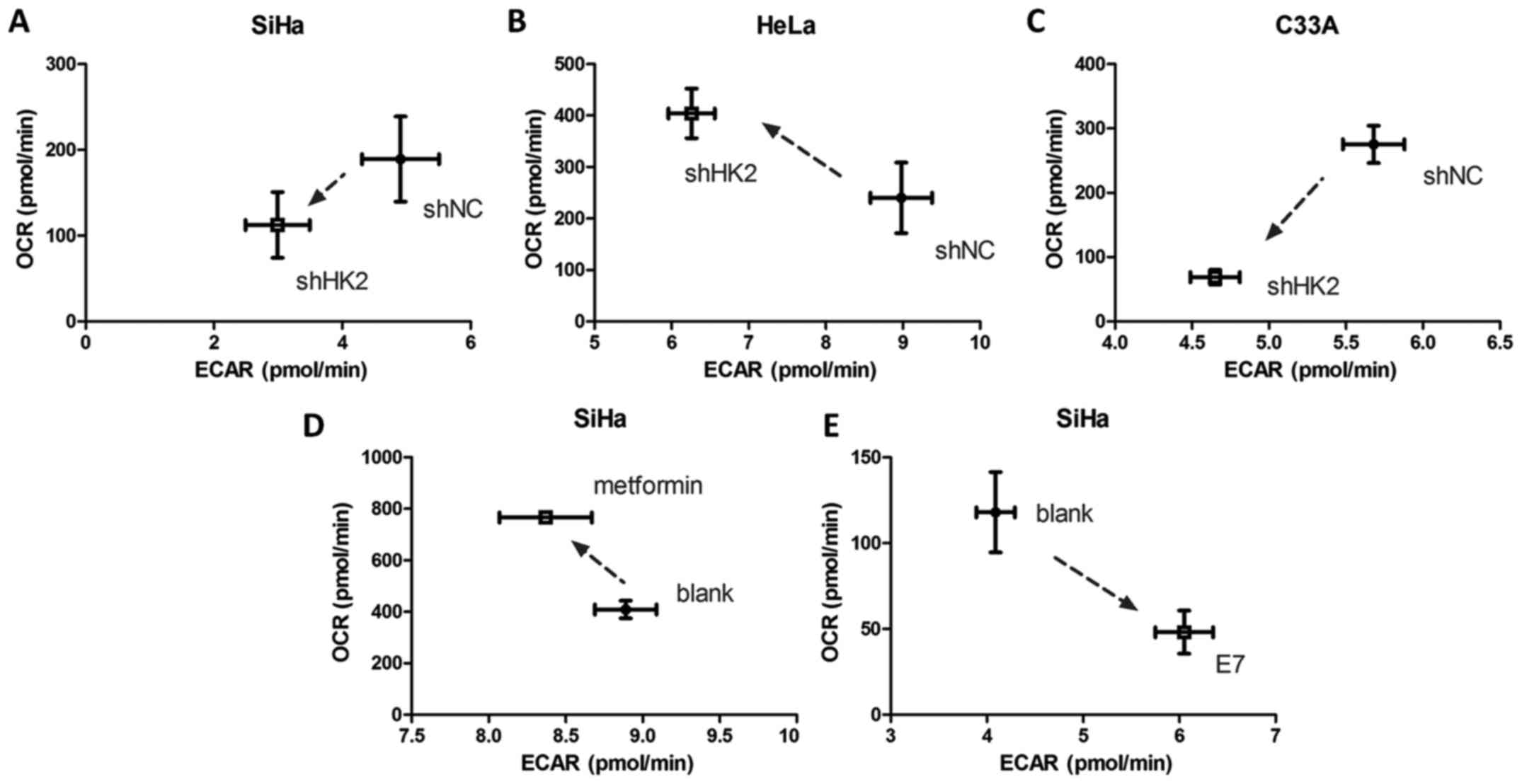
mitochondrial respiration rate (OCR) and glycolytic ECAR were
measured to study the change in metabolic profiles between shNC and
shHK2 cervical cancer cells. Intriguingly, quantitation showed
reduced ECAR and increased OCR rates upon HK2 knock-down in HeLa
cells (Fig. 10B), suggesting a
partial metabolic profile switch to oxidative phosphorylation in
HeLa-shHK2 cells. The SiHa and C33A cells showed both reduced ECAR
and OCR rates upon HK2 knock-down, which demonstrated a decreased
metabolic rate (Fig. 10A and C).
Additionally, metformin treatment of SiHa cells revealed a higher
OCR/ECAR ratio, indicating a partial metabolic alteration to
oxidative phosphorylation in SiHa cells when HK2 function was
inhibited (Fig. 10D). Finally,
SiHa cells transfected with HPV16 E7 plasmids displayed a high
ECAR/OCR ratio, demonstrating a partial metabolic switch to
glycolysis when HPV16 E7 was overexpressed (Fig. 10E).
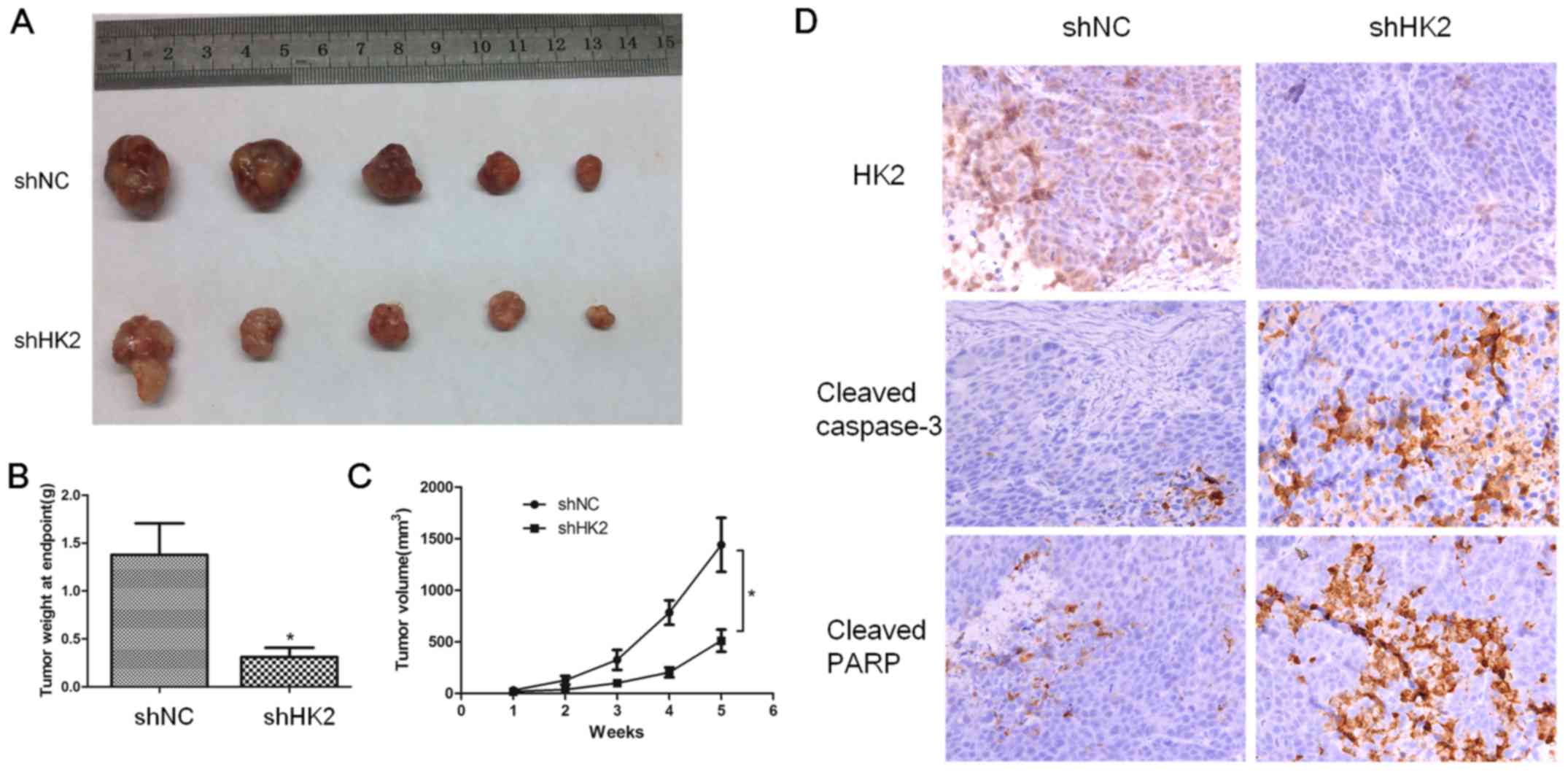
HK2 knock-down xenograft model
Finally, we performed a xenograft tumor growth assay
in nude mice to further evaluate the tumorigenic role of HK2 in
cervical cancer cells. First, mice were subcutaneously injected
with shRNA or shNC SiHa cells. After five weeks, the average size
of the tumors in the shHK2 group was substantially smaller than
that in the shNC group (Fig.
11A–C, p<0.05). To determine the effect of HK2 on apoptosis,
we performed IHC of caspase-3 and PARP in the sectioned tumor
tissues. Considerably stronger caspase-3 and PARP staining
intensities were detected in shHK2 tumor tissues relative to shNC
tissues (Fig. 11D).
Discussion
A novel hypothesis was developed by us that the
glycolytic enzyme HK2 serves as a critical step in aerobic
glycolysis inducing irradiation resistance in cervical cancer,
offering a proliferative and cell survival advantage. There are
some reports demonstrating that HK2 is aberrantly expressed in
gynecological cancers, including cervical and ovarian cancer
(40,41). For example, Huang et al
suggested that HK2, which is located in the cytoplasm of cervical
carcinoma cells, shows higher expression levels in a
radiation-resistant group than a radiation-sensitive group
(42), suggesting common roles for
HK2 as an oncoprotein and an indicator for radiation resistance in
gynecology tumors. In our study, we provided evidence demonstrating
that downregulation of HK2 restored apoptosis of cervical cancer
cells. Moreover, the levels of cleaved caspase-3 and cleaved PARP
in C33A and SiHa cells were significantly accelerated and Bcl-2
expression was inhibited by HK2 inhibition, suggesting an essential
role for HK2 in the anti-apoptotic mechanism of cervical cancer
cells. The targeted inhibition of HK2 expression by shRNA
demonstrated a suppression of tumor growth both in vitro and
in vivo. HK2 expression inhibition also attenuated the
expression of mTOR, suggesting that HK2 might modulate the
PI3K/AKT/mTOR pathway that is a crucial constituent of an adaptive
system for sensing the availability of a wide range of growth
factors and nutrients in homeostasis (43). Moreover, we found that suppression
of HK2 not only inhibited the expression of MIB, it also inhibited
the expression of MGMT. Our study demonstrated that HK2 inhibition
downregulated distinct pathway proteins including mTOR, MIB and
MGMT, that HK2 could serve as a biomarker and potential therapeutic
target of cervical cancer treatment.
In order to promote the effect of radiation therapy
to cervical cancer patients, it is most crucial to understand the
mechanism of radiation resistance. Hypoxia, as a common
microenvironment for malignant cells, is the fundamental reason
(22). HIF-1α, which is stabilized
upon hypoxia, helps the radiation resistance (23) and can encourage a variety of
functional changes including tumorigenesis and metastasis and the
glycolytic process (44,45). Glycolysis contributes to the
radioresistance for the following reasons (46–48).
Firstly, the accumulation of glycolytic products builds a redox
buffer network which removes free radicals and ROS produced by
ionizing radiation from irradiation therapy. The effect of
irradiation therapy could be significantly attenuated by the
rescuing buffer network from aerobic glycolysis in a hypoxic
environment (49,50). Secondly, glycolysis not only
produces the anabolic precursors for de novo synthesis of
nucleotides and lipids, which are necessary for high tumor growth
rate, but also supplies the tumor cells with plenty of ATP in a
hypoxic microenvironment as a vital energy contributor and
facilitate DNA repair in cells (26,27,51).
Thus, if we could attenuate cancer cell glycolysis, the rescue
network and energy contributor for cancer cell survival would be
greatly alleviated and radiasensitization would be intensified.
Unfortunately, there is limited clinical research designed to
attenuate radio-resistance through modifying hypoxia and
glycolysis. Thereby, we aimed to elicit a novel way to target the
critical enzyme of glycolysis, suppress tumor glycolysis to enhance
the ionizing radiation from irradiation therapy, block the main
energy supply, and ultimately increase the sensitivity of cervical
cancer cells to radiation therapy. HK2, being the first
irreversible critical modulator of glycolysis, is on the top of the
list of genes potentially modified and regulated. Although some HK
inhibitors are already in phase I and II clinical trials (52,53),
there are still restrictions involved in the wide range of
regulatory pathways of HK inhibitors, warranting further
research.
In the present study, we focused on HK2 inhibition
to switch cervical cancer cell metabolism to one less dependent on
glycolysis, aiming to reduce the impact of glycolysis on ionizing
radiation, block the main energy contributor, induce cancer cells
apoptosis and eventually improve cervical cancer sensitivity to
radiation therapy. We observed that HK2 inhibition with shRNA or
metformin could effectively suppress ECAR and glycolytic metabolism
in cervical cancer cell lines with dinimishing expression of LDHA
and simultaneously accelerate the OCR and enhance oxidative
phosphorylation with accelerating expression of TFAM. We indicated
that HK2 inhibition managed to impair cervical cancer cell
lactification ability and reinforce mitochondrial function. At the
same time, shNC-containing cervical cancer cell lines exhibited
superior proliferation abilities comparable to the HK2 knock-down
cell lines in both normal and hypoxic environments as well as after
radiation exposure. Irradiated cervical cancer cells displayed
significantly inferior proliferation after HK2 inhibition. Due to
the protecting shield for cancer cells created by glycolysis in
hypoxia, the anabolic precursors for tumor growth and plenty of the
ATP produced by glycolysis were severely blocked by HK2 inhibition,
HK2 knockdown cells tend to lose some of the survival chance under
irradiation circumstance and showed more sensitivity to irradiation
than shNC group. Similarly, others reported that systemic deletion
of HK2 is therapeutic (7), and
from the data displayed above, we proposed that inhibition of HK2
could prevent the glycolysis of cancer cell, suppress proliferation
of cervical cancer cells, enhance apoptosis and most importantly
intensify the sensitivity of cervical cancer cells to radiation
therapy. Metformin directly inhibits HK2 activity and subcellular
localisation inducing dissociation of HK2 from the mitochondria.
Metformin impairs glycolysis and has an inhibitory effect on AKT
phosphorylation which contributes to effects on HK2 suppression by
decreasing HK2 expression, activity and mitochondrial interaction
(35–38,45).
We showed that metformin served as an HK2 inhibitor, contributed to
the apoptosis of cervical cancer cells, suppressed the
proliferation and altered the metabolic profile of cervical cells
to less dependent on glycolysis.
Human papilloma virus (HPV) is a small, circular,
double-stranded DNA virus infecting epithelial cells and has been
reported to be necessary, but not sufficient to initiate cervical
squamous epithelial cell tumorigenesis (54,55).
HPV E7, as the vital oncoprotein of this virus, plays an important
role in the viral life cycle by impacting the tight link between
cellular proliferation and differentiation in normal epithelium,
thus leading the virus to replicate in differentiating epithelial
cells (56). In our study, we
first identified HK2 expression in different cervical cancer cell
lines. Obvious expression of HK2 was detected only in HPV(+)
cervical cancer cell line but not in the HPV(−) cell line. We
elucidated a close relationship between the HPV16 E7 oncogene and
HK2 expression. It has been reported that the HPV E7 protein
enhances HIF-1α transcriptional activity through manipulating the
response to hypoxia (57), and
displacing the histone deacetylases HDAC1, HDAC4, and HDAC7
(57,58). Both HPV E6 and E7 are independently
capable of inducing expression of HIF-1α upon DFO treatment
(59,60). It has also been shown that the
radioresistance-associated HIF1 protein upregulates many enzymes of
the glycolytic process, including HK2, through binding to the
hypoxia responsive elements (HREs) of the promoter (5,61,62).
What we discovered was HPV16 E7 was directly responsible for the
up-modulation of HK2 by a pathway independent of HIF-1α. There is a
common mechanism that HK2 expression is impacted by transcription
factors in tumor cells (63). The
key oncogenic pathways present in multiple cancers, such as
PI3K/Akt signaling, enhance the expression of the glycolytic enzyme
HK2, which further hinders cell apoptosis, facilitating tumor
growth and progression (64). In
our study, we provide the first evidence that the HPV oncoprotein
E7 as one of those transcriptional factors, could exert an
enhancing impact on HK2 expression independent of the HIF-1α
pathway. On the other hand, we found that HPV16 E7 overexpression
could effectively make SiHa cells more dependent on the glycolytic
metabolic profile through increasing ECAR and reducing OCR,
facilitating the Warburg effect in tumor cells, and knockdown of
HK2 or metformin treatment significantly abrogated glycolysis by
reducing ECAR. Thus, we postulated that HPV16 E7 increases
glycolytic metabolism and promotes HK2 expression and its
regulation on downstream glycolysis metabolism. This suggests an
underlying mechanism through which HPV E7 induces the Warburg
effect via pathways including enhancing the expression or functions
of diverse glycolytic enzymes, namely HK2. We propose an essential
role for the HPV16 E7 oncogene in the Warburg effect through
regulation of the critical rate-limiting enzyme of glycolysis, HK2.
Therefore, if we could effectively inhibit HK2 expression or
function, we could eventually abrogate the HPV16 E7-induced
glycolytic metabolism phenotype, blocking the main energy sources
of cancer cells, suppress tumor growth and progression and enhance
the sensitivity of HPV(+) cervical cancer cells to irradiation
therapy.
In conclusion, we have successfully identified an
essential role for HK2 in the HPV16 E7-induced glycolytic metabolic
profile. We further demonstrated that HK2 inhibition not only
suppress cervical cancer cell energy metabolism, which is a
hypoxia-facilitated glycolytic process, and sensitive HPV16
E7-induced cervical cancer cells to irradiation, it also suppresses
cervical cancer cell proliferation, survival and carcinogenesis,
both in vivo and in vitro. Furthermore, HPV16 E7
increases glycolytic metabolism and promotes HK2 expression and its
regulation on downstream metabolism. Our data extend the
understanding of the regulatory network of HK2 in cervical cancer
metabolism and indicate potential targets for the exploitation of
cervical cancer irradiation therapy strategies.
Acknowledgments
We thank Dr Sufang Wu for kindly providing
pCAG-myc-HPV16 E7, pCAG-myc-HPV16 E6 and pCAG-myc-blank plasmids.
This study was supported by grants from the National Natural
Science Foundation of China (NSFC nos. 81172476, 811272885 and
81472427), Shanghai Science and Technology Committee Foundation
Basic Research Field focused Project (13JC1404501), Doctoral
Specialized Fund from Ministry of Education (20120073110090).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hsu PP and Sabatini DM: Cancer cell
metabolism: Warburg and beyond. Cell. 134:703–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase II: Cancer's double-edged sword acting as both
facilitator and gatekeeper of malignancy when bound to
mitochondria. Oncogene. 25:4777–4786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mathupala SP, Rempel A and Pedersen PL:
Glucose catabolism in cancer cells: Identification and
characterization of a marked activation response of the type II
hexokinase gene to hypoxic conditions. J Biol Chem.
276:43407–43412. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Liu Z, Zhao L, Sun J, He Q, Yan W,
Lu Z and Wang A: Hexokinase 2 enhances the metastatic potential of
tongue squamous cell carcinoma via the
SOD2–H2O2 pathway. Oncotarget. 8:3344–3354.
2017.
|
|
7
|
Patra KC, Wang Q, Bhaskar PT, Miller L,
Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, et al:
Hexokinase 2 is required for tumor initiation and maintenance and
its systemic deletion is therapeutic in mouse models of cancer.
Cancer Cell. 24:213–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolf A, Agnihotri S, Micallef J, Mukherjee
J, Sabha N, Cairns R, Hawkins C and Guha A: Hexokinase 2 is a key
mediator of aerobic glycolysis and promotes tumor growth in human
glioblastoma multiforme. J Exp Med. 208:313–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bustamante E and Pedersen PL: High aerobic
glycolysis of rat hepatoma cells in culture: Role of mitochondrial
hexokinase. Proc Natl Acad Sci USA. 74:3735–3739. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JW and Dang CV: Multifaceted roles of
glycolytic enzymes. Trends Biochem Sci. 30:142–150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase-2 bound to mitochondria: Cancer's stygian link to the
'Warburg Effect' and a pivotal target for effective therapy. Semin
Cancer Biol. 19:17–24. 2009. View Article : Google Scholar
|
|
12
|
Pastorino JG, Shulga N and Hoek JB:
Mitochondrial binding of hexokinase II inhibits Bax-induced
cytochrome c release and apoptosis. J Biol Chem. 277:7610–7618.
2002. View Article : Google Scholar
|
|
13
|
Majewski N, Nogueira V, Bhaskar P, Coy PE,
Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB and Hay N:
Hexokinase-mitochondria interaction mediated by Akt is required to
inhibit apoptosis in the presence or absence of Bax and Bak. Mol
Cell. 16:819–830. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gottlob K, Majewski N, Kennedy S, Kandel
E, Robey RB and Hay N: Inhibition of early apoptotic events by
Akt/PKB is dependent on the first committed step of glycolysis and
mitochondrial hexokinase. Genes Dev. 15:1406–1418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rockwell S, Dobrucki IT, Kim EY, Marrison
ST and Vu VT: Hypoxia and radiation therapy: Past history, ongoing
research, and future promise. Curr Mol Med. 9:442–458. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimura T, Kakuda S, Ochiai Y, Nakagawa H,
Kuwahara Y, Takai Y, Kobayashi J, Komatsu K and Fukumoto M:
Acquired radioresistance of human tumor cells by
DNA-PK/AKT/GSK3beta-mediated cyclin D1 overexpression. Oncogene.
29:4826–4837. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bolderson E, Richard DJ, Zhou BB and
Khanna KK: Recent advances in cancer therapy targeting proteins
involved in DNA double-strand break repair. Clin Cancer Res.
15:6314–6320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lehmann BD, McCubrey JA, Jefferson HS,
Paine MS, Chappell WH and Terrian DM: A dominant role for
p53-dependent cellular senescence in radiosensitization of human
prostate cancer cells. Cell Cycle. 6:595–605. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergkvist GT, Argyle DJ, Pang LY, Muirhead
R and Yool DA: Studies on the inhibition of feline EGFR in squamous
cell carcinoma: Enhancement of radiosensitivity and rescue of
resistance to small molecule inhibitors. Cancer Biol Ther.
11:927–937. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baumann M, Krause M and Hill R: Exploring
the role of cancer stem cells in radioresistance. Nat Rev Cancer.
8:545–554. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown JM and Giaccia AJ: The unique
physiology of solid tumors: Opportunities (and problems) for cancer
therapy. Cancer Res. 58:1408–1416. 1998.PubMed/NCBI
|
|
22
|
Milosevic M, Warde P, Menard C, Chung P,
Toi A, Ishkanian A, McLean M, Pintilie M, Sykes J, Gospodarowicz M,
et al: Tumor hypoxia predicts biochemical failure following
radiotherapy for clinically localized prostate cancer. Clin Cancer
Res. 18:2108–2114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harada H: Hypoxia-inducible factor
1-mediated characteristic features of cancer cells for tumor
radioresistance. J Radiat Res (Tokyo). 57(Suppl 1): i99–i105. 2016.
View Article : Google Scholar
|
|
24
|
Sattler UG and Mueller-Klieser W: The
anti-oxidant capacity of tumour glycolysis. Int J Radiat Biol.
85:963–971. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bui T and Thompson CB: Cancer's sweet
tooth. Cancer Cell. 9:419–420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakashima RA, Paggi MG and Pedersen PL:
Contributions of glycolysis and oxidative phosphorylation to
adenosine 5′-triphosphate production in AS-30D hepatoma cells.
Cancer Res. 44:5702–5706. 1984.PubMed/NCBI
|
|
28
|
Meng MB, Wang HH, Guo WH, Wu ZQ, Zeng XL,
Zaorsky NG, Shi HS, Qian D, Niu ZM, Jiang B, et al: Targeting
pyruvate kinase M2 contributes to radiosensitivity of non-small
cell lung cancer cells in vitro and in vivo. Cancer Lett.
356B:985–993. 2015. View Article : Google Scholar
|
|
29
|
Bol V, Bol A, Bouzin C, Labar D, Lee JA,
Janssens G, Porporato PE, Sonveaux P, Feron O and Grégoire V:
Reprogramming of tumor metabolism by targeting mitochondria
improves tumor response to irradiation. Acta Oncol. 54:266–274.
2015. View Article : Google Scholar
|
|
30
|
Pena-Rico MA, Calvo-Vidal MN,
Villalonga-Planells R, Martínez-Soler F, Giménez-Bonafé P,
Navarro-Sabaté À, Tortosa A, Bartrons R and Manzano A: TP53 induced
glycolysis and apoptosis regulator (TIGAR) knockdown results in
radio-sensitization of glioma cells. Radiother Oncol. 101:132–139.
2011. View Article : Google Scholar
|
|
31
|
BelAiba RS, Djordjevic T, Bonello S,
Flügel D, Hess J, Kietzmann T and Görlach A: Redox-sensitive
regulation of the HIF pathway under non-hypoxic conditions in
pulmonary artery smooth muscle cells. Biol Chem. 385:249–257. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chandel NS, McClintock DS, Feliciano CE,
Wood TM, Melendez JA, Rodriguez AM and Schumacker PT: Reactive
oxygen species generated at mitochondrial complex III stabilize
hypoxia-inducible factor-1alpha during hypoxia: A mechanism of
O2 sensing. J Biol Chem. 275:25130–25138. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng Y, Chen G, Hong L, Zhou L, Hu M, Li
B, Huang J, Xia L and Li C: How does hypoxia inducible factor-1α
participate in enhancing the glycolysis activity in cervical
cancer? Ann Diagn Pathol. 17:305–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Z, Zhou D, Lai Y, Liu Y, Tao X, Wang
Q, Zhao G, Gu H, Liao H, Zhu Y, et al: Estrogen induces endometrial
cancer cell proliferation and invasion by regulating the fat mass
and obesity-associated gene via PI3K/AKT and MAPK signaling
pathways. Cancer Lett. 319:89–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Salani B, Del Rio A, Marini C, Sambuceti
G, Cordera R and Maggi D: Metformin, cancer and glucose metabolism.
Endocr Relat Cancer. 21:R461–R471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Salani B, Marini C, Rio AD, Ravera S,
Massollo M, Orengo AM, Amaro A, Passalacqua M, Maffioli S, Pfeffer
U, et al: Metformin impairs glucose consumption and survival in
Calu-1 cells by direct inhibition of hexokinase-II. Sci Rep.
3:20702013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Semenza GL: Hypoxia-inducible factor 1
(HIF-1) pathway. Sci STKE. 2007:cm82007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roberts DJ, Tan-Sah VP, Smith JM and
Miyamoto S: Akt phosphorylates HK-II at Thr-473 and increases
mitochondrial HK-II association to protect cardiomyocytes. J Biol
Chem. 288:23798–23806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Marini C, Salani B, Massollo M, Amaro A,
Esposito AI, Orengo AM, Capitanio S, Emionite L, Riondato M,
Bottoni G, et al: Direct inhibition of hexokinase activity by
metformin at least partially impairs glucose metabolism and tumor
growth in experimental breast cancer. Cell Cycle. 12:3490–3499.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peng G-Q, Yang Y, Zhong C-G, Yin H, Hu G
and Tian Y: A study of association between expression of hOGG1,
VDAC1, HK-2 and cervical carcinoma. J Exp Clin Cancer Res.
29:1292010. View Article : Google Scholar
|
|
41
|
Jin Z, Gu J, Xin X, Li Y and Wang H:
Expression of hexokinase 2 in epithelial ovarian tumors and its
clinical significance in serous ovarian cancer. Eur J Gynaecol
Oncol. 35:519–524. 2014.PubMed/NCBI
|
|
42
|
Huang X, Liu M, Sun H, Wang F, Xie X, Chen
X, Su J, He Y, Dai Y, Wu H, et al: HK2 is a radiation resistant and
independent negative prognostic factor for patients with locally
advanced cervical squamous cell carcinoma. Int J Clin Exp Pathol.
8:4054–4063. 2015.PubMed/NCBI
|
|
43
|
Husseinzadeh N and Husseinzadeh HD: mTOR
inhibitors and their clinical application in cervical, endometrial
and ovarian cancers: A critical review. Gynecol Oncol. 133:375–381.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Y, Padmanabha D, Gentile LB, Dumur CI,
Beckstead RB and Baker KD: HIF- and non-HIF-regulated hypoxic
responses require the estrogen-related receptor in Drosophila
melanogaster. PLoS Genet. 9:e10032302013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fraga A, Ribeiro R and Medeiros R: Tumor
hypoxia: The role of HIF. Actas Urol Esp. 33:941–951. 2009.In
Spanish. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pitroda SP, Wakim BT, Sood RF, Beveridge
MG, Beckett MA, MacDermed DM, Weichselbaum RR and Khodarev NN:
STAT1-dependent expression of energy metabolic pathways links
tumour growth and radioresistance to the Warburg effect. BMC Med.
7:682009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hirschhaeuser F, Sattler UG and
Mueller-Klieser W: Lactate: A metabolic key player in cancer.
Cancer Res. 71:6921–6925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shimura T, Noma N, Sano Y, Ochiai Y,
Oikawa T, Fukumoto M and Kunugita N: AKT-mediated enhanced aerobic
glycolysis causes acquired radioresistance by human tumor cells.
Radiother Oncol. 112:302–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Moeller BJ, Richardson RA and Dewhirst MW:
Hypoxia and radiotherapy: Opportunities for improved outcomes in
cancer treatment. Cancer Metastasis Rev. 26:241–248. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cullis PM, Jones GD, Symons MC and Lea JS:
Electron transfer from protein to DNA in irradiated chromatin.
Nature. 330:773–774. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bhatt AN, Chauhan A, Khanna S, Rai Y,
Singh S, Soni R, Kalra N and Dwarakanath BS: Transient elevation of
glycolysis confers radio-resistance by facilitating DNA repair in
cells. BMC Cancer. 15:3352015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pathania D, Millard M and Neamati N:
Opportunities in discovery and delivery of anticancer drugs
targeting mitochondria and cancer cell metabolism. Adv Drug Deliv
Rev. 61:1250–1275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Deng Q, Yu X, Xiao L, Hu Z, Luo X, Tao Y,
Yang L, Liu X, Chen H, Ding Z, et al: Neoalbaconol induces energy
depletion and multiple cell death in cancer cells by targeting
PDK1-PI3-K/Akt signaling pathway. Cell Death Dis. 4:e8042013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Roden R and Wu TC: How will HPV vaccines
affect cervical cancer? Nat Rev Cancer. 6:753–763. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Doorbar J: Molecular biology of human
papillomavirus infection and cervical cancer. Clin Sci (Lond).
110:525–541. 2006. View Article : Google Scholar
|
|
56
|
Münger K, Basile JR, Duensing S, Eichten
A, Gonzalez SL, Grace M and Zacny VL: Biological activities and
molecular targets of the human papillomavirus E7 oncoprotein.
Oncogene. 20:7888–7898. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rodolico V, Arancio W, Amato MC, Aragona
F, Cappello F, Di Fede O, Pannone G and Campisi G: Hypoxia
inducible factor-1 alpha expression is increased in infected
positive HPV16 DNA oral squamous cell carcinoma and positively
associated with HPV16 E7 oncoprotein. Infect Agent Cancer.
6:182011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bodily JM, Mehta KP and Laimins LA: Human
papillomavirus E7 enhances hypoxia-inducible factor 1-mediated
transcription by inhibiting binding of histone deacetylases. Cancer
Res. 71:1187–1195. 2011. View Article : Google Scholar
|
|
59
|
Nakamura M, Bodily JM, Beglin M, Kyo S,
Inoue M and Laimins LA: Hypoxia-specific stabilization of
HIF-1alpha by human papillomaviruses. Virology. 387:442–448. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Li G, He L, Zhang E, Shi J, Zhang Q, Le
AD, Zhou K and Tang X: Overexpression of human papillomavirus (HPV)
type 16 oncoproteins promotes angiogenesis via enhancing HIF-1α and
VEGF expression in non-small cell lung cancer cells. Cancer Lett.
311:160–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gao JL and Chen YG: Natural compounds
regulate glycolysis in hypoxic tumor microenvironment. BioMed Res
Int. 2015:3541432015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Marín-Hernández A, Gallardo-Pérez JC,
Ralph SJ, Rodríguez-Enríquez S and Moreno-Sánchez R: HIF-1alpha
modulates energy metabolism in cancer cells by inducing
overexpression of specific glycolytic isoforms. Mini Rev Med Chem.
9:1084–1101. 2009. View Article : Google Scholar
|
|
63
|
Wang L, Xiong H, Wu F, Zhang Y, Wang J,
Zhao L, Guo X, Chang LJ, Zhang Y, You MJ, et al: Hexokinase
2-mediated Warburg effect is required for PTEN- and
p53-deficiency-driven prostate cancer growth. Cell Rep.
8:1461–1474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhuo B, Li Y, Li Z, Qin H, Sun Q, Zhang F,
Shen Y, Shi Y and Wang R: PI3K/Akt signaling mediated Hexokinase-2
expression inhibits cell apoptosis and promotes tumor growth in
pediatric osteosarcoma. Biochem Biophys Res Commun. 464:401–406.
2015. View Article : Google Scholar : PubMed/NCBI
|