Introduction
Colorectal cancer (CRC), including colon and rectal
cancer, is the third most commonly diagnosed malignancy and the
fourth leading cause of cancer-related deaths all over the world.
Rapid increases in both CRC incidence and mortality are now
observed with the rapid social and economic changes in Eastern
Europe, Asia and South America (1). Although improved treatment strategies
involving surgery and chemo-and radio-therapy have increased the
overall survival rates in the early stages, 40-50% of all patients
with CRC present with metastasis either at the time of diagnosis or
as recurrent disease upon intended curative therapy (2). Currently, irinotecan (CPT-11) is
mainly used in CRC diagnosed patients with metastases, with
recorded relapse or progression after application of standard 5-FU
based therapy (3,4). However, the response rate to these
regimens is only in the range of ~30-55% and the 5-year survival
rate is less than 10%. Resistance to chemotherapy is a major
limitation to the outcome in CRC (5,6).
Irinotecan, a semi-synthetic analog of camptothecin, is a
broad-spectrum anticancer drug that specifically target DNA
topoisomerase I (Topo I). In humans, irinotecan is metabolized by
an endogenous carboxylesterase-mediated hydrolyzation into a highly
active metabolite, SN-38. The formation of SN-38-Topo I-DNA complex
results in lethal double-strand DNA breakage and cell death
(7,8). Numerous studies have been done to
uncover possible mechanisms for the cellular resistance to this
agent, suggesting the following general aspects: i) variable levels
of the enzymes involved in the conversion of irinotecan; ii)
reduced cellular accumulation from active drug efflux caused by ABC
transporters; iii) changed activity of Topo I that decreases levels
of the SN-38-TopoI-DNA complex; and iv) alterations in the events
downstream from the ternary complex, for example, apoptosis, cell
cycle regulation, checkpoints and DNA repair (3,7,9).
However, research on the mechanism underlying the resistance to
irinotecan is still limited and needs further investigation.
Epithelial-mesenchymal transition (EMT) is a unique
process initially characterized in embryonic development in which
cells lose epithelial features and gain mesenchymal properties. EMT
results in epithelial cells becoming spindle shaped, with loss of
cellular polarity similar to mesenchymal cells. These phenotypic
changes closely correlate with increased cellular motility,
invasion and therapeutic resistance (2,10).
Furthermore, cells with different molecular characteristics within
the same tumor respond differently to anticancer therapeutics,
leading to drug resistance. Cancer cells may also undergo adaptive
changes following therapy, exacerbating drug resistance. In
epithelial cancers, these adaptive changes may involve, at least in
part, EMT and the reverse process (MET) (11). Notably, these properties have also
been ascribed to normal stem cells and cancer stem cells. The
cancer stem cells (CSC) are specific undifferentiated cancer cells,
the tumor-initiating cells, have the ability to self-renew,
propagate and differentiate leading to cancer growth and
progression (12). Likewise, CSCs
display aggressive characteristics including increased invasion,
metastatic ability and resistance to therapy and predict poor
patient prognosis. In experimental models, CSCs are more resistant
than differentiated tumor cells to chemo- and radiotherapy, and
they can escape from the effects of conventional cytotoxic
treatments (13).
Twist is a basic helix-loop-helix transcription
factor, which is one of the master regulators of EMT process,
including Twist1 and Twist2 (14).
Our previous study indicated that Twist1 is overexpressed in colon
cancer tissue, its positive expression is related to histological
grade, TNM stage, recurrence and poor overall survival and is a
significant independent prognostic indicator in CRC patients
(15). Different studies have also
reported that high expression of Twist1 is closely associated with
more aggressive behavior of breast (16), hepatocellular (17), pancreatic (18) and esophageal squamous cell cancer
(19). In addition, Twist is
critical for the maintenance of EMT associated CSC-like
characteristics (20,21). Moreover, Twist1 is also responsible
for paclitaxel resistance in breast cancer cells (16), cisplatin resistance in lung cancer
cells (22) and 5-FU resistance in
colon cancer cells (23). However,
the relationship Twist1, CSC and resistance to irinotecan in colon
cancer is still ambiguous. A better understanding of these
underlying resistance mechanisms is a major concern for the future
development of new Topo I inhibitors and the identification of
biomarkers that could be used to predict tumor response to these
drugs clinically.
Materials and methods
Cell culture and establishment of
irinotecan-resistant LoVo cell subline
Colon cancer LoVo cells were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). The LoVo cells were cultured as monolayers in RPMI-1640
medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco, São Paulo, Brazil) and 1%
penicillin/streptomycin (Beijing Solarbio Science and Technology,
Co. Ltd., China). Cells were grown at 37°C in a humidified
atmosphere containing 5% CO2. Irinotecan-resistant LoVo
cell subline, referred to as LoVo/CPT-11R cells, were established
by gradual adaptation of the original cell line to increasing
irinotecan concentrations over a period of 10 months. The
concentrations increased as follows: 1 µg/ml ➞3 µg/ml
➞5 µg/ml ➞10 µg/ml ➞20 µg/ml ➞30 µg/ml
➞40 µg/ml ➞50 µg/ml ➞60 µg/ml ➞70
µg/ml. Cells were maintained at a particular irinotecan
concentration for ~10 passages or until they displayed, more or
less, standard growth and survival after subculture. Cells were
passaged twice a week if appropriate. Otherwise, the medium was
changed twice a week until the cell culture reached conditions
allowing passaging. Prior to the subsequent experiments, the cells
were maintained in drug-free growth medium for at least 3
weeks.
Reagents and antibodies
Irinotecan was purchased from Jiangsu Hengrui
Medicine, Co., Ltd. (Jiangsu, China), 5-fluorouracil, and cisplatin
were purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved
in phosphate-buffered saline (PBS), curcumin (Sigma-Aldrich) was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich), aliquoted
and stored at 4°C according to the manufacturer's instructions. EMT
antibody sampler kit was purchased from Cell Signaling Technology
(Danvers, MA, USA). Mouse monoclonal antibody against Twist1 was
purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Rabbit polyclonal antibodies against respectively β-actin, MMP2,
MMP9, CD44 and CD133 were purchased from Proteintech Group, Inc.
(Rosemont, IL, USA). Rabbit polyclonal antibody against P-gp and
the secondary antibodies, goat anti-rabbit IgG or goat anti-mouse
IgG, were purchased from Abcam (Cambridge, MA, USA).
Cell viability assay
Cell viability assay was performed using the Cell
Counting kit-8 assay (CCK-8; Dojindo Laboratories, Kumamoto,
Japan). Briefly, cells (8×103/well) were seeded onto a
96-well plate in 100 µl RPMI-1640 medium supplemented with
10% FBS at 37°C in 5% CO2. After 24 h, drugs diluted
with the culture medium were added to each well. The concentrations
of the drug (irinotecan, 5-fluorouracil, cisplatin or curcumin)
were 5, 10, 20 and 40 µg/ml, respectively. Following drug
treatment for 24 h, the culture medium in the 96-wells was replaced
with fresh medium containing 10% CCK-8 reagent, after 2 h, the
absorbance at 450 nm was measured and the values were corrected by
subtracting the absorbance of blank wells that did not contain
cells.
Cellular morphology and
immunofluorescence assay
Cells grown on coverslips were washed three times
with PBS. They were fixed for 10 min with cold paraformaldehyde and
washed 3 times with PBS. The coverslips were then blocked in a
solution of PBS, 10% bovine serum, and 0.1% Tween-20 for 1 h. The
cells were incubated with primary antibody (E-cadherin, 1:100;
vimentin, 1:100; CD44, 1:100) for 1 h, then washed twice with PBS,
and incubated with secondary antibodies diluted in PBS (Alexa Fluor
594 (red/green)-conjugated anti-rabbit IgG antibody (Santa Cruz
Biotechnology) diluted 1:100 in blocking buffer). After incubating
on coverslips for 1 h at 37°C the coverslips were washed as
described above and mounted on slides in medium containing DAPI.
Cells were observed and images were captured through an
epifluorescence microscope (Olympus BX51; Olympus, Tokyo,
Japan).
Lentiviruses transfection assay
Lentiviruses overexpressing human Twist1 and empty
vector were built by Shanghai Genechem, Co., Ltd. (Shanghai,
China). For lentiviral trans-fection of LoVo cells, 100
multiplicity of infection Twist1 or empty vector lentiviruses were
added to a well containing 5×104 cells, medium and 8
µg/ml polybrene. After 24 h of incubation, transfected cells
were selected with 2 µg/ml puromycin (Sigma-Aldrich). Empty
vector lentivirus was used as a control. Selected cells were
maintained in growth medium with 0.5 µg/ml puromycin.
Inhibition of Twist1 expression by
RNAi
Twist1-targeted small interfering RNA (si-Twist1)
(target DNA sequence is 5'-GGUACAUCGACUUCCUCUATT-3') and no-target
control siRNA (NC) were purchased from Guangzhou RiboBio, Co., Ltd.
(Guangzhou, China). Cells (2×105) were seeded in 6-well
plates in triplicate, after overnight incubation, the cells were
transfected with NC or si-Twist1. The siRNA (50 nmol) and
Lipofectamine 3000 (Invitrogen, Waltham, MA, USA) were mixed in 100
µl of minimal essential medium (Opti-MEM; Gibco) for 15 min,
and the siRNA/Lipofectamine 3000 mixture was added into the
serum-free culture medium. Six hours later, the medium was replaced
with complete medium, and then the cells were cultured for an
additional 48 h prior to the detection of Twist1 expression
knockdown by RT-PCR.
Migration and invasion assay
Cell migration and invasion were assessed with
Boyden chambers or modified Boyden chambers according to the
protocol of the manufacturer (Becton-Dickinson Labware, Bedford,
MA, USA). Briefly, 100 µl RPMI-1640 medium without FBS
included 100,000 cells was placed on an 8.0-µm pore size
membrane insert in 24-well plates, and 600 µl RPMI-1640
medium with 10% FBS was placed in the bottom wells. After 24 h at
37°C, cells that did not migrate were removed from the top side of
the inserts with a cotton swab. After fixed with 4%
paraformaldehyde for 10 min, cells that had migrated to the
underside of the inserts were stained with 0.1% crystal violet
solution and the cells on each insert were counted using a
microscope (Olympus BX51; Olympus). Cells were counted in five
random fields per insert. The invasion assay was done in a similar
fashion except the 8.0-µm pore size membrane inserts were
coated with Matrigel. Results were expressed as cells migrated per
field.
Quantitative real-time PCR assay
Total mRNA of the cells was extracted using the
Total RNA kit (TransGen Biotech, Inc., Beijing, China). First step
cDNA synthesis was generated from 500 ng total mRNA. RT-PCR
amplification was performed in triplicate on
LightCycler® 480 II (Roche, Berlin, Germany). The
primers used in each reaction were synthesized in Sangon Biotech,
Co., Ltd. (Shanghai, China). Expression levels for each target gene
were normalized to β-actin gene. Results were calculated using the
comparative threshold cycle (ΔΔCT) method. Data are presented as
the mean ± standard deviation (SD) from three independent
experiments.
Western blotting assay
Cells were harvested with a plastic scraper and
washed twice with cold PBS. Cells were then lysed with ice-cold
protein extract solution RIPA (Beyotime Institute of Biotechnology,
Shanghai, China) and protein concentration was quantified using the
BCA procedure (Beyotime Institute of Biotechnology). Equal amounts
(30 µg) of protein samples were separated by SDS-PAGE using
12% or 10% polyacrylamide gel and then transferred onto a PVDF
membrane (Millipore, Billerica, MA, USA). The membrane was blocked
with 5% skim milk TBST (Tris-buffered saline Tween-20) buffer for 1
h at room temperature. The membranes were incubated with primary
antibodies including EMT antibody sampler kit (1:1,000),
anti-Twist1, anti-MMP1, anti-MMP9, anti-CD44, anti-CD133 (1:500);
anti-P-gp, anti-β-actin (1:3,000) at 4°C overnight. After washed
with TBST, the blot was incubated with the appropriate horseradish
peroxidase-conjugated anti-rabbit or anti-mouse secondary
antibodies (1:5,000). The antigen was detected by WesternBright ECL
HRP substrate kit (Millipore) in gel image analysis system (Kodak,
Rochester, NY, USA). The intensities of the protein bands were
analyzed by Molecular Imaging software. β-actin protein was used as
the internal control.
Statistical analysis
Quantitative data are expressed as the mean ± SD of
three independent experiments. Comparisons among multiple groups
were performed using one-way analysis of variance (ANOVA) test
followed by Dunnett's test. P-values are two-sided, and a value of
0.05 was considered to be statistically significant. All
statistical calculations were performed using SPSS software
(version 19.0; SPSS, Inc., Chicago, IL, USA). Graphs were prepared
using GraphPad Prism 5.
Results
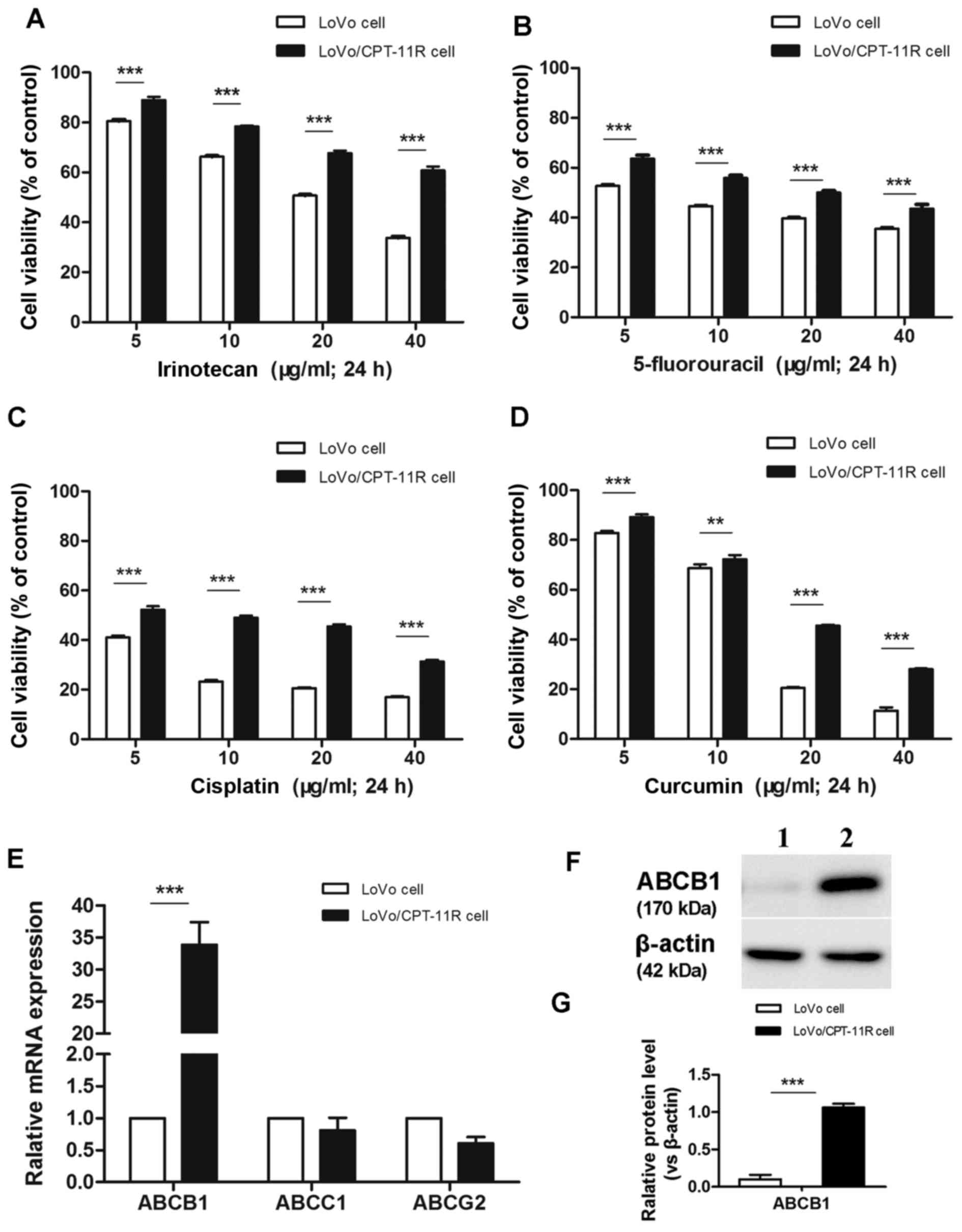
Cross-resistance to anticancer drugs and
increased expression of ABCB1 in irinotecan-resistant cells
Via long-term culturing of original
irinotecan-sensitive LoVo cells by gradual adaptation of increasing
irinotecan concentrations, we successfully established
irinotecan-resistant variant LoVo/CPT-11R cells. The resistant
cells were capable of long-term survival and proliferation in media
containing 70 µg/ml irinotecan. In order to investigate
whether the LoVo/CPT-11R cells acquired cross-resistance to other
anticancer drugs, their sensitivities to various anticancer drugs
used to treat CRC, including 5-fluorouracil, cisplatin and
curcumin, were determined using CCK-8 assay. The results showed
that cell viabilities of LoVo/CPT-11R cells treated with anticancer
drugs (irinotecan, 5-fluorouracil, cisplatin and curcumin) were
markedly increased compared with the control LoVo cells (Fig. 1A–D), herein the LoVo/CPT-11R cells
acquired multidrug resistance property.
Additionally, we compared expression of the most
known ABC transporter genes (ABCB1, ABCC1 and ABCG2) in mRNA level
in LoVo and LoVo/CPT-11R cells. Results indicated that mRNA
expression of ABCB1 was significantly increased in LoVo/CPT-11R
cells, but the changes of expression of ABCC1 and ABCG2 were not
significant (Fig. 1E). In
addition, the expression of ABCB1 protein (P-gp) was detected by
western blotting assay. Likewise, the ABCB1 was significantly
overexpressed on protein level in LoVo/CPT-11R cells (Fig. 1F and G).
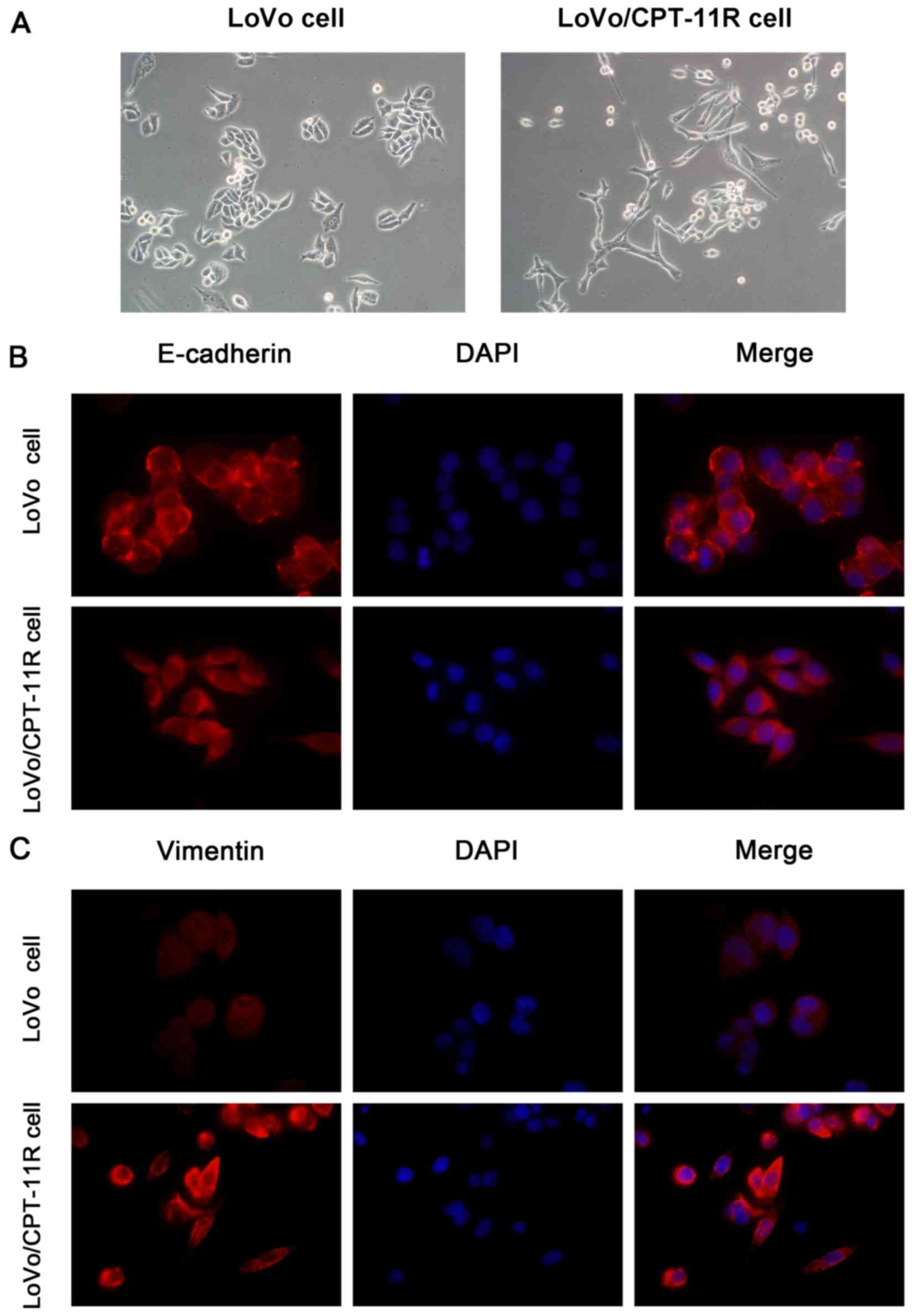
EMT-like morphology and altered
localization of EMT markers in irinotecan-resistant cells
Light microscopy revealed a marked alteration of
cellular morphology in monolayer culture. LoVo/CPT-11R cells
exhibited spindle shape, loose intercellular space, and irregular
scattering, suggested a more 'mesenchymal' phenotype than parental
LoVo cells (Fig. 2A). To further
confirm our observation, the EMT marker proteins were stained by
immunofluorescence assay. It was found that epithelial marker
E-cadherin was located mainly in cell membrane of LoVo cells, but
E-cadherin protein was located mainly in the cytoplasm of
LoVo/CPT-11R cells (Fig. 2B).
Moreover, compared to LoVo cells, the mesenchymal marker Vimentin
protein was more distinctly stained in cytoskeletal localization in
LoVo/CPT-11R cells (Fig. 2C).
Irinotecan-resistant cells overexpress
EMT markers, Twist1 and CSC markers
The observed morphological changes implied that the
irinotecan-resistant cells had transitioned to a mesenchymal
phenotype. To determine whether these morphological changes were
associated with EMT, we examined respectively the expression of EMT
markers in mRNA and protein levels. Consistent with morphological
changes, LoVo/CPT-11R cells showed downregulation of the epithelial
markers E-cadherin and ZO1, and upregulation of the mesenchymal
markers vimentin and N-cadherin (Fig.
3A–C). These results suggested that EMT could be associated
with acquired drug resistance after long-term exposure to
irinotecan. In addition, EMT-inducing transcription factors were
also detected by the above methods. The expression of Twist1 was
higher in LoVo/CPT-11R cells than LoVo cells, the changes of expres
sion of Snail, Slug and Zeb1 were not statistically significant
(Fig. 3D–F).
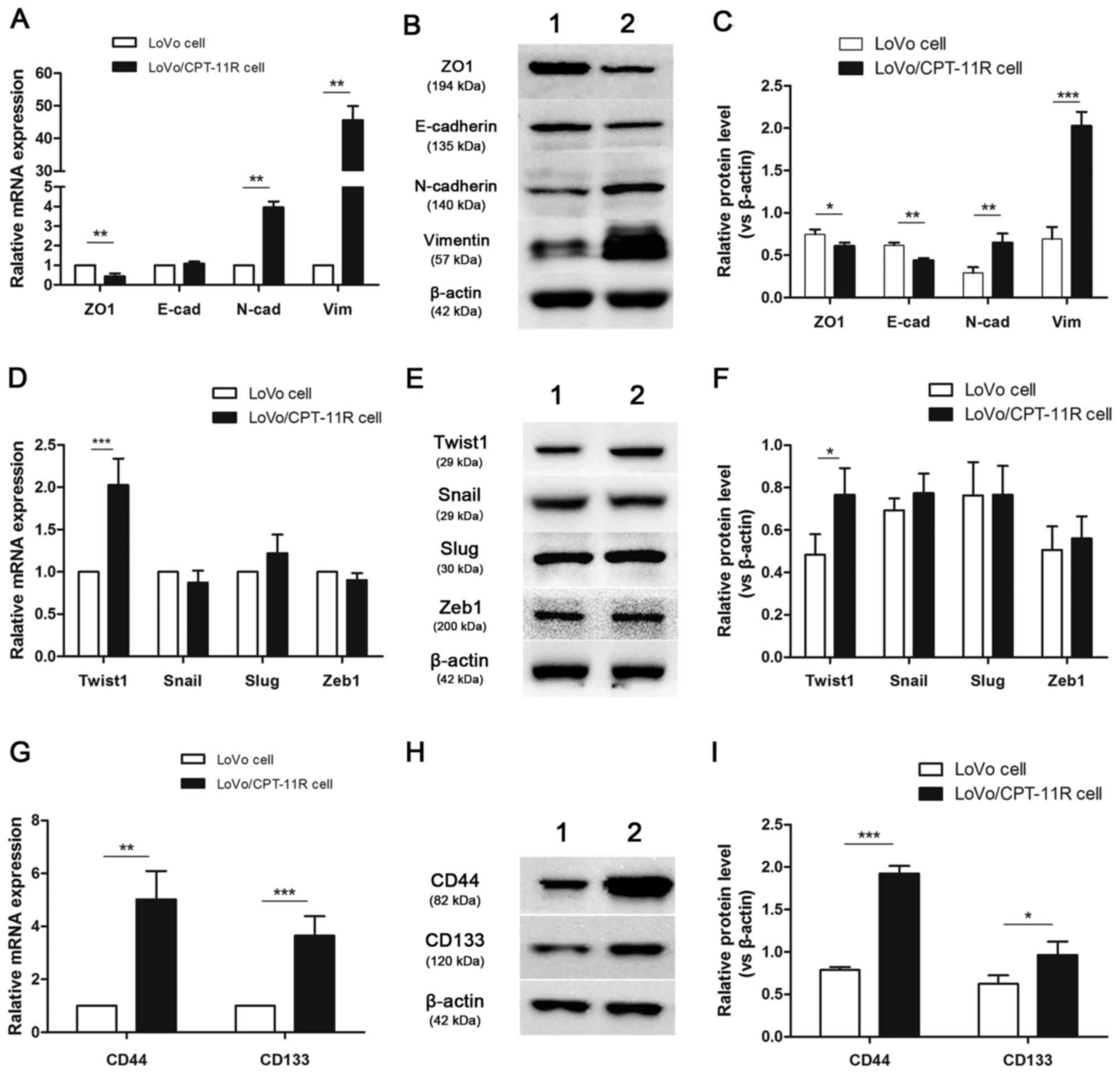 | Figure 3Expression of EMT markers,
EMT-inducing transcription factors and CSC markers. The mRNA
expression levels of EMT markers (A), EMT-inducing transcription
factors (D) and CSC markers (G) were analyzed by qRT-PCR assay in
LoVo cells and LoVo/CPT-11R cells. Protein expression and relative
protein expression levels of EMT markers (B and C), EMT-inducing
transcription factors (E and F) and CSC markers (H and I) were
detected by western blotting assay and measured by Image J. β-actin
was used as a loading control. Values present the mean ± SD of
three independent experiments. *P<0.05, **P<0.01,
***P<0.001 vs. LoVo cells. Lane 1, LoVo cells; lane
2, LoVo/CPT-11R cells. E-cad, E-cadherin; N-cad, N-cadherin; Vim,
vimentin. |
The relation of aggressive cell characteristics and
CSC continue to be under intense investigation. Therefore, we
detected respectively the expression of CSC identification markers.
It was founded that the expression of CD44 and CD133, most widely
studied CSC biomarkers, were increased in mRNA and protein levels
in LoVo/CPT-11R cells compared to LoVo cells (Fig. 3G–I).
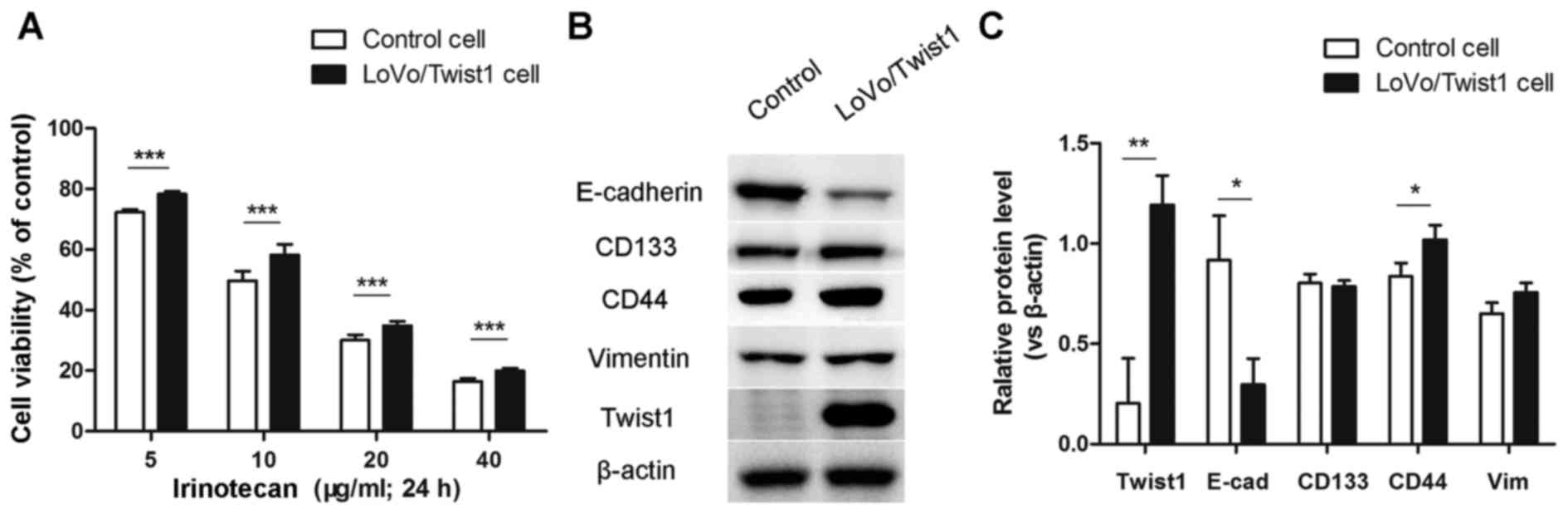
Overexpression of Twist1 contributes to
irinotecan resistance, EMT and CSC-like phenotype of LoVo
cells
Considering the expression of Twist1 was
significantly increased in LoVo/CPT-11R cells, we built
overexpressed Twist1 LoVo cells by lentivirus transfection assay,
to confirm whether overexpression of Twist1 contributed to
resistance to irinotecan, EMT and CSC-like phenotype of LoVo cells.
The control LoVo cells (transfected with empty vector lentiviruses)
and LoVo/Twist1 cells (transfected with overexpressed Twist1
lentiviruses) were treated with different concentrations of
irinotecan and CCK-8 assays were performed. The results showed that
overexpression of Twist1 led to a distinct increase in cell
viability compared with control cells (Fig. 4A). Moreover, we detected
respectively the expression of EMT and CSC markers in protein level
by western blotting assay. The results showed that the expression
of E-cadherin was decreased and that the expression of CD44 was
increased (Fig. 4B and C).
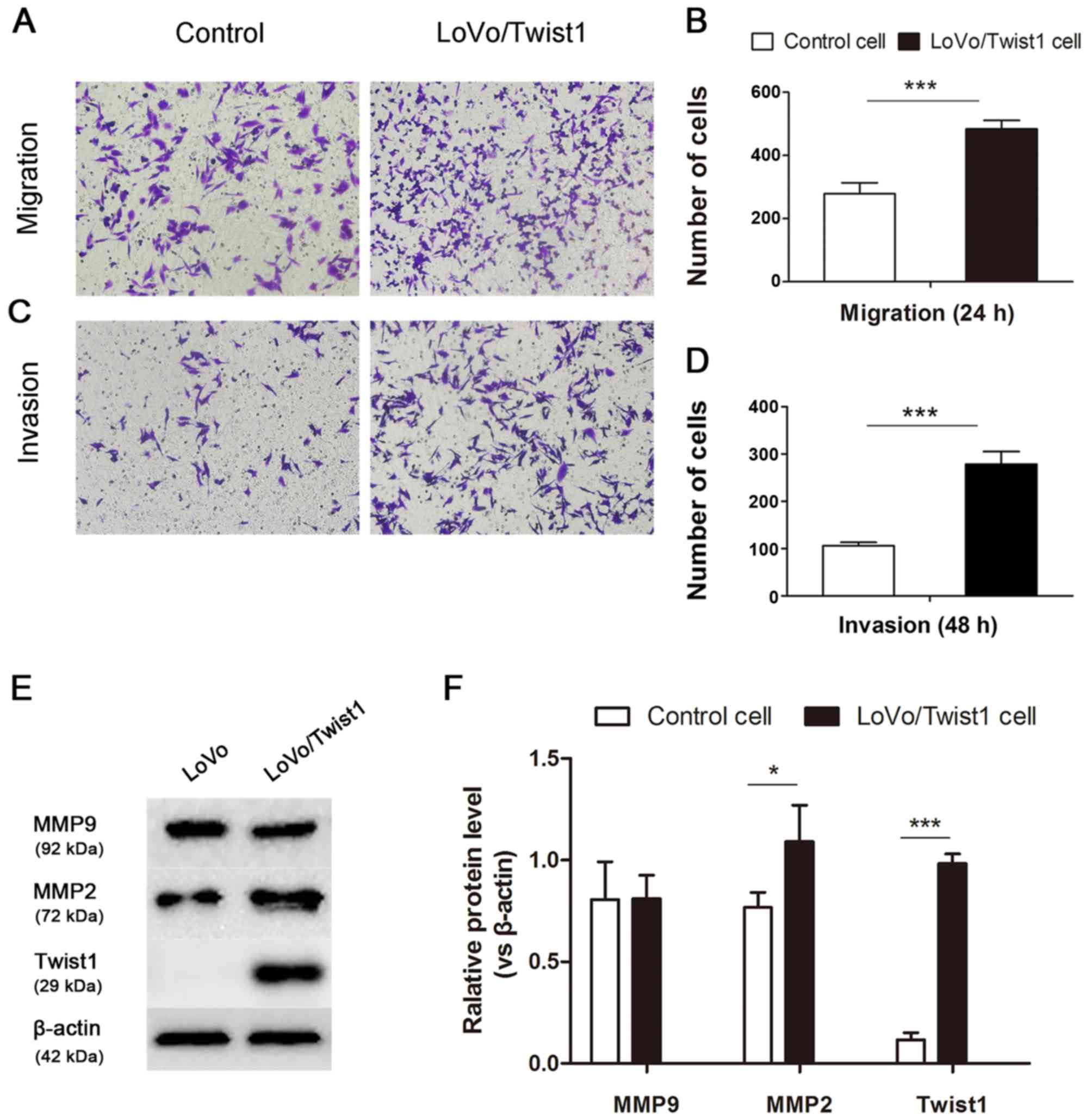
Overexpression of Twist1 enhances
migration and invasion potential by regulating MMP2 expression
To assess the migratory and invasive potentials of
overexpressed Twist1 LoVo cells, in vitro cell migration and
invasion assay were performed. Compared with the controls,
LoVo/Twist1 cells demonstrated a 1.74-fold increase at 24 h in
migration (Fig. 5A and B), and a
2.63-fold increase at 48 h in invasion (Fig. 5C and D). Metalloproteinases (MMPs)
are crucial to invasion and migration, a significant association
has been reported between tumor aggressiveness and increased levels
of MMP2 and MMP9 in many experimental and clinical studies. Our
results detected that the expression of MMP2 was significantly
increased in the protein level, but the MMP9 was not significantly
changed in LoVo/Twist1 cells compared to control cells.
Downregulation of Twist1 increases the
irinotecan sensitivity, and reverses EMT and CSC-like phenotype of
LoVo/CPT-11R cells
To furtherly discover the molecular link between
irinotecan resistance and Twist1, we inhibited the expression of
Twist1 in LoVo cells and LoVo/CPT-11R cells by RNAi. After
transfected respectively with NC and si-Twist1 in LoVo cells and
LoVo/CPT-11R cells, cells were treated with different
concentrations of irinotecan (0, 12.5, 25, 50 and 100 µg/ml)
for 24 h, then absorbance of each group at 450 nm was detected by
CCK-8 assays. The results showed that the inhibition of Twist1
expression led to a distinct decrease in absorbance (Fig. 6A). It indicated that the
downregulation of Twist1 could increase the irinotecan sensitivity
in LoVo cells and LoVo/CPT-11R cells.
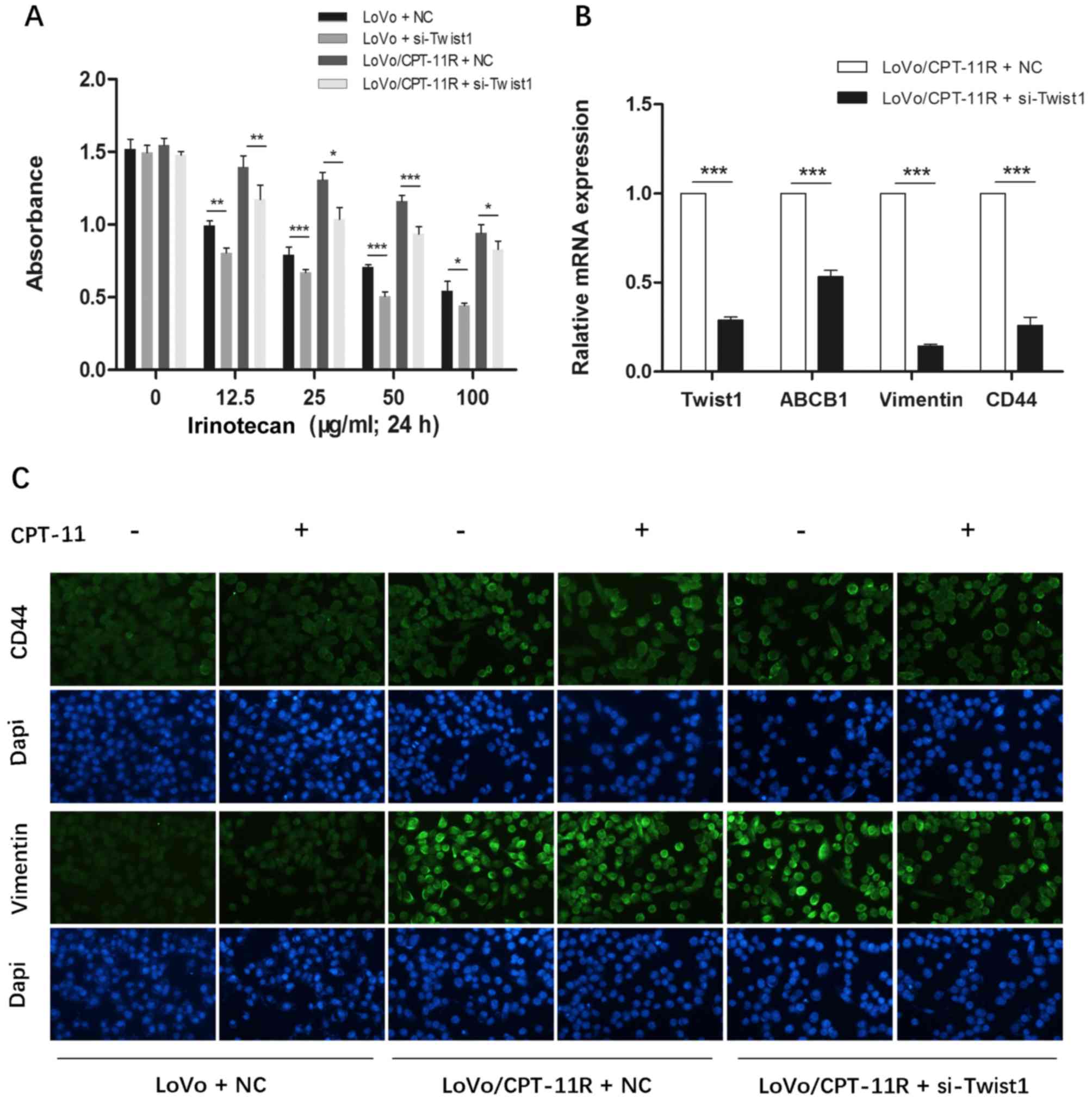 | Figure 6Downregulation of Twist1 increases
the sensitivity to irinotecan and reverses EMT and CSC-like
phenotype of the LoVo/CPT-11R cells. (A) After transfected
respectively with NC and Twist1-targeted siRNA in LoVo cells and
LoVo/CPT-11R cells, cells were treated with different
concentrations of irinotecan (0, 12.5, 25, 50 and 100 µg/ml)
for 24 h, then every group absorbance at 450 nm was detected by
CCK-8 assays. (B) The mRNA expression of Twist1, ABCB1, vimentin
and CD44 were analyzed by qRT-PCR assay. (C) The protein expression
of vimentin and CD44 were detected by immunocytochemistry assay.
Cells were stained with Dapi (blue) and second antibody (green).
(Original magnification, ×200). Each bar represents the mean ± SD
of the three independent experiments. *P<0.05,
**P<0.01, ***P<0.001. NC, cells
transfected with N Control; si-Twist1, cells transfected with
Twist1-targeted siRNA. |
Moreover, we detected respectively the expression of
EMT and CSC-like markers by RT-PCR and immunofluorescence assays.
The results showed that the inhibition of Twist1 could downregulate
the mRNA expression of vimentin, CD44 and P-gp (Fig. 6B), and distinctly decreased the
protein expression of vimentin and CD44 of LoVo/CPT-11R cells when
adding irinotecan (Fig. 6C). It
indicated that the downregulation of Twist1 could reverse EMT and
CSC-like phenotype of LoVo/CPT-11R cells.
Discussion
We established irinotecan-resistant subline of human
colon cancer LoVo cells by stepwise adaptation to increasing drug
concentrations. In addition, LoVo/CTP-11R cells also displayed
cross-resistance to non-camptothecin anticancer drugs
(5-fluorouracil, cisplatin and curcumin), indicated the presence of
the multidrug resistant (MDR) phenotype. Numerous published studies
have showed that ATP-binding cassette (ABC) transporters play a
crucial role in the development of multidrug resistance by the
efflux of anticancer agents outside the cancer cells (24). The most extensively characterized
MDR transporters included ABCB1 (also known as MDR1 or
P-glycoprotein), ABCC1 (also known as MRP1) and ABCG2 (also known
as BCRP or MXR) (25). When
compared with the genes expression in LoVo cells, we found only the
ABCB1 was significantly higher in LoVo/CPT-11R cells, and the
expression of P-gp was also increased. Indeed, ABCB1 encoded the
xenobiotic transporter P-gp that has been extensively investigated
in vitro and in vivo as a predictor of MDR in various
tumors (26,27). The expression of ABCB1 is regulated
at different levels by multiple signaling pathways, including
hypoxia-inducible factor-1α (HIF-1α), p53, chromosomal
rearrangement, methylation, acetylation and microRNA (28). Recently, a potential
transcriptional regulatory role of Twist1 has been identified in
chemotherapy drug resistance (29). In the present study, the positively
correlated downregulation of ABCB1 and Twist1 in LoVo/CPT-11R cells
was detected, which also indicate a novel role of Twist1 in
maintaining the irinotecan-resistant phenotype of colon cancer
through regulating ABCB1 expression.
EMT program is now known to facilitate the
metastatic spread and progression of cancer cells from the site of
the primary tumor to the surrounding tissues and distant organ(s).
It is also generally considered that EMT is associated with cancer
aggressiveness, invasive and metastatic behavior, and
chemotherapeutic resistance (30).
However, comprehensive studies of EMT and irinotecan resistance in
colon cancer are lacking. In the present study, we found that
LoVo/CPT-11R cells exhibited a more mesenchymal phenotype and
location alteration of epithelial marker E-cadherin from cell
membrane to cytoplasm than parental LoVo cells. To confirm the role
of EMT and resistance to irinotecan, we examined the expression of
EMT markers in mRNA and protein levels, respectively. As expected,
LoVo/CPT-11R cells showed downregulation of E-cadherin and ZO1 and
upregulation of vimentin and N-cadherin. In addition, the
expression levels of EMT-inducing transcription factors (Twist1,
Snail, Slug and Zeb1) were detected. Notably, results showed that
only Twist1 was significantly higher in LoVo/CPT-11R cells than
LoVo cells. Increasing evidence suggested that EMT not only enables
cancer cells to disseminate but also to acquire the ability to
self-renew by inducing a CSC trait (31-33).
In experimental models, CSCs are more resistant than differentiated
tumor cells to chemo- and radiotherapy and they can escape from the
effects of conventional cytotoxic treatments (34). It has been reported that CD133 and
CD44 are two proposed stem cell markers in various metastasized
cancers including colorectal cancer (35-37).
Therefore, we detected respectively the expression of CSC
identification markers. The expression of CD44 and CD133 were
increased both in mRNA and protein levels in LoVo/CPT-11R cells.
Therefore, it was identified that the irinotecan resistance cells
presented EMT-like and CSC-like phenotype. In order to confirm
whether Twist1 contributes to formation of resistance to irinotecan
and alteration of EMT, CSC-like phenotype in colon cancer cells, we
established overexpressed Twist1 LoVo cells, named LoVo/Twist1
cells, by lentivirus transfection assay. After treated with
different concentrations of irinotecan, we found that the
overexpression of Twist1 led to a distinct resistance to irinotecan
compared with control cells transfected with empty vector.
Moreover, the decreased expression of E-cadherin and the increased
expression of CD44 demonstrated the formation of EMT, CSC-like
phenotype in LoVo/Twist1 cells. In contrast, we found that
downregulation of Twist1 increased the irinotecan sensitivity,
reversed EMT and CSC-like phenotype of LoVo/CPT-11R cells
transfected with NC and si-Twist1. Therefore, it was identified
that EMT and CSC-like phenotype induced by Twist1 contribute to
acquire resistance to irinotecan.
In addition, compared with the controls, LoVo/Twist1
cells performed a significant enhancement both in migration and
invasion. It is known that MMPs are a group of matrix-degrading
proteins implicated in several pathological processes, including
invasion and metastasis of CRC (38). Increased MMP2 and MMP9 expression
have also been documented to correlate with cancer invasion
(39). Consistent with previous
reports, MMP2 protein was increased in LoVo/Twist1 cells, but MMP9
was not significantly changed compared to control cells, which
indicated that overexpressed Twist1 contribute to the migratory and
invasive potentials of colon cancer by upregulated MMP2 expression.
In summary, the development of a highly aggressive colon cancer
phenotype requires the coordination of many different molecular
changes, which are a consequence of genomic alterations. We have
demonstrated that EMT and CSC-like phenotype induced by Twist1
contribute to acquiring resistance to irinotecan, and high
expression of Twist1 enhanced migration and invasion potential. It
suggests that Twist1 may be a potential molecular target for
overcoming the irinotecan resistance and metastasis in colon
cancer. However, it would make the resistance mechanisms associated
with Twist1 more fruitful if gene expression profiling of
LoVo/CPT-11R cells was added to the analysis.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
CSC
|
cancer stem cell
|
|
CD
|
cluster of differentiation
|
|
MMP
|
metalloprotemase
|
Acknowledgments
The present study was supported by the China
Postdoctoral Science Foundation Grant to G.W. (grant no.
2015M582358) and the Project of Traditional Chinese Medicine Bureau
of Guang Dong Province to X.C. (grant no. 20141307).
References
|
1
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar
|
|
2
|
Cao H, Xu E, Liu H, Wan L and Lai M:
Epithelial-mesenchymal transition in colorectal cancer metastasis:
A system review. Pathol Res Pract. 211:557–569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Panczyk M: Pharmacogenetics research on
chemotherapy resistance in colorectal cancer over the last 20
years. World J Gastroenterol. 20:9775–9827. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanz-Garcia E, Grasselli J, Argiles G,
Elez ME and Tabernero J: Current and advancing treatments for
metastatic colorectal cancer. Expert Opin Biol Ther. 16:93–110.
2016. View Article : Google Scholar
|
|
5
|
Sievers CK, Kratz JD, Zurbriggen LD,
LoConte NK, Lubner SJ, Uboha N, Mulkerin D, Matkowskyj KA and
Deming DA: The multidisciplinary management of colorectal cancer:
Present and future paradigms. Clin Colon Rectal Surg. 29:232–238.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramesh M, Ahlawat P and Srinivas NR:
Irinotecan and its active metabolite, SN-38: Review of
bioanalytical methods and recent update from clinical pharmacology
perspectives. Biomed Chromatogr. 24:104–123. 2010. View Article : Google Scholar
|
|
8
|
Xu Y and Villalona-Calero MA: Irinotecan:
Mechanisms of tumor resistance and novel strategies for modulating
its activity. Ann Oncol. 13:1841–1851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu T, Li Z, Gao CY and Cho CH: Mechanisms
of drug resistance in colon cancer and its therapeutic strategies.
World J Gastroenterol. 22:6876–6889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zubeldia IG, Bleau AM, Redrado M, Serrano
D, Agliano A, Gil-Puig C, Vidal-Vanaclocha F, Lecanda J and Calvo
A: Epithelial to mesenchymal transition and cancer stem cell
phenotypes leading to liver metastasis are abrogated by the novel
TGFβ1-targeting peptides P17 and P144. Exp Cell Res. 319:12–22.
2013. View Article : Google Scholar
|
|
11
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitra A, Mishra L and Li S: EMT, CTCs and
CSCs in tumor relapse and drug-resistance. Oncotarget.
6:10697–10711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khan MA, Chen HC, Zhang D and Fu J: Twist:
A molecular target in cancer therapeutics. Tumour Biol.
34:2497–2506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu DJ, Chen XW, Zhang WJ, Wang JZ, Ouyang
MZ, Zhong Q and Liu CC: Twist1 is a potential prognostic marker for
colorectal cancer and associated with chemoresistance. Am J Cancer
Res. 5:2000–2011. 2015.PubMed/NCBI
|
|
16
|
Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD
and Wang LH: Twist transcriptionally up-regulates AKT2 in breast
cancer cells leading to increased migration, invasion, and
resistance to paclitaxel. Cancer Res. 67:1979–1987. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Niu RF, Zhang L, Xi GM, Wei XY, Yang Y,
Shi YR and Hao XS: Up-regulation of Twist induces angiogenesis and
correlates with metastasis in hepatocellular carcinoma. J Exp Clin
Cancer Res. 26:385–394. 2007.PubMed/NCBI
|
|
18
|
Ji H, Lu HW, Li YM, Lu L, Wang JL, Zhang
YF and Shang H: Twist promotes invasion and cisplatin resistance in
pancreatic cancer cells through growth differentiation factor 15.
Mol Med Rep. 12:3841–3848. 2015.PubMed/NCBI
|
|
19
|
Lee KW, Kim JH, Han S, Sung CO, Do IG, Ko
YH, Um SH and Kim SH: Twist1 is an independent prognostic factor of
esophageal squamous cell carcinoma and associated with its
epithelial-mesenchymal transition. Ann Surg Oncol. 19:326–335.
2012. View Article : Google Scholar
|
|
20
|
Chanmee T, Ontong P, Mochizuki N,
Kongtawelert P, Konno K and Itano N: Excessive hyaluronan
production promotes acquisition of cancer stem cell signatures
through the coordinated regulation of Twist and the transforming
growth factor β (TGF-β)-Snail signaling axis. J Biol Chem.
289:26038–26056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer. 11:492011.
View Article : Google Scholar
|
|
22
|
Zhuo WL, Wang Y, Zhuo XL, Zhang YS and
Chen ZT: Short interfering RNA directed against TWIST, a novel zinc
finger transcription factor, increases A549 cell sensitivity to
cisplatin via MAPK/mitochondrial pathway. Biochem Biophys Res
Commun. 369:1098–1102. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakowicz-Burkiewicz M, Przybyla T,
Wesserling M, Bielarczyk H, Maciejewska I and Pawelczyk T:
Suppression of TWIST1 enhances the sensitivity of colon cancer
cells to 5-fluo-rouracil. Int J Biochem Cell Biol. 78:268–278.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stavrovskaya AA and Stromskaya TP:
Transport proteins of the ABC family and multidrug resistance of
tumor cells. Biochemistry (Mosc). 73:592–604. 2008. View Article : Google Scholar
|
|
25
|
Fletcher JI, Haber M, Henderson MJ and
Norris MD: ABC transporters in cancer: More than just drug efflux
pumps. Nat Rev Cancer. 10:147–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Johnatty SE, Beesley J, Paul J, Fereday S,
Spurdle AB, Webb PM, Byth K, Marsh S, McLeod H, Harnett PR, et al
AOCS Study Group: ABCB1 (MDR 1) polymorphisms and progression-free
survival among women with ovarian cancer following
paclitaxel/carboplatin chemotherapy. Clin Cancer Res. 14:5594–5601.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu D, Lu Q and Hu X: Down-regulation of
P-glycoprotein expression in MDR breast cancer cell MCF-7/ADR by
honokiol. Cancer Lett. 243:274–280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu K, Chen L, Han X and Wang J and Wang
J: Short hairpin RNA targeting Twist1 suppresses cell proliferation
and improves chemosensitivity to cisplatin in HeLa human cervical
cancer cells. Oncol Rep. 27:1027–1034. 2012.PubMed/NCBI
|
|
29
|
Lu S, Yu L, Mu Y, Ma J, Tian J, Xu W and
Wang H: Role and mechanism of Twist1 in modulating the
chemosensitivity of FaDu cells. Mol Med Rep. 10:53–60.
2014.PubMed/NCBI
|
|
30
|
Shen W, Pang H, Liu J, Zhou J, Zhang F and
Liu L, Ma N, Zhang N, Zhang H and Liu L: Epithelial-mesenchymal
transition contributes to docetaxel resistance in human non-small
cell lung cancer. Oncol Res. 22:47–55. 2014. View Article : Google Scholar
|
|
31
|
Biddle A and Mackenzie IC: Cancer stem
cells and EMT in carcinoma. Cancer Metastasis Rev. 31:285–293.
2012. View Article : Google Scholar
|
|
32
|
Ishiwata T: Cancer stem cells and
epithelial-mesenchymal transition: Novel therapeutic targets for
cancer. Pathol Int. 66:601–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liang L, Sun H, Zhang W, Zhang M, Yang X,
Kuang R and Zheng H: Meta-analysis of EMT datasets reveals
different types of EMT. PLoS One. 11:e01568392016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carnero A, Garcia-Mayea Y, Mir C, Lorente
J, Rubio IT and LLeonart ME: The cancer stem-cell signaling network
and resistance to therapy. Cancer Treat Rev. 49:25–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J and
Li J: Cancer stem/progenitor cells are highly enriched in
CD133+CD44+ population in hepatocellular
carcinoma. Int J Cancer. 126:2067–2078. 2010. View Article : Google Scholar
|
|
36
|
Sahlberg SH, Spiegelberg D, Glimelius B,
Stenerlöw B and Nestor M: Evaluation of cancer stem cell markers
CD133, CD44, CD24: Association with AKT isoforms and radiation
resistance in colon cancer cells. PLoS One. 9:e946212014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Horst D, Kriegl L, Engel J, Kirchner T and
Jung A: Prognostic significance of the cancer stem cell markers
CD133, CD44, and CD166 in colorectal cancer. Cancer Invest.
27:844–850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elander N, Söderkvist P and Fransén K:
Matrix metalloproteinase (MMP) -1, -2, -3 and -9 promoter
polymorphisms in colorectal cancer. Anticancer Res. 26(1B):
791–795. 2006.PubMed/NCBI
|
|
39
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Patterns of MMP-2 and MMP-9 expression in
human cancer cell lines. Oncol Rep. 21:1323–1333. 2009.PubMed/NCBI
|